Combustion Chemistry of Unsaturated Hydrocarbons Mixed with NOx: A Review with a Focus on Their Interactions
Abstract
:1. Introduction
2. Fundamental Combustion Experiments
2.1. Unsaturated Hydrocarbons Mixed with NO
2.2. Unsaturated Hydrocarbons Mixed with NO2
2.3. Unsaturated Hydrocarbons Mixed with N2O
3. Chemical Kinetics
3.1. Widely Adopted Kinetic Models for Nitrogen Chemistry
3.2. Conversion between NOx Species
3.3. Important Direct Interacting Reactions between NOx and Unsaturated Hydrocarbons
3.3.1. RH + NO2 Pathways
3.3.2. RH + N2O Pathways
3.3.3. ROO + NO
4. Conclusions and Recommendations
- There is an immense need for fundamental combustion experiments, with a special emphasis on NO2 and N2O; heavier unsaturated hydrocarbons (i.e., carbon number > 3); and high-pressure conditions (e.g., >40 bar). It is still obvious based on the limited data that the effects of NOx blending on the reactivity of unsaturated hydrocarbons are diverse. In general, NO and NO2 promote fuel reactivity at low temperatures. This promoting effect diminishes as the temperature increases and can even be reversed at high temperatures, when NO and NO2 start to inhibit fuel reactivity. The blending effects of N2O are less clear, as it has mostly been used directly as an oxidizer, quickly decomposing to produce O atoms via N2O(+M) = N2 + O(+M). As such, fundamental combustion experiments over wider ranges of conditions are highly recommended to re-evaluate the effects of NOx blending on unsaturated hydrocarbon combustion characteristics in a consistent manner, and to distinguish the effects of NOx blending between unsaturated and saturated hydrocarbons.
- The diverse effects of NOx blending are further complicated by the interconversion between the NOx species, for which the available conversion mechanisms remain controversial. This is most prominent for the conversion between NO and NO2, which has been observed to exhibit both second and third reaction orders and both strong and weak dependence on the O2 concentration. Experimental and theoretical studies on NOx interconversions are urgently needed to account for their impact on the effects of NOx blending, as well as to guide future fundamental combustion experiments in properly preparing fuel/NOx/oxidizer mixtures.
- There is a unique interaction between NOx and unsaturated hydrocarbons, with NO2 and N2O able to bond directly to the carbon–carbon double and triple bonds. These interacting pathways depend highly on pressure (due to the imbalance in molar number across the reaction), which might substantially alter the effects of NOx blending under the high-pressure conditions that are relevant to advanced combustion engines. In addition, other types of direct interaction, such as RH + NO2 = R + HONO (or HNO2) and ROO + NO = RO + NO2, have also been highlighted as holding significance. Currently, the understanding of these interactions is seriously lacking, let alone their impact on the effects of NOx blending. Further investigations into these interactions are therefore highly recommended. In addition to fundamental experiments, theoretical studies such as ab initio calculations could be quite valuable to determine the specific reaction channels involved in these interactions and establish the respective rate rules.
- The existing chemical kinetic models are inadequate and inconsistent in replicating the combustion characteristics of unsaturated hydrocarbon/NOx mixtures. To date, the pathways of NO2 and N2O bonding to unsaturated carbon–carbon bonds have been rarely addressed, and NOx interconversions are insufficiently represented in existing chemical kinetic models. The situation has been made worse by the limitations of available fundamental combustion experiments and theoretical studies. Systematic efforts, such as those pointed out in (1)–(3), are necessary to address the inconsistencies and inadequacies of existing models for unsaturated hydrocarbon/NOx mixtures.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pu, W.; Sun, D.; Fan, W.; Pan, W.; Chai, Q.; Wang, X.; Lv, Y. Cu-Catalyzed atom transfer radical addition reactions of alkenes with alpha-bromoacetonitrile. Chem. Commun. 2019, 55, 4821–4824. [Google Scholar] [CrossRef] [PubMed]
- Zamir, E.; Haas, Y.; Levine, R.D. Laser enhanced addition reactions between hydrogen halides and unsaturated hydrocarbons: An information–theoretic approach. J. Chem. Phys. 1980, 73, 2680–2687. [Google Scholar] [CrossRef]
- Preston, T.J.; Dunning, G.T.; Orr-Ewing, A.J.; Vazquez, S.A. Direct and indirect hydrogen abstraction in Cl + alkene reactions. J. Phys. Chem. A 2014, 118, 5595–5607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehl, M.; Pitz, W.J.; Westbrook, C.K.; Yasunaga, K.; Conroy, C.; Curran, H.J. Autoignition behavior of unsaturated hydrocarbons in the low and high temperature regions. Proc. Combust. Inst. 2011, 33, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Seleznev, R.K.; Surzhikov, S.T.; Shang, J.S. A review of the scramjet experimental data base. Prog. Aerosp. Sci. 2019, 106, 43–70. [Google Scholar] [CrossRef]
- Zhou, C.W.; Farooq, A.; Yang, L.; Mebel, A.M. Combustion chemistry of alkenes and alkadienes. Prog. Energy Combust. Sci. 2022, 90, 100983. [Google Scholar] [CrossRef]
- Garner, S.; Sivaramakrishnan, R.; Brezinsky, K. The high-pressure pyrolysis of saturated and unsaturated C7 hydrocarbons. Proc. Combust. Inst. 2009, 32, 461–467. [Google Scholar] [CrossRef]
- Wolk, B.; Ekoto, I.; Northrop, W.F.; Moshammer, K.; Hansen, N. Detailed speciation and reactivity characterization of fuel-specific in-cylinder reforming products and the associated impact on engine performance. Fuel 2016, 185, 348–361. [Google Scholar] [CrossRef] [Green Version]
- Pool-Zobel, B.L.; Schmezer, P.; Zeller, W.J.; Klein, R.G. In vitro and ex vivo effects of the air pollutants SO2 and NOx on benzo(a)pyrene activating enzymes of the rat liver. Exp. Pathol. 1990, 39, 207–212. [Google Scholar] [CrossRef]
- Kramlich, J.C.; Linak, W.P. Nitrous oxide behavior in the atmosphere, and in combustion and industrial systems. Prog. Energy Combust. Sci. 1994, 20, 149–202. [Google Scholar] [CrossRef]
- Hill, S.C.; Douglas Smoot, L. Modeling of nitrogen oxides formation and destruction in combustion systems. Prog. Energy Combust. Sci. 2000, 26, 417–458. [Google Scholar] [CrossRef]
- Razus, D. Nitrous Oxide: Oxidizer and Promoter of Hydrogen and Hydrocarbon Combustion. Ind. Eng. Chem. Res. 2022, 61, 11329–11346. [Google Scholar] [CrossRef]
- Zakirov, V.; Sweeting, M.; Lawrence, T.; Sellers, J. Nitrous oxide as a rocket propellant. Acta Astronaut. 2001, 48, 353–362. [Google Scholar] [CrossRef]
- Sahu, A.B.; Mohamed, A.A.E.-S.; Panigrahy, S.; Saggese, C.; Patel, V.; Bourque, G.; Pitz, W.J.; Curran, H.J. An experimental and kinetic modeling study of NOx sensitization on methane autoignition and oxidation. Combust. Flame 2022, 238, 111746. [Google Scholar] [CrossRef]
- Zhai, Y.; Xu, Q.; Feng, B.; Shao, C.; Wang, Z.; Sarathy, S.M. Impacts of NO on low-temperature oxidation of n-heptane in a jet-stirred reactor. Combust. Flame 2023, 253, 112824. [Google Scholar] [CrossRef]
- Yu, B.; Kum, S.-M.; Lee, C.-E.; Lee, S. Study on the combustion characteristics of a premixed combustion system with exhaust gas recirculation. Energy 2013, 61, 345–353. [Google Scholar] [CrossRef]
- Mahla, S.K.; Dhir, A.; Gill, K.J.S.; Cho, H.M.; Lim, H.C.; Chauhan, B.S. Influence of EGR on the simultaneous reduction of NOx-smoke emissions trade-off under CNG-biodiesel dual fuel engine. Energy 2018, 152, 303–312. [Google Scholar] [CrossRef]
- Ivanov, D.; Babushkin, D.; Semikolenov, S.; Malykhin, S.; Kharitonov, A.; Dubkov, K. Effect of cis/trans isomerism on selective oxidation of olefins with nitrous oxide. Tetrahedron 2016, 72, 2501–2506. [Google Scholar] [CrossRef]
- Starokon, E.V.; Dubkov, K.A.; Babushkin, D.E.; Parmon, V.N.; Panov, G.I. Liquid Phase Oxidation of Alkenes with Nitrous Oxide to Carbonyl Compounds. Adv. Synth. Catal. 2004, 346, 268–274. [Google Scholar] [CrossRef]
- Semikolenov, S.V.; Dubkov, K.A.; Starokon, E.V.; Babushkin, D.E.; Panov, G.I. Liquid-phase noncatalytic butene oxidation with nitrous oxide. Russ. Chem. Bull. 2005, 54, 948–956. [Google Scholar] [CrossRef]
- Konnov, A.A. Detailed Reaction Mechanism for Small Hydrocarbons Combustion, Release 0.5. 2000. Available online: https://homepages.vub.ac.be/~akonnov/ (accessed on 23 May 2023).
- Coppens, F.; Deruyck, J.; Konnov, A. The effects of composition on burning velocity and nitric oxide formation in laminar premixed flames of CH4 + H2 + O2 + N2. Combust. Flame 2007, 149, 409–417. [Google Scholar] [CrossRef]
- Konnov, A.A. Implementation of the NCN pathway of prompt-NO formation in the detailed reaction mechanism. Combust. Flame 2009, 156, 2093–2105. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Panigrahy, S.; Sahu, A.B.; Bariki, C.; Liang, J.; Mohamed, A.; Dong, S.; Tang, C.; Pitsch, H.; Huang, Z.; et al. Understanding the antagonistic effect of methanol as a component in surrogate fuel models: A case study of methanol/n-heptane mixtures. Combust. Flame 2021, 226, 229–242. [Google Scholar] [CrossRef]
- Doughty, A.; Barnes, F.J.; Bromly, J.H.; Haynes, B.S. The mutually sensitied oxidation of ethylene and NO: An experimental and kinetic modeling study. Symp. Int. Combust. 1996, 26, 589–596. [Google Scholar] [CrossRef]
- Dagaut, P.; Lecomte, F.; Chevailler, S.; Cathonnet, M. The reduction of NO by ethylene in a jet-stirred reactor at 1 atm: Experimental and kinetic modelling. Combust. Flame 1999, 119, 494–504. [Google Scholar] [CrossRef]
- Dagaut, P.; Mathieu, O.; Nicolle, A.; Dayma, G. Experimental Study and Detailed Kinetic Modeling of the Mutual Sensitization of the Oxidation of Nitric Oxide, Ethylene, and Ethane. Combust. Sci. Technol. 2005, 177, 1767–1791. [Google Scholar] [CrossRef]
- Giménez-López, J.; Alzueta, M.U.; Rasmussen, C.T.; Marshall, P.; Glarborg, P. High pressure oxidation of C2H4/NO mixtures. Proc. Combust. Inst. 2011, 33, 449–457. [Google Scholar] [CrossRef]
- Atakan, B.; Hartlieb, A.T. Laser diagnostics of NO reburning in fuel-rich propene flames. Appl. Phys. B 2014, 71, 697–702. [Google Scholar] [CrossRef]
- Dagaut, P.; Luche, J.; Cathonnet, M. Experimental and kinetic modeling of the reduction of NO by propene at 1 atm. Combust. Flame 2000, 121, 651–661. [Google Scholar] [CrossRef]
- Yuan, W.; Ruwe, L.; Schwarz, S.; Cao, C.; Yang, J.; Deutschmann, O.; Kohse-Höinghaus, K.; Qi, F. Insights into the interaction kinetics between propene and NOx at moderate temperatures with experimental and modeling methods. Proc. Combust. Inst. 2021, 38, 795–803. [Google Scholar] [CrossRef]
- Gossler, S.; Ruwe, L.; Yuan, W.; Yang, J.; Chen, X.; Schmitt, S.; Maier, L.; Kohse-Höinghaus, K.; Qi, F.; Deutschmann, O. Exploring the interaction kinetics of butene isomers and NOx at low temperatures and diluted conditions. Combust. Flame 2021, 233, 111557. [Google Scholar] [CrossRef]
- Prabhu, S.K.; Bhat, R.K.; Miller, D.L.; Cernansky, N.P. 1-Pentene oxidation and its interaction with nitric oxide in the low and negative temperature coefficient regions. Combust. Flame 1996, 104, 377–390. [Google Scholar] [CrossRef]
- Quang, L.N.; Vanpee, M. A spectroscopic investigation of the premixed acetylene-nitric oxide flame. Symp. Int. Combust. 1982, 19, 293–301. [Google Scholar] [CrossRef]
- Quang Ngoc, L.; Vanpee, M. Free radical concentration measurements in nitric oxide acetylene flames. Combust. Flame 1985, 62, 193–210. [Google Scholar] [CrossRef]
- Dagaut, P.; Lecomte, F.; Chevailler, S.; Cathonnet, M. Experimental and kinetic modeling of nitric oxide reduction by acetylene in an atmospheric pressure jet-stirred reactor. Fuel 1999, 78, 1245–1252. [Google Scholar] [CrossRef]
- Guarneri, F.; Ikeda, E.; Mackie, J.C. A Study of Furan as a Model Oxygenated Reburn Fuel for Nitric Oxide Reduction. Energ Fuels 2001, 2001, 743–750. [Google Scholar] [CrossRef]
- Alexandrino, K.; Millera, Á.; Bilbao, R.; Alzueta, M.U. Interaction between 2,5-Dimethylfuran and Nitric Oxide: Experimental and Modeling Study. Energy Fuels 2014, 28, 4193–4198. [Google Scholar] [CrossRef]
- Alexandrino, K.; Millera, Á.; Bilbao, R.; Alzueta, M.U. 2-methylfuran Oxidation in the Absence and Presence of NO. Flow Flow Turbul. Combust. Flow 2015, 96, 343–362. [Google Scholar] [CrossRef] [Green Version]
- Alzueta, M.U.; Serinyel, Z.; Simmie, J.M.; Curran, H.J. Oxidation of Acetone and Its Interaction with Nitric Oxide. Energy Fuels 2010, 24, 1511–1520. [Google Scholar] [CrossRef]
- Williams, B.A.; Pasternack, L. The effect of nitric oxide on premixed flames of CH4, C2H6, C2H4, and C2H2. Combust. Flame 1997, 111, 87–110. [Google Scholar] [CrossRef]
- Glarborg, P.; Alzueta, M.U.; Dam-Johansen, K.; Miller, J.A. Kinetic Modeling of Hydrocarbon/Nitric Oxide Interactions in a Flow Reactor. Combust. Flame 1998, 115, 1–27. [Google Scholar] [CrossRef]
- Konnov, A.A.; Dyakov, I.V.; Knyazkov, D.A.; Korobeinichev, O.P. Formation and Destruction of Nitric Oxide in NO Doped Premixed Flames of C2H4, C2H6, and C3H8 at Atmospheric Pressure. Energy Fuels 2010, 24, 4833–4840. [Google Scholar] [CrossRef]
- Hori, M.; Matsunaga, N.; Marinov, N.; William, P.; Charles, W. An experimental and kinetic calculation of the promotion effect of hydrocarbons on the NO-NO2 conversion in a flow reactor. Symp. Int. Combust. 1998, 27, 389–396. [Google Scholar] [CrossRef]
- Abián, M.; Silva, S.L.; Millera, Á.; Bilbao, R.; Alzueta, M.U. Effect of operating conditions on NO reduction by acetylene–ethanol mixtures. Fuel Process. Technol. 2010, 91, 1204–1211. [Google Scholar] [CrossRef]
- Marrodán, L.; Berdusán, L.; Aranda, V.; Millera, Á.; Bilbao, R.; Alzueta, M.U. Influence of dimethyl ether addition on the oxidation of acetylene in the absence and presence of NO. Fuel 2016, 183, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Saggese, C.; Goldsborough, S.S.; Wagnon, S.W.; Pitz, W.J. Unraveling the role of EGR olefins at advanced combustion conditions in the presence of nitric oxide: Ethylene, propene and isobutene. Combust. Flame 2022, 245, 112344. [Google Scholar] [CrossRef]
- Alzueta, M.U.; Glarborg, P.; Dam-Johansen, K. Low temperature interactions between hydrocarbons and nitric oxide: An experimental study. Combust. Flame 1997, 109, 25–36. [Google Scholar] [CrossRef]
- Abián, M.; Peribáñez, E.; Millera, Á.; Bilbao, R.; Alzueta, M.U. Impact of nitrogen oxides (NO, NO2, N2O) on the formation of soot. Combust. Flame 2014, 161, 280–287. [Google Scholar] [CrossRef]
- Menon, A.V.; Lee, S.-Y.; Linevsky, M.J.; Litzinger, T.A.; Santoro, R.J. Addition of NO2 to a laminar premixed ethylene–air flame: Effect on soot formation. Proc. Combust. Inst. 2007, 31, 593–601. [Google Scholar] [CrossRef]
- Deng, F.; Zhang, Y.; Sun, W.; Huang, W.; Zhao, Q.; Qin, X.; Yang, F.; Huang, Z. Towards a kinetic understanding of the NOx sensitization effect on unsaturation hydrocarbons: A case study of ethylene/nitrogen dioxide mixtures. Proc. Combust. Inst. 2019, 37, 719–726. [Google Scholar] [CrossRef]
- Volponi, J.V.; Branch, M.C. Flame structure of C2H2-O2-Argon and C2H2-NO2- Argon laminar premixed flames. Symp. Int. Combust. 1992, 24, 823–831. [Google Scholar] [CrossRef]
- Marshall, P.; Leung, C.; Gimenez-Lopez, J.; Rasmussen, C.T.; Hashemi, H.; Glarborg, P.; Abian, M.; Alzueta, M.U. The C2H2 + NO2 reaction: Implications for high pressure oxidation of C2H2/NOx mixtures. Proc. Combust. Inst. 2019, 37, 469–476. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Ma, Z.; Wang, X.; Liu, F.; Li, X.; Chu, X. Experimental and Kinetic Study of the Effect of Nitrogen Dioxide on Ethanol Autoignition. ACS Omega 2023, 8, 8377–8387. [Google Scholar] [CrossRef]
- Ye, W.; Shi, J.C.; Zhang, R.T.; Wu, X.J.; Zhang, X.; Qi, M.L.; Luo, S.N. Experimental and Kinetic Modeling Study of CH3OCH3 Ignition Sensitized by NO2. Energy Fuels 2016, 30, 10900–10908. [Google Scholar] [CrossRef]
- Alzueta, M.U.; Muro, J.; Bilbao, R.; Glarborg, P. Oxidation of Dimethyl Ether and its Interaction with Nitrogen Oxides. Isr. J. Chem. 1999, 39, 73–86. [Google Scholar] [CrossRef]
- Atkinson, R.; Aschmann, S.M.; Winer, A.M.; Pitts, J.N., Jr. Gas phase reaction of NO2 with alkenes and dialkenes. Int. J. Chem. Kinet. 1984, 16, 697–706. [Google Scholar] [CrossRef]
- Bernard, F.; Cazaunau, M.; Mu, Y.; Wang, X.; Daele, V.; Chen, J.; Mellouki, A. Reaction of NO2 with selected conjugated alkenes. J. Phys. Chem. A 2013, 117, 14132–14140. [Google Scholar] [CrossRef]
- Ohta, T.; Nagura, H.; Suzuki, S. Rate constants for the reactions of conjugated olefins with NO2 in the gas phase. Int. J. Chem. Kinet. 1986, 18, 1–11. [Google Scholar] [CrossRef]
- Harrison, R.M.; Shi, J.P.; Grenfell, J.L. Novel nighttime free radical chemistry in severe nitrogen dioxide pollution episodes. Atmos. Environ. 1998, 32, 2769–2774. [Google Scholar] [CrossRef]
- Shi, J.P.; Harrison, R.M. Rapid NO2 formation in diluted petrol-fuelled engine exhaust—A source of NO2 in winter smog episodes. Atmos. Environ. 1997, 31, 3857–3866. [Google Scholar] [CrossRef]
- Trenwith, A.B. The kinetics of the oxidation of ethylene by nitrous oxide. J. Chem. Soc. 1960, 743, 3722–3726. [Google Scholar] [CrossRef]
- Howard, S.L.; Newberry, J.E.; Sausa, R.C.; Miziolek, A.W. Triple quadrupole mass spectrometry as applied to flame diagnostics: Study of the C2H4/N2O/Ar Flame. J. Am. Soc. Mass Spectrom. 1993, 4, 152–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werlin, L.; Felix, L.; Dominic, F.; Nicole, R.; Helmut, C.; Stefan, S. Experimental Investigation of the Flame Propagation and Flashback Behavior of a Green Propellant Consisting of N2O and C2H4. J. Energy Power Eng. 2017, 11, 735–752. [Google Scholar]
- Naumann, C.; Kick, T.; Methling, T.; Braun-Unkhoff, M.; Riedel, U. Ethene/Dinitrogen Oxide—A Green Propellant to substitute Hydrazine: Investigation on its Ignition Delay Time and Laminar Flame Speed. In Proceedings of the 26th ICDERS, Boston, MA, USA, 30 July–4 August 2017. [Google Scholar]
- Kick, T.; Starcke, J.H.; Naumann, C. Green Propellant Substituting Hydrazine: Investigation of Ignition Delay Time and Laminar Flame Speed of Ethene/Dinitrogen Oxide Mixtures. In Proceedings of the European Combustion Meeting, Dubrovnik, Croatia, 18–21 April 2017. [Google Scholar]
- Deng, F.; Pan, Y.; Sun, W. Comparative Study of the Effects of Nitrous Oxide and Oxygen on Ethylene Ignition. Energ Fuels 2017, 31, 14116–14128. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, H.Y.; Feng, J.C.; Zheng, D. Experimental investigation of auto-ignition of ethylene-nitrous oxide propellants in rapid compression machine. Fuel 2021, 288, 119688. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.; Liao, C.; Tang, C.; Zhou, C.-W.; Huang, Z. The auto-ignition boundary of ethylene/nitrous oxide as a promising monopropellant. Combust. Flame 2020, 221, 64–73. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H. Laminar burning velocities of C2H4/N2O flames: Experimental study and its chemical kinetics mechanism. Combust. Flame 2019, 202, 362–375. [Google Scholar] [CrossRef]
- Aldous, K.M.; Bailey, B.W.; Rankin, J.M. Burning velocity of the premixed nitrous-oxide/acetylene flame and its influence on burner design. Anal. Chem. 1972, 44, 191–194. [Google Scholar] [CrossRef]
- Alekseev, V.A.; Bystrov, N.; Emelianov, A.; Eremin, A.; Yatsenko, P.; Konnov, A.A. High-temperature oxidation of acetylene by N2O at high Ar dilution conditions and in laminar premixed C2H2 + O2 + N2 flames. Combust. Flame 2022, 238, 111924. [Google Scholar] [CrossRef]
- Powell, O.A.; Papas, P.; Dreyer, C. Laminar Burning Velocities for Hydrogen-, Methane-, Acetylene-, and Propane-Nitrous Oxide Flames. Combust. Sci. Technol. 2009, 181, 917–936. [Google Scholar] [CrossRef]
- Powell, O.A.; Papas, P. Flame Structure Measurements of Nitric Oxide in Hydrocarbon-Nitrous-Oxide Flames. J. Propul. Power 2012, 28, 1052–1059. [Google Scholar] [CrossRef]
- Mével, R.; Shepherd, J.E. Ignition delay-time behind reflected shock waves of small hydrocarbons–nitrous oxide(–oxygen) mixtures. Shock Waves 2014, 25, 217–229. [Google Scholar] [CrossRef]
- Naumann, C.; Kick, T.; Methling, T.; Braun-Unkhoff, M.; Riedel, U. Ethene/Nitrous Oxide Mixtures as Green Propellant to Substitute Hydrazine: Reaction Mechanism Validation. Int. J. Energ. Mater. Chem. Propul. 2020, 19, 65–71. [Google Scholar] [CrossRef]
- Tsang, W. Chemical Kinetic Data Base for Propellant Combustion. II. Reactions Involving CN, NCO, and HNCO. J. Phys. Chem. Ref. Data 1992, 21, 753–791. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.A.; Papas, P.; Nelson, H.H. Kinetics and product channels of the reaction CN + N2O. J. Phys. Chem. 1995, 99, 13471–13475. [Google Scholar] [CrossRef]
- Xu, S.; Lin, M.C. Ab initio chemical kinetics for the reactions of HNCN with O(3P) and O2. Proc. Combust. Inst. 2009, 32, 99–106. [Google Scholar] [CrossRef]
- Xu, S.; Lin, M.C. Ab Initio Chemical Kinetics for the OH + HNCN Reaction. J. Phys. Chem. A 2007, 111, 6730–6740. [Google Scholar] [CrossRef]
- Hashemi, H.; Christensen, J.M.; Gersen, S.; Levinsky, H.; Klippenstein, S.J.; Glarborg, P. High-pressure oxidation of methane. Combust. Flame 2016, 172, 349–364. [Google Scholar] [CrossRef] [Green Version]
- Gimenez-Lopez, J.; Rasmussen, C.T.; Hashemi, H.; Alzueta, M.U.; Gao, Y.; Marshall, P.; Goldsmith, C.F.; Glarborg, P. Experimental and Kinetic Modeling Study of C2H2 Oxidation at High Pressure. Int. J. Chem. Kinet. 2016, 48, 724–738. [Google Scholar] [CrossRef]
- Hashemi, H.; Jacobsen, J.G.; Rasmussen, C.T.; Christensen, J.M.; Glarborg, P.; Gersen, S.; van Essen, M.; Levinsky, H.B.; Klippenstein, S.J. High-pressure oxidation of ethane. Combust. Flame 2017, 182, 150–166. [Google Scholar] [CrossRef] [Green Version]
- Klippenstein, S.J.; Harding, L.B.; Glarborg, P.; Miller, J.A. The role of NNH in NO formation and control. Combust. Flame 2011, 158, 774–789. [Google Scholar] [CrossRef] [Green Version]
- Dagaut, P.; Glarborg, P.; Alzueta, M. The oxidation of hydrogen cyanide and related chemistry. Prog. Energy Combust. Sci. 2008, 34, 1–46. [Google Scholar] [CrossRef]
- Mendiara, T.; Glarborg, P. Ammonia chemistry in oxy-fuel combustion of methane. Combust. Flame 2009, 156, 1937–1949. [Google Scholar] [CrossRef]
- Mendiara, T.; Glarborg, P. Reburn Chemistry in Oxy-fuel Combustion of Methane. Energy Fuels 2009, 23, 3565–3572. [Google Scholar] [CrossRef]
- Zhou, C.W.; Li, Y.; Burke, U.; Banyon, C.; Somers, K.P.; Ding, S.; Khan, S.; Hargis, J.W.; Sikes, T.; Mathieu, O.; et al. An experimental and chemical kinetic modeling study of 1,3-butadiene combustion: Ignition delay time and laminar flame speed measurements. Combust. Flame 2018, 197, 423–438. [Google Scholar] [CrossRef]
- Yang, C.; Wang, W.; Li, Y.; Cheng, X. Experimental and kinetic study of NO/NO2 chemical effects on n-heptane high temperature auto-ignition. Combust. Flame 2023, 249, 112604. [Google Scholar] [CrossRef]
- Kaufman, F.; Gerri, N.J.; Bowman, R.E. Role of Nitric Oxide in the Thermal Decomposition of Nitrous Oxide. J. Chem. Phys. 1956, 25, 106–115. [Google Scholar] [CrossRef]
- Baber, S.C.; Dean, A.M. N2O Dissociation behind reflected shock waves. Int. J. Chem. Kinet. 1975, 7, 381–398. [Google Scholar] [CrossRef]
- Buczkó, N.A.; Varga, T.; Zsély, I.G.; Turányi, T. Formation of NO in High-Temperature N2/O2/H2O Mixtures: Re-evaluation of Rate Coefficients. Energy Fuels 2018, 32, 10114–10120. [Google Scholar] [CrossRef]
- Baldwin, R.R.; Gethin, A.; Plaistowe, J.; Walker, R.W. Reaction between hydrogen and nitrous oxide. J. Chem. Soc., J. Chem. Soc. Faraday Trans. 1 1975, 71, 1265–1284. [Google Scholar] [CrossRef]
- Marshall, P.; Fontijn, A.; Melius, C.F. High-temperature photochemistry and BAC-MP4 studies of the reaction between ground-state H atoms and N2O. J. Chem. Phys. 1987, 86, 5540–5549. [Google Scholar] [CrossRef] [Green Version]
- Bozzelli, J.W.; Chang, A.Y.; Dean, A.M. Analysis of the reactions H + N2O and NH + NO: Pathways and rate constants over a wide range of temperature and pressure. Symp. Int. Combust. 1994, 25, 965–974. [Google Scholar] [CrossRef]
- Roose, T.R.; Hanson, R.K.; Kruger, C.H. A shock tube study of the decomposition of no in the presence of NH3. Symp. Int. Combust. 1981, 18, 853–862. [Google Scholar] [CrossRef]
- Kovács, M.; Papp, M.; Zsély, I.G.; Turányi, T. Determination of rate parameters of key N/H/O elementary reactions based on H2/O2/NOx combustion experiments. Fuel 2020, 264, 116720. [Google Scholar] [CrossRef]
- Park, J.; Hershberger, J.F. A diode laser study of the isocyanate + nitrogen dioxide reaction. J. Phys. Chem. 1993, 97, 13647–13652. [Google Scholar] [CrossRef]
- Zhao, R.; Gao, D.; Pan, X.; Xia, W.; Yu, H.; Yu, S.; Yao, L. Theoretical studies of anharmonic effect on the main reactions involving in NO2 in fuel burning. Chem. Phys. Lett. 2018, 703, 97–105. [Google Scholar] [CrossRef]
- Kaufman, F.; Kelso, J.R. Reaction between Nitric and Nitrous Oxide. J. Chem. Phys. 1955, 23, 602–603. [Google Scholar] [CrossRef]
- Fishburne, E.S.; Edse, R. Shock-Tube Study of Nitrous Oxide Decomposition. J. Chem. Phys. 1964, 41, 1297–1304. [Google Scholar] [CrossRef]
- Mebel, A.M.; Lin, M.C.; Morokuma, K.; Melius, C.F. Theoretical study of reactions of N2O with NO and OH radicals. Int. J. Chem. Kinet. 1996, 28, 693–703. [Google Scholar] [CrossRef]
- Hanson, R.K.; Salimian, S. Survey of Rate Constants in the N/H/O System. In Combustion Chemistry; Gardiner, W.C., Ed.; Springer: New York, NY, USA, 1984; pp. 361–421. [Google Scholar]
- Ashmore, P.G.; Burnett, M.G.; Tyler, B.J. Reaction of nitric oxide and oxygen. Trans. Faraday Soc. 1962, 58, 685–691. [Google Scholar] [CrossRef]
- Greig, J.D.; Hall, P.G. Thermal oxidation of nitric oxide at low concentrations. Trans. Faraday Soc. 1967, 63, 655–661. [Google Scholar] [CrossRef]
- Tipper, C.F.H.; Williams, R.K. The effect of sulphur dioxide on the combustion of some inorganic compounds. Part 2. The nitric oxide+sulphur dioxide+oxygen system. Trans. Faraday Soc. 1961, 57, 79–86. [Google Scholar] [CrossRef]
- Park, J.; Giles, N.D.; Moore, J.; Lin, M.C. A Comprehensive Kinetic Study of Thermal Reduction of NO2 by H2. J. Phys. Chem. A 1998, 102, 10099–10105. [Google Scholar] [CrossRef]
- Röhrig, M.; Petersen, E.L.; Davidson, D.F.; Hanson, R.K. A shock tube study of the pyrolysis of NO2. Int. J. Chem. Kinet. 1997, 29, 483–493. [Google Scholar] [CrossRef]
- Zimet, E. Thermal Decomposition of N2O4 and NO2 by Shock Waves. J. Chem. Phys. 1970, 53, 515–518. [Google Scholar] [CrossRef]
- Konnov, A.A.; Ruyck, J.D. Kinetic Modeling of Nitrogen Oxides Decomposition at Flame Temperatures. Combust. Sci. Technol. 1999, 149, 53–78. [Google Scholar] [CrossRef]
- Stagni, A.; Cavallotti, C.; Arunthanayothin, S.; Song, Y.; Herbinet, O.; Battin-Leclerc, F. An experimental, theoretical and kinetic-modeling study of the gas-phase oxidation of ammonia. React. Chem. Eng. 2020, 5, 696–711. [Google Scholar] [CrossRef] [Green Version]
- Tsang, W.; Herron, J.T. Chemical Kinetic Data Base for Propellant Combustion I. Reactions Involving NO, NO2, HNO, HNO2, HCN and N2O. J. Phys. Chem. Ref. Data 1991, 20, 609–663. [Google Scholar] [CrossRef]
- Baulch, D.L.; Drysdale, D.D.; Home, D.G. Evaluated Kinetic Data for High Temperature Reactions Vol. 2, Homogeneous Gas Phase Reactions of the H2-N2-O2 System; Butterworths: London, UK, 1973. [Google Scholar]
- Brown, F.B.; Crist, R.H. Further Studies on the Oxidation of Nitric Oxide; the Rate of the Reaction between Carbon Monoxide and Nitrogen Dioxide. J. Chem. Phys. 1941, 9, 840–846. [Google Scholar] [CrossRef]
- Treacy, J.C.; Daniels, F. Kinetic Study of the Oxidation of Nitric Oxide with Oxygen in the Pressure Range 1 to 20 Mm.1. J. Am. Chem. Soc. 1955, 77, 2033–2036. [Google Scholar] [CrossRef]
- Neyrolles, E.; Lara Cruz, J.; Bassil, G.; Contamine, F.; Cezac, P.; Arpentinier, P. Kinetic study of the nitric oxide oxidation between 288 and 323 K, under pressure, focus on the oxygen influence on the reaction rate constant. Int. J. Chem. Kinet. 2020, 52, 329–340. [Google Scholar] [CrossRef]
- Šolc, M. Kinetics of the Reaction of Nitric Oxide with Molecular Oxygen. Nature 1966, 209, 706. [Google Scholar] [CrossRef]
- Chakraborty, D.; Park, J.; Lin, M.C. Theoretical study of the OH+NO2 reaction: Formation of nitric acid and the hydroperoxyl radical. Chem. Phys. 1998, 231, 39–49. [Google Scholar] [CrossRef]
- Howard, C.J. Kinetic study of the equilibrium HO2 + NO = OH + NO2 and the thermochemistry of HO2. J. Am. Chem. Soc. 1980, 102, 6937–6941. [Google Scholar] [CrossRef]
- Howard, C.J. Temperature dependence of the reaction HO2 + NO → OH + NO2. J. Chem. Phys. 1979, 71, 2352–2359. [Google Scholar] [CrossRef]
- Bardwell, M.W.; Bacak, A.; Teresa-Raventos, M.; Percival, C.J.; Sanchez-Reyna, G.; Shallcross, D.E. Kinetics of the HO2 + NO reaction: A temperature and pressure dependence study using chemical ionisation mass spectrometry. Phys. Chem. Chem. Phys. 2003, 5, 2381–2385. [Google Scholar] [CrossRef]
- Wu, H.; Sun, W.; Huang, Z.; Zhang, Y. Biphasic sensitization effect of NO2 on n-C4H10 auto-ignition. Combust. Flame 2022, 237, 111844. [Google Scholar] [CrossRef]
- Gao, Z.; Yang, M.; Tang, C.; Yang, F.; Yang, K.; Deng, F.; Huang, Z. Measurements of the High Temperature Ignition Delay Times and Kinetic Modeling Study on Oxidation of Nitromethane. Combust. Sci. Technol. 2019, 192, 313–334. [Google Scholar] [CrossRef]
- Chai, J.; Goldsmith, C.F. Rate coefficients for fuel + NO2: Predictive kinetics for HONO and HNO2 formation. Proc. Combust. Inst. 2017, 36, 617–626. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.H. Gas phase reactions of nitrogen dioxide. Part 1.-Oxidation of acetylene. Trans. Faraday Soc. 1952, 48, 1142–1149. [Google Scholar] [CrossRef]
- Sprung, J.L.; Akimoto, H.; Pitts, J.N., Jr. Nitrogen dioxide catalyzed geometric isomerization of olefins. Isomerization kinetics of the 2-butenes and 2-pentenes. J. Am. Chem. Soc. 1974, 96, 6549–6554. [Google Scholar] [CrossRef]
- Parmon, V.N.; Panov, G.I.; Uriarte, A.; Noskov, A.S. Nitrous oxide in oxidation chemistry and catalysis: Application and production. Catal. Today 2005, 100, 115–131. [Google Scholar] [CrossRef]
- Bridson-Jones, F.S.; Buckley, G.D.; Cross, L.H.; Driver, A.P. Oxidation of organic compounds by nitrous oxide. Part I. J. Chem. Soc. 1951, 666, 2999–3008. [Google Scholar] [CrossRef]
- Bridson-Jones, F.S.; Buckley, G.D. Oxidation of organic compounds by nitrous oxide. Part II. Tri- and tetra-substituted ethylenes. J. Chem. Soc. 1951, 667, 3009–3016. [Google Scholar] [CrossRef]
- Avdeev, V.I.; Ruzankin, S.F.; Zhidomirov, G.M. Molecular mechanism of direct alkene oxidation with nitrous oxide: DFT analysis. Kinet Catal. 2005, 46, 177–188. [Google Scholar] [CrossRef]
- Panov, G.I.; Dubkov, K.A.; Starokon, E.V.; Parmon, V.N. Non-catalytic liquid phase oxidation of alkenes with nitrous oxide. 1. Oxidation of cyclohexene to cyclohexanone. React. Kinet. Catal. Lett. 2002, 76, 401–406. [Google Scholar] [CrossRef]
- Dubkov, K.A.; Panov, G.I.; Starokon, E.V. Non-catalytic liquid phase oxidation of alkenes with nitrous oxide. 2. Oxidation of cyclopentene to cyclopentanone. React. Kinet. Catal. Lett. 2002, 77, 197–205. [Google Scholar] [CrossRef]
- Karami, F.; Vahedpour, M. Theoretical study on the gas phase reaction mechanism of acetylene with nitrous oxide. Struct. Chem. 2012, 24, 1513–1526. [Google Scholar] [CrossRef]
- Ess, D.H.; Houk, K.N. Activation Energies of Pericyclic Reactions: Performance of DFT, MP2, and CBS-QB3 Methods for the Prediction of Activation Barriers and Reaction Energetics of 1,3-Dipolar Cycloadditions, and Revised Activation Enthalpies for a Standard Set of Hydrocarbon Pericyclic Reactions. J. Phys. Chem. A 2005, 109, 9542–9553. [Google Scholar]
- Grimme, S.; Mück-Lichtenfeld, C.; Würthwein, E.-U.; Ehlers, A.W.; Goumans, T.P.M.; Lammertsma, K. Consistent Theoretical Description of 1,3-Dipolar Cycloaddition Reactions. J. Phys. Chem. A 2006, 110, 2583–2586. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J. Quantitative Dynamics of the N2O + C2H2 = Oxadiazole Reaction: A Model for 1,3-Dipolar Cycloadditions. ACS Omega 2020, 5, 23343–23350. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, R.; Xu, S.; Gong, X. Theoretical Study on the Gas-Phase Oxidation Mechanism of Ethylene by Nitrous Oxide. Propellants Explos. Propellants Explos. Pyrotech. 2022, 47, e202200082. [Google Scholar] [CrossRef]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Crowley, J.N.; Hampson, R.F.; Hynes, R.G.; Jenkin, M.E.; Rossi, M.J.; Troe, J. Evaluated kinetic and photochemical data for atmospheric chemistry: Volume II—Gas phase reactions of organic species. Atmos. Chem. Phys. 2006, 6, 3625–4055. [Google Scholar] [CrossRef] [Green Version]
- Maricq, M.M.; Szente, J.J. Kinetics of the Reaction between Ethylperoxy Radicals and Nitric Oxide. J. Phys. Chem. 1996, 100, 12374–12379. [Google Scholar] [CrossRef]
- Chow, J.M.; Miller, A.M.; Elrod, M.J. Kinetics of the C3H7O2 + NO Reaction: Temperature Dependence of the Overall Rate Constant and the i-C3H7ONO2 Branching Channel. J. Phys. Chem. A 2003, 107, 3040–3047. [Google Scholar] [CrossRef]
- Butkovskaya, N.I.; Kukui, A.; Bras, L.G.; Rayez, M.T.; Rayez, J.C. Pressure Dependence of Butyl Nitrate Formation in the Reaction of Butylperoxy Radicals with Nitrogen Oxide. J. Phys. Chem. A 2015, 119, 4408–4417. [Google Scholar] [CrossRef]
- Cassanelli, P.; Fox, D.J.; Cox, R.A. Temperature dependence of pentyl nitrate formation from the reaction of pentyl peroxy radicals with NO. Phys. Chem. Chem. Phys. 2007, 9, 4332–4337. [Google Scholar] [CrossRef]
- Atkinson, R.; Aschmann, S.M.; Winer, A.M. Alkyl nitrate formation from the reaction of a series of branched RO2 radicals with NO as a function of temperature and pressure. J. Atmos Chem. 1987, 5, 91–102. [Google Scholar] [CrossRef]
- Eberhard, J.; Howard, C.J. Rate Coefficients for the Reactions of Some C3 to C5 Hydrocarbon Peroxy Radicals with NO. J. Phys. Chem. A 1997, 101, 3360–3366. [Google Scholar] [CrossRef]
- Feng, B.; Sun, C.; Zhang, S. Atmospheric degradation mechanism of benzyl peroxy radical: A theoretical study. Atmos Env. 2019, 201, 18–27. [Google Scholar] [CrossRef]
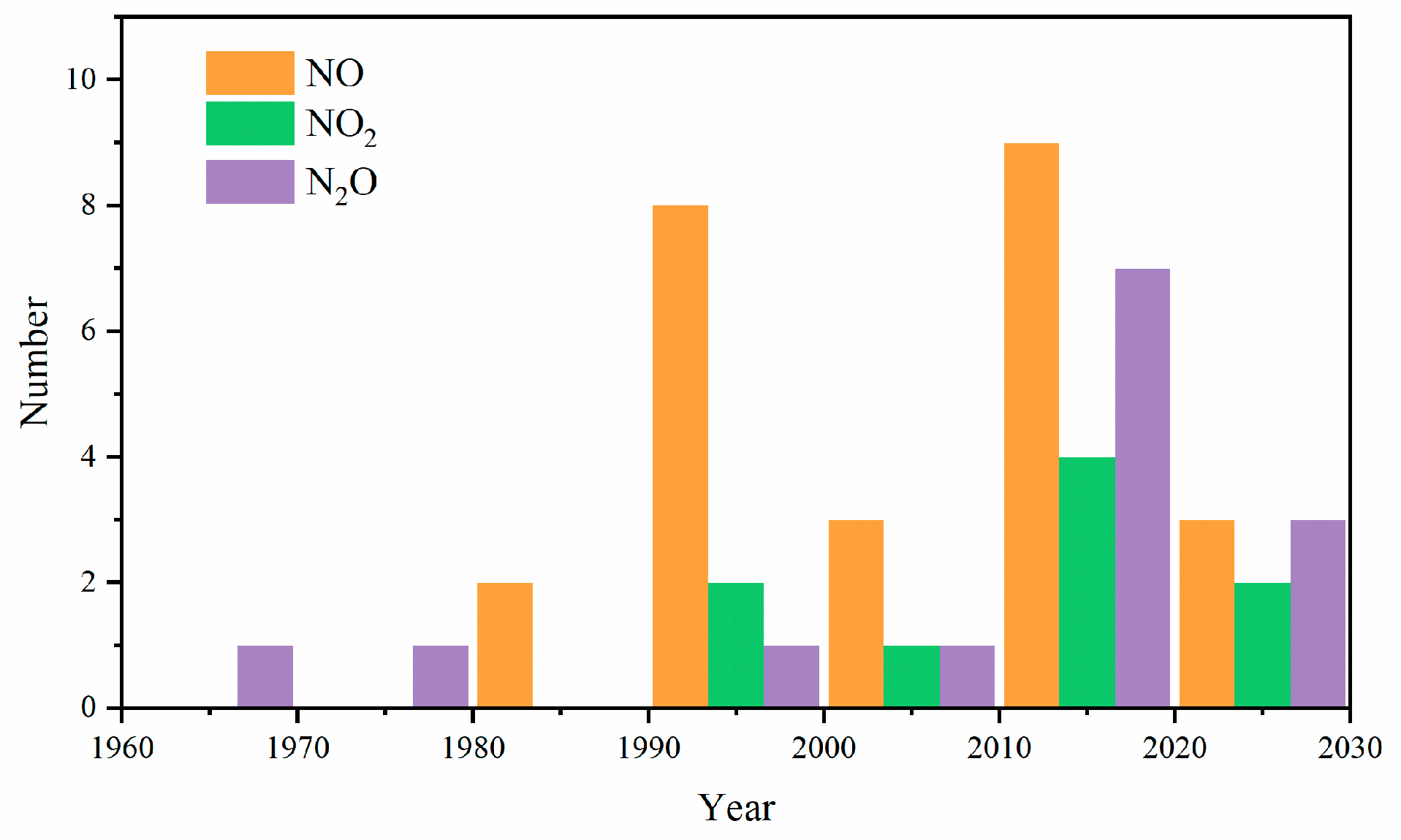

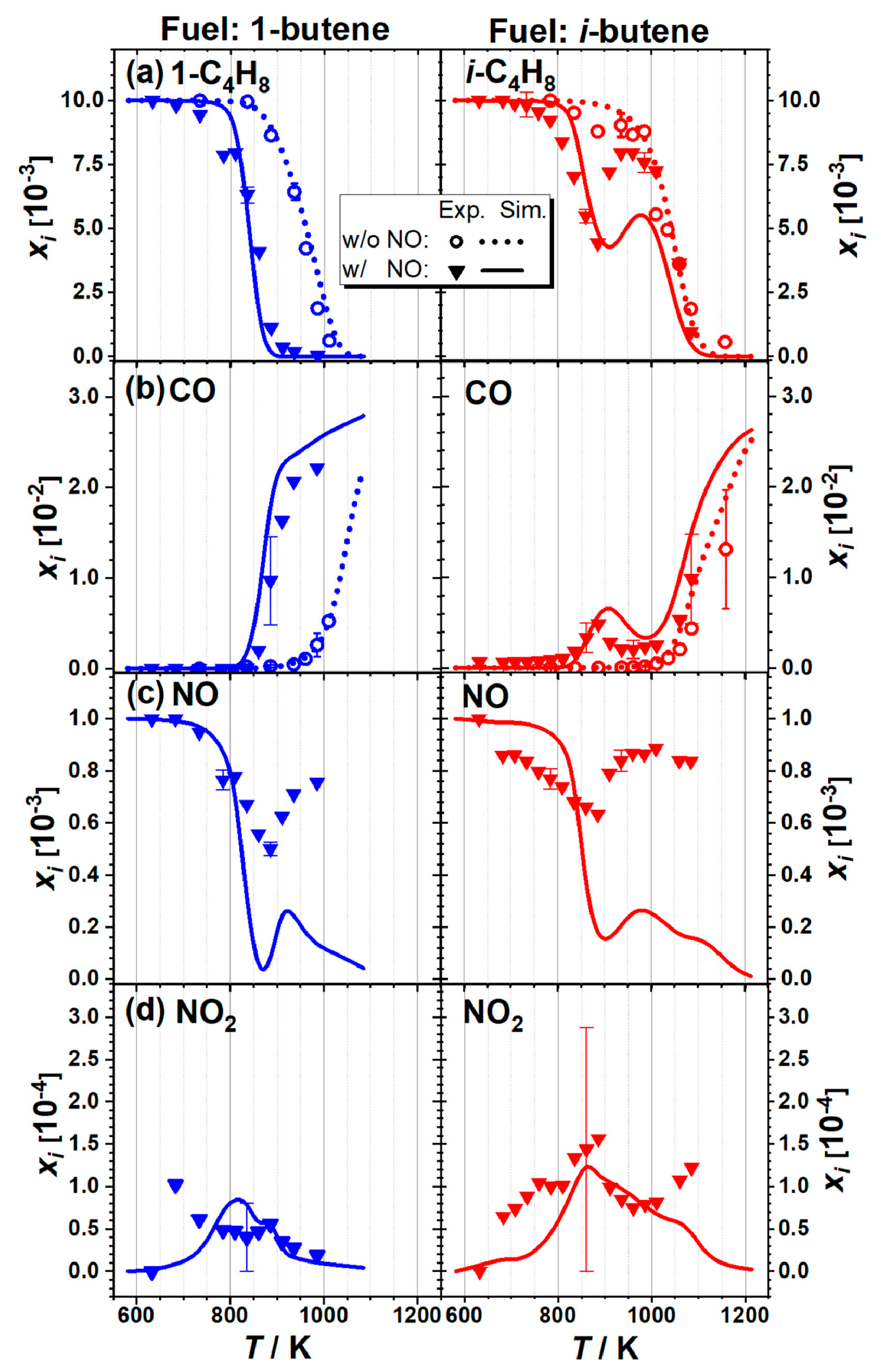
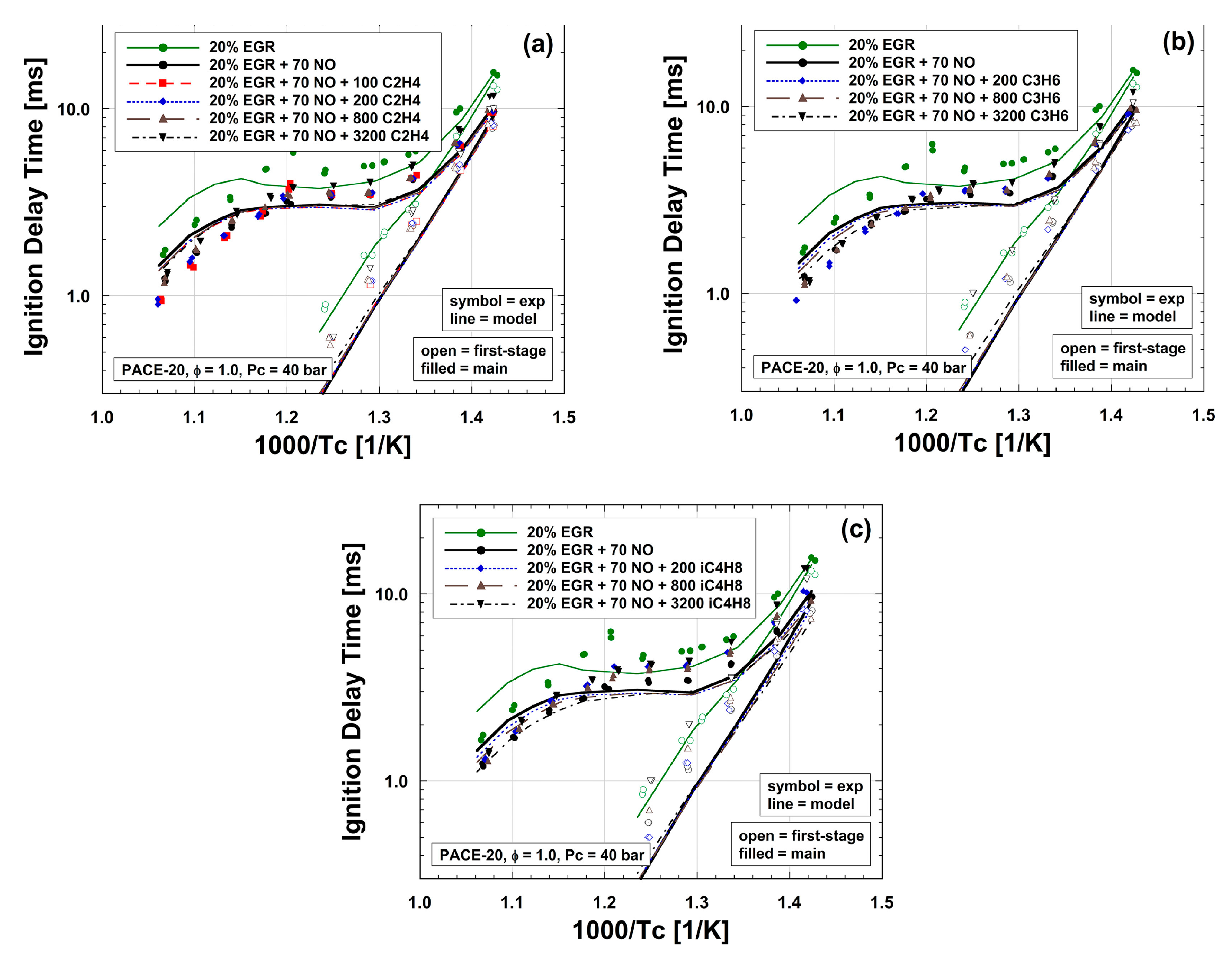



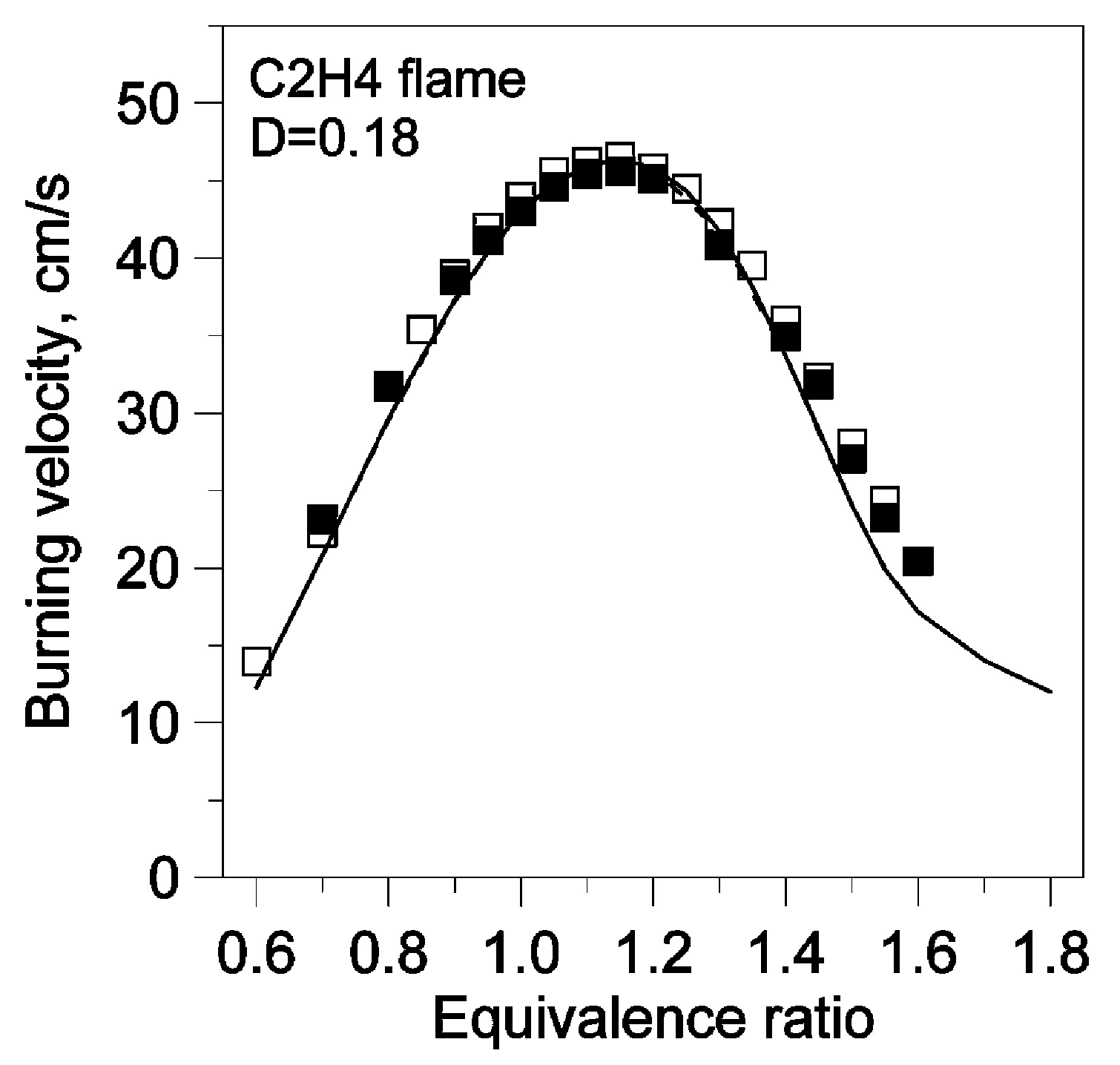
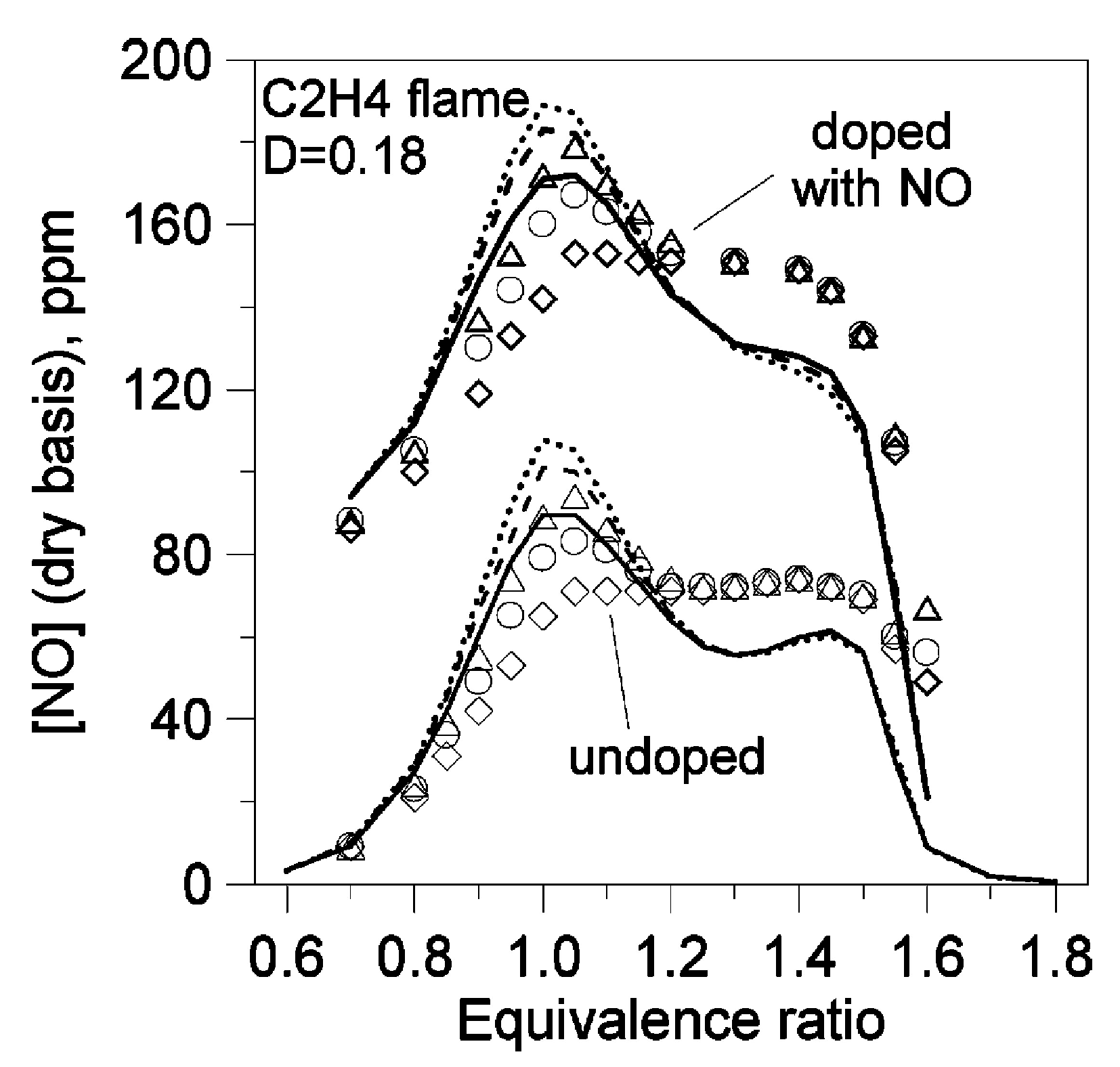

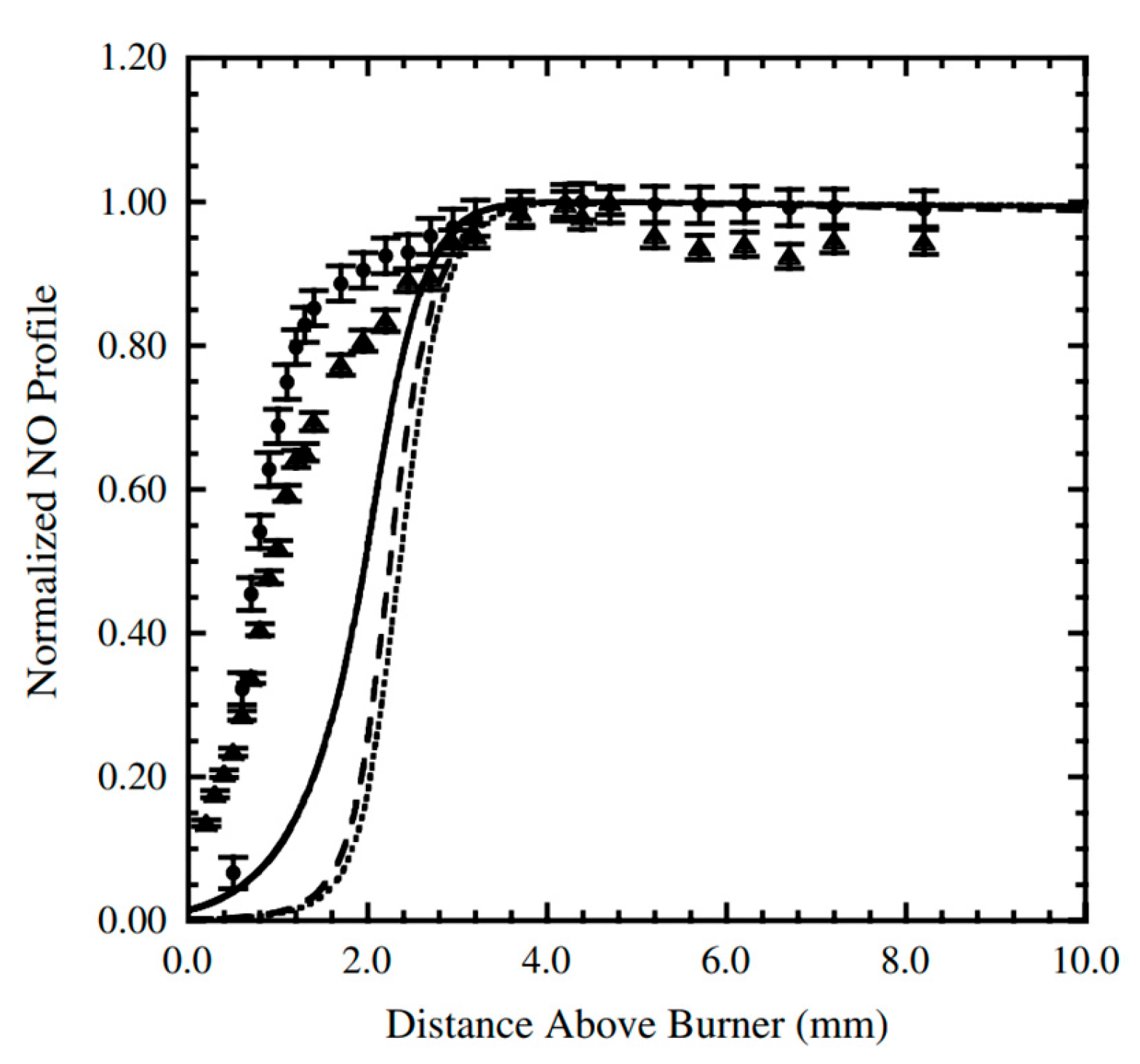
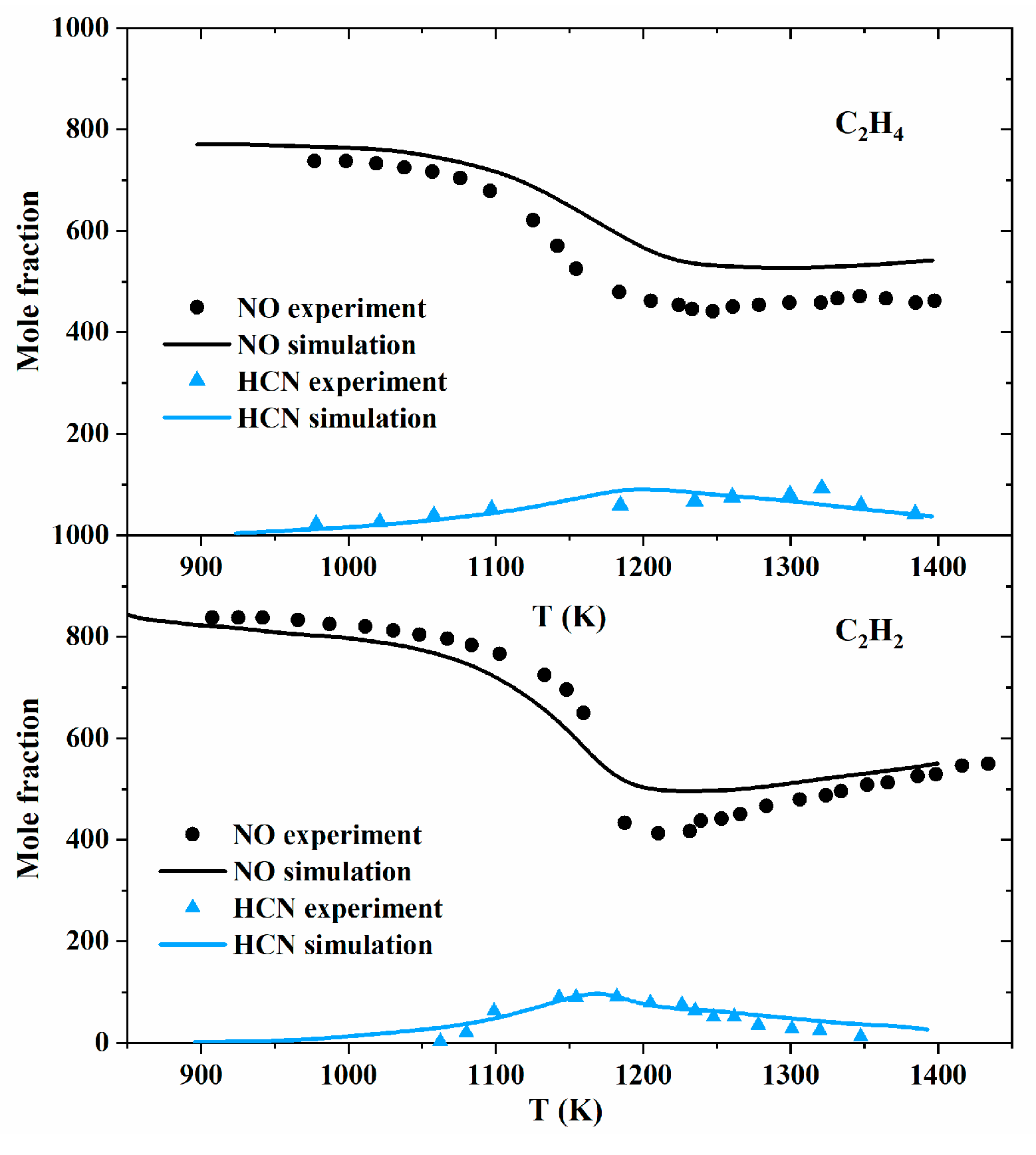
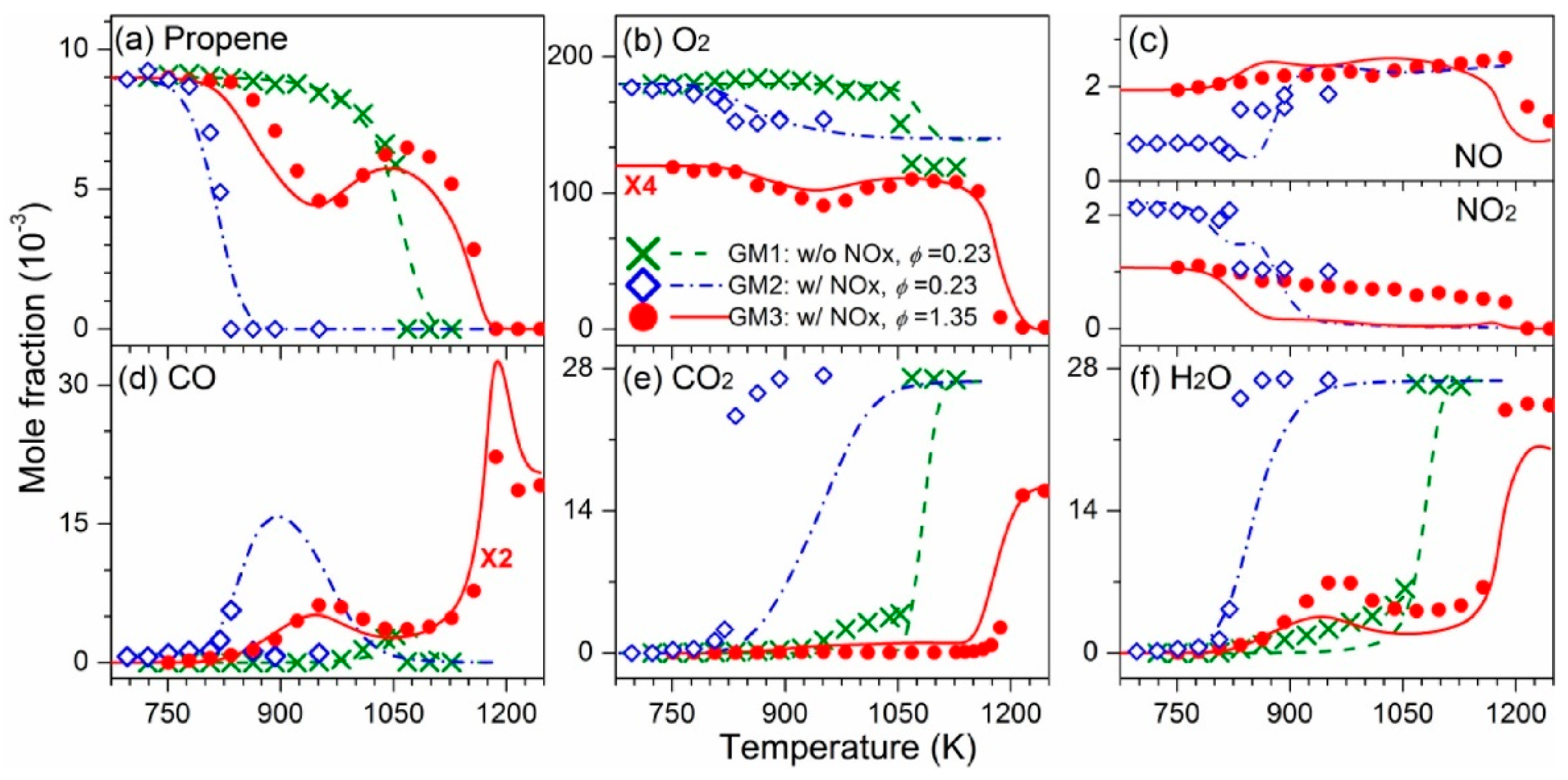
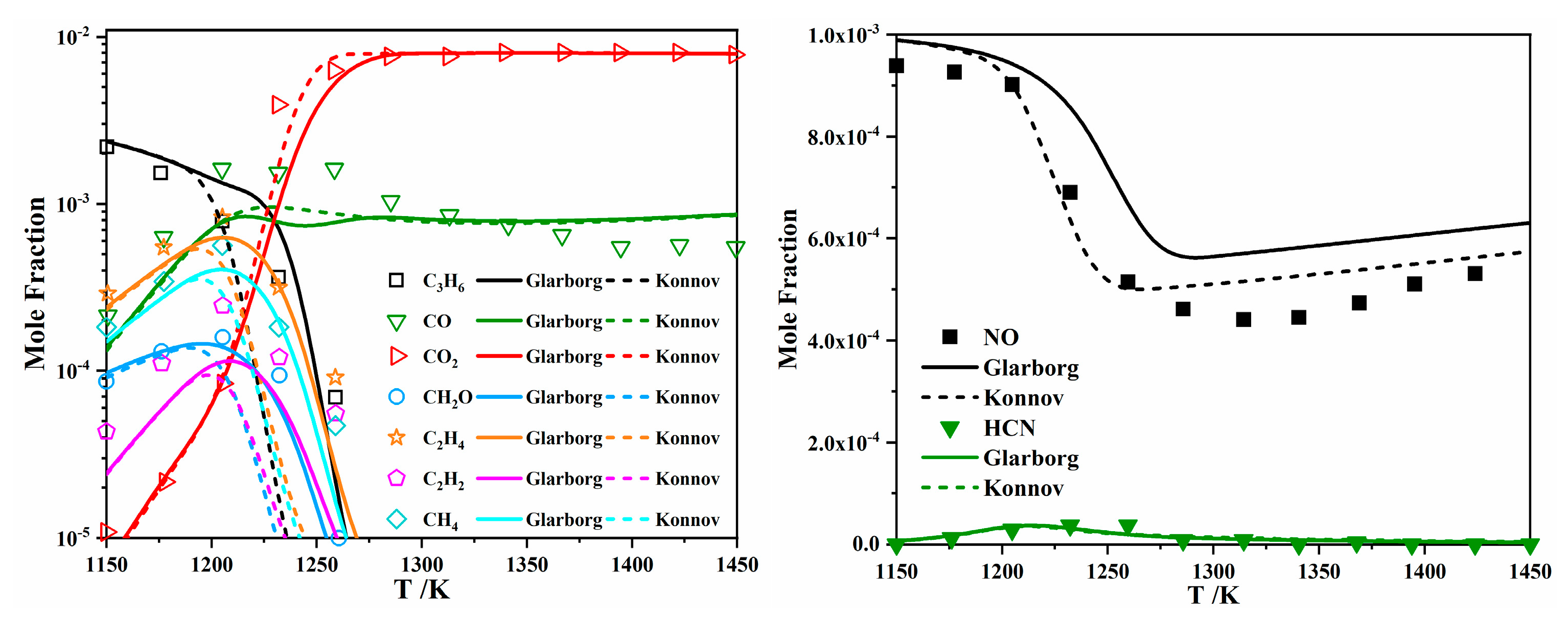
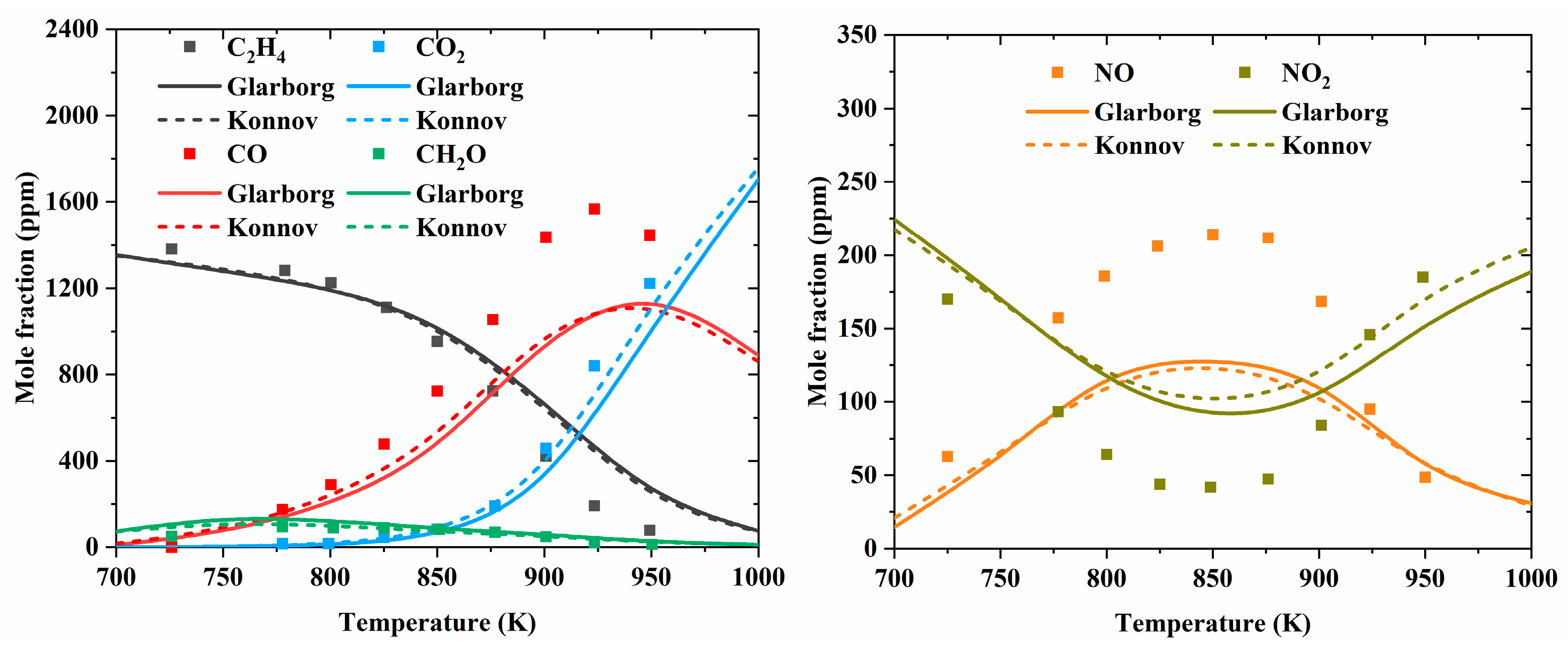

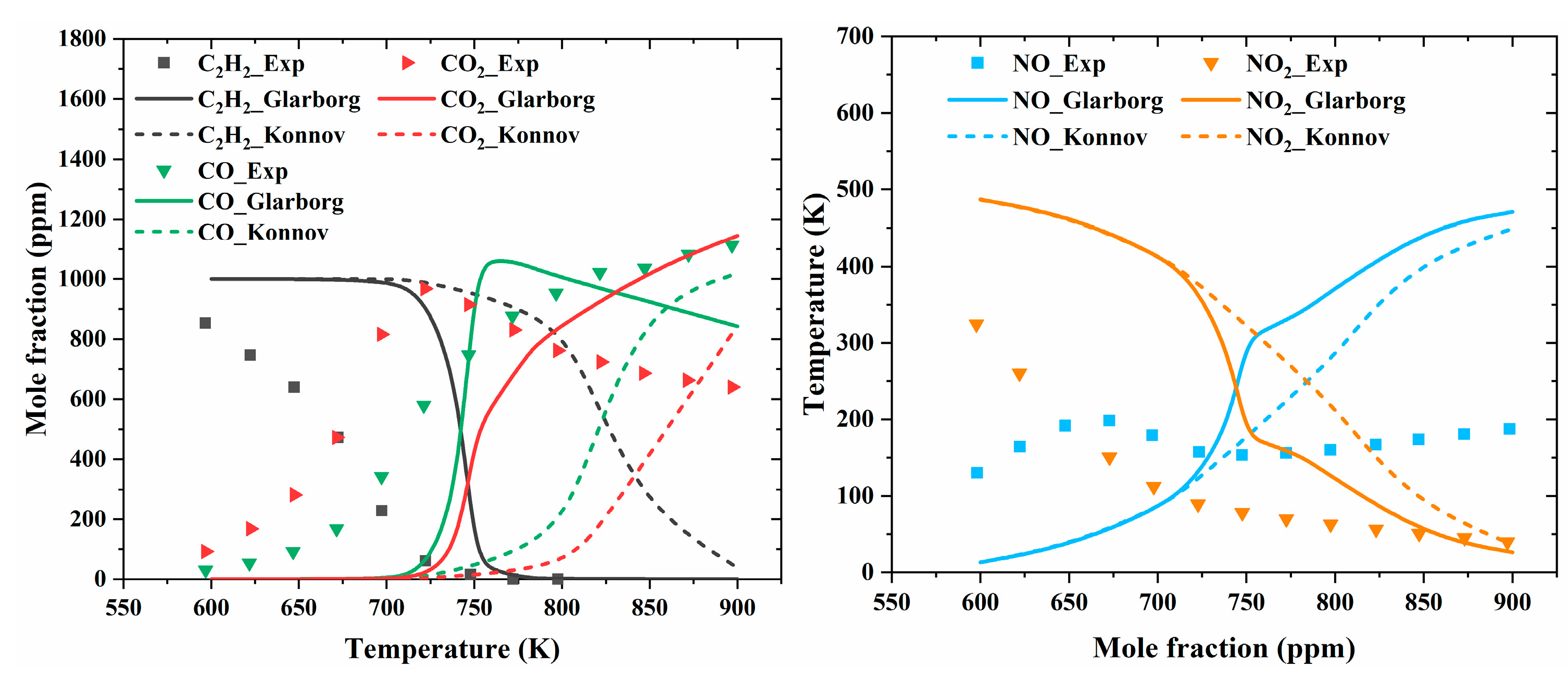
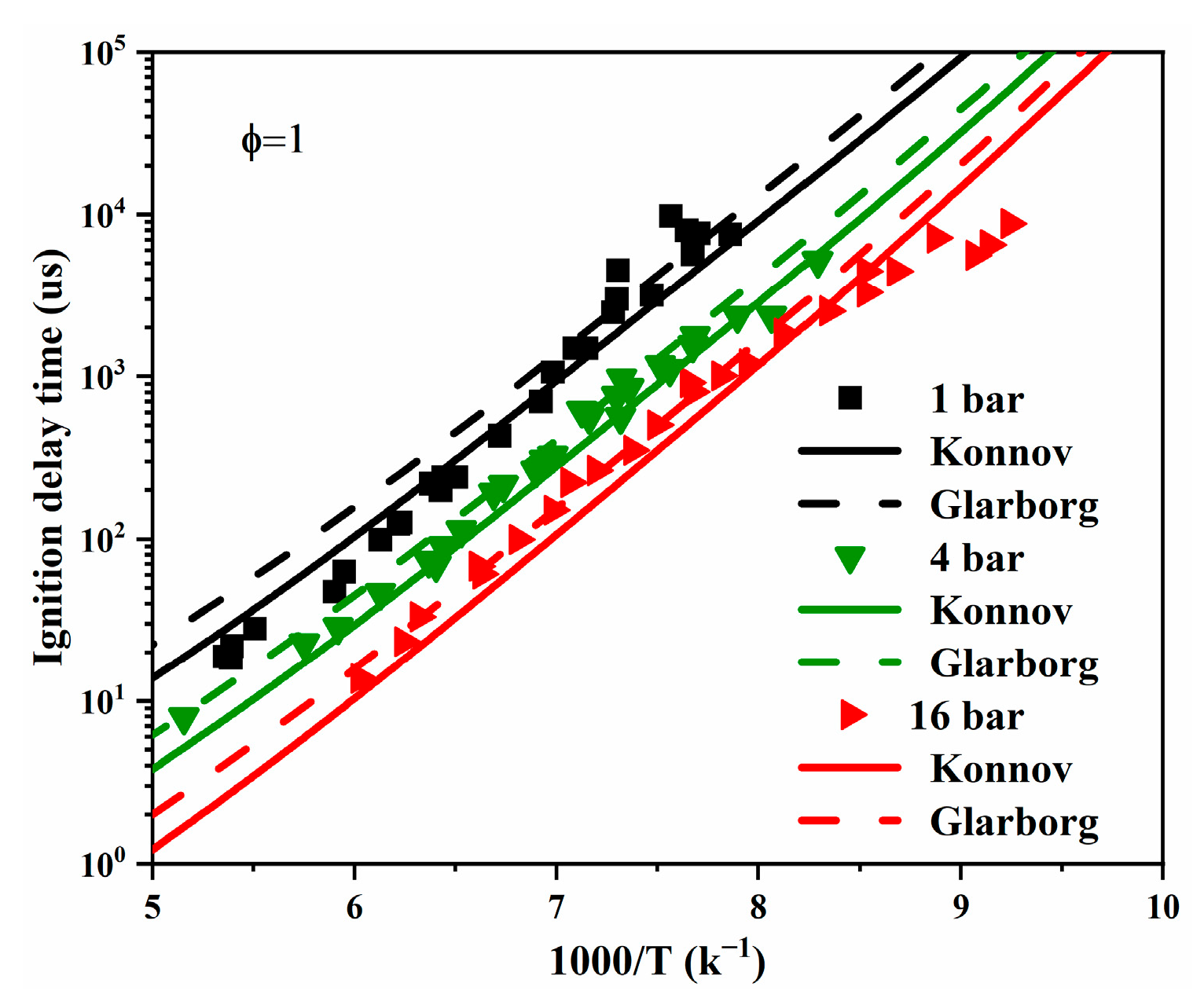
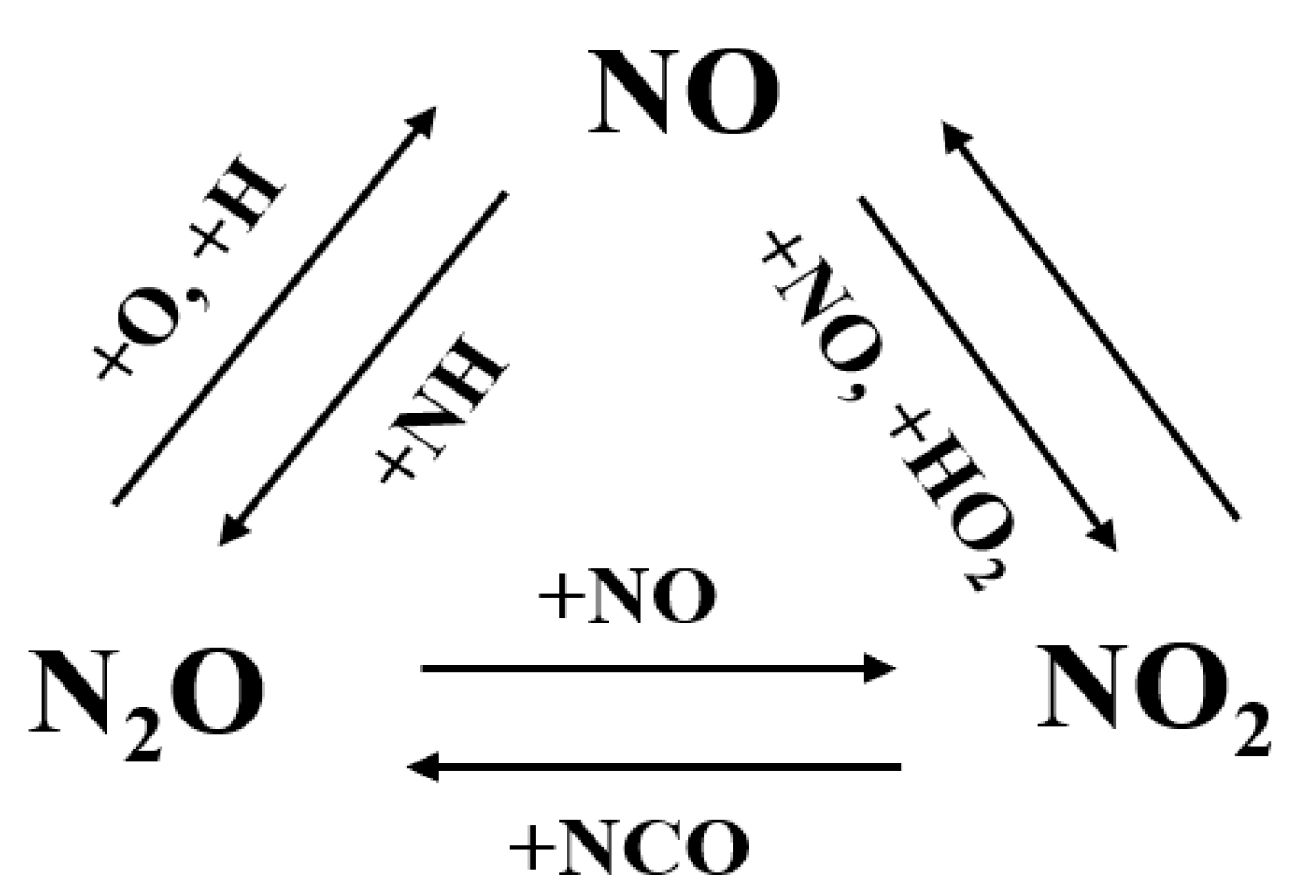
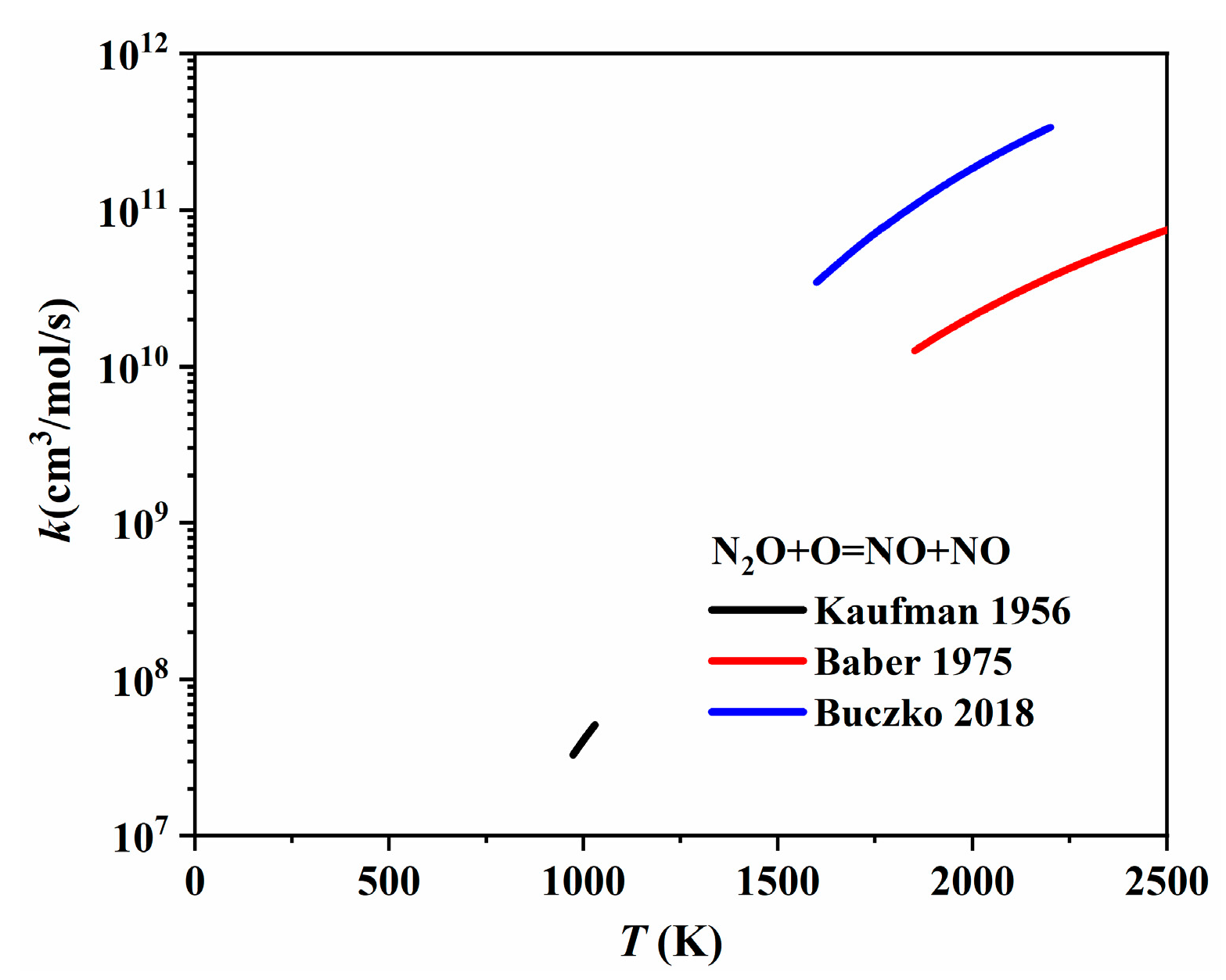
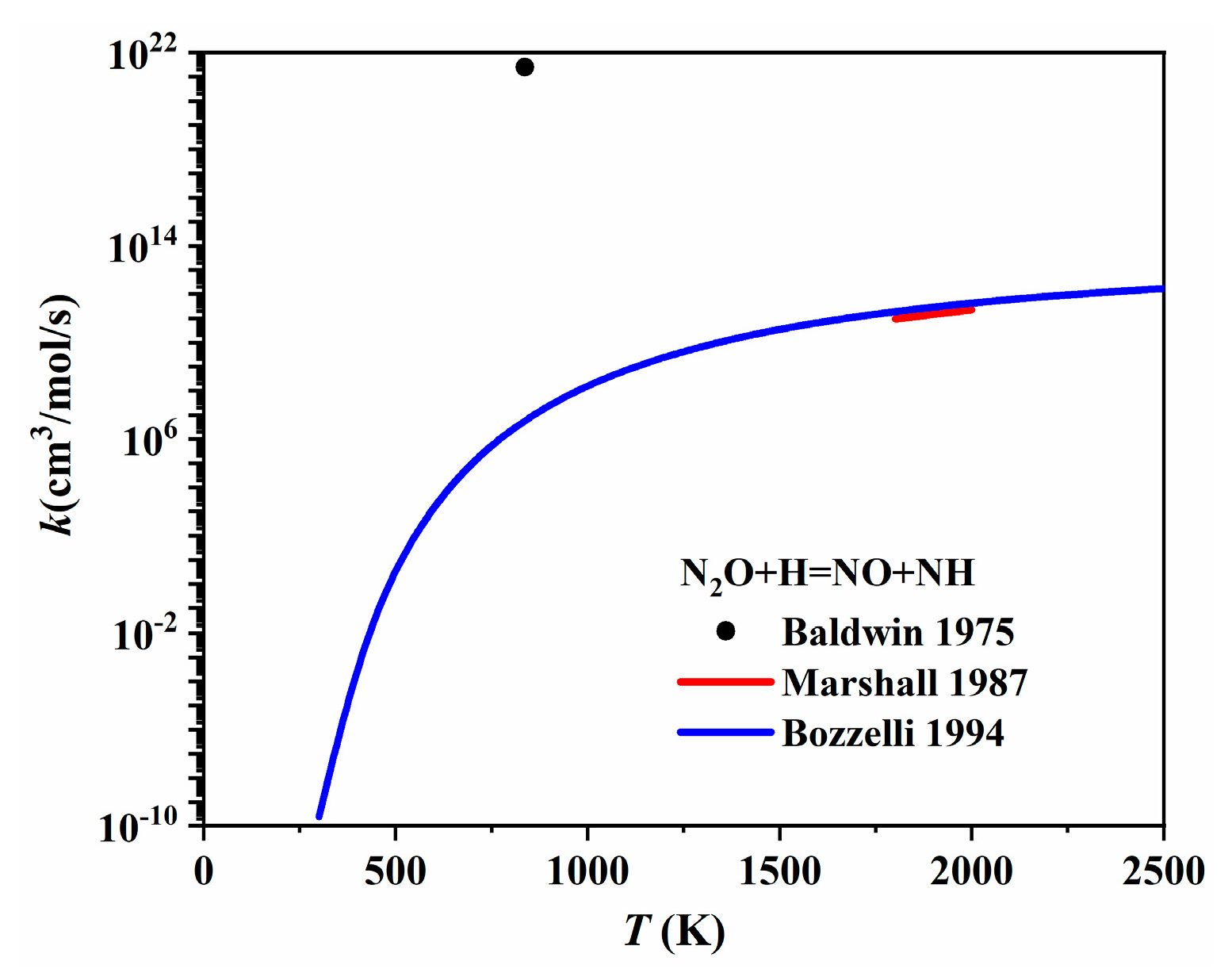
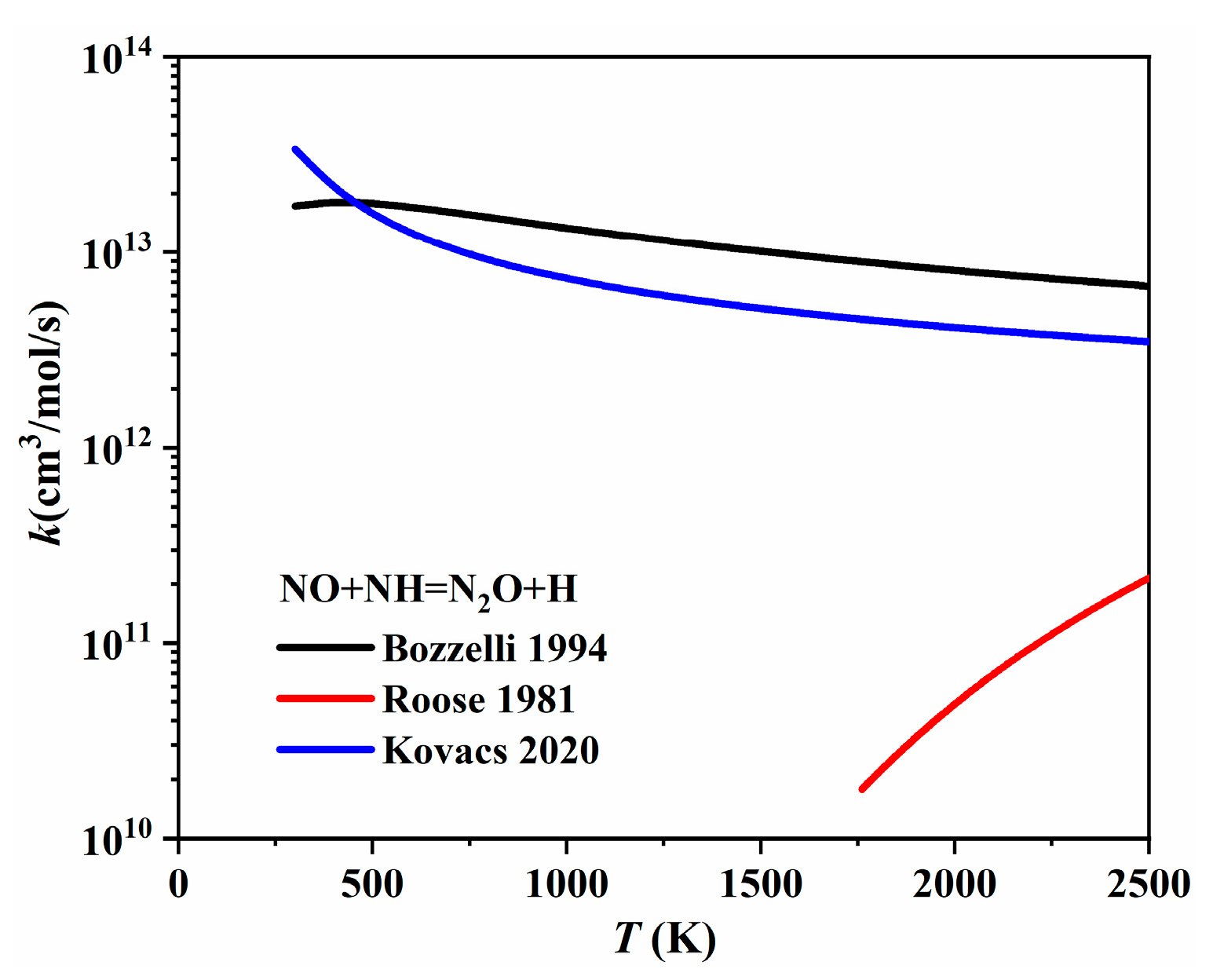
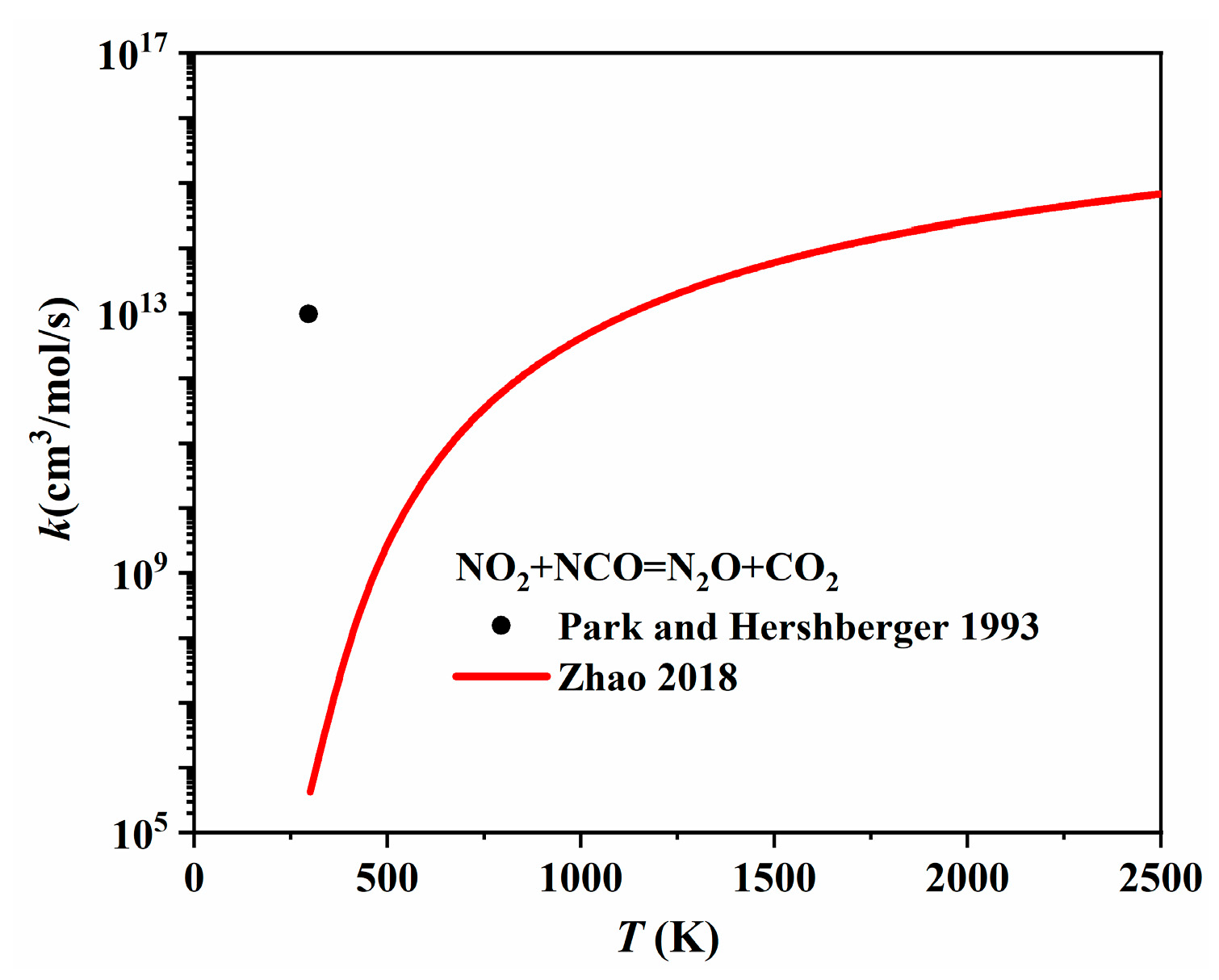
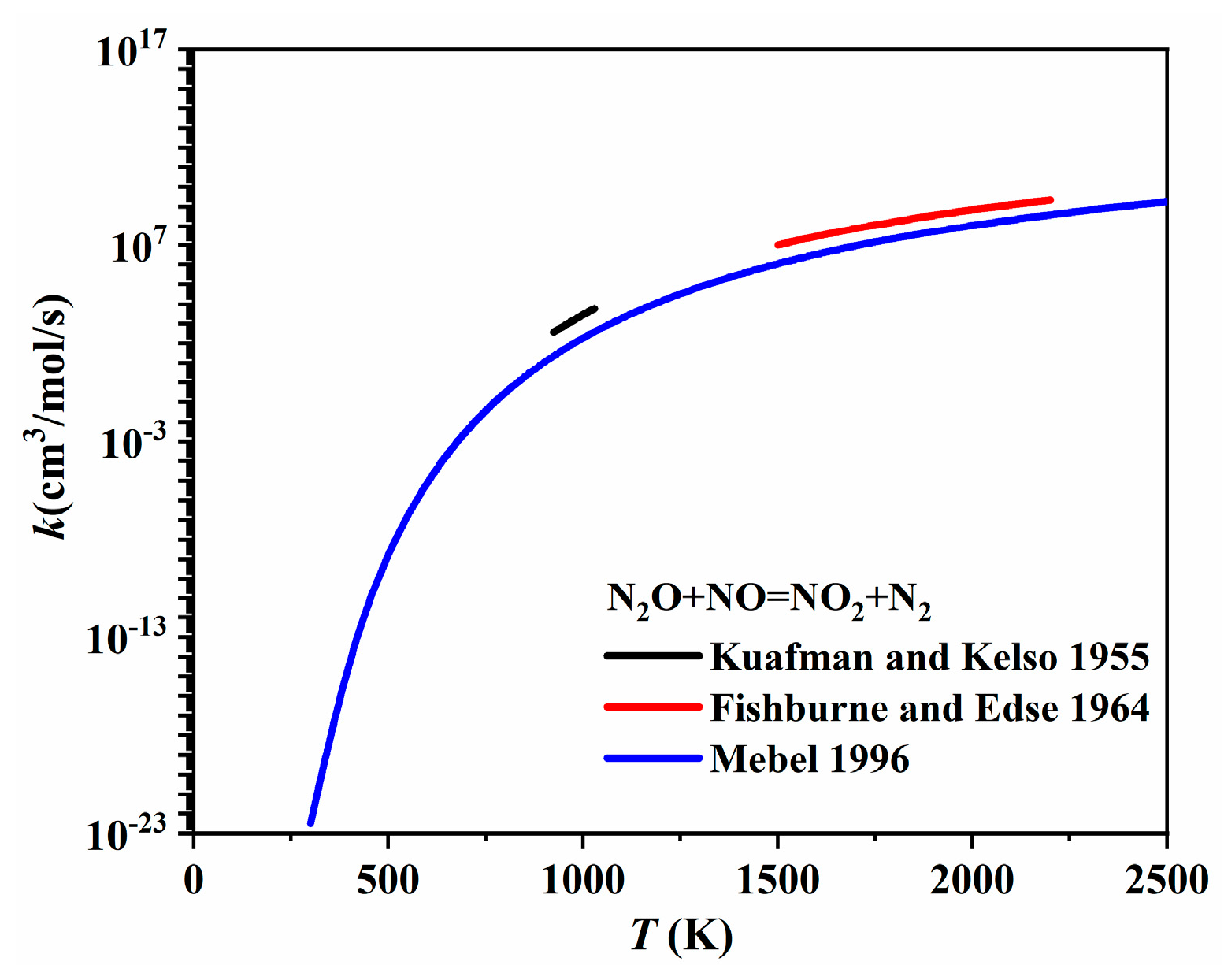
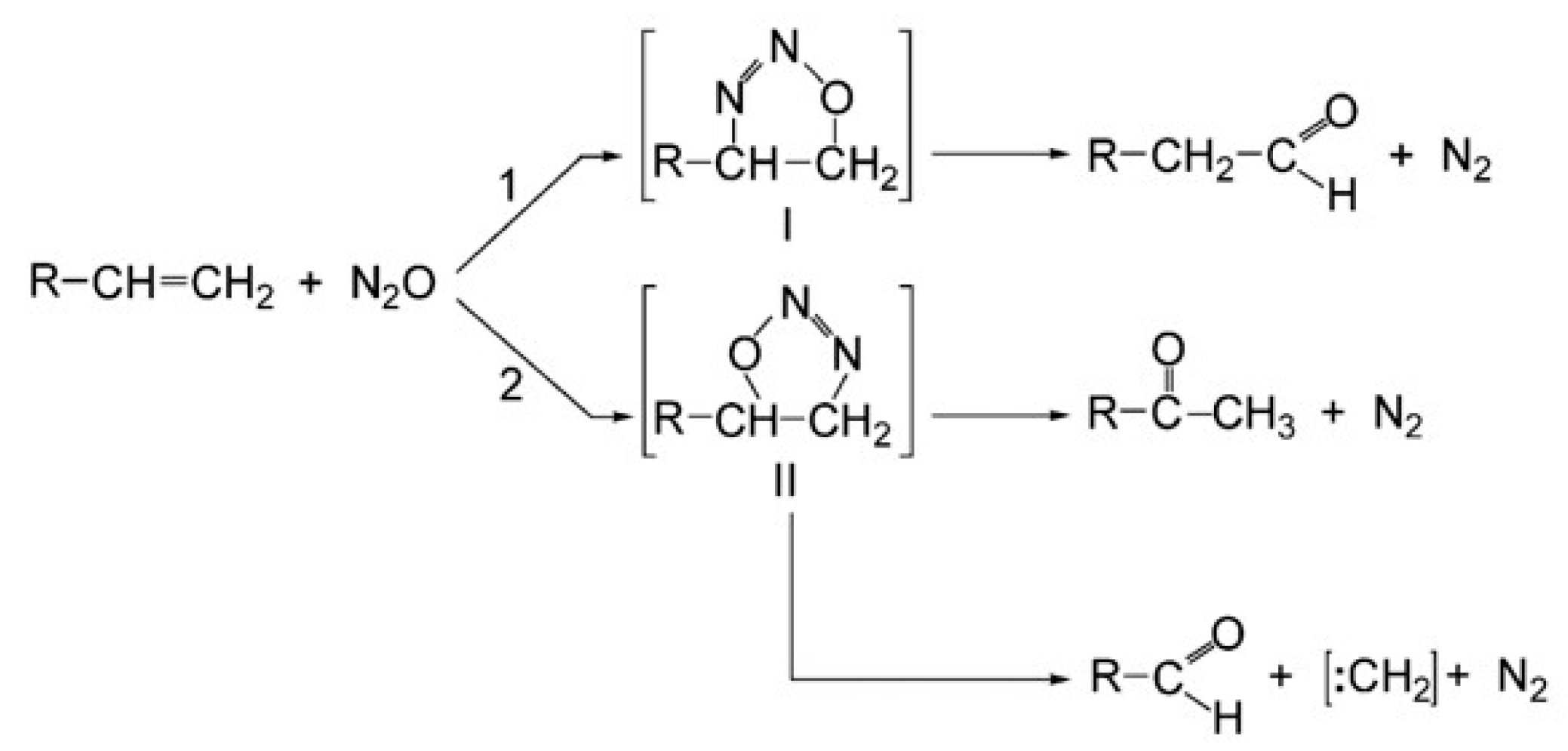
| Author | Mixture | Experimental Device | Properties | Conditions |
|---|---|---|---|---|
| Doughty et al. [26] | Ethylene (C2H4)/NO/O2/N2 | Flow reactor | Speciation | T = 650–1000 K P = 1 atm = 2–3.5 s NO: 0–200 ppm |
| Dagaut et al. [27] | Ethylene (C2H4)/NO /O2/N2 | Jet-stirred reactor | Speciation | T = 900–1400 K P = 1 atm ϕ = 0.75–2 = 0.12–0.16 s NO: 1000 ppm |
| Dagaut et al. [28] | Ethylene (C2H4) /Ethane (C2H6)/NO/O2/N2 | Jet-stirred reactor | Speciation | T = 700–1150 K P = 1 atm = 0.12–0.24 s NO: 0–1200 ppm |
| Gimenez-Lopez et al. [29] | Ethylene (C2H4)/NO/O2/N2 | Flow reactor | Speciation | T = 600–900 K P = 60 bar (s) = 8892/T NO: 500 ppm |
| Atakan and Hartlieb [30] | Propene (C3H6)/NO/O2/Ar | Burner | Speciation | T = 313 K ϕ = 1.5, 1.8, 2.3 NO: 0.2–1% |
| Dagaut et al. [31] | Propene (C3H6)/NO/O2/N2 | Jet-stirred reactor | Speciation | T = 1100–1450 K P = 1 atm ϕ = 0.75–2 = 0.12–0.16 s NO: 750–1000 ppm |
| Yuan et al. [32] | Propene (C3H6)/NO/NO2/O2 | Flow reactor | Speciation | T = 725–1250 K P = 1 bar ϕ = 0.23, 1.35 = 0.3–0.5 s NOx: 0.3% |
| Gossler et al. [33] | i-Butene (i-C4H8)/ 1-butene (1-C4H8)/ NO/O2/Ar | Flow reactor | Speciation | T = 600–1200 K P = 1 atm ϕ = 2 = 0.6–1.4 s NO: 1000 ppm |
| Prabhu et al. [34] | 1-Pentene (1-C5H10)/NO/O2/N2 | Flow reactor | Speciation | T = 600–800 K P = 6 atm ϕ = 0.4 = 30–220 ms NO: 0–500 ppm |
| Quang and Vanpee [35] | Acetylene (C2H2)/NO | Circular flat burner | Speciation | T = 297 K P = 80 torr NO: 73.1%, 83.3% |
| Quang and Vanpee [36] | Acetylene (C2H2)/NO | Circular flat burner | Speciation | T = 2800–3100 K P = 80 torr NO: 73.1%, 83.3% |
| Dagaut et al. [37] | Acetylene (C2H2)/NO/O2/N2 | Jet-stirred reactor | Speciation | T = 1050–1300 K P = 1 atm ϕ = 0.75–2 = 0.12–0.16 s NO: 1000 ppm |
| Guarneri et al. [38] | Furan (C4H4O)/NO/O2/Ar | Shock tube | Speciation | T = 1210–1950 K P = 12.4–15.3 atm ϕ = 2.4–5.0 = 570–1140 μs NO: 300–380 ppm |
| Alexandrino et al. [39] | 2,5-Dimethylfuran (2,5-DMF)/NO/O2/N2 | Flow reactor | Speciation | T = 800–1400 K P = 1 atm ϕ = 0.03–3.3 (s) = 195/T NO: 900 ppm |
| Alexandrino et al. [40] | 2-methylfuran (2-MF)/NO/O2/N2 | Flow reactor | Speciation | T = 800–1400 K P = 1 atm ϕ = 0.02–3.3 (s) = 195/T NO: 900 ppm |
| Alzueta et al. [41] | Acetone (CH3COCH3)/NO/O2/H2O/N2 | Flow reactor | Speciation | T = 700–1500 K P = 1 atm ϕ = 0.14–2.9 (s) = 400/T NO: 290 ppm, 300 ppm |
| Williams and Pasternack [42] | Methane (CH4)/acetylene (C2H2)/ethylene (C2H4)/ethane (C2H6)/NO/O2/N2 | McKenna flat flame burner | Speciation | T (peak) = 1800 K P = 10 torr ϕ = 1 NO: 1% |
| Garborg et al. [43] | Acetylene (C2H2)/ethylene (C2H4)/NO/O2/N2 | Flow reactor | Speciation | T = 800–1500 K P = 1.05 atm ϕ = 1.3 (s) = 162/T NO: 835 ppm ϕ = 1.5 (s) = 165/T NO: 790 ppm |
| Konnov et al. [44] | Ethylene (C2H4)/ethane (C2H6)/propane (C3H8)/NO/O2/N2 | Burner | LBV, speciation | T = 298 K P = 1 atm ϕ = 0.6–1.8 NO: 100 ppm |
| Hori et al. [45] | Methane (CH4)/ethylene (C2H4)/ethane (C2H6)/ propene (C3H6)/propane (C3H8)/NO/O2/N2 | Flow reactor | Speciation | T = 600–1100 K P = 1 atm = 0.16–1.46 s NO: 20 ppm |
| Abian et al. [46] | Acetylene (C2H2)/ethanol (C2H5OH)/NO/O2/N2/H2O | Flow reactor | Speciation | T = 775–1375 K P = 1 atm ϕ = 0.5–5 (s) = 195/T NO: 100, 500, 1200 ppm |
| Marrodan et al. [47] | Dimethyl ether (DME)/ acetylene (C2H2)/NO/O2/N2/H2O | Flow reactor | Speciation | T = 575–1475 K P = 1 atm ϕ = 0.05–5 (s) = 195/T NO: 500 ppm |
| Cheng et al. [48] | Surrogate/EGR/ethylene (C2H4)/propene (C3H6)/ i-butene (i-C4H8)/NO/O2/N2 | Rapid compression machine | IDT | T = 680–950 K P = 20, 40 bar ϕ = 1 NO: 70 ppm |
| Author | Mixture | Experimental Device | Properties | Conditions |
|---|---|---|---|---|
| Abian et al. [50] | Ethylene (C2H4)/NO/NO2/N2O/N2 | Flow reactor | Speciation | T = 975–1475 K P = 1 atm (s) = 4550/T NO: 590–12,200 ppm NO2: 500–11,800 ppm N2O: 480–11,900 ppm |
| Menon et al. [51] | Ethylene (C2H4)/NO2/air/N2 | Burner | Speciation, soot volume fraction | P = 1 atm ϕ = 2.34, 2.64 NO2: 5% by volume |
| Deng et al. [52] | Ethylene (C2H4)/NO2/O2/Ar | Shock tube | IDT | T = 920–1780 K P = 1.2–10 atm ϕ = 0.5–2.0 NO2: 0.5% |
| Volponi and Branch [53] | Acetylene (C2H2)/NO2/O2/Ar | Flat flame burner | Speciation | P = 25 torr |
| Marshall et al. [54] | Acetylene (C2H2)/O2/NO2/NO | Flow reactor | Speciation | T = 600–900 K P = 50–60 bar ϕ = 0.05, 1.0, 5.6 NO/NO2: 500 ppm |
| Jin et al. [55] | Ethanol (C2H5OH)/NO2/O2/Ar | Shock tube | IDT | T = 1050–1650 K P = 0.2 MPa ϕ = 0.5–1.5 NO2: 0.2%, 1% |
| Ye et al. [56] | Dimethyl ether (DME)/NO2/O2/Ar | Shock tube | IDT | T = 987–1517 K P = 4, 10 atm ϕ = 0.5, 1.0, 2.0 NO2: 0.201%, 1.722% |
| Alzueta et al. [57] | Dimethyl ether (DME)/NO2/O2/H2O/N2 | Flow reactor | Speciation | T = 600–1500 K P = 1 atm ϕ = 0.013–2.04 (s) ≈ 188/T NO: 493 ppm, 502 ppm, 508 ppm NO2: 43 ppm, 44 ppm, 72 ppm |
| Author | Mixture | Experimental Device | Properties | Conditions |
|---|---|---|---|---|
| Trenwith [63] | Ethylene (C2H4)/N2O | Cylindrical silica vessel | Speciation | T = 828–875 K P = 20–100 mm |
| Howard et al. [64] | Ethylene (C2H4)/N2O/Ar | McKenna flat flame burner | Speciation | P = 20 torr |
| Werling et al. [65] | Ethylene (C2H4)/N2O | Ignition chamber | Flame propagation, flashback behavior | T = 293 K P = 1–1.75 bar |
| Naumann et al. [66] | Ethylene (C2H4)/N2O/N2 | Shock tube | IDT | T = 1050–2000 K P = 1, 4, 16 bar N2O: 17.14% ϕ = 1.0 |
| Ethylene (C2H4)/N2O/N2 | Burner | LBV | T = 473 K P = 1–10 bar ϕ = 1.0 N2O: 42.86% | |
| Kick et al. [67] | Ethylene (C2H4)/N2O/N2/Ar/CO | Shock tube | IDT | T = 1050–2000 K P = 1, 4, 16 bar ϕ = 1.0 N2O: 17.14%, 42.86% |
| Ethylene (C2H4)/N2O/N2 | Burner | LBV | T = 473 K P = 1, 3, 6 bar ϕ = 1.0 N2O: 42.86% | |
| Deng et al. [68] | Ethylene (C2H4)/N2O/N2/Ar | Shock tube | IDT | T = 1090–1760 K P = 1.2–10 atm ϕ = 0.5–2.0 N2O: 3–12% |
| Zhang et al. [69] | Ethylene (C2H4)/N2O/Ar | Rapid compression machine | IDT | T = 885–940 K P = 2.5–4.3 MPa ϕ = 1.05–1.35 N2O: 40.8%, 42.5% |
| Yang et al. [70] | Ethylene (C2H4)/N2O/Ar | Rapid compression machine | IDT | T = 1170–1290 K P = 15–45 bar ϕ = 0.5–2 N2O: 1.5%, 3% |
| Wang and Zhang [71] | Ethylene (C2H4)/N2O/Ar | Steel cube chamber | LBV | T = 280 K P = 0.5–2.0 atm ϕ = 0.2–2.4 |
| Aldous et al. [72] | Acetylene (C2H2)/N2O | Bunsen burner | LBV | T = 400/500/600 K P = 1 atm ϕ = 0.35–2.0 |
| Alekseev et al. [73] | Acetylene (C2H2)/N2O/Ar | Shock tube | Oxygen atom profiles | T = 1688–3179 K P = 1.8–3.0 bar N2O: 10 ppm |
| Powell et al. [74] | Hydrogen (H2)/methane (CH4)/acetylene (C2H2)/propane (C3H8)/ N2O/N2 | McKenna flat flame burner | LBV | T = 295 K P = 0.8 atm ϕ = 0.56–1.6 |
| Powell and Papas [75] | methane (CH4)/acetylene (C2H2)/propane (C3H8)/ N2O/N2 | McKenna flat flame burner | NO concentration profiles | P = 0.81 atm ϕ = 1.0 N2O: 31.34% (CH4) N2O: 24.71% (C2H2) N2O: 32.88% (C3H8) |
| Mevel and Shepherd [76] | Methane (CH4)/ethane (C2H6)/ethylene (C2H4)/ acetylene (C2H2)/O2/N2O/Ar | Shock tube | IDT | T = 1269–1945 K P = 222–397 kPa ϕ = 0.78–1.80 N2O: 1.133–3.598% |
| NO2 + NO2 = NO + NO + O2 | |||
|---|---|---|---|
| A (cm3/mole·s) | n | Ea (kcal/mol) | |
| Glarborg et al. [24] | 4.50 × 1012 | 0.000 | 27.599 |
| Konnov et al. [23] | 3.95 × 1012 | 0.000 | 27.590 |
| CRECK [112] | 1.63 × 1012 | 0.000 | 26.030 |
| Baulch et al. [114] | 2.00 × 1012 | 0.000 | 26.825 |
| Author | Species | Reaction | A (cm3/mole·s) | n | Ea (kcal/mol) |
|---|---|---|---|---|---|
| Gao et al. [124] | C2H4 | C2H4 + NO2 = CH3CHO + NO | 4.34 × 1056 | −14.2 | 51.92 |
| C2H4 + NO2 = Oxirane + NO | 6.62 × 1045 | −11.3 | 37.81 | ||
| C2H4 + NO2 = C2H3 + HONO | 2.13 × 101 | 3.9 | 30.19 | ||
| C2H4 + NO2 = C2H3 + HNO2 | 4.31 × 100 | 3.9 | 30.19 | ||
| Chai and Goldsmith [125] | C3H6, 1-C4H8, trans-2-C4H8, iso-C4H8 | C3H6 + NO2 = C3H5 + cis-HONO | 4.47 × 10−4 | 4.79 | 18.16 |
| C3H6 + NO2 = C3H5 + trans-HONO | 2.63 × 10−6 | 5.32 | 25.18 | ||
| C3H6 + NO2 = C3H5 + HNO2 | 2.54 × 10−2 | 4.21 | 18.76 | ||
| 1-C4H8 + NO2 = 1-C4H7 + cis-HONO | 3.11 × 10−3 | 4.4 | 15.75 | ||
| 1-C4H8 + NO2 = 1-C4H7 + trans-HONO | 1.35 × 10−3 | 4.47 | 24.46 | ||
| 1-C4H8 + NO2 = 1-C4H7 + HNO2 | 7.83 × 10−2 | 3.93 | 15.25 | ||
| Trans-2-C4H8 + NO2 = trans-2-C4H7 + cis-HONO | 1.31 × 10−3 | 4.62 | 17.36 | ||
| Trans-2-C4H8 + NO2 = trans-2-C4H7 + trans-HONO | 1.86 × 10−6 | 5.29 | 22.58 | ||
| Trans-2-C4H8 + NO2 = trans-2-C4H7 + HNO2 | 4.89 × 10−2 | 4.06 | 17.66 | ||
| Iso-2-C4H8 + NO2 = iso-2-C4H7 + cis-HONO | 1.67× 10−3 | 4.56 | 16.46 | ||
| Iso-2-C4H8 + NO2 = iso-2-C4H7 + trans-HONO | 8.91 × 10−6 | 5.18 | 22.98 | ||
| Iso-2-C4H8 + NO2 = iso-2-C4H7+ HNO2 | 1.51 × 10−1 | 3.95 | 17.76 | ||
| Thomas [126] | C2H2 | C2H2 + NO2 = products + NO | 1.26 × 1012 | 15.05 | |
| Marshall et al. [54] | C2H2 | C2H2 + NO2 = CHCHO + NO | 1.42 × 103 | 2.79 | 16.76 |
| Sprung et al. [127] | C2H2, C2H4, C3H6, 1-C4H8, i-C4H8, cis-2-C4H8, trans-2- C4H8, 1-C5H10, cis-2-C5H10, trans-2-C5H10 | C2H2 + NO2 = adduct | 5.83 × 1012 | - | 14.40 |
| C2H4 + NO2 = adduct | 9.33 × 1012 | - | 14.00 | ||
| C3H6 + NO2 = adduct | 2.48 × 1012 | - | 27.10 | ||
| 1-C4H8 + NO2 = adduct | 1.82 × 1012 | - | 25.00 | ||
| I-C4H8 + NO2 = adduct | 4.37 × 1012 | - | 28.70 | ||
| Cis-2-C4H8 + NO2 = adduct | 4.41 × 1011 | - | 11.40 | ||
| Trans-2-C4H8 + NO2 = adduct | 7.47 × 1011 | - | 11.60 | ||
| 1-C5H10 + NO2 = adduct | 1.70 × 1012 | - | 24.70 | ||
| Cis-2-C5H10 + NO2 = adduct | 4.81 × 1011 | - | 23.20 | ||
| Trans-2-C5H10 + NO2 = adduct | 9.70 × 1011 | - | 23.40 |
| Author | Species | Reaction | A (cm3/mole·s) | n | Ea (kcal/mole) |
|---|---|---|---|---|---|
| Trenwith [64] | C2H4 | C2H4 + N2O = C2H4O + N2 | 7.95 × 109 | - | 38.13 |
| Karami and Vahedpour [134] * | C2H2 | C2H2 + N2O = CH2CO + N2 | - | - | - |
| Ess and Houk [135] * | C2H2 | C2H2 + N2O = (CH)2ON2 | - | - | - |
| C2H4 | C2H4 + N2O = (CH2)2ON2 | - | - | - | |
| Grimme et al. [136] * | C2H2 | C2H2 + N2O = (CH)2ON2 | - | - | - |
| C2H4 | C2H4 + N2O = (CH2)2ON2 | - | - | - | |
| Liu and Li [137] * | C2H2 | C2H2 + N2O = (CH)2ON2 | - | - | - |
| Li et al. [138] * | C2H4 | C2H4 + N2O = (CH2)2ON2 | - | - | - |
| Author | Species | Reaction | A (cm3/mole·s) | n | Ea (kcal/mole) |
|---|---|---|---|---|---|
| Eberhard and Howard [145] | C3H5O2 | C3H5O2 + NO = product | 6.32 × 1012 | - | - |
| Feng et al. [146] * | C6H5CH2O2 | C6H5CH2O2 + NO = C6H5CH2O+ NO2 | - | - | - |
| C6H5CH2O2 + NO = C6H5CH2ONO2 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, R.; Cheng, S. Combustion Chemistry of Unsaturated Hydrocarbons Mixed with NOx: A Review with a Focus on Their Interactions. Energies 2023, 16, 4967. https://doi.org/10.3390/en16134967
Tang R, Cheng S. Combustion Chemistry of Unsaturated Hydrocarbons Mixed with NOx: A Review with a Focus on Their Interactions. Energies. 2023; 16(13):4967. https://doi.org/10.3390/en16134967
Chicago/Turabian StyleTang, Ruoyue, and Song Cheng. 2023. "Combustion Chemistry of Unsaturated Hydrocarbons Mixed with NOx: A Review with a Focus on Their Interactions" Energies 16, no. 13: 4967. https://doi.org/10.3390/en16134967
APA StyleTang, R., & Cheng, S. (2023). Combustion Chemistry of Unsaturated Hydrocarbons Mixed with NOx: A Review with a Focus on Their Interactions. Energies, 16(13), 4967. https://doi.org/10.3390/en16134967






