Computational Studies of Energetic Property Peculiarities in Trinitrophenyl-Substituted Nitramines
Abstract
:1. Introduction
2. Materials and Methods
where φ = nM1/2Q1/2
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Name | Structural Formula | Molecular Formula | Molecular Weight | Molecular Composition |
|---|---|---|---|---|---|
| 1. | N-(3-methoxypropyl)-N-(2,4,6-trinitrophenyl)nitramine | 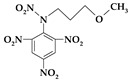 | C10H11N5O9 | 345.222 | C 34.79% H 3.21% N 20.29% O 41.71% |
| 2. | N-(2-methoxyethyl)-N-(2,4,6-trinitrophenyl)nitramine | 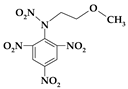 | C9H9N5O9 | 331.196 | C 32.64% H 2.74% N 21.15% O 43.48% |
| 3. | N-tert-butyl-N-(2,4,6-trinitrophenyl)nitramine |  | C10H11N5O8 | 329.223 | C 36.48% H 3.37% N 21.27% O 38.88% |
| 4. | N-prop-2-yn-1-yl-N-(2,4,6-trinitrophenyl)nitramine | 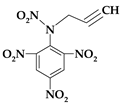 | C9H5N5O8 | 311.165 | C 34.74% H 1.62% N 22.51% O 41.13% |
| 5. | N-(2-methylpropyl)-N-(2,4,6-trinitrophenyl)nitramine |  | C10H11N5O8 | 329.223 | C 36.48% H 3.37% N 21.27% O 38.88% |
| 6. | N-butan-2-yl-N-(2,4,6-trinitrophenyl)nitramine |  | C10H11N5O8 | 329.223 | C 36.48% H 3.37% N 21.27% O 38.88% |
| 7. | N-prop-2-en-1-yl-N-(2,4,6-trinitrophenyl)nitramine | 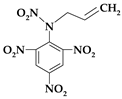 | C9H7N5O8 | 313.181 | C 34.52% H 2.25% N 22.36% O 40.87% |
| 8. | N-(trifluoromethyl)-N-(2,4,6-trinitrophenyl)nitramine |  | C7H2F3N5O8 | 341.115 | C 24.65% H 0.59% F 16.71% N 20.53% O 37.52% |
| 9. | N-[(1H-tetrazol-5-yl)methyl]-N-(2,4,6-trinitrophenyl)nitramine | 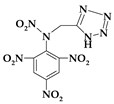 | C8H5N9O8 | 355.181 | C 27.05% H 1.42% N 35.49% O 36.04% |
| 10. | N-(2-azidoethyl)-N-(2,4,6-trinitrophenyl)ni-tramine | 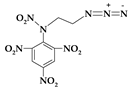 | C8H6N8O8 | 342.182 | C 28.08% H 1.77% N 32.75% O 37.41% |
| 11. | N-(2-nitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | 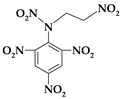 | C8H6N6O10 | 346.167 | C 27.76% H 1.75% N 24.28% O 46.22% |
| 12. | N-(trinitromethyl)-N-(2,4,6-trinitrophenyl)nitramine | 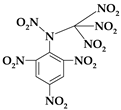 | C7H2N8O14 | 422.136 | C 19.92% H 0.48% N 26.54% O 53.06% |
| 13. | N-(2,4,6-trinitrophenyl)-N-[(3,4,5-trinitro-1H-pyrazol-1-yl)methyl]nitramine |  | C10H4N10O14 | 488.197 | C 24.60% H 0.83% N 28.69% O 45.88% |
| 14. | N-(2-fluoro-2,2-dinitroethyl)-N-(2,4,6-trinitrophenyl)nitramine |  | C8H4FN7O12 | 409.155 | C 23.48% H 0.99% F 4.64% N 23.96% O 46.92% |
| 15. | N-(2,2,2-trinitroethyl)-N-(2,4,6-trinitrophenyl)nitramine |  | C8H4N8O14 | 436.163 | C 22.03% H 0.92% N 25.69% O 51.36% |
| 16. | N-(2,2-dinitroethyl)-N-(2,4,6-trinitrophenyl)nitramine |  | C8H5N7O12 | 391.165 | C 24.56% H 1.29% N 25.07% O 49.08% |
| 17. | N-[(1,2,4,5-tetrazin-3-yl)methyl]-N-(2,4,6-trinitrophenyl)nitramine |  | C9H5N9O8 | 367.192 | C 29.44% H 1.37% N 34.33% O 34.86% |
References
- Akhavan, J. The Chemistry of Explosives, 4th ed.; RSC: London, UK, 2022; 359p, ISBN 978-1-83916-446-0.2. [Google Scholar]
- Koch, E.C. High Explosives, Propellants, Pyrotechnics; Walter de Gruyter GmbH: Berlin, Germany; Boston, MA, USA, 2021; 759p, ISBN 978-3-11-066052-4. [Google Scholar]
- Agrawal, J.P. High Energy Materials: Propellants, Explosives and Pyrotechnics; Wiley-VCH: Weinheim, Germany, 2010; 498p, ISBN 978-3-527-32610-5. [Google Scholar]
- Olah, G.A.; Squire, D.R. (Eds.) Chemistry of Energetic Materials; Academic Press: Cambridge, MA, USA, 2012; p. 212. ISBN 978-0-1239-5897-6. [Google Scholar]
- Agrawal, J.P. Recent trends in high-energy materials. Prog. Energy Combust. Sci. 1998, 24, 1–30. [Google Scholar] [CrossRef]
- Sikder, A.K.; Sikder, N. A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications. J. Hazard. Mater. 2004, 112, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Badgujar, D.M.; Talawar, M.B.; Asthana, S.N.; Mahulikar, P.P. Advances in science and technology of modern energetic materials: An overview. J. Hazard. Mater. 2008, 151, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Nair, U.R.; Asthana, S.N.; Rao, A.S.; Gandhe, B.R. Advances in high energy materials. Def. Sci. J. 2010, 60, 137. [Google Scholar] [CrossRef] [Green Version]
- Explosives: Global Markets to 2022. Available online: https://www.bccresearch.com/market-research/chemicals/explosives-global-markets.html (accessed on 1 May 2023).
- Yernaidu, J.; Patel, V.K.; Tripathi, A.K. An Overview of Explosive Energy Utilization in Mining. Int. J. Eng. Res. Technol. 2022, 9, 1110–1112. [Google Scholar]
- Khademian, A.; Bagherpour, R. Environmentally sustainable mining through proper selection of explosives in blasting operation. Environ. Earth Sci. 2017, 76, e166. [Google Scholar] [CrossRef]
- Mariz, J.L.V.; Soofastaei, A. Advanced Analytics for Rock Blasting and Explosives Engineering in Mining. In Advanced Analytics in Mining Engineering; Soofastaei, A., Ed.; Springer: Cham, Switzerland, 2022; pp. 363–477. [Google Scholar] [CrossRef]
- Singh, J.; Staples, R.J.; Shreeve, J.N.M. Balancing Energy and Stability of Nitroamino-1, 2, 4-Oxadiazoles through a Planar Bridge. Org. Lett. 2022, 24, 8832–8836. [Google Scholar] [CrossRef]
- Sinditskii, V.P.; Smirnov, S.P.; Egorshev, V.Y.; Chernyi, A.N.; Shkineva, T.K.; Palysaeva, N.V.; Suponitsky, K.Y.; Dalinger, I.L. Thermal decomposition peculiarities and combustion behavior of nitropyrazoles. Thermochim. Acta 2017, 651, 83–99. [Google Scholar] [CrossRef]
- Stepanov, R.S.; Kruglyakova, L.A. On the mechanism of autocatalytic thermal decomposition of some secondary nitramines. Russ. J. Gen. Chem. 2017, 87, 204–207. [Google Scholar] [CrossRef]
- Zeman, S.; Elbeih, A.; Hussein, A.; Elshenawy, T.; Jungova, M.; Yan, Q.L. A modified vacuum stability test in the study of initiation reactivity of nitramine explosives. Thermochim. Acta 2017, 656, 16–24. [Google Scholar] [CrossRef]
- Zeman, S.; Atalar, T. A new view of relationships of the N–N bond dissociation energies of cyclic nitramines. Part III. Relationship with detonation velocity. J. Energ. Mater. 2009, 27, 217–229. [Google Scholar] [CrossRef]
- Chakraborty, D.; Muller, R.P.; Dasgupta, S.; Goddard, W.A. A detailed model for the decomposition of nitramines: RDX and HMX. J. Comput. Aided Mol. Des. 2002, 8, 203–212. [Google Scholar] [CrossRef]
- Vogelsanger, B. Chemical stability, compatibility and shelf life of explosives. Chimia 2004, 58, 401–408. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Pflüger, C. 3-Nitramino-4-nitrofurazan: Enhancing the Stability and Energetic Properties by Introduction of Alkylnitramines. Z. Anorg. Allgem. Chem. 2017, 643, 619–624. [Google Scholar] [CrossRef]
- Wang, G.X.; Xiao, H.M.; Xu, X.J.; Ju, X.H. Detonation velocities and pressures, and their relationships with electric spark sensitivities for nitramines. Propellants Explos. Pyrotech. 2006, 31, 102–109. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Krumm, B.; Steemann, F.X. Preparation, Characterization, and Sensitivity Data of Some Azidomethyl Nitramines. Propellants Explos. Pyrotech. 2009, 34, 13–23. [Google Scholar] [CrossRef]
- Szimhardt, N.; Wurzenberger, M.H.H.; Spieß, P.; Klapötke, T.M.; Stierstorfer, J. Potassium N-Nitramino-5H-Tetrazolates—Powerful Green Primary Explosives with High Initiation Capabilities. Propellants Explos. Pyrotech. 2018, 43, 1203–1209. [Google Scholar] [CrossRef]
- Zhang, J.H.; He, C.L.; Parrish, D.A.; Shreeve, J.M. Nitramines with Varying Sensitivities: Functionalized Dipyrazolyl- N-nitromethanamines as Energetic Materials. Chem. Eur. J. 2013, 19, 8929–8936. [Google Scholar] [CrossRef]
- Fischer, D.; Klapotke, T.M.; Stierstorfer, J. 1,5-Di(nitramino)tetrazole: High Sensitivity and Superior Explosive Performance. Angew. Chem. Int. Ed. 2015, 54, 10299–10302. [Google Scholar] [CrossRef]
- Zeman, S.; Jungova, M. Sensitivity and Performance of Energetic Materials. Propellants Explos. Pyrotech. 2016, 41, 426–451. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Bernstein, E.R. Sensitivity and performance of azole based energetic materials. J. Phys. Chem. A 2013, 117, 10889–10902. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, X.D.; Wang, L.J.; Wang, G.X.; Xiao, H.M. Substituent effects on the properties related to detonation performance and sensitivity for 2,2′,4,4′,6,6′-hexanitroazobenzene derivatives. J. Phys. Chem. A 2011, 115, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, D. (Ed.) Molecular modeling of the sensitivities of energetic materials. In Theoretical and Computational Chemistry Series; Elsevier: Amsterdam, The Netherlands, 2022; Volume 22, 486p, ISBN 978-0-12822-971-2. [Google Scholar]
- Liu, W.H.; Liu, Q.J.; Zhong, M.; Gan, Y.D.; Liu, F.S.; Li, X.H.; Tang, B. Predicting impact sensitivity of energetic materials: Insights from energy transfer of carriers. Acta Mater. 2022, 236, 118137. [Google Scholar] [CrossRef]
- Mathieu, D. Toward a physically based quantitative modeling of impact sensitivities. J. Phys. Chem. A 2013, 117, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, D.; Alaime, T. Impact sensitivities of energetic materials: Exploring the limitations of a model based only on structural formulas. J. Mol. Graph. Model. 2015, 62, 81–86. [Google Scholar] [CrossRef]
- Mathieu, D.; Alaime, T. Predicting impact sensitivities of nitro compounds on the basis of a semi-empirical rate constant. J. Phys. Chem. A 2014, 118, 9720–9726. [Google Scholar] [CrossRef]
- Mathieu, D. Theoretical shock sensitivity index for explosives. J. Phys. Chem. A 2012, 116, 1794–1800. [Google Scholar] [CrossRef]
- Zeman, S. Influence of the energy content and its outputs on sensitivity of polynitroarenes. J. Energ. Mater. 2019, 37, 445–458. [Google Scholar] [CrossRef]
- Elbeih, A.; Mohamed, M.M.; Wafy, T. Sensitivity and Detonation Characteristics of Selected Nitramines Bonded by Sylgard Binder. Propellants Explos. Pyrotech. 2016, 41, 1044–1049. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Krumm, B.; Steemann, F.X.; Umland, K.D. Bis(1,3-dinitratoprop-2-yl) Nitramine, a New Sensitive Explosive Combining a Nitrate Ester with a Nitramine. Z. Anorg. Allg. Chem. 2010, 636, 2343–2346. [Google Scholar] [CrossRef] [Green Version]
- Sunahara, G.I.; Lotufo, G.; Kuperman, R.G.; Hawari, J. Ecotoxicology of Explosives; CRC Press: Boca Raton, ON, Canada, 2009; 336p. [Google Scholar] [CrossRef]
- Sunahara, G.I.; Robidoux, P.Y.; Gong, P.; Lachance, B.; Rocheleau, S.; Dodard, S.G.; Sarrazin, M.; Hawari, J.; Thiboutot, S.; Ampleman, G.; et al. Laboratory and field approaches to characterize the soil ecotoxicology of polynitro explosives. ASTM Spec. Tech. Publ. 2001, 1403, 293–312. [Google Scholar] [CrossRef]
- Fedorff, B.T. Encyclopedia of Explosives and Related Items; Picatinny Arsenal: Dover, NJ, USA, 1960; Volume I, 692p. [Google Scholar]
- Agrawal, J.P.; Hodgson, R. Organic Chemistry of Explosives; John Wiley & Sons, Ltd.: Chichester, UK, 2007; p. 273. ISBN 978-0-470-02967. [Google Scholar]
- Department of Health and Human Services; Public Health Service Agency for Toxic Substances and Disease Registry. Tetryl. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp80-c1-b.pdf (accessed on 1 May 2023).
- Liu, D.H.; Spanggord, R.J.; Bailey, H.C. Toxicity of TNT Wastewater (Pink Water) to Aquatic Organisms; Stanford Research Institute: Menlo Park, CA, USA, 1976; 34p, Available online: https://apps.dtic.mil/sti/pdfs/ADA031067.pdf (accessed on 25 May 2023).
- Newell, G.W.; Dilley, J.V.; Spanggord, R.J. Mammalian Toxicological Evaluations of TNT Wastewater (Pink Water); Stanford Research Institute: Menlo Park, CA, USA, 1976; 69p, Available online: https://apps.dtic.mil/sti/pdfs/ADA044785.pdf (accessed on 25 May 2023).
- Meng, Z.; Zhang, Q.; Xue, M.; Wang, D.; Wang, A. Removal of 2,4,6-Trinitrotoluene from “Pink Water” Using Molecularly-Imprinted Absorbent. Propellants Explos. Pyrotech. 2012, 37, 100–106. [Google Scholar] [CrossRef]
- Talawar, M.B.; Sivabalan, R.; Mukundan, T.; Muthurajan, H.; Sikder, A.K.; Gandhe, B.R.; Rao, A.S. Environmentally compatible next generation green energetic materials (GEMs). J. Hazard. Mater. 2009, 161, 589–607. [Google Scholar] [CrossRef]
- Badgujar, D.M.; Talawar, M.B.; Mahulikar, P.P. Review on greener and safer synthesis of nitro compounds. Propellants Explos. Pyrotech. 2016, 41, 24–34. [Google Scholar] [CrossRef]
- Golding, P.; Millar, R.W.; Paul, N.C.; Richards, D.H. Preparation of Nitramine-Nitrates by Ring-opening Nitration of Aziridines by Dinitrogen Pentoxide (N2O5). Tetrahedron 1993, 49, 7063–7076. [Google Scholar] [CrossRef]
- Millar, R.W.; Philbin, S.P. Clean nitrations: Novel syntheses of nitramines and nitrate esters by nitrodesilylation reactions using dinitrogen pentoxide (N2O5). Tetrahedron 1998, 53, 4371–4386. [Google Scholar] [CrossRef]
- Kuchurov, I.V.; Zharkov, M.N.; Fershtat, L.L.; Makhova, N.N.; Zlotin, S.G. Prospective Symbiosis of Green Chemistry and Energetic Materials. ChemSusChem 2017, 10, 3914–3946. [Google Scholar] [CrossRef] [PubMed]
- Cumming, A.S. (Ed.) Energetics Science and Technology: An Integrated Approach; IOP Publishing: Bristol, UK, 2022; 530p, ISBN 978-0-7503-3941-4. [Google Scholar] [CrossRef]
- Talawar, M.B.; Sivabalan, R.; Anniyappan, M.; Gore, G.M.; Asthana, S.N.; Gandhe, B.R. Emerging trends in advanced high energy materials. Combust. Explos. Shock Waves 2007, 43, 62–72. [Google Scholar] [CrossRef]
- Badgujar, D.M.; Talawar, M.B.; Mahulikar, P.P. Review of promising insensitive energetic materials. Cent. Eur. J. Energ. Mater. 2017, 14, 821–843. [Google Scholar] [CrossRef]
- Sikder, A.K.; Maddala, G.; Agrawal, J.P.; Singh, H. Important aspects of behaviour of organic energetic compounds. J. Hazard. Mater. 2001, 84, 1–26. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Q.; Shreeve, J.M. Fused heterocycle-based energetic materials (2012–2019). J. Mater. Chem. A 2020, 8, 4193–4216. [Google Scholar] [CrossRef]
- Ritter, H.; Licht, H.H. Synthesis and Characterization of Methylnitramino-Substituted pyridines and triazines. Propellants Explos. Pyrotech. 1993, 18, 81–88. [Google Scholar] [CrossRef]
- Tamuliene, J.; Sarlauskas, J. Impact of Incremental Methylene Groups on the Energetic Properties of Aromatic Nitramines. Energies 2023, 16, 3117. [Google Scholar] [CrossRef]
- Becke, A.D. Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef] [Green Version]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 W; Gaussian, Inc.: Wallingford, CT, USA, 2016; p. 139. Available online: https://www.cwu.edu/chemistry/sites/cts.cwu.edu.chemistry/files/documents/Gaussian_09_ReferenceManual.pdf (accessed on 26 March 2023).
- Cardia, R.; Malloci, G.; Mattoni, A.; Cappellini, G. Effects of TIPS-Functionalization and Perhalogenation on the Electronic, Optical, and Transport Properties of Angular and Compact Dibenzochrysene. J. Phys. Chem. A 2014, 118, 5170–5177. [Google Scholar] [CrossRef] [PubMed]
- Cardia, R.; Malloci, G.; Rignanese, G.M.; Blasé, X.; Molteni, E.; Cappellini, G. Electronic and optical properties of hexathiapentacene in the gas and crystal phases. Phys. Rev. B 2016, 93, 235132. [Google Scholar] [CrossRef]
- Dardenne, N.; Cardia, R.; Li, J.; Malloci, G.; Cappellini, G.; Blasé, X.; Charlier, J.C.; Rignanese, G. Tuning Optical Properties of Dibenzochrysenes by Functionalization: A Many-Body Perturbation Theory Study. Phys. Chem. C 2017, 121, 24480–24488. [Google Scholar] [CrossRef]
- Antidormi, A.; Aprile, G.; Cappellini, G.; Cara, E.; Cardia, R.; Colombo, L.; Farris, R.; d’Ischia, M.; Mehrabanian, M.; Melis, C.; et al. Physical and Chemical Control of Interface Stability in Porous Si–Eumelanin Hybrids. J. Phys. Chem. C 2018, 122, 28405–28415. [Google Scholar] [CrossRef]
- Mocci, P.; Cardia, R.; Cappellini, G. Inclusions of Si-atoms in Graphene nanostructures: A computational study on the ground-state electronic properties of Coronene and Ovalene. J. Phys. Conf. Ser. 2018, 956, 012020. [Google Scholar] [CrossRef]
- Mocci, P.; Cardia, R.; Cappellini, G. Si-atoms substitutions effects on the electronic and optical properties of coronene and ovalene. New J. Phys. 2018, 20, 113008. [Google Scholar] [CrossRef]
- Kumar, A.; Cardia, R.; Cappellini, G. Electronic and optical properties of chromophores from bacterial cellulose. Cellulose 2018, 25, 2191–2203. [Google Scholar] [CrossRef]
- Szafran, M.; Koput, J. Ab initio and DFT calculations of structure and vibrational spectra of pyridine and its isotopomers. J. Mol. Struct. 2001, 565, 439–448. [Google Scholar] [CrossRef]
- Begue, D.; Carbonniere, P.; Pouchan, C. Calculations of Vibrational Energy Levels by Using a Hybrid ab Initio and DFT Quartic Force Field: Application to Acetonitrile. J. Phys. Chem. A 2005, 109, 4611–4616. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Padmanabhan, J.; Subramanian, V.; Maiti, B.; Chattaraj, P.K. Toxicity analysis of 3,3′,4,4′,5-pentachloro biphenyl through chemical reactivity and selectivity profiles. Curr. Sci. 2004, 86, 535–542. Available online: https://www.jstor.org/stable/24107906 (accessed on 30 January 2023).
- Kaya, S.; Kaya, C. New equation based on ionization energies and electron affinities of atoms for calculating of group electronegativity. Comput. Theor. Chem. 2015, 1052, 42–46. [Google Scholar] [CrossRef]
- Rajakumar, B.; Arathala, P.; Muthiah, B. Thermal Decomposition of 2-Methyltetrahydrofuran behind Reflected Shock Waves over the Temperature Range of 1179–1361 K. J. Phys. Chem. A 2021, 125, 5406–54223. [Google Scholar] [CrossRef]
- Arathala, P.; Musah, R.A. Oxidation of Dipropyl Thiosulfinate Initiated by Cl Radicals in the Gas Phase: Implications for Atmospheric Chemistry. ACS Earth Space Chem. 2021, 5, 2878–2890. [Google Scholar] [CrossRef]
- Cooper, P.W. Explosives Engineering; Wiley-VCH: New York, NY, USA, 1996; 480p, ISBN 0-471-18636-8. [Google Scholar]
- Shevchenko, A.A.; Dolgoborodov, A.Y.; Brazhnikov, M.A.; Kirilenko, V.G. Pseudoideal detonation of mechanoactivated mixtures of ammonium perchlorate with nanoaluminum. J. Phys. Conf. Ser. 2018, 946, 012055. [Google Scholar] [CrossRef]
- Kozak, G.D. Measurement and calculation of the ideal detonation velocity for liquid nitrocompounds. Combust Explos. Shock Waves 1998, 34, 581–586. [Google Scholar] [CrossRef]
- Bolton, O.; Simke, L.R.; Pagoria, P.F.; Matzger, A.J. High Power Explosive with Good Sensitivity: A 2:1 Cocrystal of CL-20:HMX. Cryst. Growth Des. 2012, 12, 4311–4314. [Google Scholar] [CrossRef]
- Viswanath, D.S.; Ghosh, T.K.; Boddu, V.M. 5-Nitro-2,4-dihydro-3H-1,2,4-Triazole-3-one (NTO). In Emerging Energetic Materials: Synthesis, Physicochemical, and Detonation Properties; Springer: Dordrecht, The Netherlands, 2018; pp. 163–211. [Google Scholar] [CrossRef]
- Eaton, P.E.; Gilardi, R.G.; Zhang, M. Polynitrocubanes: Advanced high-density, high-energy materials. Adv. Mater. 2000, 12, 1143–1148. [Google Scholar] [CrossRef]
- Wen, L.; Wang, B.; Yu, T.; Lai, W.; Shi, J.; Liu, M.; Liu, Y. Accelerating the search of CHONF-containing highly energetic materials by combinatorial library design and high-throughput screening. Fuel 2022, 310, 122241. [Google Scholar] [CrossRef]
- Keshavarz, M.H.; Pouretedal, H.R. Simple empirical method for prediction of impact sensitivity of selected class of explosives. J. Hazard. Mater. 2005, 124, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.H. Prediction of impact sensitivity of nitroaliphatic, nitroaliphatic containing other functional groups and nitrate explosives. J. Hazard. Mater. 2007, 148, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.H. A new general correlation for predicting impact sensitivity of energetic compounds. Propellants Explos. Pyrotech. 2013, 38, 754–760. [Google Scholar] [CrossRef]
- Keshavarz, M.H. New method for calculating densities of nitroaromatic explosive compounds. J. Hazard. Mater. 2007, 145, 263–269. [Google Scholar] [CrossRef]
- Keshavarz, M.H. A new computer code to evaluate detonation performance of high explosives and their thermochemical properties, part I. J. Hazard. Mater. 2009, 172, 1218–1228. [Google Scholar] [CrossRef]
- Türker, L. Velocity of detonation—A mathematical model. Acta Chim. Slov. 2010, 57, 288–296. [Google Scholar]
- Kamlet, M.J.; Jacobs, S.J. Chemistry of Detonations. I. Simple Method for Calculating Detonation Properties of CHNO Explosives. J. Chem. Phys. 1968, 48, 23–55. [Google Scholar] [CrossRef]
- Mathieu, D. Sensitivity of Energetic Materials: Theoretical Relationships to Detonation Performance and Molecular Structure. Ind. Eng. Chem. Res. 2017, 56, 8191–8201. [Google Scholar] [CrossRef]
- Hooper, N.; Beeching, L.J.; Dyke, J.M.; Morris, A.; Ogden, J.S.; Dias, A.A.; Costa, M.L.; Barros, M.T.; Cabral, M.H.; Moutinho, A.M.C. A Study of the Thermal Decomposition of 2-Azidoethanol and 2-Azidoethyl Acetate by Ultraviolet Photoelectron Spectroscopy and Matrix Isolation Infrared Spectroscopy. J. Phys. Chem. A 2002, 106, 9968–9975. [Google Scholar] [CrossRef]
- Yan, Z.; Lu, T.; Liu, Y.; Liu, W.; Zhao, B.; Wang, Y.; Ge, Z. High Thermal Stability and Insensitive Fused Triazole-Triazine Trifluoromethyl-Containing Explosives (TFX). ACS Omega 2021, 6, 8591–18597. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.H. A simple approach for determining detonation velocity of high explosive at any loading density. J. Hazard. Mater. 2005, 121, 31–36. [Google Scholar] [CrossRef]
- Urizar, M.J.; James, E., Jr.; Smith, L.C. Detonation velocity of pressed TNT. Phys. Fluids 1961, 4, 262–274. [Google Scholar] [CrossRef]


| Compound | Binding Energy per Atom, eV | HOMO–LUMO Gap, eV | Hardness, eV | Softness, eV | Electronegativity, eV | Hardness Index Y |
|---|---|---|---|---|---|---|
| N-[(1,2,4,5-tetrazin-3-yl)methyl]-N-(2,4,6-trinitrophenyl)nitramine | 5.65 | 2.90 | 1.45 | 0.35 | 5.65 | 0.76 |
| N-(trifluoromethyl)-N-(2,4,6-trinitrophenyl)nitramine | 5.07 | 3.35 | 1.67 | 0.30 | 7.26 | 0.82 |
| N-(2-azidoethyl)-N-(2,4,6-trinitrophenyl)nitramine | 5.58 | 3.43 | 1.72 | 0.29 | 5.90 | 0.83 |
| N-(2-methoxyethyl)-N-(2,4,6-trinitrophenyl)nitramine | 5.08 | 3.65 | 1.82 | 0.27 | 5.90 | 0.85 |
| N-(3-methoxypropyl)-N-(2,4,6-trinitrophenyl)nitramine | 5.04 | 3.70 | 1.85 | 0.27 | 5.86 | 0.85 |
| N-prop-2-en-1-yl-N-(2,4,6-trinitrophenyl)nitramine | 5.29 | 4.20 | 2.10 | 0.24 | 6.12 | 0.89 |
| N-prop-2-yn-1-yl-N-(2,4,6-trinitrophenyl)nitramine | 5.82 | 4.23 | 2.11 | 0.24 | 6.18 | 0.89 |
| N-butan-2-yl-N-(2,4,6-trinitrophenyl)nitramine | 5.08 | 4.26 | 2.13 | 0.24 | 6.12 | 0.89 |
| N-(2-methylpropyl)-N-(2,4,6-trinitrophenyl)nitramine | 5.08 | 4.28 | 2.14 | 0.23 | 6.18 | 0.89 |
| N-tert-butyl-N-(2,4,6-trinitrophenyl)nitramine | 5.44 | 4.32 | 2.16 | 0.23 | 6.12 | 0.89 |
| N-(2-nitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | 5.19 | 4.33 | 2.17 | 0.23 | 6.47 | 0.89 |
| Tetryl | 4.84 | 4.31 | 2.17 | 0.23 | 6.19 | 0.89 |
| N-(2-nitroethyl)-N-(2,4,6-trinitrophenyl)nitramine 1 | 5.42 | 4.43 | 2.21 | 0.23 | 6.52 | 0.9 |
| N-(2,4,6-trinitrophenyl)-N-[(3,4,5-trinitro-1H-pyrazol-1-yl)methyl]nitramine | 5.44 | 4.50 | 2.25 | 0.22 | 6.70 | 0.9 |
| N-[(1H-tetrazol-5-yl)methyl]-N-(2,4,6-trinitrophenyl)nitramine | 5.58 | 4.58 | 2.29 | 0.22 | 6.54 | 0.9 |
| N-[(1H-tetrazol-5-yl)methyl]-N-(2,4,6-trinitrophenyl)nitramine | 5.19 | 4.62 | 2.31 | 0.22 | 6.83 | 0.91 |
| N-(2,2-dinitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | 5.18 | 4.62 | 2.31 | 0.22 | 6.46 | 0.91 |
| N-(2-fluoro-2,2-dinitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | 5.19 | 4.63 | 2.32 | 0.22 | 6.80 | 0.91 |
| N-(trinitromethyl)-N-(2,4,6-trinitrophenyl)nitramine | 4.94 | 4.72 | 2.36 | 0.21 | 6.88 | 0.91 |
| Compound | Activation Energy, eV |
|---|---|
| N-(2-methoxyethyl)-N-(2,4,6 trinitrophenyl)nitramine | 0.06 |
| N-(3-methoxypropyl)-N-(2,4,6-rinitrophenyl)nitramine | 0.09 |
| N-butan-2-yl-N-(2,4,6-trinitrophenyl)nitramine | 0.08 |
| N-(2-methylpropyl)-N-(2,4,6-trinitrophenyl)nitramine | 0.11 |
| N-prop-2-yn-1-yl-N-(2,4,6-trinitrophenyl)nitramine | 0.03 |
| N-tert-butyl-N-(2,4,6-trinitrophenyl)nitramine | 0.10 |
| N-(2-azidoethyl)-N-(2,4,6-trinitrophenyl)nitramine | 0.0003 |
| N-(2-nitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | 0.02 |
| N-(2-fluoro-2,2-dinitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | 0.09 |
| N-(2,2-dinitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | 0.05 |
| N-(2,2,2-trinitroethyl)-N-(2,4,6 trinitrophenyl)nitramine | 0.12 |
| N-(trinitromethyl)-N-(2,4,6-trinitrophenyl)nitramine | 0.05 |
| N-[(1H-tetrazol-5-yl)methyl]-N-(2,4,6-trinitrophenyl)nitramine | 0.17 |
| N-(trifluoromethyl)-N-(2,4,6-trinitrophenyl)nitramine | 0.91 |
| N-[(1,2,4,5-tetrazin-3-yl)methyl]-N-(2,4,6-trinitrophenyl)nitramine | 0.14 |
| Compound | logh1 | logh2 | OxB, % |
|---|---|---|---|
| N-(trinitromethyl)-N-(2,4,6-trinitrophenyl)nitramine | 1.212 | 0.904 | −3.79 |
| N-(2-nitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | 1.460 | 1.091 | −14.67 |
| N-(2-fluoro-2,2-dinitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | −21.51 | ||
| N-(trifluoromethyl)-N-(2,4,6-trinitrophenyl)nitramine | −25.80 | ||
| N-(2,4,6-trinitrophenyl)-N-[(3,4,5-trinitro-1H-pyrazol-1-yl)methyl]nitramine | 1.422 | 1.179 | −26.22 |
| N-(2,2-dinitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | 1.658 | 1.277 | −26.59 |
| N-(2,2,2-trinitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | 1.908 | 1.510 | −41.60 |
| N-[(1H-tetrazol-5-yl)methyl]-N-(2,4,6-trinitrophenyl)nitramine | 1.848 | 1.432 | −47.30 |
| N-[(1,2,4,5-tetrazin-3-yl)methyl]-N-(2,4,6-trinitrophenyl)nitramine | 1.845 | 1.484 | −49.24 |
| N-(2-azidoethyl)-N-(2,4,6-trinitrophenyl)nitramine. | 2.020 | 1.548 | −51.43 |
| N-prop-2-yn-1-yl-N-(2,4,6-trinitrophenyl)nitramine | 1.770 | 1.756 | −64.27 |
| N-prop-2-en-1-yl-N-(2,4,6-trinitrophenyl)nitramine | 2.153 | 1.895 | −68.97 |
| N-(2-methoxyethyl)-N-(2,4,6-trinitrophenyl)nitramine | 2.363 | 2.001 | −72.26 |
| N-(3-methoxypropyl)-N-(2,4,6-rinitrophenyl)nitramine | 2.706 | 2.125 | −76.47 |
| N-(2-methylpropyl)-N-(2,4,6-trinitrophenyl)nitramine | 2.803 | 2.232 | −85.05 |
| N-tert-butyl-N-(2,4,6-trinitrophenyl)nitramine | 2.803 | 2.232 | −85.05 |
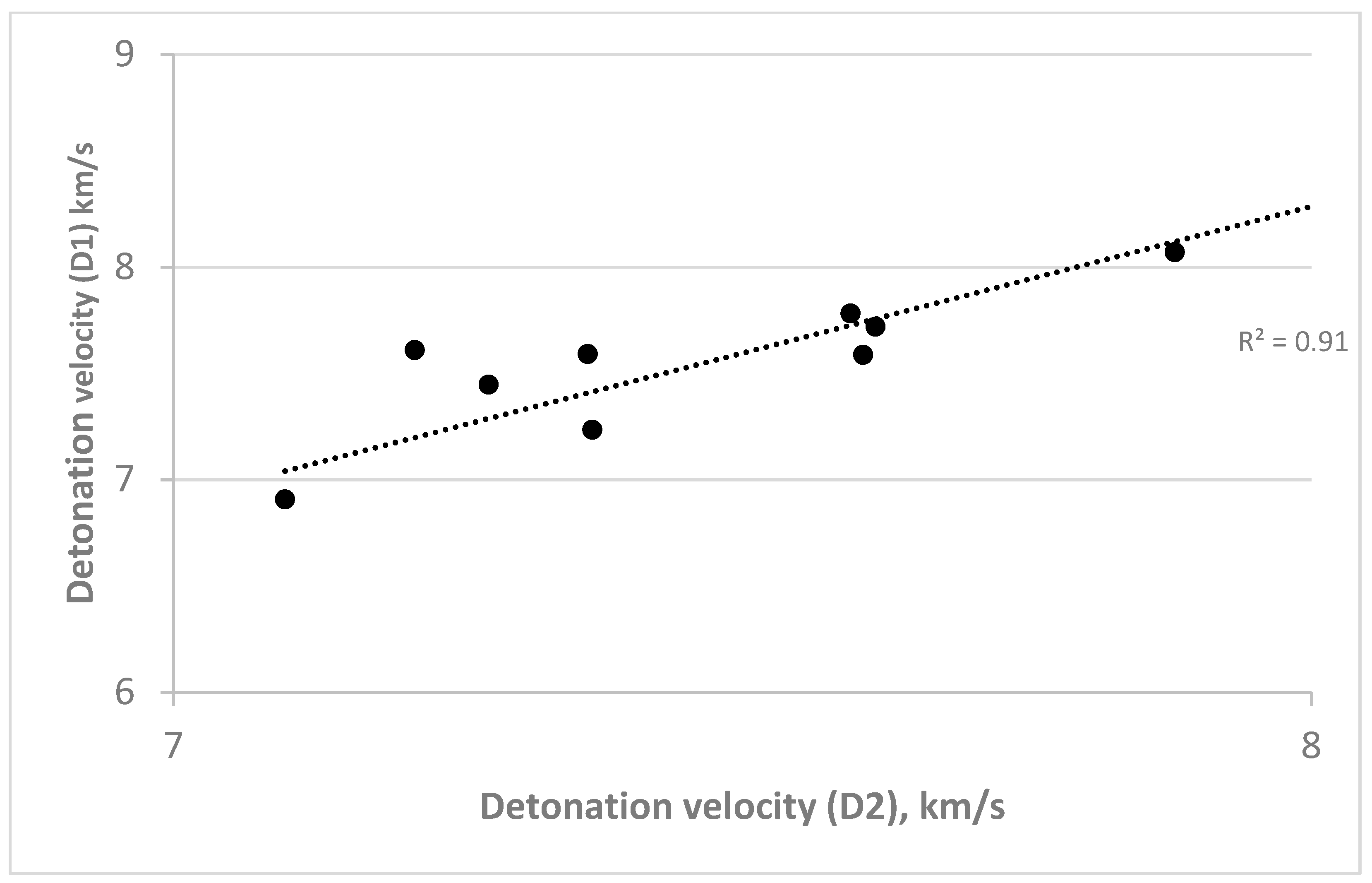
| Compounds | Detonation Velocity, km/s | |
|---|---|---|
| D1 | D2 | |
| N-(trifluoromethyl)-N-(2,4,6-trinitrophenyl)nitramine | * | 6.935 |
| N-(2-fluoro-2,2-dinitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | * | 7.338 |
| N-(2-methylpropyl)-N-(2,4,6-trinitrophenyl)nitramine | 6.908 | 7.098 |
| N-butan-2-yl-N-(2,4,6-trinitrophenyl)nitramine | 6.908 | 7.098 |
| N-tert-butyl-N-(2,4,6-trinitrophenyl)nitramine | 6.908 | 7.098 |
| N-prop-2-yn-1-yl-N-(2,4,6-trinitrophenyl)nitramine | 7.610 | 7.212 |
| N-prop-2-en-1-yl-N-(2,4,6-trinitrophenyl)nitramine | 7.447 | 7.277 |
| N-[(1,2,4,5-tetrazin-3-yl)methyl]-N-(2,4,6-trinitrophenyl)nitramine | 7.592 | 7.364 |
| N-(3-methoxypropyl)-N-(2,4,6-trinitrophenyl)nitramine | 7.235 | 7.368 |
| N-[(1H-tetrazol-5-yl)methyl]-N-(2,4,6-trinitrophenyl)nitramine | 7.782 | 7.595 |
| N-(2-methoxyethyl)-N-(2,4,6-trinitrophenyl)nitramine | 7.588 | 7.606 |
| N-(2-azidoethyl)-N-(2,4,6-trinitrophenyl)nitramine | 7.720 | 7.617 |
| Tetryl | 8.070 | 7.880 |
| N-(2-nitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | 8.305 | 8.088 |
| N-(2,4,6-trinitrophenyl)-N-[(3,4,5-trinitro-1H-pyrazol-1-yl)methyl]nitramine | 8.767 | 8.265 |
| N-(2,2-dinitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | 8.778 | 8.473 |
| N-(2,2,2-trinitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | 9.144 | 8.780 |
| N-(trinitromethyl)-N-(2,4,6-trinitrophenyl)nitramine | 9.421 | 9.012 |
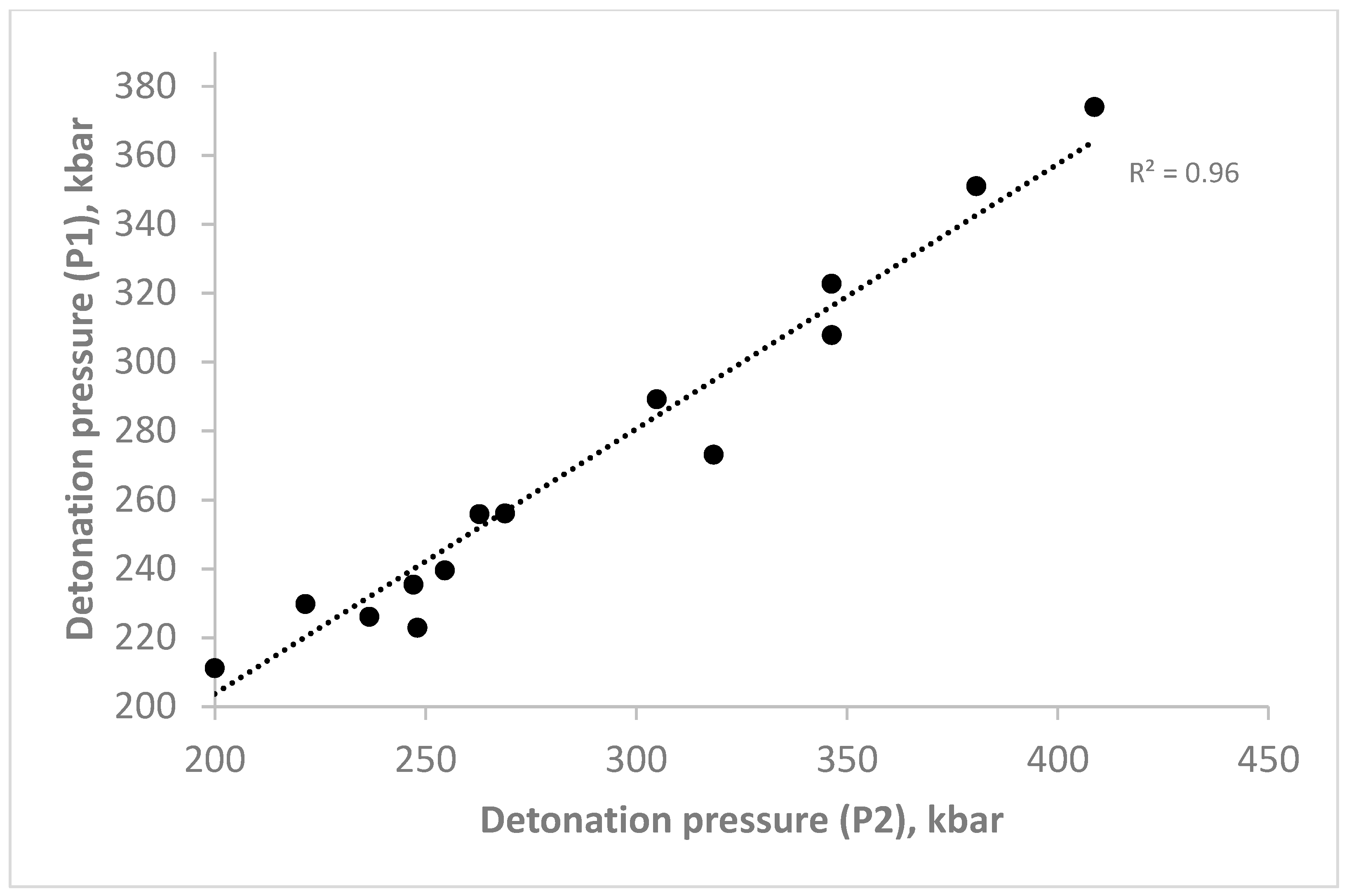
| Compounds | Detonation Pressure, kbar | |
|---|---|---|
| p(D1) | p(D2) | |
| N-(trifluoromethyl)-N-(2,4,6-trinitrophenyl)nitramine | * | 216.882 |
| N-(2-fluoro-2,2-dinitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | * | 220.918 |
| N-tert-butyl-N-(2,4,6-trinitrophenyl)nitramine | 199.991 | 211.144 |
| N-(2-methylpropyl)-N-(2,4,6-trinitrophenyl)nitramine | 200.000 | 211.145 |
| N-butan-2-yl-N-(2,4,6-trinitrophenyl)nitramine | 199.998 | 211.145 |
| N-prop-2-yn-1-yl-N-(2,4,6-trinitrophenyl)nitramine | 248.109 | 222.866 |
| N-prop-2-en-1-yl-N-(2,4,6-trinitrophenyl)nitramine | 236.714 | 226.074 |
| N-(3-methoxypropyl)-N-(2,4,6-rinitrophenyl)nitramine | 221.521 | 229.753 |
| N-(2-methoxyethyl)-N-(2,4,6-trinitrophenyl)nitramine | 247.144 | 235.389 |
| N-[(1,2,4,5-tetrazin-3-yl)methyl]-N-(2,4,6-trinitrophenyl)nitramine | 254.580 | 239.506 |
| N-(2-azidoethyl)-N-(2,4,6-trinitrophenyl)nitramine | 262.825 | 255.849 |
| N-[(1H-tetrazol-5-yl)methyl]-N-(2,4,6-trinitrophenyl)nitramine | 268.861 | 256.065 |
| Tetryl | 318.370 | 273.110 |
| N-(2-nitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | 304.896 | 289.190 |
| N-(2,4,6-trinitrophenyl)-N-[(3,4,5-trinitro-1H-pyrazol-1-yl)methyl]nitramine | 346.394 | 307.834 |
| N-(2,2-dinitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | 346.370 | 322.740 |
| N-(2,2,2-trinitroethyl)-N-(2,4,6-trinitrophenyl)nitramine | 380.715 | 351.033 |
| N-(trinitromethyl)-N-(2,4,6-trinitrophenyl)nitramine | 408.716 | 374.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamuliene, J.; Sarlauskas, J. Computational Studies of Energetic Property Peculiarities in Trinitrophenyl-Substituted Nitramines. Energies 2023, 16, 5180. https://doi.org/10.3390/en16135180
Tamuliene J, Sarlauskas J. Computational Studies of Energetic Property Peculiarities in Trinitrophenyl-Substituted Nitramines. Energies. 2023; 16(13):5180. https://doi.org/10.3390/en16135180
Chicago/Turabian StyleTamuliene, Jelena, and Jonas Sarlauskas. 2023. "Computational Studies of Energetic Property Peculiarities in Trinitrophenyl-Substituted Nitramines" Energies 16, no. 13: 5180. https://doi.org/10.3390/en16135180





