Microbial Oil Production from Alkali Pre-Treated Giant Reed (Arundo donax L.) by Selected Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Giant Reed Biomass
2.1.2. Fungal Strains
2.2. Methods
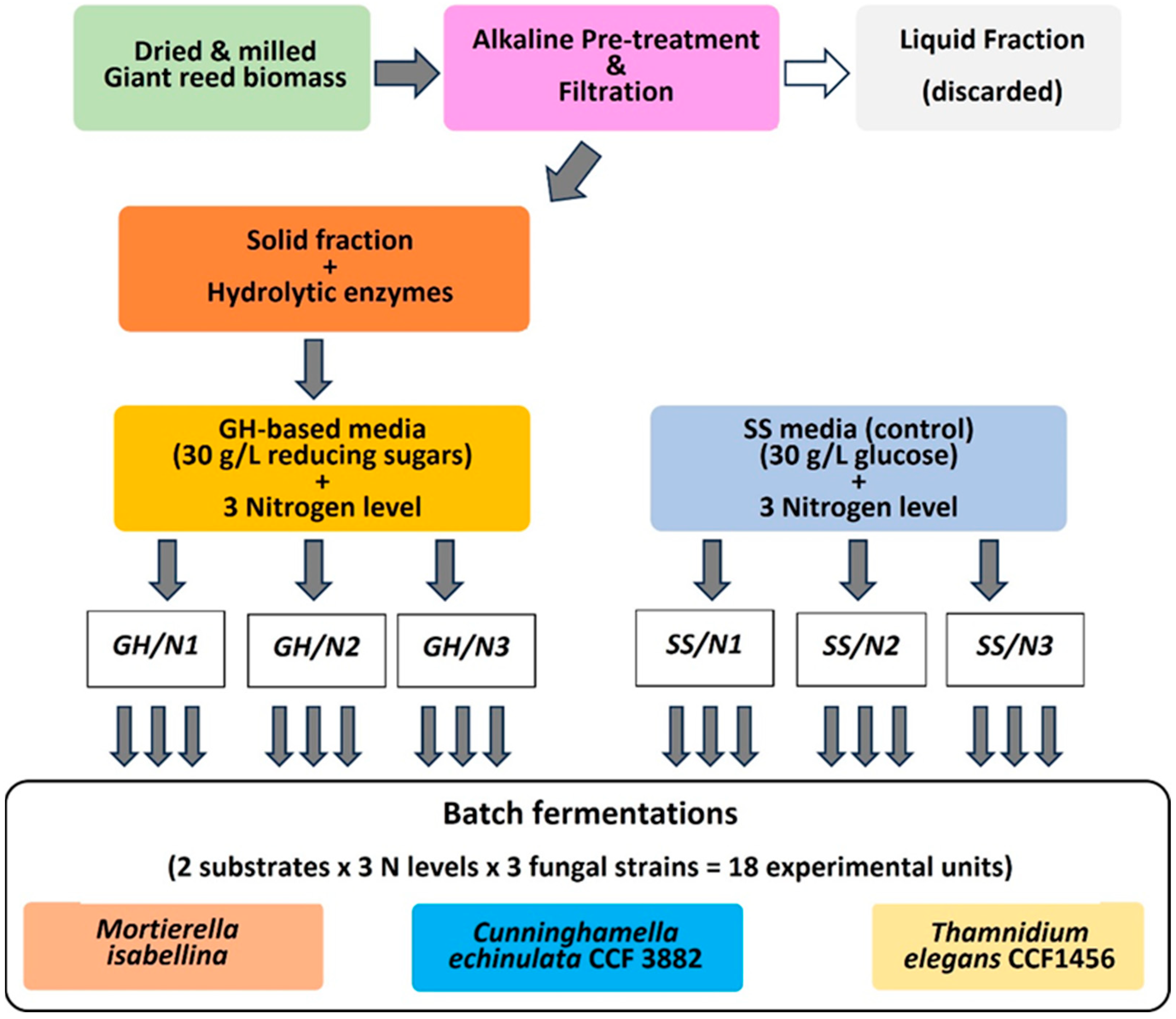
2.2.1. Experimental Design
- (i)
- Alkaline pre-treatment of the plant biomass, followed by solid/liquid separation by filtration and pre-treated solid fraction recovery. The liquid fraction containing potential inhibitors (residual alkali and solubilized lignin) was discarded.
- (ii)
- Enzymatic hydrolysis of the pre-treated giant reed biomass.
- (iii)
- Formulation of fermentation media based on the giant reed hydrolysate (GH), with 3 levels of N supplementation (N1, N2, N3), in comparison to semi-synthetic media (SS) as the control, for a total of 6 media: GH/N1, GH/N2, GH/N3, and SS/N1, SS/N2, SS/N3).
- (iv)
- Batch fermentation (monitoring sugar consumption) with the 3 fungal strains in the 6 fermentation media and determination of the final cell biomass and oil concentrations, cellular oil content, and FA profiles. Two independent experiments involving batch fermentation were carried out with two replications.
2.2.2. Alkaline Pre-Treatment and Recovery of the Pre-Treated Biomass
2.2.3. Enzymatic Hydrolysate Preparation
2.2.4. Sugar Determination
2.2.5. Formulation of Fermentation Media
2.2.6. Fermentation Experiments
2.2.7. Oil Extraction and Analysis of the Fatty Acid Content and Composition
2.2.8. Assessment of Biodiesel Quality Parameters
2.2.9. Statistical Analysis
3. Results
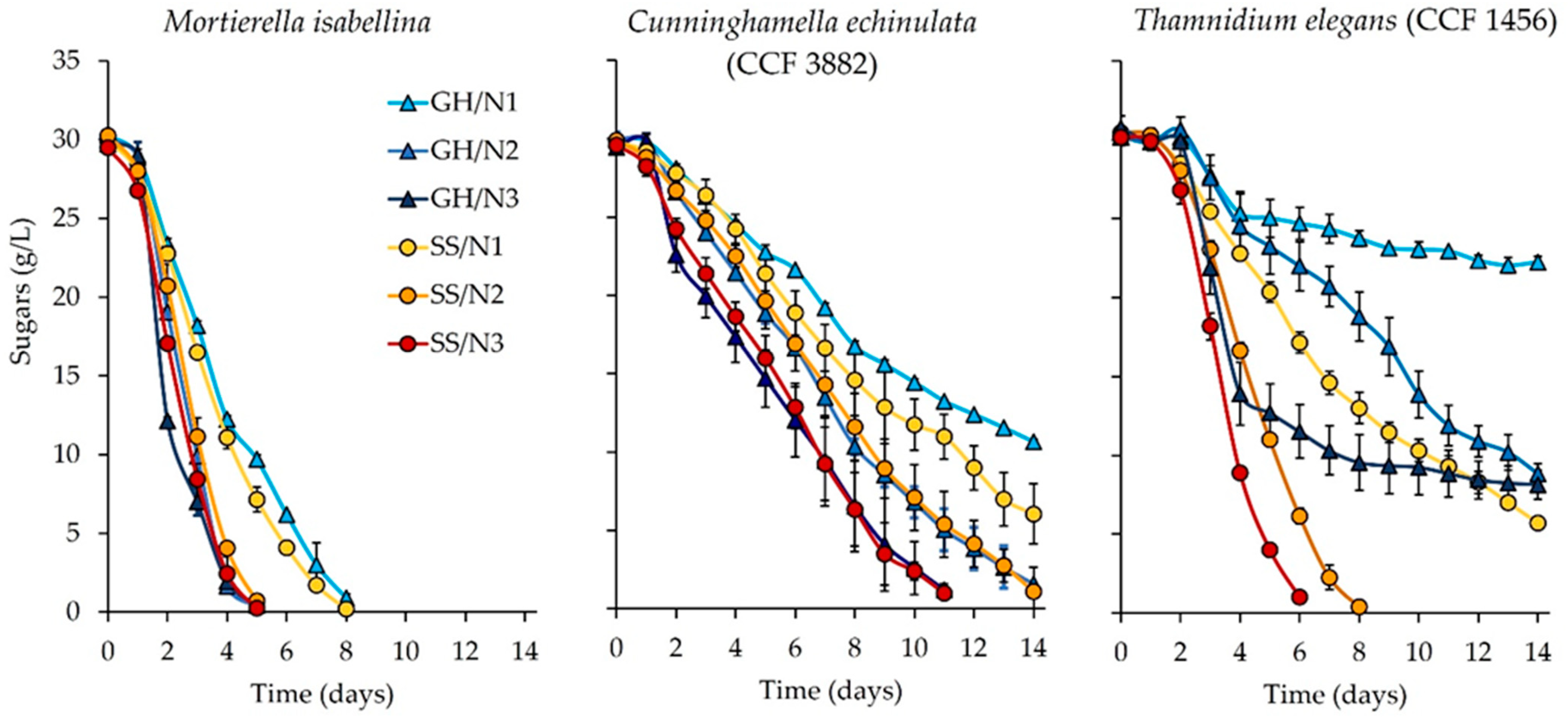
3.1. Setting the Scene: Sugar Content of the Hydrolysate and Sugar Consumption in the Fungal Cultures
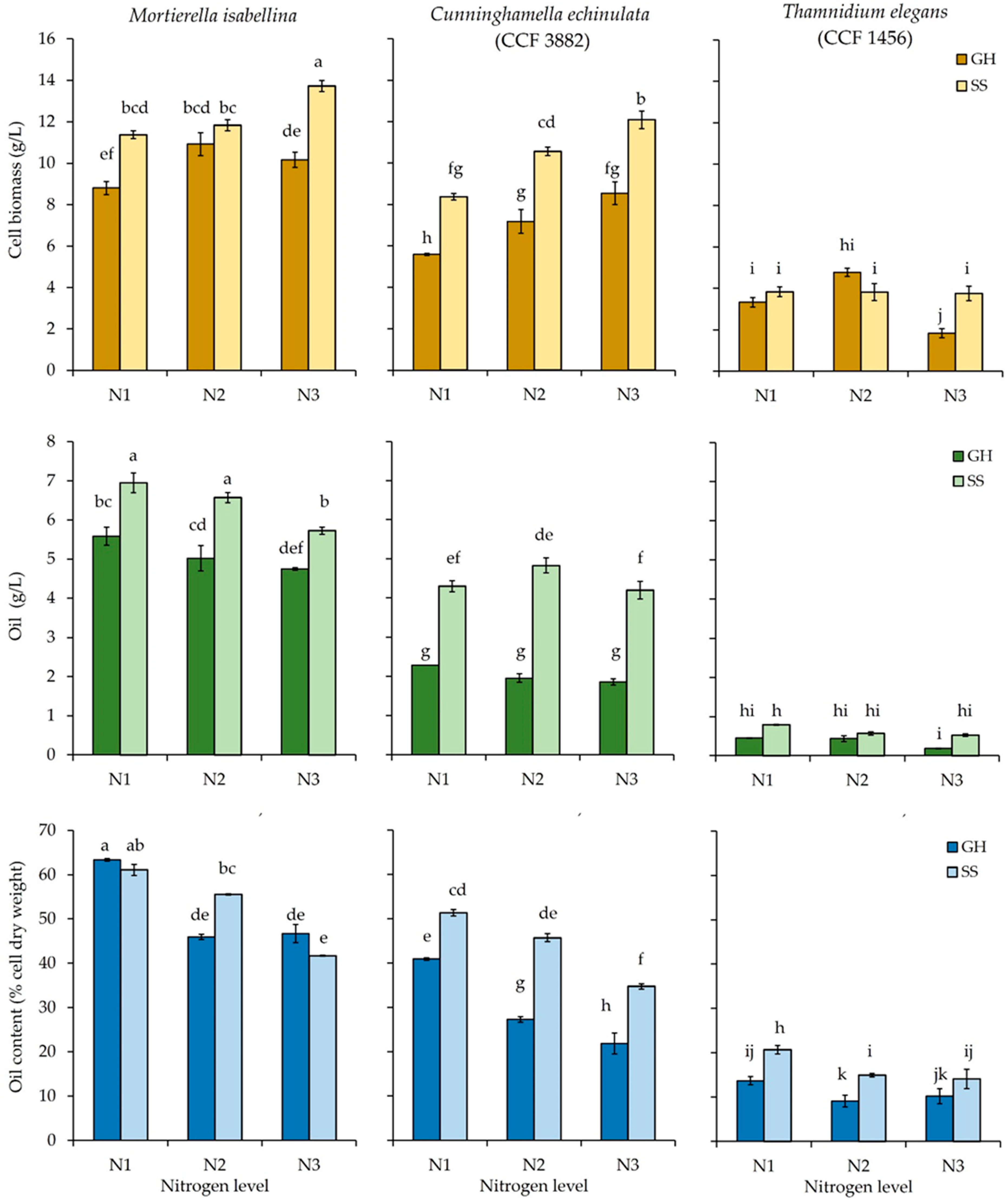
3.2. Cell Biomass and Oil Concentrations, and Cellular Oil Content at the End of the Fermentation
3.3. Fatty Acid Profiles
3.4. Oil Yield and Productivity and γ-Linolenic Acid Concentration
3.5. Prediction of Biodiesel Parameters
4. Discussion
4.1. Sugar Content of the Hydrolysate and Sugar Consumption of the Fungal Cultures
4.2. Cell Biomass and Oil Concentrations, and Cellular Oil Content at the End of the Fermentation
4.3. Fatty Acid Profile
4.4. Oil Yield and Productivity and γ-Linolenic Acid Concentration
4.5. Assessment of Biodiesel Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dragone, G.; Fernandes, B.; Vicente, A.A.; Teixeira, J.A. Third Generation Biofuels from Microalgae. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2010; Volume 2, pp. 1355–1366. [Google Scholar]
- Mota, M.; Múgica, P.; Sá-Correia, I. Exploring Yeast Diversity to Produce Lipid-Based Biofuels from Agro-Forestry and Industrial Organic Residues. J. Fungi 2022, 8, 687. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zong, M.; Wu, H.; Liu, Q. Microbial Oil Production from Rice Straw Hydrolysate by Trichosporon fermentans. Bioresour. Technol. 2009, 100, 4535–4538. [Google Scholar] [CrossRef] [PubMed]
- Ceotto, E.; Vasmara, C.; Marchetti, R.; Cianchetta, S.; Galletti, S. Biomass and Methane Yield of Giant Reed (Arundo donax L.) as Affected by Single and Double Annual Harvest. GCB Bioenergy 2021, 13, 393–407. [Google Scholar] [CrossRef]
- Valdes, G.; Mendonca, R.T.; Aggelis, G. Lignocellulosic Biomass as a Substrate for Oleaginous Microorganisms: A Review. Appl. Sci. 2020, 10, 7698. [Google Scholar] [CrossRef]
- Van Dyk, J.S.; Pletschke, B.I. A Review of Lignocellulose Bioconversion Using Enzymatic Hydrolysis and Synergistic Cooperation between Enzymes—Factors Affecting Enzymes, Conversion and Synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef]
- Cianchetta, S.; Di Maggio, B.; Burzi, P.L.; Galletti, S. Evaluation of Selected White-Rot Fungal Isolates for Improving the Sugar Yield from Wheat Straw. Appl. Biochem. Biotechnol. 2014, 173, 609–623. [Google Scholar] [CrossRef]
- Rahman, S.; Arbter, P.; Popovic, M.; Bajpai, R.; Subramaniam, R. Microbial Lipid Production from Lignocellulosic Hydrolyzates: Effect of Carbohydrate Mixtures and Acid-Hydrolysis Byproducts on Cell Growth and Lipid Production by Lipomyces starkeyi: Microbial Lipid Production from Lignocellulosic Hydrolyzates. J. Chem. Technol. Biotechnol. 2017, 92, 1980–1989. [Google Scholar] [CrossRef]
- McIntosh, S.; Vancov, T. Optimisation of Dilute Alkaline Pretreatment for Enzymatic Saccharification of Wheat Straw. Biomass Bioenergy 2011, 35, 3094–3103. [Google Scholar] [CrossRef]
- Cianchetta, S.; Bregoli, L.; Galletti, S. Microplate-Based Evaluation of the Sugar Yield from Giant Reed, Giant Miscanthus and Switchgrass after Mild Chemical Pre-Treatments and Hydrolysis with Tailored Trichoderma Enzymatic Blends. Appl. Biochem. Biotechnol. 2017, 183, 876–892. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Cianchetta, S.; Nota, M.; Polidori, N.; Galletti, S. Alkali Pre-Treatment and Enzymatic Hydrolysis of Arundo donax for Single Cell Oil Production. Environ. Eng. Manag. J. 2019, 18, 1693–1701. [Google Scholar]
- Cianchetta, S.; Polidori, N.; Vasmara, C.; Ceotto, E.; Marchetti, R.; Galletti, S. Single Cell Oil Production from Hydrolysates of Alkali Pre-Treated Giant Reed: High Biomass-to-Lipid Yields with Selected Yeasts. Ind. Crops Prod. 2022, 178, 114596. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Sridharan, S.; Sowmya, V.; Yuvaraj, D.; Praveenkumar, R. Microbial Oil—A Plausible Alternate Resource for Food and Fuel Application. Bioresour. Technol. 2017, 233, 423–432. [Google Scholar] [CrossRef]
- Sitepu, I.R.; Garay, L.A.; Sestric, R.; Levin, D.; Block, D.E.; German, J.B.; Boundy-Mills, K.L. Oleaginous Yeasts for Biodiesel: Current and Future Trends in Biology and Production. Biotechnol. Adv. 2014, 32, 1336–1360. [Google Scholar] [CrossRef]
- Karatay, S.E.; Demiray, E.; Donmez, G. Efficient Approaches to Convert Coniochaeta hoffmannii Lipids into Biodiesel by In-Situ Transesterification. Bioresour. Technol. 2019, 285, 121321. [Google Scholar] [CrossRef]
- Sergeeva, Y.E.; Galanina, L.A.; Andrianova, D.A.; Feofilova, E.P. Lipids of Filamentous Fungi as a Material for Producing Biodiesel Fuel. Appl. Biochem. Microbiol. 2008, 44, 523–527. [Google Scholar] [CrossRef]
- Ruan, Z.; Zanotti, M.; Wang, X.; Ducey, C.; Liu, Y. Evaluation of Lipid Accumulation from Lignocellulosic Sugars by Mortierella isabellina for Biodiesel Production. Bioresour. Technol. 2012, 110, 198–205. [Google Scholar] [CrossRef]
- Šantek, M.I.; Grubišić, M.; Perečinec, M.G.; Beluhan, S.; Šantek, B. Lipid Production by Mortierella isabellina from Pretreated Corn Cobs and Effect of Lignocellulose Derived Inhibitors on Growth and Lipid Synthesis. Process Biochem. 2021, 109, 46–58. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of Oleaginous Yeasts. Part II: Technology and Potential Applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Ratledge, C.; Wynn, J.P. The Biochemistry and Molecular Biology of Lipid Accumulation in Oleaginous Microorganisms. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2002; Volume 51, pp. 1–52. ISBN 978-0-12-002653-1. [Google Scholar]
- Di Fidio, N.; Ragaglini, G.; Dragoni, F.; Antonetti, C.; Raspolli Galletti, A.M. Integrated Cascade Biorefinery Processes for the Production of Single Cell Oil by Lipomyces starkeyi from Arundo donax L. Hydrolysates. Bioresour. Technol. 2021, 325, 124635. [Google Scholar] [CrossRef]
- Di Fidio, N.; Liuzzi, F.; Mastrolitti, S.; Albergo, R.; De Bari, I. Single Cell Oil Production from Undetoxified Arundo donax L. Hydrolysate by Cutaneotrichosporon curvatus. J. Microbiol. Biotechnol. 2019, 29, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, D.; Fiorentino, N.; Impagliazzo, A.; Sannino, F.; Yousuf, A.; Zuccaro, G.; Fagnano, M. Lipid Production from Arundo donax Grown under Different Agronomical Conditions. Renew. Energy 2015, 77, 456–462. [Google Scholar] [CrossRef]
- Zuccaro, G.; Travaglini, G.; Caputo, G.; Pirozzi, D. Enzymatic Hydrolysis and Oleaginous Fermentation of Steam-Exploded Arundo donax and Lipomyces starkeyi in a Single Bioreactor for Microbial Oil Accumulation. J. Multidiscip. Eng. Sci. Stud. 2019, 5, 2577–2586. [Google Scholar]
- Galletti, S.; Cianchetta, S.; Righini, H.; Roberti, R. A Lignin-Rich Extract of Giant Reed (Arundo donax L.) as a Possible Tool to Manage Soilborne Pathogens in Horticulture: A Preliminary Study on a Model Pathosystem. Horticulturae 2022, 8, 589. [Google Scholar] [CrossRef]
- Vasmara, C.; Cianchetta, S.; Marchetti, R.; Ceotto, E.; Galletti, S. Hydrogen Production from Enzymatic Hydrolysates of Alkali Pre-Treated Giant Reed (Arundo donax L.). Energies 2022, 15, 4876. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Cianchetta, S.; Galletti, S.; Burzi, P.L.; Cerato, C. A Novel Microplate-Based Screening Strategy to Assess the Cellulolytic Potential of Trichoderma Strains. Biotechnol. Bioeng. 2010, 107, 461–468. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Galiotou-Panayotou, M.; Fakas, S.; Komaitis, M.; Aggelis, G. Lipid Production by Oleaginous Mucorales Cultivated on Renewable Carbon Sources. Eur. J. Lipid Sci. Technol. 2007, 109, 1060–1070. [Google Scholar] [CrossRef]
- Hara, A.; Radin, N.S. Lipid Extraction of Tissues with a Low-Toxicity Solvent. Anal. Biochem. 1978, 90, 420–426. [Google Scholar] [CrossRef]
- Matteo, R.; D’Avino, L.; Ramirez-Cando, L.J.; Pagnotta, E.; Angelini, L.G.; Spugnoli, P.; Tavarini, S.; Ugolini, L.; Foschi, L.; Lazzeri, L. Camelina (Camelina sativa L. Crantz) under Low-Input Management Systems in Northern Italy: Yields, Chemical Characterization and Environmental Sustainability. Ital. J. Agron. 2020, 15, 132–143. [Google Scholar] [CrossRef]
- Kalayasiri, P.; Jeyashoke, N.; Krisnangkura, K. Survey of Seed Oils for Use as Diesel Fuels. J. Am. Oil Chem. Soc. 1996, 73, 471–474. [Google Scholar] [CrossRef]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, Á. Influence of Fatty Acid Composition of Raw Materials on Biodiesel Properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, D.-K.; Lee, J.-P.; Park, S.-C.; Kim, Y.-J.; Lee, J.-S. Blending Effects of Biodiesels on Oxidation Stability and Low Temperature Flow Properties. Bioresour. Technol. 2008, 99, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Verduzco, L.F.; Rodríguez-Rodríguez, J.E.; Jaramillo-Jacob, A.D.R. Predicting Cetane Number, Kinematic Viscosity, Density and Higher Heating Value of Biodiesel from Its Fatty Acid Methyl Ester Composition. Fuel 2012, 91, 102–111. [Google Scholar] [CrossRef]
- SAS. SAS/STAT 14.1 User’s Guide. 2015. Available online: Https://Support.Sas.Com/Documentation/Onlinedoc/Stat/141/Intro.Pdf (accessed on 20 May 2023).
- ASTM U.S. Department of Energy. ASTM D6751 Biodiesel Specifications. Available online: Https://Afdc.Energy.Gov/Fuels/Biodiesel_specifications.Html (accessed on 24 February 2023).
- EN 14214; European Standard EN 14214: 2012+A1. European Committee for Standardization: Brussels, Belgium, 2014; pp. 1–21.
- Lemoes, J.S.; Lemons e Silva, C.F.; Farias Avila, S.P.; Scherrer Montero, C.R.; dos Anjos e Silva, S.D.; Samios, D.; Ruaro Peralba, M.d.C. Chemical Pretreatment of Arundo donax L. for Second-Generation Ethanol Production. Electron. J. Biotechnol. 2018, 31, 67–74. [Google Scholar] [CrossRef]
- Vasmara, C.; Cianchetta, S.; Marchetti, R.; Ceotto, E.; Galletti, S. Potassium Hydroxyde Pre-Treatment Enhances Methane Yield from Giant Reed (Arundo donax L.). Energies 2021, 14, 630. [Google Scholar] [CrossRef]
- Fakas, S.; Papanikolaou, S.; Batsos, A.; Galiotou-Panayotou, M.; Mallouchos, A.; Aggelis, G. Evaluating Renewable Carbon Sources as Substrates for Single Cell Oil Production by Cunninghamella echinulata and Mortierella isabellina. Biomass Bioenergy 2009, 33, 573–580. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Fakas, S.; Galiotou-Panayotou, M.; Komaitis, M.; Aggelis, G.; Papanikolaou, S. Commercial Sugars as Substrates for Lipid Accumulation in Cunninghamella echinulata and Mortierella isabellina Fungi. Eur. J. Lipid Sci. Technol. 2010, 112, 1048–1057. [Google Scholar] [CrossRef]
- Bellou, S.; Makri, A.; Sarris, D.; Michos, K.; Rentoumi, P.; Celik, A.; Papanikolaou, S.; Aggelis, G. The Olive Mill Wastewater as Substrate for Single Cell Oil Production by Zygomycetes. J. Biotechnol. 2014, 170, 50–59. [Google Scholar] [CrossRef]
- Fakas, S.; Čertik, M.; Papanikolaou, S.; Aggelis, G.; Komaitis, M.; Galiotou-Panayotou, M. γ-Linolenic Acid Production by Cunninghamella echinulata Growing on Complex Organic Nitrogen Sources. Bioresour. Technol. 2008, 99, 5986–5990. [Google Scholar] [CrossRef]
- Ruan, Z.; Zanotti, M.; Zhong, Y.; Liao, W.; Ducey, C.; Liu, Y. Co-Hydrolysis of Lignocellulosic Biomass for Microbial Lipid Accumulation. Biotechnol. Bioeng. 2013, 110, 1039–1049. [Google Scholar] [CrossRef]
- Vamvakaki, A.-N.; Kandarakis, I.; Kaminarides, S.; Komaitis, M.; Papanikolaou, S. Cheese Whey as a Renewable Substrate for Microbial Lipid and Biomass Production by Zygomycetes. Eng. Life Sci. 2010, 10, 348–360. [Google Scholar] [CrossRef]
- Economou, C.N.; Aggelis, G.; Pavlou, S.; Vayenas, D.V. Single Cell Oil Production from Rice Hulls Hydrolysate. Bioresour. Technol. 2011, 102, 9737–9742. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, X.; Zeng, J.; Chen, S. Feasibility of Filamentous Fungi for Biofuel Production Using Hydrolysate from Dilute Sulfuric Acid Pretreatment of Wheat Straw. Biotechnol. Biofuels 2012, 5, 50. [Google Scholar] [CrossRef]
- Fang, H.; Zhao, C.; Chen, S. Single Cell Oil Production by Mortierella Isabellina from Steam Exploded Corn Stover Degraded by Three-Stage Enzymatic Hydrolysis in the Context of on-Site Enzyme Production. Bioresour. Technol. 2016, 216, 988–995. [Google Scholar] [CrossRef]
- Kosa, G.; Zimmermann, B.; Kohler, A.; Ekeberg, D.; Afseth, N.K.; Mounier, J.; Shapaval, V. High-Throughput Screening of Mucoromycota Fungi for Production of Low- and High-Value Lipids. Biotechnol. Biofuels 2018, 11, 66. [Google Scholar] [CrossRef]
- Chen, H.-C.; Chang, C.-C. Production of γ-Linolenic Acid by the Fungus Cunninghamella echinulata CCRC 31840. Biotechnol. Prog. 1996, 12, 338–341. [Google Scholar] [CrossRef]
- Gardeli, C.; Athenaki, M.; Xenopoulos, E.; Mallouchos, A.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Lipid Production and Characterization by Mortierella (Umbelopsis) isabellina Cultivated on Lignocellulosic Sugars. J. Appl. Microbiol. 2017, 123, 1461–1477. [Google Scholar] [CrossRef]
- Alakhras, R.; Bellou, S.; Fotaki, G.; Stephanou, G.; Demopoulos, N.A.; Papanikolaou, S.; Aggelis, G. Fatty Acid Lithium Salts from Cunninghamella echinulata Have Cytotoxic and Genotoxic Effects on HL-60 Human Leukemia Cells. Eng. Life Sci. 2015, 15, 243–253. [Google Scholar] [CrossRef]
- Bogdan, V.I.; Koklin, A.E.; Krasovsky, V.G.; Lunin, V.V.; Sergeeva, Y.E.; Ivashechkin, A.A.; Feofilova, E.P. Production of Fatty Acid Methyl Esters That Are the Basis for Biodiesel Fuel from Mycelial Fungi Lipids Extracted by Supercritical CO2. Russ. J. Phys. Chem. B 2014, 8, 1004–1008. [Google Scholar] [CrossRef]
- Fakas, S.; Bellou, S.; Makri, A.; Aggelis, G. Single Cell Oil and Gamma-Linolenic Acid Production by Thamnidium elegans Grown on Raw Glycerol. In Microbial Conversions of Raw Glycerol; Aggelis G.: New York, NY, USA, 2009; pp. 85–99. ISBN 1-60692-392-7. [Google Scholar]
- Radwan, S.S.; Zreik, M.M.; Mulder, J.L. Distribution of Arachidonic Acid among Lipid Classes during Culture Ageing of Five Zygomycete Species. Mycol. Res. 1996, 100, 113–116. [Google Scholar] [CrossRef]
- Kavadia, A.; Komaitis, M.; Chevalot, I.; Blanchard, F.; Marc, I.; Aggelis, G. Lipid and γ-Linolenic Acid Accumulation in Strains of Zygomycetes Growing on Glucose. J. Am. Oil Chem. Soc. 2001, 78, 341–346. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Reader, S.L.; Davies, R.J. Fatty Acid Production Characteristics of Fungi with Particular Emphasis on Gamma Linolenic Acid Production. Biotechnol. Bioeng. 1993, 42, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Fakas, S.; Papanikolaou, S.; Galiotou-Panayotou, M.; Komaitis, M.; Aggelis, G. Lipids of Cunninghamella echinulata with Emphasis to γ-Linolenic Acid Distribution among Lipid Classes. Appl. Microbiol. Biotechnol. 2006, 73, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Kumar, P.; Kumar, D.; Syed, N.; Gupta, M.; Chanotiya, C.S.; Rout, P.K.; Khare, S.K. An Innovative Prosopis cineraria Pod Aqueous Waste as Natural Inhibitor for Enhancing Unsaturated Lipids Production in Yeast Cell Using Banana Peel. Waste Biomass Valorization 2022, 13, 3113–3126. [Google Scholar] [CrossRef]
- Yu, X.; Zeng, J.; Zheng, Y.; Chen, S. Effect of Lignocellulose Degradation Products on Microbial Biomass and Lipid Production by the Oleaginous Yeast Cryptococcus curvatus. Process Biochem. 2014, 49, 457–465. [Google Scholar] [CrossRef]
- Passoth, V.; Brandenburg, J.; Chmielarz, M.; Martín-Hernández, G.C.; Nagaraj, Y.; Müller, B.; Blomqvist, J. Oleaginous Yeasts for Biochemicals, Biofuels and Food from Lignocellulose-hydrolysate and Crude Glycerol. Yeast 2023, 1–13. [Google Scholar] [CrossRef]
- Vasmara, C.; Cianchetta, S.; Marchetti, R.; Ceotto, E.; Galletti, S. Co-Digestion of Pig Slurry and KOH Pre-Treated Giant Reed (Arundo donax L.) Enhances Methane Yield and Digestate Characteristics. Environ. Technol. Innov. 2023, 31, 103204. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Zhang, X.; Hu, F.; Ryu, D.D.Y.; Bao, J. Screening of Oleaginous Yeast Strains Tolerant to Lignocellulose Degradation Compounds. Appl. Biochem. Biotechnol. 2009, 159, 591–604. [Google Scholar] [CrossRef]
- Fei, Q.; Chang, H.N.; Shang, L.; Choi, J.; Kim, N.; Kang, J. The Effect of Volatile Fatty Acids as a Sole Carbon Source on Lipid Accumulation by Cryptococcus albidus for Biodiesel Production. Bioresour. Technol. 2011, 102, 2695–2701. [Google Scholar] [CrossRef]
- Harde, S.M.; Wang, Z.; Horne, M.; Zhu, J.Y.; Pan, X. Microbial Lipid Production from SPORL-Pretreated Douglas Fir by Mortierella isabellina. Fuel 2016, 175, 64–74. [Google Scholar] [CrossRef]
- Papanikolaou, S. Single Cell Oil (SCO) Production by Mortierella isabellina Grown on High-Sugar Content Media. Bioresour. Technol. 2004, 95, 287–291. [Google Scholar] [CrossRef]
- Chen, H.-C.; Liu, T.-M. Inoculum Effects on the Production of γ-Linolenic Acid by the Shake Culture of Cunninghamella echinulata CCRC 31840. Enzyme Microb. Technol. 1997, 21, 137–142. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Fakas, S.; Fick, M.; Chevalot, I.; Galiotou-Panayotou, M.; Komaitis, M.; Marc, I.; Aggelis, G. Biotechnological Valorisation of Raw Glycerol Discharged after Bio-Diesel (Fatty Acid Methyl Esters) Manufacturing Process: Production of 1,3-Propanediol, Citric Acid and Single Cell Oil. Biomass Bioenergy 2008, 32, 60–71. [Google Scholar] [CrossRef]
- Stredansky, M.; Conti, E.; Stredanska, S.; Zanetti, F. γ-Linolenic Acid Production with Thamnidium elegans by Solid-State Fermentation on Apple Pomace. Bioresour. Technol. 2000, 73, 41–45. [Google Scholar] [CrossRef]
- Zahan, K.; Kano, M. Biodiesel Production from Palm Oil, Its By-Products, and Mill Effluent: A Review. Energies 2018, 11, 2132. [Google Scholar] [CrossRef]
- Carota, E.; Crognale, S.; D’Annibale, A.; Petruccioli, M. Bioconversion of Agro-Industrial Waste into Microbial Oils by Filamentous Fungi. Process Saf. Environ. Prot. 2018, 117, 143–151. [Google Scholar] [CrossRef]
- Lujaji, F.; Bereczky, A.; Janosi, L.; Novak, C.; Mbarawa, M. Cetane Number and Thermal Properties of Vegetable Oil, Biodiesel, 1-Butanol and Diesel Blends. J. Therm. Anal. Calorim. 2010, 102, 1175–1181. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of Biodiesel Composition, Properties, and Specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]

| N Level | Yeast Extract (g/L) | (NH4)2SO4 (g/L) | Poly-Peptone (g/L) | Total N Supplemented (g/L) |
|---|---|---|---|---|
| N1 | 0.6 | 0.2 | 0.2 | 0.14 |
| N2 | 1.2 | 0.4 | 0.2 | 0.25 |
| N3 | 2.4 | 0.8 | 0.2 | 0.47 |
| Factor | [Cell Biomass] | [Oil] | Cellular Oil Content |
|---|---|---|---|
| Strain | *** | **** | ** |
| Substrate | * | * | * |
| Strain × Substrate | ** | ** | * |
| N supplementation | ** | ** | **** |
| Strain × N supplementation | ** | ** | ** |
| Substrate × N supplementation | ns | ns | ns |
| Strain × Substrate × N supplement. | * | ns | ** |
| Factor | Fatty Acid | |||||||
|---|---|---|---|---|---|---|---|---|
| Myristic Acid (C14:0) | Palmitic Acid (C16:0) | Palmitoleic Acid (C16:1) | Stearic Acid (C18:0) | Oleic Acid (C18:1) | Linoleic Acid (C18:2) | γ-Linolenic Acid (C18:3) | Other Fatty Acids | |
| Strain | ** | ** | * | * | ** | * | ** | ** |
| Substrate | ns | * | ns | ns | ns | ns | * | ns |
| Strain × Substrate | * | ** | ns | ns | ** | * | ns | ns |
| N suppl. | ns | * | * | * | ns | ns | *** | ns |
| Strain × N suppl. | ** | ** | ns | ns | ns | **** | ns | ns |
| Substrate × N suppl. | ns | ns | ns | ns | ns | ns | ns | ns |
| Strain × Subs. × N suppl. | ns | ns | ** | * | ** | * | ** | ns |
| Strain | Medium Id | Myristic Acid (C14:0) (%) | Palmitic Acid (C16:0) (%) | Palmitoleic Acid (C16:1) (%) | Stearic Acid (C18:0) (%) | Oleic Acid (C18:1) (%) | Linoleic Acid (C18:2) (%) | γ-Linolenic Acid (C18:3) (%) | Other Fatty Acids 1 (%) |
|---|---|---|---|---|---|---|---|---|---|
| Mortierella | GH/N1 | 0.7 d | 20.3 bc | 4.1 a | 2.0 b | 50.3 a–d | 17.3 cde | 3.5 g | 2.1 cd |
| isabellina | GH/N2 | 0.7 d | 22.3 ab | 3.1 abc | 2.8 ab | 51.8 abc | 14.2 ef | 3.2 g | 2.2 cd |
| GH/N3 | 0.8 d | 24.3 a | 2.2 a–d | 4.0 ab | 53.3 a | 10.7 g | 3.0 g | 1.8 d | |
| SS/N1 | 0.7 d | 20.7 bc | 4.0 a | 2.2 b | 52.7 ab | 14.6 ef | 3.1 g | 2.2 cd | |
| SS/N2 | 0.8 d | 21.3 abc | 4.0 a | 2.3 b | 52.5 ab | 14.1 efg | 3.1 g | 2.1 cd | |
| SS/N3 | 0.8 d | 23.5 ab | 3.2 ab | 2.8 ab | 52.6 ab | 12.5 fg | 3.1 g | 1.6 d | |
| MEAN | 0.7 B | 22.1 A | 3.4 A | 2.7 B | 52.2 A | 13.9 C | 3.1 C | 1.9 C | |
| Cunninghamella | GH/N1 | 0.5 d | 15.7 ef | 2.0 bcd | 4.1 ab | 40.6 ef | 20.1 bc | 13.4 ef | 3.5 a–d |
| echinulata | GH/N2 | 0.6 d | 14.4 efg | 1.5 cde | 4.9 a | 34.8 fg | 22.1 ab | 18.0 b-e | 3.8 a–d |
| (CCF 3882) | GH/N3 | 0.5 d | 12.4 g | 1.7 b-e | 4.3 ab | 32.4 g | 26.4 a | 20.2 a-d | 2.2 cd |
| SS/N1 | 0.5 d | 14.6 efg | 2.3 a-d | 2.8 ab | 44.9 cde | 19.4 bcd | 12.3 f | 3.3 a–d | |
| SS/N2 | 0.5 d | 14.7 efg | 1.9 bcd | 3.5 b ab | 45.4 b–e | 17.8 cde | 13.1 ef | 3.2 a–d | |
| SS/N3 | 0.6 d | 14.6 efg | 1.3 de | 5.4 a | 43.9 de | 16.9 cde | 14.9 def | 2.6 bcd | |
| MEAN | 0.5 B | 14.4 C | 1.8 AB | 4.2 A | 40.3 B | 20.4 A | 15.3 B | 3.1 B | |
| Thamnidium | GH/N1 | 3.4 bc | 20.4 bc | 0.8 e | 3.5 ab | 30.5 g | 19.3 bcd | 19.7 bcd | 2.4 cd |
| elegans | GH/N2 | 2.7 bc | 17.1 de | 1.2 de | 1.9 b | 28.7 g | 16.9 cde | 26.3 a | 5.2 abc |
| (CCF 1456) | GH/N3 | 0.9 d | 13.3 fg | 1.0 de | 3.0 ab | 34.5 fg | 16.7 cde | 23.7 ab | 6.8 a |
| SS/N1 | 5.5 a | 22.8 ab | 2.3 a–d | 2.6 ab | 30.4 g | 16.8 cde | 16.4 c–f | 3.2 a–d | |
| SS/N2 | 4.2 ab | 18.8 cd | 2.0 bcd | 3.5 ab | 30.6 g | 15.9 def | 20.9 abc | 4.0 a–d | |
| SS/N3 | 2.1 c | 14.6 efg | 1.3 de | 3.5 ab | 30.8 g | 18.6 bcd | 22.6 abc | 6.5 ab | |
| MEAN | 3.1 A | 17.8 B | 1.5 B | 3.0 B | 30.9 C | 17.4 B | 21.6 A | 4.8 A |
| Factor | Oil Yield per g Sugar Consumed | Oil Productivity | [γ-Linolenic Acid] |
|---|---|---|---|
| Strain | *** | *** | ** |
| Substrate | ns | ns | ns |
| Strain × Substrate | * | * | * |
| N supplementation | ** | *** | ns |
| Strain × N suppl. | * | **** | ns |
| Substrate × N suppl. | * | ns | ns |
| Strain × Subs. × N suppl. | * | ns | ns |
| Strain | γ-Linolenic Acid (g/L) | |
|---|---|---|
| GH | SS | |
| Mortierella isabellina | 166 cd | 196 c |
| Cunninghamella echinulata (CCF 3882) | 345 b | 595 a |
| Thamnidium elegans (CCF 1456) | 83 e | 122 de |
| Strain | Medium Id | IV | SV | DU | OS | LCSF | CFPP | HHV | D | KV | CN | C18:3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g I2/100 g | mg KOH/g | % | h | % | °C | MJ/kg | g/cm3 | mm2/s | min | % | ||
| Mortierella | GH/N1 | 88.4 | 193.8 | 98.2 | 8.1 | 3.7 | −3.8 | 39.8 | 0.875 | 4.31 | 58.6 | 4.2 |
| isabellina | GH/N2 | 82.8 | 193.8 | 91.9 | 9.1 | 4.7 | −0.4 | 39.8 | 0.875 | 4.36 | 59.9 | 4.1 |
| GH/N3 | 76.6 | 194.1 | 85.0 | 10.7 | 5.3 | 1.8 | 39.8 | 0.874 | 4.40 | 61.2 | 3.9 | |
| SS/N1 | 84.8 | 193.8 | 94.4 | 9.1 | 3.9 | −3.3 | 39.8 | 0.875 | 4.34 | 59.4 | 4.2 | |
| SS/N2 | 83.7 | 193.9 | 93.2 | 9.2 | 4.0 | −2.8 | 39.8 | 0.875 | 4.34 | 59.6 | 3.9 | |
| SS/N3 | 79.7 | 194.2 | 88.7 | 9.9 | 4.4 | −1.5 | 39.8 | 0.874 | 4.36 | 60.4 | 3.8 | |
| MEAN | 82.7 | 193.9 | 91.9 | 9.2 | 4.3 | −1.7 | 39.8 | 0.875 | 4.35 | 59.8 | 4.0 | |
| CV% | 5 | 0 | 5 | 10 | 14 | 1 a | 0 | 0 | 1 | 2 | 4 | |
| Cunninghamella | GH/N1 | 109.0 | 192.4 | 112.3 | 6.1 | 6.0 | 3.9 | 39.8 | 0.878 | 4.21 | 54.5 | 13.6 |
| echinulata | GH/N2 | 119.0 | 192.1 | 119.3 | 5.5 | 6.7 | 6.4 | 39.7 | 0.879 | 4.14 | 52.4 | 19.3 |
| (CCF 3882) | GH/N3 | 129.0 | 192.1 | 128.9 | 5.1 | 5.1 | 0.9 | 39.7 | 0.881 | 4.05 | 50.0 | 18.9 |
| SS/N1 | 108.6 | 192.3 | 113.3 | 6.3 | 4.4 | −1.4 | 39.8 | 0.878 | 4.21 | 54.6 | 12.8 | |
| SS/N2 | 107.8 | 192.2 | 111.5 | 6.4 | 5.1 | 0.8 | 39.8 | 0.878 | 4.23 | 54.8 | 12.9 | |
| SS/N3 | 108.5 | 192.1 | 110.6 | 6.3 | 5.8 | 3.5 | 39.8 | 0.878 | 4.22 | 54.7 | 13.6 | |
| MEAN | 113.6 | 192.2 | 116.0 | 5.9 | 5.5 | 2.4 | 39.8 | 0.879 | 4.18 | 53.5 | 15.2 | |
| CV% | 8 | 0 | 6 | 9 | 15 | 1 | 0 | 0 | 2 | 4 | 20 | |
| Thamnidium | GH/N1 | 112.9 | 194.5 | 110.7 | 5.6 | 6.1 | 4.2 | 39.7 | 0.879 | 4.07 | 52.7 | 20.0 |
| elegans | GH/N2 | 127.0 | 193.4 | 119.6 | 5.3 | 7.4 | 8.7 | 39.7 | 0.881 | 4.01 | 50.2 | 27.3 |
| (CCF 1456) | GH/N3 | 126.2 | 191.8 | 121.2 | 5.5 | 7.5 | 9.0 | 39.7 | 0.880 | 4.09 | 51.0 | 26.8 |
| SS/N1 | 101.8 | 195.8 | 100.9 | 6.1 | 6.5 | 5.6 | 39.7 | 0.877 | 4.10 | 54.8 | 17.2 | |
| SS/N2 | 112.8 | 194.7 | 109.0 | 5.8 | 6.5 | 5.7 | 39.7 | 0.878 | 4.06 | 52.7 | 20.7 | |
| SS/N3 | 123.5 | 192.6 | 119.2 | 5.5 | 7.9 | 10.5 | 39.7 | 0.880 | 4.08 | 51.3 | 24.7 | |
| MEAN | 117.3 | 193.8 | 113.4 | 5.6 | 6.9 | 7.2 | 39.7 | 0.879 | 4.07 | 52.1 | 22.8 | |
| CV% | 8 | 1 | 7 | 5 | 10 | 1 | 0 | 0 | 1 | 3 | 18 | |
| U.S. (ASTM D6751) | >3 | 1.9–6.0 | >47 | |||||||||
| E.U. (EN 14214) | <120 | >8 | 0.86–0.9 | 3.5–5.0 | >51 | <12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cianchetta, S.; Ceotto, E.; Galletti, S. Microbial Oil Production from Alkali Pre-Treated Giant Reed (Arundo donax L.) by Selected Fungi. Energies 2023, 16, 5398. https://doi.org/10.3390/en16145398
Cianchetta S, Ceotto E, Galletti S. Microbial Oil Production from Alkali Pre-Treated Giant Reed (Arundo donax L.) by Selected Fungi. Energies. 2023; 16(14):5398. https://doi.org/10.3390/en16145398
Chicago/Turabian StyleCianchetta, Stefano, Enrico Ceotto, and Stefania Galletti. 2023. "Microbial Oil Production from Alkali Pre-Treated Giant Reed (Arundo donax L.) by Selected Fungi" Energies 16, no. 14: 5398. https://doi.org/10.3390/en16145398
APA StyleCianchetta, S., Ceotto, E., & Galletti, S. (2023). Microbial Oil Production from Alkali Pre-Treated Giant Reed (Arundo donax L.) by Selected Fungi. Energies, 16(14), 5398. https://doi.org/10.3390/en16145398







