Towards the Development of Miniature Scale Liquid Fuel Combustors for Power Generation Application—A Review
Abstract
:1. Background
1.1. Need for Liquid Fuel Power Systems
1.2. Application and Different Power Ranges Required
1.3. Different Scale of Combustors
1.4. Central Theme of Preceding and Current Review
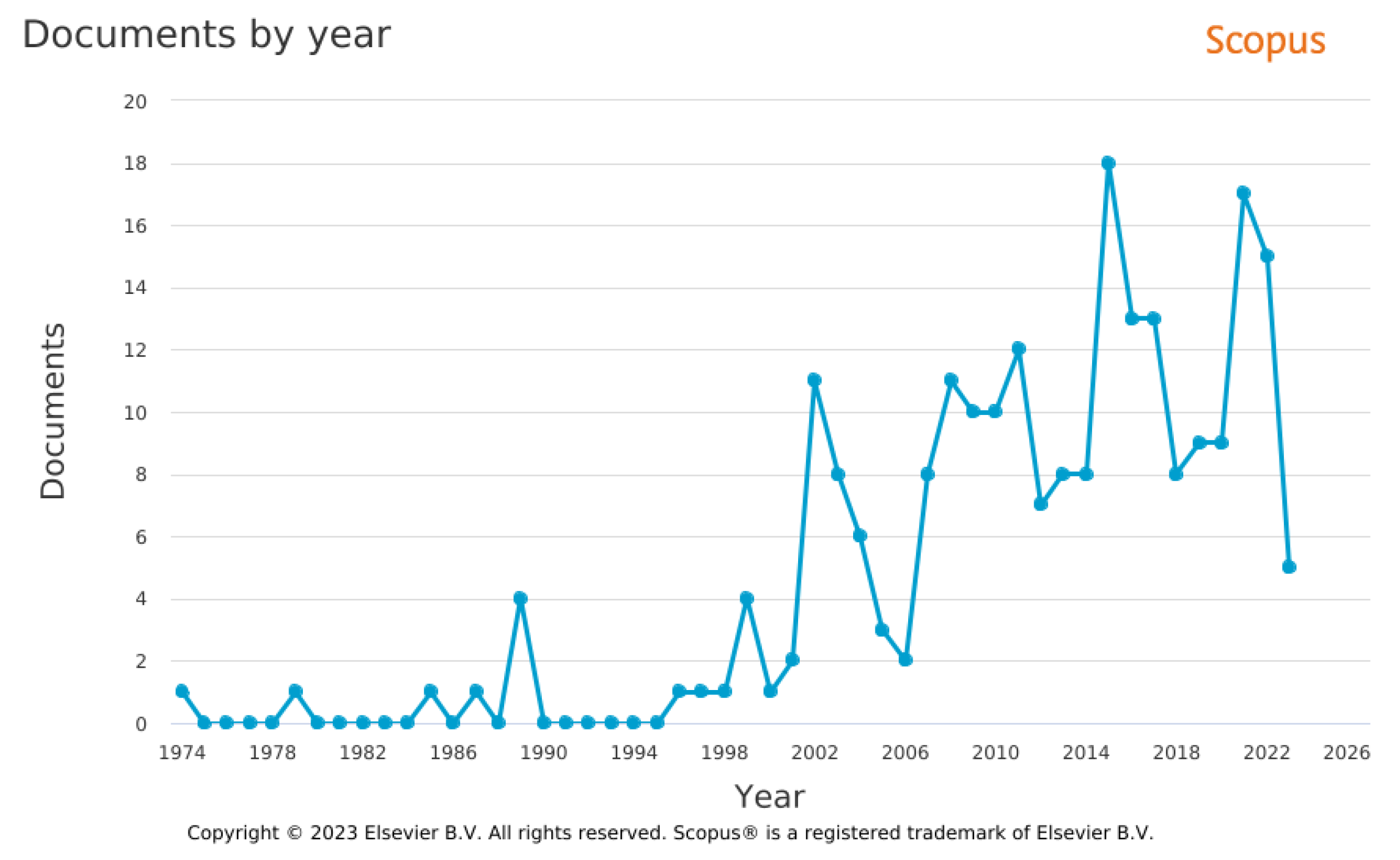
2. Mixture Preparation
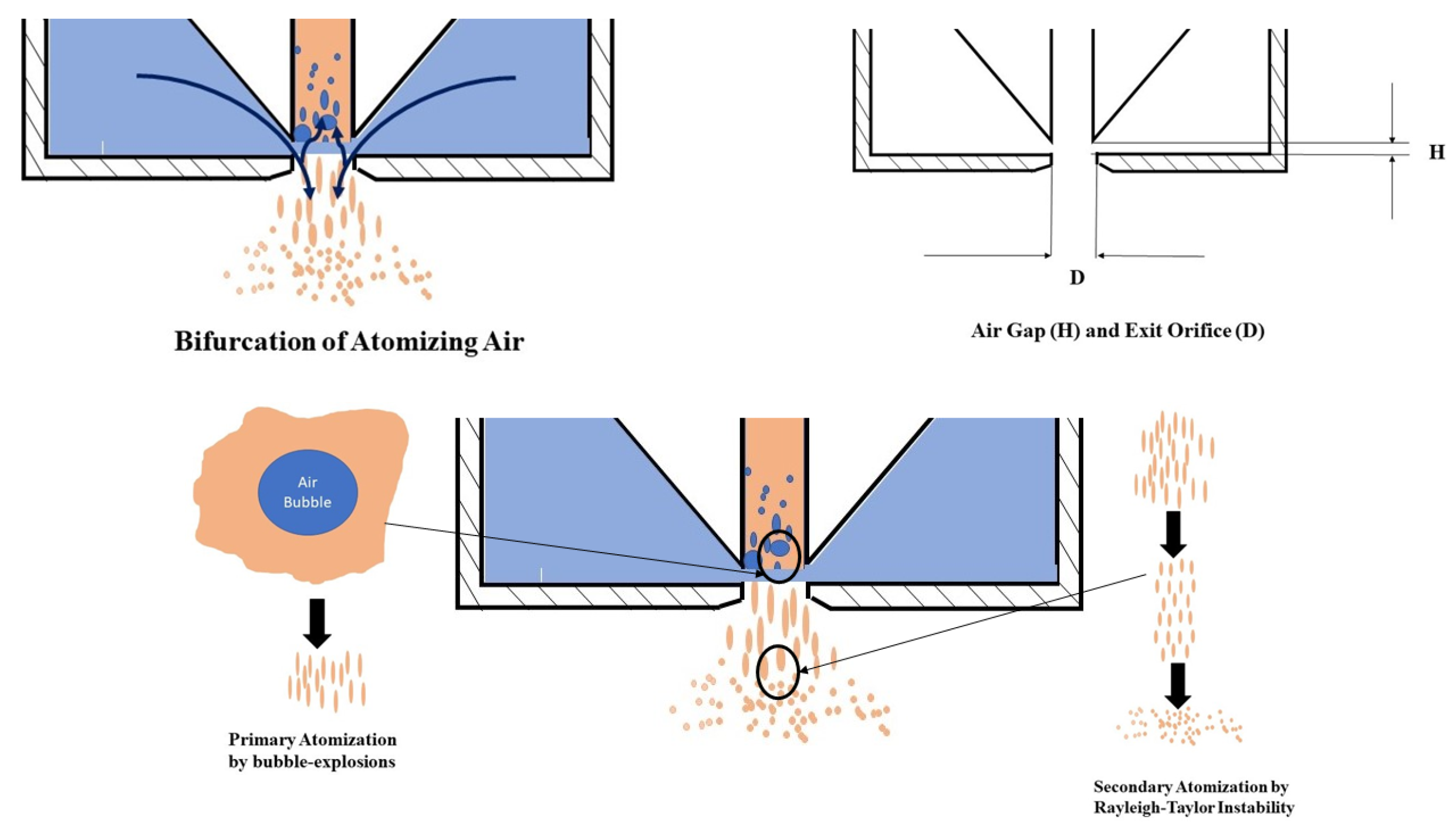
2.1. Injectors
| Previous Works | Liquid | Liquid Flow Rate (mg/s) | Air Flow Rate (g/s) |
|---|---|---|---|
| Simmons et al. [27] | Water | 80–830 | 0.2–0.8 |
| Jiang et al. [28] | Water | 200 | 0.4 |
| Azevedo et al. [29] | Water | 120–470 | 0.037–0.238 |
| Azevedo et al. [30] | soy methyl ester biodiesel | 110–560 | 0.026–0.107 |
| Azevedo et al. [31] | Ethanol | 80–420 | 0.082–0.24 |
| Azad et al. [19] | Water, Glycerine-water, Ethanol | 2.22–50.3 | 0.01–0.1 |
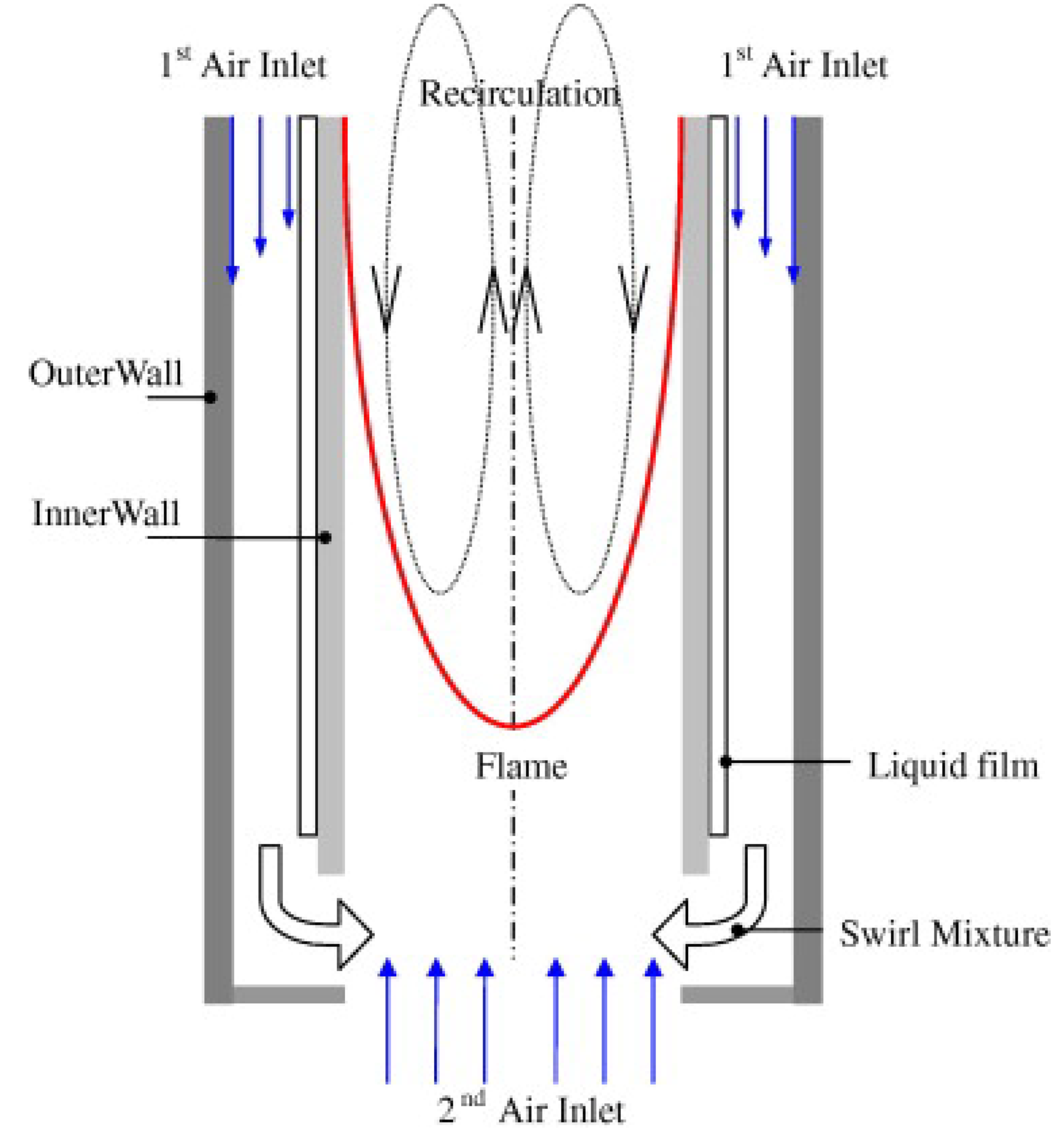
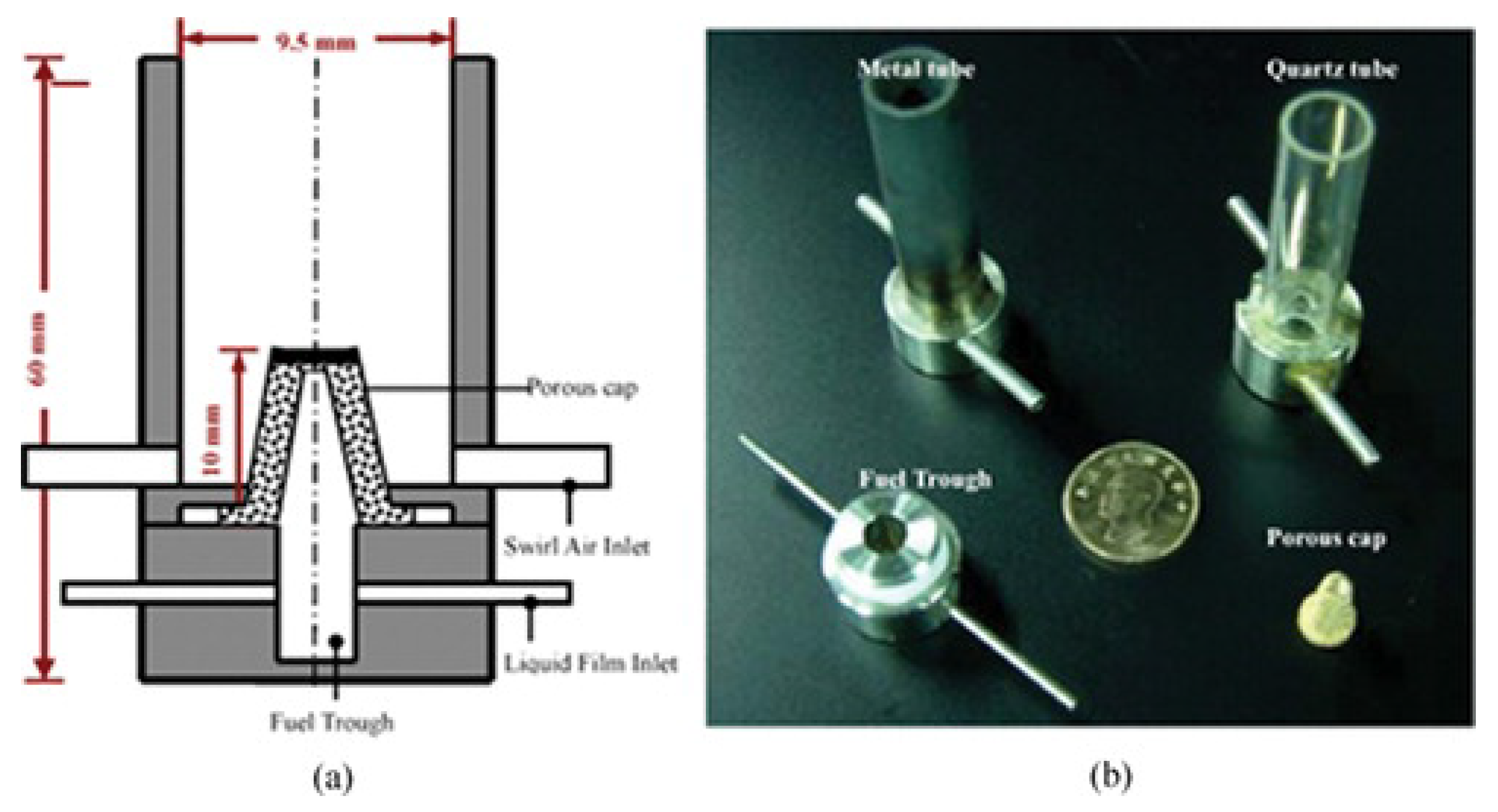
2.2. Fuel Film
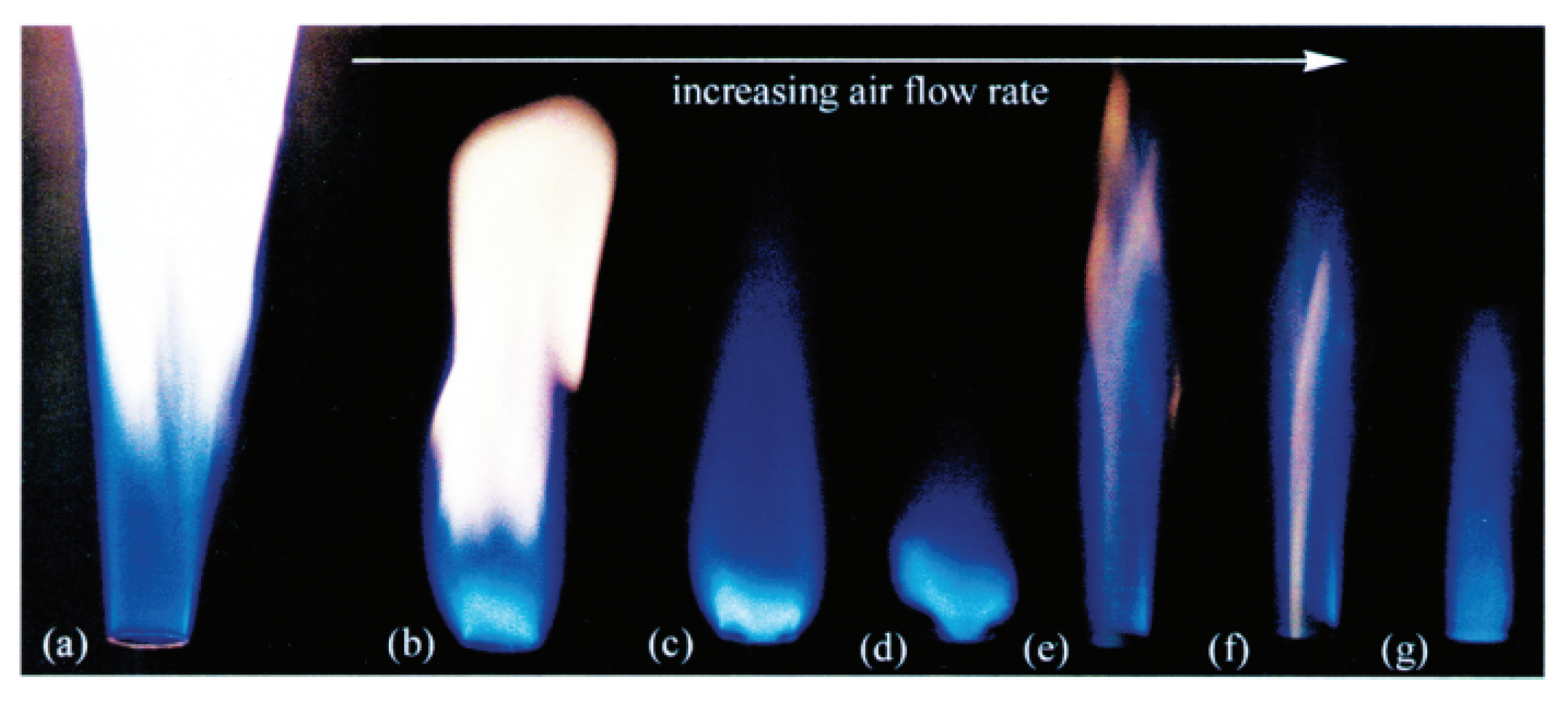
2.3. Electrospray
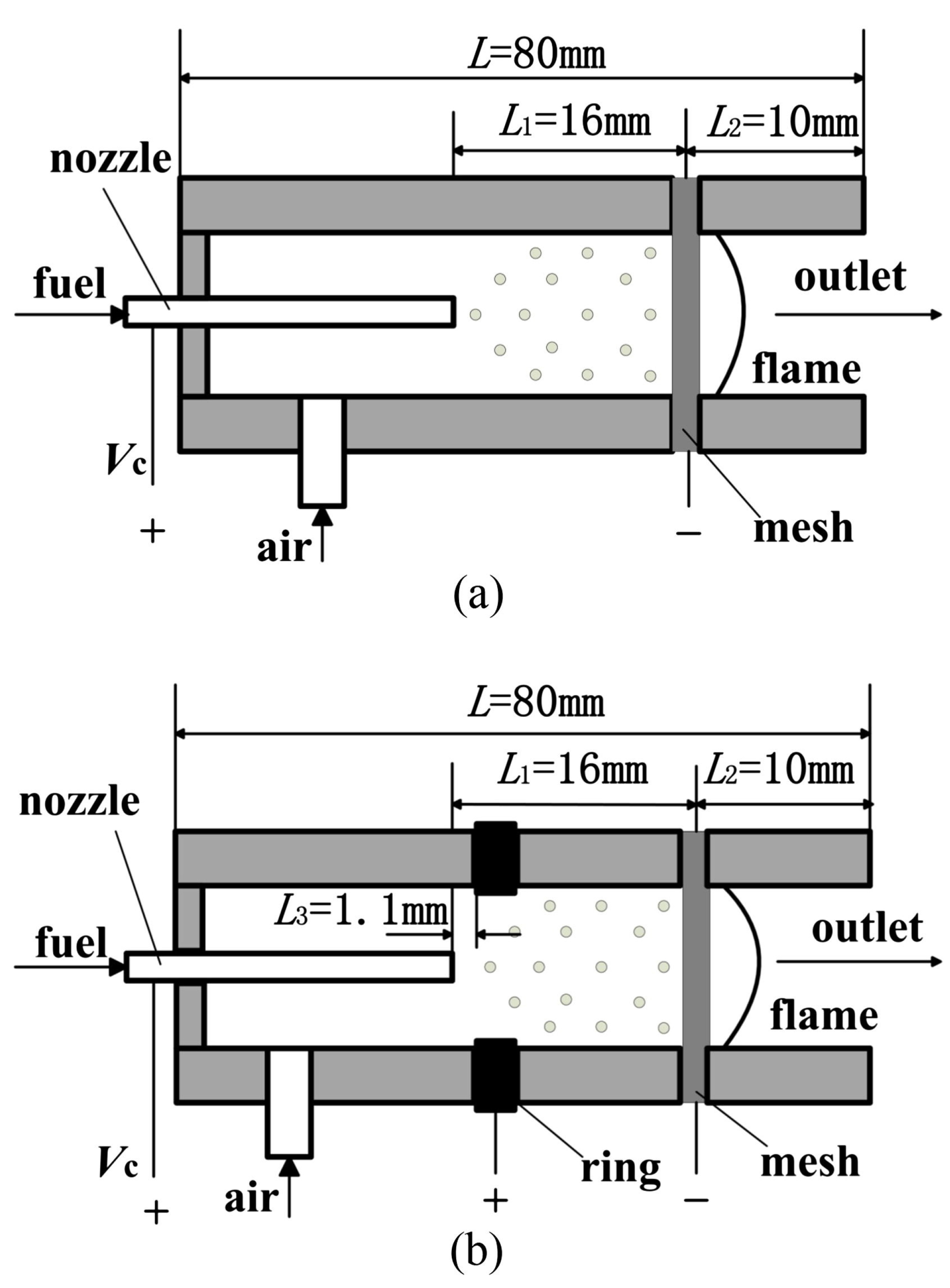
2.4. Porous Medium
2.5. Miscellaneous Designs
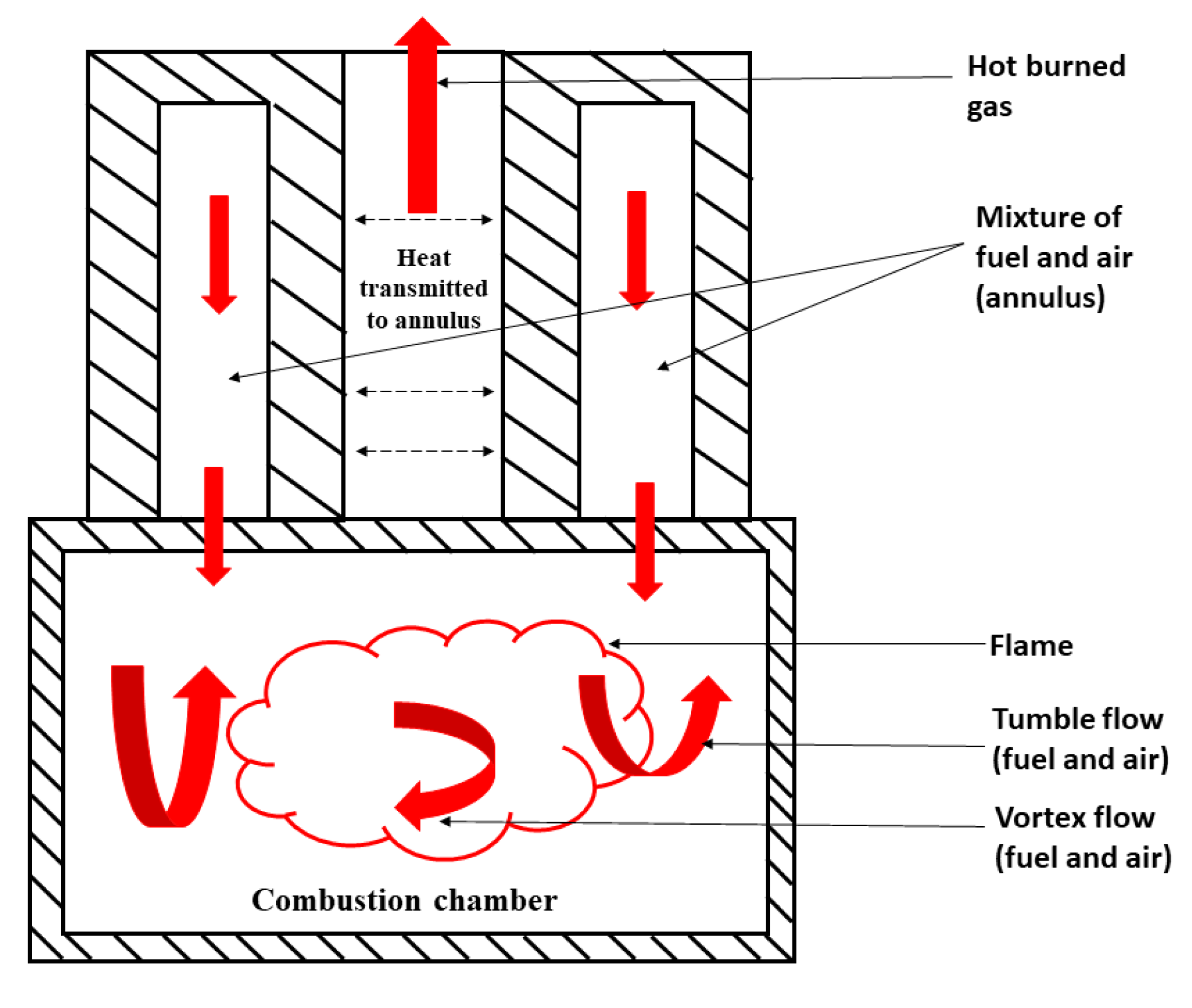
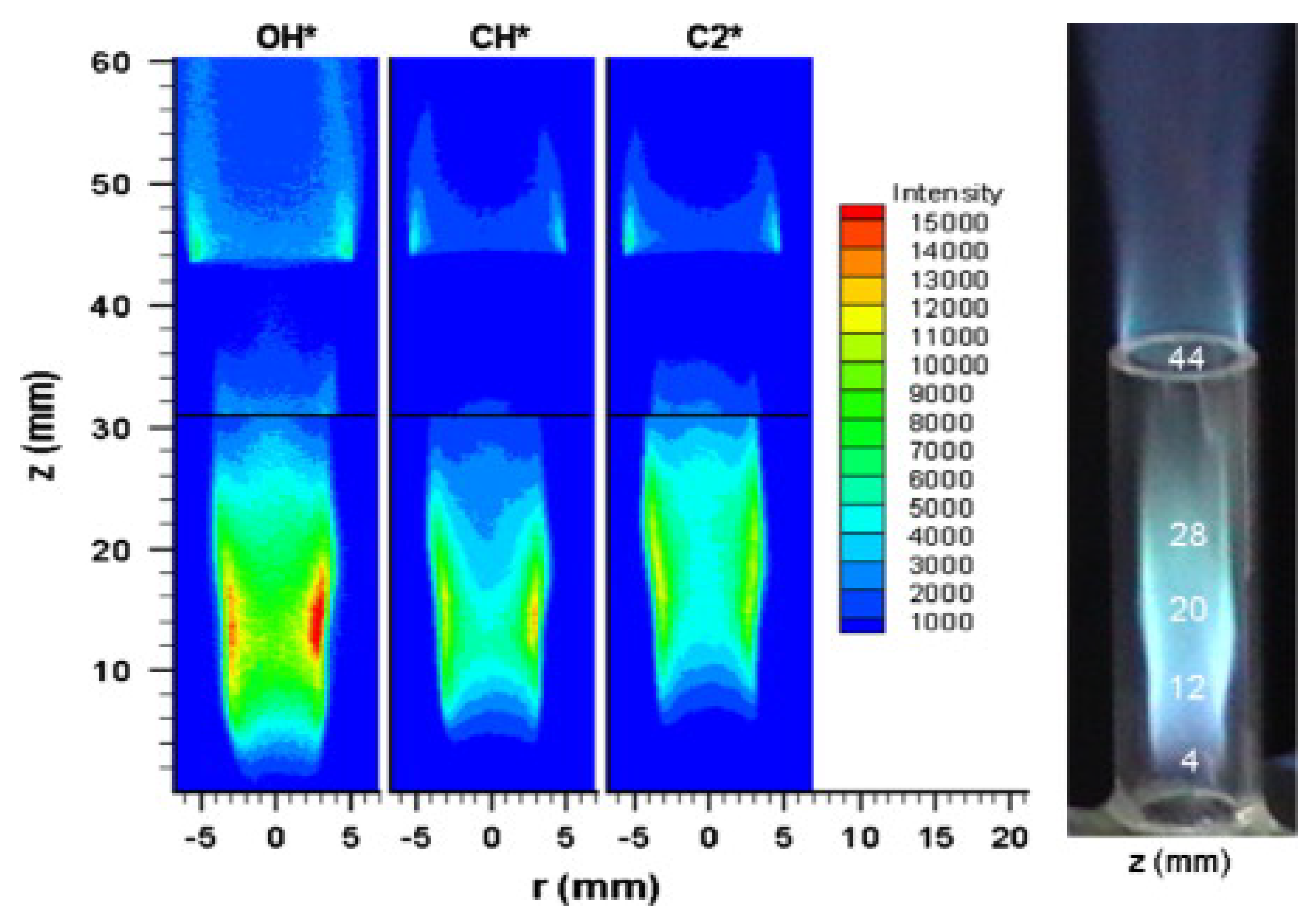
3. Combustion
4. Power Generation
5. Instabilities for Liquid Fuel Combustion at Small Scale
6. Summary and Outlook
- Flow-blurring atomizer is an ideal choice for ultra-low flow rate combustion systems as it can produce a fine spray without the need for high fuel injection pressure.
- Injecting liquid fuel into porous media, which possesses unique characteristics such as a large specific surface area, excellent heat transport capability, and flow permeability, was found to be one of the most promising techniques for facilitating liquid fuel combustion in compact combustors.
- The utilization of hydro-static pressure-driven pumping systems in miniature scale combustors is evidently a promising methodology for the development of standalone type power generators, because of the absence of electrical power-driven auxiliary components.
- The technique of utilizing exhaust gas enthalpy to preheat and evaporate incoming liquid fuel holds great potential for the development of liquid-fuelled miniature combustors.
- Investigations on electric power generation techniques from liquid-fueled miniature combustors are limited, and further investigation is necessary to advance the power generation methodology.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Micro electromechanical systems | |
| Thermoelectric generator | |
| Thermophotovoltaic systems | |
| Free flame | |
| Zirconia foam | |
| Nickel foam | |
| Inner diameter | |
| Outer diameter | |
| Flame repetitive extinction and ignition | |
| Micro flow reactor |
References
- Aravind, B.; Raghuram, G.K.; Kishore, V.R.; Kumar, S. Compact design of planar stepped micro combustor for portable thermoelectric power generation. Energy Convers. Manag. 2018, 156, 224–234. [Google Scholar] [CrossRef]
- Aravind, B.; Khandelwal, B.; Ramakrishna, P.; Kumar, S. Towards the development of a high power density, high efficiency, micro power generator. Appl. Energy 2020, 261, 114386. [Google Scholar] [CrossRef]
- Aravind, B.; Khandelwal, B.; Kumar, S. Recent Advancements in Microcombustion-Based Power Generators. Int. J. Energy Clean Environ. 2022, 23. [Google Scholar] [CrossRef]
- Aravind, B.; Hiranandani, K.; Kumar, S. Development of an ultra-high capacity hydrocarbon fuel based micro thermoelectric power generator. Energy 2020, 206, 118099. [Google Scholar] [CrossRef]
- Aravind, B.; Hiranandani, K.; Kumar, S. Experimental and Numerical Studies on Combustion-Based Small-Scale Power Generators. In Sustainable Development for Energy, Power, and Propulsion; Springer: Singapore, 2021; pp. 221–247. [Google Scholar]
- Behrens, D.A.; Lee, I.C.; Waits, C.M. Catalytic combustion of alcohols for microburner applications. J. Power Sources 2010, 195, 2008–2013. [Google Scholar] [CrossRef]
- Yang, X.; Yu, B.; Peng, X.; Zhou, H. Investigation of thermal performance and energy conversion in a novel planar micro-combustor with four-corner entrances for thermo-photovoltaic power generators. J. Power Sources 2021, 515, 230625. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Dunn-Rankin, D.; Leal, E.M.; Walther, D.C. Personal power systems. Prog. Energy Combust. Sci. 2005, 31, 422–465. [Google Scholar] [CrossRef]
- Kaisare, N.S.; Vlachos, D.G. A review on microcombustion: Fundamentals, devices and applications. Prog. Energy Combust. Sci. 2012, 38, 321–359. [Google Scholar] [CrossRef]
- Ju, Y.; Maruta, K. Microscale combustion: Technology development and fundamental research. Prog. Energy Combust. Sci. 2011, 37, 669–715. [Google Scholar] [CrossRef]
- Fernandez-Pello, A.C. Micropower generation using combustion: Issues and approaches. Proc. Combust. Inst. 2002, 29, 883–899. [Google Scholar] [CrossRef]
- Walther, D.C.; Ahn, J. Advances and challenges in the development of power-generation systems at small scales. Prog. Energy Combust. Sci. 2011, 37, 583–610. [Google Scholar] [CrossRef]
- Chou, S.; Yang, W.; Chua, K.; Li, J.; Zhang, K. Development of micro power generators—A review. Appl. Energy 2011, 88, 1–16. [Google Scholar] [CrossRef]
- Shirsat, V.; Gupta, A. A review of progress in heat recirculating meso-scale combustors. Appl. Energy 2011, 88, 4294–4309. [Google Scholar] [CrossRef]
- Nakamura, Y.; Gao, J.; Matsuoka, T. Progress in small-scale combustion. J. Therm. Sci. Technol. 2017, 12, JTST0001. [Google Scholar] [CrossRef]
- Wan, J.; Fan, A. Recent progress in flame stabilization technologies for combustion-based micro energy and power systems. Fuel 2021, 286, 119391. [Google Scholar] [CrossRef]
- Khan, M.A.; Gadgil, H.; Kumar, S. Influence of liquid properties on atomization characteristics of flow-blurring injector at ultra-low flow rates. Energy 2019, 171, 1–13. [Google Scholar] [CrossRef]
- Sadasivuni, V.; Agrawal, A.K. A novel meso-scale combustion system for operation with liquid fuels. Proc. Combust. Inst. 2009, 32, 3155–3162. [Google Scholar] [CrossRef]
- Gañán-Calvo, A.M. Enhanced liquid atomization: From flow-focusing to flow-blurring. Appl. Phys. Lett. 2005, 86, 214101. [Google Scholar] [CrossRef]
- Qavi, I.; Jiang, L. Optical characterization of near-field sprays for various alternative and conventional jet fuels using a flow-blurring injector. Flow Turbul. Combust. 2022, 108, 599–624. [Google Scholar] [CrossRef]
- Peck, J. Development of a Liquid-Fueled Micro-Combustor. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2008. [Google Scholar]
- Sirignano, W.A.; Pham, T.K.; Dunn-Rankin, D. Miniature-scale liquid-fuel-film combustor. Proc. Combust. Inst. 2002, 29, 925–931. [Google Scholar] [CrossRef]
- Vijayan, V.; Gupta, A. Thermal performance of a meso-scale liquid-fuel combustor. Appl. Energy 2011, 88, 2335–2343. [Google Scholar] [CrossRef]
- Giovannoni, V.; Sharma, R.N.; Raine, R.R. Experimental investigation of a small-scale combustion chamber fuelled with vegetable oil. Combust. Sci. Technol. 2019, 192, 240–259. [Google Scholar] [CrossRef]
- Simmons, B.M.; Panchasara, H.V.; Agrawal, A.K. A comparison of air-blast and flow-blurring injectors using phase Doppler particle analyzer technique. In Proceedings of the Turbo Expo: Power for Land, Sea, and Air, Orlando, FL, USA, 8–12 June 2009; Volume 48838, pp. 981–992. [Google Scholar]
- Jiang, L.; Agrawal, A.K. Spray features in the near field of a flow-blurring injector investigated by high-speed visualization and time-resolved PIV. Exp. Fluids 2015, 56, 1–13. [Google Scholar] [CrossRef]
- Azevedo, C.G.; de Andrade, J.C.; de Souza Costa, F. Effects of nozzle exit geometry on spray characteristics of a blurry injector. At. Sprays 2013, 23, 193–209. [Google Scholar] [CrossRef]
- de Azevedo, C.G.; de Andrade, J.C.; de Souza Costa, F. Effects of injector tip design on the spray characteristics of soy methyl ester biodiesel in a blurry injector. Renew. Energy 2016, 85, 287–294. [Google Scholar] [CrossRef]
- de Azevedo, C.G.; de Andrade, J.C.; de Souza, F.C. Experimental valuation diagnostics of hydrous ethanol sprays formed by a blurry injector. J. Aerosp. Technol. Manag. 2013, 5, 197–204. [Google Scholar] [CrossRef]
- Pham, T.K.; Amade, N.S.; Dunn-Rankin, D.; Sirignano, W. Liquid film combustion in small cylindrical chambers. In Proceedings of the Fourth Joint Meeting of the US Sections of the Combustion Institute, Philadelphia, PA, USA, 20–23 March 2005. [Google Scholar]
- Pham, T.K.; Dunn-Rankin, D.; Sirignano, W.A. Flame structure in small-scale liquid film combustors. Proc. Combust. Inst. 2007, 31, 3269–3275. [Google Scholar] [CrossRef]
- Li, Y.H.; Chao, Y.C.; Amadé, N.S.; Dunn-Rankin, D. Progress in miniature liquid film combustors: Double chamber and central porous fuel inlet designs. Exp. Therm. Fluid Sci. 2008, 32, 1118–1131. [Google Scholar] [CrossRef]
- Jaworek, A.; Krupa, A. Classification of the modes of EHD spraying. J. Aerosol Sci. 1999, 30, 873–893. [Google Scholar] [CrossRef]
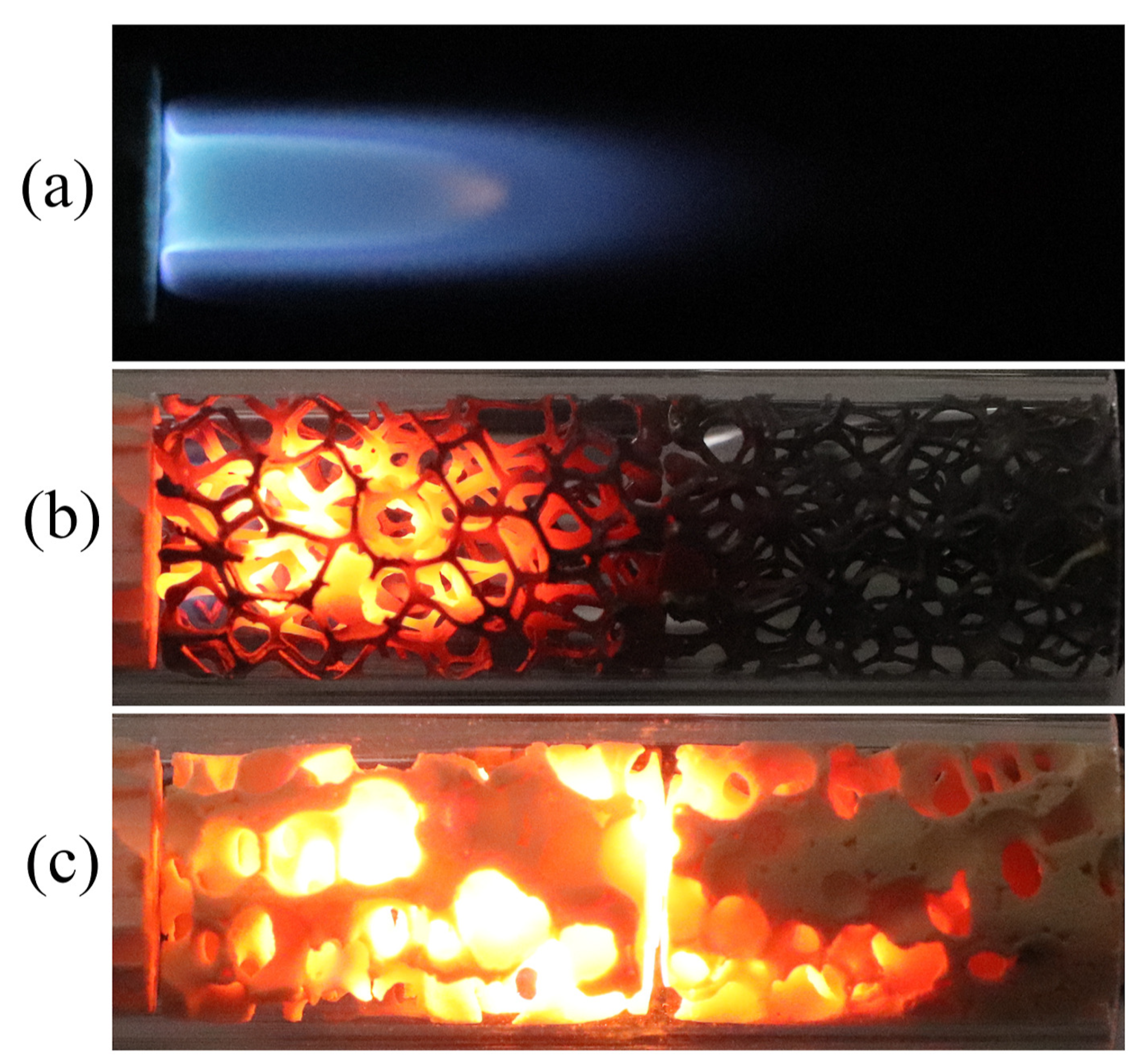
- Chen, X.; Li, J.; Feng, M.; Zhao, D.; Shi, B.; Wang, N. Flame stability and combustion characteristics of liquid fuel in a meso-scale burner with porous media. Fuel 2019, 251, 249–259. [Google Scholar] [CrossRef]
- Weclas, M. Potential of porous-media combustion technology as applied to internal combustion engines. J. Thermodyn. 2010, 2010, 789262. [Google Scholar] [CrossRef]
- Gharehghani, A.; Ghasemi, K.; Siavashi, M.; Mehranfar, S. Applications of porous materials in combustion systems: A comprehensive and state-of-the-art review. Fuel 2021, 304, 121411. [Google Scholar] [CrossRef]
- Mujeebu, M.A.; Abdullah, M.; Bakar, M.A.; Mohamad, A.; Abdullah, M. A review of investigations on liquid fuel combustion in porous inert media. Prog. Energy Combust. Sci. 2009, 35, 216–230. [Google Scholar] [CrossRef]
- Li, Y.H.; Chao, Y.C.; Dunn-Rankin, D. Combustion in a meso-scale liquid-fuel-film combustor with central-porous fuel inlet. Combust. Sci. Technol. 2008, 180, 1900–1919. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.; Yan, M.; Zhao, D.; Zhao, J.; Wei, Z.; Wang, N. Experimental study of n-heptane/air combustion in meso-scale burners with porous media. Exp. Therm. Fluid Sci. 2014, 52, 47–58. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Zhao, D.; Rashid, M.T.; Zhou, X.; Wang, N. Effects of porous media on partially premixed combustion and heat transfer in meso-scale burners fuelled with ethanol. Energy 2021, 224, 120191. [Google Scholar] [CrossRef]
- Majid, N.A.; Idris, A.C.; Faizal, H.M.; Rahman, M.R.A.; Hosseini, S.E. Characteristics of liquid fuel combustion in a novel miniature vortex combustor. J. Therm. Anal. Calorim. 2020, 140, 1569–1578. [Google Scholar] [CrossRef]
- Shi, B.; Cao, Q.; Xie, D.; Peng, W.; Wang, N. A novel combustion system for liquid fuel evaporating and burning. Proc. Combust. Inst. 2019, 37, 4329–4336. [Google Scholar] [CrossRef]
- Shimokuri, D.; Taomoto, Y.; Matsumoto, R. Development of a powerful miniature power system with a meso-scale vortex combustor. Proc. Combust. Inst. 2017, 36, 4253–4260. [Google Scholar] [CrossRef]
- Khan, M.A.; Kumar, S. Prototype development of a new self-aspirating liquid-fueled microcombustor. Combust. Sci. Technol. 2022, 1–21. [Google Scholar] [CrossRef]
- Kyritsis, D.C.; Roychoudhury, S.; McEnally, C.S.; Pfefferle, L.D.; Gomez, A. Mesoscale combustion: A first step towards liquid fueled batteries. Exp. Therm. Fluid Sci. 2004, 28, 763–770. [Google Scholar] [CrossRef]
- Yuliati, L.; Seo, T.; Mikami, M. Liquid-fuel combustion in a narrow tube using an electrospray technique. Combust. Flame 2012, 159, 462–464. [Google Scholar] [CrossRef]
- Gan, Y.; Luo, Z.; Cheng, Y.; Xu, J. The electro-spraying characteristics of ethanol for application in a small-scale combustor under combined electric field. Appl. Therm. Eng. 2015, 87, 595–604. [Google Scholar] [CrossRef]
- Gan, Y.; Tong, Y.; Ju, Y.; Zhang, X.; Li, H.; Chen, X. Experimental study on electro-spraying and combustion characteristics in meso-scale combustors. Energy Convers. Manag. 2017, 131, 10–17. [Google Scholar] [CrossRef]
- Gan, Y.; Tong, Y.; Jiang, Z.; Chen, X.; Li, H.; Jiang, X. Electro-spraying and catalytic combustion characteristics of ethanol in meso-scale combustors with steel and platinum meshes. Energy Convers. Manag. 2018, 164, 410–416. [Google Scholar] [CrossRef]
- Gan, Y.; Chen, X.; Tong, Y.; Zhang, X.; Zhang, Y. Thermal performance of a meso-scale combustor with electrospray technique using liquid ethanol as fuel. Appl. Therm. Eng. 2018, 128, 274–281. [Google Scholar] [CrossRef]
- Jiang, Z.; Gan, Y.; Ju, Y.; Liang, J.; Zhou, Y. Experimental study on the electrospray and combustion characteristics of biodiesel-ethanol blends in a meso-scale combustor. Energy 2019, 179, 843–849. [Google Scholar] [CrossRef]
- Stanchi, S.; Dunn-Rankin, D.; Sirignano, W. Combustor Miniaturization with Liquid-Fuel Filming. In Proceedings of the 41st Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 6–9 January 2003; p. 1163. [Google Scholar]
- Li, Y.H.; Chao, Y.C.; Dunn-Rankin, D. Combustion in Small-Scale Central-Porous-Media Liquid Film Combustors. In Proceedings of the 21st International Colloquium on Dynamics of Explosions and Reactive Systems, Poitiers, France, 23–27 July 2007; pp. 23–27. [Google Scholar]
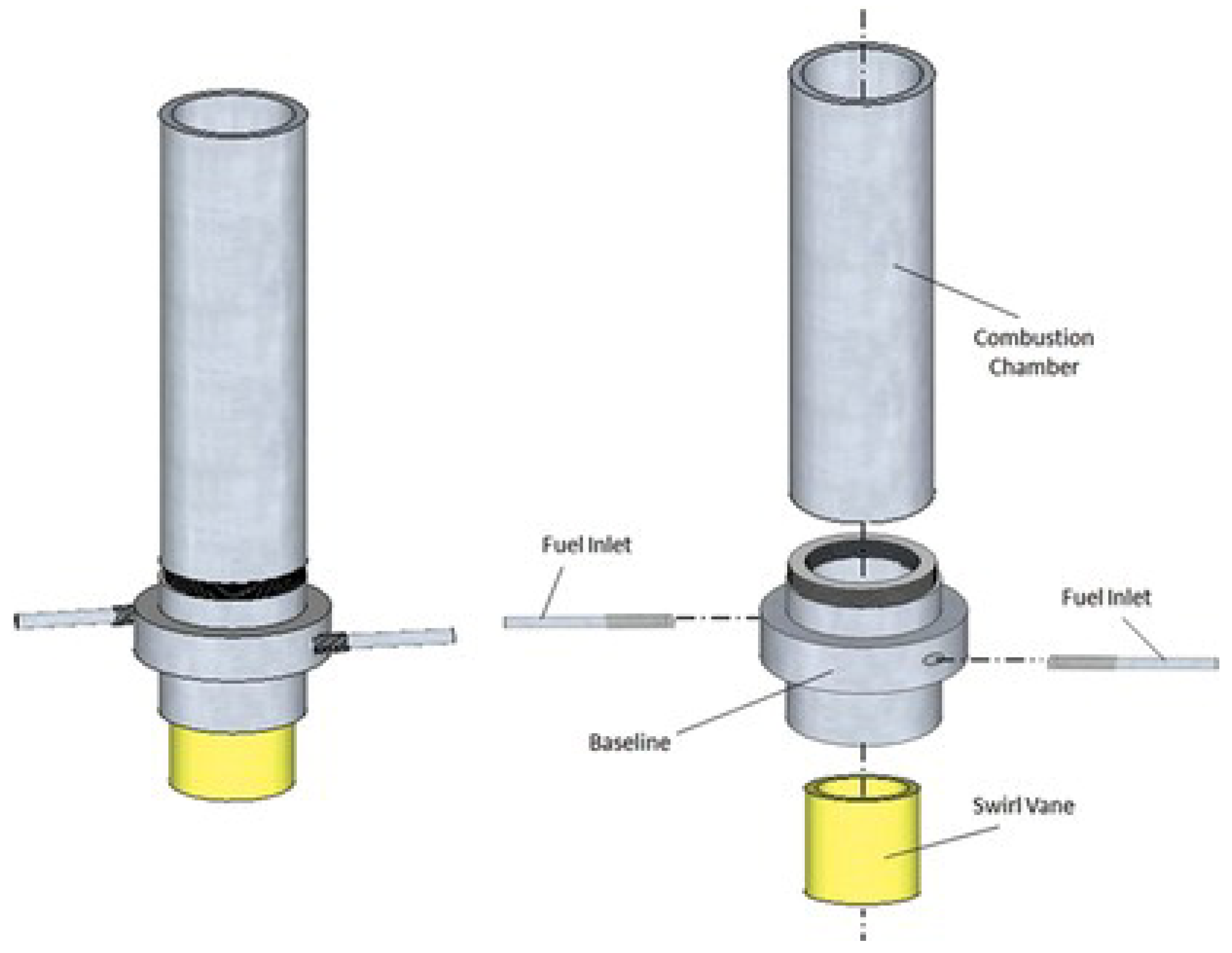
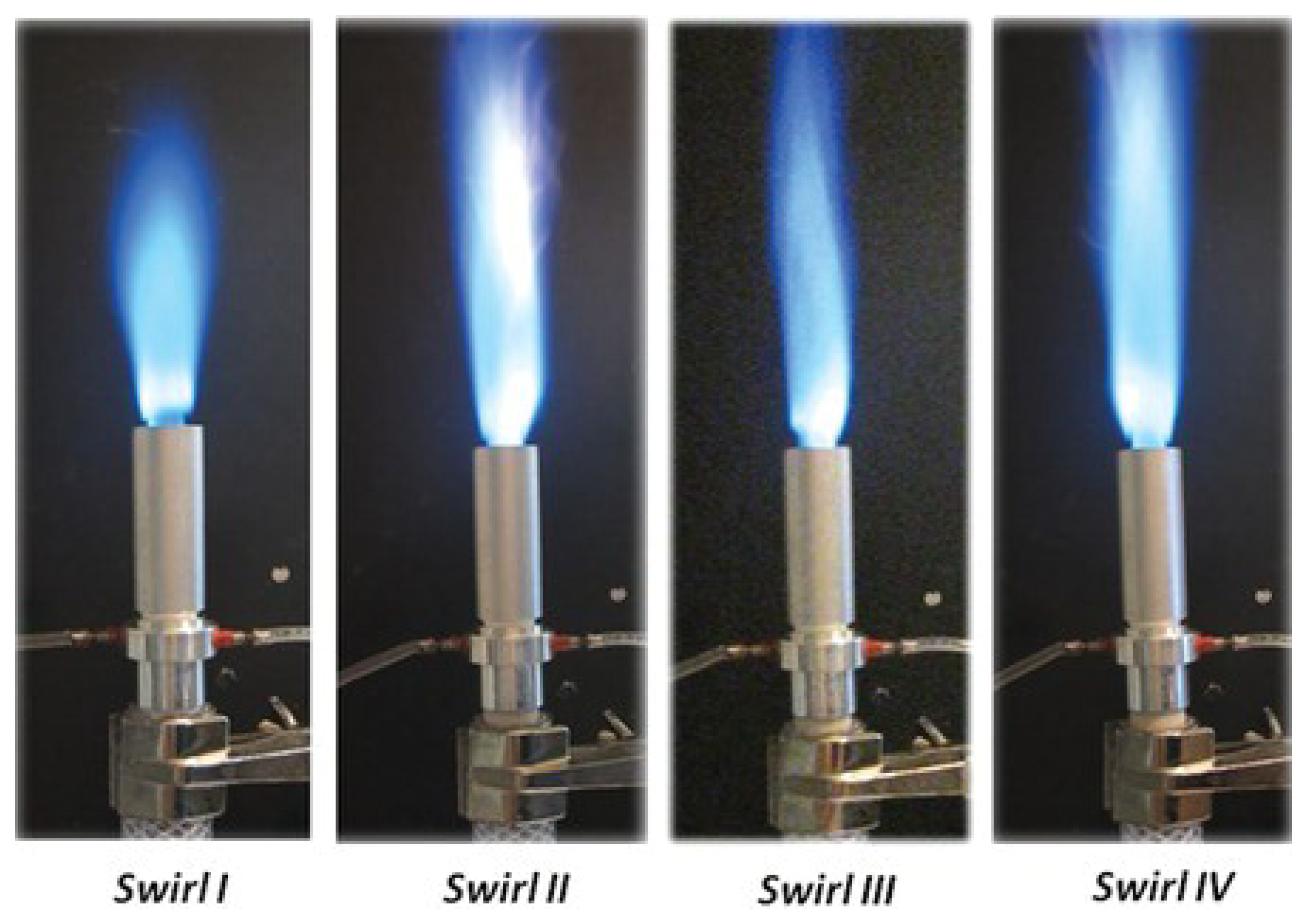
- Giani, C.; Dunn-Rankin, D. Miniature fuel film combustor: Swirl vane design and combustor characterization. Combust. Sci. Technol. 2013, 185, 1464–1481. [Google Scholar] [CrossRef]
- da Silva, A.P.; Sauer, V.M.; Dunn-Rankin, D. Experimental evaluation of a miniature liquid film combustor with secondary air injection. In Proceedings of the Fall 2015 Meeting of the Western States Section of the Combustion Institute, Provo, UT, USA, 5–6 October 2015; pp. 1–9. [Google Scholar]
- Sauer, V.M.; Dunn-Rankin, D. Liquid fuel nonpremixed swirl-type tubular flame burner. Combust. Sci. Technol. 2018, 195, 1–12. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.; Zhao, D.; Zhao, J.; Yan, M.; Wang, N. Diffusion combustion of liquid heptane in a small tube with and without heat recirculating. Combust. Sci. Technol. 2012, 184, 1591–1607. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.; Chen, X.; Zhao, D.; Shi, B.; Wei, Z.; Wang, N. Effects of heat recirculation on combustion characteristics of n-heptane in micro combustors. Appl. Therm. Eng. 2016, 109, 697–708. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.; Chen, X.; Yan, M.; Zhao, D.; Wei, Z.; Wang, N. Experimental study on flame stability and thermal performance of an n-heptane-fueled microscale combustor. Combust. Sci. Technol. 2017, 189, 1198–1215. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Feng, M.; Wang, N. Effects of external heating on flame stability in a micro porous combustor fuelled with heptane. Combust. Sci. Technol. 2019, 191, 311–324. [Google Scholar] [CrossRef]
- Bharadwaz, N.A.; Jain, N.; Arghode, V.K. Development of a standalone, liquid fuelled miniature power generation system. J. Energy Resour. Technol. 2020, 142, 042004. [Google Scholar] [CrossRef]
- Li, Y.H.; Lien, Y.S.; Chao, Y.C.; Dunn-Rankin, D. Performance of a mesoscale liquid fuel-film combustion-driven TPV power system. Prog. Photovoltaics Res. Appl. 2009, 17, 327–336. [Google Scholar] [CrossRef]
- Li, Y.H.; Li, H.Y.; Dunn-Rankin, D.; Chao, Y.C. Enhancing thermal, electrical efficiencies of a miniature combustion-driven thermophotovoltaic system. Prog. Photovoltaics Res. Appl. 2009, 17, 502–512. [Google Scholar] [CrossRef]
- Jain, N.; Arghode, V. Development of a Standalone, Liquid Fuelled Miniature Power Generation System. In Proceedings of the ASME Power Conference. American Society of Mechanical Engineers, Charlotte, NC, USA, 26–30 June 2017; Volume 57601, p. V001T04A028. [Google Scholar]
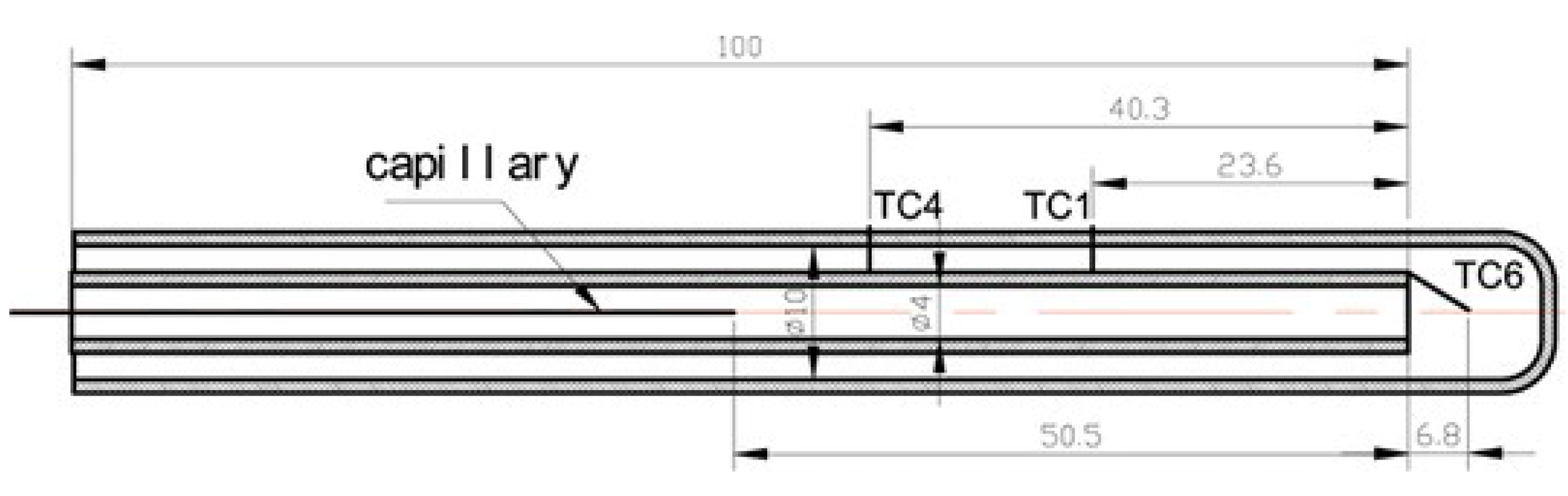
- Singh, S.; Veetil, J.E.; Kumbhakarna, N.; Velamati, R.K.; Kumar, S. Flame dynamics of premixed CH4/H2/air flames in a microchannel with a wall temperature gradient. Combust. Theory Model. 2022, 26, 989–1013. [Google Scholar] [CrossRef]
- Nair, A.; Kishore, V.R.; Kumar, S. Dynamics of premixed hydrogen-air flames in microchannels with a wall temperature gradient. Combust. Sci. Technol. 2015, 187, 1620–1637. [Google Scholar] [CrossRef]
- Veetil, J.E.; Kumbhakarna, N.; Singh, S.; Velamati, R.K.; Kumar, S. Effect of hydrogen addition on the dynamics of premixed C1–C4 alkane-air flames in a microchannel with a wall temperature gradient. Int. J. Hydrogen Energy 2022, 47, 30660–30670. [Google Scholar] [CrossRef]
- Kishore, V.R.; Minaev, S.; Akram, M.; Kumar, S. Dynamics of premixed methane/air mixtures in a heated microchannel with different wall temperature gradients. RSC Adv. 2017, 7, 2066–2073. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Zhao, D.; Song, A.; Zhou, X.; Wang, N. Experimental investigation on propagation characteristics of n-heptane/air combustion wave in foamed porous media. Fuel 2021, 306, 121742. [Google Scholar] [CrossRef]
- Chen, J.; Peng, X.; Yang, Z.; Cheng, J. Characteristics of liquid ethanol diffusion flames from mini tube nozzles. Combust. Flame 2009, 156, 460–466. [Google Scholar] [CrossRef]
- Akita, K.; Morii, Y.; Nakamura, H.; Tezuka, T.; Maruta, K. 2D computations of FREI with cool flames for n-heptane/air mixture. Proc. Combust. Inst. 2021, 38, 2247–2255. [Google Scholar] [CrossRef]
- Akita, K.; Morii, Y.; Murakami, Y.; Nakamura, H.; Tezuka, T.; Maruta, K. Dynamics of FREI with/without cool flame interaction. Proc. Combust. Inst. 2022. [Google Scholar] [CrossRef]

























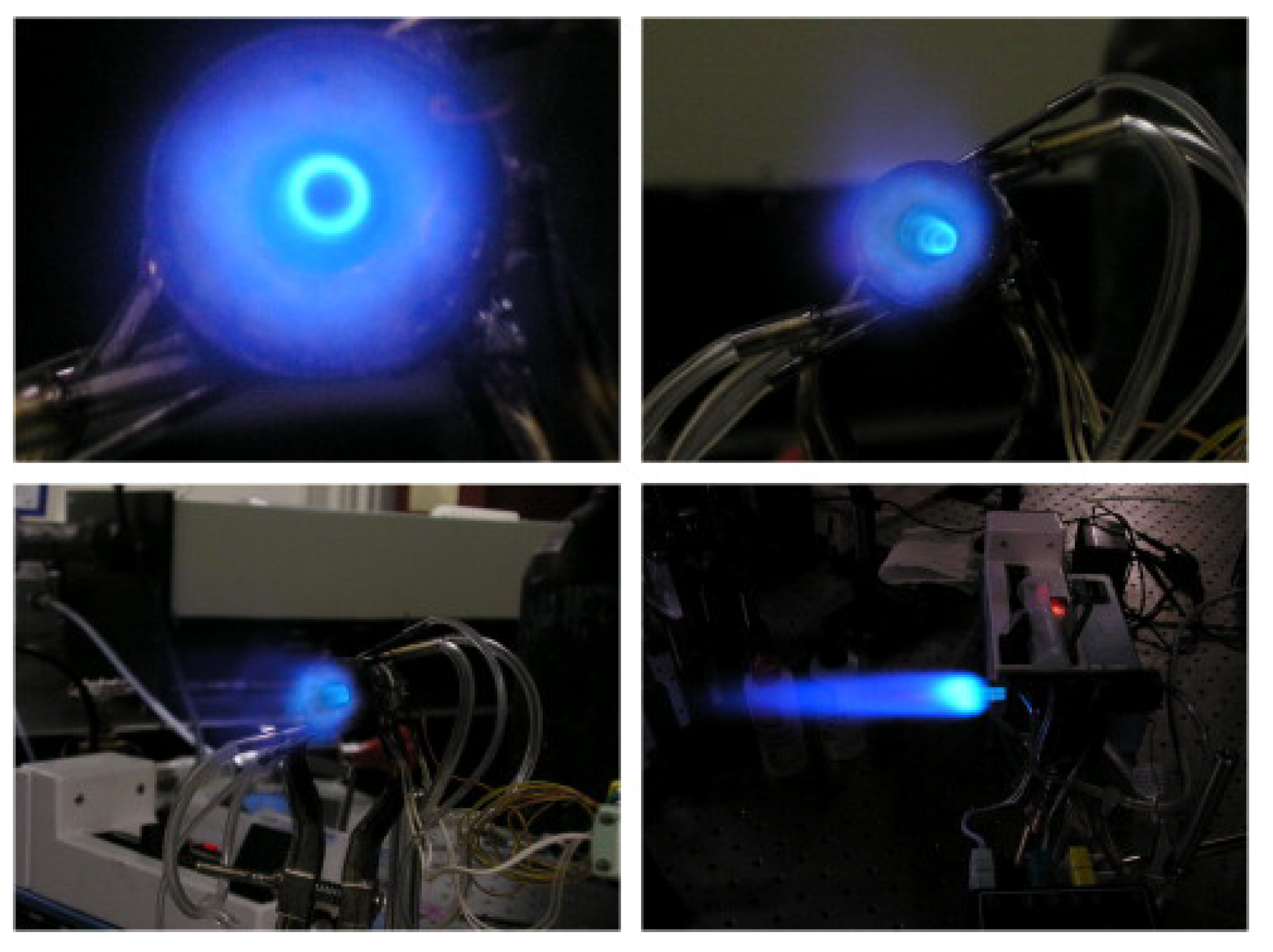
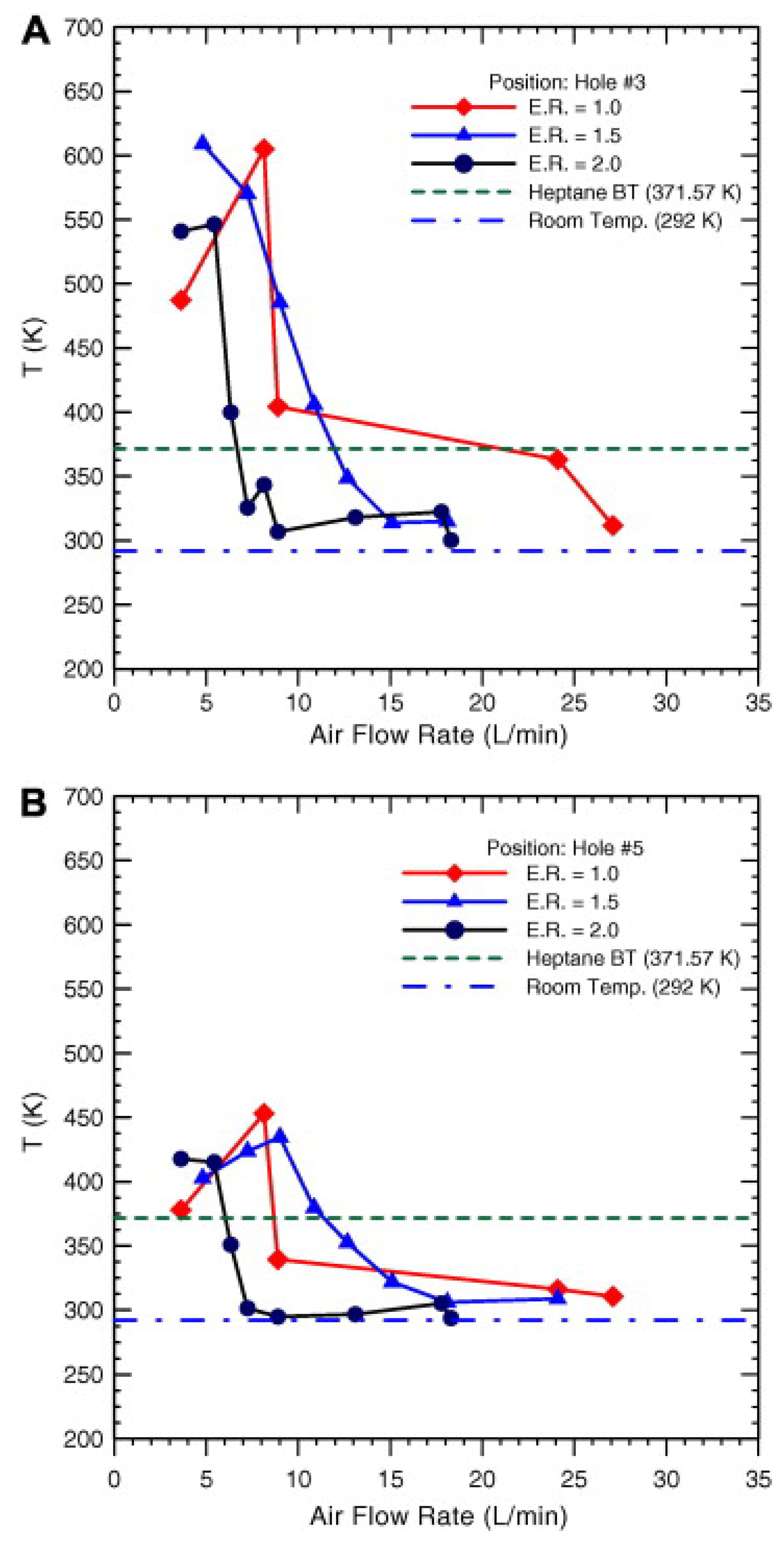
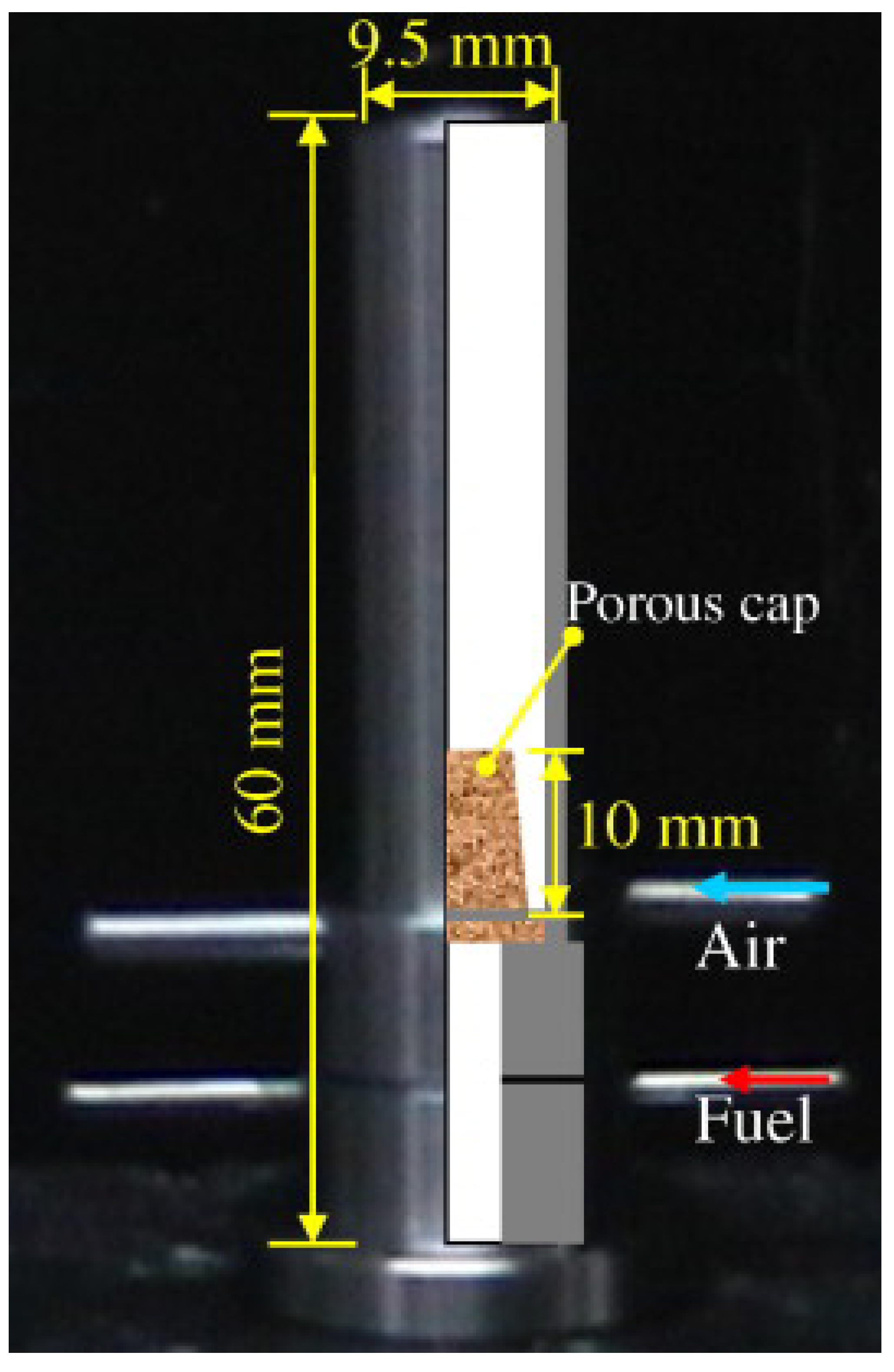
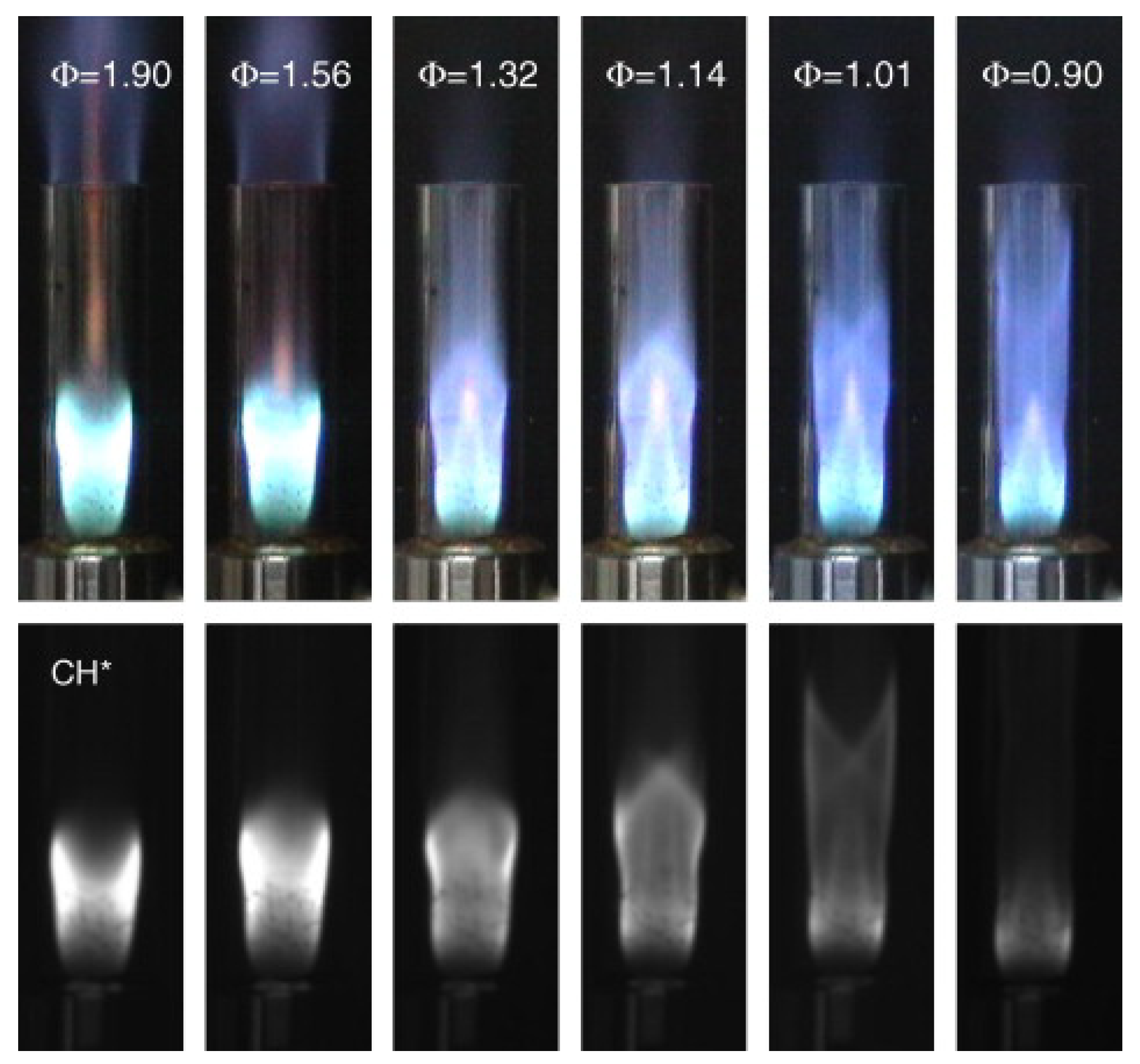






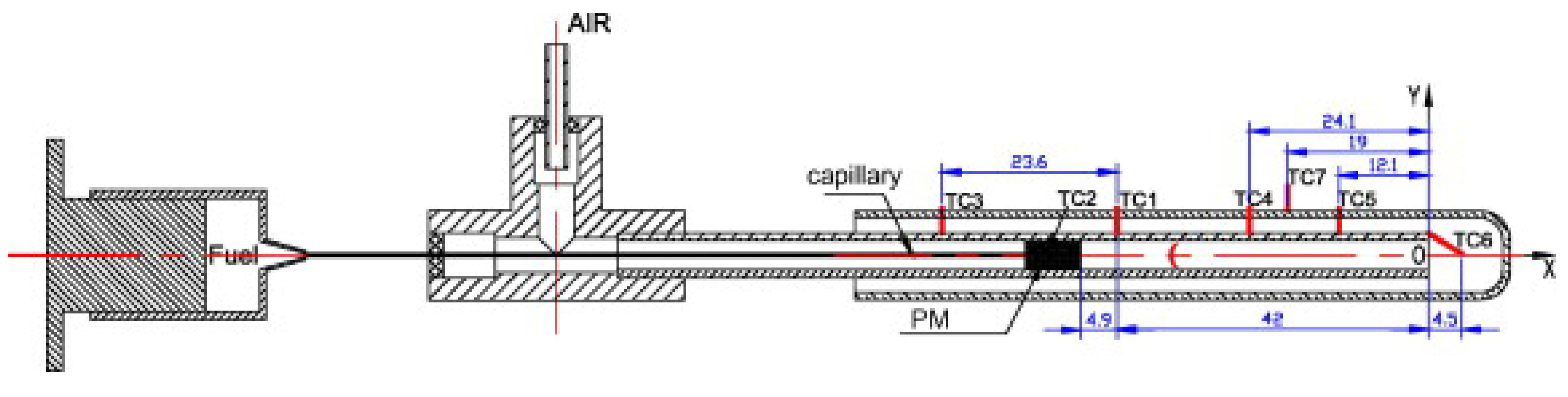

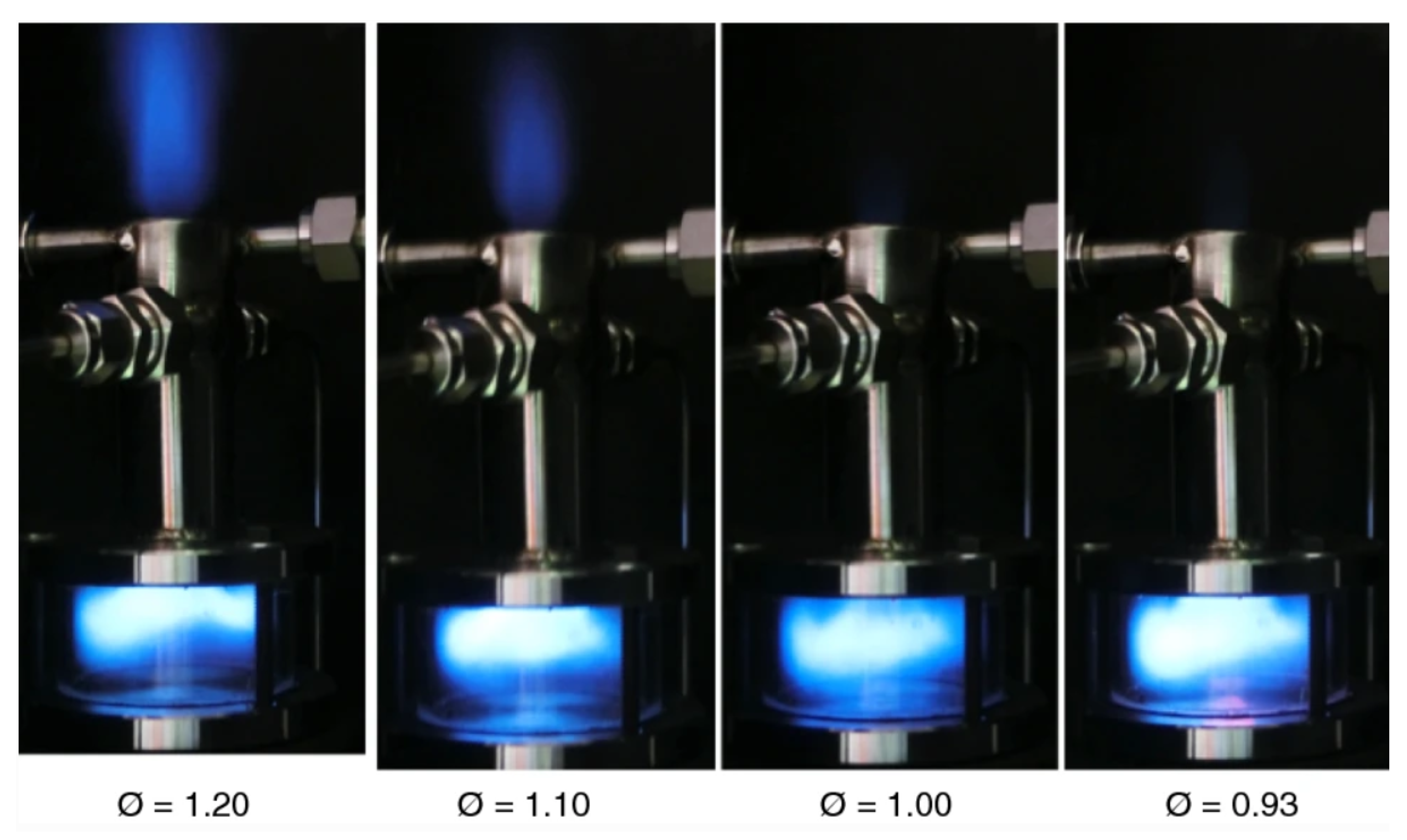



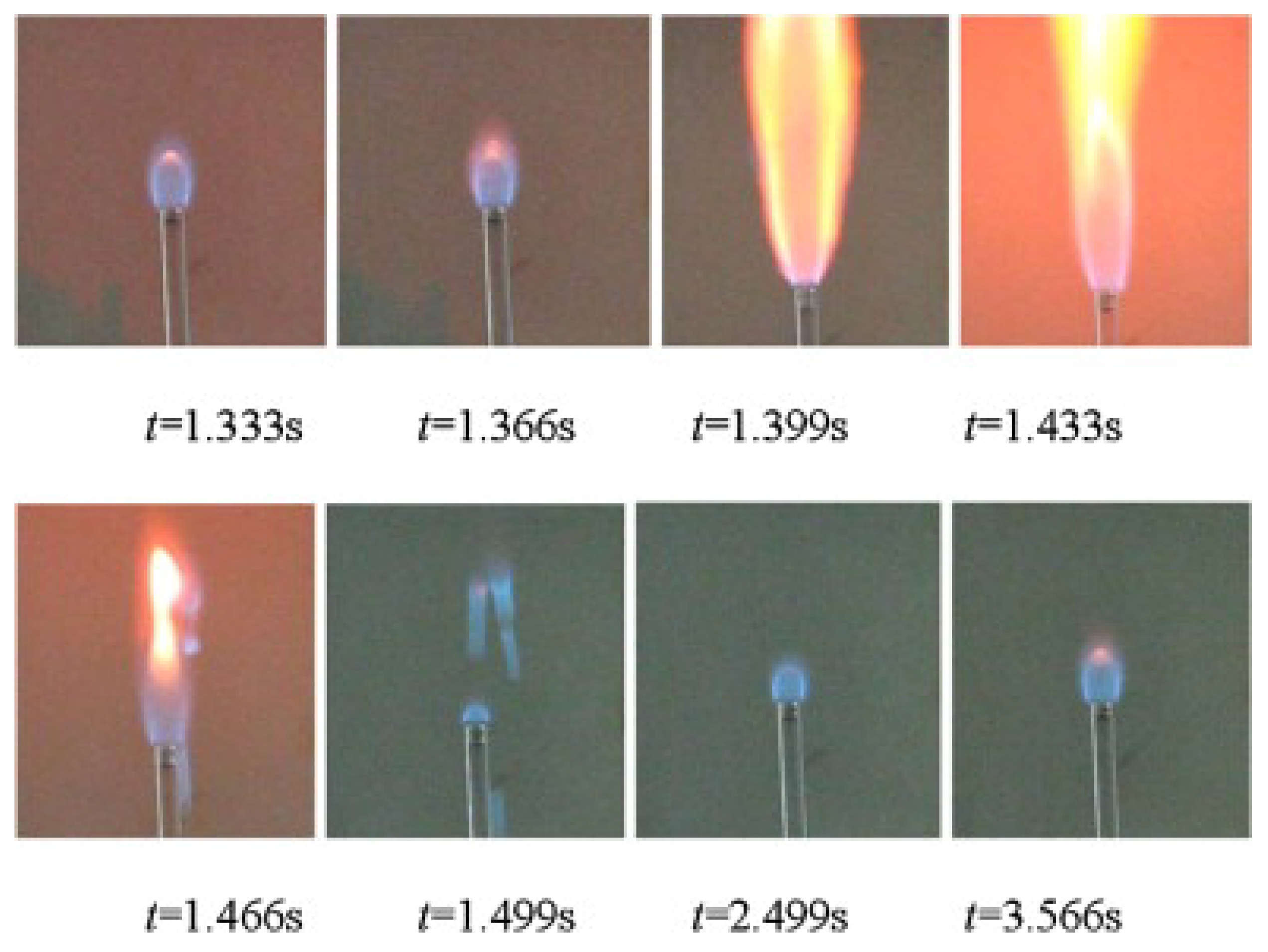
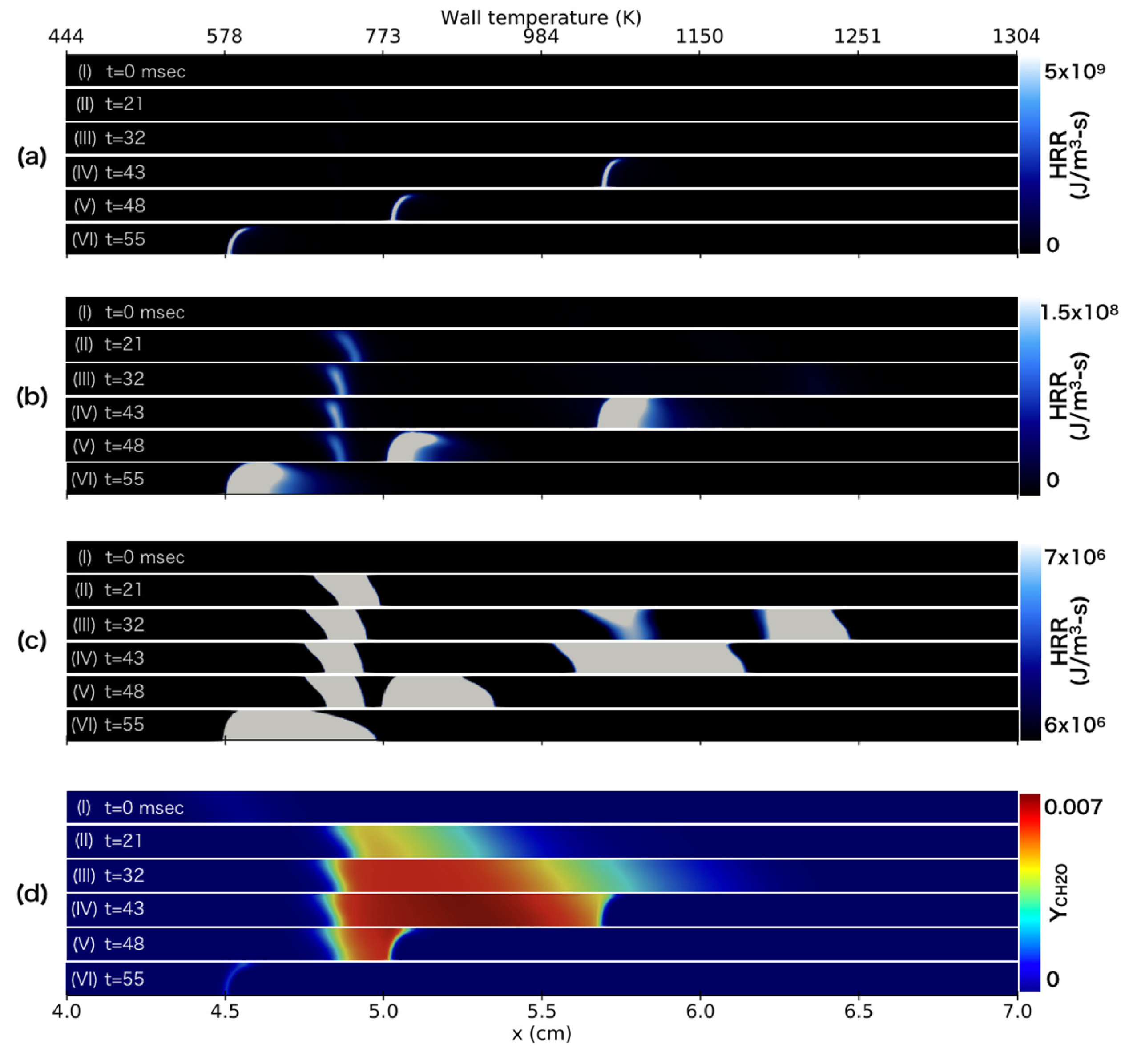

| Reference Length Scale | Combustion Regime | Length Scale |
|---|---|---|
| Physical length | Mesoscale | 1–10 mm |
| Microscale | 1–1000 m | |
| Quenching diameter | Mesoscale | ∼Quenching diameter |
| Microscale | Quenching diameter ∼ mean free path | |
| Device scale | Microscale | Smaller than conventional device size |
| Previous Works | Liquid Fuel | Porous Medium Used | Porosity |
|---|---|---|---|
| Heng Li et al. [40] | n-Heptane | Sintered powder | 35.6 |
| Agarwal et al. [20] | Kerosene | Silicon carbide coated carbon foam | 85 |
| Junwei Li et al. [41] | n-Heptane | Graphite fiber | 87 |
| Junwei Li et al. [36] | n-Heptane | Nickel foam | 95 |
| Chen et al. [42] | Ethanol | Nickel foam, Zirconia foam | 95, 96, 80.7 |
| Previous Works | Liquid Fuel | Thermal Capacity (W) | Combustor Size (cc) | CO (ppm) | NOx (ppm) |
|---|---|---|---|---|---|
| Kyritsis et al. [47] | JP8 | 119 | 43.4 | 1000–5000 | - |
| Agarwal et al. [20] | Kerosene | 180–460 | 6.8 | 220–250 | 240–310 |
| Li et al. [64] | n-Heptane | 460–580 | 4.3 | 100–10,000 | 20–60 |
| Jiang et al. [53] | Biodiesel-ethanol blends | - | 11.3 | 3900–8500 | - |
| Chen et al. [36] | n-heptane | 45–76 | 8.46 | 20–200 | 40–75 |
| Giovannoni et al. [26] | Vegetable Oil | 194–554 | 58.9 | 1000–5000 | 10–90 |
| Chen et al. [42] | Ethanol | - | 8.46 | 22–13,694 | 5–55 |
| Azad et al. [46] | Ethanol | 6–17 | 18.3 | 224–423 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sankar, V.; Sudarsanan, S.; Mukhopadhyay, S.; Selvaraj, P.; Balakrishnan, A.; Velamati, R.K. Towards the Development of Miniature Scale Liquid Fuel Combustors for Power Generation Application—A Review. Energies 2023, 16, 4035. https://doi.org/10.3390/en16104035
Sankar V, Sudarsanan S, Mukhopadhyay S, Selvaraj P, Balakrishnan A, Velamati RK. Towards the Development of Miniature Scale Liquid Fuel Combustors for Power Generation Application—A Review. Energies. 2023; 16(10):4035. https://doi.org/10.3390/en16104035
Chicago/Turabian StyleSankar, Vinay, Sreejith Sudarsanan, Sudipto Mukhopadhyay, Prabhu Selvaraj, Aravind Balakrishnan, and Ratna Kishore Velamati. 2023. "Towards the Development of Miniature Scale Liquid Fuel Combustors for Power Generation Application—A Review" Energies 16, no. 10: 4035. https://doi.org/10.3390/en16104035
APA StyleSankar, V., Sudarsanan, S., Mukhopadhyay, S., Selvaraj, P., Balakrishnan, A., & Velamati, R. K. (2023). Towards the Development of Miniature Scale Liquid Fuel Combustors for Power Generation Application—A Review. Energies, 16(10), 4035. https://doi.org/10.3390/en16104035








