Non-Thermal Plasma Pyrolysis of Fuel Oil in the Liquid Phase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Raw Material
2.2. Experimental Setup
2.3. Sample Analysis and Characterization
3. Results and Discussion
3.1. Characteristics of NTP Pyrolysis and Analysis of Gaseous Products
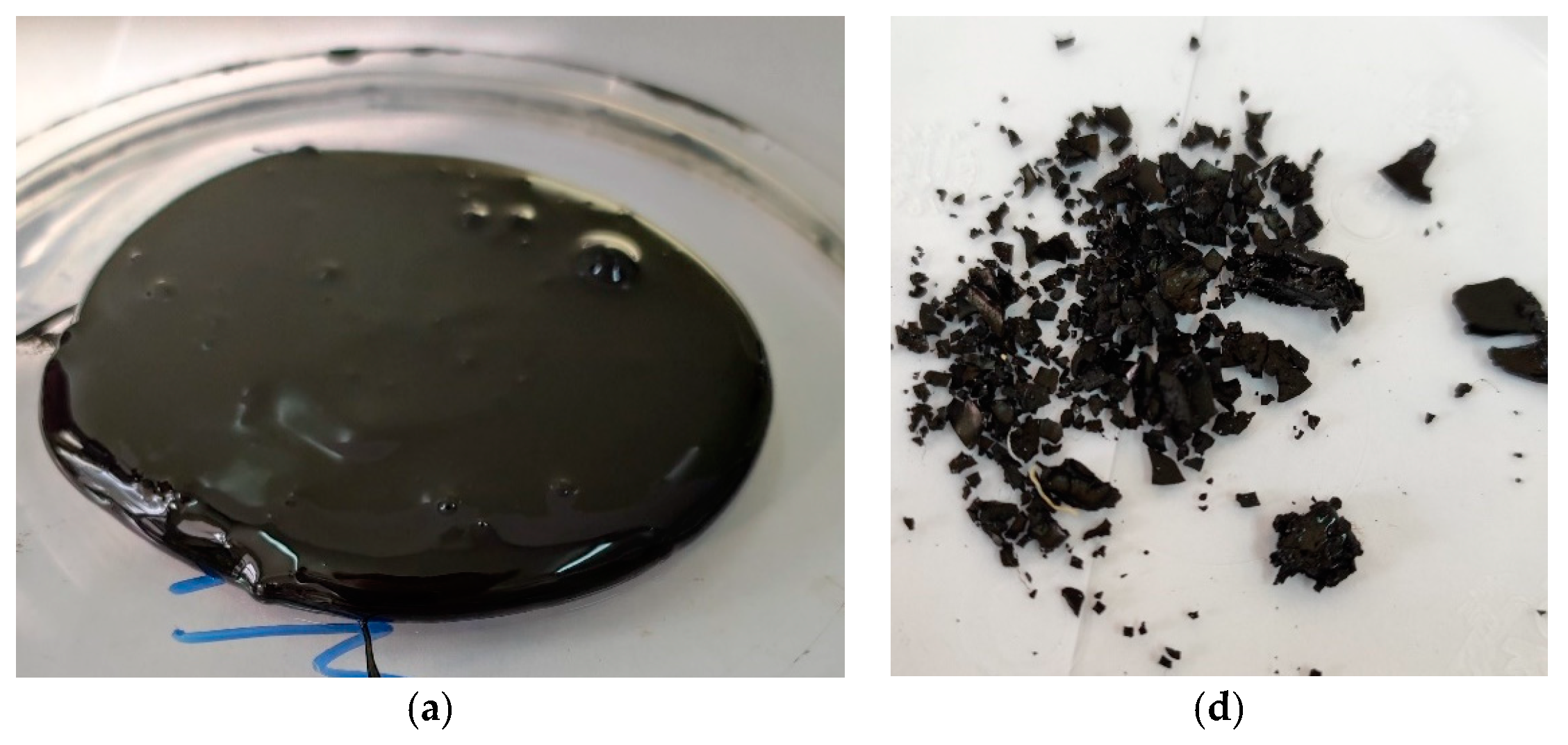
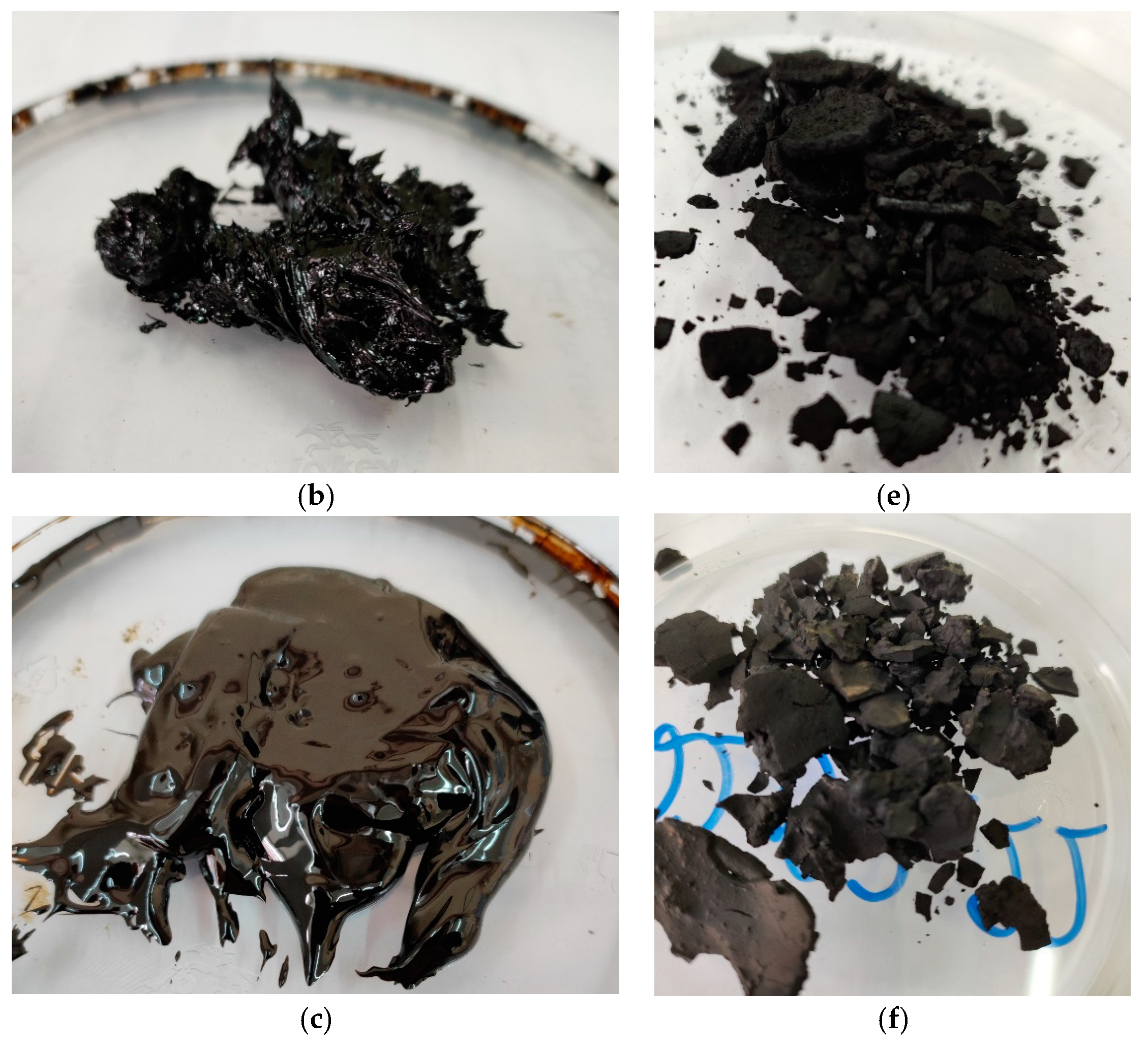
3.2. Still Bottoms Characteristics from the NTP Pyrolysis of Fuel Oil
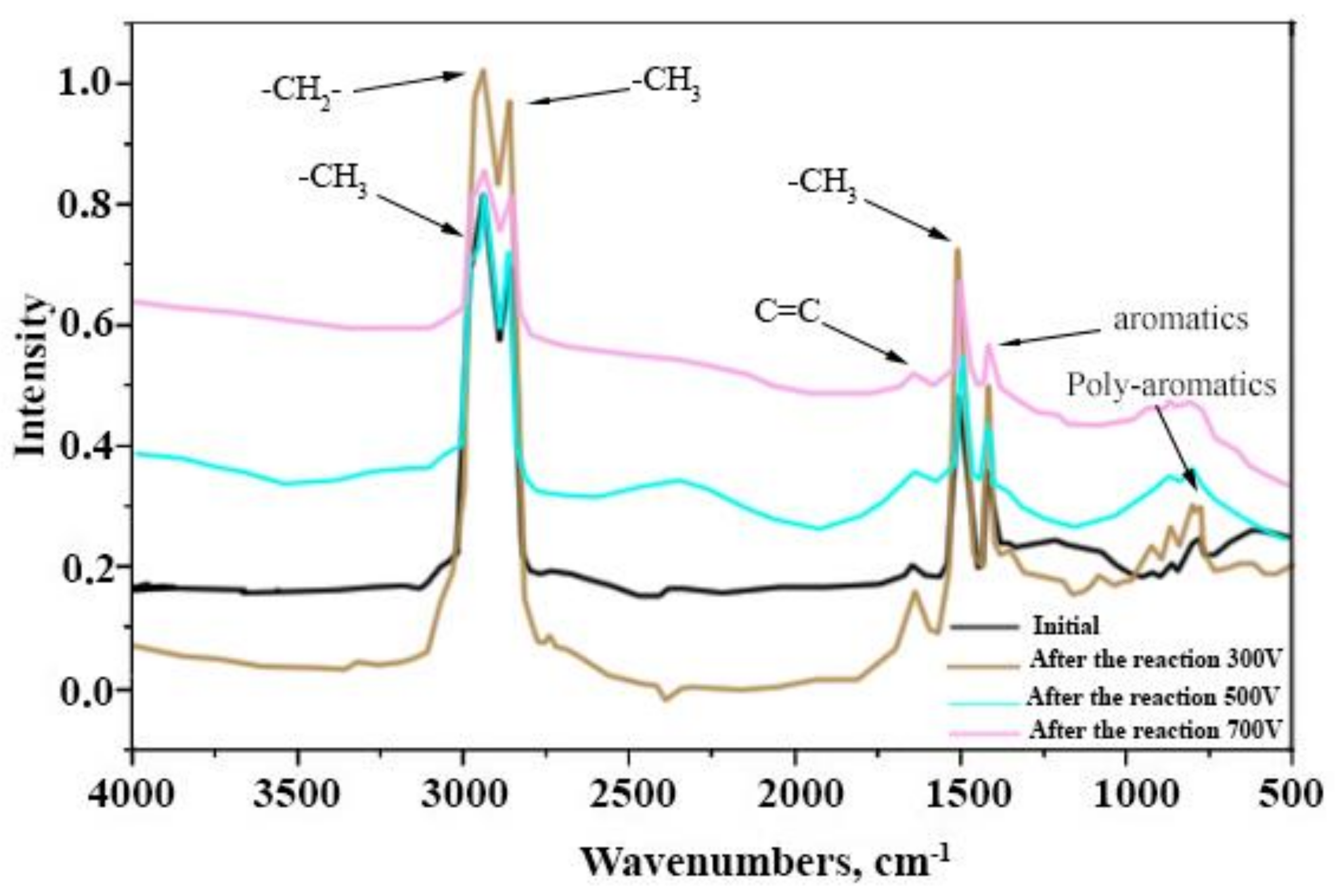
3.3. IR and NMR Spectroscopy of Still Bottoms of the NTP Pyrolysis of Fuel Oil
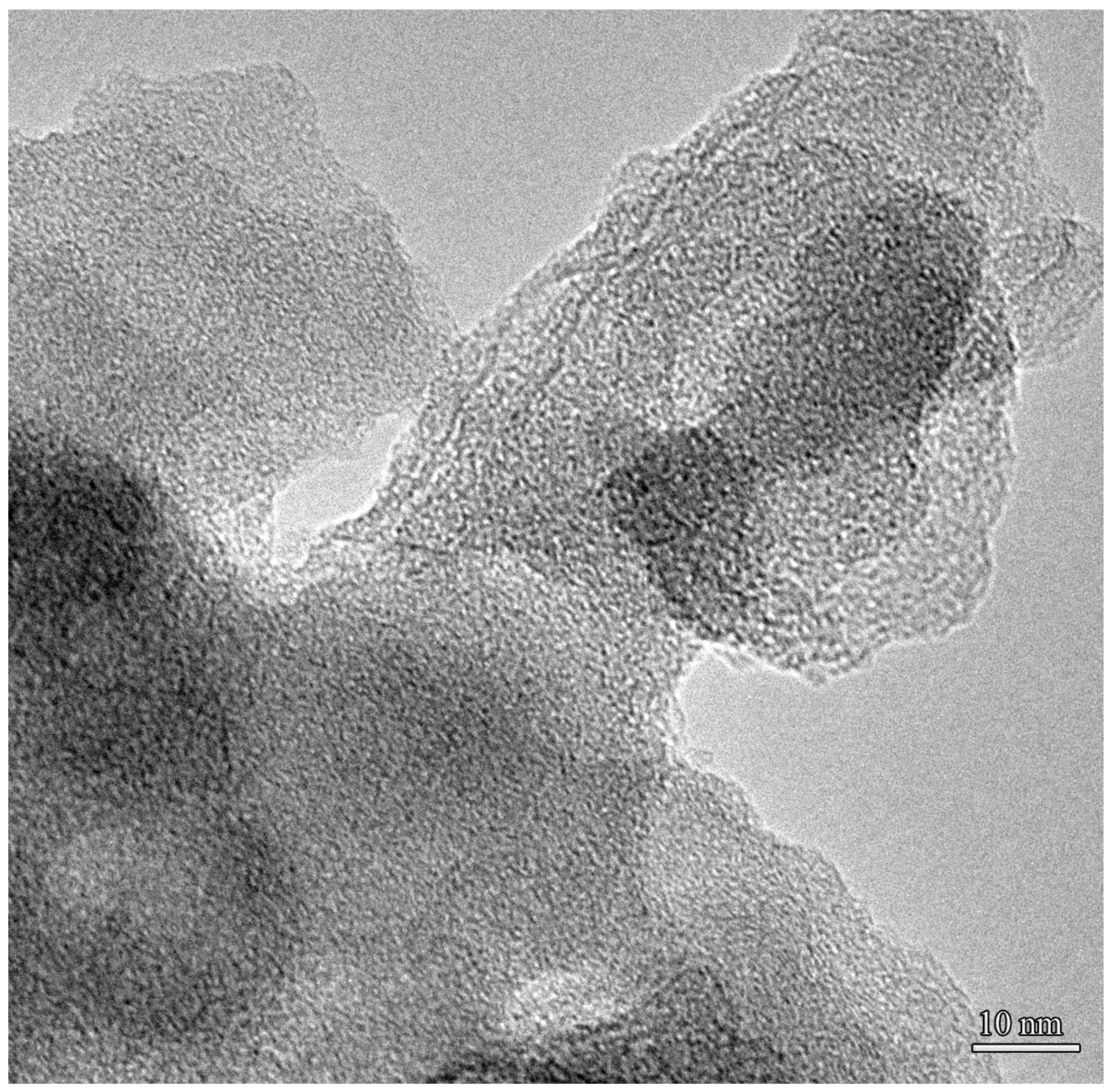
3.4. Characteristics of Solid Products of the NTP Pyrolysis of Fuel Oil
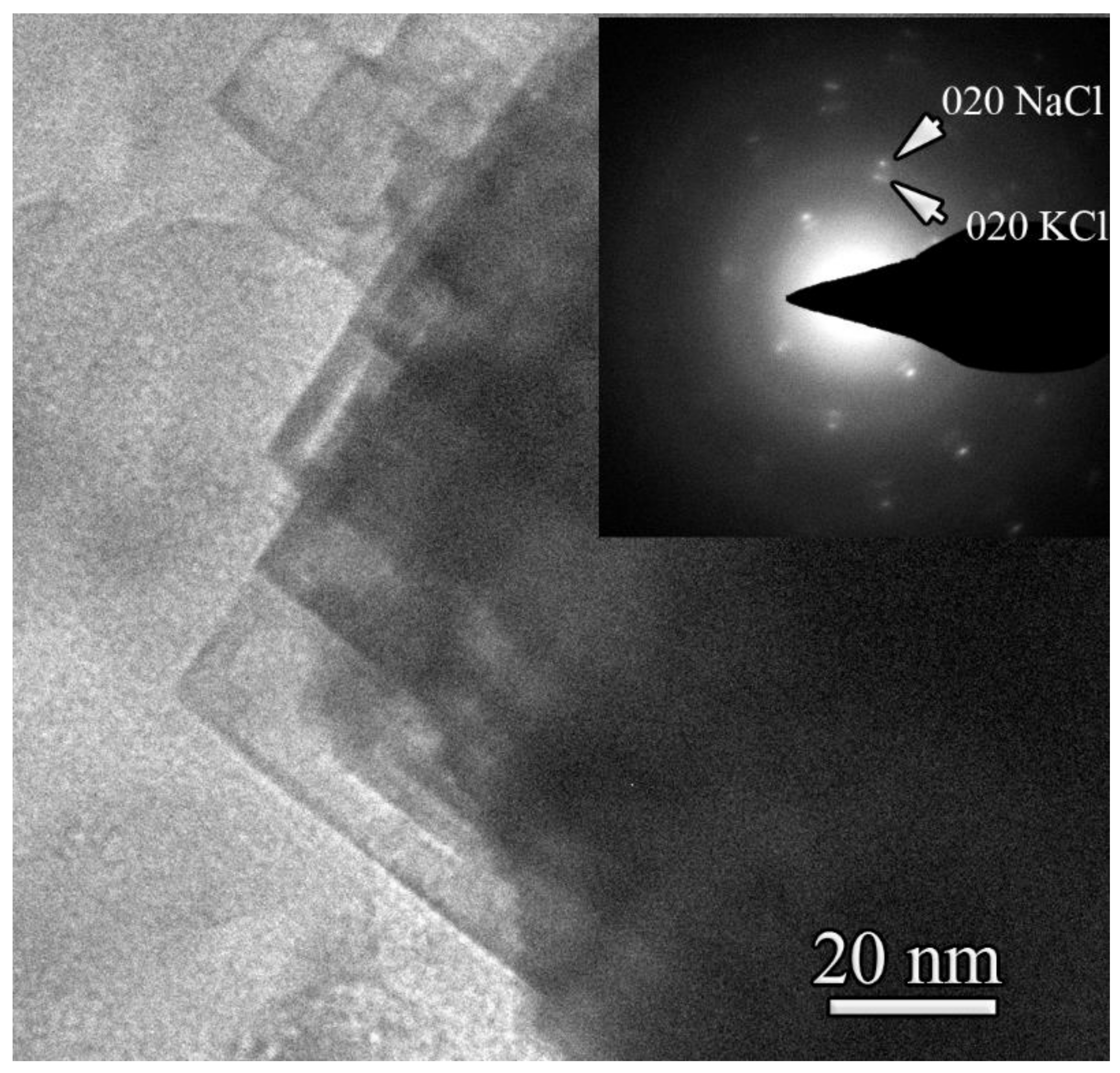
3.4.1. Transmission Electron Microscopy and Microanalysis
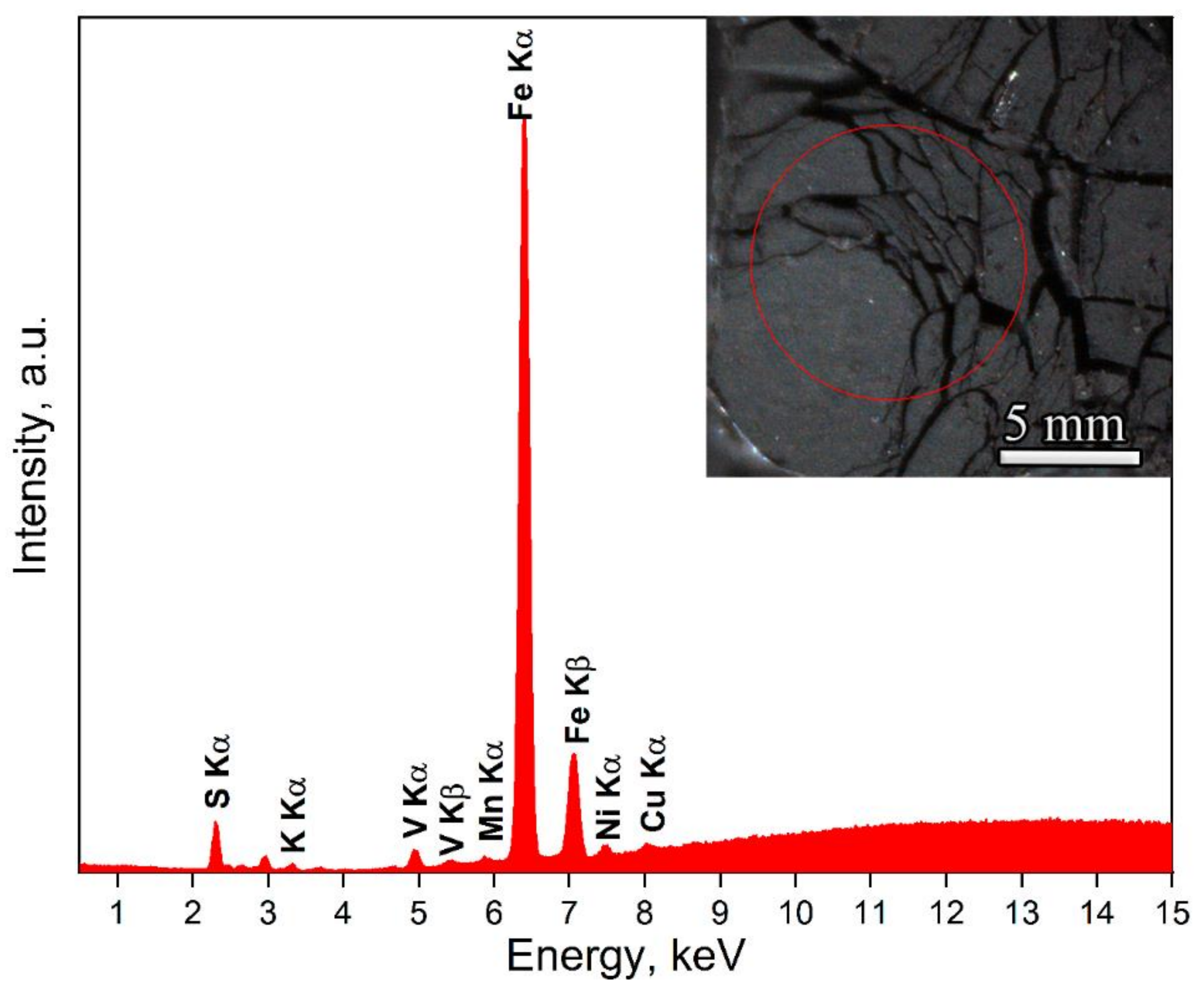
3.4.2. X-ray Fluorescence Analysis
3.4.3. X-ray Phase Analysis
3.4.4. Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Li, M.; Zhang, Y.; Zhang, W.; Qiao, P. Research on the pyrolysis characteristics and kinetics of two typical inferior heavy oils. Fuel 2022, 328, 125330. [Google Scholar] [CrossRef]
- Ganapathi, R.; Henni, A.; Shirif, E. Solubility of carbon dioxide and ethane in Lloydminster heavy oil: Experimental study and modeling. Can. J. Chem. Eng. 2022, 100, 1235–1243. [Google Scholar] [CrossRef]
- Santos, R.G.; Loh, W.; Bannwart, A.C.; Trevisan, O.V. An overview of heavy oil properties and its recovery and transportation methods. Braz. J. Chem. Eng. 2014, 31, 571–590. [Google Scholar] [CrossRef]
- Houda, S.; Lancelot, C.; Blanchard, P.; Poinel, L.; Lamonier, C. Oxidative desulfurization of heavy oils with high sulfur content: A review. Catalysts 2018, 8, 344. [Google Scholar] [CrossRef]
- Al-Samhan, M.; Al-Fadhli, J.; Al-Otaibi, A.M.; Al-Attar, F.; Bouresli, R.; Rana, M.S. Prospects of refinery switching from conventional to integrated: An opportunity for sustainable investment in the petrochemical industry. Fuel 2022, 310, 122161. [Google Scholar] [CrossRef]
- Moud, A.A. Asphaltene induced changes in rheological properties: A review. Fuel 2022, 316, 123372. [Google Scholar] [CrossRef]
- Ampah, J.D.; Liu, X.; Sun, X.; Pan, X.; Xu, L.; Jin, C.; Sun, T.; Geng, G.; Afrane, S.; Liu, H. Study on characteristics of marine heavy fuel oil and low carbon alcohol blended fuels at different temperatures. Fuel 2022, 310, 122307. [Google Scholar] [CrossRef]
- Xia, Y.; Ma, C.; Ju, R.; Zhao, C.; Zheng, F.; Sun, X.; Li, Z.; Wang, C.; Shi, D.; Lin, X.; et al. Characterization of nitrogen-containing compounds in petroleum fractions by online reversed-phase liquid chromatography-electrospray ionization Orbitrap mass spectrometry. Fuel 2021, 284, 119035. [Google Scholar] [CrossRef]
- Gao, N.; Li, J.; Quan, C.; Tan, H. Product property and environmental risk assessment of heavy metals during pyrolysis of oily sludge with fly ash additive. Fuel 2020, 266, 117090. [Google Scholar] [CrossRef]
- Rana, M.S.; Ancheyta, J.; Maity, S.K.; Marroquin, G. Comparison between refinery processes for heavy oil upgrading: A future fuel demand. Int. J. Oil Gas Coal Technol. 2008, 1, 250–282. [Google Scholar] [CrossRef]
- Bello, S.S.; Wang, C.; Zhang, M.; Gao, H.; Han, Z.; Shi, L.; Su, F.; Xu, G. A review on the reaction mechanism of hydrodesulfurization and hydrodenitrogenation in heavy oil upgrading. Energy Fuels 2021, 35, 10998–11016. [Google Scholar] [CrossRef]
- Prajapati, R.; Kohli, K.; Maity, S.K. Slurry phase hydrocracking of heavy oil and residue to produce lighter fuels: An experimental review. Fuel 2021, 288, 119686. [Google Scholar] [CrossRef]
- Tirado, A.; Yuan, C.; Varfolomeev, M.A.; Ancheyta, J. Kinetic modeling of aquathermolysis for upgrading of heavy oils. Fuel 2022, 310, 122286. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Sher, F.; Jamil, M.A.; Murtaza, D.; Al Qubeissi, M.; UI Hassan, M.; Mujtaba, M.A. Current Status and Potential of Tire Pyrolysis Oil Production as an Alternative Fuel in Developing Countries. Sustainability 2021, 13, 3214. [Google Scholar] [CrossRef]
- Fairuzov, D.; Gerzeliev, I.; Maximov, A.; Naranov, E. Catalytic dehydrogenation of ethane: A mini-review of recent advances and perspective of chemical looping technology. Catalysts 2021, 11, 833. [Google Scholar] [CrossRef]
- Dolganov, I.; Bunaev, A.; Dolganova, I. Unsteady-state mathematical modeling of hydrocarbon feedstock pyrolysis. Processes 2020, 8, 1394. [Google Scholar] [CrossRef]
- Honegger, M.; Burns, W.; Morrow, D.R. Is carbon dioxide removal ‘mitigation of climate change’? Rev. Eur. Comp. Int. Environ. Law 2021, 30, 327–335. [Google Scholar] [CrossRef]
- Wéry, F.; Geerts, M.; Vandewalle, L.A.; Reyniers, P.A.; Heynderickx, G.J.; Marin, G.B.; Van Geem, K.M. Assessing the CO2 emission reduction potential of steam cracking furnace innovations via computational fluid dynamics: From high-emissivity coatings, over coil modifications to firing control. Chem. Eng. Res. Des. 2023, 190, 129–142. [Google Scholar] [CrossRef]
- Rajabloo, T.; De Ceuninck, W.; Van Wortswinkel, L.; Rezakazemi, M.; Aminabhavi, T. Environmental management of industrial decarbonization with focus on chemical sectors: A review. J. Environ. Manag. 2022, 302, 114055. [Google Scholar] [CrossRef]
- Hao, H.; Wu, B.S.; Yang, J.; Guo, Q.; Yang, Y.; Li, Y.W. Non-thermal plasma enhanced heavy oil upgrading. Fuel 2015, 149, 162–173. [Google Scholar] [CrossRef]
- Rathore, K.; Bhuiyan, S.I.; Slavens, S.M.; Staack, D. Microplasma ball reactor for JP-8 liquid hydrocarbon conversion to lighter fuels. Fuel 2021, 285, 118943. [Google Scholar] [CrossRef]
- Khosravi, A.; Khani, M.R.; Goy, E.D.; Shokri, B. The processing of pyrolysis fuel oil by dielectric barrier discharge plasma torch. Plasma Chem. Plasma Process. 2018, 38, 365–378. [Google Scholar] [CrossRef]
- Delikonstantis, E.; Cameli, F.; Scapinello, M.; Rosa, V.; Van Geem, K.M.; Stefanidis, G.D. Low-carbon footprint chemical manufacturing using plasma technology. Curr. Opin. Chem. Eng. 2022, 38, 100857. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E. Plasma-liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Huang, W.; Jin, J.; Wen, G.; Yang, Q.; Su, B.; Ren, Q. Effect of nitrogen/oxygen substances on the pyrolysis of alkane-rich gases to acetylene by thermal plasma. Energies 2018, 11, 351. [Google Scholar] [CrossRef]
- Bilera, I.V.; Lebedev, Y.A. Plasma-chemical production of acetylene from hydrocarbons: History and current status (a review). Pet. Chem. 2022, 62, 329–351. [Google Scholar] [CrossRef]
- Shao, T.; Wang, R.; Zhang, C.; Yan, P. Atmospheric-pressure pulsed discharges and plasmas: Mechanism, characteristics, and applications. High Volt. 2018, 3, 14–20. [Google Scholar] [CrossRef]
- Lebedev, Y.A.; Krashevskaya, G.V.; Batukaev, T.S.; Mikhaylyuk, A.V. Time-resolved study of ignition of microwave discharge in liquid hydrocarbons. Plasma Process. Polym. 2022, 19, 2100215. [Google Scholar] [CrossRef]
- Matsui, Y.; Kawakami, S.; Takashima, K.; Katsura, S.; Mizuno, A. Liquid-phase fuel re-forming at room temperature using nonthermal plasma. Energy Fuels 2005, 19, 1561. [Google Scholar] [CrossRef]
- Bodrikov, I.V.; Kut’in, A.M.; Titov, E.Y.; Titov, D.Y.; Kurskii, Y.A.; Gazizullin, R.R. Fragmentation of thiophene and 3-methyl-2-thiophenecarboxaldehyde by direct liquid phase low-voltage discharges. Plasma Process. Polym. 2018, 15, 1800094. [Google Scholar] [CrossRef]
- Bodrikov, I.V.; Ivanova, A.G.; Vasiliev, A.L.; Titov, E.Y.; Titov, D.Y.; Serov, A.I. Influence of low-voltage discharge energy on the morphology of carbon nanostructures in induced benzene transformation. RSC Adv. 2021, 11, 39428–39437. [Google Scholar] [CrossRef] [PubMed]
- Delikonstantis, E.; Scapinello, M.; Stefanidis, G.D. Process modeling and evaluation of plasma-assisted ethylene production from methane. Processes 2019, 7, 68. [Google Scholar] [CrossRef]
- Rabinovich, A.; Nirenberg, G.; Kocagoz, S.; Surace, M.; Sales, C.; Fridman, A. Scaling up of non-thermal gliding arc plasma systems for industrial applications. Plasma Chem. Plasma Process. 2022, 42, 35–50. [Google Scholar] [CrossRef]
- Mehta, P.; Barboun, P.; Go, D.B.; Hicks, J.C.; Schneider, W.F. Catalysis enabled by plasma activation of strong chemical bonds: A review. ACS Energy Lett. 2019, 4, 1115–1133. [Google Scholar] [CrossRef]
- Das, A.; Peu, S.D. A Comprehensive Review on Recent Advancements in Thermochemical Processes for Clean Hydrogen Production to Decarbonize the Energy Sector. Sustainability 2022, 14, 11206. [Google Scholar] [CrossRef]
- Park, D.K.; Kim, J.-H.; Kim, H.-S.; Kim, J.-H.; Ryu, J.-H. Possibility Study in CO2 Free Hydrogen Production Using Dodecane (C12H26) from Plasma Reaction. Energies 2023, 16, 1589. [Google Scholar] [CrossRef]
- Barkhordari, A.; Mirzaei, S.I.; Falahat, A.; Krawczyk, D.A.; Rodero, A. Experimental Study of a Rotating Electrode Plasma Reactor for Hydrogen Production from Liquid Petroleum Gas Conversion. Appl. Sci. 2022, 12, 4045. [Google Scholar] [CrossRef]
- Korányi, T.I.; Németh, M.; Beck, A.; Horváth, A. Recent Advances in Methane Pyrolysis: Turquoise Hydrogen with Solid Carbon Production. Energies 2022, 15, 6342. [Google Scholar] [CrossRef]
- Stryczewska, H.D.; Boiko, O. Applications of Plasma Produced with Electrical Discharges in Gases for Agriculture and Biomedicine. Appl. Sci. 2022, 12, 4405. [Google Scholar] [CrossRef]
- Ali, M.; Cheng, Y.; Li, Y.; An, H.; Jin, Y. Direct conversion of poor-quality residual oil to light gases in electricity-driven thermal plasma reactor. Can. J. Chem. Eng. 2023, 101, 137. [Google Scholar] [CrossRef]
- Oost, G.V. Applications of Thermal Plasmas for the Environment. Appl. Sci. 2022, 12, 7185. [Google Scholar] [CrossRef]
- Barkhordari, A.; Karimian, S.; Rodero, A.; Krawczyk, D.A.; Mirzaei, S.I.; Falahat, A. Carbon Dioxide Decomposition by a Parallel-Plate Plasma Reactor: Experiments and 2-D Modelling. Appl. Sci. 2021, 11, 10047. [Google Scholar] [CrossRef]
- Kaufmann, S.J.; Rößner, P.; Renninger, S.; Lambarth, M.; Raab, M.; Stein, J.; Seithümmer, V.; Birke, K.P. Techno-Economic Potential of Plasma-Based CO2 Splitting in Power-to-Liquid Plants. Appl. Sci. 2023, 13, 4839. [Google Scholar] [CrossRef]
- Titov, E.Y.; Bodrikov, I.V.; Serov, A.I.; Kurskii, Y.A.; Titov, D.Y.; Bodrikova, E.R. Liquid-phase non-thermal plasma discharge for fuel oil processing. Energies 2022, 15, 3400. [Google Scholar] [CrossRef]
- Bodrikov, I.; Titov, E.Y.; Vasiliev, A.; Titov, D.; Ivanova, A.; Subbotin, A. Nonthermal plasma in the induction of polycondensation processes and intermolecular dehydrochlorination of chloroethanes in the liquid phase. Plasma Process. Polym. 2022, 19, 2200008. [Google Scholar] [CrossRef]
- Titov, E.Y.; Titov, D.Y.; Bodrikov, I.V.; Kut’in, A.M.; Kurskii, Y.A.; Gazizzulin, R.R. A device for generation of low-voltage discharges in liquid dielectric media. High Energy Chem. 2018, 52, 512–513. [Google Scholar] [CrossRef]
- Titov, E.; Bodrikov, I.; Titov, D. Control of the energy impact of electric discharges in a liquid phase. Energies 2023, 16, 1683. [Google Scholar] [CrossRef]
- Sanchez-Minero, F.; Ancheytab, J.; Silva-Olivera, G.; Flores-Vallec, S. Predicting SARA composition of crude oil by means of NMR. Fuel 2012, 110, 318–321. [Google Scholar] [CrossRef]
- Rakhmatullin, I.Z.; Efimov, S.V.; Tyurin, V.A.; Al-Muntaser, A.A.; Klimovitskii, A.E.; Varfolomeev, M.A.; Klochkov, V.V. Application of high-resolution NMR (1H and 13C) and FTIR spectroscopy for characterization of light and heavy crude oils. J. Pet. Sci. Eng. 2018, 168, 256–262. [Google Scholar] [CrossRef]
- Djimasbe, R.; Varfolomeev, M.A.; Al-Muntaser, A.A.; Yuan, C.; Feoktistov, D.A.; Suwaid, M.A.; Kirgizov, A.J.; Davletshin, R.R.; Zinnatullin, A.L.; Fatou, S.D.; et al. Oil-dispersed nickel-based catalyst for catalytic upgrading of heavy oil using supercritical water. Fuel 2022, 313, 122702. [Google Scholar] [CrossRef]
- Cao, Y.B.; Zhang, L.L.; Xia, D.H. Catalytic aquathermolysis of Shengli heavy crude oil with an amphiphilic cobalt catalyst. Pet. Sci. 2016, 13, 463–475. [Google Scholar] [CrossRef]
- Chen, M.; Li, C.; Li, G.R.; Chen, Y.L.; Zhou, C.G. In situ preparation of well-dispersed CuO nanocatalysts in heavy oil for catalytic aquathermolysis. Pet. Sci. 2019, 16, 439–446. [Google Scholar] [CrossRef]
- Chen, Q.; Yin, M.; Zhang, L.; Liu, H.; Qin, Z.; Wang, Z. Hydrogen-donated thermal upgrading of Venezuela extra-heavy oil: Identifying the role of hydrogen donor. Pet. Sci. Technol. 2020, 38, 550–555. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Chichkan, A.S. Effect of catalysts on tar carbonization. Catal. Today 2021, 379, 28–35. [Google Scholar] [CrossRef]
- Jung, H.; Bielawski, C.W. Asphaltene oxide promotes a broad range of synthetic transformations. Commun. Chem. 2019, 2, 113. [Google Scholar] [CrossRef]
- Petrova, Y.Y.; Frantsina, E.V.; Grin’ko, A.A.; Pak, A.Y.; Arkachenkova, V.V.; Povalyaev, P.V. Investigation of the process and products of plasma treatment of asphaltenes. Mater. Today Commun. 2022, 33, 104669. [Google Scholar] [CrossRef]
- Yakubov, M.R.; Milordov, D.V.; Yakubova, S.G.; Borisov, D.N.; Ivanov, V.T.; Sinyashin, K.O. Concentrations of vanadium and nickel and their ratio in heavy oil asphaltenes. Pet. Chem. 2016, 56, 16–20. [Google Scholar] [CrossRef]
- Trucano, P.; Chen, R. Structure of graphite by neutrondifraction. Nature 1975, 258, 136. [Google Scholar] [CrossRef]
- Alhreez, M.; Wen, D. Molecular structure characterization of asphaltene in the presence of inhibitors with nanoemulsions. RSC Adv. 2019, 34, 19560. [Google Scholar] [CrossRef]
- Induchoodan, G.; Jansson, H.; Swenson, J. Influence of graphene oxide on asphaltene nanoaggregates. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127614. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]












| Voltage, V | 300 | 500 | 700 |
|---|---|---|---|
| Average pulse duration, ms | 2.3 | 1.8 | 2.1 |
| Average pulse frequency, Hz | 53.3 | 43.5 | 40.2 |
| Average pulse amplitude, A | 41.4 | 64.6 | 75.4 |
| Average pulse energy, J | 1.5 | 2.4 | 4.6 |
| Voltage, V | 300 | 500 | 700 |
|---|---|---|---|
| Experiment time, min | 440 | 150 | 114 |
| Conversion rate, wt% | 28.3 | 49.0 | 46.2 |
| Gas yield, wt% | 29.9 | 46.5 | 41.1 |
| Solid product yield, wt% | 70.1 | 53.5 | 58.9 |
| Energy consumption, kWh/kg of fuel oil | 11.9 | 3.9 | 4.8 |
| Energy consumption, kWh/kg of gas | 39.7 | 8.3 | 11.7 |
| Gas flow, mL/min | 7.3 | 555.2 | 709.9 |
| H2 | 46.5 | 48.4 | 50.0 |
| CH4 | 4.8 | 4.2 | 6.2 |
| C2H4 | 7.6 | 7.8 | 8.3 |
| C2H6 | 0.3 | 0.4 | 0.4 |
| C2H2 | 34.3 | 30.4 | 27.8 |
| C3H8 | 1.9 | 2.4 | 2.1 |
| C3H4 | 1.0 | 1.0 | 0.9 |
| 1,3-C4H6 | 0.7 | 0.9 | 1.0 |
| C4H10 | 0.5 | 0.5 | 0.8 |
| C5H12 | 2.0 | 3.2 | 2.1 |
| C6+ | 0.4 | 0.8 | 0.4 |
| Fuel Oil | Still Bottom | |||
|---|---|---|---|---|
| Voltage, V | 300 | 500 | 700 | |
| Density at 20 °C, g/cm3 | 0.955 | 0.968 | - | - |
| Kinematic viscosity at 100 °C, mm2/s | 31.169 | 448.26 | - | - |
| Solid structures content, wt% | - | 21.66 | 33.9 | 33.6 |
| Sulfur content, % | 2.675 | 2.713 | 2.836 | 2.630 |
| Non-volatile residue, % | 30.6 | 56.4 | 55.7 | 56.3 |
| Initial boiling point, °C | 298.5 | 297.2 | 289.9 | 269.3 |
| End-boiling point, °C | 671.3 | 649.1 | 659.3 | 673.4 |
| Evaporated, % | 69.4 | 43.6 | 44.3 | 43.7 |
| Range NMR | 9.0–8.3 ppm | 8.3–7.3 ppm | 7.24–6.5 ppm | 4.4–2.4 ppm | 2.4–2.1 ppm | 2.1–1.05 ppm | 1.05–0.3 ppm | FHA | |
|---|---|---|---|---|---|---|---|---|---|
| Voltage, V | Har, % | Hal, % | |||||||
| - | 0.19 | 2.26 | 2.55 | 7.85 | 2.78 | 62.83 | 21.55 | 0.0499 | |
| 300 | 0.35 | 3.03 | 2.09 | 8.34 | 2.71 | 62.03 | 21.44 | 0.0548 | |
| 500 | 0.57 | 4.23 | 2.18 | 9.77 | 2.74 | 60.08 | 20.44 | 0.0697 | |
| 700 | 0.44 | 3.57 | 2.18 | 8.57 | 2.72 | 61.25 | 21.68 | 0.0613 | |
| Range NMR | 132–155 ppm | 115–132 ppm | 5–60 ppm | FCA | |
|---|---|---|---|---|---|
| Voltage, V | Car, % | Cal, % | |||
| - | 3.27 | 7.12 | 89.61 | 0.1039 | |
| 300 | 4.64 | 9.29 | 86.08 | 0.1392 | |
| 500 | 4.98 | 16.14 | 78.88 | 0.2112 | |
| 700 | 5.66 | 8.67 | 85.67 | 0.1433 | |
| Element | wt% | at% | Error, in wt% (3 Sigma) |
|---|---|---|---|
| Carbon | 83.5 | 93 | 8.74 |
| Sulfur | 2.4 | 1 | 0.58 |
| Potassium | 8.1 | 3 | 1.20 |
| Calcium | 0.1 | <1 | 0.22 |
| Chlorine | 3.0 | 1 | 0.66 |
| Sodium | 1.7 | 1 | 0.46 |
| Oxygen | 1.2 | 1 | 0.43 |
| Total | 100 | 100 |
| Element | wt% | at% |
|---|---|---|
| S | 59 | 71 |
| K | 2 | 2 |
| V | 1 | 1 |
| Mn | <1 | <1 |
| Fe | 37 | 25 |
| Ni | 0.5 | <1 |
| Cu | <1 | <1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titov, E.Y.; Bodrikov, I.V.; Vasiliev, A.L.; Kurskii, Y.A.; Ivanova, A.G.; Golovin, A.L.; Shirokov, D.A.; Titov, D.Y.; Bodrikova, E.R. Non-Thermal Plasma Pyrolysis of Fuel Oil in the Liquid Phase. Energies 2023, 16, 4017. https://doi.org/10.3390/en16104017
Titov EY, Bodrikov IV, Vasiliev AL, Kurskii YA, Ivanova AG, Golovin AL, Shirokov DA, Titov DY, Bodrikova ER. Non-Thermal Plasma Pyrolysis of Fuel Oil in the Liquid Phase. Energies. 2023; 16(10):4017. https://doi.org/10.3390/en16104017
Chicago/Turabian StyleTitov, Evgeniy Yurevich, Ivan Vasilevich Bodrikov, Alexander Leonidovich Vasiliev, Yuriy Alekseevich Kurskii, Anna Gennadievna Ivanova, Andrey Leonidovich Golovin, Dmitry Alekseevich Shirokov, Dmitry Yurievich Titov, and Evgenia Ruslanovna Bodrikova. 2023. "Non-Thermal Plasma Pyrolysis of Fuel Oil in the Liquid Phase" Energies 16, no. 10: 4017. https://doi.org/10.3390/en16104017





