Review on Salt Hydrate Thermochemical Heat Transformer
Abstract
:1. Introduction
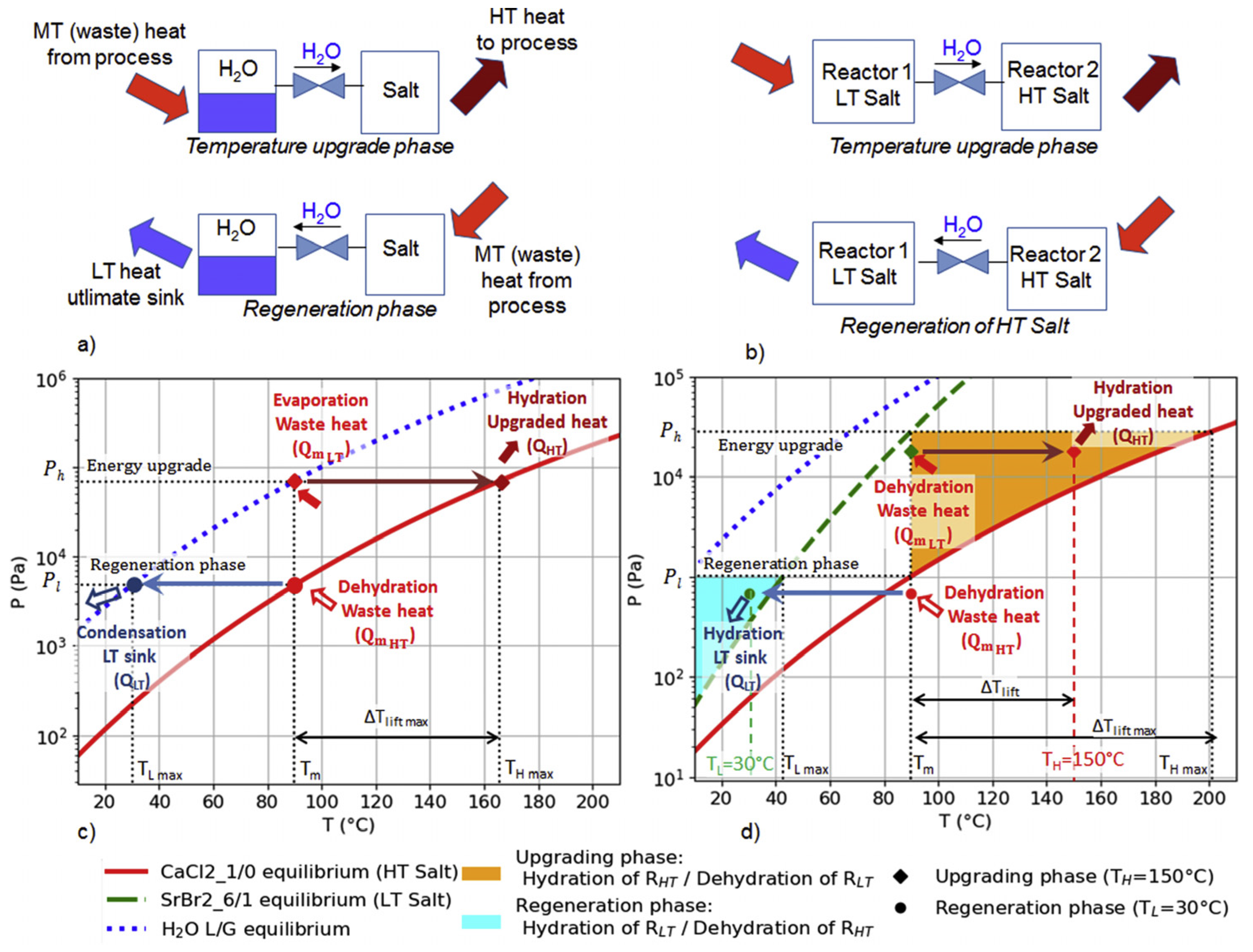
2. Thermochemical Heat Transformer (THT)
2.1. Working Principle
2.2. Reaction Phase and Working Pairs
2.3. Open vs. Closed System Solid-Gas THT
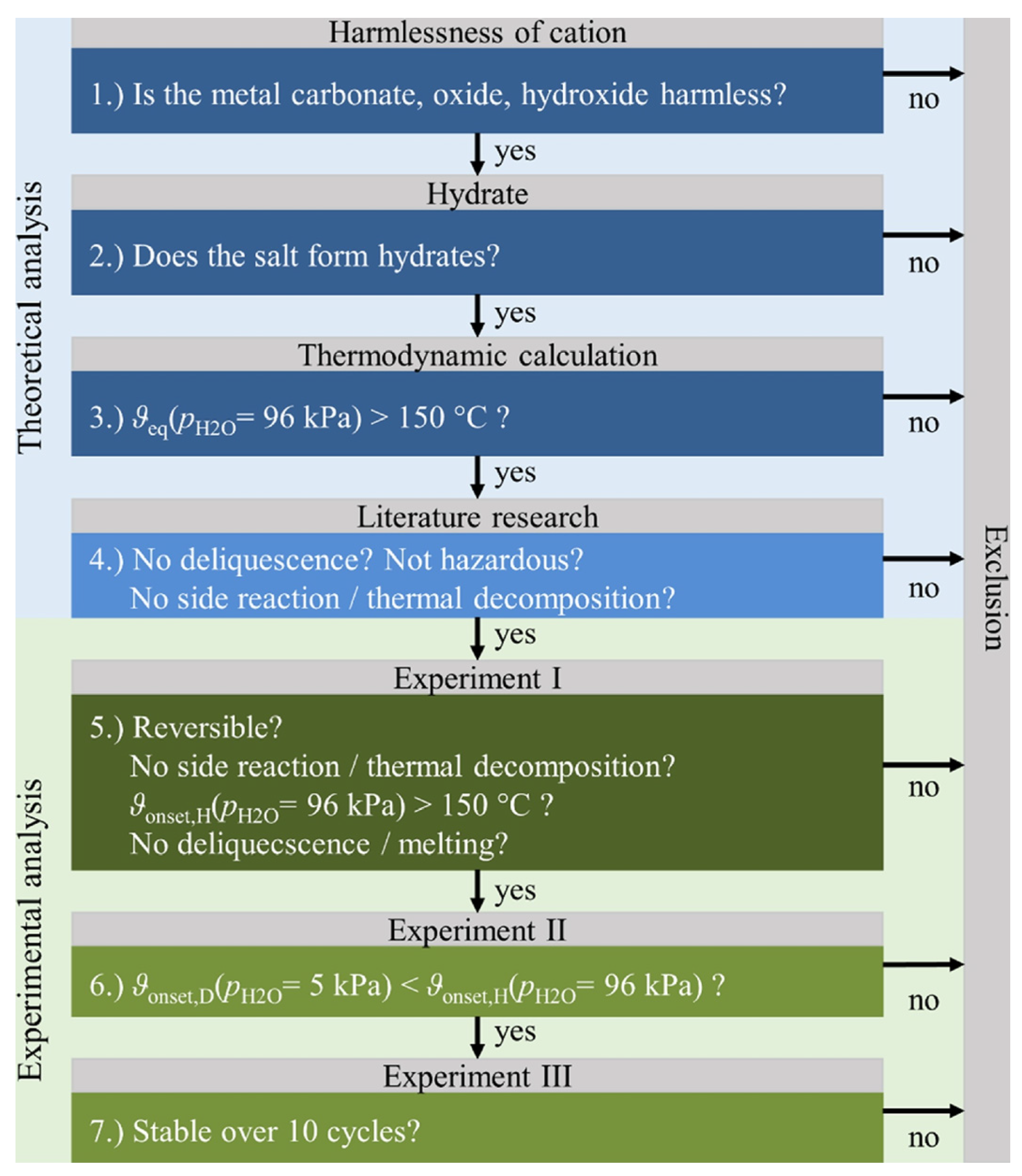
3. Salt Hydrate Characteristics Required for THT Application
- Improve mechanical strength to stabilize the salt [77].
- Accommodates the material swelling and shrinking over cycles [37]
- Improve the thermal conductivity of the reactor bed, leading to a more efficient heat transfer [75].
- Practical pressure drop through composite material may speed up the reaction [75].
4. Recent Advancement of Salt Hydrate-Based THT Research
4.1. The 1-Salt and 2-Salt THT System
4.2. Salt Hydrate THT Experiments
4.3. Alternative Material and Application
5. Research Gaps, Challenges, and Opportunities
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| AC | Activated carbon |
| BET | Brunauer-Emmet-Teller |
| BTU | British thermal unit |
| CNF | Cellulose nanofibrils |
| COP | Coefficient of performance |
| EECA | Energy Efficiency and Conservation Authority |
| EIA | Energy Information Administration |
| ENG | Expanded natural graphite |
| ENG TSA | Expanded Natural Graphite Treated with Sulfuric Acid |
| EU | European Union |
| GHG | Greenhouse gas |
| GTL | Gross temperature lift |
| HTF | Heat transfer fluid |
| IPCC | Intergovernmental Panel on climate change |
| LNG | Liquefied natural gas |
| MVC | Mechanical vapour compression |
| MVR | Mechanical vapour recompression |
| MWCNT | Multi-wall carbon nanotubes |
| PDAC | Polydiallyldimethylammonium chloride |
| PVA | Polyvynyl alcohol |
| SEM | Scanning electron microscopy |
| STA | Simultaneous thermal analysis |
| TCES | Thermochemical energy storage |
| TCHS | Thermochemical heat storage |
| THT | Thermochemical heat transformer |
| TCM | Thermochemical material |
| TES | Thermal energy storage |
| TGA | Thermogravimetric analysis |
| TRL | Technology Readiness Level |
| XRF | X-ray Fluorescence |
| ZMS | Zeolite molecular sieve |
References
- US EIA. International Energy Outlook 2021; US EIA: Washington, DA, USA, 2021. Available online: https://www.eia.gov/outlooks/ieo/tables_side_xls.php (accessed on 24 January 2023).
- Johnson, I.; Choate, W.T.; Davidson, A. Waste Heat Recovery: Technology and Opportunities in US Industry; U.S. Department of Energy: Washington, DA, USA, 2008. Available online: https://www.osti.gov/biblio/1218716 (accessed on 16 January 2023).
- Brueckner, S.; Miró, L.; Cabeza, L.F.; Pehnt, M.; Laevemann, E. Methods to Estimate the Industrial Waste Heat Potential of Regions—A Categorization and Literature Review. Renew. Sustain. Energy Rev. 2014, 38, 164–171. [Google Scholar] [CrossRef]
- Miró, L.; Brückner, S.; Cabeza, L.F. Mapping and Discussing Industrial Waste Heat (IWH) Potentials for Different Countries. Renew. Sustain. Energy Rev. 2015, 51, 847–855. [Google Scholar] [CrossRef] [Green Version]
- McKenna, R.C.; Norman, J.B. Spatial Modelling of Industrial Heat Loads and Recovery Potentials in the UK. Energy Policy 2010, 38, 5878–5891. [Google Scholar] [CrossRef]
- Brueckner, S.; Arbter, R.; Pehnt, M.; Laevemann, E. Industrial Waste Heat Potential in Germany—A Bottom-up Analysis. Energy Effic. 2017, 10, 513–525. [Google Scholar] [CrossRef]
- Hong, G.B.; Pan, T.C.; Chan, D.Y.L.; Liu, I.H. Bottom-up Analysis of Industrial Waste Heat Potential in Taiwan. Energy 2020, 198, 117393. [Google Scholar] [CrossRef]
- Papapetrou, M.; Kosmadakis, G.; Cipollina, A.; La Commare, U.; Micale, G. Industrial Waste Heat: Estimation of the Technically Available Resource in the EU per Industrial Sector, Temperature Level and Country. Appl. Therm. Eng. 2018, 138, 207–216. [Google Scholar] [CrossRef]
- Blesl, M.; Kempe, S.; Ohl, M.; Fahl, U.; König, A.; Jenssen, T.; Eltrop, L. Wärmeatlas Baden-Württemberg—Erstellung Eines Leitfadens und Umsetzung für Modellregionen (Heat Atlas Baden-Württemberg—Creation of a Guide and Implementation for Model Regions); University of Stuttgart: Stuttgart, Germany, 2009. [Google Scholar]
- Blesl, M.; Ohl, M.; Fahl, U. Ganzheitliche Bewertung Innovativer Mobiler Thermischer Energiespeicherkonzepte für Baden-Württemberg auf Basis Branchen- und Betriebsspezifischer Wärmebedarfsstrukturen (Holistic Assessment of Innovative Mobile Thermal Energy Storage Concepts for Baden-Württemberg Based on Industry and Company-Specific Heat Demand Structures); University of Stuttgart: Stuttgart, Germany, 2011. [Google Scholar]
- Richter, M.; Bouché, M.; Linder, M. Heat Transformation Based on CaCl2/H2O—Part A: Closed Operation Principle. Appl. Therm. Eng. 2016, 102, 615–621. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Zhang, M.; Xu, P.; Zhao, Z.; Wang, Z.; Huang, H.; Ouyang, T. Opportunities and Strategies for Multigrade Waste Heat Utilization in Various Industries: A Recent Review. Energy Convers. Manag. 2021, 229, 113769. [Google Scholar] [CrossRef]
- Hung, T.C.; Shai, T.Y.; Wang, S.K. A Review of Organic Rankine Cycles (ORCs) for The Recovery of Low-Grade Waste Heat. Energy 1997, 22, 661–667. [Google Scholar] [CrossRef]
- Kishore, R.A.; Priya, S. A Review on Low-Grade Thermal Energy Harvesting: Materials, Methods and Devices. Materials 2018, 11, 1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinçer, İ.; Rosen, M.A. Thermal Energy Storage and Energy Savings. In Thermal Energy Storage; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 211–231. [Google Scholar]
- N’Tsoukpoe, K.E.; Osterland, T.; Opel, O.; Ruck, W.K.L. Cascade Thermochemical Storage with Internal Condensation Heat Recovery for Better Energy and Exergy Efficiencies. Appl. Energy 2016, 181, 562–574. [Google Scholar] [CrossRef]
- Solé, A.; Martorell, I.; Cabeza, L.F. State of the Art on Gas-Solid Thermochemical Energy Storage Systems and Reactors for Building Applications. Renew. Sustain. Energy Rev. 2015, 47, 386–398. [Google Scholar] [CrossRef] [Green Version]
- Stutz, B.; Le Pierres, N.; Kuznik, F.; Johannes, K.; Palomo Del Barrio, E.; Bédécarrats, J.P.; Gibout, S.; Marty, P.; Zalewski, L.; Soto, J.; et al. Storage of Thermal Solar Energy. Comptes Rendus Phys. 2017, 18, 401–414. [Google Scholar] [CrossRef]
- Farid, M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A Review on Phase Change Energy Storage Materials and Applications. In Thermal Energy Storage with Phase Change Materials; Farid, M., Auckaili, A., Gholamibozanjani, G., Eds.; Taylor & Francis: Abingdon, UK, 2021; pp. 4–23. [Google Scholar]
- Schmerse, E.; Ikutegbe, C.A.; Auckaili, A.; Farid, M.M. Using Pcm in Two Proposed Residential Buildings in Christchurch, New Zealand. Energy 2020, 13, 6025. [Google Scholar] [CrossRef]
- N’Tsoukpoe, K.E.; Schmidt, T.; Rammelberg, H.U.; Watts, B.A.; Ruck, W.K.L. A Systematic Multi-Step Screening of Numerous Salt Hydrates for Low Temperature Thermochemical Energy Storage. Appl. Energy 2014, 124, 1–16. [Google Scholar] [CrossRef]
- Bouché, M.; Richter, M.; Linder, M. Heat Transformation Based on CaCl2/H2O—Part B: Open Operation Principle. Appl. Therm. Eng. 2016, 102, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Esaki, T.; Yasuda, M.; Kobayashi, N. Experimental Evaluation of the Heat Output/Input and Coefficient of Performance Characteristics of a Chemical Heat Pump in the Heat Upgrading Cycle of CaCl2 Hydration. Energy Convers. Manag. 2017, 150, 365–374. [Google Scholar] [CrossRef]
- Yu, Y.Q.; Zhang, P.; Wu, J.Y.; Wang, R.Z. Energy Upgrading by Solid-Gas Reaction Heat Transformer: A Critical Review. Renew. Sustain. Energy Rev. 2008, 12, 1302–1324. [Google Scholar] [CrossRef]
- Wongsuwan, W.; Kumar, S.; Neveu, P.; Meunier, F. A Review of Chemical Heat Pump Technology and Applications. Appl. Therm. Eng. 2001, 21, 1489–1519. [Google Scholar] [CrossRef]
- Fadhel, M.I.; Sopian, K.; Daud, W.R.W.; Alghoul, M.A. Review on Advanced of Solar Assisted Chemical Heat Pump Dryer for Agriculture Produce. Renew. Sustain. Energy Rev. 2011, 15, 1152–1168. [Google Scholar] [CrossRef]
- Cot-Gores, J.; Castell, A.; Cabeza, L.F. Thermochemical Energy Storage and Conversion: A-State-of-the-Art Review of the Experimental Research under Practical Conditions. Renew. Sustain. Energy Rev. 2012, 16, 5207–5224. [Google Scholar] [CrossRef]
- Chan, C.W.; Ling-Chin, J.; Roskilly, A.P. A Review of Chemical Heat Pumps, Thermodynamic Cycles and Thermal Energy Storage Technologies for Low Grade Heat Utilisation. In Applied Thermal Engineering; Elsevier: Amsterdam, The Netherlands, 2013; Volume 50, pp. 1257–1273. [Google Scholar]
- N’Tsoukpoe, K.E.; Liu, H.; Le Pierrès, N.; Luo, L. A Review on Long-Term Sorption Solar Energy Storage. Renew. Sustain. Energy Rev. 2009, 13, 2385–2396. [Google Scholar] [CrossRef]
- Clark, R.J.; Farid, M. Experimental Investigation into the Performance of Novel SrCl2-Based Composite Material for Thermochemical Energy Storage. J. Energy Storage 2021, 36, 102390. [Google Scholar] [CrossRef]
- Park, D.; Hayatina, I.; Farid, M.; Auckaili, A. Experimental Work on Salt-Based Cooling Systems. Thermo 2023, 3, 200–215. [Google Scholar] [CrossRef]
- Ferrucci, F.; Stitou, D.; Ortega, P.; Lucas, F. Mechanical Compressor-Driven Thermochemical Storage for Cooling Applications in Tropical Insular Regions. Concept and Efficiency Analysis. Appl. Energy 2018, 219, 240–255. [Google Scholar] [CrossRef]
- Fitó, J.; Coronas, A.; Mauran, S.; Mazet, N.; Perier-Muzet, M.; Stitou, D. Hybrid System Combining Mechanical Compression and Thermochemical Storage of Ammonia Vapor for Cold Production. Energy Convers. Manag. 2019, 180, 709–723. [Google Scholar] [CrossRef]
- Park, J.-G.; Han, S.-C.; Jang, H.-Y.; Lee, S.-M.; Lee, P.S.; Lee, J.-Y. The Development of Compressor-Driven Metal Hydride Heat Pump (CDMHHP) System as an Air Conditioner. Int. J. Hydrogen Energy 2002, 27, 941–944. [Google Scholar] [CrossRef]
- Desai, F.; Atayo, A.; Prasad, J.S.; Muthukumar, P.; Rahman, M.; Asmatulu, E. Experimental Studies on Endothermic Reversible Reaction of Salts for Cooling. Heat Transf. Eng. 2021, 42, 1107–1116. [Google Scholar] [CrossRef]
- Li, T.X.; Wang, R.Z.; Yan, T. Solid-Gas Thermochemical Sorption Thermal Battery for Solar Cooling and Heating Energy Storage and Heat Transformer. Energy 2015, 84, 745–758. [Google Scholar] [CrossRef]
- Clark, R.J.; Mehrabadi, A.; Farid, M. State of the Art on Salt Hydrate Thermochemical Energy Storage Systems for Use in Building Applications. J. Energy Storage 2020, 27, 101145. [Google Scholar] [CrossRef]
- Spinner, B. Ammonia-Based Thermochemical Transformers. Heat Recovery Syst. CHP 1993, 13, 301–307. [Google Scholar] [CrossRef]
- Stitou, D.; Mazet, N.; Bonnissel, M. Performance of a High Temperature Hydrate Solid/Gas Sorption Heat Pump Used as Topping Cycle for Cascaded Sorption Chillers. Energy 2004, 29, 267–285. [Google Scholar] [CrossRef]
- Scapino, L.; Zondag, H.A.; Van Bael, J.; Diriken, J.; Rindt, C.C.M. Sorption Heat Storage for Long-Term Low-Temperature Applications: A Review on the Advancements at Material and Prototype Scale. Appl. Energy 2017, 190, 920–948. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Wang, R.Z.; Li, T.X. Experimental Investigation on an Open Sorption Thermal Storage System for Space Heating. Energy 2017, 141, 2421–2433. [Google Scholar] [CrossRef]
- De Boer, R.; Haije, W.G.; Veldhuis, J.B.J.; Smeding, S.F. Solid-Sorption Cooling with Integrated Thermal Storage: The SWEAT Prototype. In Proceedings of the International Conference of Heat Powered Cycles, Larnaca, Cyprus, 11–13 October 2004. [Google Scholar]
- Zondag, H.; Kikkert, B.; Smeding, S.; de Boer, R.; Bakker, M. Prototype Thermochemical Heat Storage with Open Reactor System. Appl. Energy 2013, 109, 360–365. [Google Scholar] [CrossRef]
- Linnow, K.; Niermann, M.; Bonatz, D.; Posern, K.; Steiger, M. Experimental Studies of the Mechanism and Kinetics of Hydration Reactions. In Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 48, pp. 394–404. [Google Scholar]
- Michel, B.; Mazet, N.; Neveu, P. Experimental Investigation of an Innovative Thermochemical Process Operating with a Hydrate Salt and Moist Air for Thermal Storage of Solar Energy: Global Performance. Appl. Energy 2014, 129, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Korhammer, K.; Druske, M.M.; Fopah-Lele, A.; Rammelberg, H.U.; Wegscheider, N.; Opel, O.; Osterland, T.; Ruck, W. Sorption and Thermal Characterization of Composite Materials Based on Chlorides for Thermal Energy Storage. Appl. Energy 2016, 162, 1462–1472. [Google Scholar] [CrossRef]
- Li, S.; Huang, H.; Yang, X.; Bai, Y.; Li, J.; Kobayashi, N.; Kubota, M. Hydrophilic Substance Assisted Low Temperature LiOH·H2O Based Composite Thermochemical Materials for Thermal Energy Storage. Appl. Therm. Eng. 2018, 128, 706–711. [Google Scholar] [CrossRef]
- Rammelberg, H.U.; Osterland, T.; Priehs, B.; Opel, O.; Ruck, W.K.L. Thermochemical Heat Storage Materials—Performance of Mixed Salt Hydrates. Sol. Energy 2016, 136, 571–589. [Google Scholar] [CrossRef]
- Rehman, A.U.; Khan, M.; Maosheng, Z. Hydration Behavior of MgSO4–ZnSO4 Composites for Long-Term Thermochemical Heat Storage Application. J. Energy Storage 2019, 26, 101026. [Google Scholar] [CrossRef]
- Li, W.; Zeng, M.; Wang, Q. Development and Performance Investigation of MgSO4/SrCl2 Composite Salt Hydrate for Mid-Low Temperature Thermochemical Heat Storage. Sol. Energy Mater. Sol. Cells 2020, 210, 110509. [Google Scholar] [CrossRef]
- Ousaleh, H.A.; Said, S.; Zaki, A.; Faik, A.; El Bouari, A. Silica Gel/Inorganic Salts Composites for Thermochemical Heat Storage: Improvement of Energy Storage Density and Assessment of Cycling Stability. In Materials Today: Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 30, pp. 937–941. [Google Scholar]
- Jabbari-Hichri, A.; Bennici, S.; Auroux, A. Enhancing the Heat Storage Density of Silica–Alumina by Addition of Hygroscopic Salts (CaCl2, Ba(OH)2, and LiNO3). Sol. Energy Mater. Sol. Cells 2015, 140, 351–360. [Google Scholar] [CrossRef]
- Casey, S.P.; Elvins, J.; Riffat, S.; Robinson, A. Salt Impregnated Desiccant Matrices for “open” Thermochemical Energy Storage—Selection, Synthesis and Characterisation of Candidate Materials. Energy Build. 2014, 84, 412–425. [Google Scholar] [CrossRef]
- Shkatulov, A.I.; Houben, J.; Fischer, H.; Huinink, H.P. Stabilization of K2CO3 in Vermiculite for Thermochemical Energy Storage. Renew. Energy 2020, 150, 990–1000. [Google Scholar] [CrossRef]
- Xu, J.X.; Li, T.X.; Chao, J.W.; Yan, T.S.; Wang, R.Z. High Energy-Density Multi-Form Thermochemical Energy Storage Based on Multi-Step Sorption Processes. Energy 2019, 185, 1131–1142. [Google Scholar] [CrossRef]
- Whiting, G.; Grondin, D.; Bennici, S.; Auroux, A. Heats of Water Sorption Studies on Zeolite-MgSO4 Composites as Potential Thermochemical Heat Storage Materials. Sol. Energy Mater. Sol. Cells 2013, 112, 112–119. [Google Scholar] [CrossRef]
- Clark, R.J.; Farid, M. Experimental Investigation into Cascade Thermochemical Energy Storage System Using SrCl2-Cement and Zeolite-13X Materials. Appl. Energy 2022, 316, 119145. [Google Scholar] [CrossRef]
- Mauran, S.; Lahmidi, H.; Goetz, V. Solar Heating and Cooling by a Thermochemical Process. First Experiments of a Prototype Storing 60 KW h by a Solid/Gas Reaction. Sol. Energy 2008, 82, 623–636. [Google Scholar] [CrossRef]
- Li, W.; Klemeš, J.J.; Wang, Q.; Zeng, M. Characterisation and Sorption Behaviour of LiOH-LiCl@EG Composite Sorbents for Thermochemical Energy Storage with Controllable Thermal Upgradeability. Chem. Eng. J. 2021, 421, 129586. [Google Scholar] [CrossRef]
- Xueling, Z.; Feifei, W.; Qi, Z.; Xudong, L.; Yanling, W.; Yeqiang, Z.; Chuanxiao, C.; Tingxiang, J. Heat Storage Performance Analysis of ZMS-Porous Media/CaCl2/MgSO4 Composite Thermochemical Heat Storage Materials. Sol. Energy Mater. Sol. Cells 2021, 230, 111246. [Google Scholar] [CrossRef]
- Yu, N.; Wang, R.Z.; Lu, Z.S.; Wang, L.W. Study on Consolidated Composite Sorbents Impregnated with LiCl for Thermal Energy Storage. Int. J. Heat Mass Transf. 2015, 84, 660–670. [Google Scholar] [CrossRef]
- Salviati, S.; Carosio, F.; Saracco, G.; Fina, A. Hydrated Salt/Graphite/Polyelectrolyte Organic-Inorganic Hybrids for Efficient Thermochemical Storage. Nanomaterials 2019, 9, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salviati, S.; Carosio, F.; Cantamessa, F.; Medina, L.; Berglund, L.A.; Saracco, G.; Fina, A. Ice-Templated Nanocellulose Porous Structure Enhances Thermochemical Storage Kinetics in Hydrated Salt/Graphite Composites. Renew. Energy 2020, 160, 698–706. [Google Scholar] [CrossRef]
- Donkers, P.A.J.; Sögütoglu, L.C.; Huinink, H.P.; Fischer, H.R.; Adan, O.C.G. A Review of Salt Hydrates for Seasonal Heat Storage in Domestic Applications. Appl. Energy 2017, 199, 45–68. [Google Scholar] [CrossRef]
- Bales, C.; Gantenbein, P.; Hauer, A.; Henning, H.-M.; Jaenig, D.; Kerskes, H.; Núñez, T.; Visscher, K. Thermal Properties of Materials for Thermo-Chemical Storage of Solar Heat; International Energy Agency: Paris, France, 2005. [Google Scholar]
- Afflerbach, S.; Trettin, R. A Systematic Screening Approach for New Materials for Thermochemical Energy Storage and Conversion Based on the Strunz Mineral Classification System. Thermochim. Acta 2019, 674, 82–94. [Google Scholar] [CrossRef]
- Li, W.; Klemeš, J.J.; Wang, Q.; Zeng, M. Salt Hydrate–Based Gas-Solid Thermochemical Energy Storage: Current Progress, Challenges, and Perspectives. Renew. Sustain. Energy Rev. 2022, 154, 111846. [Google Scholar] [CrossRef]
- Fopah Lele, A.; N’Tsoukpoe, K.E.; Osterland, T.; Kuznik, F.; Ruck, W.K.L. Thermal Conductivity Measurement of Thermochemical Storage Materials. Appl. Therm. Eng. 2015, 89, 916–926. [Google Scholar] [CrossRef]
- Fumey, B.; Weber, R.; Baldini, L. Sorption Based Long-Term Thermal Energy Storage—Process Classification and Analysis of Performance Limitations: A Review. Renew. Sustain. Energy Rev. 2019, 111, 57–74. [Google Scholar] [CrossRef]
- Scapino, L.; De Servi, C.; Zondag, H.A.; Diriken, J.; Rindt, C.C.M.; Sciacovelli, A. Techno-Economic Optimization of an Energy System with Sorption Thermal Energy Storage in Different Energy Markets. Appl. Energy 2020, 258, 114063. [Google Scholar] [CrossRef]
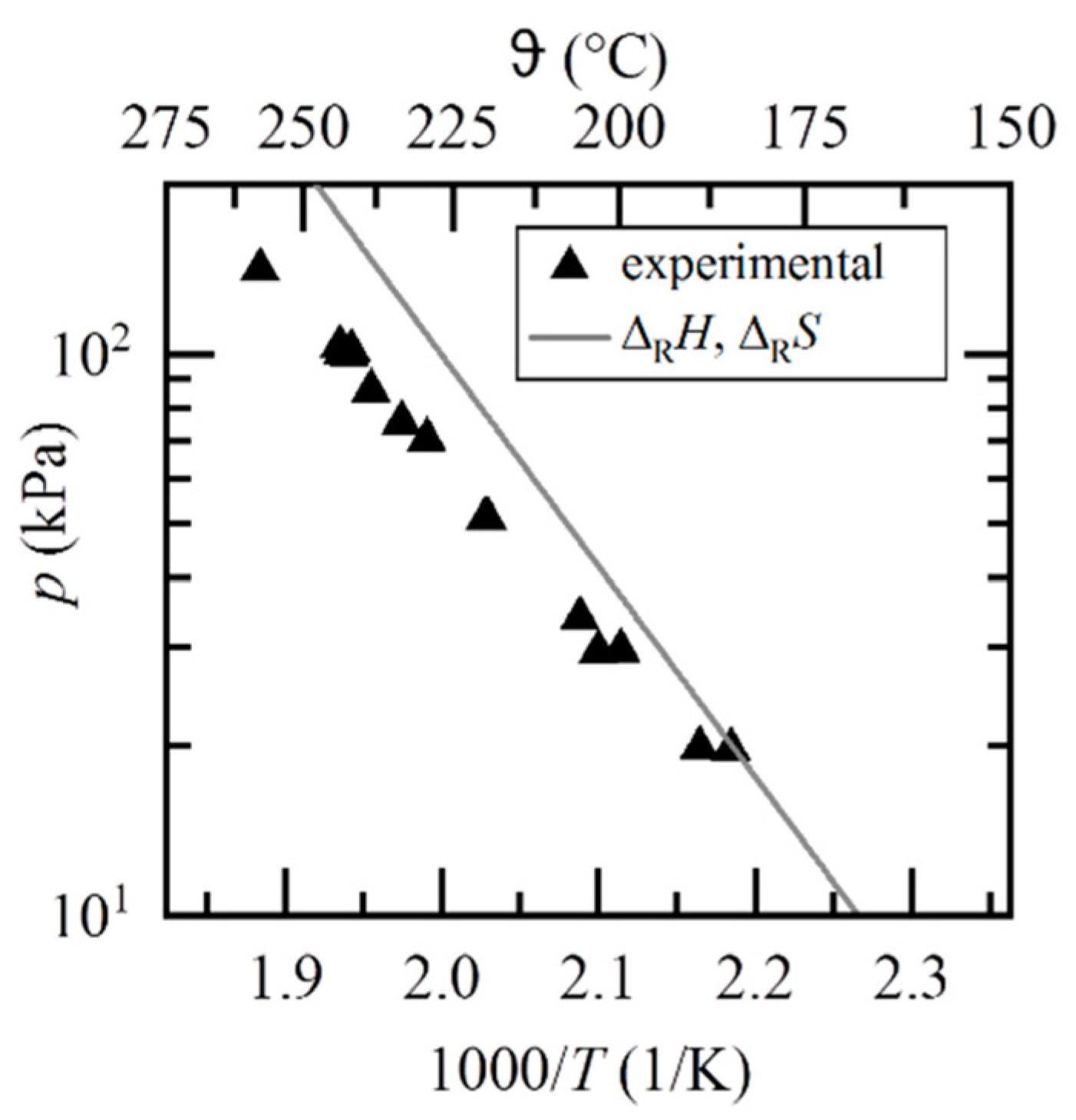
- Richter, M.; Habermann, E.M.; Siebecke, E.; Linder, M. A Systematic Screening of Salt Hydrates as Materials for a Thermochemical Heat Transformer. Thermochim. Acta 2018, 659, 136–150. [Google Scholar] [CrossRef]
- Neveu, P.; Castaing, J. Solid-Gas Chemical Heat Pumps: Field of Application and Performance of the Internal Heat of Reaction Recovery Process. Heat Recovery Syst. CHP 1993, 13, 233–251. [Google Scholar] [CrossRef]
- Trausel, F.; De Jong, A.J.; Cuypers, R. A Review on the Properties of Salt Hydrates for Thermochemical Storage. In Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 48, pp. 447–452. [Google Scholar]
- Zbair, M.; Bennici, S. Survey Summary on Salts Hydrates and Composites Used in Thermochemical Sorption Heat Storage: A Review. Energies 2021, 14, 3105. [Google Scholar] [CrossRef]
- Lin, J.; Zhao, Q.; Huang, H.; Mao, H.; Liu, Y.; Xiao, Y. Applications of Low-Temperature Thermochemical Energy Storage Systems for Salt Hydrates Based on Material Classification: A Review. Sol. Energy 2021, 214, 149–178. [Google Scholar] [CrossRef]
- Kazanci, S.; Qasim, O.A.; Bahauldin, Y.; Samanci, A. Renewable Energy Sources Energy Policy and Energy Management Journal Homepage Thermochemical Heat Storage System for Domestic Application: A Review. Renew. Energy Sources Energy Policy Energy Manag. 2021, 2, 1–11. [Google Scholar]
- Roelands, M.; Cuypers, R.; Kruit, K.D.; Oversloot, H.; De Jong, A.J.; Duvalois, W.; Van Vliet, L.; Hoegaerts, C. Preparation & Characterization of Sodium Sulfide Hydrates for Application in Thermochemical Storage Systems. In Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 70, pp. 257–266. [Google Scholar]
- Michel, B.; Clausse, M. Design of Thermochemical Heat Transformer for Waste Heat Recovery: Methodology for Reactive Pairs Screening and Dynamic Aspect Consideration. Energy 2020, 211, 118042. [Google Scholar] [CrossRef]
- Li, T.; Wang, R.; Kiplagat, J.K. A Target-Oriented Solid-Gas Thermochemical Sorption Heat Transformer for Integrated Energy Storage and Energy Upgrade. AIChE J. 2013, 59, 1334–1347. [Google Scholar] [CrossRef]
- Stengler, J.; Ascher, T.; Linder, M. High Temperature Thermochemical Heat Transformation Based on SrBr2. In Proceedings of the 12th IEA Heat Pump Conference 2017, Rotterdam, The Netherlands, 15–18 May 2017. [Google Scholar]
- Stengler, J.; Bürger, I.; Linder, M. Thermodynamic and Kinetic Investigations of the SrBr2 Hydration and Dehydration Reactions for Thermochemical Energy Storage and Heat Transformation. Appl. Energy 2020, 277, 115432. [Google Scholar] [CrossRef]
- Stengler, J.; Linder, M. Thermal Energy Storage Combined with a Temperature Boost: An Underestimated Feature of Thermochemical Systems. Appl. Energy 2020, 262, 114530. [Google Scholar] [CrossRef]
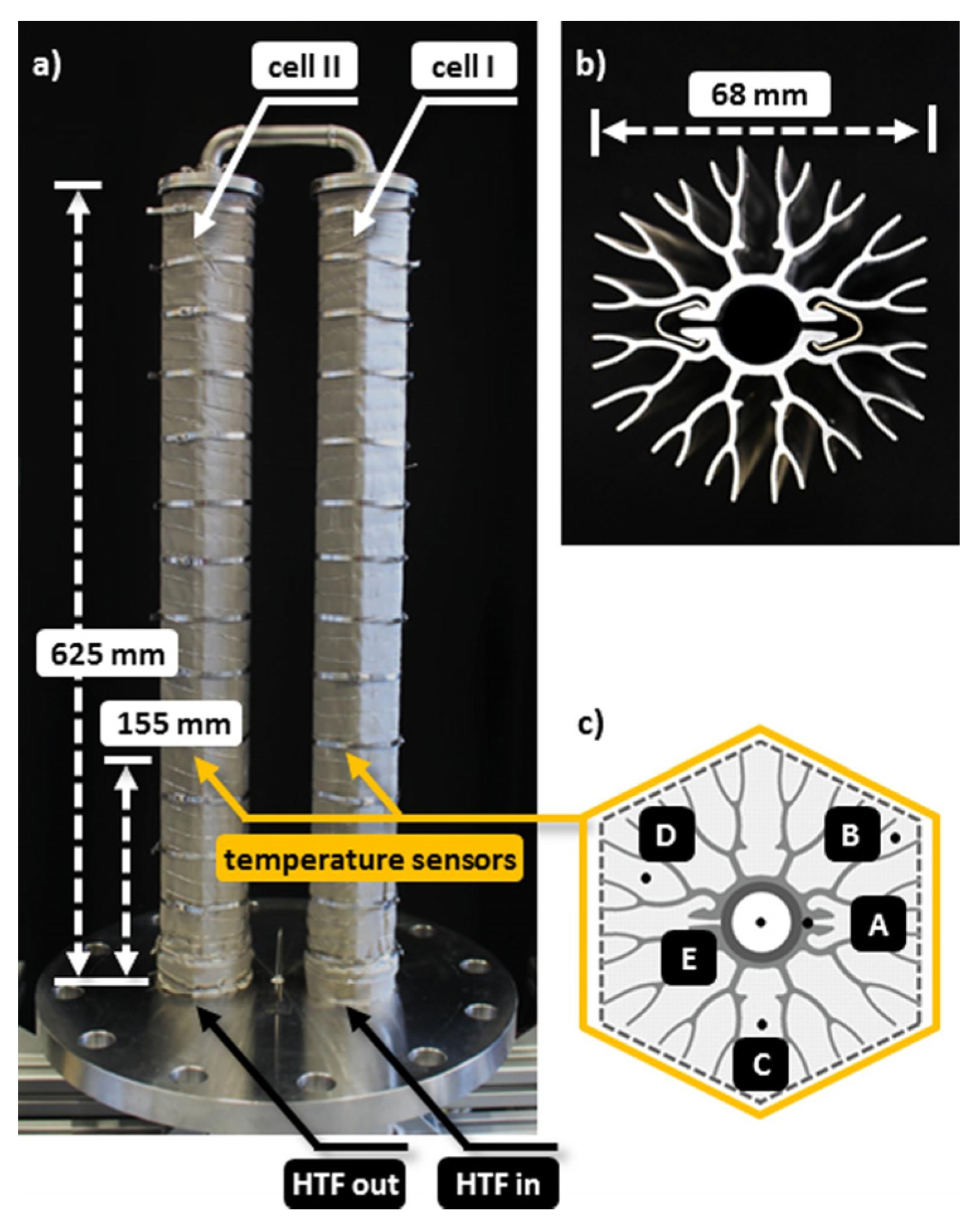
- Stengler, J.; Weiss, J.; Linder, M. Analysis of a Lab-Scale Heat Transformation Demonstrator Based on a Gas–Solid Reaction. Energy 2019, 12, 2234. [Google Scholar] [CrossRef] [Green Version]
- Michel, B.; Dufour, N.; Börtlein, C.; Zoude, C.; Prud’homme, E.; Gremillard, L.; Clausse, M. First Experimental Characterization of CaCl2 Coated Heat Exchanger for Thermochemical Heat Transformer Applications in Industrial Waste Heat Recovery. Appl. Therm. Eng. 2023, 227, 120400. [Google Scholar] [CrossRef]
- Stengler, J.; Bürger, I.; Linder, M. Performance Analysis of a Gas-Solid Thermochemical Energy Storage Using Numerical and Experimental Methods. Int. J. Heat Mass Transf. 2021, 167, 120797. [Google Scholar] [CrossRef]
- Srivastava, N.C.; Eames, I.W. A Review of Adsorbents and Adsorbates in Solid–Vapour Adsorption Heat Pump Systems. Appl. Therm. Eng. 1998, 18, 707–714. [Google Scholar] [CrossRef]
- Saren, S.; Mitra, S.; Miyazaki, T.; Ng, K.C.; Thu, K. Adsorption Heat Transformer Cycle Using Multiple Adsorbent + Water Pairs for Waste Heat Upgrade. J. Therm. Anal. Calorim. 2023, 148, 3059–3071. [Google Scholar] [CrossRef]
- Engelpracht, M.; Driesel, J.; Nießen, O.; Henninger, M.; Seiler, J.; Bardow, A. Closed-Loop Adsorption-Based Upgrading of Heat from 90 to 110 °C: Experimental Demonstration and Insights for Future Development. Energy Technol. 2022, 10, 2200251. [Google Scholar] [CrossRef]
- Frazzica, A.; Palomba, V.; Dawoud, B. Thermodynamic Performance of Adsorption Working Pairs for Low-temperature Waste Heat Upgrading in Industrial Applications. Appl. Sci. 2021, 11, 3389. [Google Scholar] [CrossRef]
- Xue, B.; Ye, S.; Zhang, L.; Wei, X.; Nakaso, K.; Fukai, J. High-Temperature Steam Generation from Low-Grade Waste Heat from an Adsorptive Heat Transformer with Composite Zeolite-13X/CaCl2. Energy Convers. Manag. 2019, 186, 93–102. [Google Scholar] [CrossRef]
- Oktariani, E.; Noda, A.; Nakashima, K.; Tahara, K.; Xue, B.; Nakaso, K.; Fukai, J. Potential of a Direct Contact Adsorption Heat Pump System for Generating Steam from Waste Water. Int. J. Energy Res. 2011, 36, 1077–1087. [Google Scholar] [CrossRef]
- Ye, S.; Xue, B.; Meng, X.; Wei, X.; Nakaso, K.; Fukai, J. Experimental Study of Heat and Mass Recovery on Steam Generation in an Adsorption Heat Pump with Composite Zeolite-CaCl2. Sustain. Cities Soc. 2020, 52, 101808. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, S.; Xue, B.; Wei, X.; Nakaso, K.; Fukai, J. Pre-Adsorption of Low-Grade Waste Steam for High-Temperature Steam Generation by Using Composite Zeolite and Long-Term Heat Storage Salt of MgSO4. Int. J. Energy Res. 2020, 44, 5634–5648. [Google Scholar] [CrossRef]
- Hayatina, I.; Auckaili, A.; Farid, M. Review on the Life Cycle Assessment of Thermal Energy Storage Used in Building Applications. Energy 2023, 16, 1170. [Google Scholar] [CrossRef]






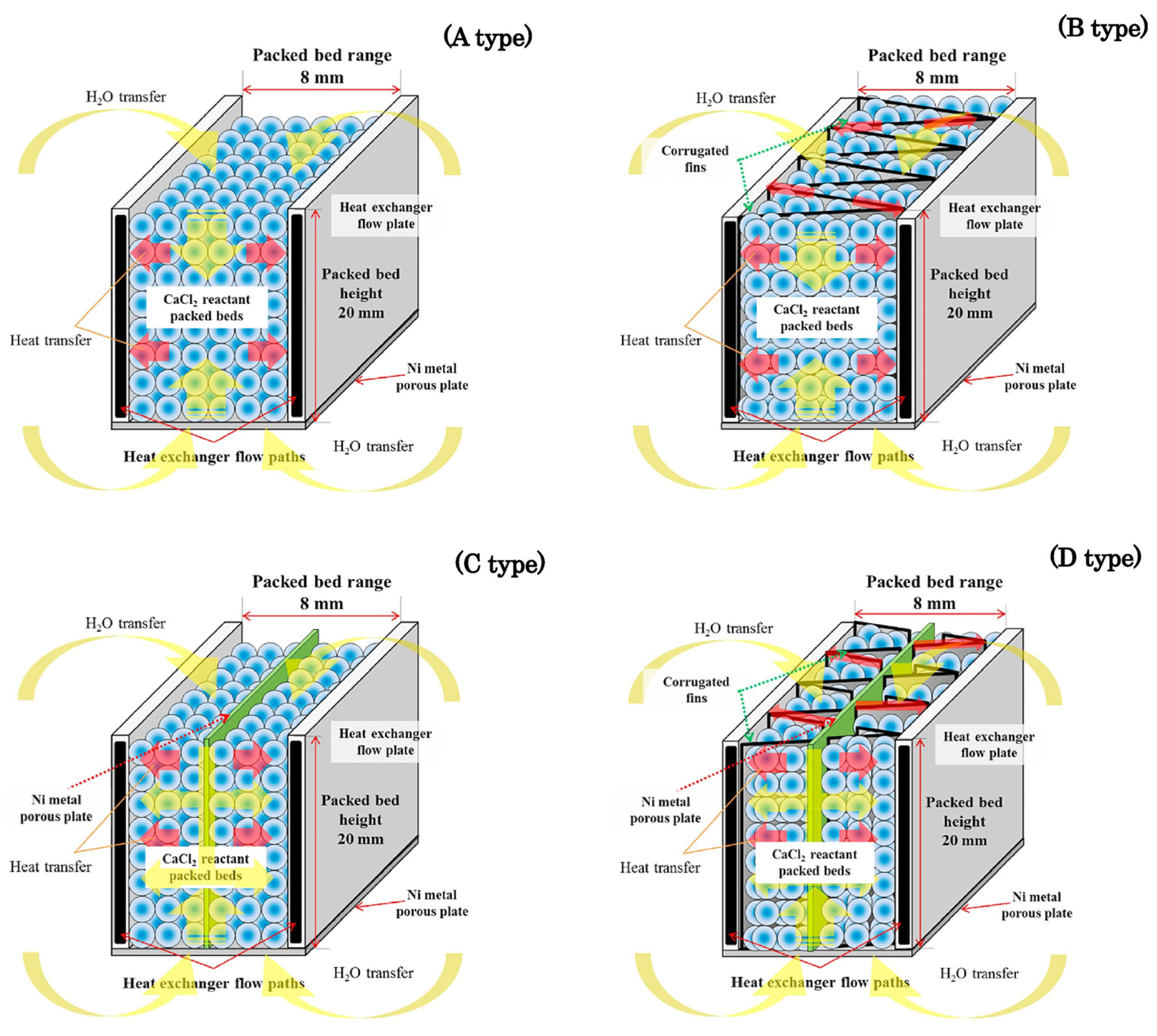

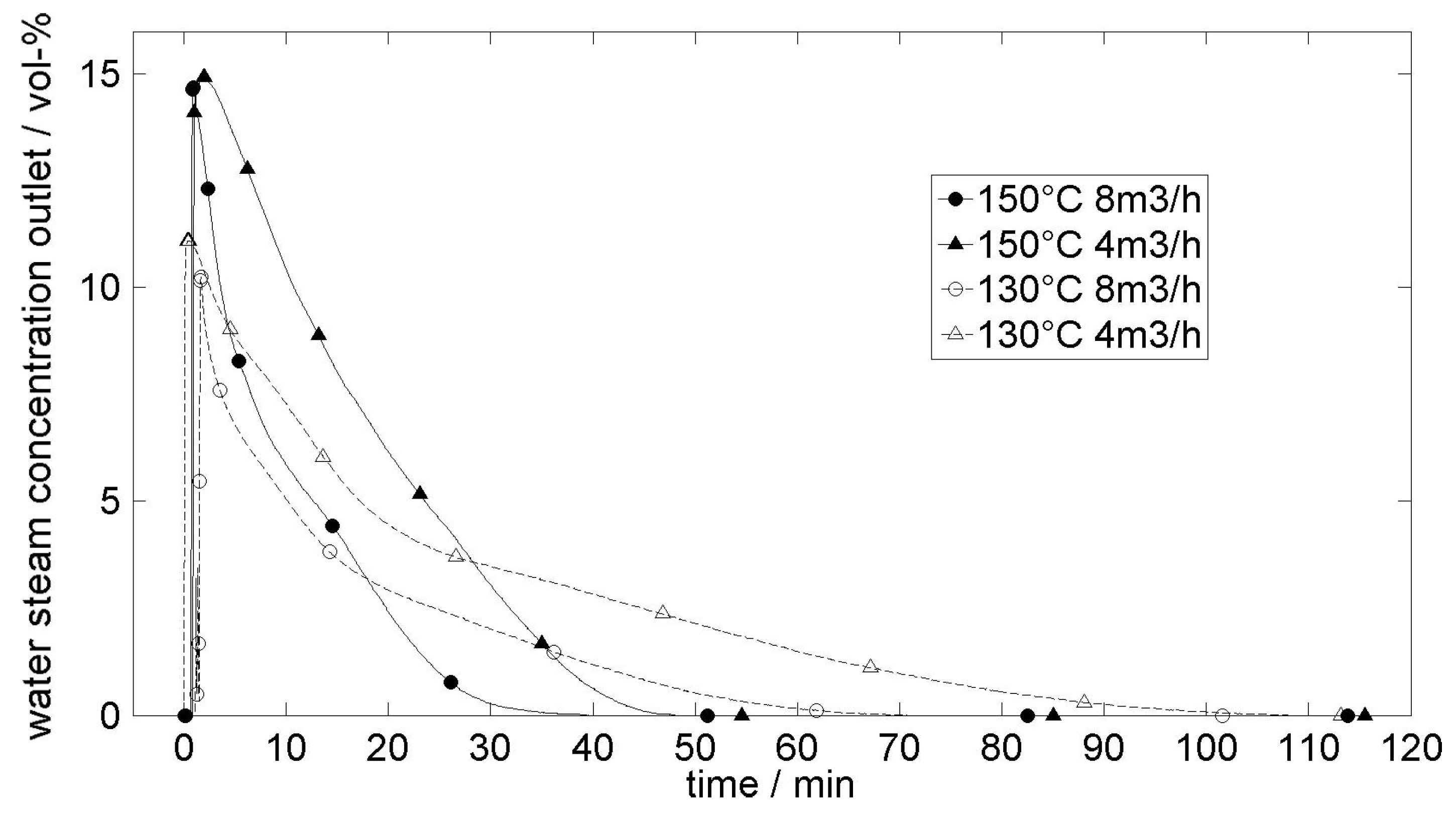

| Ammonia System | Sulfur Dioxide System | Water Vapor System | Carbon Dioxide System | Hydrogen System |
|---|---|---|---|---|
| Alkaline salts (e.g., NaOH)/NH3 Alkaline earth salts (e.g., CaCl2)/NH3 Metalic halides (e.g., MnCl2, NiCl2)/NH3 Nitrates, Phosphates/NH3 Monomethylamine/NH3 | Oxides (e.g., CaO)/SO2 | Oxides (e.g., MgO, CaO, Na2O)/H2O Salt Hydrates (e.g., CaCl2, Na2S)/H2O | Oxides (e.g., CaO, BaO, PbO)/CO2 | Metals (e.g., Ca, Ni, Mn, Al)/H2 Metal alloys (e.g., LaNi5)/H2 |
| Salt Hydrate Form | Example of Experimented Materials |
|---|---|
| A single pure salt hydrate | LiCl [41], Na2S [42], MgCl2 [43], MgSO4 [44], SrBr2 [45] |
| Combination/mixture of salt hydrates | KCl/CaCl2 [46], LiOH/LiCl [47], MgCl2/CaCl2, MgSO4/CaCl2, MgSO4/MgCl2 [48], MgSO4/ZnSO4 [49], MgSO4/SrCl2 [50] |
| Composite of salt hydrate(s) and porous matrix | Al2(SO4)3/Silica gel [51], Ba(OH)2/Silica alumina, LiNO3/Silica alumina [52], CaCl2/Vermiculite, LiBr/Vermiculite [53], LiCl/Activated alumina [41], LiOH/Zeolite 13-X [47], K2CO3/Vermiculite [54], MgCl2/Zeolite [55], MgSO4/Zeolite Na-Y [56], SrCl2/Cement [57], SrBr2/ENG [58], LiCl and LiOH/Expanded graphite [59], MgSO4 and CaCl2/ZMS [60] |
| Salt hydrate composite with additive materials | LiCl/AC and ENG TSA/Silica solution [61], SrBr2/ENG/PDAC [62], SrBr2/ENG/CNF [63] |
| System Type (Open/Closed) | Salt Hydrate | Reactor | Operating Pressure | Initial Reactor Temperature | Thermal Upgrade | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Condenser | Evaporator | Dehydration | Hydration | Reported (1) | Adjusted (1) | ||||
| Closed | SrBr2 | Pillow-plates reactor (capacity 1 kg) | 53 kPa | 70 kPa | 210 °C | 150–210 °C | 20–50 °C | 19–54 °C | [80] |
| (2) | 18–145 kPa | (2) | 132–210 °C | - | 46 °C | [83] | |||
| (3) | SrBr2 | (3) | 0 kPa | 30 kPa | 170 °C | 170 °C | - | - | [82] |
| Closed | SrBr2 | Scalable cells reactor (capacity 4.7 kg) | 1–10 kPa | 146–560 kPa | 179–251 °C | 208–231 °C | 100 °C | 21–44 °C | [81] |
| Closed | CaCl2 | Shell and tube HE (capacity 0.7 kg) | 2 kPa | 75–100 kPa | 130–150 °C | 160–185 °C | 35 °C | 10–25 °C | [11] |
| Closed | CaCl2 | Plate-tube HE (capacity 0.5 kg) | (4) | (4) | 90–120 °C | 155 °C | 55 °C | 3.4–7 °C | [23] |
| Closed | CaCl2 | HE plate—Packed Bed (capacity 60–80 g) | (2) | 70 kPa | (2) | 140 °C | 60 °C | 28 °C/20 °C (5) | [84] |
| CaCl2/PVA | HE Plate—Coated (capacity 20 g) | (2) | 70 kPa | (2) | 140 °C | 63 °C | 24 °C/23 °C (5) | ||
| Open | CaCl2 | Bundle tube exchanger (capacity 0.63 kg) | (6) | (6) | 100–150 °C | (7) | 65 °C (8) | - | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayatina, I.; Auckaili, A.; Farid, M. Review on Salt Hydrate Thermochemical Heat Transformer. Energies 2023, 16, 4668. https://doi.org/10.3390/en16124668
Hayatina I, Auckaili A, Farid M. Review on Salt Hydrate Thermochemical Heat Transformer. Energies. 2023; 16(12):4668. https://doi.org/10.3390/en16124668
Chicago/Turabian StyleHayatina, Isye, Amar Auckaili, and Mohammed Farid. 2023. "Review on Salt Hydrate Thermochemical Heat Transformer" Energies 16, no. 12: 4668. https://doi.org/10.3390/en16124668









