Laser Ablation Synthesis of Silver Nanoparticles for Polymer Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
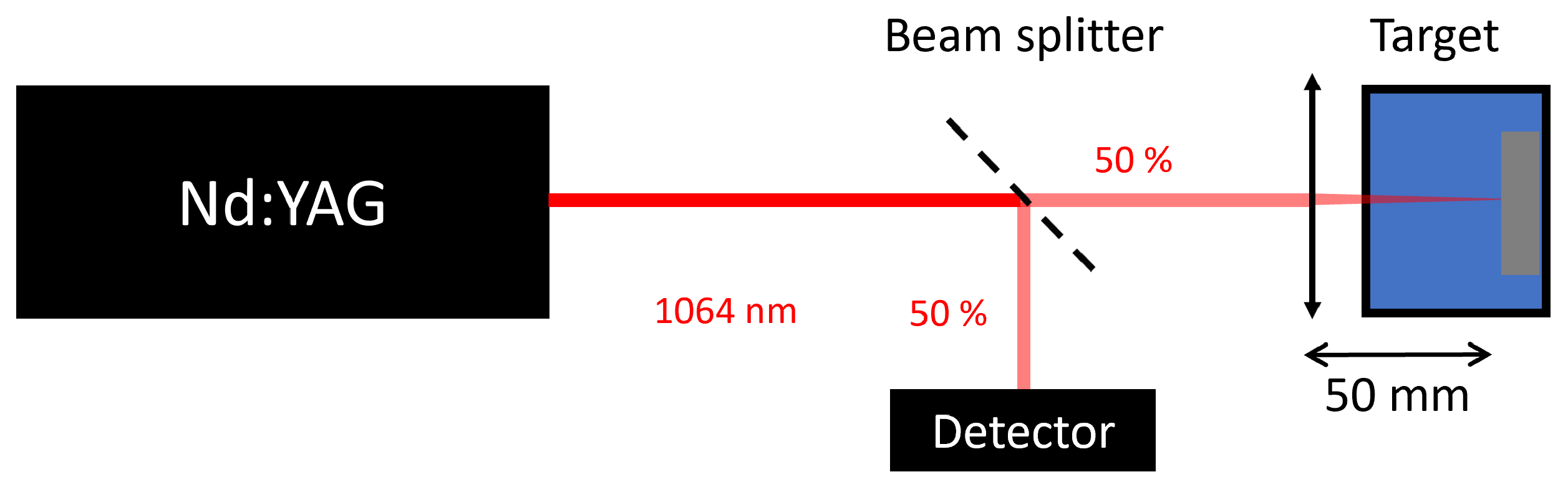
2.2. Laser Ablation in Liquids
2.3. Silver Nanoparticles Characterisation by Ultraviolet–Visible Spectroscopy
2.4. Substrate Cleaning and Nanocomposite Sample Preparation
2.5. Thickness Control of the Composite Films
2.6. Scanning Probe Microscopy Measurements
3. Results and Discussion
3.1. AgNPs Characterization
3.2. Ag-PS Film Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscope |
| AgNPS | Silver Nanoparticles |
| Ag-THF | Tetrahydofuran Ablated Silver Nanoparticles |
| Ag-Tol | Toluene Ablated Silver Nanoparticles |
| LAL | Laser Ablation in Liquids |
| LSPR | Localized Surface Plasmon Resonance |
| PS | polystyrene |
| PFT QNM | Peak Force Tapping Quantitative Nanomechanical |
| SE | Spectroscopic Ellipsometry |
| SEM | Scanning Electron Microscopy |
| SPM | Scanning Probe Microscopy |
| THF | Tetrahydrofuran |
References
- Lewis, T. Nanometric Dielectrics. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 812–825. [Google Scholar] [CrossRef]
- Tanaka, T.; Montanari, G.; Mulhaupt, R. Polymer Nanocomposites as Dielectrics and Electrical Insulation-perspectives for Processing Technologies, Material Characterization and Future Applications. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 763–784. [Google Scholar] [CrossRef]
- Green, C.; Vaughan, A. Nanodielectrics—How Much Do We Really Understand? IEEE Electr. Insul. Mag. 2008, 24, 6–16. [Google Scholar] [CrossRef]
- Zhong, S.; Dang, Z.; Zhou, W.; Cai, H. Past and Future on Nanodielectrics. IET Nanodielectr. 2018, 1, 41–47. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Mostafa, M.H.; Al-Harbi, L.M. Polymeric Nanocomposites for Environmental and Industrial Applications. Int. J. Mol. Sci. 2022, 23, 1023. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Khan, I.; Saeed, K.; Ali, N.; Zada, N.; Khan, A.; Ali, F.; Bilal, M.; Akhter, M.S. 7—Polymer nanocomposites: An overview. In Smart Polymer Nanocomposites; Ali, N., Bilal, M., Khan, A., Nguyen, T.A., Gupta, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 167–184. [Google Scholar] [CrossRef]
- Yang, K.; Huang, X.; Xie, L.; Wu, C.; Jiang, P.; Tanaka, T. Core-Shell Structured Polystyrene/BaTiO3 Hybrid Nanodielectrics Prepared by In Situ RAFT Polymerization: A Route to High Dielectric Constant and Low Loss Materials with Weak Frequency Dependence. Macromol. Rapid Commun. 2012, 33, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Zhang, C.; Zhou, G.; Zhang, T.; Feng, Y.; Chi, Q.; Lei, Q. Enhanced Energy Storage Characteristics in PVDF-Based Nanodielectrics With Core-Shell Structured and Optimized Shape Fillers. IEEE Access 2020, 8, 81542–81550. [Google Scholar] [CrossRef]
- Tanaka, T.; Vaughan, A. (Eds.) Tailoring of Nanocomposite Dielectrics: From Fundamentals to Devices and Applications; Pan Stanford Publishing: Singapore, 2017. [Google Scholar]
- Seiler, J.; Kindersberger, J. Insight into the Interphase in Polymer Nanocomposites. IEEE Trans. Dielect. Electr. Insul. 2014, 21, 537–547. [Google Scholar] [CrossRef]
- Tanaka, T. Interpretation of Several Key Phenomena Peculiar to Nano Dielectrics in terms of a Multi-core Model. In Proceedings of the 2006 IEEE Conference on Electrical Insulation and Dielectric Phenomena, Kansas City, MO, USA, 15–18 October 2006; pp. 298–301. [Google Scholar] [CrossRef]
- Raetzke, S.; Kindersberger, J. The Effect of Interphase Structures in Nanodielectrics. IEEJ 2006, 126, 1044–1049. [Google Scholar] [CrossRef] [Green Version]
- Andritsch, T.; Kochetov, R.; Morshuis, P.H.F.; Smit, J.J. Proposal of the Polymer Chain Alignment Model. In Proceedings of the 2011 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Cancun, Mexico, 16–19 October 2011; pp. 624–627. [Google Scholar] [CrossRef]
- Lewis, T.J. Interfaces are the Dominant Feature of Dielectrics at the Nanometric Level. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 739–753. [Google Scholar] [CrossRef]
- Tsagaropoulos, G.; Eisenberg, A. Dynamic Mechanical Study of the Factors Affecting the Two Glass Transition Behavior of Filled Polymers. Similarities and Differences with Random Ionomers. Macromolecules 1995, 28, 6067–6077. [Google Scholar] [CrossRef]
- Ghasem Zadeh Khorasani, M.; Silbernagl, D.; Platz, D.; Sturm, H. Insights into Nano-scale Physical and Mechanical Properties of Epoxy/boehmite Nanocomposite using Different AFM Modes. Polymers 2019, 11, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houssat, M.; Villeneuve-Faure, C.; Dignat, N.L.; Cambronne, J. A Multiscale Characterization of Nanodielectrics: The Case of PI/Si3N4 Nanocomposite. In Proceedings of the 2020 IEEE 3rd International Conference on Dielectrics (ICD), Valencia, Spain, 5–31 July 2020; pp. 221–224. [Google Scholar] [CrossRef]
- Sengwa, R.; Dhatarwal, P.; Choudhary, S. A Comparative Study of Different Metal Oxide Nanoparticles Dispersed PVDF/PEO Blend Matrix-based Advanced Multifunctional Nanodielectrics for Flexible Electronic Devices. Mater. Today Commun. 2020, 25, 101380. [Google Scholar] [CrossRef]
- Lau, K.Y.; Vaughan, A.S.; Chen, G. Nanodielectrics: Opportunities and Challenges. IEEE Electr. Insul. Mag. 2015, 31, 45–54. [Google Scholar] [CrossRef]
- Yang, G. (Ed.) Laser Ablation in Liquids: Principles and Applications in the Preparation of Nanomaterials; Pan Stanford Publishing: Singapore, 2012. [Google Scholar]
- Zhang, D.; Li, Z.; Sugioka, K. Laser Ablation in Liquids for Nanomaterial Synthesis: Diversities of Targets and Liquids. J. Phys. Photonics 2021, 3, 042002. [Google Scholar] [CrossRef]
- Mansour, Y.; Battie, Y.; Naciri, A.E.; Chaoui, N. Monitoring the Aspect Ratio Distribution of Colloidal Gold Nanoparticles under Pulsed-Laser Exposure. Opt. Express 2020, 28, 34501. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, U.; Jewrajka, S.K.; Guha, S. Dispersion of Functionalized Silver Nanoparticles in Polymer Matrices: Stability, Characterization, and Physical Properties. Polym. Compos. 2009, 30, 827–834. [Google Scholar] [CrossRef]
- Barcikowski, S.; Amendola, V.; Marzun, G.; Rehbock, C.; Reichenberger, S.; Zhang, D.; Gökce, B. Handbook of Laser Synthesis of Colloids; DuEPublico: Essen, Germany, 2016. [Google Scholar]
- Amendola, V.; Polizzi, S.; Meneghetti, M. Free Silver Nanoparticles Synthesized by Laser Ablation in Organic Solvents and Their Easy Functionalization. Langmuir 2007, 23, 6766–6770. [Google Scholar] [CrossRef]
- Amendola, V.; Polizzi, S.; Meneghetti, M. Laser Ablation Synthesis of Silver Nanoparticles Embedded in Graphitic Carbon Matrix. Sci. Adv. Mater. 2012, 4, 497–500. [Google Scholar] [CrossRef]
- Azzam, R.M.A.; Bashara, N.M. Ellipsometry and Polarized Light; North-Holland: Amsterdam, The Netherlands, 1977. [Google Scholar]
- Abargues, R.; Abderrafi, K.; Pedrueza, E.; Gradess, R.; Marqués-Hueso, J.; Valdés, J.L.; Martínez-Pastor, J. Optical properties of different polymer thin films containing in situ synthesized Ag and Au nanoparticles. New J. Chem. 2009, 33, 1720–1725. [Google Scholar] [CrossRef]
- Guyot, C.; Voué, M. Intrinsic optical properties of Ag-doped poly-(vinyl alcohol) nanocomposites: An analysis of the film thickness effect on the plasmonic resonance parameters. Appl. Phys. A 2020, 126, 870. [Google Scholar] [CrossRef]
- Kaemmer, S.B. Introduction to Bruker’s ScanAsyst and PeakForce Tapping AFM Technology; Bruker Nano Inc.: Santa Barbara, CA, USA, 2011. [Google Scholar]
- Enrriques, A.E.; Howard, S.; Timsina, R.; Khadka, N.K.; Hoover, A.N.; Ray, A.E.; Ding, L.; Onwumelu, C.; Nordeng, S.; Mainali, L.; et al. Atomic Force Microscopy Cantilever-Based Nanoindentation: Mechanical Property Measurements at the Nanoscale in Air and Fluid. J. Vis. Exp. 2022, 190, 64497. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zheng, W.; Singh, D.J. Light scattering and surface plasmons on small spherical particles. Light Sci. Appl. 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.B.; Christy, R.W. Optical Constants of the Noble Metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]
- Delmée, M.; Mertz, G.; Bardon, J.; Marguier, A.; Ploux, L.; Roucoules, V.; Ruch, D. Laser Ablation of Silver in Liquid Organic Monomer: Influence of Experimental Parameters on the Synthesized Silver Nanoparticles/Graphite Colloids. J. Phys. Chem. B 2017, 121, 6646–6654. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Muijlder, T.; Voué, M.; Leclère, P. Laser Ablation Synthesis of Silver Nanoparticles for Polymer Nanocomposites. Energies 2023, 16, 4625. https://doi.org/10.3390/en16124625
De Muijlder T, Voué M, Leclère P. Laser Ablation Synthesis of Silver Nanoparticles for Polymer Nanocomposites. Energies. 2023; 16(12):4625. https://doi.org/10.3390/en16124625
Chicago/Turabian StyleDe Muijlder, Thomas, Michel Voué, and Philippe Leclère. 2023. "Laser Ablation Synthesis of Silver Nanoparticles for Polymer Nanocomposites" Energies 16, no. 12: 4625. https://doi.org/10.3390/en16124625





