Pyrolysis and Extraction of Bark in a Biorefineries Context: A Critical Review
Abstract
1. Introduction
2. Tree Barks and Biorefinery Research
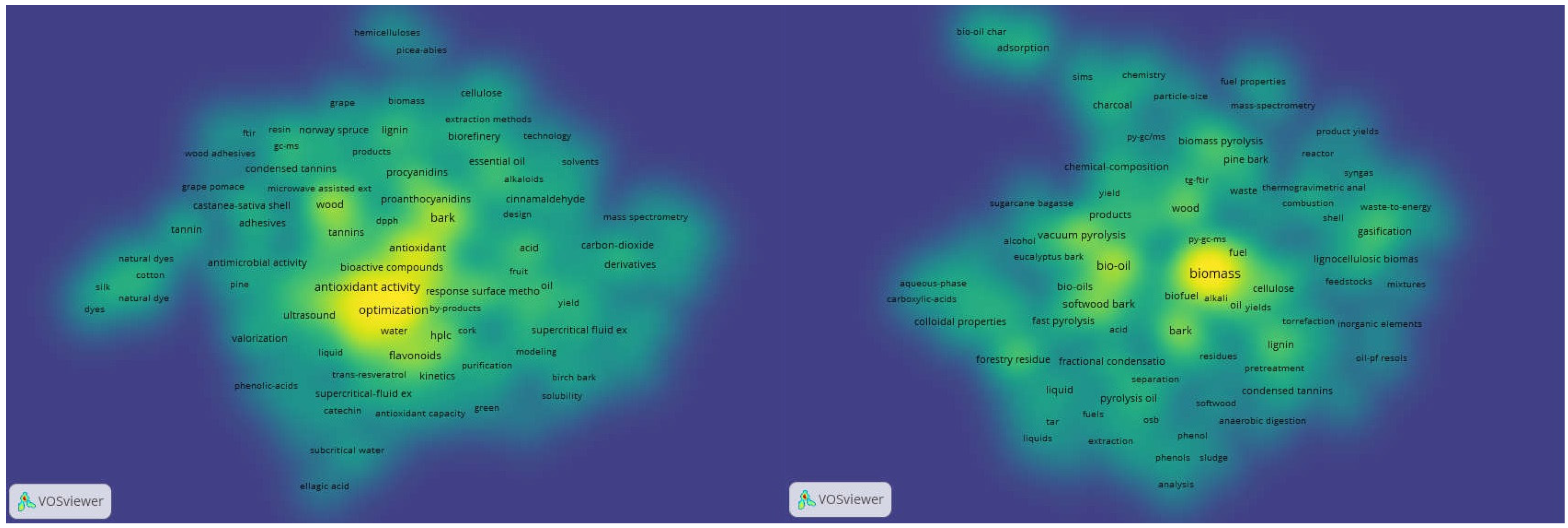
2.1. Trends in Bark-Based Biorefineries
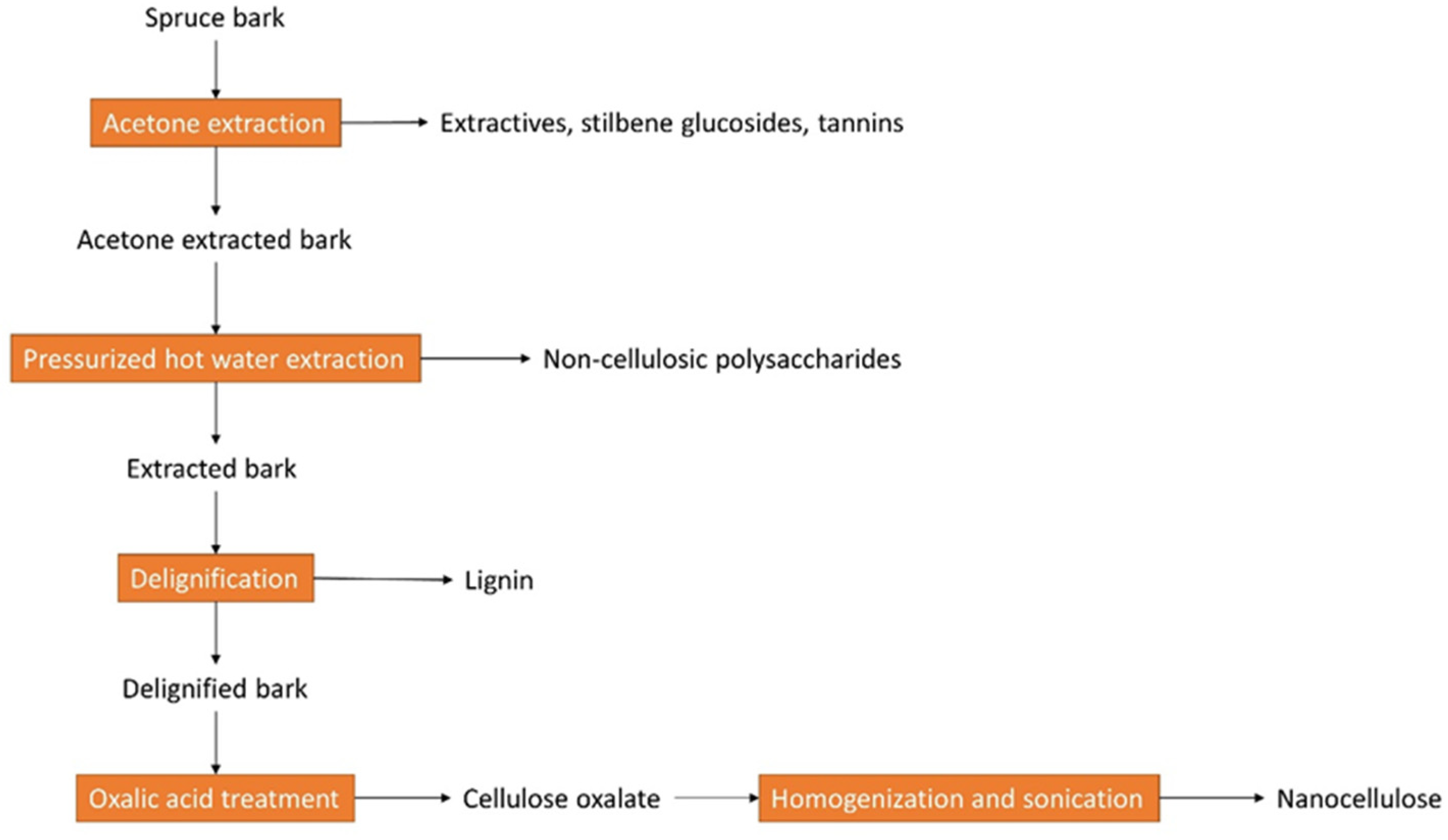
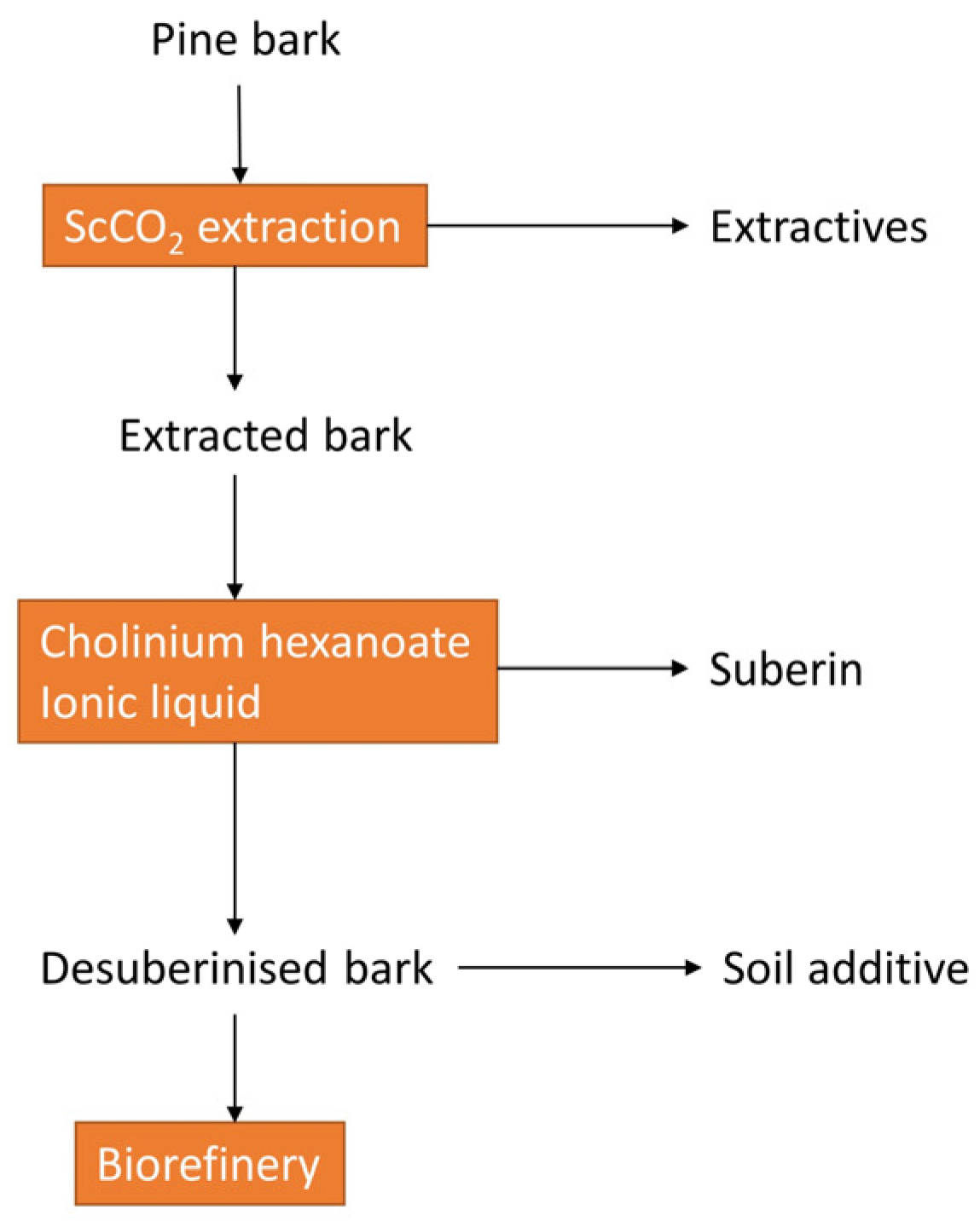
2.2. Current Bark-Based Biorefinery Applications
3. Pyrolysis of Bark
3.1. Torrefaction
3.2. Slow Pyrolysis
3.3. Hydrothermal Carbonization (HTC)
4. Extraction of Bark
4.1. Sequential Solvent Extraction
4.2. Alternative Extraction Methods
- The extraction yield is not optimized, and it is limited because extractions are performed under atmospheric pressure and below 100 °C.
- The usage of organic solvents renders the method not environmentally friendly.
- It usually takes a long time, from days to weeks, to obtain the extracts.
- It has low selectivity to target compounds.
- The thermally sensitive extracts are lost during the hot water extraction, which may not be appropriate to produce bioactive compounds.
5. Critical Evaluation of Bark-Based Biorefineries
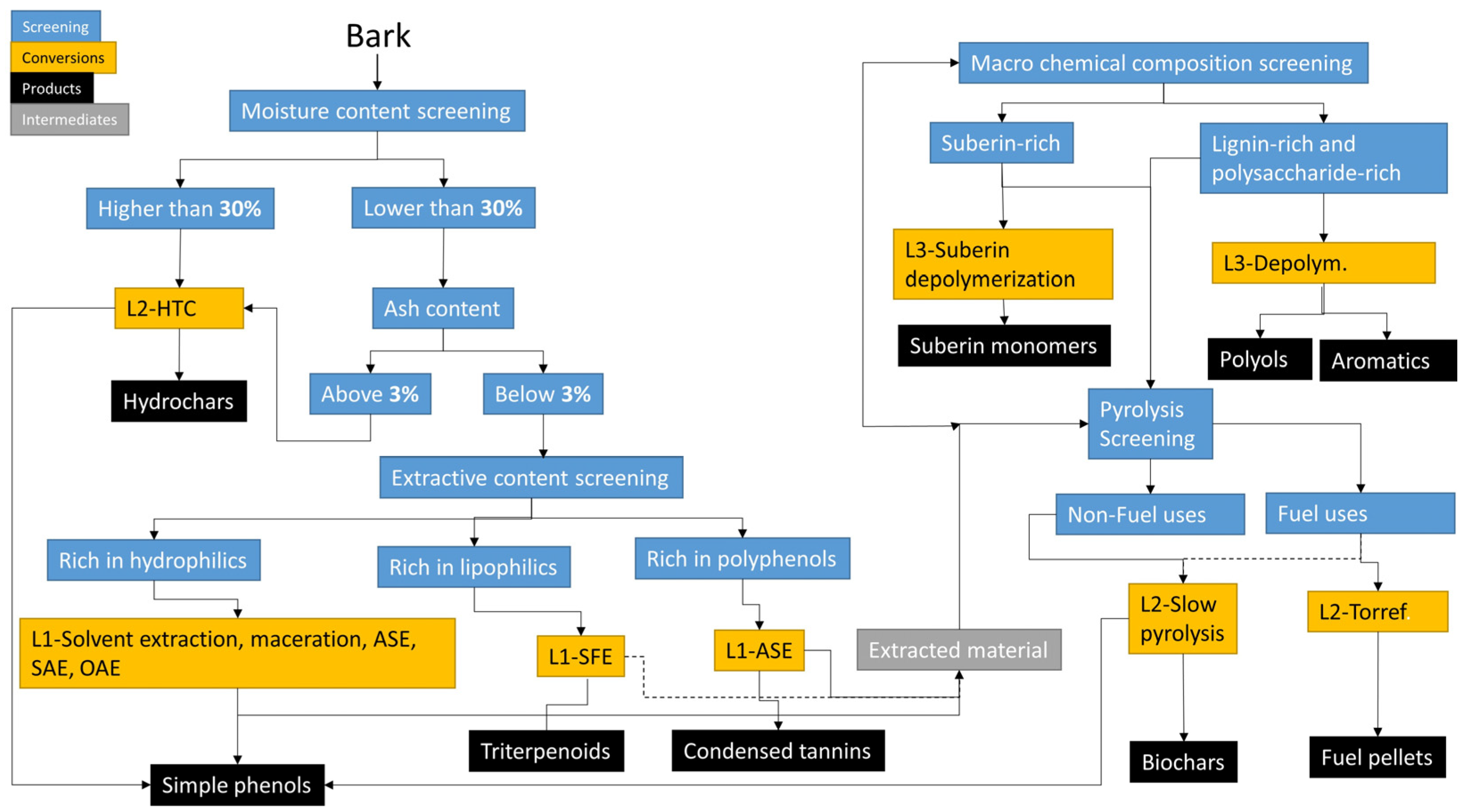
5.1. Screening Barks for Biorefinery
5.2. Development of a Biorefinery Scheme
5.3. Current Knowledge Gaps and Future Directions
- The number of bark characterization studies is insufficient: only a total of 21 articles investigated the extract composition of different barks (Table 2).
- The applied pyrolysis conditions are non-standardized: a total of 13 articles applied different conditions (Table 1), which complicates the evaluation of the most economic processing conditions.
- The applied extraction conditions were also variable. Since the extract composition, properties and yield depend on the applied solvent and method, we limited our discussion to cases with similar extraction procedures (Table 2).
- Energy balance is an important parameter in the evaluation of different processing paths and the scaling up of biorefinery processes. However, the pyrolysis and extraction conditions, as well as chemical properties, of different barks are highly variable which make the energy balance an unhelpful tool. More research is needed to evaluate the energy balance of different bark conversion processes.
- There is a lack of studies on the environmental impact of bark valorization.
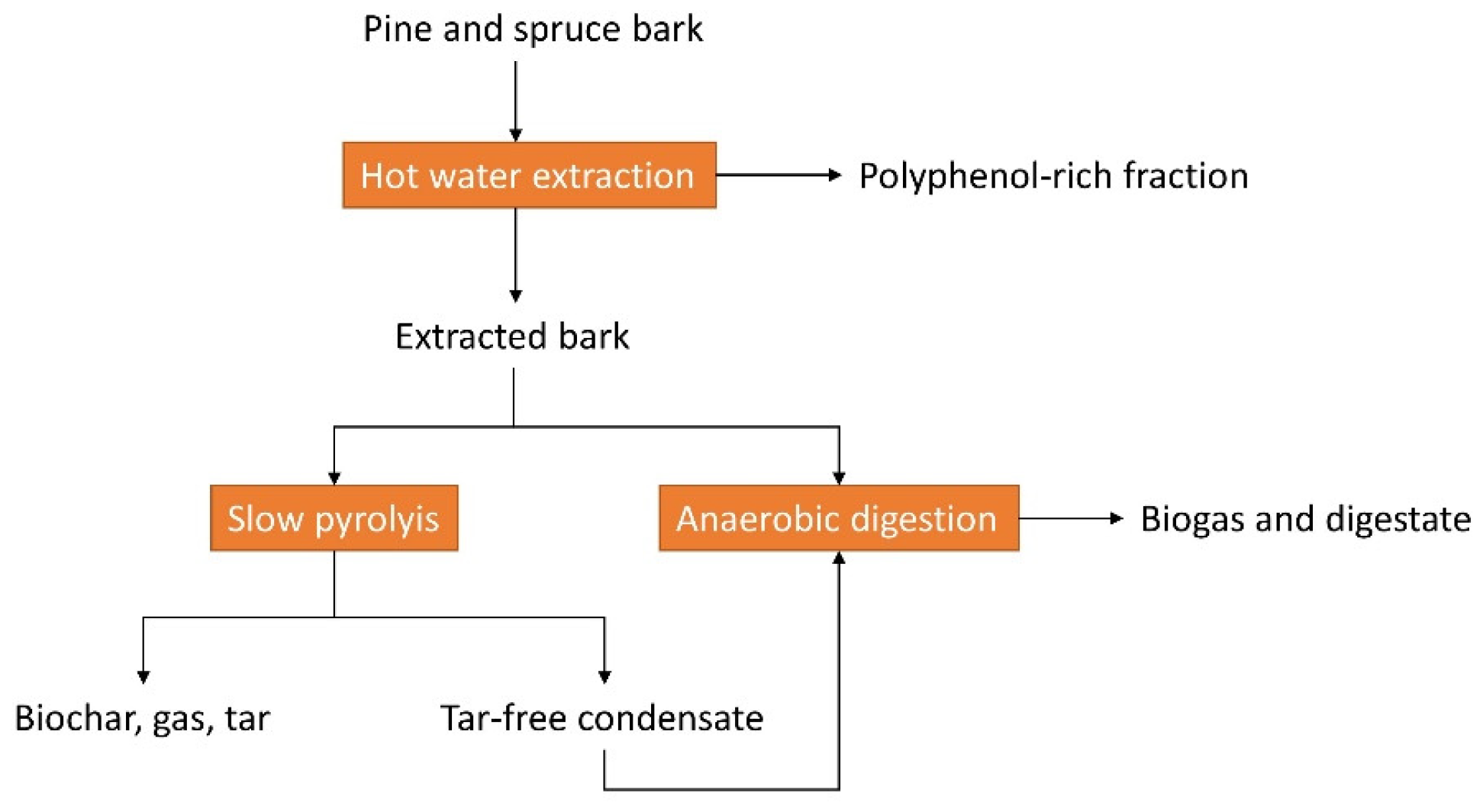
- Studies combining different processing routes are insufficient: only Rasi and co-workers (2019) studied a cascade processing method consisting of hot water extraction, pyrolysis, and anaerobic digestion for pine and spruce barks [27].
- There is a lack of studies investigating the production of bark-based platform chemicals such as simple phenols via pyrolysis or extraction.
- More research is needed on the chemical and pyrolysis properties of barks. Possibly fewer than 100 barks have been considered for valorization, which is a very small number regarding the huge potential for bark valorization given the existing number of tree species. Future bark valorization studies should apply standardized methods.
- The bark valorization studies should consider cascade processing, combining different valorization processes instead of a single process.
- The environmental impact of the different applied conversion processes is largely unknown, as is their economic evaluation. Energy balances should be provided in bark conversion studies.
- Different bark extracts should be screened for antioxidant or nutraceutical potential, including pharmacokinetic profiles and drug-like properties.
- The production of phenolic substances and simple phenols should target extractive-rich barks after chemical screening. Barks may be a source of platform chemicals such as simple phenols, as in earlier studies before their replacement with petroleum-based products. It is therefore necessary to re-consider bark for the production of platform chemicals to be used in the food, fragrance, or pharma industries. Efficient and selective production of these chemicals through extraction, pyrolysis, or depolymerization may open up new possibilities for bark valorization, namely using optimized and environmentally friendly conversion methods and improved catalysts [192].
6. Conclusions
- The number of bark valorization studies has been increasing in recent years, since barks are widely available feedstocks and may be processed to produce sustainable and environmentally friendly products, including chars, antioxidants, and platform chemicals.
- Adsorption applications and antioxidant production are predicted to be the most important topics in bark valorization in the near future.
- More studies are required to screen different barks for their chemical composition, extractives profiles, and drug properties.
- Bark valorization studies should be designed in the form of cascade processing and should combine different processing paths, including pyrolysis, extraction, and enzymatic digestion or chemical fractioning.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trockenbrodt, M. Survey and discussion of the terminology used in bark anatomy. IAWA J. 1990, 11, 141–166. [Google Scholar] [CrossRef]
- Harkin, J.M.; Rowe, J.W. Bark and Its Possible Uses; Forest Products Laboratory: Madison, WI, USA, 1971; Volume 91. [Google Scholar]
- Şen, A.U.; Pereira, H. State-of-the-Art Char Production with a Focus on Bark Feedstocks: Processes, Design, and Applications. Processes 2021, 9, 87. [Google Scholar] [CrossRef]
- Pasztory, Z.; Mohácsiné, I.R.; Gorbacheva, G.; Börcsök, Z. The utilization of tree bark. BioResources 2016, 11, 7859–7888. [Google Scholar] [CrossRef]
- Pereira, H. Cork: Biology, Production and Uses; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-444-52967-1. [Google Scholar]
- Silva, S.P.; Sabino, M.A.; Fernandes, E.M.; Correlo, V.M.; Boesel, L.F.; Reis, R.L. Cork: Properties, capabilities and applications. Int. Mater. Rev. 2005, 50, 345–365. [Google Scholar] [CrossRef]
- Menor, M.C.P.; Ros, P.S.; García, A.M.; Caballero, M.J.A. Granulated cork with bark characterised as environment-friendly lightweight aggregate for cement based materials. J. Clean. Prod. 2019, 229, 358–373. [Google Scholar] [CrossRef]
- Lakreb, N.; Sen, U.; Beddiar, A.; Zitoune, R.; Nobre, C.; Gomes, M.G.; Pereira, H. Properties of eco-friendly mortars produced by partial cement replacement with waste cork particles: A feasibility study. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Krasutsky, P.A. Birch bark research and development. Nat. Prod. Rep. 2006, 23, 919–942. [Google Scholar] [CrossRef] [PubMed]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure Reactions; Walter de Gruyter: Berlin, NY, USA, 1984. [Google Scholar]
- Norn, S.; Permin, H.; Kruse, P.R.; Kruse, E. From willow bark to acetylsalicylic acid. Dan Med. Arb. 2009, 37, 79–98. [Google Scholar]
- Luo, J.; Preciado, S.; Xie, P.; Larrosa, I. Carboxylation of phenols with CO2 at atmospheric pressure. Chem. Eur. J. 2016, 22, 6798–6802. [Google Scholar] [CrossRef] [PubMed]
- Cazzolla Gatti, R.; Reich, P.B.; Gamarra, J.G.P.; Crowther, T.; Hui, C.; Morera, A.; Bastin, J.-F.; De-Miguel, S.; Nabuurs, G.-J.; Svenning, J.-C. The number of tree species on Earth. Proc. Natl. Acad. Sci. USA 2022, 119, e2115329119. [Google Scholar] [CrossRef] [PubMed]
- Atabani, A.E.; Ali, I.; Naqvi, S.R.; Badruddin, I.A.; Aslam, M.; Mahmoud, E.; Almomani, F.; Juchelková, D.; Atelge, M.R.; Khan, T.M.Y. A state-of-the-art review on spent coffee ground (SCG) pyrolysis for future biorefinery. Chemosphere 2022, 286, 131730. [Google Scholar] [CrossRef]
- Velvizhi, G.; Balakumar, K.; Shetti, N.P.; Ahmad, E.; Pant, K.K.; Aminabhavi, T.M. Integrated biorefinery processes for conversion of lignocellulosic biomass to value added materials: Paving a path towards circular economy. Bioresour. Technol. 2022, 343, 126151. [Google Scholar] [CrossRef]
- Fiorentino, G.; Ripa, M.; Ulgiati, S. Chemicals from biomass: Technological versus environmental feasibility: A review. Biofuels Bioprod. Biorefin. 2017, 11, 195–214. [Google Scholar] [CrossRef]
- Şen, A.; Leite, C.; Lima, L.; Lopes, P.; Pereira, H. Industrial valorization of Quercus cerris bark: Pilot scale fractionation. Ind. Crops Prod. 2016, 92, 42–49. [Google Scholar] [CrossRef]
- Barbini, S.; Sriranganadane, D.; España Orozco, S.; Kabrelian, A.; Karlström, K.; Rosenau, T.; Potthast, A. Tools for bark biorefineries: Studies toward improved characterization of lipophilic lignocellulosic extractives by combining supercritical fluid and gas chromatography. ACS Sustain. Chem. Eng. 2020, 9, 1323–1332. [Google Scholar] [CrossRef]
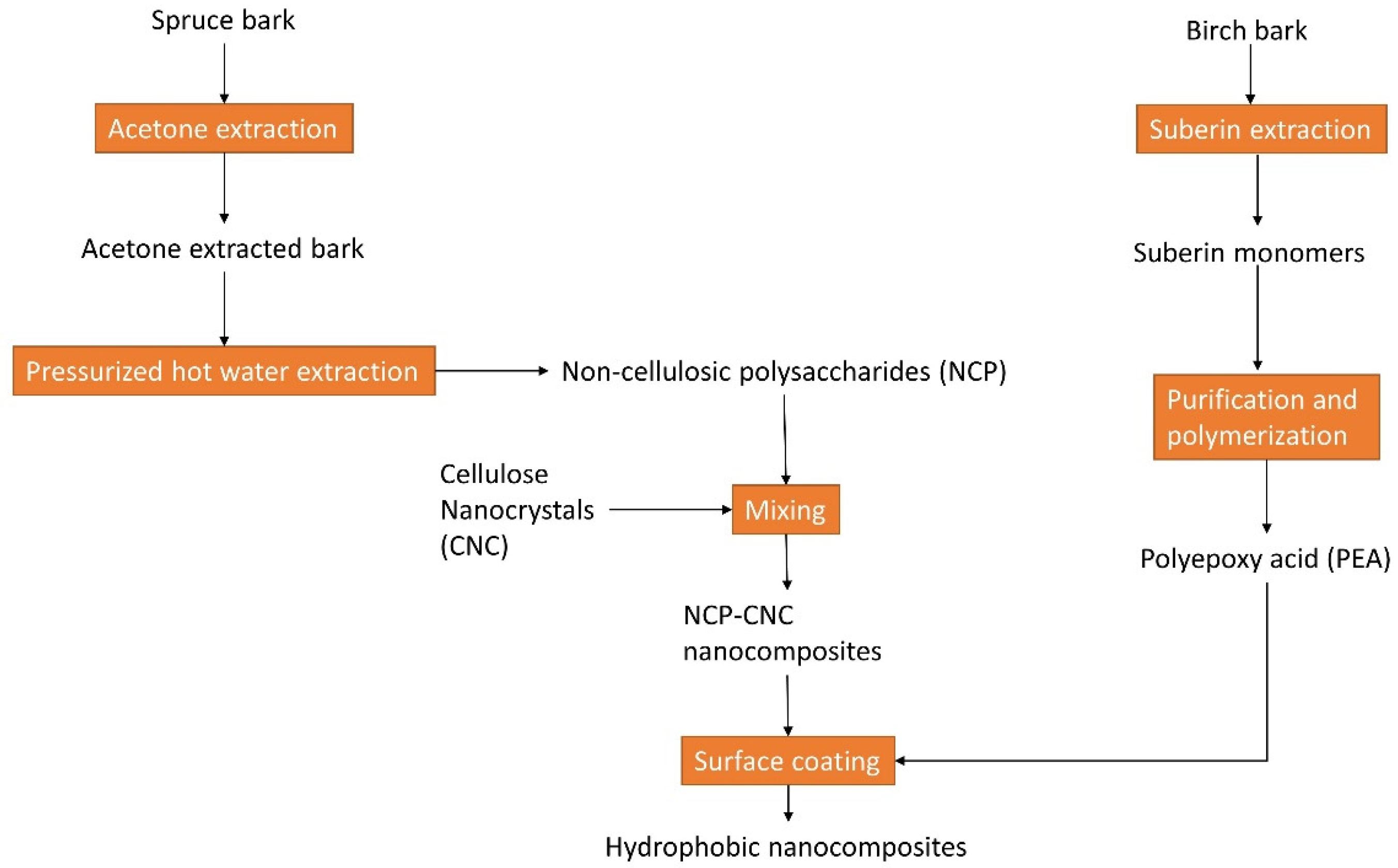
- Le Normand, M.; Moriana, R.; Ek, M. Isolation and characterization of cellulose nanocrystals from spruce bark in a biorefinery perspective. Carbohydr. Polym. 2014, 111, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Le Normand, M.; Moriana, R.; Ek, M. The bark biorefinery: A side-stream of the forest industry converted into nanocomposites with high oxygen-barrier properties. Cellulose 2014, 21, 4583–4594. [Google Scholar] [CrossRef]
- Neiva, D.M.; Araujo, S.; Gominho, J.; de Cássia Carneiro, A.; Pereira, H. Potential of Eucalyptus globulus industrial bark as a biorefinery feedstock: Chemical and fuel characterization. Ind. Crops Prod. 2018, 123, 262–270. [Google Scholar] [CrossRef]
- Ruiz, J.A.; Juárez, M.C.; Morales, M.P.; Muñoz, P.; Mendívil, M.A. Biomass gasification for electricity generation: Review of current technology barriers. Renew. Sustain. Energy Rev. 2013, 18, 174–183. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. More efficient biomass gasification via torrefaction. Energy 2006, 31, 3458–3470. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Sheeba, K.N. Combined slow pyrolysis and steam gasification of biomass for hydrogen generation—A review. Int. J. Energy Res. 2015, 39, 147–164. [Google Scholar] [CrossRef]
- Erlach, B.; Harder, B.; Tsatsaronis, G. Combined hydrothermal carbonization and gasification of biomass with carbon capture. Energy 2012, 45, 329–338. [Google Scholar] [CrossRef]
- Sjostrom, E. Wood Chemistry: Fundamentals and Applications; Gulf Professional Publishing: Houston, TX, USA; Academic Press: New York, NY, USA, 1993; ISBN 0126474818. [Google Scholar]
- Rasi, S.; Kilpeläinen, P.; Rasa, K.; Korpinen, R.; Raitanen, J.-E.; Vainio, M.; Kitunen, V.; Pulkkinen, H.; Jyske, T. Cascade processing of softwood bark with hot water extraction, pyrolysis and anaerobic digestion. Bioresour. Technol. 2019, 292, 121893. [Google Scholar] [CrossRef] [PubMed]
- Van Eck, N.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Rietzler, B.; Ek, M. Adding value to spruce bark by the isolation of nanocellulose in a biorefinery concept. ACS Sustain. Chem. Eng. 2021, 9, 1398–1405. [Google Scholar] [CrossRef]
- Rietzler, B.; Karlsson, M.; Kwan, I.; Lawoko, M.; Ek, M. Fundamental Insights on the Physical and Chemical Properties of Organosolv Lignin from Norway Spruce Bark. Biomacromolecules 2022, 23, 3349–3358. [Google Scholar] [CrossRef] [PubMed]
- Bento, A.; Escórcio, R.; Tomé, A.S.; Robertson, M.; Gaugler, E.C.; Malthus, S.J.; Raymond, L.G.; Hill, S.J.; Pereira, C.S. Pinus radiata bark sequentially processed using scCO2 and an ionic liquid catalyst yields plentiful resin acids and alkanoic acids enriched suberin. Ind. Crops Prod. 2022, 185, 115172. [Google Scholar] [CrossRef]
- Wijeyekoon, S.; Suckling, I.; Fahmy, M.; Hall, P.; Bennett, P. Techno-economic analysis of tannin and briquette co-production from bark waste: A case study quantifying symbiosis benefits in biorefinery. Biofuels Bioprod. Biorefin. 2021, 15, 1332–1344. [Google Scholar] [CrossRef]
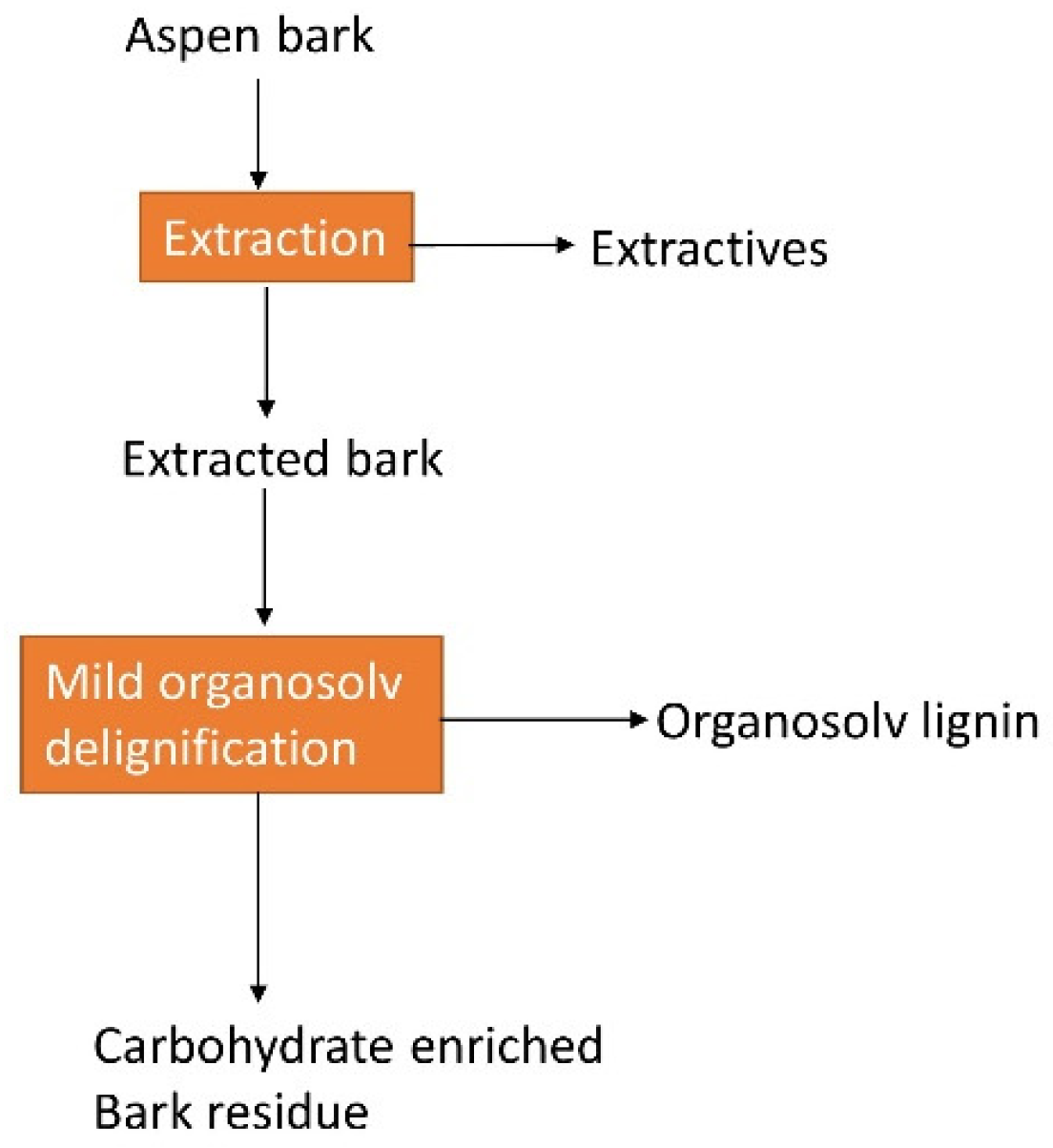
- Pals, M.; Lauberts, M.; Zijlstra, D.S.; Ponomarenko, J.; Arshanitsa, A.; Deuss, P.J. Mild organosolv delignification of residual aspen bark after extractives isolation as a step in biorefinery processing schemes. Molecules 2022, 27, 3185. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Moriana, R.; Ek, M. From forest residues to hydrophobic nanocomposites with high oxygen-barrier properties. Nord. Pulp Pap. Res. J. 2016, 31, 261–269. [Google Scholar] [CrossRef]
- Le Normand, M.; Mélida, H.; Holmbom, B.; Michaelsen, T.E.; Inngjerdingen, M.; Bulone, V.; Paulsen, B.S.; Ek, M. Hot-water extracts from the inner bark of Norway spruce with immunomodulating activities. Carbohydr. Polym. 2014, 101, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Le Normand, M.; Edlund, U.; Holmbom, B.; Ek, M. Hot-water extraction and characterization of spruce bark non-cellulosic polysaccharides. Nord. Pulp Pap. Res. J. 2012, 27, 18–23. [Google Scholar] [CrossRef]
- Ferreira, R.; Garcia, H.; Sousa, A.F.; Guerreiro, M.; Duarte, F.J.S.; Freire, C.S.R.; Calhorda, M.J.; Silvestre, A.J.D.; Kunz, W.; Rebelo, L.P.N. Unveiling the dual role of the cholinium hexanoate ionic liquid as solvent and catalyst in suberin depolymerisation. RSC Adv. 2014, 4, 2993–3002. [Google Scholar] [CrossRef]
- Şen, A.U.; Nobre, C.; Durão, L.; Miranda, I.; Pereira, H.; Gonçalves, M. Low-temperature biochars from cork-rich and phloem-rich wastes: Fuel, leaching, and methylene blue adsorption properties. Biomass Convers. Biorefin. 2020, 12, 3899–3909. [Google Scholar] [CrossRef]
- Chen, W.-H.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Principles and practice of biomass fast pyrolysis processes for liquids. J. Anal. Appl. Pyrolysis 1999, 51, 3–22. [Google Scholar] [CrossRef]
- Shoji, T.; Kawamoto, H.; Saka, S. Boiling point of levoglucosan and devolatilization temperatures in cellulose pyrolysis measured at different heating area temperatures. J. Anal. Appl. Pyrolysis 2014, 109, 185–195. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Upgrading fast pyrolysis liquids. In Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and Power; Wiley: Hoboken, NJ, USA, 2011; pp. 157–199. [Google Scholar]
- van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Trinh, T.N.; Jensen, P.A.; Dam-Johansen, K.; Knudsen, N.O.; Sørensen, H.R.; Hvilsted, S. Comparison of lignin, macroalgae, wood, and straw fast pyrolysis. Energy Fuels 2013, 27, 1399–1409. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of wood: Part 2. Analysis of products. J. Anal. Appl. Pyrolysis 2006, 77, 35–40. [Google Scholar] [CrossRef]
- Benavente, V.; Fullana, A. Torrefaction of olive mill waste. Biomass Bioenergy 2015, 73, 186–194. [Google Scholar] [CrossRef]
- Ciolkosz, D.; Wallace, R. A review of torrefaction for bioenergy feedstock production. Biofuels Bioprod. Biorefin. 2011, 5, 317–329. [Google Scholar] [CrossRef]
- Niu, Y.; Lv, Y.; Lei, Y.; Liu, S.; Liang, Y.; Wang, D. Biomass torrefaction: Properties, applications, challenges, and economy. Renew. Sustain. Energy Rev. 2019, 115, 109395. [Google Scholar] [CrossRef]
- Kung, K.S.; Shanbhogue, S.; Slocum, A.H.; Ghoniem, A.F. A decentralized biomass torrefaction reactor concept. Part I: Multi-scale analysis and initial experimental validation. Biomass Bioenergy 2019, 125, 196–203. [Google Scholar] [CrossRef]
- Chen, W.-H.; Cheng, C.-L.; Show, P.-L.; Ong, H.C. Torrefaction performance prediction approached by torrefaction severity factor. Fuel 2019, 251, 126–135. [Google Scholar] [CrossRef]
- Chew, J.J.; Doshi, V. Recent advances in biomass pretreatment—Torrefaction fundamentals and technology. Renew. Sustain. Energy Rev. 2011, 15, 4212–4222. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, B.J.; Lin, Y.Y.; Chu, Y.S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.S.; Ho, S.H.; Culaba, A.B.; et al. Progress in biomass torrefaction: Principles, applications and challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Safar, M.; Lin, B.-J.; Chen, W.-H.; Langauer, D.; Chang, J.-S.; Raclavska, H.; Pétrissans, A.; Rousset, P.; Pétrissans, M. Catalytic effects of potassium on biomass pyrolysis, combustion and torrefaction. Appl. Energy 2019, 235, 346–355. [Google Scholar] [CrossRef]
- Thengane, S.K.; Kung, K.S.; Gupta, A.; Ateia, M.; Sanchez, D.L.; Mahajani, S.M.; Lim, C.J.; Sokhansanj, S.; Ghoniem, A.F. Oxidative torrefaction for cleaner utilization of biomass for soil amendment. Clean. Eng. Technol. 2020, 1, 100033. [Google Scholar] [CrossRef]
- Adams, P.W.R.; Shirley, J.E.J.; McManus, M.C. Comparative cradle-to-gate life cycle assessment of wood pellet production with torrefaction. Appl. Energy 2015, 138, 367–380. [Google Scholar] [CrossRef]
- Brownsort, P.A. Biomass Pyrolysis Processes: Review of Scope, Control and Variability; United Kingdom Biochar Reserch Centre: London, UK, 2009; Volume 1, pp. 1–39. [Google Scholar]
- Brown, T.R.; Wright, M.M.; Brown, R.C. Estimating profitability of two biochar production scenarios: Slow pyrolysis vs. fast pyrolysis. Biofuels Bioprod. Biorefin. 2011, 5, 54–68. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Anupam, K.; Sharma, A.K.; Lal, P.S.; Dutta, S.; Maity, S. Preparation, characterization and optimization for upgrading Leucaena leucocephala bark to biochar fuel with high energy yielding. Energy 2016, 106, 743–756. [Google Scholar] [CrossRef]
- Liu, Z.; Han, G. Production of solid fuel biochar from waste biomass by low temperature pyrolysis. Fuel 2015, 158, 159–165. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Lévesque, V.; Palacios, J.H.; Raghavan, V.; Ahmed, A.; Hogue, R.; Jeanne, T.; Verma, M. Biochar for soil amendment. In Char and Carbon Materials Derived from Biomass; Elsevier: Amsterdam, The Netherlands, 2019; pp. 109–146. [Google Scholar]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, W.; Dong, J.; Yang, T.; Shangguan, Z.; Qu, J.; Li, X.; Tan, X. The effects of biochar and its applications in the microbial remediation of contaminated soil: A review. J. Hazard. Mater. 2022, 129557. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef]
- Mandal, A.; Singh, N.; Purakayastha, T.J. Characterization of pesticide sorption behaviour of slow pyrolysis biochars as low cost adsorbent for atrazine and imidacloprid removal. Sci. Total Environ. 2017, 577, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Ronsse, F.; Van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. Gcb Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Tsoumis, G. Science and Technology of Wood: Structure, Properties, Utilization; Van Nostrand Reinhold: New York, NY, USA, 1991; Volume 115. [Google Scholar]
- Xiang, S.; Wu, Q.; Ren, W.; Guo, W.; Ren, N. Mechanism of powdered activated carbon enhancing caproate production. Chin. Chem. Lett. 2023, 34, 107714. [Google Scholar] [CrossRef]
- Manyà, J.J.; Azuara, M.; Manso, J.A. Biochar production through slow pyrolysis of different biomass materials: Seeking the best operating conditions. Biomass Bioenergy 2018, 117, 115–123. [Google Scholar] [CrossRef]
- Patwa, D.; Bordoloi, U.; Dubey, A.A.; Ravi, K.; Sekharan, S.; Kalita, P. Energy-efficient biochar production for thermal backfill applications. Sci. Total Environ. 2022, 833, 155253. [Google Scholar] [CrossRef]
- Sen, U.; Martins, M.; Santos, E.; Lemos, M.A.; Lemos, F.; Pereira, H. Slow Pyrolysis of Quercus cerris Cork: Characterization of Biochars and Pyrolysis Volatiles. Environments 2023, 10, 4. [Google Scholar] [CrossRef]
- Muñoz, E.; Curaqueo, G.; Cea, M.; Vera, L.; Navia, R. Environmental hotspots in the life cycle of a biochar-soil system. J. Clean. Prod. 2017, 158, 1–7. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Hoekman, S.K.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Koprivica, M.; Petrović, J.; Ercegović, M.; Simić, M.; Milojković, J.; Šoštarić, T.; Dimitrijević, J. Improvement of combustible characteristics of Paulownia leaves via hydrothermal carbonization. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Wang, J.; Li, X.; Cheng, J.; Yang, H.; Chen, H. Effect of residence time on chemical and structural properties of hydrochar obtained by hydrothermal carbonization of water hyacinth. Energy 2013, 58, 376–383. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.; Zhu, M.; Sun, G.; Zhang, T.; Kang, K. Comparative evaluation of hydrothermal carbonization and low temperature pyrolysis of eucommia ulmoides oliver for the production of solid biofuel. Sci. Rep. 2019, 9, 5535. [Google Scholar] [CrossRef] [PubMed]
- Monedero, E.; Lapuerta, M.; Pazo, A.; Díaz-Robles, L.A.; Pino-Cortés, E.; Campos, V.; Vallejo, F.; Cubillos, F.; Gómez, J. Effect of hydrothermal carbonization on the properties, devolatilization, and combustion kinetics of Chilean biomass residues. Biomass Bioenergy 2019, 130, 105387. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Mendecka, B.; Lombardi, L.; Micali, F.; De Risi, A. Energy recovery from olive pomace by hydrothermal carbonization on hypothetical industrial scale: A LCA perspective. Waste Biomass Valorization 2020, 11, 5503–5519. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Kalemba-Rec, I.; Szymańska-Chargot, M. Upgrading of green waste into carbon-rich solid biofuel by hydrothermal carbonization: The effect of process parameters on hydrochar derived from acacia. Energy 2020, 202, 117717. [Google Scholar] [CrossRef]
- Alves, O.; Nobre, C.; Durão, L.; Monteiro, E.; Brito, P.; Gonçalves, M. Effects of dry and hydrothermal carbonisation on the properties of solid recovered fuels from construction and municipal solid wastes. Energy Convers. Manag. 2021, 237, 114101. [Google Scholar] [CrossRef]
- Nobre, C.; Alves, O.; Durão, L.; Şen, A.; Vilarinho, C.; Gonçalves, M. Characterization of hydrochar and process water from the hydrothermal carbonization of Refuse Derived Fuel. Waste Manag. 2021, 120, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Barta-Rajnai, E.; Skreiberg, Ø.; Khalil, R.; Czégény, Z.; Jakab, E.; Barta, Z.; Grønli, M. Effect of torrefaction on physiochemical characteristics and grindability of stem wood, stump and bark. Appl. Energy 2018, 227, 137–148. [Google Scholar] [CrossRef]
- Butnaru, E.; Brebu, M. The Thermochemical Conversion of Forestry Residues from Silver Fir (Abies alba Mill.) by Torrefaction and Pyrolysis. Energies 2022, 15, 3483. [Google Scholar] [CrossRef]
- Almeida, G.; Brito, J.O.; Perré, P. Alterations in energy properties of eucalyptus wood and bark subjected to torrefaction: The potential of mass loss as a synthetic indicator. Bioresour. Technol. 2010, 101, 9778–9784. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Pérez, L.E.; Segura, C.; Bustamante-García, V.; Cápiro, O.G.; Jiménez, R. Torrefaction of wood and bark from Eucalyptus globulus and Eucalyptus nitens: Focus on volatile evolution vs. feasible temperatures. Energy 2015, 93, 1731–1741. [Google Scholar] [CrossRef]
- Sessa, F.; Merlin, G.; Canu, P. Pine bark valorization by activated carbons production to be used as VOCs adsorbents. Fuel 2022, 318, 123346. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Ryu, C.; Gang, K.S.; Yang, W.; Park, Y.-K.; Jung, J.; Hyun, S. Comparison of biochar properties from biomass residues produced by slow pyrolysis at 500 °C. Bioresour. Technol. 2013, 148, 196–201. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Chesnokov, N.V.; Ivanov, I.P.; Kuznetsova, S.A.; Ivanchenko, N.M. Production of porous carbon materials from bark. Solid Fuel Chem. 2015, 49, 278–288. [Google Scholar] [CrossRef]
- Costa, P.A.; Barreiros, M.A.; Mouquinho, A.I.; Silva, O.E.; Paradela, F.; Oliveira, F.A.C. Slow pyrolysis of cork granules under nitrogen atmosphere: By-products characterization and their potential valorization. Biofuel Res. J. 2022, 9, 1562–1572. [Google Scholar] [CrossRef]
- Saletnik, B.; Saletnik, A.; Zaguła, G.; Bajcar, M.; Puchalski, C. Oak Biomass in the Form of Wood, Bark, Brushwood, Leaves and Acorns in the Production Process of Multifunctional Biochar. Molecules 2022, 27, 7191. [Google Scholar] [CrossRef] [PubMed]
- Paczkowski, S.; Comi, J.; Küttner, I.; Knappe, V.; Russ, M.; Robles, L.A.D.; Jaeger, D.; Pelz, S. Hydrothermal treatment (HTT) for improving the fuel properties of biomass residues. Biomass Convers. Biorefin. 2022, 13, 6257–6279. [Google Scholar] [CrossRef]
- Gao, P.; Zhou, Y.; Meng, F.; Zhang, Y.; Liu, Z.; Zhang, W.; Xue, G. Preparation and characterization of hydrochar from waste eucalyptus bark by hydrothermal carbonization. Energy 2016, 97, 238–245. [Google Scholar] [CrossRef]
- Rodríguez Correa, C.; Stollovsky, M.; Hehr, T.; Rauscher, Y.; Rolli, B.; Kruse, A. Influence of the carbonization process on activated carbon properties from lignin and lignin-rich biomasses. ACS Sustain. Chem. Eng. 2017, 5, 8222–8233. [Google Scholar] [CrossRef]
- Kemppainen, K.; Siika-aho, M.; Pattathil, S.; Giovando, S.; Kruus, K. Spruce bark as an industrial source of condensed tannins and non-cellulosic sugars. Ind. Crops Prod. 2014, 52, 158–168. [Google Scholar] [CrossRef]
- Umezawa, T. Chemistry of extractives. In Wood and Cellulosic Chemistry; CRC Press: Boca Raton, FL, USA, 2000; pp. 213–241. [Google Scholar]
- Sakai, K. Chemistry of bark. In Wood and Cellulosic Chemistry; CRC Press: Boca Raton, FL, USA, 2000; pp. 243–274. [Google Scholar]
- Şen, A.; Miranda, I.; Esteves, B.; Pereira, H. Chemical characterization, bioactive and fuel properties of waste cork and phloem fractions from Quercus cerris L. bark. Ind. Crops Prod. 2020, 157, 112909. [Google Scholar] [CrossRef]
- Şen, A.; Miranda, I.; Santos, S.; Graça, J.; Pereira, H. The chemical composition of cork and phloem in the rhytidome of Quercus cerris bark. Ind. Crops Prod. 2010, 31, 417–422. [Google Scholar] [CrossRef]
- Pereira, H. Variability of the chemical composition of cork. BioResources 2013, 8, 2246–2256. [Google Scholar] [CrossRef]
- Sen, A.; Zhianski, M.; Glushkova, M.; Petkova, K.; Ferreira, J.; Pereira, H. Chemical composition and cellular structure of corks from Quercus suber trees planted in Bulgaria and Turkey. Wood Sci. Technol. 2016, 50, 1261–1276. [Google Scholar] [CrossRef]
- Ferreira, J.; Miranda, I.; Şen, U.; Pereira, H. Chemical and cellular features of virgin and reproduction cork from Quercus variabilis. Ind. Crops Prod. 2016, 94, 638–648. [Google Scholar] [CrossRef]
- Sousa, V.; Ferreira, J.P.A.; Miranda, I.; Quilhó, T.; Pereira, H. Quercus rotundifolia bark as a source of polar extracts: Structural and chemical characterization. Forests 2021, 12, 1160. [Google Scholar] [CrossRef]
- Ferreira, J.P.A.; Miranda, I.; Sousa, V.B.; Pereira, H. Chemical composition of barks from Quercus faginea trees and characterization of their lipophilic and polar extracts. PLoS ONE 2018, 13, e0197135. [Google Scholar] [CrossRef]
- Sillero, L.; Prado, R.; Andrés, M.A.; Labidi, J. Characterisation of bark of six species from mixed Atlantic forest. Ind. Crops Prod. 2019, 137, 276–284. [Google Scholar] [CrossRef]
- Vangeel, T.; Neiva, D.M.; Quilho, T.; Costa, R.A.; Sousa, V.; Sels, B.F.; Pereira, H. Tree bark characterization envisioning an integrated use in a biorefinery. Biomass Convers. Biorefin. 2021, 13, 2029–2043. [Google Scholar] [CrossRef]
- Carmo, J.F.; Miranda, I.; Quilhó, T.; Sousa, V.B.; Carmo, F.H.D.J.; Latorraca, J.V.F.; Pereira, H. Chemical and structural characterization of the bark of Albizia niopoides trees from the Amazon. Wood Sci. Technol. 2016, 50, 677–692. [Google Scholar] [CrossRef]
- Mota, G.S.; Sartori, C.J.; Miranda, I.; Quilho, T.; Mori, F.A.; Pereira, H. Bark anatomy, chemical composition and ethanol-water extract composition of Anadenanthera peregrina and Anadenanthera colubrina. PLoS ONE 2017, 12, e0189263. [Google Scholar] [CrossRef]
- Ferreira, J.P.A.; Quilhó, T.; Pereira, H. Characterization of Betula pendula outer bark regarding cork and phloem components at chemical and structural levels in view of biorefinery integration. J. Wood Chem. Technol. 2017, 37, 10–25. [Google Scholar] [CrossRef]
- Carmo, J.F.; Miranda, I.; Quilhó, T.; Sousa, V.B.; Cardoso, S.; Carvalho, A.M.; Carmo, F.H.D.J.; Latorraca, J.V.F.; Pereira, H. Copaifera langsdorffii bark as a source of chemicals: Structural and chemical characterization. J. Wood Chem. Technol. 2016, 36, 305–317. [Google Scholar] [CrossRef]
- Miranda, I.; Lima, L.; Quilhó, T.; Knapic, S.; Pereira, H. The bark of Eucalyptus sideroxylon as a source of phenolic extracts with anti-oxidant properties. Ind. Crops Prod. 2016, 82, 81–87. [Google Scholar] [CrossRef]
- Carmo, J.F.; Miranda, I.; Quilhó, T.; Carvalho, A.M.; Carmo, F.H.D.J.; de Figueiredo Latorraca, J.V.; Pereira, H. Bark characterisation of the Brazilian hardwood Goupia glabra in terms of its valorisation. BioResources 2016, 11, 4794–4807. [Google Scholar] [CrossRef]
- Rios, P.; Cabral, V.; Santos, S.; Mori, F.; Graça, J. The chemistry of Kielmeyera coriacea outer bark: A potential source for cork. Eur. J. Wood Wood Prod. 2014, 72, 509–519. [Google Scholar] [CrossRef]
- Sousa, T.B.; da Mota, G.S.; da Araujo, E.S.; Carréra, J.C.; Silva, E.P.; Souza, S.G.; Lorenço, M.S.; Mori, F.A. Chemical and structural characterization of Myracrodruon urundeuva barks aiming at their potential use and elaboration of a sustainable management plan. Biomass Convers. Biorefinery 2022, 12, 1583–1593. [Google Scholar] [CrossRef]
- Mota, G.S.; Sartori, C.J.; Ferreira, J.; Miranda, I.; Quilhó, T.; Mori, F.A.; Pereira, H. Cellular structure and chemical composition of cork from Plathymenia reticulata occurring in the Brazilian Cerrado. Ind. Crops Prod. 2016, 90, 65–75. [Google Scholar] [CrossRef]
- Baptista, I.; Miranda, I.; Quilhó, T.; Gominho, J.; Pereira, H. Characterisation and fractioning of Tectona grandis bark in view of its valorisation as a biorefinery raw-material. Ind. Crops Prod. 2013, 50, 166–175. [Google Scholar] [CrossRef]
- Miranda, I.; Gominho, J.; Mirra, I.; Pereira, H. Chemical characterization of barks from Picea abies and Pinus sylvestris after fractioning into different particle sizes. Ind. Crops Prod. 2012, 36, 395–400. [Google Scholar] [CrossRef]
- Miranda, I.; Mirra, I.; Gominho, J.; Pereira, H. Fractioning of bark of Pinus pinea by milling and chemical characterization of the different fractions. Maderas. Cienc. Tecnol. 2017, 19, 185–194. [Google Scholar] [CrossRef]
- Ferreira, J.P.A.; Miranda, I.; Gominho, J.; Pereira, H. Chemical characterization of cork and phloem from Douglas fir outer bark. Holzforschung 2016, 70, 475–483. [Google Scholar] [CrossRef]
- Leite, C.; Pereira, H. Cork-containing barks—A review. Front. Mater. 2017, 3, 63. [Google Scholar] [CrossRef]
- Pettersen, R.C. The chemical composition of wood. Chem. Solid Wood 1984, 207, 57–126. [Google Scholar]
- Rowell, R.M. Handbook of Wood Chemistry and Wood Composites; CRC press: Boca Raton, LA, USA, 2012; ISBN 0429109091. [Google Scholar]
- Gomes, S.V.F.; Portugal, L.A.; dos Anjos, J.P.; de Jesus, O.N.; de Oliveira, E.J.; David, J.P.; David, J.M. Accelerated solvent extraction of phenolic compounds exploiting a Box-Behnken design and quantification of five flavonoids by HPLC-DAD in Passiflora species. Microchem. J. 2017, 132, 28–35. [Google Scholar] [CrossRef]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated solvent extraction: A technique for sample preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Wang, G.; Lee, A.S.; Lewis, M.; Kamath, B.; Archer, R.K. Accelerated solvent extraction and gas chromatography/mass spectrometry for determination of polycyclic aromatic hydrocarbons in smoked food samples. J. Agric. Food Chem. 1999, 47, 1062–1066. [Google Scholar] [CrossRef]
- Ghitescu, R.-E.; Volf, I.; Carausu, C.; Bühlmann, A.-M.; Gilca, I.A.; Popa, V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef]
- Alves, T.P.; Triques, C.C.; Palsikowski, P.A.; da Silva, C.; Fiorese, M.L.; da Silva, E.A.; Fagundes-Klen, M.R. Improved extraction of bioactive compounds from Monteverdia aquifolia leaves by pressurized-liquid and ultrasound-assisted extraction: Yield and chemical composition. J. Supercrit. Fluids 2022, 181, 105468. [Google Scholar] [CrossRef]
- Mohammad Azmin, S.N.H.; Abdul Manan, Z.; Wan Alwi, S.R.; Chua, L.S.; Mustaffa, A.A.; Yunus, N.A. Herbal processing and extraction technologies. Sep. Purif. Rev. 2016, 45, 305–320. [Google Scholar] [CrossRef]
- Chupin, L.; Maunu, S.L.; Reynaud, S.; Pizzi, A.; Charrier, B.; Bouhtoury, F.C. Microwave assisted extraction of maritime pine (Pinus pinaster) bark: Impact of particle size and characterization. Ind. Crop. Prod. 2015, 65, 142–149. [Google Scholar] [CrossRef]
- Bouras, M.; Chadni, M.; Barba, F.J.; Grimi, N.; Bals, O.; Vorobiev, E. Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Ind. Crops Prod. 2015, 77, 590–601. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Rodrigues, V.H.; de Melo, M.M.R.; Portugal, I.; Silva, C.M. Extraction of Eucalyptus leaves using solvents of distinct polarity. Cluster analysis and extracts characterization. J. Supercrit. Fluids 2018, 135, 263–274. [Google Scholar] [CrossRef]
- Şen, A.; De Melo, M.M.R.; Silvestre, A.J.D.; Pereira, H.; Silva, C.M. Prospective pathway for a green and enhanced friedelin production through supercritical fluid extraction of Quercus cerris cork. J. Supercrit. Fluids 2015, 97, 247–255. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Şen, A.; Silvestre, A.J.D.; Pereira, H.; Silva, C.M. Experimental and modeling study of supercritical CO2 extraction of Quercus cerris cork: Influence of ethanol and particle size on extraction kinetics and selectivity to friedelin. Sep. Purif. Technol. 2017, 187, 34–45. [Google Scholar] [CrossRef]
- Vieira, P.G.; de Melo, M.M.R.; Şen, A.; Simões, M.M.Q.; Portugal, I.; Pereira, H.; Silva, C.M. Quercus cerris extracts obtained by distinct separation methods and solvents: Total and friedelin extraction yields, and chemical similarity analysis by multidimensional scaling. Sep. Purif. Technol. 2020, 232, 115924. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Vieira, P.G.; Şen, A.; Pereira, H.; Portugal, I.; Silva, C.M. Optimization of the supercritical fluid extraction of Quercus cerris cork towards extraction yield and selectivity to friedelin. Sep. Purif. Technol. 2020, 238, 116395. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Oliveira, E.L.G.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of triterpenic acids from Eucalyptus globulus bark. J. Supercrit. Fluids 2012, 70, 137–145. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; de Melo, M.M.R.; Oliveira, E.L.G.; Neto, C.P.; Silvestre, A.J.D.; Silva, C.M. Optimization of the supercritical fluid extraction of triterpenic acids from Eucalyptus globulus bark using experimental design. J. Supercrit. Fluids 2013, 74, 105–114. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Oliveira, E.L.G.; Freire, C.S.R.; Couto, R.M.; Simões, P.C.; Neto, C.P.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of Eucalyptus globulus Bark—A promising approach for triterpenoid production. Int. J. Mol. Sci. 2012, 13, 7648–7662. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.H.; de Melo, M.M.R.; Portugal, I.; Silva, C.M. Simulation and techno-economic optimization of the supercritical CO2 extraction of Eucalyptus globulus bark at industrial scale. J. Supercrit. Fluids 2019, 145, 169–180. [Google Scholar] [CrossRef]
- Rodrigues, V.H.; de Melo, M.M.R.; Portugal, I.; Silva, C.M. Lupane-type triterpenoids from Acacia dealbata bark extracted by different methods. Ind. Crops Prod. 2021, 170, 113734. [Google Scholar] [CrossRef]
- Yu-hong, Z.; Tao, Y.U.; Yang, W. Extraction of betulin from bark of Betula platyphylla by supercritical carbon dioxide extraction. J. For. Res. 2003, 14, 202–204. [Google Scholar] [CrossRef]
- Ubando, A.T.; Ng, E.A.S.; Chen, W.-H.; Culaba, A.B.; Kwon, E.E. Life cycle assessment of microalgal biorefinery: A state-of-the-art review. Bioresour. Technol. 2022, 360, 127615. [Google Scholar] [CrossRef]
- Cherubini, F.; Bargigli, S.; Ulgiati, S. Life cycle assessment (LCA) of waste management strategies: Landfilling, sorting plant and incineration. Energy 2009, 34, 2116–2123. [Google Scholar] [CrossRef]
- Carlqvist, K.; Wallberg, O.; Lidén, G.; Börjesson, P. Life cycle assessment for identification of critical aspects in emerging technologies for the extraction of phenolic compounds from spruce bark. J. Clean. Prod. 2022, 333, 130093. [Google Scholar] [CrossRef]
- Hytönen, J.; Beuker, E.; Viherä-Aarnio, A. Clonal variation in basic density, moisture content and heating value of wood, bark and branches in hybrid aspen. Silva Fenn. 2018, 52, 9938. [Google Scholar] [CrossRef]
- Saka, S. Chemical composition and distribution. In Wood and Cellulosic Chemistry; CRC Press: Boca Raton, FL, USA, 2000; pp. 51–81. [Google Scholar]
- Priyanto, D.E.; Ueno, S.; Sato, N.; Kasai, H.; Tanoue, T.; Fukushima, H. Ash transformation by co-firing of coal with high ratios of woody biomass and effect on slagging propensity. Fuel 2016, 174, 172–179. [Google Scholar] [CrossRef]
- Teixeira, P.; Lopes, H.; Gulyurtlu, I.; Lapa, N.; Abelha, P. Evaluation of slagging and fouling tendency during biomass co-firing with coal in a fluidized bed. Biomass Bioenergy 2012, 39, 192–203. [Google Scholar] [CrossRef]
- Bandara, Y.W.; Gamage, P.; Gunarathne, D.S. Hot water washing of rice husk for ash removal: The effect of washing temperature, washing time and particle size. Renew. Energy 2020, 153, 646–652. [Google Scholar] [CrossRef]
- Miranda, T.; Montero, I.; Sepúlveda, F.J.; Arranz, J.I.; Rojas, C.V.; Nogales, S. A review of pellets from different sources. Materials 2015, 8, 1413–1427. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, K.; Inkinen, J.; Uusitalo, J.; Nakari-Setälä, T.; Siika-aho, M. Hot water extraction and steam explosion as pretreatments for ethanol production from spruce bark. Bioresour. Technol. 2012, 117, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.; Yang, H.; Gentili, F.G.; Söderlind, U.; Wang, X.; Zhang, W.; Chen, H. Hydrothermal carbonization of natural microalgae containing a high ash content. Fuel 2019, 249, 441–448. [Google Scholar] [CrossRef]
- Lynam, J.G.; Reza, M.T.; Yan, W.; Vásquez, V.R.; Coronella, C.J. Hydrothermal carbonization of various lignocellulosic biomass. Biomass Convers. Biorefinery 2015, 5, 173–181. [Google Scholar]
- Harun, J.; Labosky, P. Chemical constituents of five northeastern barks. Wood Fiber Sci. 2007, 17, 274–280. [Google Scholar]
- Ferreira, R.; Garcia, H.; Sousa, A.F.; Freire, C.S.R.; Silvestre, A.J.D.; Rebelo, L.P.N.; Pereira, C.S. Isolation of suberin from birch outer bark and cork using ionic liquids: A new source of macromonomers. Ind. Crops Prod. 2013, 44, 520–527. [Google Scholar] [CrossRef]
- Ekman, R. The Suberin Monomers and Triterpenoids from the Outer Bark of Betula verrucosa Ehrh. Birch Bark Suberin Triterpenoids 1983, 37, 205–211. [Google Scholar]
- Gandini, A.; Neto, C.P.; Silvestre, A.J.D. Suberin: A promising renewable resource for novel macromolecular materials. Prog. Polym. Sci. 2006, 31, 878–892. [Google Scholar] [CrossRef]
- Moiteiro, C.; Marcelo Curto, M.J.; Mohamed, N.; Bailén, M.; Martínez-Díaz, R.; González-Coloma, A. Biovalorization of friedelane triterpenes derived from cork processing industry byproducts. J. Agric. Food Chem. 2006, 54, 3566–3571. [Google Scholar] [CrossRef]
- Castola, V.; Marongiu, B.; Bighelli, A.; Floris, C.; Laï, A.; Casanova, J. Extractives of cork (Quercus suber L.): Chemical composition of dichloromethane and supercritical CO2 extracts. Ind. Crops Prod. 2005, 21, 65–69. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C.C. Depolymerization of lignins and their applications for the preparation of polyols and rigid polyurethane foams: A review. Renew. Sustain. Energy Rev. 2016, 60, 317–329. [Google Scholar] [CrossRef]
- D’Souza, J.; Yan, N. Producing bark-based polyols through liquefaction: Effect of liquefaction temperature. ACS Sustain. Chem. Eng. 2013, 1, 534–540. [Google Scholar] [CrossRef]
- D’Souza, J.; Camargo, R.; Yan, N. Polyurethane foams made from liquefied bark-based polyols. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Esteves, B.; Dulyanska, Y.; Costa, C.; Ferreira, J.V.; Domingos, I.; Pereira, H.; de Lemos, L.T.; Cruz-Lopes, L.V. Cork liquefaction for polyurethane foam production. BioResources 2017, 12, 2339–2353. [Google Scholar] [CrossRef]
- Mateus, M.M.; Acero, N.F.; Bordado, J.C.; dos Santos, R.G. Sonication as a foremost tool to improve cork liquefaction. Ind. Crops Prod. 2015, 74, 9–13. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Bordado, J.C.; Mateus, M.M. Microwave-assisted liquefaction of cork—From an industrial waste to sustainable chemicals. Ind. Eng. Manag. 2015, 4, 173. [Google Scholar]
- Huang, Y.; Duan, Y.; Qiu, S.; Wang, M.; Ju, C.; Cao, H.; Fang, Y.; Tan, T. Lignin-first biorefinery: A reusable catalyst for lignin depolymerization and application of lignin oil to jet fuel aromatics and polyurethane feedstock. Sustain. Energy Fuels 2018, 2, 637–647. [Google Scholar] [CrossRef]
- Karnaouri, A.; Rova, U.; Christakopoulos, P. Effect of different pretreatment methods on birch outer bark: New biorefinery routes. Molecules 2016, 21, 427. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; He, J.; Kong, X.; Zheng, J.; Han, L.; Liu, Y.; Zhu, Z.; Zhang, Z. From agricultural cellulosic waste to food delivery packaging: A mini-review. Chin. Chem. Lett. 2023, 34, 107407. [Google Scholar] [CrossRef]
- Sun, C.; Wu, W.; Chang, H.; Wang, R.; Wang, K.; Zhong, N.; Zhang, T.; He, X.; Sun, F.; Zhang, E. A tailored bifunctional carbon catalyst for efficient glycosidic bond fracture and selective hemicellulose fractionation. Bioresour. Technol. 2022, 362, 127861. [Google Scholar] [CrossRef] [PubMed]
- Ince, P.J. Estimating Effective Heating Value of Wood or Bark Fuels at Various Moisture Contents; US Department of Agriculture, US Forest Service: Madison, WI, USA, 1977; Volume 13. [Google Scholar]
- Nunes, L.J.R.; Matias, J.C.O.; Catalão, J.P.S. Energy recovery from cork industrial waste: Production and characterisation of cork pellets. Fuel 2013, 113, 24–30. [Google Scholar] [CrossRef]
- Lehtikangas, P. Quality properties of pelletised sawdust, logging residues and bark. Biomass Bioenergy 2001, 20, 351–360. [Google Scholar] [CrossRef]
- Kaliyan, N.; Morey, R.V. Factors affecting strength and durability of densified biomass products. Biomass Bioenergy 2009, 33, 337–359. [Google Scholar] [CrossRef]
- Tahirović, A.; Bašić, N.; Avdibegović, S. Antioxidant capacity and phenolic content of Fraxinus ornus L. and Fraxinus pennsylvanica Marsch. leaves and bark extracts. Rad. Šumar. Fak. Univ. Sarajev. 2017, 47, 1–12. [Google Scholar] [CrossRef]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Jablonsky, M.; Nosalova, J.; Sladkova, A.; Haz, A.; Kreps, F.; Valka, J.; Miertus, S.; Frecer, V.; Ondrejovic, M.; Sima, J. Valorisation of softwood bark through extraction of utilizable chemicals: A review. Biotechnol. Adv. 2017, 35, 726–750. [Google Scholar] [CrossRef]
- Foti, M.C. Antioxidant properties of phenols. J. Pharm. Pharmacol. 2007, 59, 1673–1685. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, J.; Song, W.; Xiao, L.-P. Integrated cascade biorefinery processes to transform woody biomass into phenolic monomers and carbon quantum dots. Front. Bioeng. Biotechnol. 2021, 9, 803138. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Maplesden, F. Commercial production of tannins from radiata pine bark for wood adhesives. Trans. Inst. Prof. Eng. N. Z. Electr. Eng. Sect. 1998, 25, 46–51. [Google Scholar]
- Yazaki, Y. Utilization of flavonoid compounds from bark and wood: A review. Nat. Prod. Commun. 2015, 10, 1934578X1501000333. [Google Scholar] [CrossRef]
- Pizzi, A. Condensed tannins for adhesives. Ind. Eng. Chem. Prod. Res. Dev. 1982, 21, 359–369. [Google Scholar] [CrossRef]
- Jablonsky, M.; Haz, A.; Sladkova, A.; Strizincova, P.; Skulcova, A.; Majova, V.; Jablonsky, J. Nutraceuticals as phenolic bioactive compounds analysis of softwood bark and their possibilities of industry applications. J. Hyg. Eng. Des. 2019, 26, 93–99. [Google Scholar]
- Song, Y.; Mobley, J.K.; Motagamwala, A.H.; Isaacs, M.; Dumesic, J.A.; Ralph, J.; Lee, A.F.; Wilson, K.; Crocker, M. Gold-catalyzed conversion of lignin to low molecular weight aromatics. Chem. Sci. 2018, 9, 8127–8133. [Google Scholar] [CrossRef]




| Process | Bark | Temperature (°C) | Time (min) | Char Yield (%) | HHV (MJ kg−1) | Reference |
|---|---|---|---|---|---|---|
| Torrefaction | Picea abies | 225–300 | 30–60 | 61–90 | 20–24 | [91] |
| Abies alba | 250 | 60 | 71 | 32 * | [92] | |
| Eucalyptus saligna and E. grandis | 220–280 | 60–300 | 71–91 | 17–23 | [93] | |
| Eucalyptus globulus and E. nitens | 250–280 | 15–30 | 60–95 | 19 | [94] | |
| Quercus cerris | 200–300 | 30–60 | 62–90 | - | [38] | |
| Slow pyrolysis | Leucaena leucocephala | 350–550 | 60–180 | 47 ** | 23 * | |
| Pine | 300–850 | 60 | 35–68 | - | [95] | |
| Maesopsis eminii | 500 | 60 | 32 | - | [96] | |
| Larix sibirica | 500–700 | 30–60 | 28–63 | - | [97] | |
| Quercus suber | 400–700 | - | - | 32 | [98] | |
| Quercus cerris | 325 | 30–60 | 52–73 | - | [38] | |
| Quercus petraea | 400–500 | 10 | - | 20 | [99] | |
| HTC | Conifer mixture (80% fir, 15% spruce, 5% pine) and Robinia pseudoacacia | 150–185 | 30 | 71–93 | 20–22 | [100] |
| Eucalyptus | 220–300 | 120–600 | 40–46 | 20–29 | [101] | |
| Oak and pine | 220 | 300 | 67–73 | - | [102] |
| Bark | Extraction Yield, % | ||||
|---|---|---|---|---|---|
| DCM | EtOH | Hot Water | RHL | Reference | |
| Quercus cerris | 10.9 | 3.4 | 2.4 | 0.53 | [107] |
| Quercus suber—Portugal | 5.8 | 5.9 | 4.5 | 1.79 | [108] |
| Quercus suber—Bulgaria | 4.4 | 5.4 | 2.7 | 1.84 | [109] |
| Quercus suber—Turkey | 7.1 | 3.6 | 1.2 | 0.68 | [109] |
| Quercus variabilis | 5.3 | 2.8 | 1.1 | 0.74 | [110] |
| Quercus rotundifolia | 1.6 | 6.4 | 9.3 | 9.81 | [111] |
| Quercus faginea | 1.9 | 4.9 | 6.4 | 5.95 | [112] |
| Quercus robur | 1.1 | 7.4 | 14.5 | 19.91 | [113] |
| Quercus rubra | 2.7 | 2.1 | 7.3 | 3.48 | [113] |
| Quercus rubra | 7.4 | 12.7 | 4.1 | 2.27 | [114] |
| Albiza niopoides | 2.7 | 3.9 | 5.2 | 3.37 | [115] |
| Anadenanthera peregrina | 3.0 | 21.0 | 4.7 | 8.57 | [116] |
| Anadenanthera colubrina | 2.8 | 22.5 | 4.1 | 9.50 | [116] |
| Betula pendula | 32.2 | 0.6 | 0.5 | 0.03 | [117] |
| Betula celtiberica | 2.7 | 4.1 | 7.5 | 4.30 | [113] |
| Castanea sativa | 2.0 | 9.5 | 20.4 | 14.95 | [113] |
| Copaifera langsdorffii | 2.1 | 17.4 | 1.8 | 9.14 | [118] |
| Eucalpytus sideroxylon | 1.7 | 20.1 | 33.9 | 31.76 | [119] |
| Fraxinus excelsior | 4.3 | 18.5 | 6.6 | 5.84 | [113] |
| Goupia glabra | 3.4 | 15.0 | 6.2 | 6.24 | [120] |
| Kiyelmeyera coriacea | 7.7 | 8.2 | 2.0 | 1.32 | [121] |
| Myracrodruon urundeuva | 4.2 | 9.3 | 13.5 | 5.43 | [122] |
| Plathymenia reticulata | 3.4 | 5.8 | 3.6 | 2.76 | [123] |
| Populus x canadensis | 3.0 | 13.7 | 5.1 | 6.27 | [114] |
| Robinia pseudoacacia | 3.6 | 7.2 | 5.5 | 3.53 | [114] |
| Robinia pseudoacacia | 3.8 | 3.9 | 5.0 | 2.34 | [113] |
| Salix | 3.3 | 19.9 | 5.9 | 7.82 | [114] |
| Tectona grandis | 2.2 | 2.9 | 7.3 | 4.64 | [124] |
| Pinus nigra subsp. laricio | 4.0 | 5.5 | 4.5 | 2.50 | [114] |
| Pinus slyvestris | 4.2 | 5.0 | 9.2 | 3.38 | [125] |
| Pinus pinea | 1.8 | 9.4 | 9.4 | 10.44 | [126] |
| Larix decidua | 2.8 | 19.1 | 8.2 | 9.75 | [114] |
| Picea abies | 4.8 | 5.3 | 11.2 | 3.44 | [125] |
| Pseudotsuga menziesii | 5.6 | 21.1 | 2.4 | 4.20 | [127] |
| Average | 4.7 | 9.5 | 7.0 | ||
| Std. | 5.3 | 6.7 | 6.3 | ||
| Average Extractive Content (%) | ||||
|---|---|---|---|---|
| Lipophilic | Hydrophilic | Hydrophilic/Lipophilic Ratio (RHL) | Reference | |
| Barks | 4.7 | 16.5 | 3.52 | This work |
| Cork-rich barks | 10.1 | 9.2 | 0.91 | [107,108,110,117,121,123,127,128] |
| Hardwoods | 0.7 | 8.6 | 12.29 | [129,130] |
| Softwoods | 1.5 | 7.9 | 5.27 | [129,130] |
| Target Extractive Efficiency | |||
|---|---|---|---|
| Extraction Methods | Hydrophilic Compounds | Lipophilic Compounds | Limitations |
| ASE | Efficient | Efficient | High cost, use of organic solvents, and loss of thermally sensitive compounds |
| UAE | Efficient | Not efficient | Possible low extract yields, high energy consumption |
| MAE | Efficient | Efficient | Possible low extract yields, loss of thermally sensitive compounds, and high energy consumption |
| MAC | Least efficient | Least efficient | Longer extraction time, use of organic solvents, and low extract yields |
| SFE | Efficient | Most efficient | High cost |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şen, U.; Esteves, B.; Pereira, H. Pyrolysis and Extraction of Bark in a Biorefineries Context: A Critical Review. Energies 2023, 16, 4848. https://doi.org/10.3390/en16134848
Şen U, Esteves B, Pereira H. Pyrolysis and Extraction of Bark in a Biorefineries Context: A Critical Review. Energies. 2023; 16(13):4848. https://doi.org/10.3390/en16134848
Chicago/Turabian StyleŞen, Umut, Bruno Esteves, and Helena Pereira. 2023. "Pyrolysis and Extraction of Bark in a Biorefineries Context: A Critical Review" Energies 16, no. 13: 4848. https://doi.org/10.3390/en16134848
APA StyleŞen, U., Esteves, B., & Pereira, H. (2023). Pyrolysis and Extraction of Bark in a Biorefineries Context: A Critical Review. Energies, 16(13), 4848. https://doi.org/10.3390/en16134848








