Molecular Dynamics Simulation of High Temperature Mechanical Properties of Nano-Polycrystalline Beryllium Oxide and Relevant Experimental Verification
Abstract
:1. Introduction
2. Materials and Methods
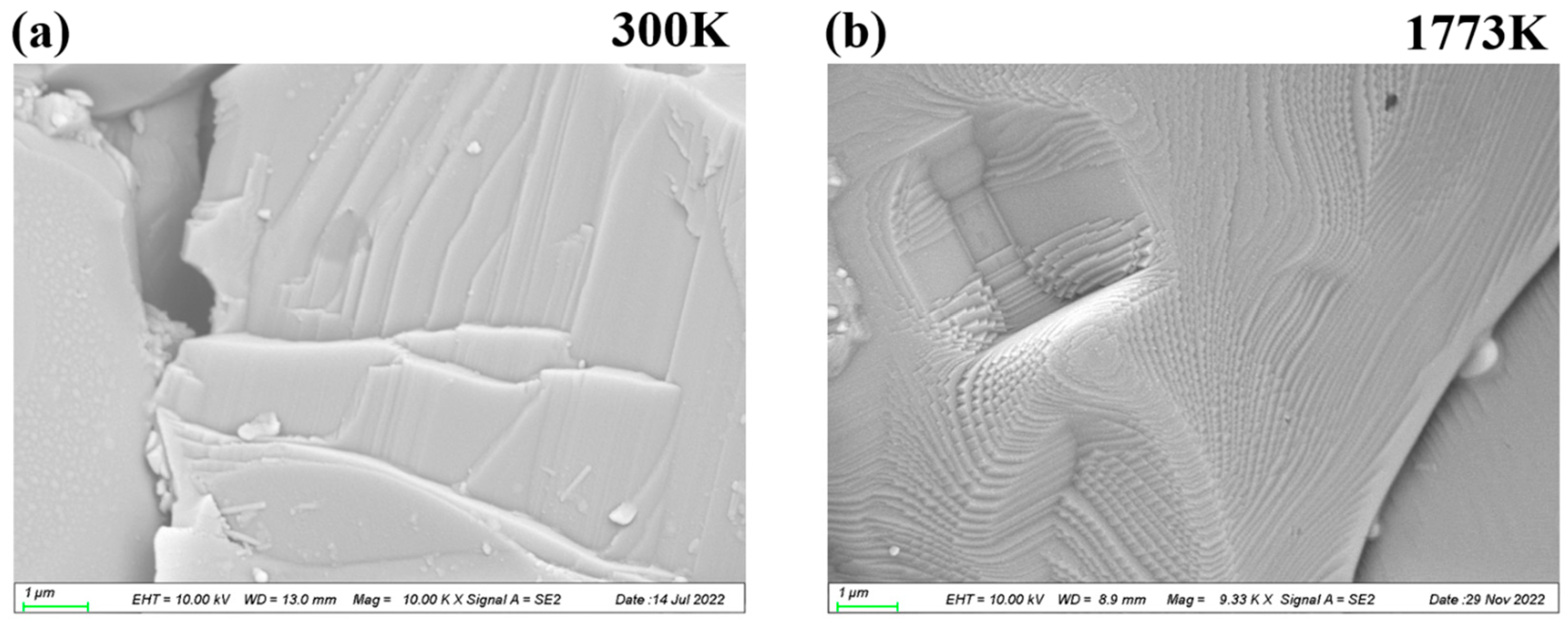
3. Results and Discussions
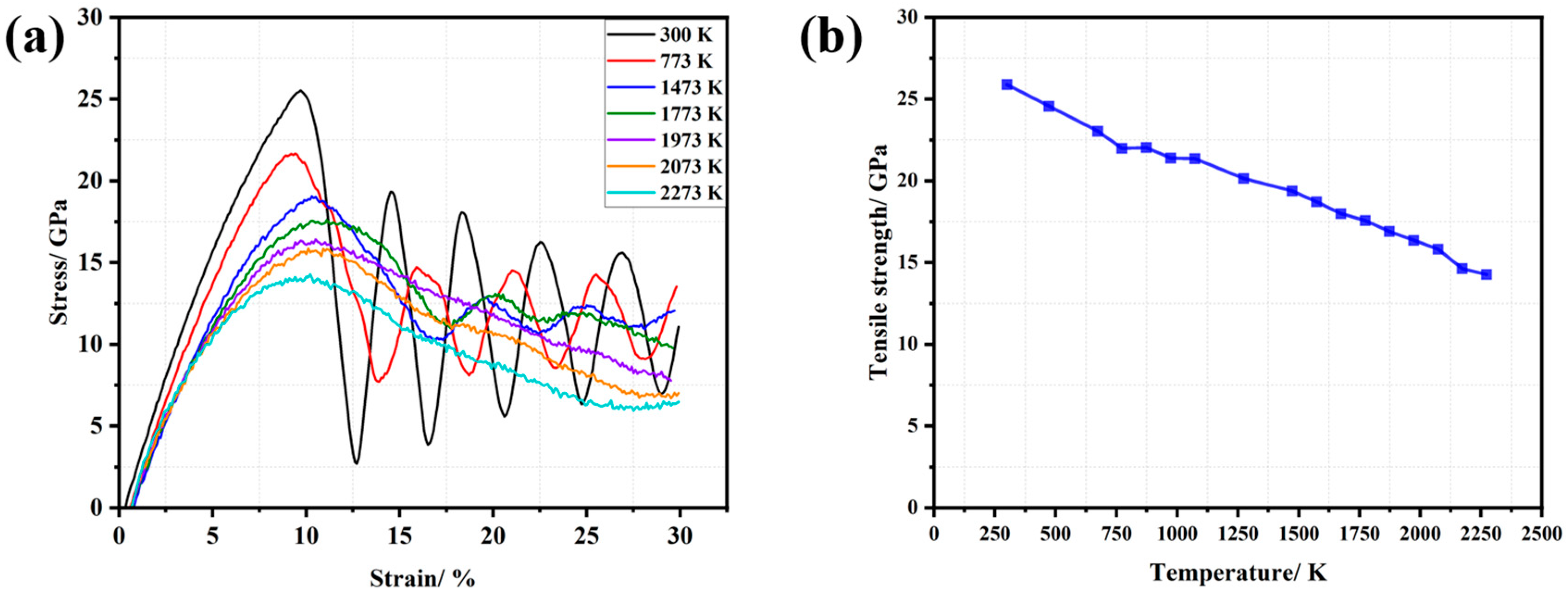
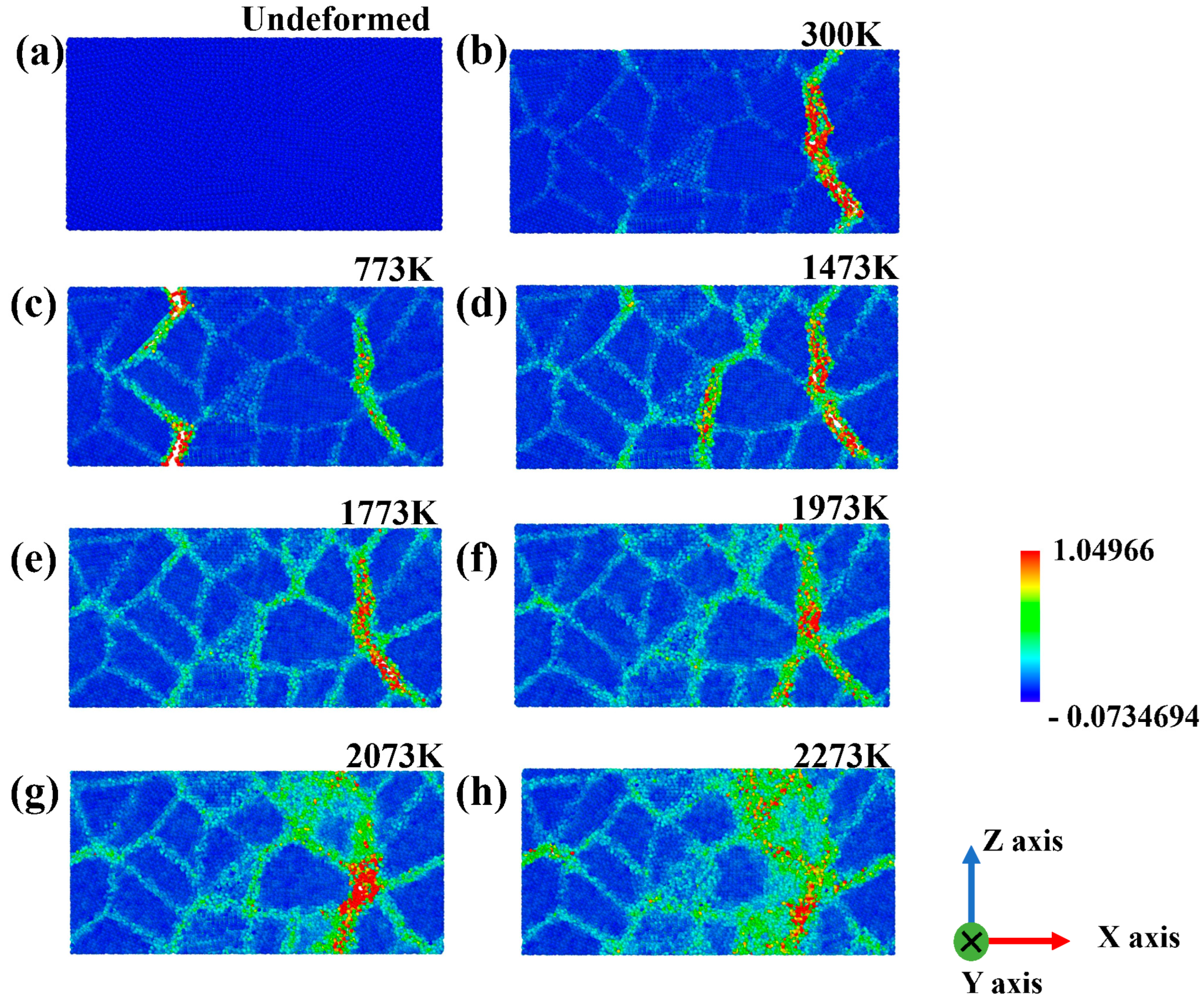
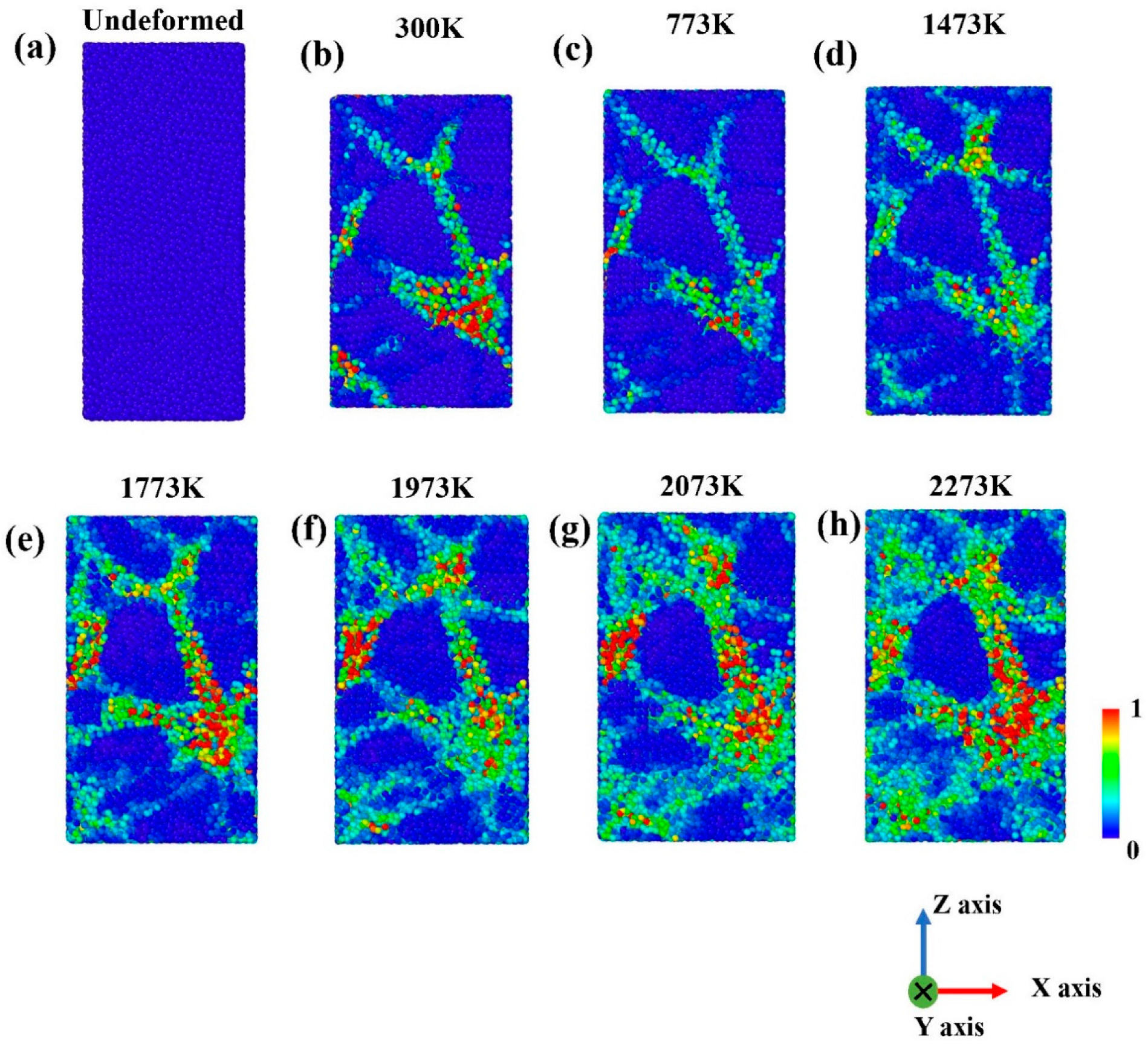
3.1. Tensile Tests
3.2. Compressive Tests
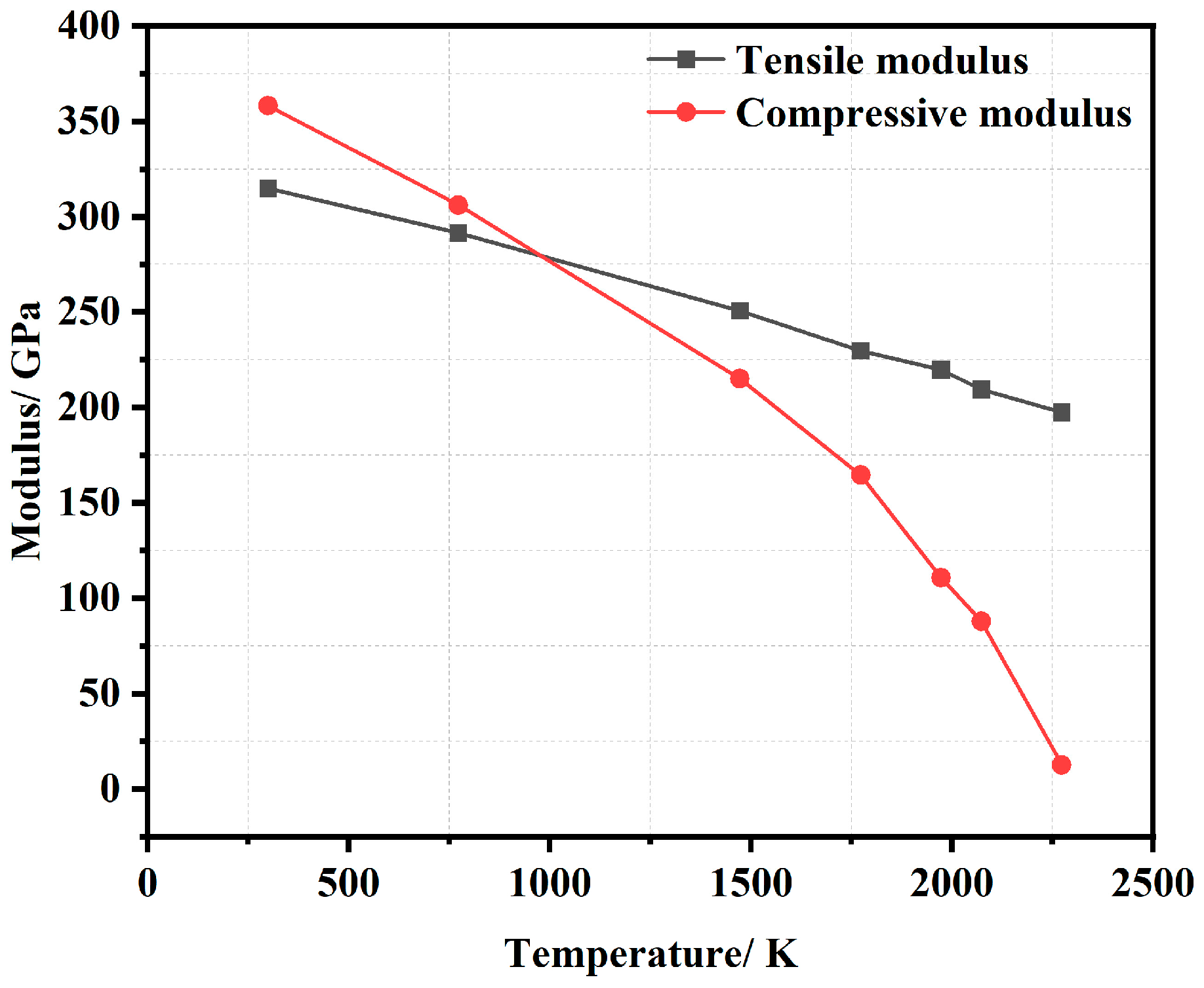
3.3. Modulus
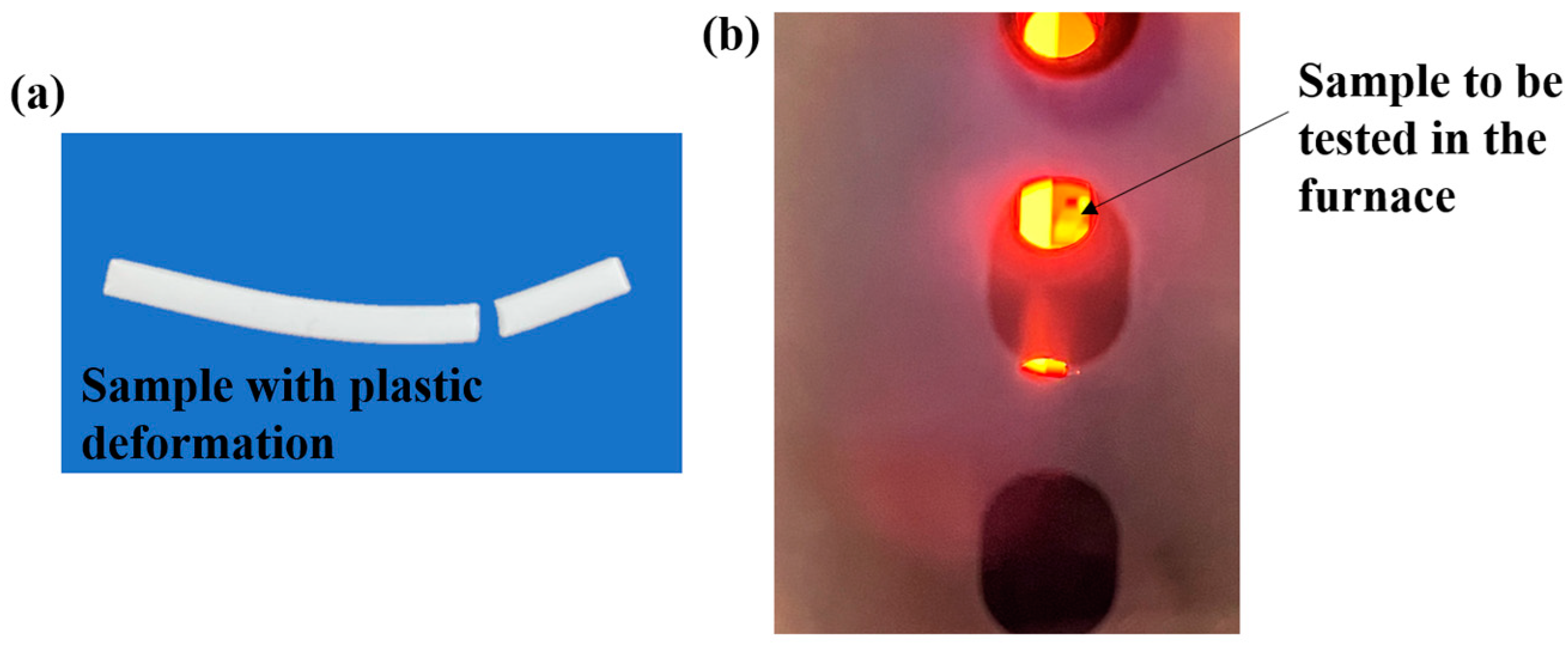
3.4. Experimental Verification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manly, W.D. Utilization of BeO in reactors. J. Nucl. Mater. 1964, 14, 3–18. [Google Scholar] [CrossRef]
- Hou, M.; Zhou, X.; Liu, B. Beryllium oxide utilized in nuclear reactors: Part I: Application history, thermal properties, mechanical properties, corrosion behavior and fabrication methods. Nucl. Eng. Technol. 2022, 54, 4393–4411. [Google Scholar] [CrossRef]
- Petrovic, J.J.; Haertling, C.L. Beryllium Oxide (BeO) Handbook; Report LA-UR-20-24561; Los Alamos National Laboratory: Los Alamos, NM, USA, 2020. [Google Scholar]
- Barmore, W.L.; Vandervoort, R.R. High-temperature creep and dislocation etch pits in polycrystalline beryllium oxide. J. Am. Ceram. Soc. 1967, 50, 316–320. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The Open Visualization Tool. Model. Simul. Mat. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in’t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Byggmästar, J.; Hodille, E.A.; Ferro, Y.; Nordlund, K. Analytical bond order potential for simulations of BeO 1D and 2D nanostructures and plasma-surface interactions. J. Phys. Condens. Matter. 2018, 30, 135001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodille, E.A.; Byggmästar, J.; Safi, E.; Nordlund, K. Molecular dynamics simulation of beryllium oxide irradiated by deuterium ions: Sputtering and reflection. J. Phys. Condens. Matter. 2019, 31, 185001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodille, E.A.; Byggmästar, J.; Safi, E.; Nordlund, K. Sputtering of beryllium oxide by deuterium at various temperatures simulated with molecular dynamics. Phys. Scr. 2020, T171, 014024. [Google Scholar] [CrossRef]
- Hodille, E.A.; Byggmästar, J.; Ferro, Y.; Nordlund, K. Molecular dynamics study of hydrogen isotopes at the Be/BeO interface. J. Phys. Condens. Matter. 2022, 34, 405001. [Google Scholar] [CrossRef] [PubMed]
- Tersoff, J. New empirical approach for the structure and energy of covalent systems. Phys. Rev. B 1988, 37, 6991–7000. [Google Scholar] [CrossRef] [PubMed]
- Tersoff, J. Modeling solid-state chemistry: Interatomic potentials for multicomponent systems. Phys. Rev. B 1989, 39, 5566–5568. [Google Scholar] [CrossRef] [PubMed]
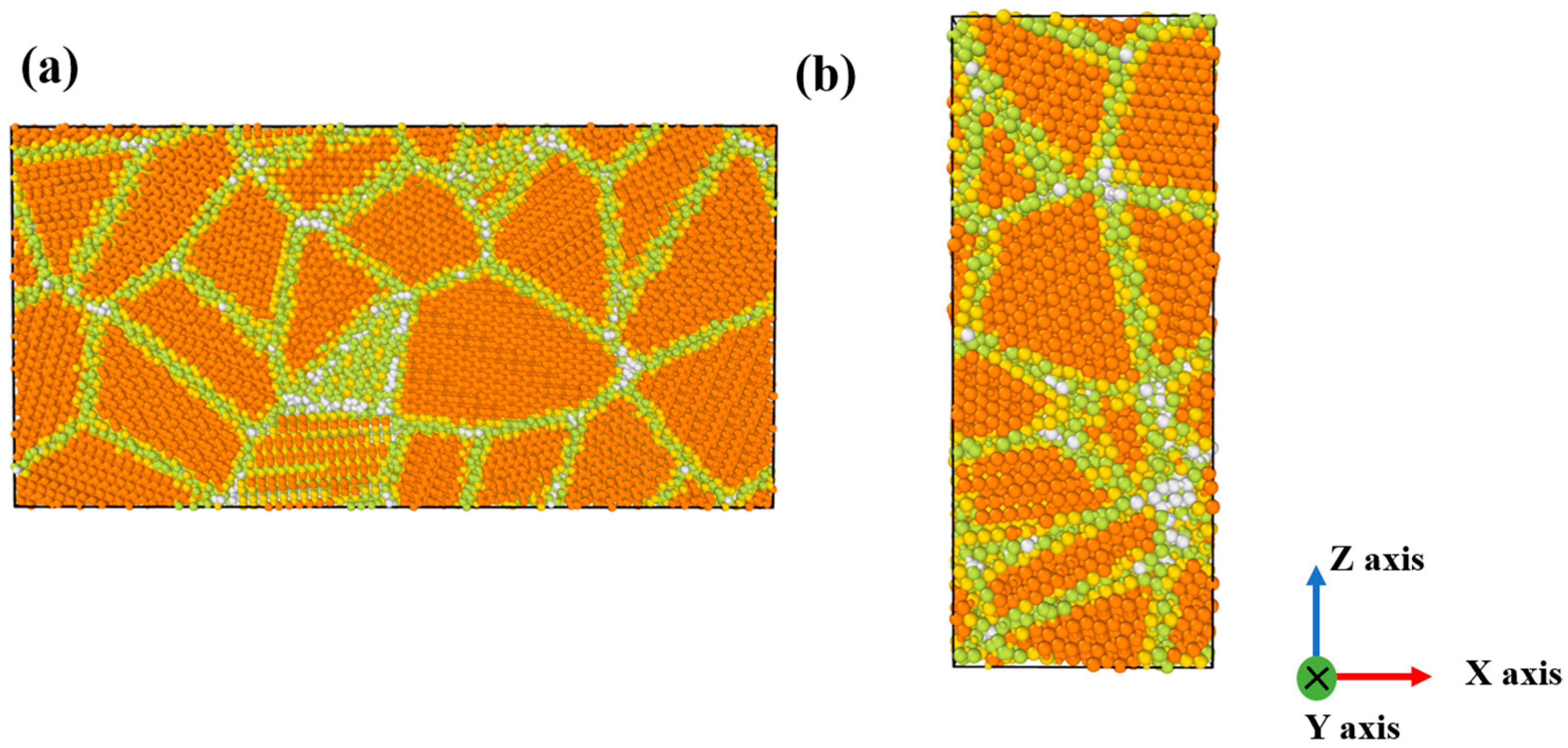




| Tensile Test Model | Compressive Test Model | |
|---|---|---|
| Length in X axis/nm | 20 | 5 |
| Length in Y axis/nm | 3 | 5 |
| Length in Z axis/nm | 10 | 12.5 |
| The number of grains | 20 | 20 |
| The number of atoms | 85,888 | 44,651 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, M.-D.; Zhou, X.-W.; Liu, M.; Liu, B. Molecular Dynamics Simulation of High Temperature Mechanical Properties of Nano-Polycrystalline Beryllium Oxide and Relevant Experimental Verification. Energies 2023, 16, 4927. https://doi.org/10.3390/en16134927
Hou M-D, Zhou X-W, Liu M, Liu B. Molecular Dynamics Simulation of High Temperature Mechanical Properties of Nano-Polycrystalline Beryllium Oxide and Relevant Experimental Verification. Energies. 2023; 16(13):4927. https://doi.org/10.3390/en16134927
Chicago/Turabian StyleHou, Ming-Dong, Xiang-Wen Zhou, Malin Liu, and Bing Liu. 2023. "Molecular Dynamics Simulation of High Temperature Mechanical Properties of Nano-Polycrystalline Beryllium Oxide and Relevant Experimental Verification" Energies 16, no. 13: 4927. https://doi.org/10.3390/en16134927





