Stability and Performance Enhancement of Perovskite Solar Cells: A Review
Abstract
:1. Introduction
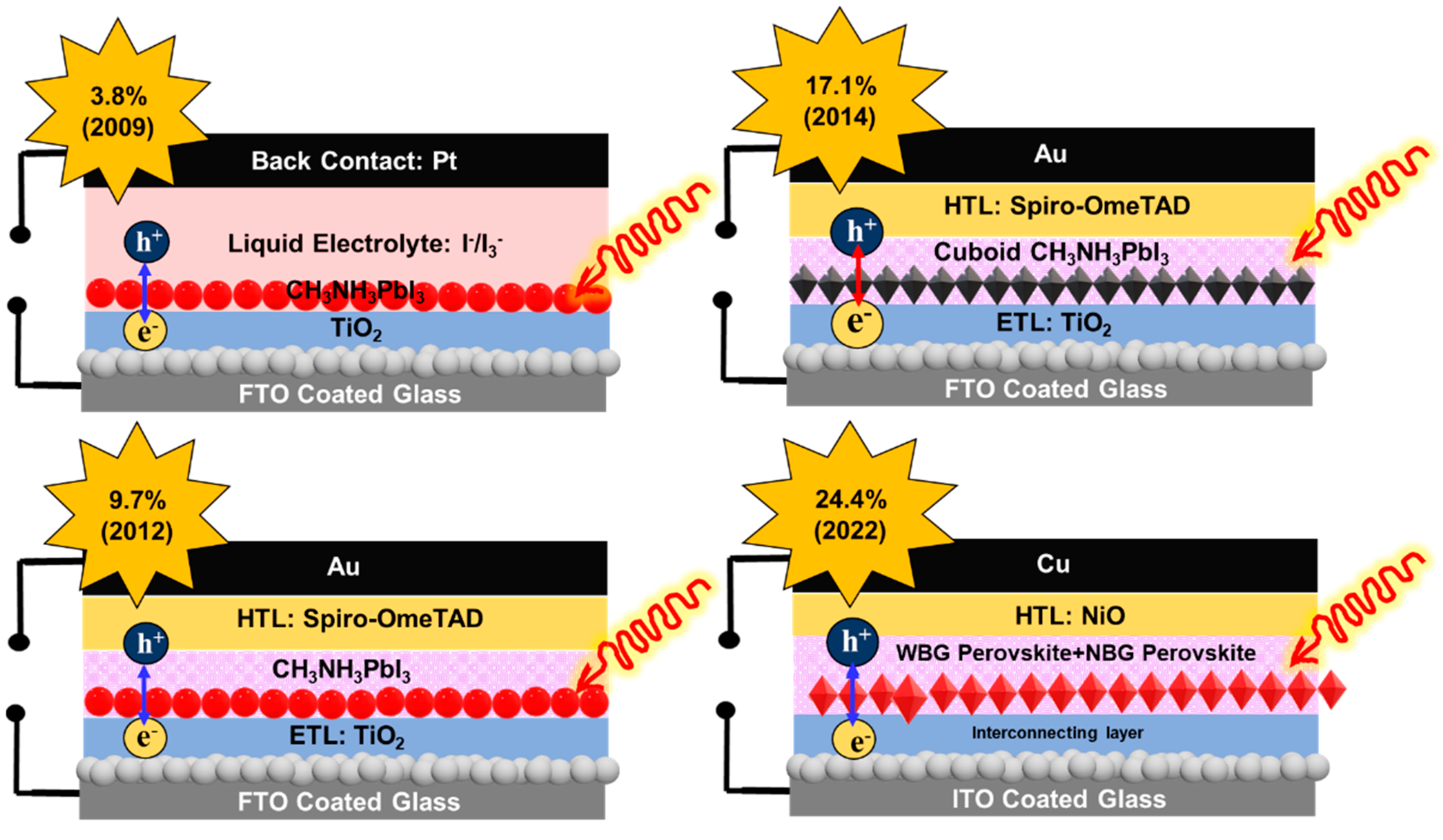
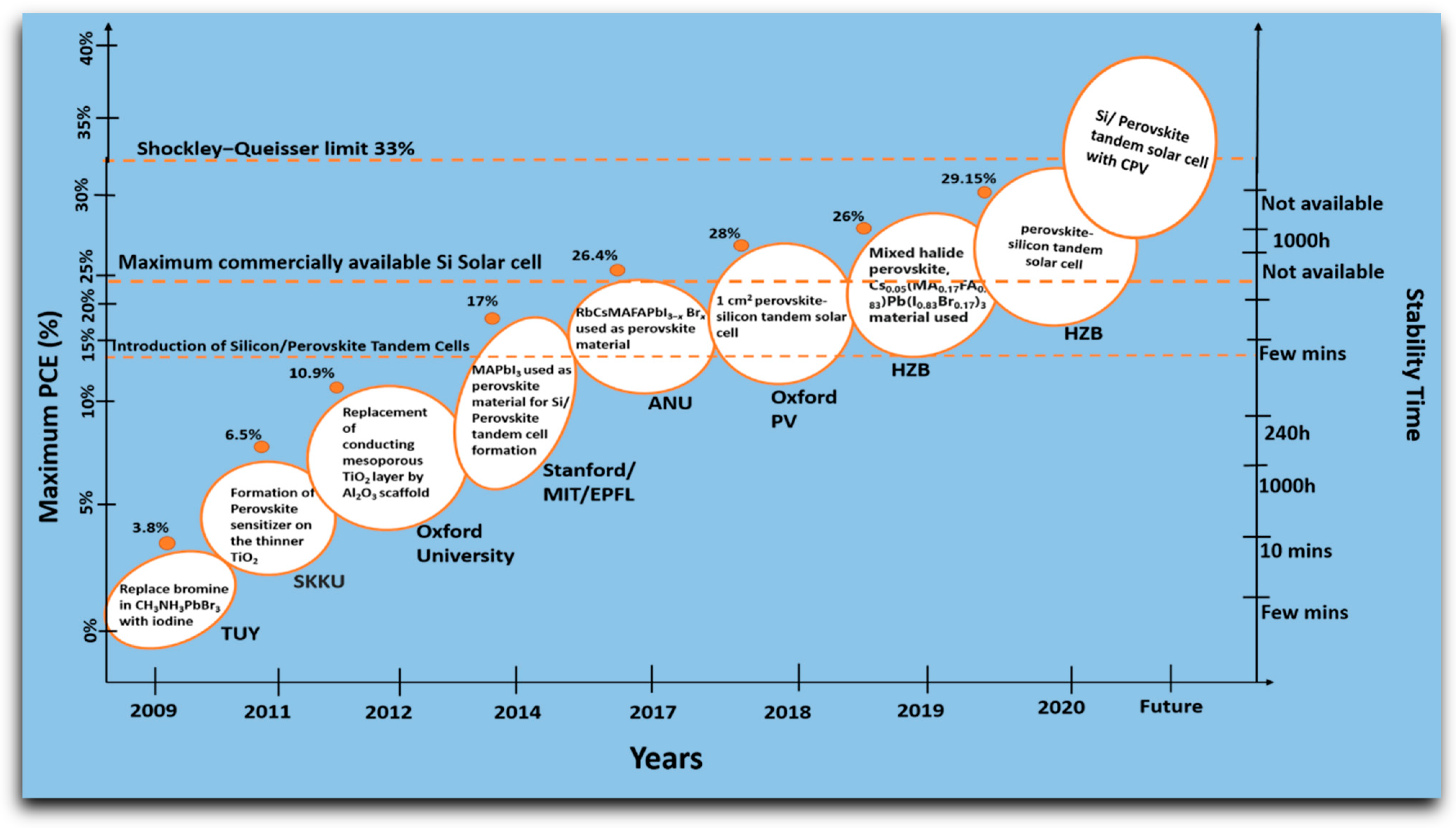
2. Evolution of the Perovskite Solar Cell
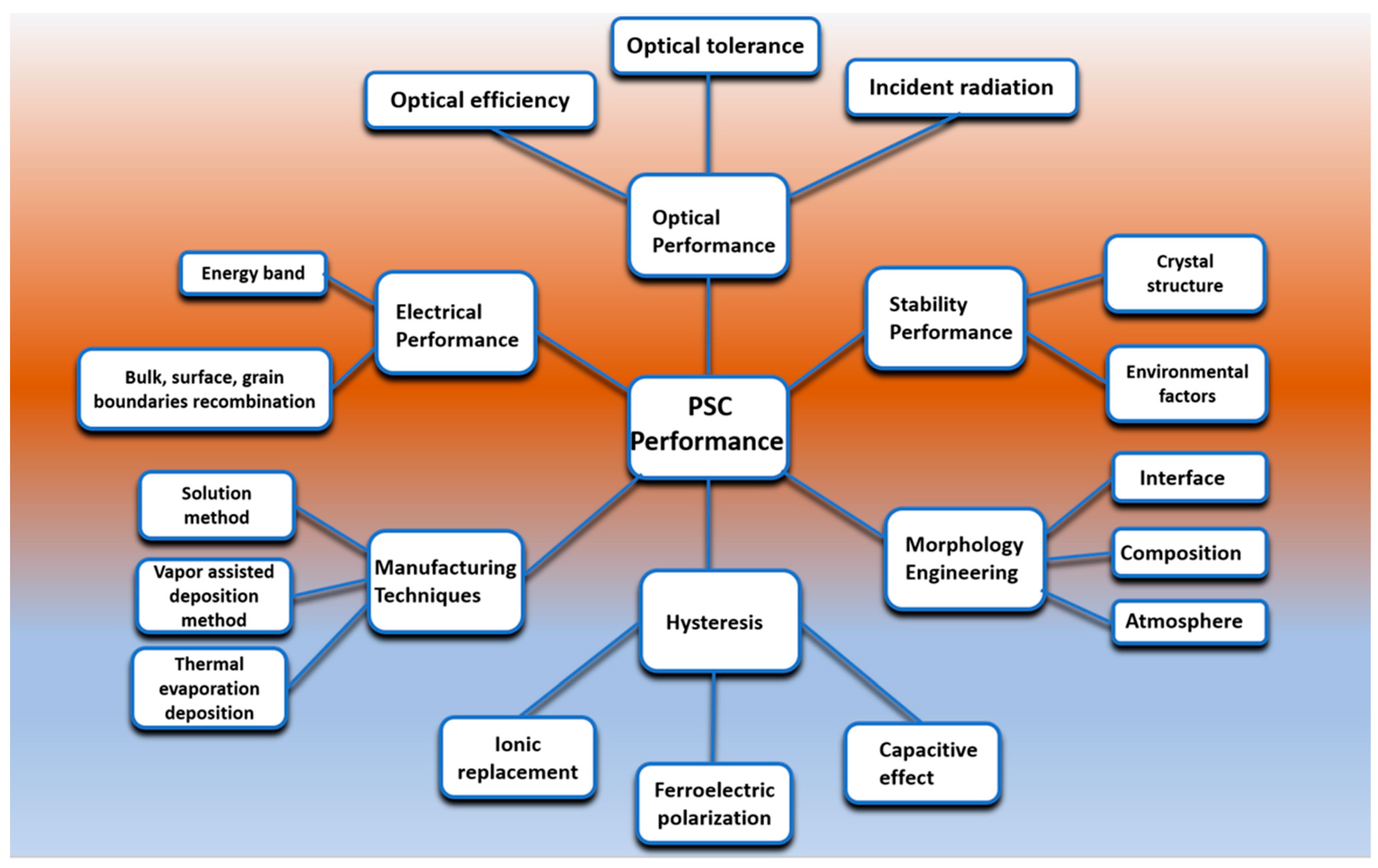
2.1. Characteristics of Perovskite Solar Cells
2.2. Improving Efficiency through Structure Modulation
2.2.1. A-Site Cation
2.2.2. B-Site Cation
2.2.3. X-Site Anion
2.3. Stability Studies of PSC
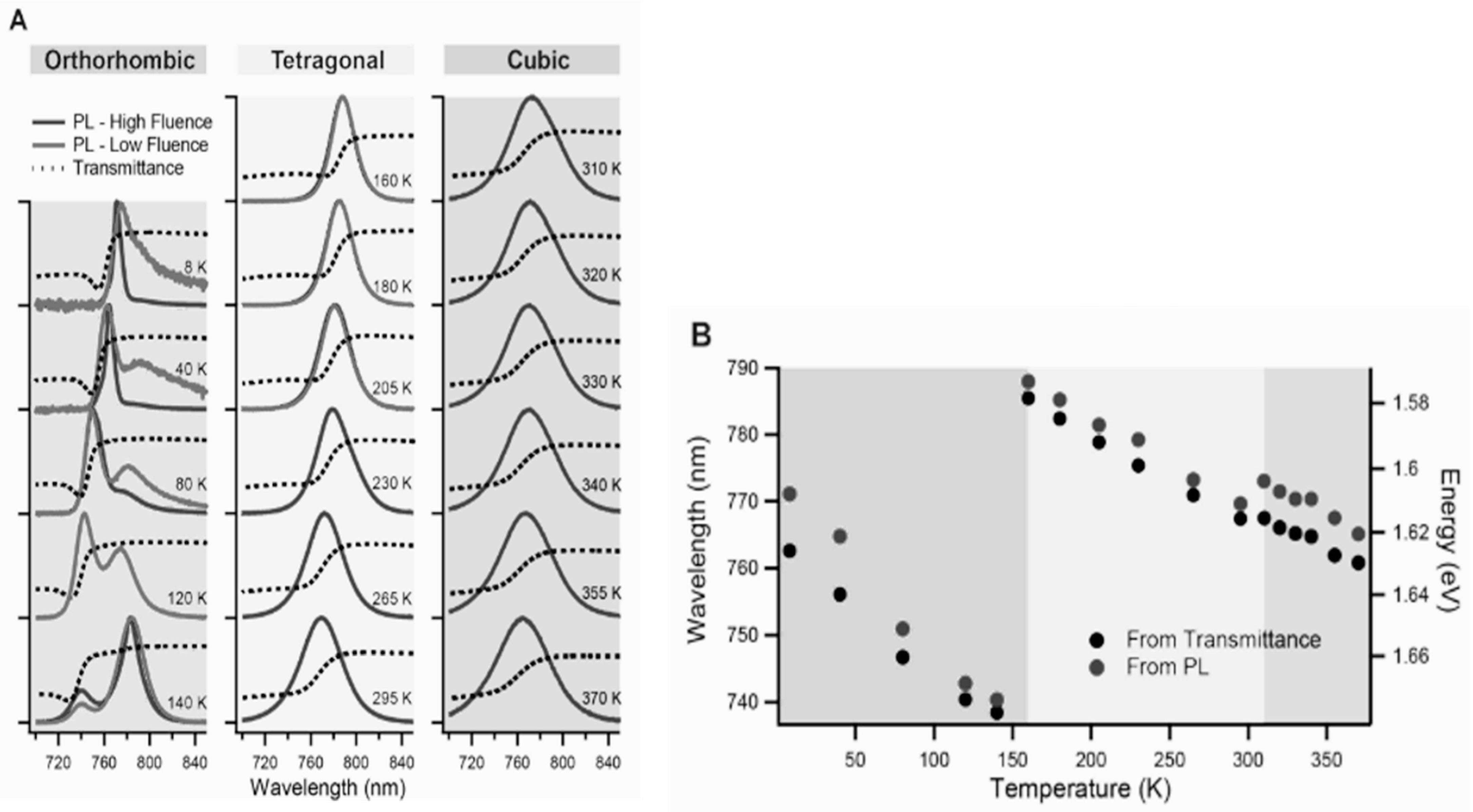
2.3.1. Crystal Structural Stability
2.3.2. Effect of Humidity
2.3.3. UV Light Stability
2.3.4. Thermal Stability
2.4. Hysteresis
3. Pathways to Improve Stability through Concentrated Perovskite Solar Cells
4. Cost-Effectiveness
5. Conclusions
Funding
Conflicts of Interest
References
- Anaya, M.; Lozano, G.; Calvo, M.E.; Míguez, H. ABX3 Perovskites for Tandem Solar Cells. Joule 2017, 1, 769–793. [Google Scholar] [CrossRef]
- Mailoa, J.P.; Bailie, C.D.; Johlin, E.C.; Hoke, E.T.; Akey, A.J.; Nguyen, W.H.; McGehee, M.D.; Buonassisi, T. A 2-Terminal Perovskite/Silicon Multijunction Solar Cell Enabled by a Silicon Tunnel Junction. Appl. Phys. Lett. 2015, 106, 121105. [Google Scholar] [CrossRef]
- Cotal, H.; Fetzer, C.; Boisvert, J.; Kinsey, G.; King, R.; Hebert, P.; Yoon, H.; Karam, N. III-V Multijunction Solar Cells for Concentrating Photovoltaics. Energy Environ. Sci. 2009, 2, 174–192. [Google Scholar] [CrossRef]
- Li, C.; Xu, F.; Li, Y.; Li, N.; Yu, H.; Yuanb, B.; Chen, Z.; Li, L.; Cao, B. An Ultrahigh 84.3% Fill Factor for Efficient CH3NH3PbI3 P-i-N Perovskite Film Solar Cell. Sol. Energy 2022, 233, 271–277. [Google Scholar] [CrossRef]
- NREL Transforming Energy. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.20200708.pdf (accessed on 7 August 2022).
- Sha, W.E.I.; Ren, X.; Chen, L.; Choy, W.C.H. The Efficiency Limit of CH3NH3PbI3 Perovskite Solar Cells. Appl. Phys. Lett. 2015, 106, 221104. [Google Scholar] [CrossRef]
- NREL Transforming Energy. Available online: https://www.nrel.gov/news/press/2022/nrel-led-breakthrough-pushes-perovskite-cell-to-greater-stability-efficiency.html (accessed on 6 December 2022).
- Park, J.; Kim, J.; Yun, H.-S.; Paik, M.J.; Noh, E.; Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S.I. Controlled Growth of Perovskite Layers with Volatile Alkylammonium Chlorides. Nature 2023, 616, 724–730. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Jose da Silva, W.; Soultati, A.; Kim, H.P.; Kim, B.S.; Reo, Y.; Ximim Gavim, A.E.; Conforto, J.; Schneider, F.K.; Felippi, M.; et al. Photonic Nanostructures Mimicking Floral Epidermis for Perovskite Solar Cells. Cell Reports Phys. Sci. 2022, 3, 101019. [Google Scholar] [CrossRef]
- EMILIANO BELLINI UNIST, EPFL Claim 25.6% Efficiency World Record for Perovskite Solar Cell. 2021. Available online: https://www.pv-magazine.com/2021/04/06/unist-epfl-claim-25-6-efficiency-world-record-for-perovskite-solar-cell/#:~:text=Scientists%20have%20set%20a%20new,efficiencies%20of%20more%20than%2010%25.&text=The%20solar%20cell%20developed%20by%20the%20Korean%2DSwiss%20research%20group (accessed on 6 April 2023).
- Wang, Z.; Lin, Q.; Wenger, B.; Christoforo, M.G.; Lin, Y.H.; Klug, M.T.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. High Irradiance Performance of Metal Halide Perovskites for Concentrator Photovoltaics. Nat. Energy 2018, 3, 855–861. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, F. Recent Advances in Lead Chemisorption for Perovskite Solar Cells. Trans. Tianjin Univ. 2022, 28, 341–357. [Google Scholar] [CrossRef]
- Soykan, C.; Gocmez, H. The Physical Properties of Bismuth Replacement in Lead Halogen Perovskite Solar Cells: CH3NH3Pb1−xBixI3 Compounds by Ab-Initio Calculations. Results Phys. 2019, 13, 102278. [Google Scholar] [CrossRef]
- Zhang, Q.; Hao, F.; Li, J.; Zhou, Y.; Wei, Y.; Lin, H. Perovskite Solar Cells: Must Lead Be Replaced–and Can It Be Done? Sci. Technol. Adv. Mater. 2018, 19, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Novel Photoelectrochemical Cell with Mesoscopic Electrodes Sensitized by Lead-Halide Compounds (11). ECS Meet. Abstr. 2008, MA2008-02, 27. [Google Scholar] [CrossRef]
- Ansari, M.I.H.; Qurashi, A.; Nazeeruddin, M.K. Frontiers, Opportunities, and Challenges in Perovskite Solar Cells: A Critical Review. J. Photochem. Photobiol. C Photochem. Rev. 2018, 35, 1–24. [Google Scholar] [CrossRef]
- Kumar, H.; Kumar, Y.; Rawat, G.; Kumar, C.; Mukherjee, B.; Pal, B.N.; Jit, S. Heating Effects of Colloidal ZnO Quantum Dots Heating Effects of Colloidal ZnO Quantum Dots. IEEE Trans. Nanotechnol. 2017, 16, 1073–1080. [Google Scholar] [CrossRef]
- Geng, W.; Zhang, L.; Zhang, Y.N.; Lau, W.M.; Liu, L.M. First-Principles Study of Lead Iodide Perovskite Tetragonal and Orthorhombic Phases for Photovoltaics. J. Phys. Chem. C 2014, 118, 19565–19571. [Google Scholar] [CrossRef]
- Foley, B.J.; Marlowe, D.L.; Sun, K.; Saidi, W.A.; Scudiero, L.; Gupta, M.C.; Choi, J.J. Temperature Dependent Energy Levels of Methylammonium Lead Iodide Perovskite. Appl. Phys. Lett. 2015, 106, 243904. [Google Scholar] [CrossRef]
- Milot, R.L.; Eperon, G.E.; Snaith, H.J.; Johnston, M.B.; Herz, L.M. Temperature-Dependent Charge-Carrier Dynamics in CH3NH3PbI3 Perovskite Thin Films. Adv. Funct. Mater. 2015, 25, 6218–6227. [Google Scholar] [CrossRef]
- Xin, C.; Veber, P.; Guennou, M.; Toulouse, C.; Valle, N.; Ciomaga Hatnean, M.; Balakrishnan, G.; Haumont, R.; Saint Martin, R.; Velazquez, M.; et al. Single Crystal Growth of BaZrO3 from the Melt at 2700 °C Using Optical Floating Zone Technique and Growth Prospects from BaB 2 O 4 Flux at 1350 °C. CrystEngComm 2019, 21, 502–512. [Google Scholar] [CrossRef]
- Pellet, N.; Gao, P.; Gregori, G.; Yang, T.Y.; Nazeeruddin, M.K.; Maier, J.; Grätzel, M. Mixed-Organic-Cation Perovskite Photovoltaics for Enhanced Solar-Light Harvesting. Angew. Chem. Int. Ed. 2014, 53, 3151–3157. [Google Scholar] [CrossRef]
- Sadhanala, A.; Deschler, F.; Thomas, T.H.; Dutton, S.E.; Goedel, K.C.; Hanusch, F.C.; Lai, M.L.; Steiner, U.; Bein, T.; Docampo, P.; et al. Preparation of Single-Phase Films of CH3NH3Pb(I1-XBrx)3 with Sharp Optical Band Edges. J. Phys. Chem. Lett. 2014, 5, 2501–2505. [Google Scholar] [CrossRef]
- Neeraj, S.; Kijima, N.; Cheetham, A.K. Novel Red Phosphors for Solid-State Lighting: The System NaM(WO4)2-x(MoO4)x:Eu3+ (M=Gd, Y, Bi). Chem. Phys. Lett. 2004, 387, 2–6. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Graẗzel, M.; Mhaisalkar, S.; Sum, T.C. Long-Range Balanced Electron-and Hole-Transport Lengths in Organic-Inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Subbiah, A.S.; Halder, A.; Ghosh, S.; Mahuli, N.; Hodes, G.; Sarkar, S.K. Inorganic Hole Conducting Layers for Perovskite-Based Solar Cells. J. Phys. Chem. Lett. 2014, 5, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Snaith, H.J.; Abate, A.; Ball, J.M.; Eperon, G.E.; Leijtens, T.; Noel, N.K.; Stranks, S.D.; Wang, J.T.W.; Wojciechowski, K.; Zhang, W. Anomalous Hysteresis in Perovskite Solar Cells. J. Phys. Chem. Lett. 2014, 5, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Biesold-Mcgee, G.V.; Liu, Y.; Kang, Z.; Lin, Z. Doping and Ion Substitution in Colloidal Metal Halide Perovskite Nanocrystals. Chem. Soc. Rev. 2020, 49, 4953–5007. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, L.; Zhu, T.; Yao, X.; Yi, C.; Zhang, X.; Cao, Y.; Liu, L.; Hu, W.; Gong, X. Efficient Perovskite Solar Cells by Hybrid Perovskites Incorporated with Heterovalent Neodymium Cations. Nano Energy 2019, 61, 352–360. [Google Scholar] [CrossRef]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva, C. Multi-Phase Semicrystalline Microstructures Drive Exciton Dissociation in Neat Plastic Semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Lin, X.; Wu, T.; Han, Q.; Zhang, Y.; Han, L. Effects of A Site Doping on the Crystallization of Perovskite Films. J. Mater. Chem. A 2021, 9, 1372–1394. [Google Scholar] [CrossRef]
- Poglitsch, A.; Weber, D. Dynamic Disorder in Methylammoniumtrihalogenoplumbates (II) Observed by Millimeter-Wave Spectroscopy. J. Chem. Phys. 1987, 87, 6373–6378. [Google Scholar] [CrossRef]
- McMeekin, D.P.; Sadoughi, G.; Rehman, W.; Eperon, G.E.; Saliba, M.; Hörantner, M.T.; Haghighirad, A.; Sakai, N.; Korte, L.; Rech, B.; et al. A Mixed-Cation Lead Mixed-Halide Perovskite Absorber for Tandem Solar Cells. Science 2015, 351, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Seol, D.J.; Cho, A.N.; Park, N.G. High-Efficiency Perovskite Solar Cells Based on the Black Polymorph of HC(NH2)2PbI3. Adv. Mater. 2014, 26, 4991–4998. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Hu, H.; Zhang, J.; Lv, S.; Yu, Y.; Wei, F.; Qin, T.; Xu, H.; Liu, Z.; Cui, G. NH2CH=NH2PbI3: An Alternative Organolead Iodide Perovskite Sensitizer for Mesoscopic Solar Cells. Chem. Mater. 2014, 26, 1485–1491. [Google Scholar] [CrossRef]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium Lead Trihalide: A Broadly Tunable Perovskite for Efficient Planar Heterojunction Solar Cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar] [CrossRef]
- Choi, H.; Jeong, J.; Kim, H.B.; Kim, S.; Walker, B.; Kim, G.H.; Kim, J.Y. Cesium-Doped Methylammonium Lead Iodide Perovskite Light Absorber for Hybrid Solar Cells. Nano Energy 2014, 7, 80–85. [Google Scholar] [CrossRef]
- Kim, H.S.; Hagfeldt, A.; Park, N.G. Morphological and Compositional Progress in Halide Perovskite Solar Cells. Chem. Commun. 2019, 55, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yun, J.S.; Ma, Q.; Zheng, J.; Lau, C.F.J.; Deng, X.; Kim, J.; Kim, D.; Seidel, J.; Green, M.A.; et al. High-Efficiency Rubidium-Incorporated Perovskite Solar Cells by Gas Quenching. ACS Energy Lett. 2017, 2, 438–444. [Google Scholar] [CrossRef]
- Beal, R.E.; Slotcavage, D.J.; Leijtens, T.; Bowring, A.R.; Belisle, R.A.; Nguyen, W.H.; Burkhard, G.F.; Hoke, E.T.; McGehee, M.D. Cesium Lead Halide Perovskites with Improved Stability for Tandem Solar Cells. J. Phys. Chem. Lett. 2016, 7, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, C.; Wang, Y.; Xu, Z.; Lu, Z.; Ma, Y.; Zhu, H.; Hu, Y.; Xiao, C.; Yi, X.; et al. All-Inorganic Perovskite Solar Cells. J. Am. Chem. Soc. 2016, 138, 15829–15832. [Google Scholar] [CrossRef]
- Gu, S.; Lin, R.; Han, Q.; Gao, Y.; Tan, H.; Zhu, J. Tin and Mixed Lead–Tin Halide Perovskite Solar Cells: Progress and Their Application in Tandem Solar Cells. Adv. Mater. 2020, 32, 1907392. [Google Scholar] [CrossRef]
- Hao, F.; Stoumpos, C.C.; Cao, D.H.; Chang, R.P.H.; Kanatzidis, M.G. Lead-Free Solid-State Organic-Inorganic Halide Perovskite Solar Cells. Nat. Photonics 2014, 8, 489–494. [Google Scholar] [CrossRef]
- Feng, J.; Xiao, B. Effective Masses and Electronic and Optical Properties of Nontoxic MASnX3 (X = Cl, Br, and I) Perovskite Structures as Solar Cell Absorber: A Theoretical Study Using HSE06. J. Phys. Chem. C 2014, 118, 19655–19660. [Google Scholar] [CrossRef]
- Zuo, F.; Williams, S.T.; Liang, P.W.; Chueh, C.C.; Liao, C.Y.; Jen, A.K.Y. Binary-Metal Perovskites Toward High-Performance Planar-Heterojunction Hybrid Solar Cells. Adv. Mater. 2014, 26, 6454–6460. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, W.; Li, H.; Zhang, C.; Fan, J.; Mai, Y. C60 Additive-Assisted Crystallization in CH3NH3Pb0.75Sn0.25I3 Perovskite Solar Cells with High Stability and Efficiency. Nanoscale 2017, 9, 13967–13975. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, H.; Zhou, Q.; Wei, Q.; Wei, M.; Jiang, L.; Wang, Z.; Peng, Z.; Wang, F.; Zang, Z.; et al. One-Step Synthesis of SnI2·(DMSO)XAdducts for High-Performance Tin Perovskite Solar Cells. J. Am. Chem. Soc. 2021, 143, 10970–10976. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zang, Z.; Zhou, Y.; Li, H.; Wei, Q.; Ning, Z. Tin Halide Perovskite Solar Cells: An Emerging Thin-Film Photovoltaic Technology. Acc. Mater. Res. 2021, 2, 210–219. [Google Scholar] [CrossRef]
- Marshall, K.P.; Walker, M.; Walton, R.I.; Hatton, R.A. Enhanced Stability and Efficiency in Hole-Transport Layer Free CsSnI3. Nat. Energy 2016, 1, 16178. [Google Scholar] [CrossRef]
- Krishnamoorthy, T.; Ding, H.; Yan, C.; Leong, W.L.; Baikie, T.; Zhang, Z.; Sherburne, M.; Li, S.; Asta, M.; Mathews, N.; et al. Lead-Free Germanium Iodide Perovskite Materials for Photovoltaic Applications. J. Mater. Chem. A 2015, 3, 23829–23832. [Google Scholar] [CrossRef]
- Gao, X.X.; Luo, W.; Zhang, Y.; Hu, R.; Zhang, B.; Züttel, A.; Feng, Y.; Nazeeruddin, M.K. Stable and High-Efficiency Methylammonium-Free Perovskite Solar Cells. Adv. Mater. 2020, 32, 1905502. [Google Scholar] [CrossRef]
- Williams, S.T.; Zuo, F.; Chueh, C.C.; Liao, C.Y.; Liang, P.W.; Jen, A.K.Y. Role of Chloride in the Morphological Evolution of Organo-Lead Halide Perovskite Thin Films. ACS Nano 2014, 8, 10640–10654. [Google Scholar] [CrossRef]
- Zhu, W.; Bao, C.; Li, F.; Zhou, X.; Yang, J.; Yu, T.; Zou, Z. An Efficient Planar-Heterojunction Solar Cell Based on Wide-Bandgap CH3NH3PbI2.1Br0.9 Perovskite Film for Tandem Cell Application. Chem. Commun. 2016, 52, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S. Chemical Management for Colorful, Efficient, and Stable Inorganic–Organic Hybrid Nanostructured Solar Cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, E.; Amat, A.; Nazeeruddin, M.K.; Grätzel, M.; De Angelis, F. First-Principles Modeling of Mixed Halide Organometal Perovskites for Photovoltaic Applications. J. Phys. Chem. C 2013, 117, 13902–13913. [Google Scholar] [CrossRef]
- Chung, I.; Lee, B.; He, J.; Chang, R.P.H.; Kanatzidis, M.G. All-Solid-State Dye-Sensitized Solar Cells with High Efficiency. Nature 2012, 485, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tschumi, M.; Han, H.; Babkair, S.S.; Alzubaydi, R.A.; Ansari, A.A.; Habib, S.S.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Grätzel, M. Outdoor Performance and Stability under Elevated Temperatures and Long-Term Light Soaking of Triple-Layer Mesoporous Perovskite Photovoltaics. Energy Technol. 2015, 3, 551–555. [Google Scholar] [CrossRef]
- Liu, T.; Chen, K.; Hu, Q.; Zhu, R.; Gong, Q. Inverted Perovskite Solar Cells: Progresses and Perspectives. Adv. Energy Mater. 2016, 6, 1600457. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Forgács, D.; Rivera, T. Industrial Opportunities and Challenges for Perovskite Photovoltaic Technology. Sol. RRL 2019, 3, 1900144. [Google Scholar] [CrossRef]
- Niu, G.; Guo, X.; Wang, L. Review of Recent Progress in Chemical Stability of Perovskite Solar Cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Tiep, N.H.; Ku, Z.; Fan, H.J. Recent Advances in Improving the Stability of Perovskite Solar Cells. Adv. Energy Mater. 2016, 6, 1501420. [Google Scholar] [CrossRef]
- Berhe, T.A.; Su, W.N.; Chen, C.H.; Pan, C.J.; Cheng, J.H.; Chen, H.M.; Tsai, M.C.; Chen, L.Y.; Dubale, A.A.; Hwang, B.J. Organometal Halide Perovskite Solar Cells: Degradation and Stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
- Kim, H.S.; Seo, J.Y.; Park, N.G. Material and Device Stability in Perovskite Solar Cells. ChemSusChem 2016, 9, 2528–2540. [Google Scholar] [CrossRef] [PubMed]
- Bouclé, J.; Herlin-Boime, N. The Benefits of Graphene for Hybrid Perovskite Solar Cells. Synth. Met. 2016, 222, 3–16. [Google Scholar] [CrossRef]
- Piralaee, M.; Asgari, A. Nitride/Perovskite Tandem Solar Cell with High Stability: Analytical Study of Adjusting Current Matching Condition. Int. J. Energy Res. 2023, 2023, 2925434. [Google Scholar] [CrossRef]
- Leijtens, T.; Bush, K.; Cheacharoen, R.; Beal, R.; Bowring, A.; McGehee, M.D. Towards Enabling Stable Lead Halide Perovskite Solar Cells; Interplay between Structural, Environmental, and Thermal Stability. J. Mater. Chem. A 2017, 5, 11483–11500. [Google Scholar] [CrossRef]
- Kour, R.; Arya, S.; Verma, S.; Gupta, J.; Bandhoria, P.; Bharti, V.; Datt, R.; Gupta, V. Potential Substitutes for Replacement of Lead in Perovskite Solar Cells: A Review. Glob. Chall. 2019, 3, 1900050. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Tanaka, S.; Manabe, K.; Nishino, H. Effects of Surface Blocking Layer of Sb2S3 on Nanocrystalline TiO2 for CH3NH3PbI3 Perovskite Solar Cells. J. Phys. Chem. C 2014, 118, 16995–17000. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting Tin and Lead Iodide Perovskites with Organic Cations: Phase Transitions, High Mobilities, and near-Infrared Photoluminescent Properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Kawamura, Y.; Mashiyama, H.; Hasebe, K. Structural Study on Cubic-Tetragonal Transition of CH3NH3PbI3. J. Phys. Soc. Jpn 2002, 71, 1694–1697. [Google Scholar] [CrossRef]
- Whitfield, P.S.; Herron, N.; Guise, W.E.; Page, K.; Cheng, Y.Q.; Milas, I.; Crawford, M.K. Structures, Phase Transitions and Tricritical Behavior of the Hybrid Perovskite Methyl Ammonium Lead Iodide. Sci. Rep. 2016, 6, 35685. [Google Scholar] [CrossRef]
- Smecca, E.; Numata, Y.; Deretzis, I.; Pellegrino, G.; Boninelli, S.; Miyasaka, T.; La Magna, A.; Alberti, A. Stability of Solution-Processed MAPbI3 and FAPbI3 Layers. Phys. Chem. Chem. Phys. 2016, 18, 13413–13422. [Google Scholar] [CrossRef]
- Xia, X.; Wu, W.; Li, H.; Zheng, B.; Xue, Y.; Xu, J.; Zhang, D.; Gao, C.; Liu, X. Spray Reaction Prepared FA1-XCsxPbI3 Solid Solution as a Light Harvester for Perovskite Solar Cells with Improved Humidity Stability. RSC Adv. 2016, 6, 14792–14798. [Google Scholar] [CrossRef]
- Alberti, A.; Deretzis, I.; Pellegrino, G.; Bongiorno, C.; Smecca, E.; Mannino, G.; Giannazzo, F.; Condorelli, G.G.; Sakai, N.; Miyasaka, T.; et al. Similar Structural Dynamics for the Degradation of CH3NH3PbI3 in Air and in Vacuum. ChemPhysChem 2015, 16, 3064–3071. [Google Scholar] [CrossRef]
- Zhou, Y.; Kwun, J.; Garces, H.F.; Pang, S.; Padture, N.P. Observation of Phase-Retention Behavior of the HC(NH2)2PbI3 Black Perovskite Polymorph upon Mesoporous TiO2 Scaffolds. Chem. Commun. 2016, 52, 7273–7275. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Park, J.S.; Wei, S.H.; Berry, J.J.; Zhu, K. Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Yi, C.; Luo, J.; Meloni, S.; Boziki, A.; Ashari-Astani, N.; Grätzel, C.; Zakeeruddin, S.M.; Röthlisberger, U.; Grätzel, M. Entropic Stabilization of Mixed A-Cation ABX3 Metal Halide Perovskites for High Performance Perovskite Solar Cells. Energy Environ. Sci. 2016, 9, 656–662. [Google Scholar] [CrossRef]
- Xiang, F.-X.; Wang, X.-L.; Dou, S.-X. Transport Evidence for the Coexistence of the Topological Surface State and a Two-Dimensional Electron Gas in BiSbTe3 Topological Insulator. arXiv 2014, 1404, 11. [Google Scholar] [CrossRef]
- Law, C.; Miseikis, L.; Dimitrov, S.; Shakya-Tuladhar, P.; Li, X.; Barnes, P.R.F.; Durrant, J.; O’Regan, B.C. Performance and Stability of Lead Perovskite/TiO2, Polymer/PCBM, and Dye Sensitized Solar Cells at Light Intensities up to 70 Suns. Adv. Mater. 2014, 26, 6268–6273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; McMeekin, D.P.; Sakai, N.; van Reenen, S.; Wojciechowski, K.; Patel, J.B.; Johnston, M.B.; Snaith, H.J. Efficient and Air-Stable Mixed-Cation Lead Mixed-Halide Perovskite Solar Cells with n-Doped Organic Electron Extraction Layers. Adv. Mater. 2017, 29, 1604186. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Hong, Z.; Bin Song, T.; Meng, L.; Liu, Y.; Jiang, C.; Zhou, H.; Chang, W.H.; Li, G.; Yang, Y. Moisture Assisted Perovskite Film Growth for High Performance Solar Cells. Appl. Phys. Lett. 2014, 105, 183902. [Google Scholar] [CrossRef]
- Yang, J.; Siempelkamp, B.D.; Liu, D.; Kelly, T.L. Investigation of CH3NH3PbI3degradation Rates and Mechanisms in Controlled Humidity Environments Using in Situ Techniques. ACS Nano 2015, 9, 1955–1963. [Google Scholar] [CrossRef]
- Christians, J.A.; Miranda Herrera, P.A.; Kamat, P.V. Transformation of the Excited State and Photovoltaic Efficiency of CH3NH3PbI3 Perovskite upon Controlled Exposure to Humidified Air. J. Am. Chem. Soc. 2015, 137, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.S.; Lee, J.W.; Park, N.G. 15.76% Efficiency Perovskite Solar Cells Prepared under High Relative Humidity: Importance of PbI2 Morphology in Two-Step Deposition of CH3NH3PbI3. J. Mater. Chem. A 2015, 3, 8808–8815. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential Deposition as a Route to High-Performance Perovskite-Sensitized Solar Cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient Planar Heterojunction Perovskite Solar Cells by Vapour Deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Lim, J.; Yun, H.J.; Kim, Y.H.; Park, T. A Diketopyrrolopyrrole-Containing Hole Transporting Conjugated Polymer for Use in Efficient Stable Organic-Inorganic Hybrid Solar Cells Based on a Perovskite. Energy Environ. Sci. 2014, 7, 1454–1460. [Google Scholar] [CrossRef]
- Shirayama, M.; Kato, M.; Miyadera, T.; Sugita, T.; Fujiseki, T.; Hara, S.; Kadowaki, H.; Murata, D.; Chikamatsu, M.; Fujiwara, H. Degradation Mechanism of CH3NH3PbI3 Perovskite Materials upon Exposure to Humid Air. J. Appl. Phys. 2016, 119, 115501. [Google Scholar] [CrossRef]
- Ledinský, M.; Löper, P.; Niesen, B.; Holovský, J.; Moon, S.J.; Yum, J.H.; De Wolf, S.; Fejfar, A.; Ballif, C. Raman Spectroscopy of Organic-Inorganic Halide Perovskites. J. Phys. Chem. Lett. 2015, 6, 401–406. [Google Scholar] [CrossRef]
- Hu, L.; Shao, G.; Jiang, T.; Li, D.; Lv, X.; Wang, H.; Liu, X.; Song, H.; Tang, J.; Liu, H. Investigation of the Interaction between Perovskite Films with Moisture via in Situ Electrical Resistance Measurement. ACS Appl. Mater. Interfaces 2015, 7, 25113–25120. [Google Scholar] [CrossRef]
- Mosconi, E.; Azpiroz, J.M.; De Angelis, F. Ab Initio Molecular Dynamics Simulations of Methylammonium Lead Iodide Perovskite Degradation by Water. Chem. Mater. 2015, 27, 4885–4892. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, H.; Liu, R.; Sun, Y.; Huang, W. Composite Encapsulation Enabled Superior Comprehensive Stability of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 27277–27285. [Google Scholar] [CrossRef]
- Cao, F.; Zhang, P.; Li, L. Multidimensional Perovskite Solar Cells. Fundam. Res. 2022, 2, 237–253. [Google Scholar] [CrossRef]
- Aristidou, N.; Sanchez-Molina, I.; Chotchuangchutchaval, T.; Brown, M.; Martinez, L.; Rath, T.; Haque, S.A. The Role of Oxygen in the Degradation of Methylammonium Lead Trihalide Perovskite Photoactive Layers. Angew. Chem. Int. Ed. 2015, 54, 8208–8212. [Google Scholar] [CrossRef]
- Li, W.; Zhang, W.; Van Reenen, S.; Sutton, R.J.; Fan, J.; Haghighirad, A.A.; Johnston, M.B.; Wang, L.; Snaith, H.J. Enhanced UV-Light Stability of Planar Heterojunction Perovskite Solar Cells with Caesium Bromide Interface Modification. Energy Environ. Sci. 2016, 9, 490–498. [Google Scholar] [CrossRef]
- Dong, X.; Fang, X.; Lv, M.; Lin, B.; Zhang, S.; Wang, Y.; Yuan, N.; Ding, J. Method for Improving Illumination Instability of Organic–Inorganic Halide Perovskite Solar Cells. Sci. Bull. 2016, 61, 236–244. [Google Scholar] [CrossRef]
- Leijtens, T.; Eperon, G.E.; Pathak, S.; Abate, A.; Lee, M.M.; Snaith, H.J. Overcoming Ultraviolet Light Instability of Sensitized TiO2 with Meso-Superstructured Organometal Tri-Halide Perovskite Solar Cells. Nat. Commun. 2013, 4, 2885. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, F.T.F.; Lee, Y.H.; Jellett, C.; Dmitrov, S.; Bryant, D.T.J.; Durrant, J.R.; O’Regan, B.C.; Graetzel, M.; Nazeeruddin, M.K.; Haque, S.A. Improved Environmental Stability of Organic Lead Trihalide Perovskite-Based Photoactive-Layers in the Presence of Mesoporous TiO2. J. Mater. Chem. A 2015, 3, 7219–7223. [Google Scholar] [CrossRef]
- Pearson, A.J.; Eperon, G.E.; Hopkinson, P.E.; Habisreutinger, S.N.; Wang, J.T.W.; Snaith, H.J.; Greenham, N.C. Oxygen Degradation in Mesoporous Al2O3/CH3NH3PbI3-XClx Perovskite Solar Cells: Kinetics and Mechanisms. Adv. Energy Mater. 2016, 6, 1600014. [Google Scholar] [CrossRef]
- Matsui, T.; Tress, W.; Saliba, M.; Abate, A.; Gra, M.; Hagfeldt, A. Highly Efficient and Stable Planar Perovskite Solar Cells by Solution-Processed Tin Oxide. Energy Environ. Sci. 2016, 9, 3128–3134. [Google Scholar]
- Correa Baena, J.P.; Steier, L.; Tress, W.; Saliba, M.; Neutzner, S.; Matsui, T.; Giordano, F.; Jacobsson, T.J.; Srimath Kandada, A.R.; Zakeeruddin, S.M.; et al. Highly Efficient Planar Perovskite Solar Cells through Band Alignment Engineering. Energy Environ. Sci. 2015, 8, 2928–2934. [Google Scholar] [CrossRef]
- Zhu, Z.; Bai, Y.; Liu, X.; Chueh, C.C.; Yang, S.; Jen, A.K.Y. Enhanced Efficiency and Stability of Inverted Perovskite Solar Cells Using Highly Crystalline SnO2Nanocrystals as the Robust Electron-Transporting Layer. Adv. Mater. 2016, 28, 6478–6484. [Google Scholar] [CrossRef]
- Turedi, B.; Yeddu, V.; Zheng, X.; Kim, D.Y.; Bakr, O.M.; Saidaminov, M.I. Perovskite Single-Crystal Solar Cells: Going Forward. ACS Energy Lett. 2021, 6, 631–642. [Google Scholar] [CrossRef]
- Conings, B.; Drijkoningen, J.; Gauquelin, N.; Babayigit, A.; D’Haen, J.; D’Olieslaeger, L.; Ethirajan, A.; Verbeeck, J.; Manca, J.; Mosconi, E.; et al. Intrinsic Thermal Instability of Methylammonium Lead Trihalide Perovskite. Adv. Energy Mater. 2015, 5, 1500477. [Google Scholar] [CrossRef]
- Dualeh, A.; Tétreault, N.; Moehl, T.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Effect of Annealing Temperature on Film Morphology of Organic-Inorganic Hybrid Pervoskite Solid-State Solar Cells. Adv. Funct. Mater. 2014, 24, 3250–3258. [Google Scholar] [CrossRef]
- Ciccioli, A.; Latini, A. Thermodynamics and the Intrinsic Stability of Lead Halide Perovskites CH3NH3PbX3. J. Phys. Chem. Lett. 2018, 9, 3756–3765. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.W.; Moore, D.T.; Saliba, M.; Sai, H.; Estroff, L.A.; Hanrath, T.; Snaith, H.J.; Wiesner, U. Evolution and Performance of Solar Cells. ACS Nano 2014, 8, 4730–4739. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Xiao, H.; Wang, Y.; Zhao, Z.; Lin, Z.; Cheng, H.C.; Lee, S.J.; Wang, G.; Feng, Z.; Goddard, W.A.; et al. Layer-by-Layer Degradation of Methylammonium Lead Tri-Iodide Perovskite Microplates. Joule 2017, 1, 548–562. [Google Scholar] [CrossRef]
- Han, Y.; Meyer, S.; Dkhissi, Y.; Weber, K.; Pringle, J.M.; Bach, U.; Spiccia, L.; Cheng, Y.B. Degradation Observations of Encapsulated Planar CH3NH3PbI3 Perovskite Solar Cells at High Temperatures and Humidity. J. Mater. Chem. A 2015, 3, 8139–8147. [Google Scholar] [CrossRef]
- Wei, Z.; Yan, K.; Chen, H.; Yi, Y.; Zhang, T.; Long, X.; Li, J.; Zhang, L.; Wang, J.; Yang, S. Cost-Efficient Clamping Solar Cells Using Candle Soot for Hole Extraction from Ambipolar Perovskites. Energy Environ. Sci. 2014, 7, 3326–3333. [Google Scholar] [CrossRef]
- Tress, W.; Marinova, N.; Moehl, T.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Grätzel, M. Understanding the Rate-Dependent J-V Hysteresis, Slow Time Component, and Aging in CH3NH3PbI3 Perovskite Solar Cells: The Role of a Compensated Electric Field. Energy Environ. Sci. 2015, 8, 995–1004. [Google Scholar] [CrossRef]
- Gonzalez Pedro, V.; Juarez-Perez, E.J.; Arsyad, W.-S.; Barea, E.M.; Santiago, F.F.; Mora-Sero, I.; Bisquert, J. General Working Principles of CH3NH3PbX3 Perovskite Solar Cells. Nano Lett. 2014, 14, 888–893. [Google Scholar] [CrossRef]
- Almora, O.; Zarazua, I.; Mas-Marza, E.; Mora-Sero, I.; Bisquert, J.; Garcia-Belmonte, G. Capacitive Dark Currents, Hysteresis, and Electrode Polarization in Lead Halide Perovskite Solar Cells. J. Phys. Chem. Lett. 2015, 6, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Buin, A.; Ip, A.H.; Li, W.; Voznyy, O.; Comin, R.; Yuan, M.; Jeon, S.; Ning, Z.; McDowell, J.J.; et al. Perovskite-Fullerene Hybrid Materials Suppress Hysteresis in Planar Diodes. Nat. Commun. 2015, 6, 7081. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Wang, Q.; Shao, Y.; Yuan, Y.; Xiao, Z.; Huang, J. Non-Wetting Surface-Driven High-Aspect-Ratio Crystalline Grain Growth for Efficient Hybrid Perovskite Solar Cells. Nat. Commun. 2015, 6, 7747. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, Y.; Li, H.; Li, G.; Pan, J.; Xu, D.; Zhao, Q.; Yu, D. Hysteresis Analysis Based on the Ferroelectric Effect in Hybrid Perovskite Solar Cells. J. Phys. Chem. Lett. 2014, 5, 3937–3945. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, N.K.; Uddin, A. Hysteresis in Organic-Inorganic Hybrid Perovskite Solar Cells. Sol. Energy Mater. Sol. Cells 2016, 157, 476–509. [Google Scholar] [CrossRef]
- Berry, J.; Buonassisi, T.; Egger, D.A.; Hodes, G.; Kronik, L.; Loo, Y.L.; Lubomirsky, I.; Marder, S.R.; Mastai, Y.; Miller, J.S.; et al. Hybrid Organic-Inorganic Perovskites (HOIPs): Opportunities and Challenges. Adv. Mater. 2015, 27, 5102–5112. [Google Scholar] [CrossRef]
- Unger, E.L.; Hoke, E.T.; Bailie, C.D.; Nguyen, W.H.; Bowring, A.R.; Heumüller, T.; Christoforo, M.G.; McGehee, M.D. Hysteresis and Transient Behavior in Current-Voltage Measurements of Hybrid-Perovskite Absorber Solar Cells. Energy Environ. Sci. 2014, 7, 3690–3698. [Google Scholar] [CrossRef]
- Fu, A.; Yang, P. Lower Threshold for Nanowire Lasers. Nat. Mater. 2015, 14, 557–558. [Google Scholar] [CrossRef] [PubMed]
- Mettan, X.; Pisoni, R.; Matus, P.; Pisoni, A. Tuning of the Thermoelectric Figure of Merit of CH3NH3MI3 (M = Pb, Sn) Photovoltaic Perovskites. J. Phys. Chem. C 2015, 119, 11506–11510. [Google Scholar] [CrossRef]
- Kegelmann, L.; Wolff, C.M.; Awino, C.; Lang, F.; Unger, E.L.; Korte, L.; Dittrich, T.; Neher, D.; Rech, B.; Albrecht, S. It Takes Two to Tango-Double-Layer Selective Contacts in Perovskite Solar Cells for Improved Device Performance and Reduced Hysteresis. ACS Appl. Mater. Interfaces 2017, 9, 17245–17255. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, T.; Wang, N.; Luo, Q.; Lin, H.; Li, J.; Jiang, Q.; Wu, L.; Guo, Z. Ni-Doped α-Fe2O3 as Electron Transporting Material for Planar Heterojunction Perovskite Solar Cells with Improved Efficiency, Reduced Hysteresis and Ultraviolet Stability. Nano Energy 2017, 38, 193–200. [Google Scholar] [CrossRef]
- Van Reenen, S.; Kemerink, M.; Snaith, H.J. Modeling Anomalous Hysteresis in Perovskite Solar Cells. J. Phys. Chem. Lett. 2015, 6, 3808–3814. [Google Scholar] [CrossRef]
- Kutes, Y.; Ye, L.; Zhou, Y.; Pang, S.; Huey, B.D.; Padture, N.P. Direct Observation of Ferroelectric Domains in Solution-Processed CH3NH3PbI3 Perovskite Thin Films. J. Phys. Chem. Lett. 2014, 5, 3335–3339. [Google Scholar] [CrossRef]
- Tao, C.; Neutzner, S.; Colella, L.; Marras, S.; Srimath Kandada, A.R.; Gandini, M.; de Bastiani, M.; Pace, G.; Manna, L.; Caironi, M.; et al. 17.6% Stabilized Efficiency in Low-Temperature Processed Planar Perovskite Solar Cells. Energy Environ. Sci. 2015, 8, 2365–2370. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.H.; Lee, J.H.; Hong, K.H. The Role of Intrinsic Defects in Methylammonium Lead Iodide Perovskite. J. Phys. Chem. Lett. 2014, 5, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.J.; Shi, T.; Yan, Y. Unusual Defect Physics in CH3NH3PbI3 Perovskite Solar Cell Absorber. Appl. Phys. Lett. 2014, 104, 063903. [Google Scholar] [CrossRef]
- Sanchez, R.S.; Gonzalez-Pedro, V.; Lee, J.W.; Park, N.G.; Kang, Y.S.; Mora-Sero, I.; Bisquert, J. Slow Dynamic Processes in Lead Halide Perovskite Solar Cells. Characteristic Times and Hysteresis. J. Phys. Chem. Lett. 2014, 5, 2357–2363. [Google Scholar] [CrossRef]
- Pockett, A.; Eperon, G.E.; Peltola, T.; Snaith, H.J.; Walker, A.; Peter, L.M.; Cameron, P.J. Characterization of Planar Lead Halide Perovskite Solar Cells by Impedance Spectroscopy, Open-Circuit Photovoltage Decay, and Intensity-Modulated Photovoltage/Photocurrent Spectroscopy. J. Phys. Chem. C 2015, 119, 3456–3465. [Google Scholar] [CrossRef]
- Xiao, Z.; Yuan, Y.; Shao, Y.; Wang, Q.; Dong, Q.; Bi, C.; Sharma, P.; Gruverman, A.; Huang, J. Giant Switchable Photovoltaic Effect in Organometal Trihalide Perovskite Devices. Nat. Mater. 2015, 14, 193–197. [Google Scholar] [CrossRef]
- Shao, Y.; Xiao, Z.; Bi, C.; Yuan, Y.; Huang, J. Origin and Elimination of Photocurrent Hysteresis by Fullerene Passivation in CH3NH3PbI3 Planar Heterojunction Solar Cells. Nat. Commun. 2014, 5, 5784. [Google Scholar] [CrossRef]
- Duan, H.S.; Zhou, H.; Chen, Q.; Sun, P.; Luo, S.; Bin Song, T.; Bob, B.; Yang, Y. The Identification and Characterization of Defect States in Hybrid Organic-Inorganic Perovskite Photovoltaics. Phys. Chem. Chem. Phys. 2015, 17, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Green, M.; Dunlop, E.; Kopidakis, N.; Hao, X.; Yoshita, M. Solar Cell Efficiency Tables (Version 57). Prog. Photovolt. Res. Appl. 2021, 29, 3–15. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Guo, Q.; Yang, Y.; Luo, H.; Liu, Q. Pyrrole: An Additive for Improving the e Ffi Ciency and Stability of Perovskite Solar Cells †. J. Mater. Chem. A 2019, 7, 11764–11770. [Google Scholar] [CrossRef]
- Chang, X.; Fang, J.; Fan, Y.; Luo, T.; Su, H.; Zhang, Y.; Lu, J.; Tsetseris, L.; Anthopoulos, T.D.; Liu, S.F.; et al. Printable CsPbI 3 Perovskite Solar Cells with PCE of 19% via an Additive Strategy. Adv. Mater. 2020, 32, 2001243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, J.; Wang, S.; Pan, W.; Zhang, M.; Wang, X.; Lan, Z.; Lin, J. Additive Engineering by 6-Aminoquinoline Monohydrochloride for High-Performance Perovskite Solar Cells. ACS Appl. Energy Mater. 2021, 4, 7083–7090. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, Y.; Eickemeyer, F.T.; Pan, L.; Ren, D.; Ruiz-Preciado, M.A.; Carlsen, B.; Yang, B.; Dong, X.; Wang, Z.; et al. Tailored Amphiphilic Molecular Mitigators for Stable Perovskite Solar Cells with 23.5% Efficiency. Adv. Mater. 2020, 32, 1907757. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeong, S.; Seo, S.; Shin, H.; Ma, C.; Park, N.G. Acid Dissociation Constant: A Criterion for Selecting Passivation Agents in Perovskite Solar Cells. ACS Energy Lett. 2021, 6, 1612–1621. [Google Scholar] [CrossRef]
- Shu, H.; Xia, J.; Yang, H.; Luo, J.; Wan, Z.; Malik, H.A.; Han, F.; Yao, X.; Jia, C. Self-Assembled Hydrophobic Molecule-Based Surface Modification: A Strategy to Improve Efficiency and Stability of Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2020, 8, 10859–10869. [Google Scholar] [CrossRef]
- Lian, X.; Chen, J.; Shan, S.; Wu, G.; Chen, H. Polymer Modification on the NiOx Hole Transport Layer Boosts Open-Circuit Voltage to 1.19 V for Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 46340–46347. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, H.; Larson, B.W.; Xiao, C.; Dunfield, S.P.; Reid, O.G.; Chen, X.; Yang, M.; Berry, J.J.; Beard, M.C.; et al. Surface Lattice Engineering through Three-Dimensional Lead Iodide Perovskitoid for High-Performance Perovskite Solar Cells. Chem 2021, 7, 774–785. [Google Scholar] [CrossRef]
- Pu, X.; Han, J.; Wang, S.; Zhou, H.; Cao, Q.; Yang, J.; He, Z.; Li, X. Surface Modification with Ionic Liquid for Efficient CsPbI2Br Perovskite Solar Cells. J. Mater. 2021, 7, 1039–1048. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, Z.; Snaith, H.J.; Johnston, M.B.; Herz, L.M. Hybrid Perovskites: Prospects for Concentrator Solar Cells. Adv. Sci. 2018, 5, 1700792. [Google Scholar] [CrossRef]
- Cherif, F.E.; Sammouda, H. Optoelectronic Simulation and Optimization of Tandem and Multi-Junction Perovskite Solar Cells Using Concentrating Photovoltaic Systems. Energy Rep. 2021, 7, 5895–5908. [Google Scholar] [CrossRef]
- Baig, H.; Kanda, H.; Asiri, A.M.; Nazeeruddin, M.K.; Mallick, T. Increasing Efficiency of Perovskite Solar Cells Using Low Concentrating Photovoltaic Systems. Sustain. Energy Fuels 2020, 4, 528–537. [Google Scholar] [CrossRef]
- Bertoluzzi, L.; Boyd, C.C.; Rolston, N.; Xu, J.; Prasanna, R.; O’Regan, B.C.; McGehee, M.D. Mobile Ion Concentration Measurement and Open-Access Band Diagram Simulation Platform for Halide Perovskite Solar Cells. Joule 2020, 4, 109–127. [Google Scholar] [CrossRef]
- Bush, K.A.; Palmstrom, A.F.; Yu, Z.J.; Boccard, M.; Cheacharoen, R.; Mailoa, J.P.; McMeekin, D.P.; Hoye, R.L.Z.; Bailie, C.D.; Leijtens, T.; et al. 23.6%-Efficient Monolithic Perovskite/Silicon Tandem Solar Cells with Improved Stability. Nat. Energy 2017, 2, 17009. [Google Scholar] [CrossRef]
- Duong, T.; Wu, Y.L.; Shen, H.; Peng, J.; Fu, X.; Jacobs, D.; Wang, E.C.; Kho, T.C.; Fong, K.C.; Stocks, M.; et al. Rubidium Multication Perovskite with Optimized Bandgap for Perovskite-Silicon Tandem with over 26% Efficiency. Adv. Energy Mater. 2017, 7, 1700228. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Wang, X.; Sun, Y.; Zhao, Z.; Li, Y.; Zhou, H.; Chen, Q. Cost Analysis of Perovskite Tandem Photovoltaics. Joule 2018, 2, 1559–1572. [Google Scholar] [CrossRef]
- Werner, J.; Niesen, B.; Ballif, C. Perovskite/Silicon Tandem Solar Cells: Marriage of Convenience or True Love Story?—An Overview. Adv. Mater. Interfaces 2018, 5, 1700731. [Google Scholar] [CrossRef]
- Yu, Z.J.; Carpenter, J.V.; Holman, Z.C. Techno-Economic Viability of Silicon-Based Tandem Photovoltaic Modules in the United States. Nat. Energy 2018, 3, 747–753. [Google Scholar] [CrossRef]
- Todorov, T.; Gunawan, O.; Guha, S. A Road towards 25% Efficiency and beyond: Perovskite Tandem Solar Cells. Mol. Syst. Des. Eng. 2016, 1, 370–376. [Google Scholar] [CrossRef]
- Shen, H.; Walter, D.; Wu, Y.; Fong, K.C.; Jacobs, D.A.; Duong, T.; Peng, J.; Weber, K.; White, T.P.; Catchpole, K.R. Monolithic Perovskite/Si Tandem Solar Cells: Pathways to Over 30% Efficiency. Adv. Energy Mater. 2020, 10, 1902840. [Google Scholar] [CrossRef]
- Adjogri, S.J.; Meyer, E.L. Chalcogenide Perovskites and Perovskite-Based Chalcohalide as Photoabsorbers: A Study of Their Properties, and Potential Photovoltaic Applications. Materials 2021, 14, 7857. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, R.; Wang, X.; Liu, C.; Ahmed, Y.; Huang, Z.; Zhang, Z.; Li, H.; Zhang, M.; Gao, Y.; et al. Oxidation-Resistant All-Perovskite Tandem Solar Cells in Substrate Configuration. Nat. Commun. 2023, 14, 1819. [Google Scholar] [CrossRef]
- Jhou, J.C.; Gaurav, A.; Chang, C.H.; Lin, C.F. Enhanced Efficiency of Semitransparent Perovskite Solar Cells via Double-Sided Sandwich Evaporation Technique for Four Terminal Perovskite-Silicon Tandem Application. Nanomaterials 2022, 12, 1569. [Google Scholar] [CrossRef]
- Kumar, A.; Sayyed, M.I.; Sabugaa, M.M.; Singh, S.; Gavilán, J.C.O.; Sharma, A. Additive Engineering with Sodium Azide Material for Efficient Carbon-Based Perovskite Solar Cells. New J. Chem. 2023, 47, 7765–7773. [Google Scholar] [CrossRef]
- Sha, N.; Bala, H.; Chen, C.; Zhang, B.; Zhang, W.; An, X.; Chen, D.; Zhao, Z. Perovskite Solar Cells Using Naf Additive with Enhanced Stability Under Air Environment. Electrochim. Acta 2023, 456, 142409. [Google Scholar] [CrossRef]
- Shakher, C. Probing the Photodegradation of MAPI Perovskite with Concentrated Sunlight. Opt. Mater. 2022, 133, 113012. [Google Scholar] [CrossRef]
- Alnuaimi, A.; Almansouri, I.; Nayfeh, A. Performance of Planar Heterojunction Perovskite Solar Cells under Light Concentration. AIP Adv. 2016, 6, 115012. [Google Scholar] [CrossRef]
- Cai, M.; Wu, Y.; Chen, H.; Yang, X.; Qiang, Y.; Han, L. Cost-Performance Analysis of Perovskite Solar Modules. Adv. Sci. 2017, 4, 1600269. [Google Scholar] [CrossRef] [PubMed]






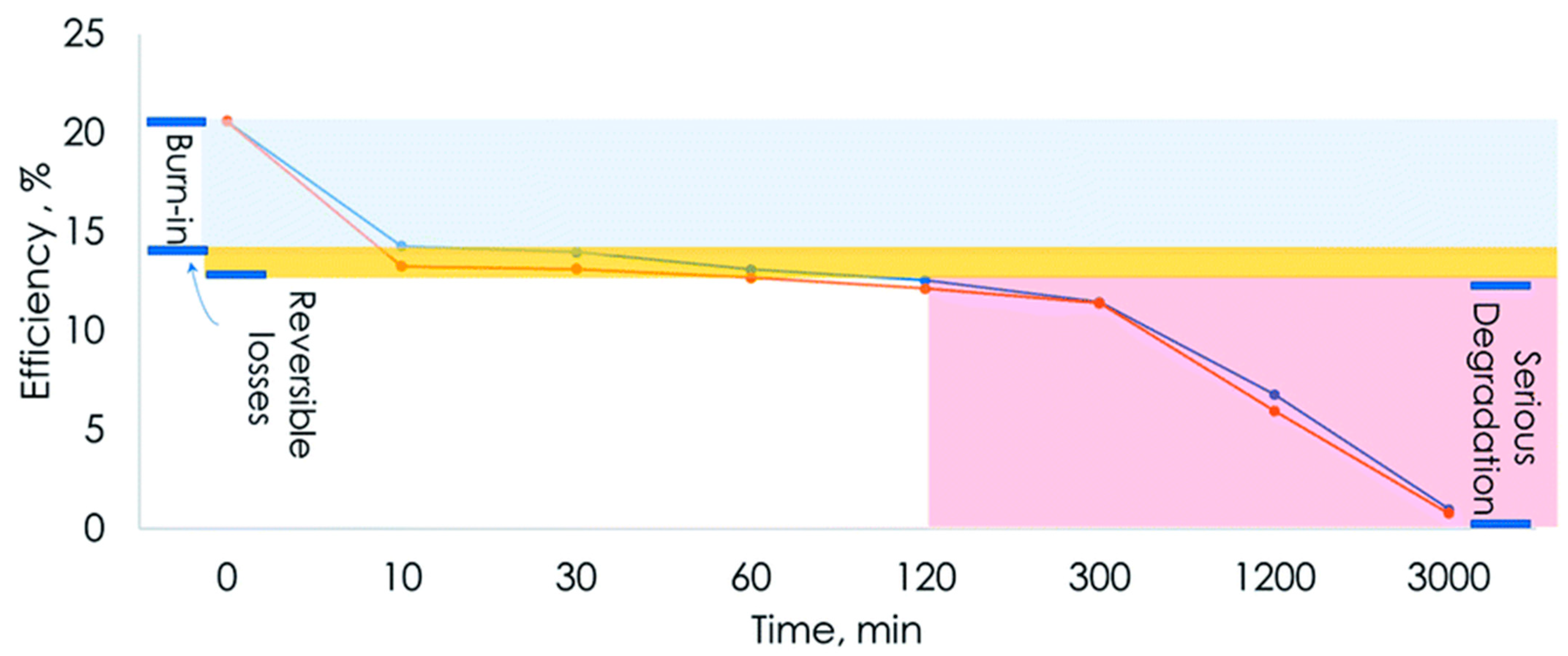
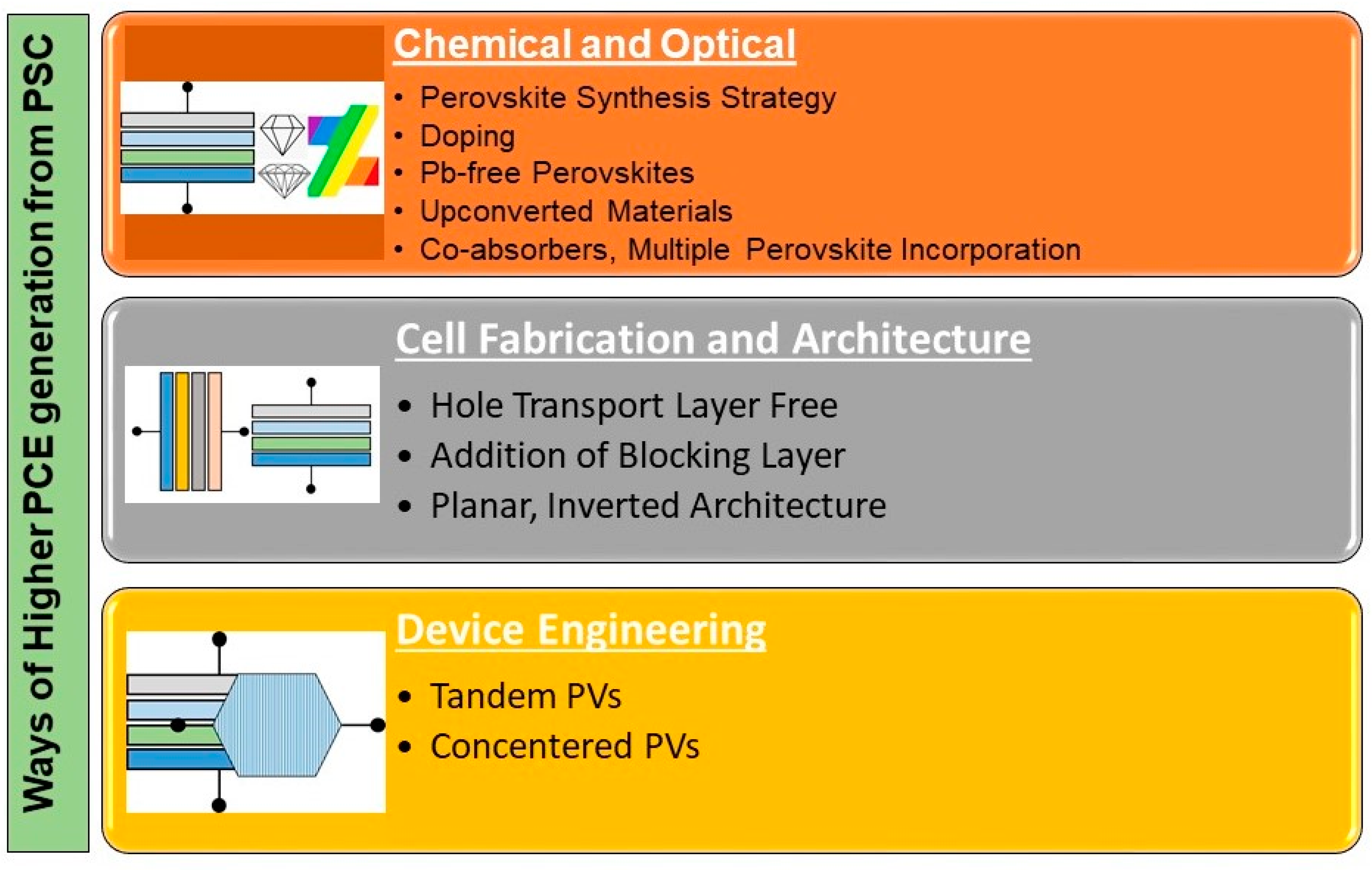

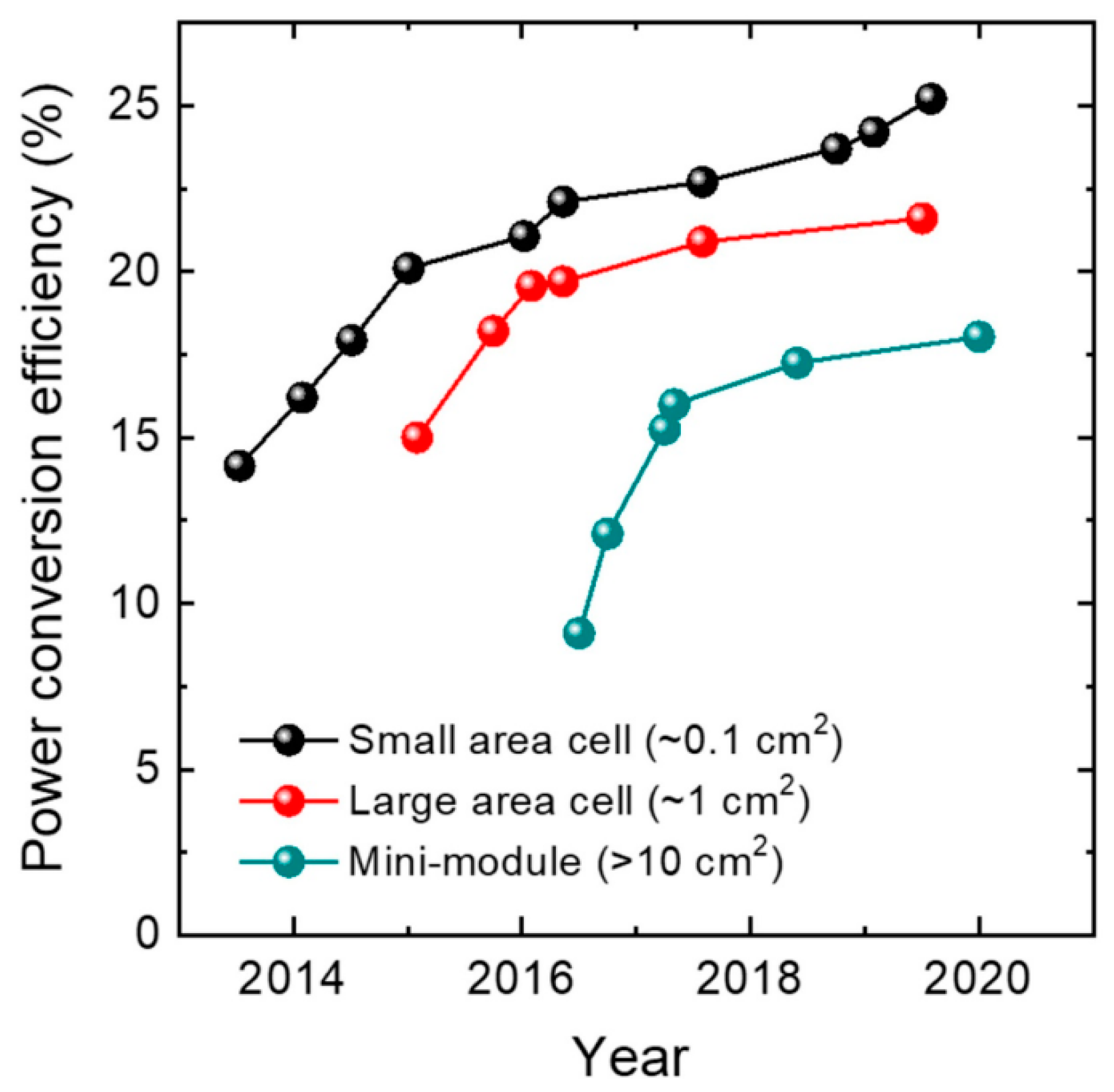
| Perovskite Material | Perovskite Composition | Efficiency | Year | References | ||||
|---|---|---|---|---|---|---|---|---|
| CH3NH3 mixed | (PEAI) on HC (NH2)2–CH3NH3 mixed | 23.32% | 2019 | [135] | ||||
| 25.5% | 2021 | [136] | ||||||
| Additive | ||||||||
| Perovskite Material | Additive | Efficiency | Year | References | ||||
| MAPbI3 | Pyrrole | 20.07% | 2019 | [137] | ||||
| CsPbI3 | Bis(pentafluorophenyl)zinc [Zn(C6F5)2] | 19% | 2020 | [138] | ||||
| Cs0.05(MA0.12FA0.88)0.95Pb (I0.88Br0.12)3 | 6-Aminoquinoline monohydrochloride (AQCl) | 21.66% | 2021 | [139] | ||||
| Defect passivation implement | ||||||||
| Passivation Material | Perovskite Absorbing Layer | Efficiency | Year | References | ||||
| Phenethylammonium iodide (PEAI) | (FAPbI3)1− x(MAPbBr3) | 23.32% | 2019 | [135] | ||||
| 4-Tert-butyl benzylammonium iodide(tBBAI) | Cs0.05FA0.85MA0.10Pb(I0.97Br0.03)3 | 23.50% | 2020 | [140] | ||||
| Cyclohexylammonium chloride (CYCl) | FAPbI3 | 23.34% | 2021 | [141] | ||||
| Surface modification | ||||||||
| Device architecture | Fabrication strategy | Efficiency | Year | References | ||||
| ITO/SnO2/perovskite/PTAA/Metal | Self-assembled facile strategy | 20.30 | 2019 | [142] | ||||
| ITO/NiOx/PTAA/(MAPbI3)0.95(MAPbBr2Cl)0.05/PCBM/BCP/Ag | Spin coating | 21.56 | 2020 | [143] | ||||
| FTO/TiO2/perovskite/(Me-PDA)Pb2I6/Spiro-OMETAD/Au | Perovskitoid surface engineering | 22.0 | 2021 | [144] | ||||
| FTO/TiO2/CsPbI2Br [PEVIM]Cl modified/Spiro-OMETAD/Ag | Surface modification | 14.19 | 2021 | [145] | ||||
| ITO/Cs0.05(FA0.92MA0.08)0.95Pb(I0.92Br0.08)3/ETL/BCP/Cu | Surface modification | 22.0 | 2022 | [66] | ||||
| CPV-based PSC | ||||||||
| Perovskite Material | Strategy | Device Area | Lifetime | Efficiency | No. of Suns | Stability | Year | References |
| FA0.83Cs0.17PbI2.7Br0.3 | Concentrated light | 9.19 mm2 | 150 h | 23.6% | 14 | 90% of initial η at 10 suns | 2018 | [11] |
| Concentrated light | 26.5% | Theoretical | 2018 | [146] | ||||
| Concentrated light | 24.93% | Theoretical | 2019 | [147] | ||||
| (FAPbI3)0.875 (MAPbBr3)0.125(CsPbI3)0.1 | Concentrated light | 9 mm2 | 5 h | 21.6% | 1.78 | 19% of initial η at 1.78 suns | 2020 | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, M.; Mallick, T.K. Stability and Performance Enhancement of Perovskite Solar Cells: A Review. Energies 2023, 16, 4031. https://doi.org/10.3390/en16104031
Khalid M, Mallick TK. Stability and Performance Enhancement of Perovskite Solar Cells: A Review. Energies. 2023; 16(10):4031. https://doi.org/10.3390/en16104031
Chicago/Turabian StyleKhalid, Maria, and Tapas Kumar Mallick. 2023. "Stability and Performance Enhancement of Perovskite Solar Cells: A Review" Energies 16, no. 10: 4031. https://doi.org/10.3390/en16104031
APA StyleKhalid, M., & Mallick, T. K. (2023). Stability and Performance Enhancement of Perovskite Solar Cells: A Review. Energies, 16(10), 4031. https://doi.org/10.3390/en16104031








