A Critical Review of Growth Media Recycling to Enhance the Economics and Sustainability of Algae Cultivation
Abstract
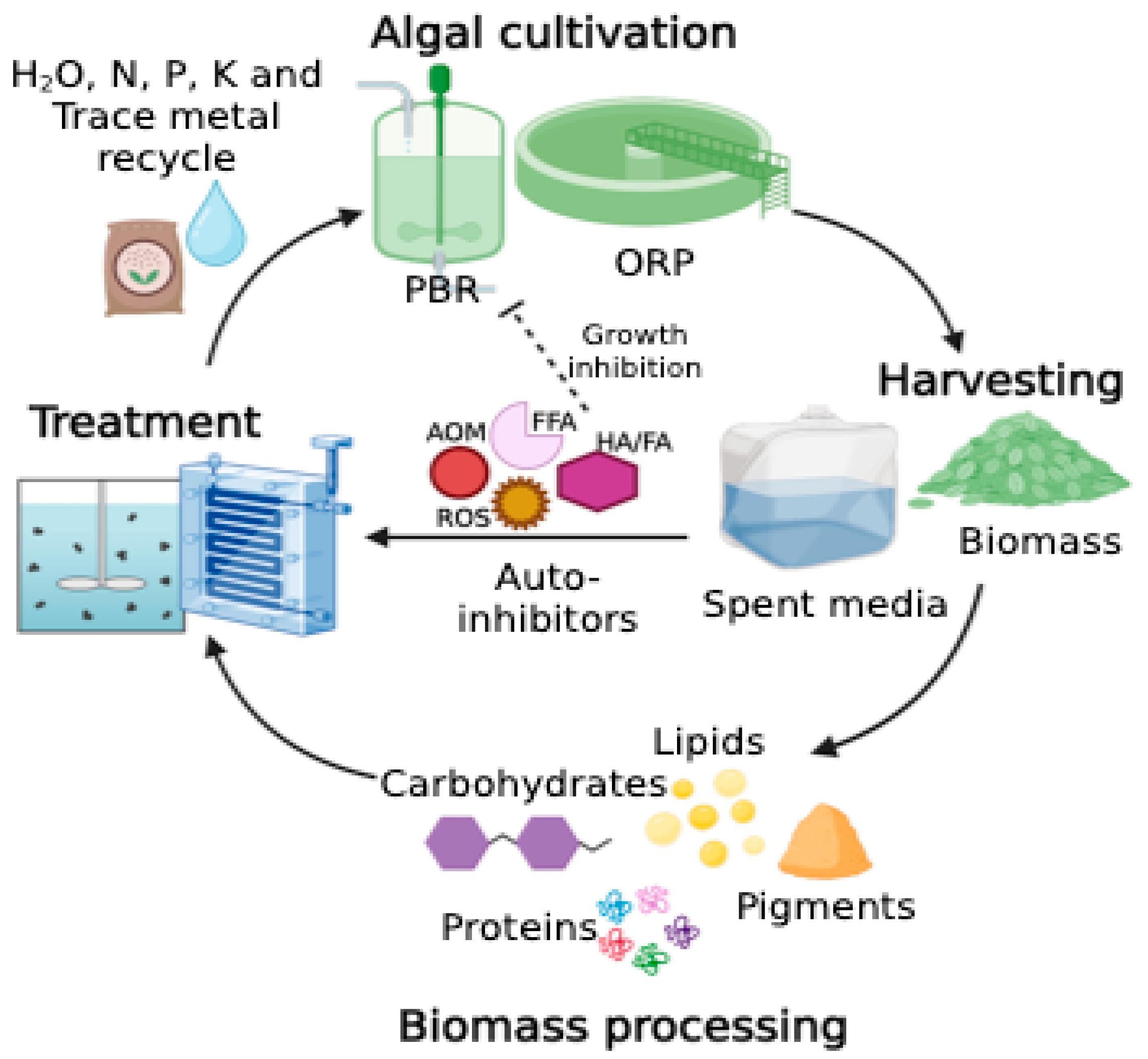
:1. Introduction
2. Effect of Media Recycling on Algal Growth and Biochemical Composition
3. Impact of Auto-Inhibitors on Algal Growth
4. Strategies to Increase Growth Media Recycling
4.1. Ultraviolet Advanced Oxidation
4.2. Activated Carbon
4.3. Ultrasonication
4.4. Microfiltration
4.5. Crop Rotation
4.6. Nutrient Replenishment
5. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loke Show, P. Global market and economic analysis of microalgae technology: Status and perspectives. Bioresour. Technol. 2022, 357, 127329. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Philippidis, G.P. The prospects of algae-derived vitamins and their precursors for sustainable cosmeceuticals. Processes 2023, 11, 587. [Google Scholar] [CrossRef]
- Martin, M.; Harris, S. Prospecting the sustainability implications of an emerging industrial symbiosis network. Resour. Conserv. Recycl. 2018, 138, 246–256. [Google Scholar] [CrossRef]
- Guieysse, B.; Bechet, Q.; Shilton, A. Variability and uncertainty in water demand and water footprint assessments of fresh algae cultivation based on case studies from five climatic regions. Bioresour. Technol. 2013, 128, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Batan, L.; Quinn, J.C.; Bradley, T.H. Analysis of water footprint of a photobioreactor microalgae biofuel production system from blue, green and lifecycle perspectives. Algal Res. 2013, 2, 196–203. [Google Scholar] [CrossRef]
- Hoekstra, A.Y. Water Footprint Assessment: Evolvement of a New Research Field. Water Resour. Manag. 2017, 31, 3061–3081. [Google Scholar] [CrossRef] [Green Version]
- Gerbens-Leenes, P.W.; Xu, L.; de Vries, G.J.; Hoekstra, A.Y. The blue water footprint and land use of biofuels from algae. Water Resour. Res. 2014, 50, 8549–8563. [Google Scholar] [CrossRef]
- Banerjee, A.; Gautam, R.; Mudliar, S.; Bhaskar, T.; Ghosh, D. Water footprint and wastewater quality assessment of yeast single cell oil production: Gate to gate approach for industrial water sustainability. Sci. Total Environ. 2023, 866, 161127. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Nagappan, S.; Bhosale, R.R.; Tsai, P.-C.; Natarajan, S.; Devendran, S.; Al-Haj, L.; Ponnusamy, V.K.; Kumar, G. Various potential techniques to reduce the water footprint of microalgal biomass production for biofuel—A review. Sci. Total Environ. 2020, 749, 142218. [Google Scholar] [CrossRef]
- Quiroz, D.; Greene, J.M.; McGowen, J.; Quinn, J.C. Geographical assessment of open pond algal productivity and evaporation losses across the United States. Algal Res. 2021, 60, 102483. [Google Scholar] [CrossRef]
- Feng, P.-Z.; Zhu, L.-D.; Qin, X.-X.; Li, Z.-H. Water footprint of biodiesel production from microalgae cultivated in photobioreactors. J. Environ. Eng. 2016, 142, 04016067. [Google Scholar] [CrossRef]
- Sun, J.; Yang, L.; Xiao, S.; Chu, H.; Jiang, S.; Yu, Z.; Zhou, X.; Zhang, Y. A promising microalgal wastewater cyclic cultivation technology: Dynamic simulations, economic viability, and environmental suitability. Water Res. 2022, 217, 118411. [Google Scholar] [CrossRef]
- Yang, J.; Xu, M.; Zhang, X.; Hu, Q.; Sommerfeld, M.; Chen, Y. Life-cycle analysis on biodiesel production from microalgae: Water footprint and nutrients balance. Bioresour. Technol. 2011, 102, 159–165. [Google Scholar] [CrossRef]
- Lu, Z.; Sha, J.; Wang, W.; Li, Y.; Wang, G.; Chen, Y.; Hu, Q.; Zhang, X. Identification of auto-inhibitors in the reused culture media of the Chlorophyta Scenedesmus acuminatus. Algal Res. 2019, 44, 101665. [Google Scholar] [CrossRef]
- Loftus, S.E.; Hunt, D.E.; Johnson, Z.I. Reused cultivation water from a self-inhibiting alga does not inhibit other algae but alters their microbiomes. Algal Res. 2020, 51, 102067. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Sacchi, R.; Masi, P. Techno-economic assessment of DHA-rich Aurantiochytrium sp. production using food industry by-products and waste streams as alternative growth media. Bioresour. Technol. Rep. 2022, 18, 100997. [Google Scholar] [CrossRef]
- Kwan, T.H.; Pleissner, D.; Lau, K.Y.; Venus, J.; Pommeret, A.; Lin, C.S.K. Techno-economic analysis of a food waste valorization process via microalgae cultivation and co-production of plasticizer, lactic acid and animal feed from algal biomass and food waste. Bioresour. Technol. 2015, 198, 292–299. [Google Scholar] [CrossRef]
- Lane, T.W. Barriers to microalgal mass cultivation. Curr. Opin. Biotechnol. 2022, 73, 323–328. [Google Scholar] [CrossRef]
- Molina-Miras, A.; López-Rosales, L.; Sánchez-Mirón, A.; López-Rodríguez, M.; Cerón-García, M.C.; García-Camacho, F.; Molina-Grima, E. Influence of culture medium recycling on the growth of a marine dinoflagellate microalga and bioactives production in a raceway photobioreactor. Algal Res. 2020, 47, 101820. [Google Scholar] [CrossRef]
- Sukkrom, K.; Bunnag, B.; Powtongsook, S.; Siangdung, W.; Pavasant, P. Biomass and lipid enhancement in Ankistrodesmus sp. cultured with reused and minimal nutrients media. Prep. Biochem. Biotechnol. 2016, 46, 467–473. [Google Scholar] [CrossRef]
- Morocho-Jácome, A.L.; Mascioli, G.F.; Sato, S.; de Carvalho, J.C.M. Evaluation of physicochemical treatment conditions for the reuse of a spent growth medium in Arthrospira platensis cultivation. Algal Res. 2016, 13, 159–166. [Google Scholar] [CrossRef]
- Depraetere, O.; Pierre, G.; Noppe, W.; Vandamme, D.; Foubert, I.; Michaud, P.; Muylaert, K. Influence of culture medium recycling on the performance of Arthrospira platensis cultures. Algal Res. 2015, 10, 48–54. [Google Scholar] [CrossRef]
- Bagul, V.P.; Annapure, U.S. Effect of sequential recycling of spent media wastewater on docosahexaenoic acid production by newly isolated strain Aurantiochytrium sp. ICTFD5. Bioresour. Technol. 2020, 306, 123153. [Google Scholar] [CrossRef]
- Kumaran, J.; Singh, I.S.B.; Joseph, V. Effective biomass harvesting of marine diatom Chaetoceros muelleri by chitosan-induced flocculation, preservation of biomass, and recycling of culture medium for aquaculture feed application. J. Appl. Phycol. 2021, 33, 1605–1619. [Google Scholar] [CrossRef]
- Delrue, F.; Imbert, Y.; Fleury, G.; Peltier, G.; Sassi, J.-F. Using coagulation–flocculation to harvest Chlamydomonas reinhardtii: Coagulant and flocculant efficiencies, and reuse of the liquid phase as growth medium. Algal Res. 2015, 9, 283–290. [Google Scholar] [CrossRef]
- Yu, Z.; Pei, H.; Hou, Q.; Nie, C.; Zhang, L.; Yang, Z.; Wang, X. The effects of algal extracellular substances on algal growth, metabolism and long-term medium recycle, and inhibition alleviation through ultrasonication. Bioresour. Technol. 2018, 267, 192–200. [Google Scholar] [CrossRef]
- Daiek, C.; Liao, W.; Liu, Y. Effects of water recirculation on microalgae assemblage and corresponding sustainability of the photobioreactor cultivation system. Biomass Bioenergy 2022, 157, 106326. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, Y.; Huang, W.; Zhang, C.; Li, T.; Zhang, Y.; Li, A. Evaluation of flocculation induced by pH increase for harvesting microalgae and reuse of flocculated medium. Bioresour. Technol. 2012, 110, 496–502. [Google Scholar] [CrossRef]
- Hadj-Romdhane, F.; Jaouen, P.; Pruvost, J.; Grizeau, D.; Van Vooren, G.; Bourseau, P. Development and validation of a minimal growth medium for recycling Chlorella vulgaris culture. Bioresour. Technol. 2012, 123, 366–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, W.; Naqvi, S.R.; Sajid, M.; Shrivastav, A.; Kumar, K. Monitoring lipids profile, CO2 fixation, and water recyclability for the economic viability of microalgae Chlorella vulgaris cultivation at different initial nitrogen. J. Biotechnol. 2022, 345, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Hadj-Romdhane, F.; Zheng, X.; Jaouen, P.; Pruvost, J.; Grizeau, D.; Croue, J.P.; Bourseau, P. The culture of Chlorella vulgaris in a recycled supernatant: Effects on biomass production and medium quality. Bioresour. Technol. 2013, 132, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z.; Duan, Y.; Wang, H. The effect of recycling culture medium after harvesting of Chlorella vulgaris biomass by flocculating bacteria on microalgal growth and the functionary mechanism. Bioresour. Technol. 2019, 280, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Lo, E.; Arora, N.; Philippidis, G.P. Deciphering metabolic alterations in algae cultivated in spent media as means for enhancing algal biorefinery sustainability. Bioresour. Technol. 2021, 342, 125890. [Google Scholar] [CrossRef] [PubMed]
- Farooq, W.; Moon, M.; Ryu, B.-G.; Suh, W.I.; Shrivastav, A.; Park, M.S.; Mishra, S.K.; Yang, J.-W. Effect of harvesting methods on the reusability of water for cultivation of Chlorella vulgaris, its lipid productivity and biodiesel quality. Algal Res. 2015, 8, 1–7. [Google Scholar] [CrossRef]
- Chu, R.; Hu, D.; Zhu, L.; Li, S.; Yin, Z.; Yu, Y. Recycling spent water from microalgae harvesting by fungal pellets to re-cultivate Chlorella vulgaris under different nutrient loads for biodiesel production. Bioresour. Technol. 2022, 344, 126227. [Google Scholar] [CrossRef]
- Dasan, Y.K.; Lam, M.K.; Lim, J.W.; Tan, I.S.; Foo, H.C.Y.; Kiew, P.L.; Show, P.L.; Lee, K.T. The impact of using recycled culture medium to grow Chlorella vulgaris in a sequential flow system: Evaluation on growth, carbon removal, and biochemical compositions. Biomass Bioenergy 2022, 159, 106412. [Google Scholar] [CrossRef]
- Augustine, A.; Tanwar, A.; Tremblay, R.; Kumar, S. Flocculation processes optimization for reuse of culture medium without pH neutralization. Algal Res. 2019, 39, 101437. [Google Scholar] [CrossRef]
- Nigam, H.; Jain, R.; Malik, A.; Singh, V. Comparative Life-Cycle assessment of microalgal biomass production in conventional growth media versus newly developed nanoemulsion media. Bioresour. Technol. 2022, 352, 127069. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, L.; Hu, D.; Li, S.; Chu, R.; Liu, C.; Lv, Y.; Bao, J.; Xiang, M.; Zhu, L. Biocompatible magnetic flocculant for efficient harvesting of microalgal cells: Isotherms, mechanisms and water recycling. Sep. Purif. Technol. 2021, 279, 119679. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Z.; Hiltunen, E. Microalgae Chlorella vulgaris biomass harvesting by natural flocculant: Effects on biomass sedimentation, spent medium recycling and lipid extraction. Biotechnol. Biofuels 2018, 11, 183. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.K.; Chakraborty, S. Multiscale integration of mixotrophic microalgal cultivation, lipid synthesis, rapid biomass harvesting, and nutrient recycling in pilot-scale photobioreactors. Algal Res. 2021, 53, 102146. [Google Scholar] [CrossRef]
- Zhu, L.D.; Takala, J.; Hiltunen, E.; Wang, Z.M. Recycling harvest water to cultivate Chlorella zofingiensis under nutrient limitation for biodiesel production. Bioresour. Technol. 2013, 144, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Monte, J.; Sá, M.; Parreira, C.; Galante, J.; Serra, A.R.; Galinha, C.F.; Costa, L.; Pereira, V.J.; Brazinha, C.; Crespo, J.G. Recycling of Dunaliella salina cultivation medium by integrated membrane filtration and advanced oxidation. Algal Res. 2019, 39, 101460. [Google Scholar] [CrossRef]
- Chuka-ogwude, D.; Nafisi, M.; Vadiveloo, A.; Taher, H.; Bahri, P.A.; Moheimani, N.R. Effect of medium recycling, culture depth, and mixing duration on D. salina growth. Algal Res. 2021, 60, 102495. [Google Scholar] [CrossRef]
- Salim, S.; Shi, Z.; Vermue, M.H.; Wijffels, R.H. Effect of growth phase on harvesting characteristics, autoflocculation and lipid content of Ettlia texensis for microalgal biodiesel production. Bioresour. Technol. 2013, 138, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Garzon-Sanabria, A.J.; Ramirez-Caballero, S.S.; Moss, F.E.; Nikolov, Z.L. Effect of algogenic organic matter (AOM) and sodium chloride on Nannochloropsis salina flocculation efficiency. Bioresour. Technol. 2013, 143, 231–237. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Z.; Wang, Y.; Wensel, P.; Sommerfeld, M.; Hu, Q. Recycling Nannochloropsis oceanica culture media and growth inhibitors characterization. Algal Res. 2016, 20, 282–290. [Google Scholar] [CrossRef]
- Fret, J.; Roef, L.; Blust, R.; Diels, L.; Tavernier, S.; Vyverman, W.; Michiels, M. Reuse of rejuvenated media during laboratory and pilot scale cultivation of Nannochloropsis sp. Algal Res. 2017, 27, 265–273. [Google Scholar] [CrossRef]
- Fret, J.; Roef, L.; Diels, L.; Tavernier, S.; Vyverman, W.; Michiels, M. Combining medium recirculation with alternating the microalga production strain: A laboratory and pilot scale cultivation test. Algal Res. 2020, 46, 101763. [Google Scholar] [CrossRef]
- Sabia, A.; Baldisserotto, C.; Biondi, S.; Marchesini, R.; Tedeschi, P.; Maietti, A.; Giovanardi, M.; Ferroni, L.; Pancaldi, S. Re-cultivation of Neochloris oleoabundans in exhausted autotrophic and mixotrophic media: The potential role of polyamines and free fatty acids. Appl. Microbiol. Biotechnol. 2015, 99, 10597–10609. [Google Scholar] [CrossRef]
- Wang, W.; Sha, J.; Lu, Z.; Shao, S.; Sun, P.; Hu, Q.; Zhang, X. Implementation of UV-based advanced oxidation processes in algal medium recycling. Sci. Total Environ. 2018, 634, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Grabski, K.; Tukaj, Z. Autoinduction activity of a conditioned medium obtained from high density cultures of the green alga Scenedesmus subspicatus. J. Appl. Phycol. 2007, 20, 323–330. [Google Scholar] [CrossRef]
- Pandey, A.; Shah, R.; Yadav, P.; Verma, R.; Srivastava, S. Harvesting of freshwater microalgae Scenedesmus sp. by electro-coagulation-flocculation for biofuel production: Effects on spent medium recycling and lipid extraction. Environ. Sci. Pollut. Res. Int. 2020, 27, 3497–3507. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Thaher, M.I.; Abdul Hakim, M.A.; Al-Jabri, H.M.; Alghasal, G.S. Microalgae harvesting by pH adjusted coagulation-flocculation, recycling of the coagulant and the growth media. Bioresour. Technol. 2016, 216, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.S.; Pinto, F.H.V.; Melão, M.G.G.; Lombardi, A.T. Growing Scenedesmus quadricauda in used culture media: Is it viable? J. Appl. Phycol. 2014, 27, 171–178. [Google Scholar] [CrossRef]
- Fon Sing, S.; Isdepsky, A.; Borowitzka, M.A.; Lewis, D.M. Pilot-scale continuous recycling of growth medium for the mass culture of a halotolerant Tetraselmis sp. in raceway ponds under increasing salinity: A novel protocol for commercial microalgal biomass production. Bioresour. Technol. 2014, 161, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Dzuman, M.J.; Severo, I.A.; Moreira, M.A.C.; de Lima Luz Junior, L.F.; Mitchell, D.A.; Vargas, J.V.C.; Mariano, A.B. Microalgae culture medium recycling: Improved production of biomass and lipids, biodiesel properties and cost reduction. Bioenergy Res. 2022, 15, 2076–2089. [Google Scholar] [CrossRef]
- Oh, H.-M.; Lee, S.J.; Park, M.-H.; Kim, H.-S.; Kim, H.-C.; Yoon, J.-H.; Kwon, G.-S.; Yoon, B.-D. Harvesting of Chlorella vulgaris using a bioflocculant from Paenibacillus sp. AM49. Biotechnol. Lett. 2001, 23, 1229–1234. [Google Scholar] [CrossRef]
- Song, Q.; Kong, F.; Liu, B.-F.; Song, X.; Ren, N.-Q.; Ren, H.-Y. Insights into the effect of Rhamnolipids on the anaerobic fermentation and microalgae lipid production of waste activated sludge: Performance and mechanisms. ACS EST Eng. 2023, 3, 438–448. [Google Scholar] [CrossRef]
- Song, X.; Kong, F.; Liu, B.F.; Song, Q.; Ren, N.Q.; Ren, H.Y. Thallium-mediated NO signaling induced lipid accumulation in microalgae and its role in heavy metal bioremediation. Water Res. 2023, 239, 120027. [Google Scholar] [CrossRef]
- Higgins, B.T.; Wang, Q.; Du, S.; Hennebelle, M.; Taha, A.Y.; Fiehn, O.; VanderGheynst, J.S. Impact of thiamine metabolites and spent medium from Chlorella sorokiniana on metabolism in the green algae Auxenochlorella prototheciodes. Algal Res. 2018, 33, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Klok, A.; Lamers, P.; Martens, D.; Draaisma, R.; Wijffels, R. Edible oils from microalgae: Insights in TAG accumulation. Trends Biotechnol. 2014, 32, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Abalde, J.; Fabregas, J.; Herrero, C. β-Carotene, vitamin C and vitamin E content of the marine microalga Dunaliella tertiolecta cultured with different nitrogen sources. Bioresour. Technol. 1991, 38, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.K.; Hietala, D.C.; Cardinale, B.J. Tolerance to allelopathic inhibition by free fatty acids in five biofuel candidate microalgae strains. Bioresour. Technol. Rep. 2023, 21, 101321. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Fraeye, I.; Muylaert, K. Influence of organic matter generated by Chlorella vulgaris on five different modes of flocculation. Bioresour. Technol. 2012, 124, 508–511. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Liang, L.; Liu, K.; Xie, L.; Huang, L.; He, W.; Chen, Y.; Xue, T. Spent yeast as an efficient medium supplement for fucoxanthin and eicosapentaenoic acid (EPA) production by Phaeodactylum tricornutum. J. Appl. Phycol. 2019, 32, 59–69. [Google Scholar] [CrossRef]
- Pratt, R. Studies on Chlorella Vulgaris. V. Some Properties of the Growth-Inhibitor Formed by Chlorella Cells. Am. J. Bot. 1942, 29, 142–148. [Google Scholar] [CrossRef]
- Hellebust, J.A. Excretion of some organic compounds by marine phytoplankton. Limnol. Oceanogr. 1965, 10, 192–206. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, M.; Liu, J. Auto-signals in Haematococcus pluvialis. Trans. Oceanol. Limnol. 2001, 3, 22–28. [Google Scholar]
- Imada, N.; Kobayashi, K.; Tahara, K.; Oshima, Y. Production of an autoinhibitor by Skeletonema costatum and its effect on the growth of other phytoplankton. Nippon Suisan Gakkaishi 1991, 57, 2285–2290. [Google Scholar] [CrossRef]
- Yingying, S.; Changhai, W.; Jing, C. Growth inhibition of the eight species of microalgae by growth inhibitor from the culture of Isochrysis galbana and its isolation and identification. J. Appl. Phycol. 2008, 20, 315–321. [Google Scholar] [CrossRef]
- Chaïb, S.; Pistevos, J.C.A.; Bertrand, C.; Bonnard, I. Allelopathy and allelochemicals from microalgae: An innovative source for bio-herbicidal compounds and biocontrol research. Algal Res. 2021, 54, 102213. [Google Scholar] [CrossRef]
- Wu, J.-T.; Chiang, Y.-R.; Huang, W.-Y.; Jane, W.-N. Cytotoxic effects of free fatty acids on phytoplankton algae and cyanobacteria. Aquat. Toxicol. 2006, 80, 338–345. [Google Scholar] [CrossRef]
- Sun, B.K.; Tanji, Y.; Unno, H. Influences of iron and humic acidon the growth of the cyanobacterium Anabaena circinalis. Biochem. Eng. J. 2005, 24, 195–201. [Google Scholar] [CrossRef]
- Sun, B.-K.; Tanji, Y.; Unno, H. Extinction of cells of cyanobacterium Anabaena circinalis in the presence of humic acid under illumination. Appl. Microbiol. Biotechnol. 2006, 72, 823–828. [Google Scholar] [CrossRef] [Green Version]
- Sha, J.; Lu, Z.; Ye, J.; Wang, G.; Hu, Q.; Chen, Y.; Zhang, X. The inhibition effect of recycled Scenedesmus acuminatus culture media: Influence of growth phase, inhibitor identification and removal. Algal Res. 2019, 42, 101612. [Google Scholar] [CrossRef]
- Ribalet, F.; Berges, J.A.; Ianora, A.; Casotti, R. Growth inhibition of cultured marine phytoplankton by toxic algal-derived polyunsaturated aldehydes. Aquat. Toxicol. 2007, 85, 219–227. [Google Scholar] [CrossRef]
- Casotti, R.; Mazza, S.; Brunet, C.; Vantrepotte, V.; Ianora, A.; Miralto, A. Growth inhibition and toxicity of the diatom Aldehyde 2-trans, 4-trans-decadienal on Thalassiosira weissflogii (bacillariophyceae). J. Phycol. 2005, 41, 7–20. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- M’Arimi, M.M.; Mecha, C.A.; Kiprop, A.K.; Ramkat, R. Recent trends in applications of advanced oxidation processes (AOPs) in bioenergy production: Review. Renew. Sustain. Energy Rev. 2020, 121, 109669. [Google Scholar] [CrossRef]
- Sher, F.; Hanif, K.; Rafey, A.; Khalid, U.; Zafar, A.; Ameen, M.; Lima, E.C. Removal of micropollutants from municipal wastewater using different types of activated carbons. J. Environ. Manag. 2021, 278, 111302. [Google Scholar] [CrossRef] [PubMed]
- Agarwalla, A.; Mishra, S.; Mohanty, K. Treatment and recycle of harvested microalgal effluent using powdered activated carbon for reducing water footprint and enhancing biofuel production under a biorefinery model. Bioresour. Technol. 2022, 360, 127598. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yang, Z.; Lu, Z.; Wang, Q.; Liu, J.; Song, L. Combination of utilization of CO2 from flue gas of biomass power plant and medium recycling to enhance cost-effective Spirulina production. J. Appl. Phycol. 2019, 31, 2175–2185. [Google Scholar] [CrossRef]
- Servais, P.; Billen, G.; Bouillot, P. Biological colonization of granular activated carbon filters in drinking-water treatment. J. Environ. Eng. 1994, 120, 888–899. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, H.; Gao, B.; Liu, Y.; Zhai, J.; Zhang, S.; Xu, B. Ultrasound-initiated synthesis of cationic polyacrylamide for oily wastewater treatment: Enhanced interaction between the flocculant and contaminants. Ultrason. Sonochem. 2018, 42, 31–41. [Google Scholar] [CrossRef]
- Barzegar, G.; Jorfi, S.; Zarezade, V.; Khatebasreh, M.; Mehdipour, F.; Ghanbari, F. 4-Chlorophenol degradation using ultrasound/peroxymonosulfate/nanoscale zero valent iron: Reusability, identification of degradation intermediates and potential application for real wastewater. Chemosphere 2018, 201, 370–379. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Takdastan, A.; Jorfi, S.; Ghanbari, F.; Ahmadi, M.; Barzegar, G. The performance study on ultrasonic/Fe3O4/H2O2 for degradation of azo dye and real textile wastewater treatment. J. Mol. Liq. 2018, 256, 462–470. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Rittmann, B.E. Effect of permeate recycling and light intensity on growth kinetics of Synechocystis sp. PCC 6803. Algal Res. 2017, 27, 170–176. [Google Scholar] [CrossRef]
- Ahmad, T.; Guria, C.; Mandal, A. A review of oily wastewater treatment using ultrafiltration membrane: A parametric study to enhance the membrane performance. J. Water Process. Eng. 2020, 36, 101289. [Google Scholar] [CrossRef]
- Rodolfi, L.; Zittelli, G.C.; Barsanti, L.; Rosati, G.; Tredici, M.R. Growth medium recycling in Nannochloropsis sp. mass cultivation. Biomol. Eng. 2003, 20, 243–248. [Google Scholar] [CrossRef]
| Algae strain | Cultivation Media | Growth Cultivation Conditions | Biomass Harvesting Method | Pretreatment of Media before Recycling | Percentage of Recycled Media (%) | Number of Recycling Cycles | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | pH | Light Cycle | |||||||
| Amphidinium carterae Dn241EHU | Mediterranean seawater | 21 | 8.5 | 12:12 | centrifugation | 0.2 μm filtration and autoclave | 75 | 4 | [19] |
| Ankistrodesmus sp. | BG11 media | 30 | NR | natural light | NR | 100 | 2 | [20] | |
| Arthrospira platensis UTEX1296 | Modified Schlösser medium | 32 | 9.5 | continuous | flocculation and absorption | Whatman filter paper no.3 | 1 | [21] | |
| Arthrospira platensis 21.99 | Modified Zarrouk medium | 20 | NR | 16:8 | micro-strainer | NR | 4 | [22] | |
| Aurantiochytrium sp. ICTFD5 | Glucose and Yeast media | 25 | 6.8 | NR | centrifugation | 50 | 3 | [23] | |
| Chaetoceros muelleri | Modified F/2 medium | 8.0 | 16:8 | flocculation (chitosan) | 100 | 1 | [24] | ||
| Chlamydomonas reinhardtii DW15 | Minimal medium | 7.2 | continuous | flocculation | 0.2 μm filtration | 1 | [25] | ||
| Chlorella SDEC-18 | BG11 media | NR | centrifugation | ultrasonication | 4 | [26] | |||
| Chlorella sorokiniana MSU | Modified TAP medium | 6.2 | NR | 50 | - | [27] | |||
| Chlorella vulgaris | BG11 media | 7.5 | pH Flocculation | 100 | 1 | [28] | |||
| Chlorella vulgaris 221/19SAG | HAMGM media | centrifugation | 16 | [29] | |||||
| Chlorella vulgaris UTEX265 | BG11 media | 7.0 | 3 | [30] | |||||
| Chlorella vulgaris 211/19 SAG | HAMGM media | 7.5 | 16 | [31] | |||||
| Chlorella vulgaris | BG11 media | NR | 12:12 | bacterial flocculation | sterile media washes | NR | 2 | [32] | |
| Chlorella vulgaris UTEX 395 | BBM media | 7.5 | continuous | centrifugation | NR | 100 and 50 | 2 | [33] | |
| Chlorella vulgaris UTEX 265 | BG11 media | 25 | NR | centrifugation or flocculation | 0.2 μm filtration | 100 | 3 | [34] | |
| Chlorella vulgaris | 28 | - | 12:12 | fungi flocculation | autoclave | 1 | [35] | ||
| BBM media | 25 | 3 | continuous | gravity sedimentation | NR | 3 | [36] | ||
| Parachlorella kessleri | f/2 medium | 7.5 | 16:8 | chemo magnetic flocculation | 0.2 μm filtration | 1 | [37] | ||
| Chlorella pyrenoidosa | Nanoemulsion media | - | 12:12 | centrifugation | NR | 50 | 2 | [38] | |
| Chlorella vulgaris | BBM media | 28 | 7.2 | 12:12 | magnetic flocculant | 100 and 50 | 1 | [39] | |
| Modified BBM media | 23 | 6.8 | continuous | flocculation | 100 | 1 | [40] | ||
| Chlorella sorokiniana | TAP media (mixotrophic growth) | 27 | 7.0 | 18:6 | centrifugation | 2 | [41] | ||
| Chlorella zofingiensis | BG11 media | 25 | 6.8 | continuous | 100 and 50 | 3 | [42] | ||
| Chlorococcum sp. | 7.5 | pH flocculation | 100 | 1 | [28] | ||||
| Dunaliella salina DF40 | A4F industrial media | 21 | 8 | - | membrane processing | integrative membrane filtration | 50 | 2 | [43] |
| Dunaliella salina (MUR 8) | Modified F-medium | outdoor | - | outdoor | centrifugation | NaClO treatment | 100 | 6 | [44] |
| Ettlia texensis SAG79.80 | Modified freshwater medium | 26 | 6.5 | continuous | flocculation | NR | 2 | [45] | |
| Nannochloropsis salina | Modified f/2 medium | 25 | 8.0–8.8 | continuous | flocculation | [46] | |||
| Nannochloropsis oceanica | 23–28 | 7.6–8.2 | ultrafiltration membrane | 3 | [47] | ||||
| Nannochloropsis sp. CCAP278 | Artificial seawater | outdoor cultivation | centrifugation | sand filtration | 10 | [48] | |||
| Nannochloropsis sp. CCAP221 | 22 | 8.7 | 16:8 | centrifugation | 0.22 μm filtration | 1 | [49] | ||
| Nannochloropsis oculata | Modified artificial seawater | 25 | 7.6 | continuous | pH flocculation | NR | 1 | [28] | |
| Neochloris oleoabundans UTEX 1185 | Brackish medium | 24 | - | 16:8 | centrifugation | 1 | [50] | ||
| Phaeodactylum tricornutum | Modified artificial seawater | 25 | 7.8 | continuous | pH flocculation | 1 | [28] | ||
| Scenedesmus acuminatus GT-2 | BG11 media | 26–28 | 6.5–7.0 | ultrafiltration membrane | UV-based AOP | 100 | 1 | [51] | |
| Scenedesmus acuminatus | 25 | 6.5–7.0 | NR | 3 | [14] | ||||
| Scenedesmus subspicatus UTEX 2594 | BBM media | 26 | 6.9 | centrifugation | 0.30 μm filtration | 50, 20, 10 | [52] | ||
| Scenedesmus SDEC-8 | BG11 media | 25 | - | ultrasonication | 100 | 4 | [26] | ||
| Scenedesmus sp. | 7.5 | pH flocculation | NR | 1 | [28] | ||||
| Scenedesmus sp. | 30 | 7.0 | continuous | electro–coagulation–flocculation | 0.2 μm filtration | 5 | [53] | ||
| 25 | 6.5–8.0 | 12:12 | flocculation and centrifugation | NR | 2 | [54] | |||
| Scenedesmus quadricauda FWAC 276 | LC Oligo medium | 20 | 7.0 | centrifugation | 2 | [55] | |||
| Tisochrysis lutea CCAP927 | Artificial seawater | 25 | 9.1 | 22:2 | centrifugation | 0.22 μm filtration | 1 | [49] | |
| Tetraselmis sp. MUR 233 | Seawater-based media | outdoor cultivation | electro-flocculation, and centrifugation | NR | 50 | continuous | [56] | ||
| Tetradesmus obliquus | Chu synthetic medium | 20 | - | 12:12 | gravity sedimentation | 3 | [57] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arora, N.; Lo, E.; Legall, N.; Philippidis, G.P. A Critical Review of Growth Media Recycling to Enhance the Economics and Sustainability of Algae Cultivation. Energies 2023, 16, 5378. https://doi.org/10.3390/en16145378
Arora N, Lo E, Legall N, Philippidis GP. A Critical Review of Growth Media Recycling to Enhance the Economics and Sustainability of Algae Cultivation. Energies. 2023; 16(14):5378. https://doi.org/10.3390/en16145378
Chicago/Turabian StyleArora, Neha, Enlin Lo, Noah Legall, and George P. Philippidis. 2023. "A Critical Review of Growth Media Recycling to Enhance the Economics and Sustainability of Algae Cultivation" Energies 16, no. 14: 5378. https://doi.org/10.3390/en16145378





