Thermal Performance Analysis of Composite Phase Change Material of Myristic Acid-Expanded Graphite in Spherical Thermal Energy Storage Unit
Abstract
:1. Introduction
2. Experimental Methods and Procedures
2.1. Preparation and Packaging of CPCMs
2.2. Experimental Process
2.3. Uncertainty Analysis
3. Problem Statement
3.1. Governing Relations
- (1)
- The PCM within the heat storage unit is assumed to be isotropic and uniform.
- (2)
- The melted PCM is treated as an incompressible laminar fluid.
- (3)
- The volume change of the PCM during phase change is negligible.
- (4)
- Physical properties, except for density, are assumed to remain constant with temperature. The study focuses on the constrained melting of PCM in the heat storage unit and employs the Boussinesq hypothesis to define density. Density changes are only considered when buoyancy is taken into account.
- (5)
- Density changes in the liquid state, which drive natural convection, are considered only in the momentum term.
3.2. Boundary Conditions and Computational Methodology
3.3. Characteristic Parameters and Evaluation Index
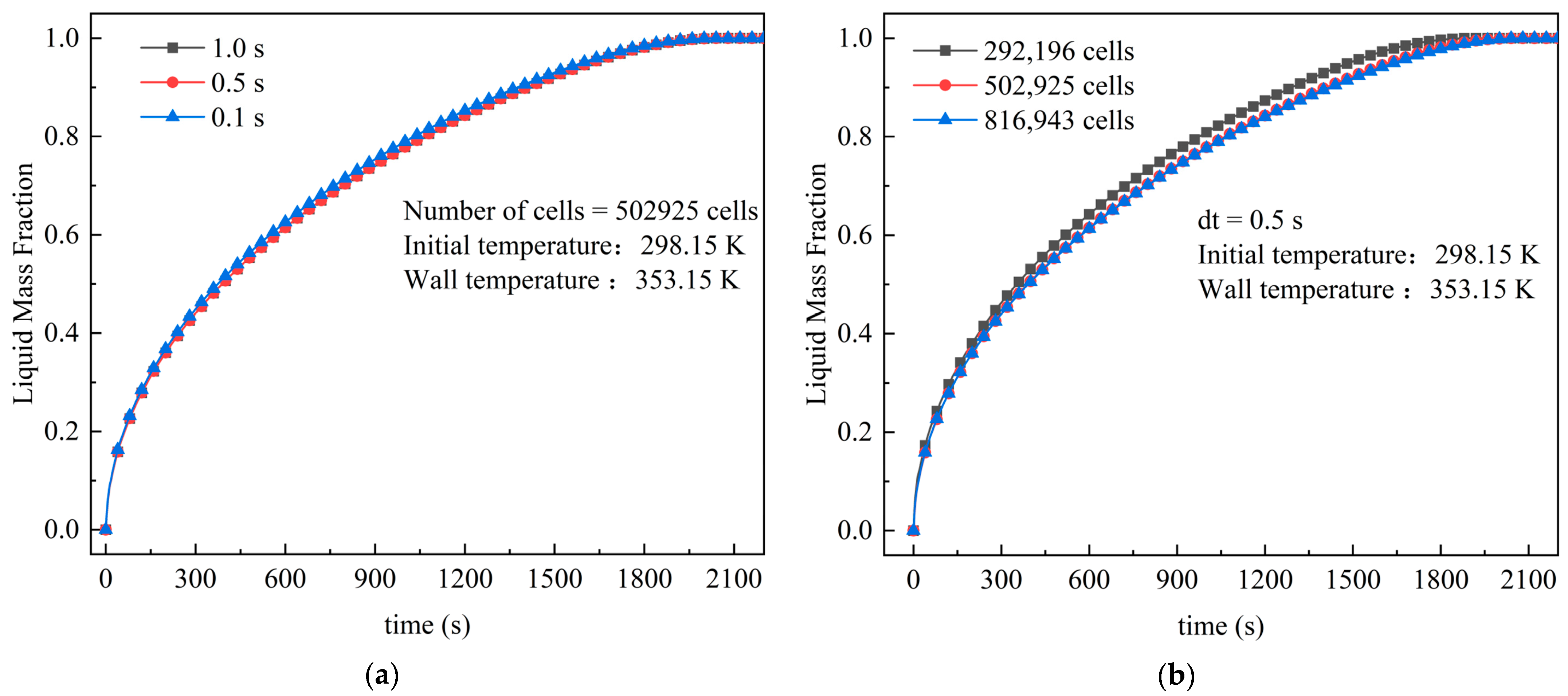
3.4. Grid and Step-Length Independence Verification
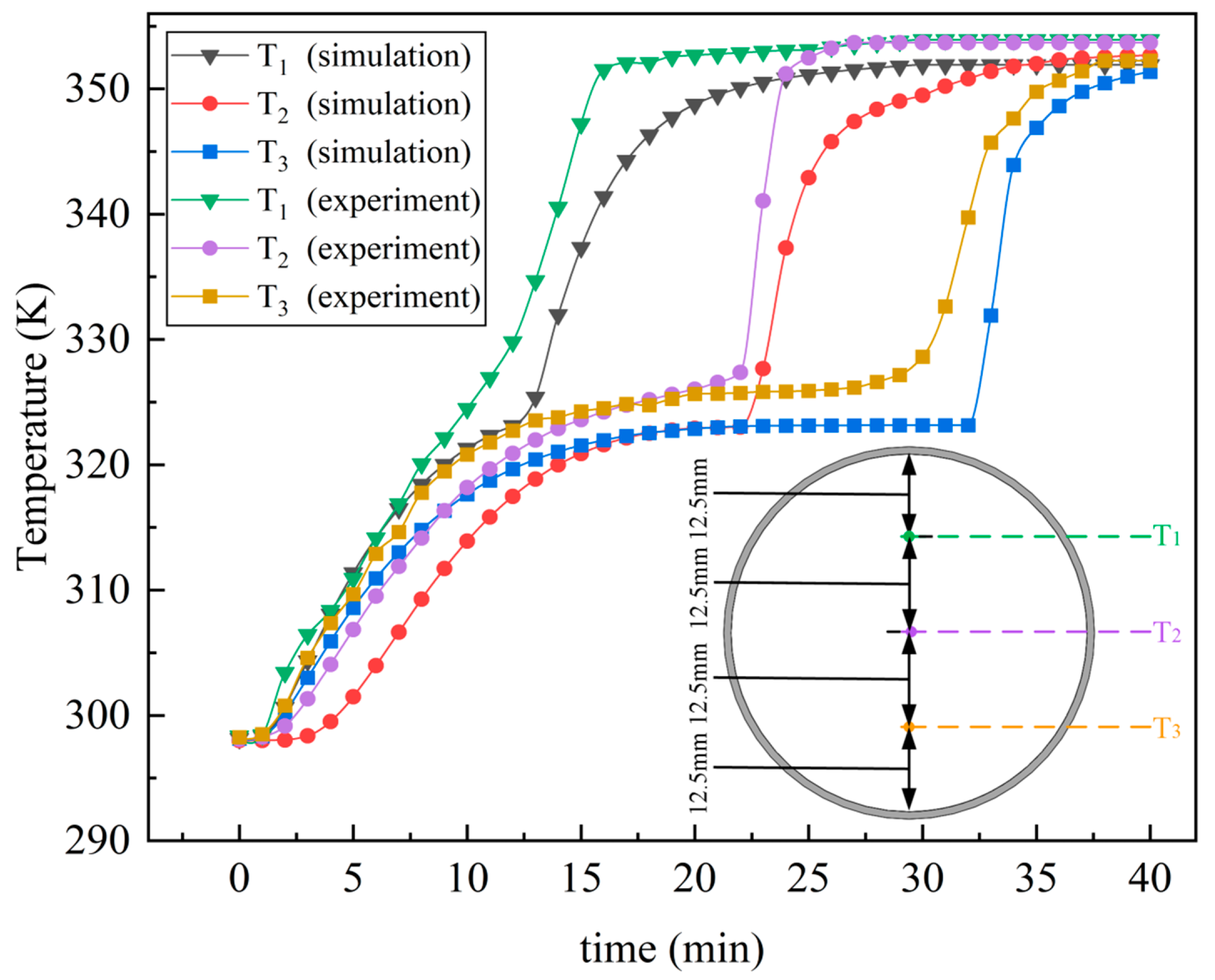
3.5. Model Validation
4. Results and Discussion
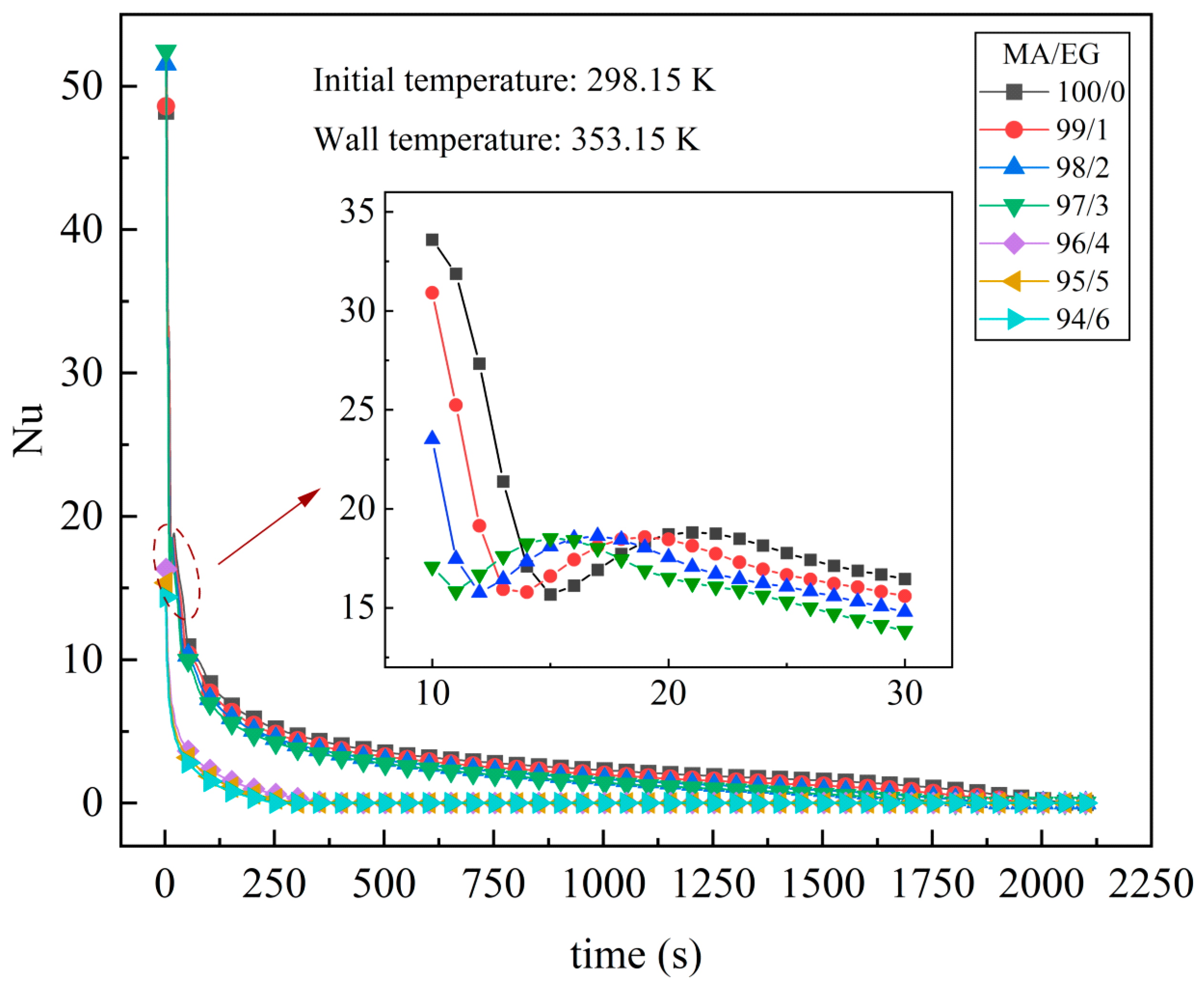
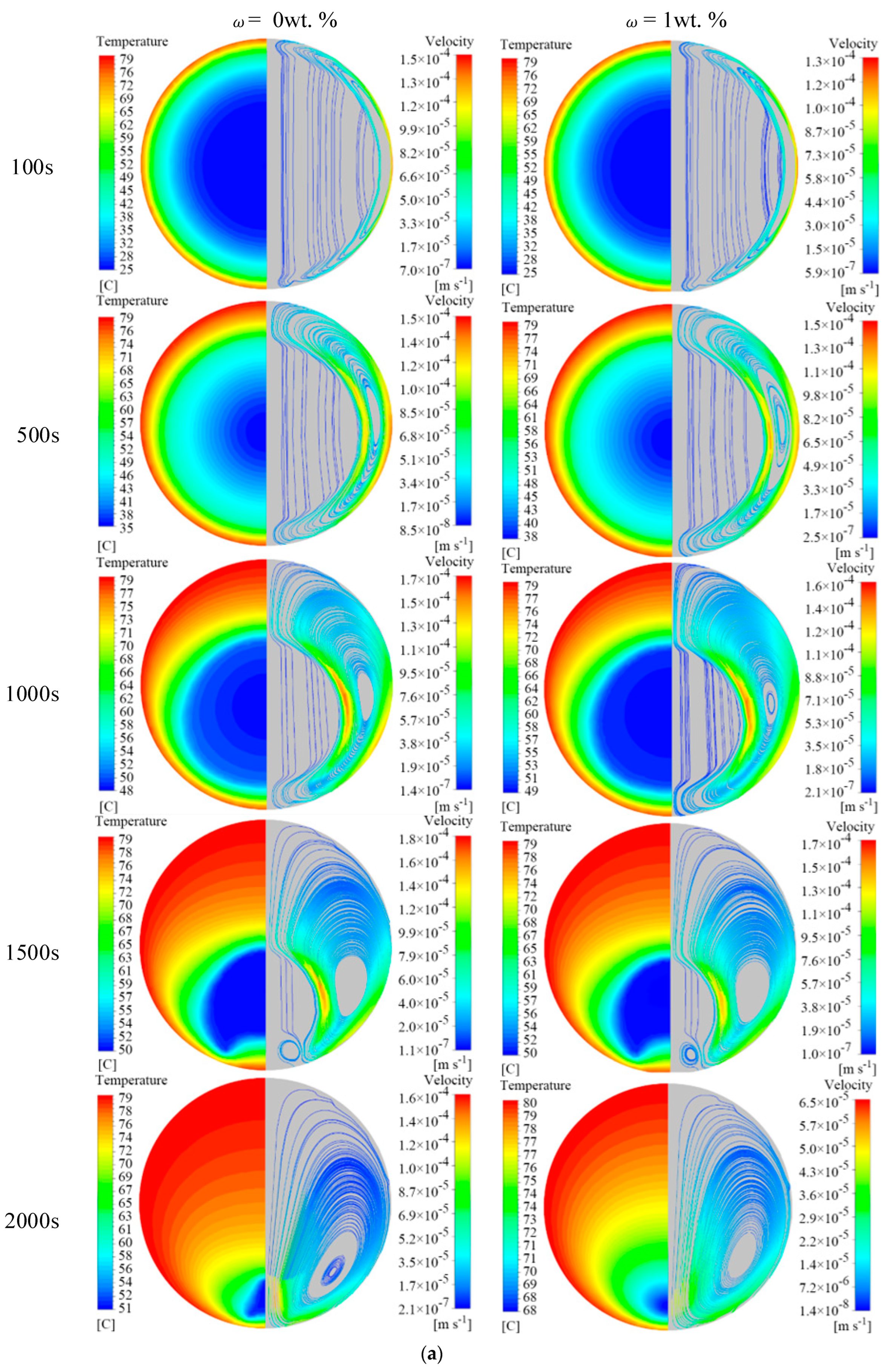
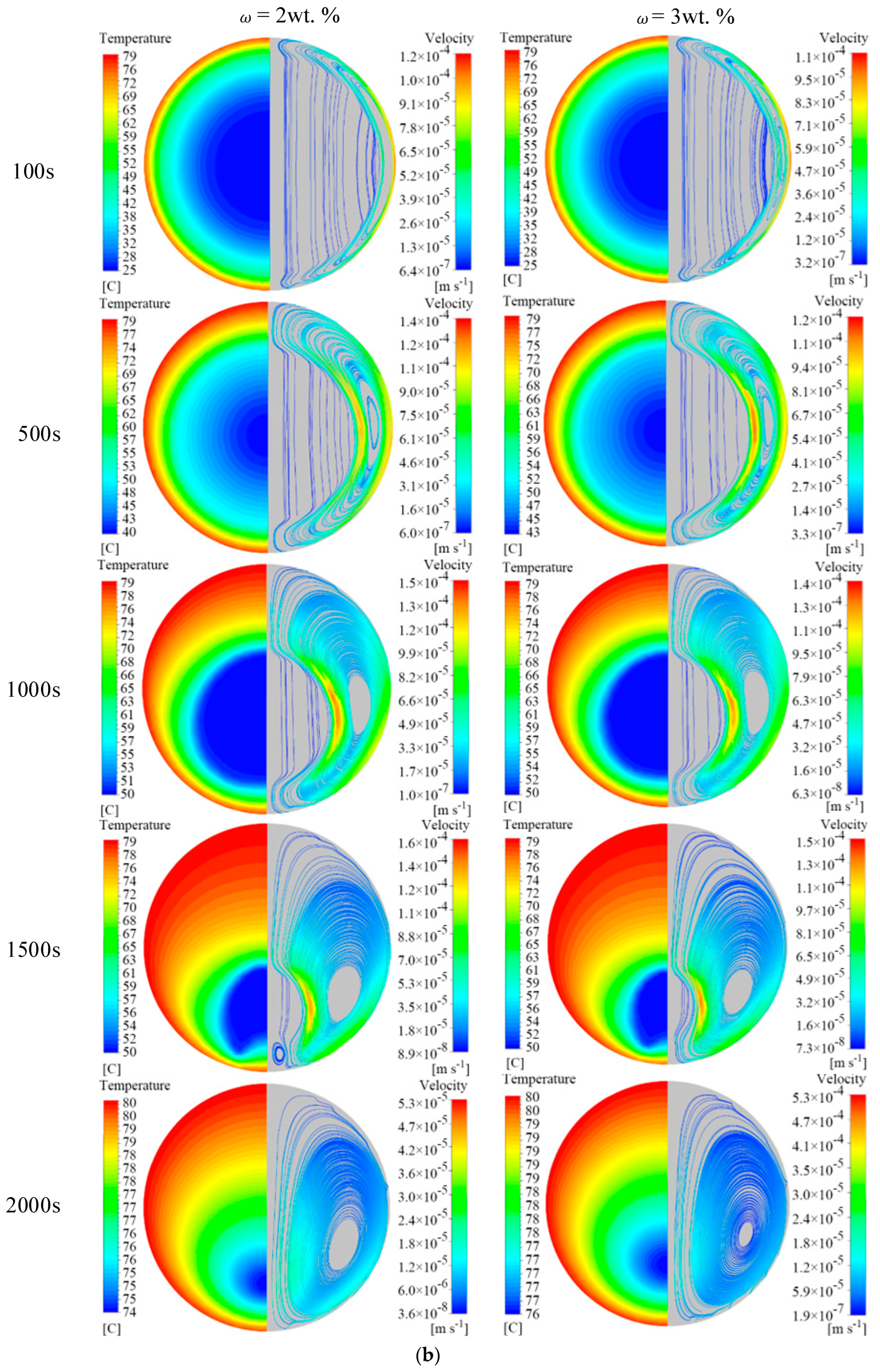
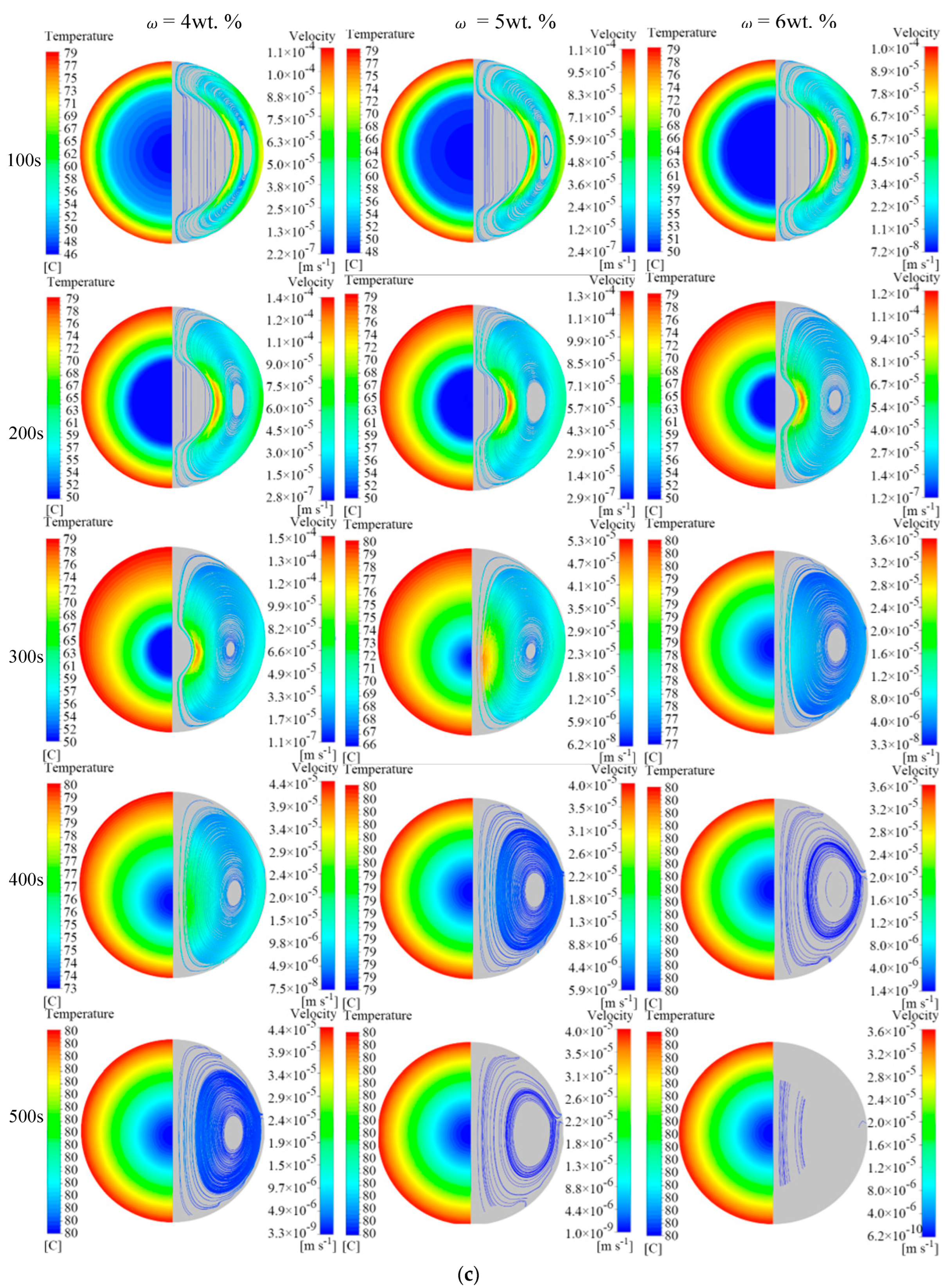
4.1. Effect of EG Content on Melting Properties
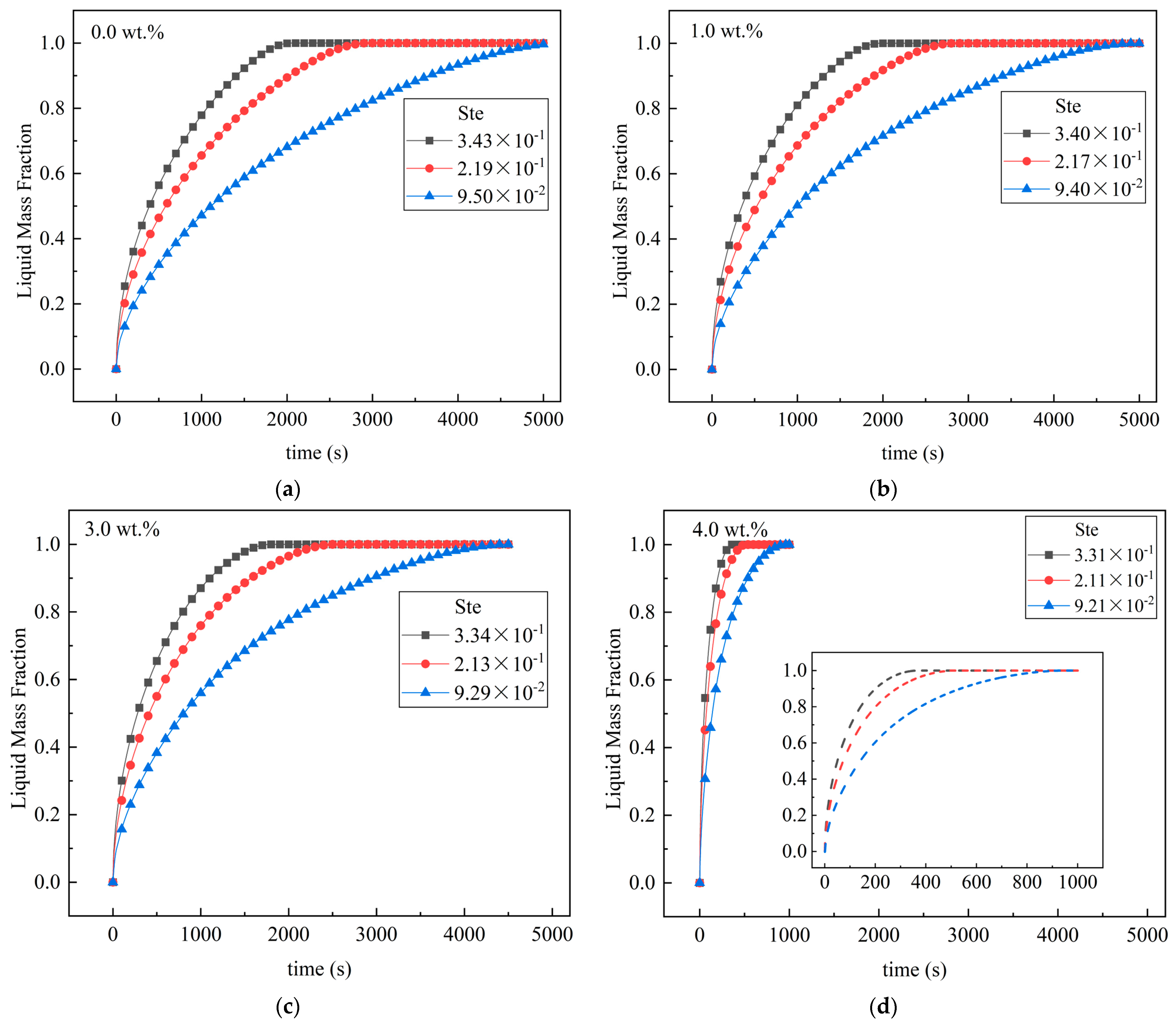
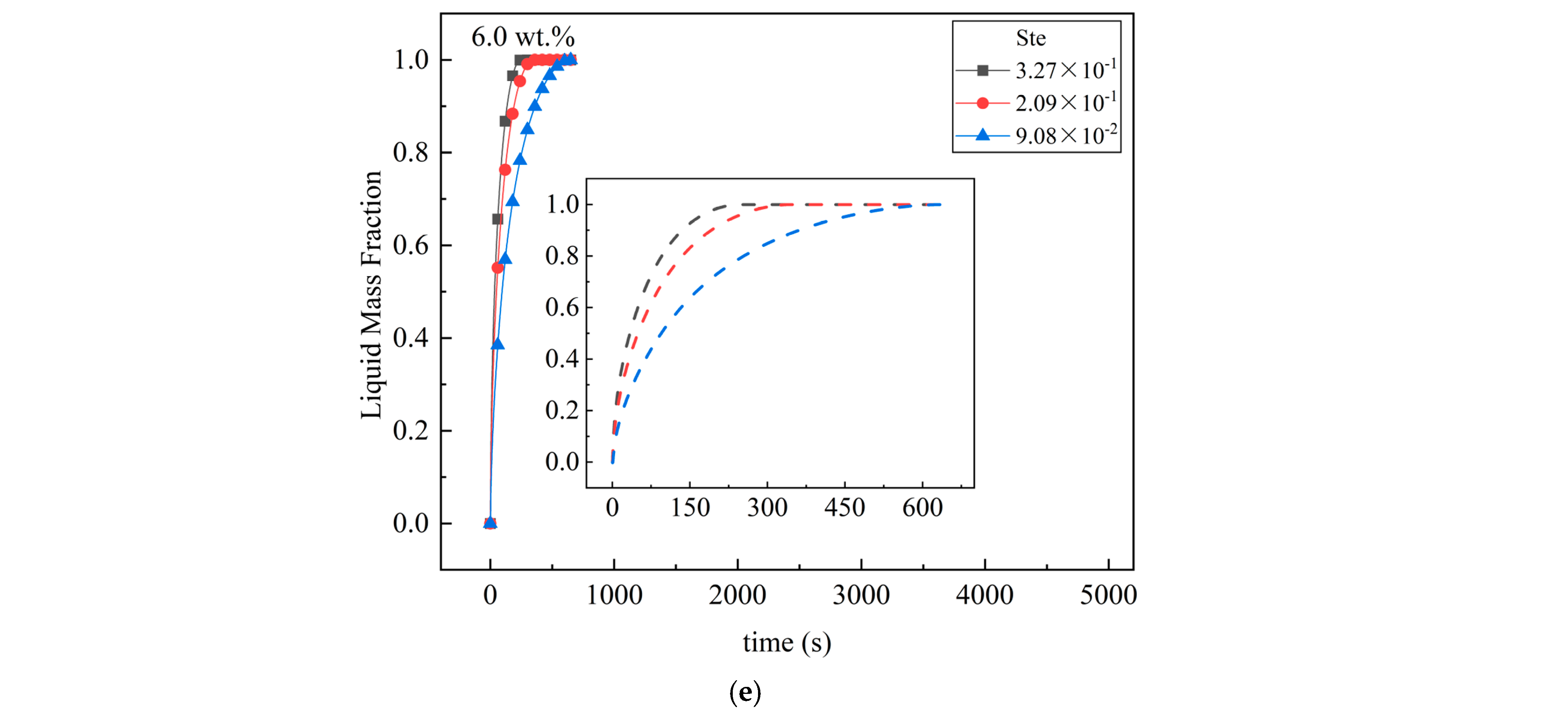
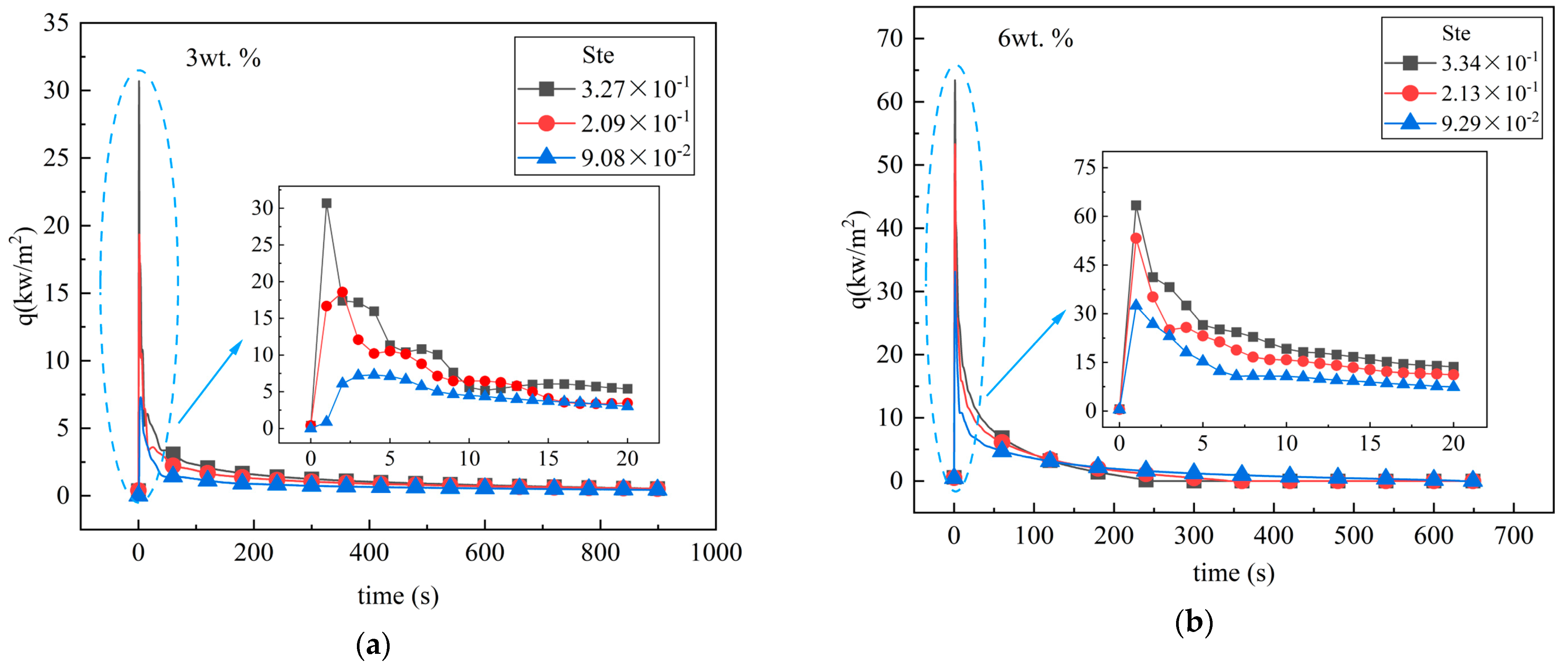
4.2. Influence of Ste Number on Melting
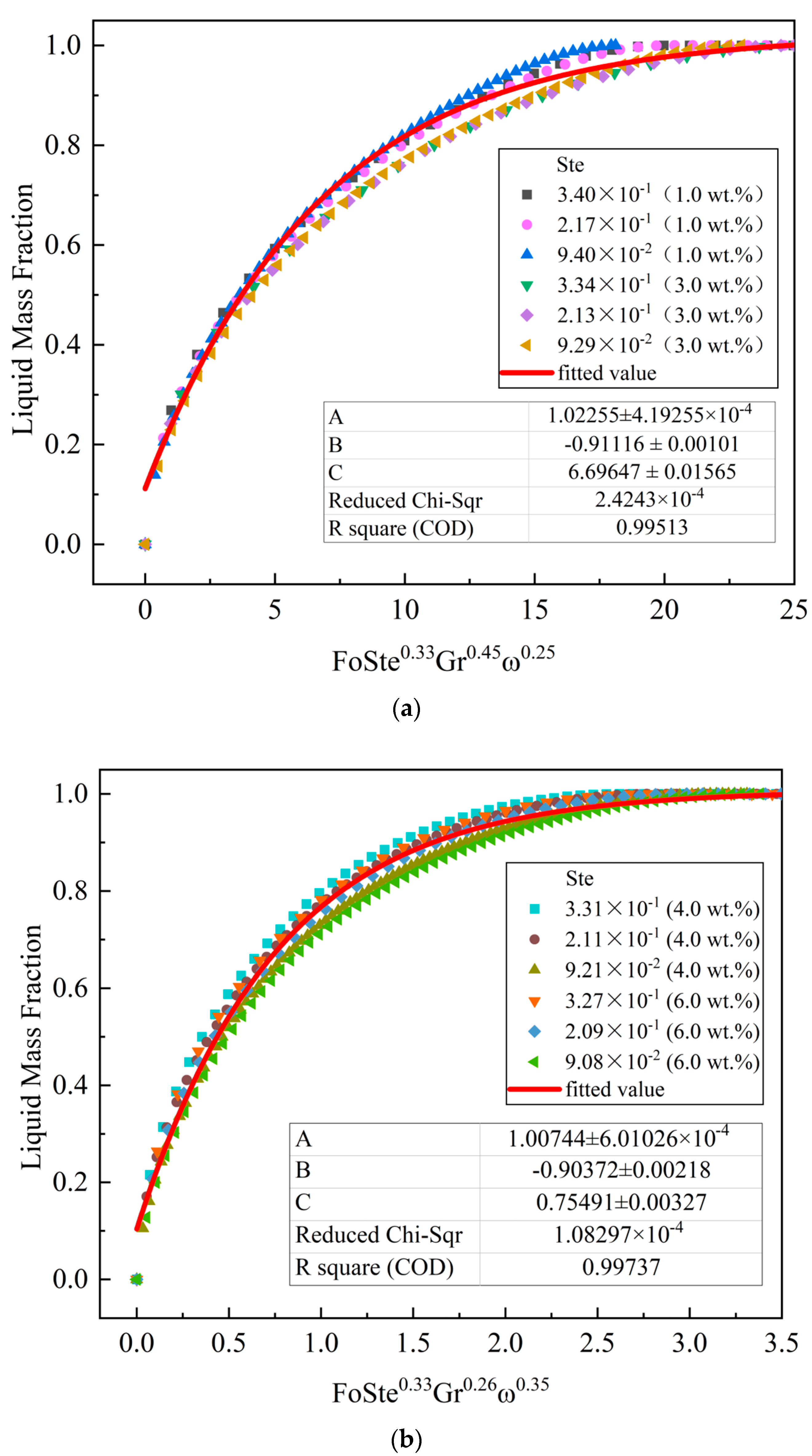
4.3. Dimensionless Analysis
5. Conclusions
- The addition of EG material promotes the melting of the PCM in the unit. When EG contents are 4 wt.%, 5 wt.%, and 6 wt.%, the time required to complete melting in the unit is reduced by 82.2%, 85.6%, and 88.0%, respectively, compared with pure MA. During the melting process of the phase change unit, the addition of EG suppresses the natural convective effect inside the unit. In the middle and late stages of melting, the natural convection effect dominates the heat transfer when the EG content is low, and heat conduction dominates the heat transfer when the EG content is high.
- An increase in the Ste number can effectively reduce the melting time of the unit. For the pure MA unit, when the Ste number decreased from 3.43 × 10−1 to 9.50 × 10−2, the complete melting time of the material in the unit increased by 58.9%. When Ste numbers were 9.21 × 10−2 (4 wt.%) and 9.50 × 10−2 (0 wt.%), the melting time was reduced by 81.2%. It was also found that when the mass fraction of EG is small, the change in the Ste number has a greater impact on the liquid mass fraction of the PCM.
- The dimensionless relationship between the liquid mass fraction and Fo, Ste, and Gr numbers and EG mass fraction ω was obtained by fitting. By comparing the power exponents of various dimensionless numbers, it was found that during the melting of the EG–MA composite PCM with ω ≤ 3 wt.%, the proportion of Gr was relatively large; when ω ≥ 4 wt.%, the impact of ω on the process was greater. The simulation data for each working condition converged well to the fitting curve, and the deviation was within 6%. The correlation equation can be used to guide the engineering applications of latent heat-packed bed heat exchangers.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Roman symbols | |
| c | coefficient of association |
| cp | constant-pressure specific heat, J kg−1 K−1 |
| e | root mean square deviation |
| Fo | Fourier number |
| Gr | Grashof number |
| g | acceleration of gravity, m/s2 |
| H | sensible heat enthalpy, kJ/kg |
| hl | convection heat transfer coefficient, W/m2 K |
| Htot | total enthalpy, kJ/kg |
| kl | thermal conductivity, W m−1 K−1 |
| L | latent heat, kJ/kg |
| m | instantaneous melting mass, kg |
| M | total mass, kg |
| Nu | Nusselt number |
| Pr | Prandtl number of the fluid |
| P | pressure, Pa |
| R | radius of sphericity |
| Ste | Stefan number |
| t | time, min |
| T | temperature, K |
| T0 | ambient temperature, K |
| Tm | phase change temperature, K |
| v | velocity, m/s |
| ΔT | heat transfer temperature difference, K |
| Greek symbols | |
| β | coefficient of thermal expansion, 1/K |
| ϕ | volume fraction, % |
| ω | mass fraction, % |
| ρ | density, kg/m3 |
| μ | dynamic viscosity, kg/m s |
| λ | liquid mass fraction, % |
| Abbreviations | |
| EG | expanded graphite |
| FEM | finite element method |
| MA | myristic acid |
| PCM | phase change material |
| POM | polarizing optical microscope |
| Subscripts | |
| cpcm | composite phase change material |
| h | wall |
| o | initial state |
| c | continuous phase |
| d | dispersed phase |
| EG | expanded graphite |
| p | PCM |
| s | spherical shell |
References
- IRENA. Available online: https://www.irena.org/Data/View-data-by-topic/Capacity-and-Generation/Statistics-Time-Series (accessed on 17 April 2023).
- Han, X.; Li, Y.; Nie, L.; Huang, X.; Deng, Y.; Yan, J.; Kourkoumpas, D.; Karellas, S. Comparative Life Cycle Greenhouse Gas Emissions Assessment Of Battery Energy Storage Technologies For Grid Applications. J. Clean. Prod. 2023, 392, 136251. [Google Scholar] [CrossRef]
- Sun, B.; Liu, Z.; Ji, X.; Gao, L.; Che, D. Thermal energy storage characteristics of packed bed encapsulating spherical capsules with composite phase change materials. Appl. Therm. Eng. 2022, 201, 117659. [Google Scholar] [CrossRef]
- Sun, B.; Sun, M.; Gao, L.; Che, D.; Deng, Y. Thermal performance analysis and optimization of a double-layer spherical phase-change material capsule with annular fins. J. Energy Storage 2023, 57, 106280. [Google Scholar] [CrossRef]
- Leng, G.; Qiao, G.; Jiang, Z.; Xu, G.; Qin, Y.; Chang, C.; Ding, Y. Micro encapsulated & form-stable phase change materials for high temperature thermal energy storage. Appl. Energy 2018, 217, 212–220. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Cong, L.; Jiang, F.; Zhao, Y.; Liu, C.; Xiong, Y.; Chang, C.; Ding, Y. MgO based composite phase change materials for thermal energy storage: The effects of MgO particle density and size on microstructural characteristics as well as thermophysical and mechanical properties. Appl. Energy 2019, 250, 81–91. [Google Scholar] [CrossRef]
- Lee, S.L.; Saidur, R.; Sabri, M.F.M.; Min, T.K. Effects of the particle size and temperature on the efficiency of nanofluids using molecular dynamic simulation. Numer. Heat Transf. Part A Appl. 2016, 69, 996–1013. [Google Scholar] [CrossRef]
- Joshi, V.; Rathod, M.K. Constructal enhancement of thermal transport in latent heat storage systems assisted with fins. Int. J. Therm. Sci. 2019, 145, 105984. [Google Scholar] [CrossRef]
- Jia, X.; Zhai, X.; Cheng, X. Thermal performance analysis and optimization of a spherical PCM capsule with pin-fins for cold storage. Appl. Therm. Eng. 2019, 148, 929–938. [Google Scholar] [CrossRef]
- Meghari, Z.; Bouhal, T.; Benghoulam, M.; El Rhafiki, T.; El Khattabi, E.M.; Doghmi, H.; Mohammed, O.J. Numerical simulation of a phase change material in a spherical capsule with a hollow fin. J. Energy Storage 2021, 43, 103024. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Z.; Zhou, J.; Zeng, C.; Lyu, W.; Lu, L.; Yuan, Y. Heat transfer analysis of hollow channel in phase change materials spherical capsule. Appl. Therm. Eng. 2023, 219, 119390. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, R.; Gao, Z.; Yu, X. Effect of nanoparticles and metal foams on heat transfer properties of PCMs. Int. J. Therm. Sci. 2022, 179, 107567. [Google Scholar] [CrossRef]
- Prabakaran, R.; Kumar, J.P.N.; Lal, D.M.; Selvam, C.; Harish, S. Constrained melting of graphene-based phase change nanocomposites inside a sphere. J. Therm. Anal. Calorim. 2019, 139, 941–952. [Google Scholar] [CrossRef]
- Mayilvelnathan, V.; Valan Arasu, A. Experimental investigation on thermal behavior of graphene dispersed erythritol PCM in a shell and helical tube latent energy storage system. Int. J. Therm. Sci. 2020, 155, 106446. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Z.; Huang, S.; Huang, X.; Liang, K.; Yang, Y.; Zhang, H.; Tian, M.; Akrami, M.; Wen, C. Improving the melting performance of phase change materials using novel fins and nanoparticles in tubular energy storage systems. Appl. Energy 2022, 322, 119416. [Google Scholar] [CrossRef]
- Jirawattanapanit, A.; Abderrahmane, A.; Mourad, A.; Guedri, K.; Younis, O.; Bouallegue, B.; Subkrajang, K.; Rajchakit, G.; Shah, N.A. A Numerical Investigation of a Melting Rate Enhancement inside a Thermal Energy Storage System of Finned Heat Pipe with Nano-Enhanced Phase Change Material. Nanomaterials 2022, 12, 2519. [Google Scholar] [CrossRef]
- Abderrahmane, A.; Qasem, N.A.A.; Mourad, A.; Al-Khaleel, M.; Said, Z.; Guedri, K.; Younis, O.; Marzouki, R. Enhancing the Melting Process of Shell-and-Tube PCM Thermal Energy Storage Unit Using Modified Tube Design. Nanomaterials 2022, 12, 3078. [Google Scholar] [CrossRef]
- Masoumi, H.; Haghighi Khoshkhoo, R. Investigation of melting of nanoparticle-enhanced phase change materials (NePCMs) in a shell-and-tube heat exchanger with longitudinal fins. Heat Mass Transf. 2020, 57, 681–701. [Google Scholar] [CrossRef]
- Eanest Jebasingh, B.; Valan Arasu, A. A detailed review on heat transfer rate, supercooling, thermal stability and reliability of nanoparticle dispersed organic phase change material for low-temperature applications. Mater. Today Energy 2020, 16, 100408. [Google Scholar] [CrossRef]
- Gao, L.; Sun, X.; Sun, B.; Che, D.; Li, S.; Liu, Z. Preparation and thermal properties of palmitic acid/expanded graphite/carbon fiber composite phase change materials for thermal energy storage. J. Therm. Anal. Calorim. 2019, 141, 25–35. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y.; Wang, X.; Cao, X.; Yang, X.; Hu, S. Preparation and characterization of Lauric–myristic–palmitic acid ternary eutectic mixtures/expanded graphite composite phase change material for thermal energy storage. Chem. Eng. J. 2013, 231, 214–219. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, N.; Tao, W.; Cao, X.; He, Y. Fatty acids as phase change materials: A Review. Renew. Sustain. Energy Rev. 2014, 29, 482–498. [Google Scholar] [CrossRef]
- Gao, Z.; Yao, Y.; Wu, H. A visualization study on the unconstrained melting of paraffin in spherical container. Appl. Therm. Eng. 2019, 155, 428–436. [Google Scholar] [CrossRef]
- Venkitaraj, K.; Suresh, S. Experimental study on thermal and chemical stability of pentaerythritol blended with low melting alloy as possible PCM for latent heat storage. Exp. Therm. Fluid Sci. 2017, 88, 73–87. [Google Scholar] [CrossRef]
- Venkitaraj, K.; Suresh, S.; Praveen, B.; Nair, S.C. Experimental heat transfer analysis of macro packed neopentylglycol with CuO nano additives for building cooling applications. J. Energy Storage 2018, 17, 1–10. [Google Scholar] [CrossRef]
- Voller, V.R.; Prakash, C. A fixed grid numerical modelling methodology for convection-diffusion mushy region phase-change problems. Int. J. Heat Mass Transf. 1987, 30, 1709–1719. [Google Scholar] [CrossRef]
- Fan, L.-W.; Zhu, Z.-Q.; Zeng, Y.; Ding, Q.; Liu, M.-J. Unconstrained melting heat transfer in a spherical container revisited in the presence of nano-enhanced phase change materials (nepcm). Int. J. Heat Mass Transf. 2016, 95, 1057–1069. [Google Scholar] [CrossRef]
- Alktranee, M.; Shehab, M.A.; Németh, Z.; Bencs, P.; Hernadi, K. Effect of zirconium oxide nanofluid on the behaviour of photovoltaic–thermal system: An experimental study. Energy Rep. 2023, 9, 1265–1277. [Google Scholar] [CrossRef]
- Zhou, X.; Duan, W.; Chi, F.; Jiang, Y. Numerical Investigation of Nano-PCM melting enhanced by thermocapillary convection under microgravity condition. Microgravity Sci. Technol. 2022, 34, 41. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Z.; Zhang, H.; Sun, X.; Dou, B.; Wu, W.; Zhang, G.; Jiang, L. Experimental investigation of the comprehensive heat transfer performance of pcms filled with CMF in a heat storage device. Int. J. Heat Mass Transf. 2022, 188, 122582. [Google Scholar] [CrossRef]




| Thermophysical Property | MA | EG | 304-Stainless Steel |
|---|---|---|---|
| Density (kg/m3) | 862/990 | 269 | 7930 |
| Specific heat capacity (J/kg·K) | 1700/2264 | 874.5 | 460 |
| Thermal conductivity (W/m·K) | 0.22 | 17.5 | 16.2 |
| Melting temperature (K) | 327.15 | - | - |
| Latent heat (kJ/kg) | 182.6 | - | - |
| Dynamic viscosity (kg/m·s) | 0.00506 | - | - |
| Coefficient of thermal expansion (1/K) | 0.00062 | - | - |
| Thermophysical Property | ω = 1 wt.% | ω = 2 wt.% | ω = 3 wt.% | ω = 4 wt.% | ω = 5 wt.% | ω = 6 wt.% |
|---|---|---|---|---|---|---|
| Density (kg/m3) | 843.4 | 825.6 | 808.5 | 792.1 | 776.4 | 761.3 |
| Specific heat capacity (J/kg·K) | 2220.4 | 2178.7 | 2138.7 | 2100.3 | 2063.5 | 2028.1 |
| Thermal conductivity (W/m·K) | 0.24 | 0.26 | 0.28 | 1.64 | 1.98 | 2.31 |
| Latent heat (kJ/kg) | 180.8 | 178.9 | 177.1 | 175.3 | 173.5 | 171.6 |
| Dynamic viscosity (kg/m·s) | 0.0055 | 0.0059 | 0.0064 | 0.0069 | 0.0075 | 0.0081 |
| (wt.%) | Th (°C) | Ste | Gr |
|---|---|---|---|
| 0.0 | 80 70 60 | 3.43 × 10−1 2.19 × 10−1 9.53 × 10−2 | 1.62 × 105 1.03 × 105 4.51 × 104 |
| 1.0 | 80 70 60 | 3.40 × 10−1 2.17 × 10−1 9.44 × 10−2 | 1.33 × 105 8.47 × 104 3.68 × 104 |
| 3.0 | 80 70 60 | 3.34 × 10−1 2.13 × 10−1 9.29 × 10−2 | 8.91 × 104 5.69 × 104 2.47 × 104 |
| 4.0 | 80 70 60 | 3.31 × 10−1 2.11 × 10−1 9.21 × 10−2 | 7.33 × 104 4.68 × 104 2.04 × 104 |
| 6.0 | 80 70 60 | 3.27 × 10−1 2.09 × 10−1 9.08 × 10−2 | 4.99 × 104 3.19 × 104 1.39 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, W.; Deng, Y.; Gao, L.; Bai, J.; Xu, L.; Chen, J.; Yuan, Z. Thermal Performance Analysis of Composite Phase Change Material of Myristic Acid-Expanded Graphite in Spherical Thermal Energy Storage Unit. Energies 2023, 16, 4527. https://doi.org/10.3390/en16114527
Li J, Wang W, Deng Y, Gao L, Bai J, Xu L, Chen J, Yuan Z. Thermal Performance Analysis of Composite Phase Change Material of Myristic Acid-Expanded Graphite in Spherical Thermal Energy Storage Unit. Energies. 2023; 16(11):4527. https://doi.org/10.3390/en16114527
Chicago/Turabian StyleLi, Ji, Weiqing Wang, Yimin Deng, Long Gao, Junchao Bai, Lei Xu, Jun Chen, and Zhi Yuan. 2023. "Thermal Performance Analysis of Composite Phase Change Material of Myristic Acid-Expanded Graphite in Spherical Thermal Energy Storage Unit" Energies 16, no. 11: 4527. https://doi.org/10.3390/en16114527
APA StyleLi, J., Wang, W., Deng, Y., Gao, L., Bai, J., Xu, L., Chen, J., & Yuan, Z. (2023). Thermal Performance Analysis of Composite Phase Change Material of Myristic Acid-Expanded Graphite in Spherical Thermal Energy Storage Unit. Energies, 16(11), 4527. https://doi.org/10.3390/en16114527







