Current Research on Green Ammonia (NH3) as a Potential Vector Energy for Power Storage and Engine Fuels: A Review
Abstract
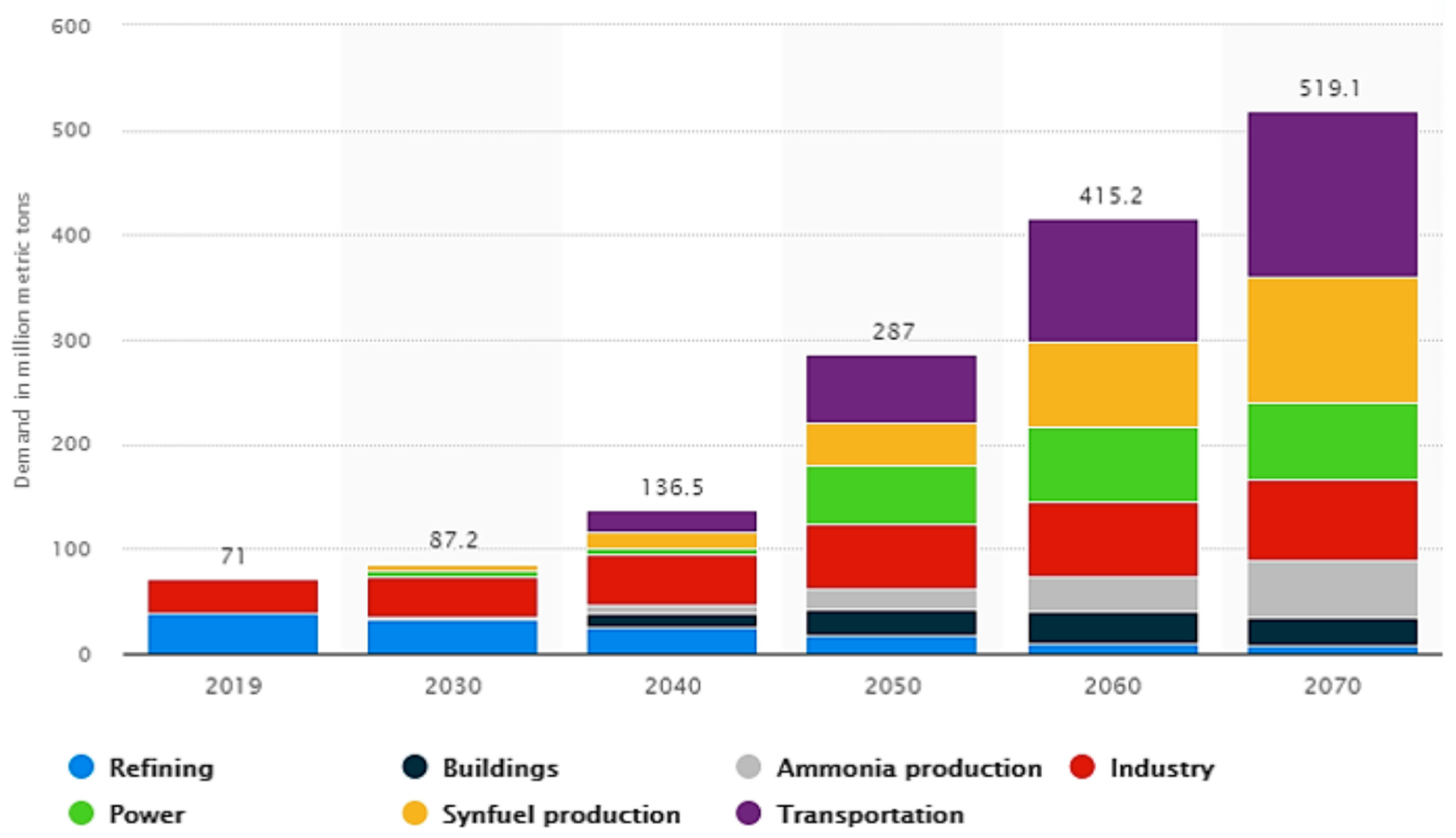
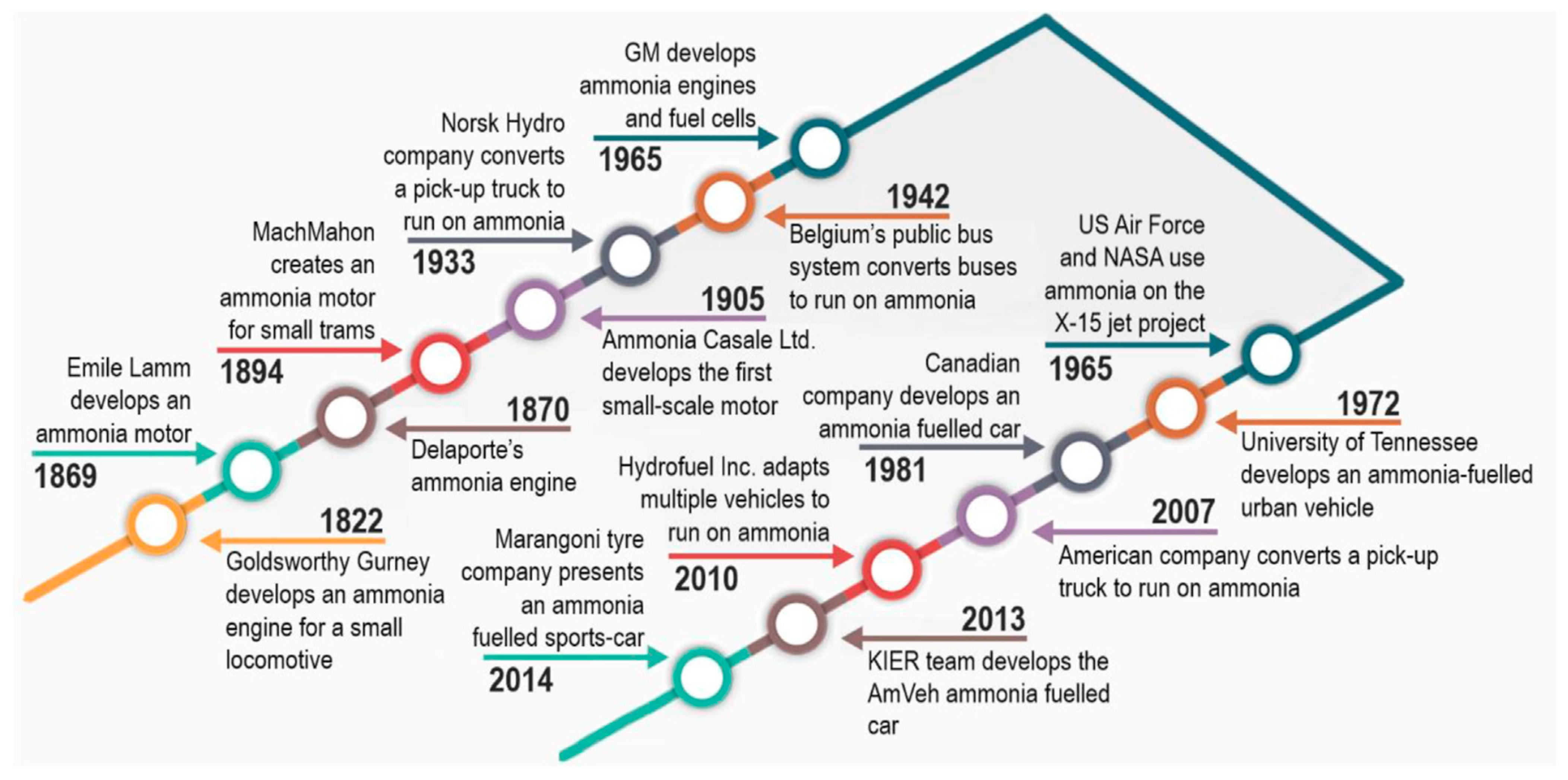
:1. Introduction
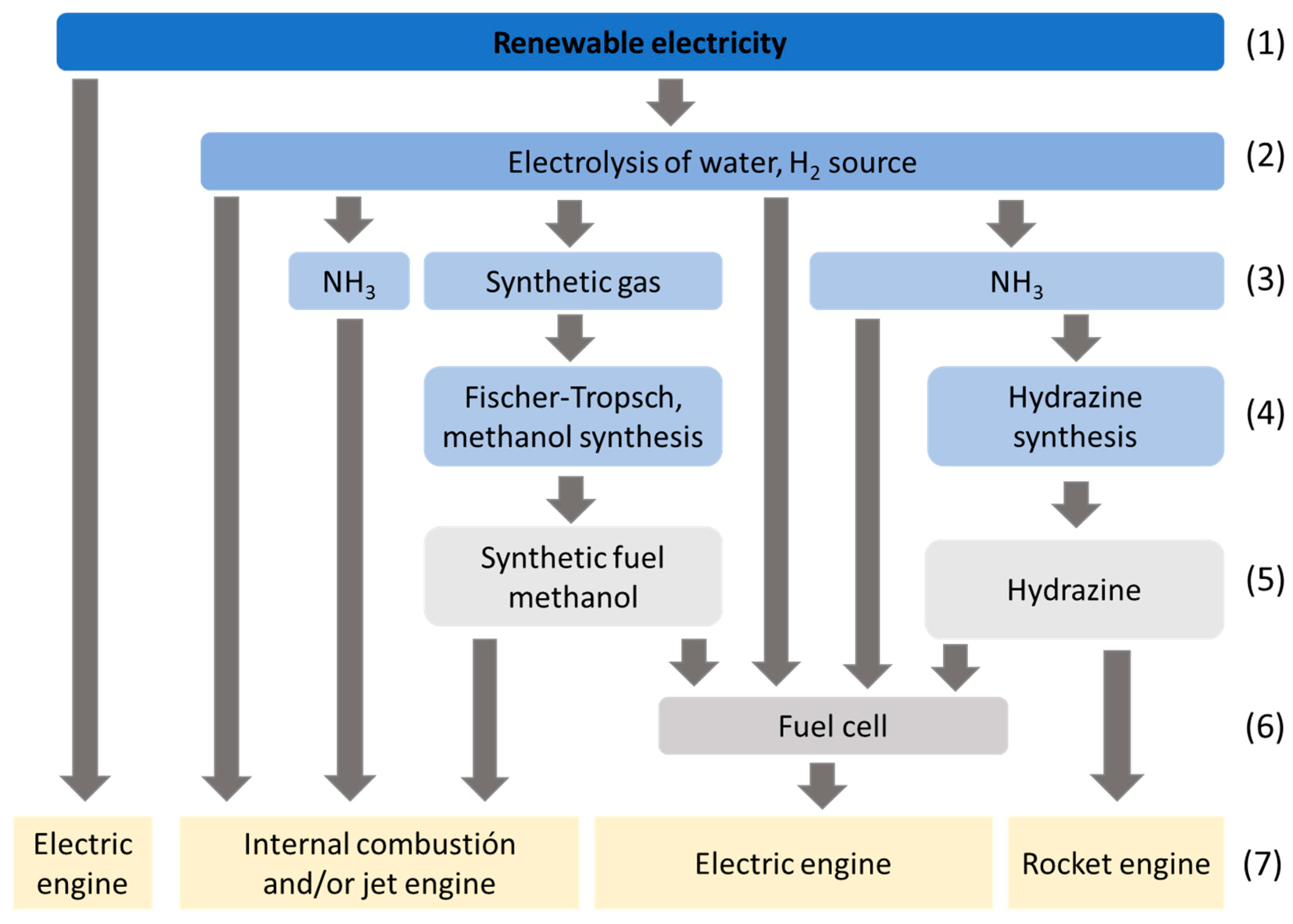
2. Ammonia as an Energy Carrier Vector
3. Ammonia as an Electricity Generator in Combined Cycle Gas Turbines
4. Green Ammonia as E-Fuel in the Transportation Sector
4.1. Green Ammonia as Fuel
4.2. Green Ammonia as a Fuel for Spark Ignition (SI) Engines
4.3. Green Ammonia as a Fuel for Compression Ignition (CI) Engines
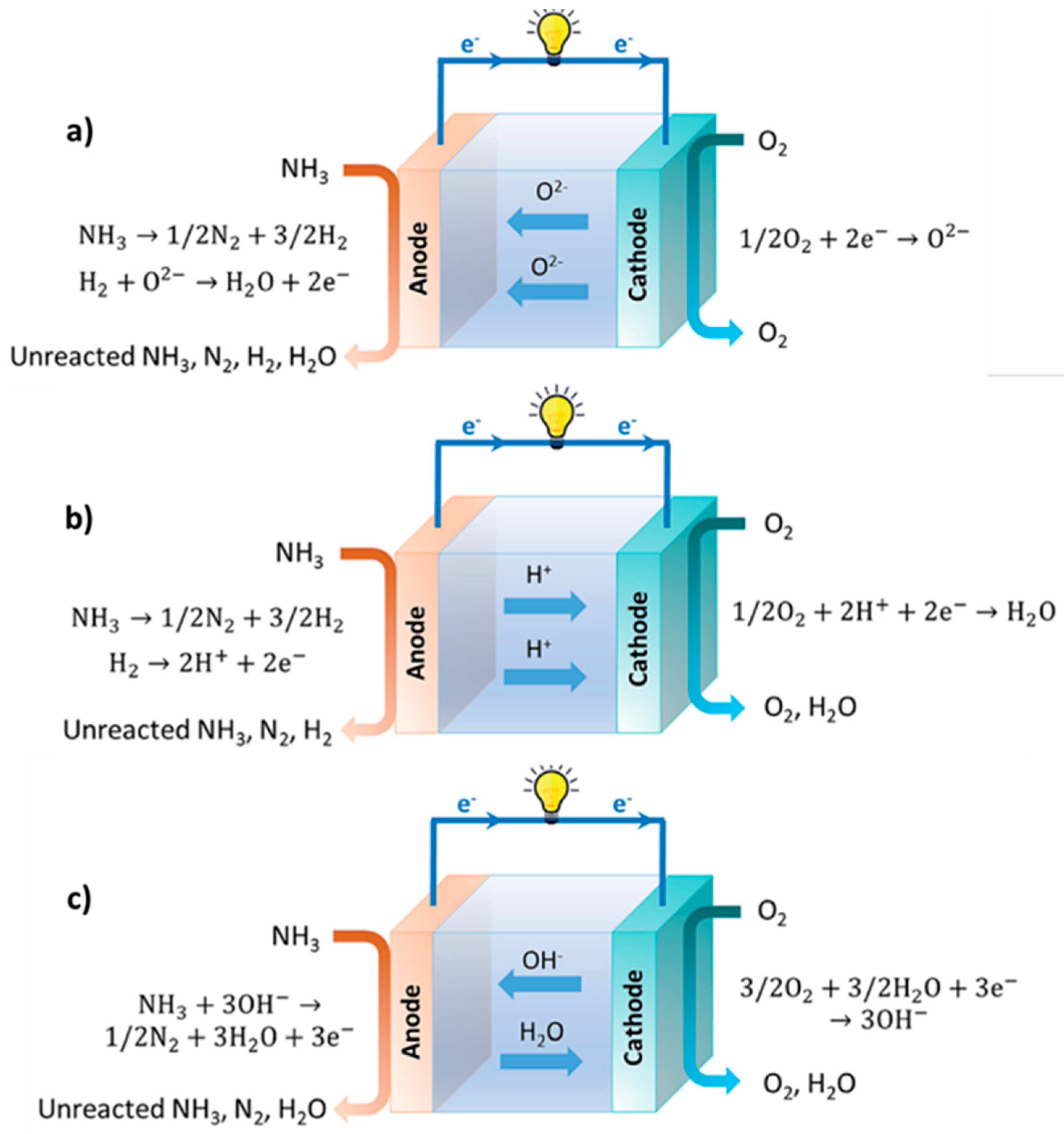
4.4. Green Ammonia as a Fuel-Cell for Clean Engines
5. Nitrogen-Based Fuels: Compounds Derived from Green Ammonia as Fuels for Engines
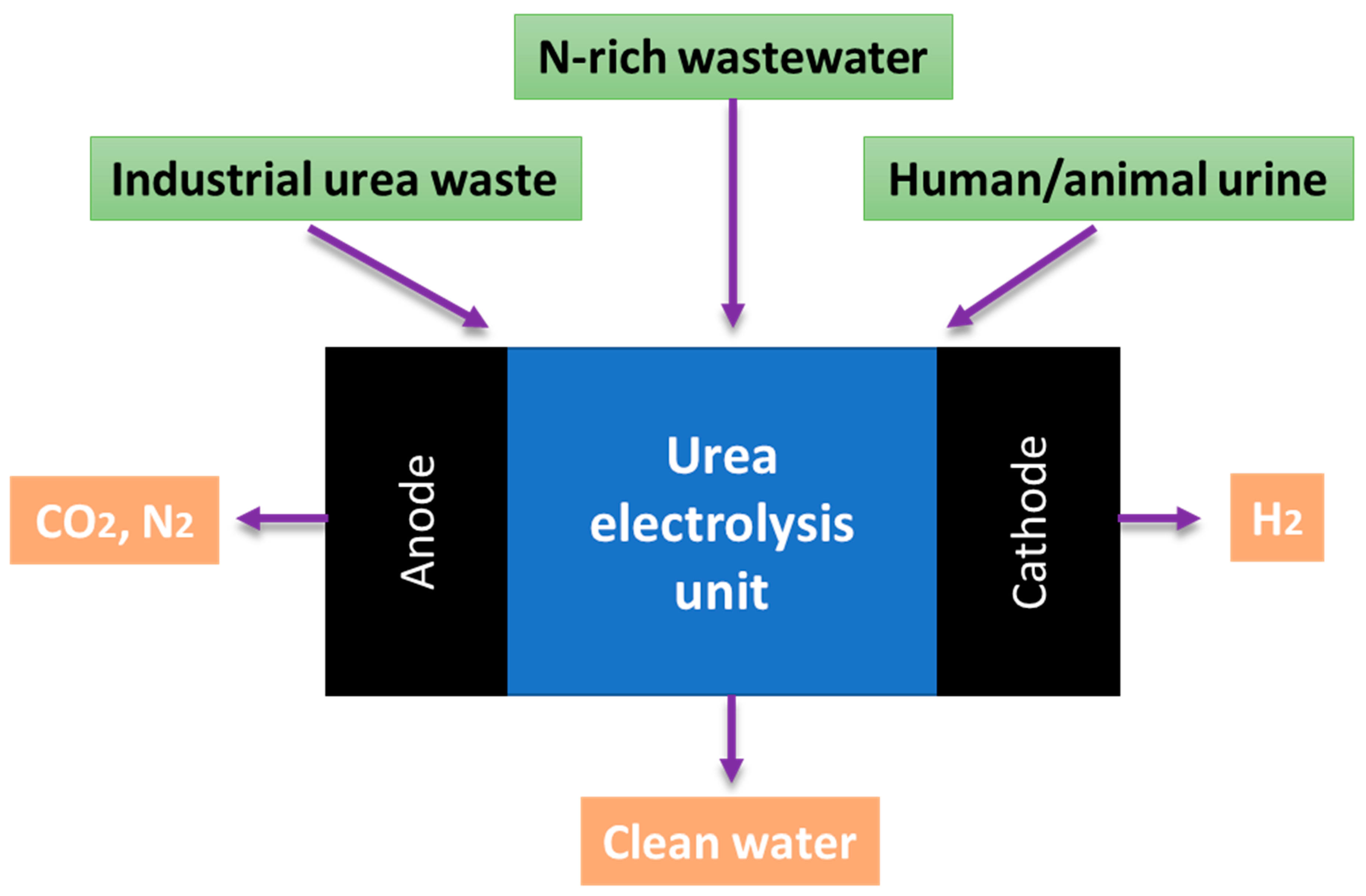
5.1. Green Urea as Fuel
5.2. Green Hydrazine as a Fuel
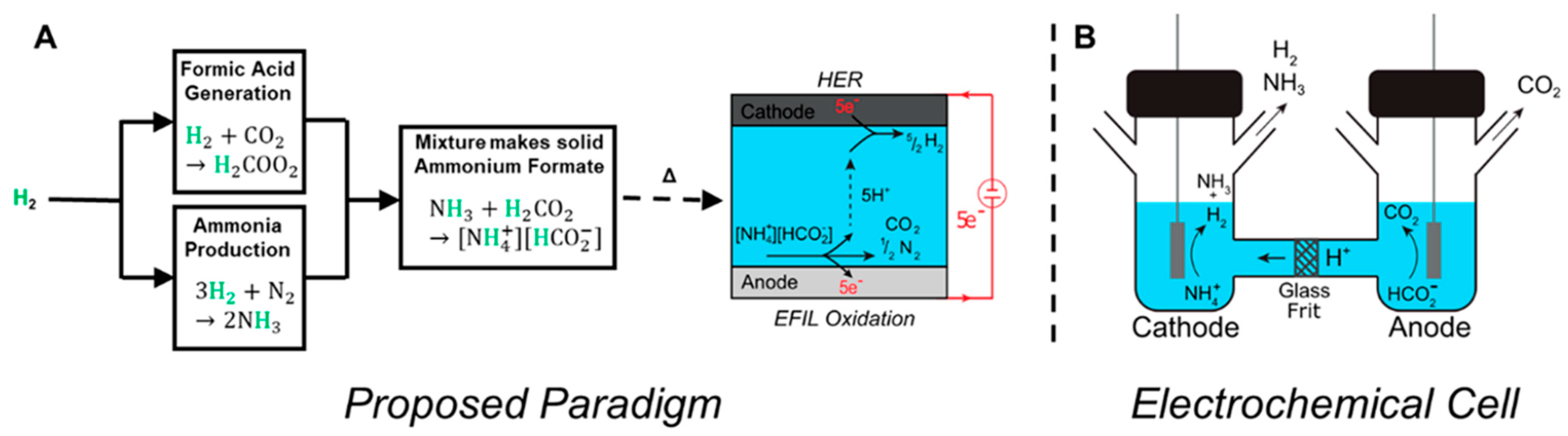
5.3. Green Ammonium Nitrate as Fuel: Aqueous Solutions of Urea, Ammonium Nitrate and Their Mixtures (UAN) as Fuels
5.4. Green Ammonium Nitrate as a Fuel: Aqueous Solutions of Ammonium Hydroxide and Ammonium Nitrate (AAN)
5.5. Other Synthetic Nitrogen-Based Fuels
6. Storage, Transportation, Safety, Production, Decomposition and Consumption of Green Ammonia
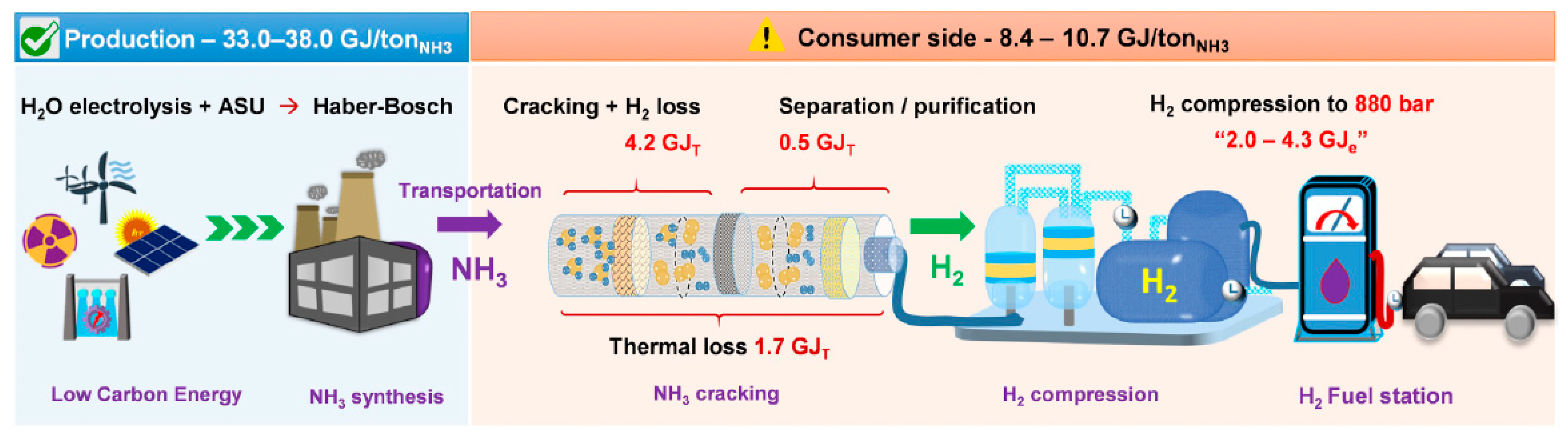
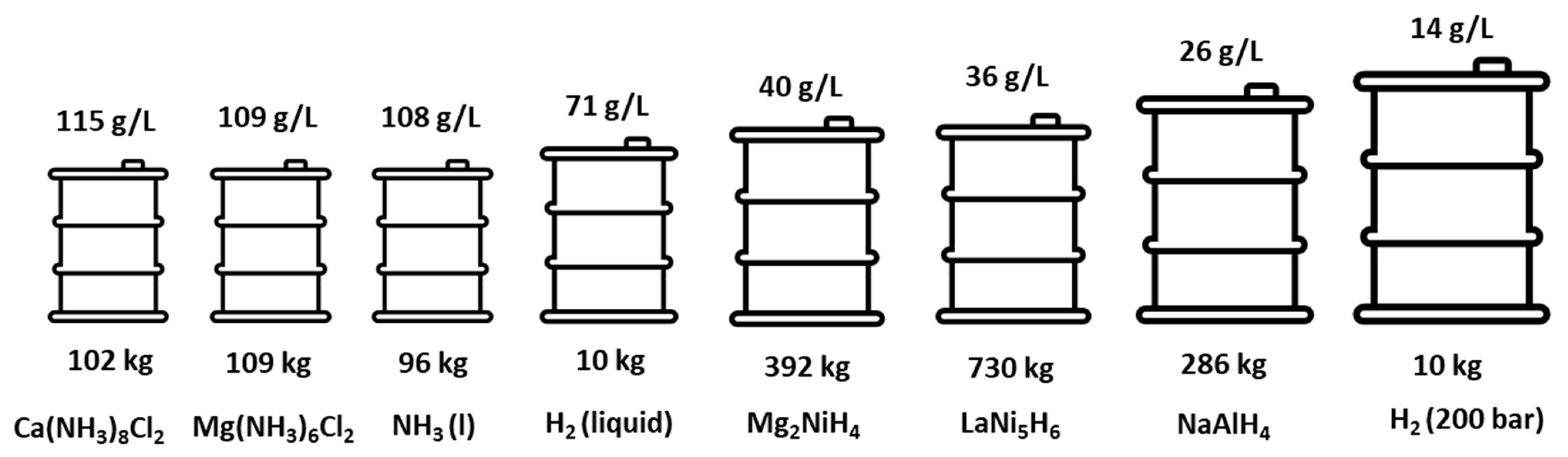
6.1. Storage and Transportation of Ammonia Fuels
6.2. Production of Ammonia Fuels
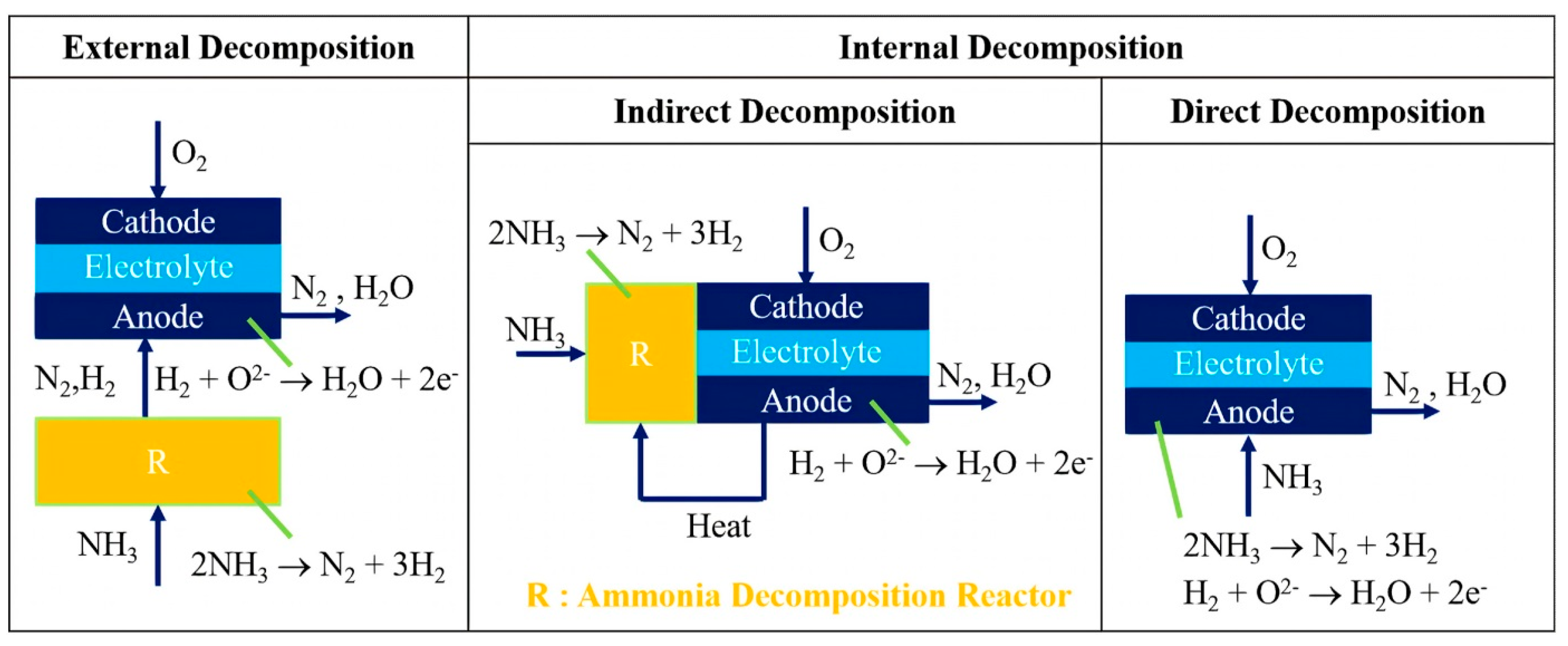
6.3. Decomposition and Consumption of Green Ammonia
7. Summary and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brynolf, S.; Hansson, J.; Anderson, J.E.; Skov, I.R.; Wallington, T.J.; Grahn, M.; Korberg, A.D.; Malmgren, E.; Taljegard, M.J. Review of electrofuel feasibility-Prospects for road, ocean, and air transport. Prog. Energy 2022, 4, 042007. [Google Scholar] [CrossRef]
- Van Renssen, S. The hydrogen solution? Nat. Clim. Chang. 2020, 10, 799–801. [Google Scholar] [CrossRef]
- Kovač, A.; Paranos, M.; Marciuš, D. Hydrogen in energy transition: A review. Int. J. Hydrogen Energy 2021, 46, 10016–10035. [Google Scholar] [CrossRef]
- Cheng, W.; Lee, S. How green are the national hydrogen strategies? Sustainability 2022, 14, 1930. [Google Scholar] [CrossRef]
- Lebrouhi, B.; Djoupo, J.; Lamrani, B.; Benabdelaziz, K.; Kousksou, T. Global hydrogen development-A technological and geopolitical overview. Int. J. Hydrogen Energy 2022, 47, 7016–7048. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Ugbeh-Johnson, J.; Okeke, N.E.; Ogbonnaya, C. Present and projected developments in hydrogen production: A technological review. Carbon Capture Sci. Technol. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrogen Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Hong, X.; Thaore, V.B.; Karimi, I.A.; Farooq, S.; Wang, X.; Usadi, A.K.; Chapman, B.R.; Johnson, R.A. Techno-enviro-economic analyses of hydrogen supply chains with an ASEAN case study. Int. J. Hydrogen Energy 2021, 46, 32914–32928. [Google Scholar] [CrossRef]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S. Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.A.H.; Rooney, D.W. Hydrogen production, storage, utilisation and environmental impacts: A review. Environ. Chem. Lett 2022, 20, 153–188. [Google Scholar] [CrossRef]
- Megía, P.J.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen production technologies: From fossil fuels toward renewable sources. A mini review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Martino, M.; Ruocco, C.; Meloni, E.; Pullumbi, P.; Palma, V. Main hydrogen production processes: An overview. Catalysts 2021, 11, 547. [Google Scholar] [CrossRef]
- Longden, T.; Beck, F.J.; Jotzo, F.; Andrews, R.; Prasad, M. ‘Clean’hydrogen?–Comparing the emissions and costs of fossil fuel versus renewable electricity based hydrogen. Appl. Energy 2022, 306, 118145. [Google Scholar] [CrossRef]
- Guilbert, D.; Vitale, G. Hydrogen as a clean and sustainable energy vector for global transition from fossil-based to zero-carbon. Clean Technol. 2021, 3, 881–909. [Google Scholar] [CrossRef]
- Xiang, H.; Ch, P.; Nawaz, M.A.; Chupradit, S.; Fatima, A.; Sadiq, M. Integration and economic viability of fueling the future with green hydrogen: An integration of its determinants from renewable economics. Int. J. Hydrogen Energy 2021, 46, 38145–38162. [Google Scholar] [CrossRef]
- Liu, W.; Wan, Y.; Xiong, Y.; Gao, P. Green hydrogen standard in China: Standard and evaluation of low-carbon hydrogen, clean hydrogen, and renewable hydrogen. Int. J. Hydrogen Energy 2022, 47, 24584–24591. [Google Scholar] [CrossRef]
- Dong, H.; Wu, Y.; Zhou, J.; Chen, W. Optimal selection for wind power coupled hydrogen energy storage from a risk perspective, considering the participation of multi-stakeholder. J. Clean. Prod. 2022, 356, 131853. [Google Scholar] [CrossRef]
- Li, L.; Feng, L.; Manier, H.; Manier, M.-A. Life cycle optimization for hydrogen supply chain network design. Int. J. Hydrogen Energy 2022, in press. [Google Scholar] [CrossRef]
- Alkawsi, G.; Baashar, Y.; Abbas, U.D.; Alkahtani, A.A.; Tiong, S.K. Review of renewable energy-based charging infrastructure for electric vehicles. Appl. Sci. 2021, 11, 3847. [Google Scholar] [CrossRef]
- Rego de Vasconcelos, B.; Lavoie, J.-M. Recent advances in power-to-X technology for the production of fuels and chemicals. Front. Chem. 2019, 7, 392. [Google Scholar] [CrossRef] [Green Version]
- Cano, M.H.; Agbossou, K.; Kelouwani, S.; Dubé, Y. Experimental evaluation of a power management system for a hybrid renewable energy system with hydrogen production. Renew. Energy 2017, 113, 1086–1098. [Google Scholar] [CrossRef]
- Dadak, A.; Mehrpooya, M.; Kasaeian, A. Design and development of an innovative integrated structure for the production and storage of energy and hydrogen utilizing renewable energy. Sustain. Energy Technol. Assess. 2021, 45, 101123. [Google Scholar] [CrossRef]
- Hannan, M.; Wali, S.; Ker, P.; Abd Rahman, M.; Mansor, M.; Ramachandaramurthy, V.; Muttaqi, K.; Mahlia, T.; Dong, Z. Battery energy-storage system: A review of technologies, optimization objectives, constraints, approaches, and outstanding issues. J. Energy Storage 2021, 42, 103023. [Google Scholar] [CrossRef]
- de Siqueira, L.M.S.; Peng, W. Control strategy to smooth wind power output using battery energy storage system: A review. J. Energy Storage 2021, 35, 102252. [Google Scholar] [CrossRef]
- Jurczyk, M.; Węcel, D.; Uchman, W.; Skorek-Osikowska, A. Assessment of operational performance for an integrated ‘power to synthetic natural gas’ system. Energies 2022, 15, 74. [Google Scholar] [CrossRef]
- Skov, I.R.; Schneider, N. Incentive structures for power-to-X and e-fuel pathways for transport in EU and member states. Energy Policy 2022, 168, 113121. [Google Scholar] [CrossRef]
- Oh, S.; Park, C.; Kim, S.; Kim, Y.; Choi, Y.; Kim, C. Natural gas–ammonia dual-fuel combustion in spark-ignited engine with various air–fuel ratios and split ratios of ammonia under part load condition. Fuel 2021, 290, 120095. [Google Scholar] [CrossRef]
- Galimova, T.; Ram, M.; Bogdanov, D.; Fasihi, M.; Khalili, S.; Gulagi, A.; Karjunen, H.; Mensah, T.N.O.; Breyer, C. Global demand analysis for carbon dioxide as raw material from key industrial sources and direct air capture to produce renewable electricity-based fuels and chemicals. J. Clean. Prod. 2022, 373, 133920. [Google Scholar] [CrossRef]
- Desport, L.; Selosse, S. An overview of CO2 capture and utilization in energy models. Resour. Conserv. Recycl. 2022, 180, 106150. [Google Scholar] [CrossRef]
- Breuer, J.L.; Scholten, J.; Koj, J.C.; Schorn, F.; Fiebrandt, M.; Samsun, R.C.; Albus, R.; Görner, K.; Stolten, D.; Peters, R. An Overview of Promising Alternative Fuels for Road, Rail, Air, and Inland Waterway Transport in Germany. Energies 2022, 15, 1443. [Google Scholar] [CrossRef]
- Roy, S.; Cherevotan, A.; Peter, S.C. Thermochemical CO2 hydrogenation to single carbon products: Scientific and technological challenges. ACS Energy Lett. 2018, 3, 1938–1966. [Google Scholar] [CrossRef] [Green Version]
- Angeles, D.A.; Tan, R.R.; Aviso, K.B.; Are, K.R.A.G.; Razon, L.F. Fuzzy optimization of the automotive ammonia fuel cycle. J. Clean. Prod. 2018, 186, 877–882. [Google Scholar] [CrossRef]
- Liu, R.; Ting, D.S.-K.; Checkel, M.D. Ammonia as a Fuel for SI Engine; SAE Technical Paper 0148-7191; SAE International: Warrendale, PA, USA, 2003. [Google Scholar]
- Westlye, F.R.; Ivarsson, A.; Schramm, J. Experimental investigation of nitrogen based emissions from an ammonia fueled SI-engine. Fuel 2013, 111, 239–247. [Google Scholar] [CrossRef]
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as effective hydrogen storage: A review on production, storage and utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Hasan, M.H.; Mahlia, T.M.I.; Mofijur, M.; Rizwanul Fattah, I.; Handayani, F.; Ong, H.C.; Silitonga, A. A comprehensive review on the recent development of ammonia as a renewable energy carrier. Energies 2021, 14, 3732. [Google Scholar] [CrossRef]
- Chatterjee, S.; Parsapur, R.K.; Huang, K.-W. Limitations of ammonia as a hydrogen energy carrier for the transportation sector. ACS Energy Lett. 2021, 6, 4390–4394. [Google Scholar] [CrossRef]
- Sánchez, A.; Castellano, E.; Martín, M.; Vega, P. Evaluating ammonia as green fuel for power generation: A thermo-chemical perspective. Appl. Energy 2021, 293, 116956. [Google Scholar] [CrossRef]
- Ikäheimo, J.; Kiviluoma, J.; Weiss, R.; Holttinen, H. Power-to-ammonia in future North European 100% renewable power and heat system. Int. J. Hydrogen Energy 2018, 43, 17295–17308. [Google Scholar] [CrossRef]
- Wu, S.; Salmon, N.; Li, M.M.-J.; Bañares-Alcántara, R.; Tsang, S.C.E. Energy decarbonization via green H2 or NH3? ACS Energy Lett. 2022, 7, 1021–1033. [Google Scholar] [CrossRef]
- Kojima, Y.; Yamaguchi, M. Ammonia as a hydrogen energy carrier. Int. J. Hydrogen Energy 2022, 47, 22832–22839. [Google Scholar] [CrossRef]
- Salmon, N.; Bañares-Alcántara, R. Green ammonia as a spatial energy vector: A review. Sustain. Energy Fuels 2021, 5, 2814–2839. [Google Scholar] [CrossRef]
- Morlanés, N.; Katikaneni, S.P.; Paglieri, S.N.; Harale, A.; Solami, B.; Sarathy, S.M.; Gascon, J. A technological roadmap to the ammonia energy economy: Current state and missing technologies. Chem. Eng. J. 2021, 408, 127310. [Google Scholar] [CrossRef]
- Del Pozo, C.A.; Cloete, S. Techno-economic assessment of blue and green ammonia as energy carriers in a low-carbon future. Energy Convers. Manag. 2022, 255, 115312. [Google Scholar] [CrossRef]
- Cesaro, Z.; Ives, M.; Nayak-Luke, R.; Mason, M.; Bañares-Alcántara, R. Ammonia to power: Forecasting the levelized cost of electricity from green ammonia in large-scale power plants. Appl. Energy 2021, 282, 116009. [Google Scholar] [CrossRef]
- Liu, F.; Ding, D.; Duan, C. Protonic ceramic electrochemical cells for synthesizing sustainable chemicals and fuels. Adv. Sci. 2023, 10, 2206478. [Google Scholar] [CrossRef]
- Huo, X.; Van Hoomissen, D.J.; Liu, J.; Vyas, S.; Strathmann, T.J. Hydrogenation of aqueous nitrate and nitrite with ruthenium catalysts. Appl. Catal. B Environ. 2017, 211, 188–198. [Google Scholar] [CrossRef] [Green Version]
- Boasiako, C.A.; Zhou, Z.; Huo, X.; Ye, T. Development of Pd-based catalysts for hydrogenation of nitrite and nitrate in water: A review. J. Hazard. Mater. 2023, 446, 130661. [Google Scholar] [CrossRef]
- Desai, P.D.; Turley, M.; Robinson, R.; Zimmerman, W.B. Hot microbubble injection in thin liquid film layers for ammonia separation from ammonia rich-wastewater. Chem. Eng. Process.-Process Intensif. 2022, 180, 108693. [Google Scholar] [CrossRef]
- De la Rubia, M.Á.; Walker, M.; Heaven, S.; Banks, C.J.; Borja, R. Preliminary trials of in situ ammonia stripping from source segregated domestic food waste digestate using biogas: Effect of temperature and flow rate. Bioresour. Technol. 2010, 101, 9486–9492. [Google Scholar] [CrossRef] [Green Version]
- Wen, D.; Aziz, M. Design and analysis of biomass-to-ammonia-to-power as an energy storage method in a renewable multi-generation system. Energy Convers. Manag. 2022, 261, 115611. [Google Scholar] [CrossRef]
- Wiseman, S.; Rieth, M.; Gruber, A.; Dawson, J.R.; Chen, J.H. A comparison of the blow-out behavior of turbulent premixed ammonia/hydrogen/nitrogen-air and methane–air flames. Proc. Combust. Inst. 2021, 38, 2869–2876. [Google Scholar] [CrossRef]
- Vigueras-Zuniga, M.-O.; Tejeda-del-Cueto, M.-E.; Vasquez-Santacruz, J.-A.; Herrera-May, A.-L.; Valera-Medina, A. Numerical predictions of a swirl combustor using complex chemistry fueled with ammonia/hydrogen blends. Energies 2020, 13, 288. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Yang, C.; Wang, H.; Hu, D.; Wang, Y. Effect of Diesel-Ignited Ammonia/Hydrogen mixture fuel combustion on engine combustion and emission performance. Fuel 2023, 331, 125865. [Google Scholar] [CrossRef]
- Chai, W.S.; Bao, Y.; Jin, P.; Tang, G.; Zhou, L. A review on ammonia, ammonia-hydrogen and ammonia-methane fuels. Renew. Sustain. Energy Rev. 2021, 147, 111254. [Google Scholar] [CrossRef]
- Zhang, M.; An, Z.; Wei, X.; Wang, J.; Huang, Z.; Tan, H. Emission analysis of the CH4/NH3/air co-firing fuels in a model combustor. Fuel 2021, 291, 120135. [Google Scholar] [CrossRef]
- Ji, L.; Wang, J.; Hu, G.; Mao, R.; Zhang, W.; Huang, Z. Experimental study on structure and blow-off characteristics of NH3/CH4 co-firing flames in a swirl combustor. Fuel 2022, 314, 123027. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Albalawi, A.M.; Wang, S.; Roberts, W.L. Stability and characteristics of NH3/CH4/air flames in a combustor fired by a double swirl stabilized burner. Proc. Combust. Inst. 2022, 39, 4205–4213. [Google Scholar] [CrossRef]
- Sun, J.; Yang, Q.; Zhao, N.; Chen, M.; Zheng, H. Numerically study of CH4/NH3 combustion characteristics in an industrial gas turbine combustor based on a reduced mechanism. Fuel 2022, 327, 124897. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Wang, S.; Guiberti, T.F.; Roberts, W.L. Review on the recent advances on ammonia combustion from the fundamentals to the applications. Fuel Commun. 2022, 10, 100053. [Google Scholar] [CrossRef]
- He, C.; Jiang, J.; Sun, M.; Yu, Y.; Liu, K.; Zhang, B. Analysis of the NH3 blended ratio on the impinging flame structure in non-premixed CH4/NH3/air combustion. Fuel 2022, 330, 125559. [Google Scholar] [CrossRef]
- Okafor, E.C.; Somarathne, K.K.A.; Ratthanan, R.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Tsujimura, T.; Furutani, H.; Kobayashi, H. Control of NOx and other emissions in micro gas turbine combustors fuelled with mixtures of methane and ammonia. Combust. Flame 2020, 211, 406–416. [Google Scholar] [CrossRef]
- Zhu, R.; Han, X.; Zhang, Z.; He, Y.; Wang, Z. Experimental and kinetic study on laminar burning velocities of NH3/CH4/H2S/air flames. Fuel 2023, 332, 126174. [Google Scholar] [CrossRef]
- Mikulčić, H.; Baleta, J.; Wang, X.; Wang, J.; Qi, F.; Wang, F. Numerical simulation of ammonia/methane/air combustion using reduced chemical kinetics models. Int. J. Hydrogen Energy 2021, 46, 23548–23563. [Google Scholar] [CrossRef]
- Xiao, H.; Howard, M.; Valera-Medina, A.; Dooley, S.; Bowen, P. Reduced chemical mechanisms for ammonia/methane co-firing for gas turbine applications. Energy Procedia 2017, 105, 1483–1488. [Google Scholar] [CrossRef]
- Ayaz, S.K.; Altuntas, O.; Caliskan, H. Enhanced life cycle modelling of a micro gas turbine fuelled with various fuels for sustainable electricity production. Renew. Sustain. Energy Rev. 2021, 149, 111323. [Google Scholar] [CrossRef]
- Kurata, O.; Iki, N.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Kobayashi, H.; Hayakawa, A. Performances and emission characteristics of NH3–air and NH3CH4–air combustion gas-turbine power generations. Proc. Combust. Inst. 2017, 36, 3351–3359. [Google Scholar] [CrossRef]
- Mong, G.R.; Chiong, M.-C.; Chong, C.T.; Ng, J.-H.; Mashruk, S.; Tran, M.-V.; Lee, K.M.; Samiran, N.A.; Wong, K.Y.; Valera-Medina, A. Fuel-lean ammonia/biogas combustion characteristics under the reacting swirl flow conditions. Fuel 2023, 331, 125983. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; Costa, M.; Sun, Z.; He, Y.; Cen, K. Experimental and kinetic modeling study of laminar burning velocities of NH3/air, NH3/H2/air, NH3/CO/air and NH3/CH4/air premixed flames. Combust. Flame 2019, 206, 214–226. [Google Scholar] [CrossRef]
- Xiao, H.; Valera-Medina, A.; Bowen, P.J. Study on premixed combustion characteristics of co-firing ammonia/methane fuels. Energy 2017, 140, 125–135. [Google Scholar] [CrossRef]
- Shu, T.; Xue, Y.; Zhou, Z.; Ren, Z. An experimental study of laminar ammonia/methane/air premixed flames using expanding spherical flames. Fuel 2021, 290, 120003. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Experimental and numerical study of the laminar burning velocity of CH4–NH3–air premixed flames. Combust. Flame 2018, 187, 185–198. [Google Scholar] [CrossRef]
- Somarathne, K.D.K.A.; Okafor, E.C.; Sugawara, D.; Hayakawa, A.; Kobayashi, H. Effects of OH concentration and temperature on NO emission characteristics of turbulent non-premixed CH4/NH3/air flames in a two-stage gas turbine like combustor at high pressure. Proc. Combust. Inst. 2021, 38, 5163–5170. [Google Scholar] [CrossRef]
- Okafor, E.C.; Yamashita, H.; Hayakawa, A.; Somarathne, K.K.A.; Kudo, T.; Tsujimura, T.; Uchida, M.; Ito, S.; Kobayashi, H. Flame stability and emissions characteristics of liquid ammonia spray co-fired with methane in a single stage swirl combustor. Fuel 2021, 287, 119433. [Google Scholar] [CrossRef]
- Yin, G.; Li, J.; Zhou, M.; Li, J.; Wang, C.; Hu, E.; Huang, Z. Experimental and kinetic study on laminar flame speeds of ammonia/dimethyl ether/air under high temperature and elevated pressure. Combust. Flame 2022, 238, 111915. [Google Scholar] [CrossRef]
- Issayev, G.; Giri, B.R.; Elbaz, A.M.; Shrestha, K.P.; Mauss, F.; Roberts, W.L.; Farooq, A. Ignition delay time and laminar flame speed measurements of ammonia blended with dimethyl ether: A promising low carbon fuel blend. Renew. Energy 2022, 181, 1353–1370. [Google Scholar] [CrossRef]
- Shrestha, K.P.; Giri, B.R.; Elbaz, A.M.; Issayev, G.; Roberts, W.L.; Seidel, L.; Mauss, F.; Farooq, A. A detailed chemical insights into the kinetics of diethyl ether enhancing ammonia combustion and the importance of NOx recycling mechanism. Fuel Commun. 2022, 10, 100051. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, M.; Zhao, C.; Tian, H.; Tian, J.; Long, W.; Bi, M. Study of combustion and NO chemical reaction mechanism in ammonia blended with DME. Fuel 2022, 319, 123832. [Google Scholar] [CrossRef]
- Issayev, G.; Giri, B.R.; Elbaz, A.M.; Shrestha, K.P.; Mauss, F.; Roberts, W.L.; Farooq, A. Combustion behavior of ammonia blended with diethyl ether. Proc. Combust. Inst. 2021, 38, 499–506. [Google Scholar] [CrossRef]
- Cai, T.; Zhao, D. Enhancing and assessing ammonia-air combustion performance by blending with dimethyl ether. Renew. Sustain. Energy Rev. 2022, 156, 112003. [Google Scholar] [CrossRef]
- Chen, J.; Gou, X. Experimental and kinetic study on the extinction characteristics of ammonia-dimethyl ether diffusion flame. Fuel 2023, 334, 126743. [Google Scholar] [CrossRef]
- Mei, B.; Ma, S.; Zhang, Y.; Zhang, X.; Li, W.; Li, Y. Exploration on laminar flame propagation of ammonia and syngas mixtures up to 10 atm. Combust. Flame 2020, 220, 368–377. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, W.; Tan, H.; An, Q.; Wang, J.; Dai, H.; Wang, X.; Wang, X.; Deng, S. Experimental and kinetic modeling study on NH3/syngas/air and NH3/bio-syngas/air premixed laminar flames at elevated temperature. Combust. Flame 2021, 233, 111594. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Elbaz, A.M.; Han, X.; He, Y.; Costa, M.; Konnov, A.A.; Roberts, W.L. Experimental study and kinetic analysis of the laminar burning velocity of NH3/syngas/air, NH3/CO/air and NH3/H2/air premixed flames at elevated pressures. Combust. Flame 2020, 221, 270–287. [Google Scholar] [CrossRef]
- Xu, H.; Wang, J.; Zhang, C.; Dai, L.; He, Z.; Wang, Q. Numerical study on laminar burning velocity of ammonia flame with methanol addition. Int. J. Hydrogen Energy 2022, 47, 28152–28164. [Google Scholar] [CrossRef]
- Giri, B.R.; Shrestha, K.P.; Mai, T.V.T.; Mauss, F.; Huynh, L.K. A theoretical kinetic study of the reactions of NH2 radicals with methanol and ethanol and their implications in kinetic modeling. Int. J. Chem. Kinet. 2023, 55, 3–14. [Google Scholar] [CrossRef]
- Ronan, P.; Pierre, B.; Christine, M.-R.; Guillaume, D.; Fabien, H. Laminar flame speed of ethanol/ammonia blends–An experimental and kinetic study. Fuel Commun. 2022, 10, 100052. [Google Scholar] [CrossRef]
- Lavadera, M.L.; Pelucchi, M.; Konnov, A.A. The influence of ammonia on the laminar burning velocities of methylcyclohexane and toluene: An experimental and kinetic modeling study. Combust. Flame 2022, 237, 111839. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Giri, B.R.; Issayev, G.; Shrestha, K.P.; Mauss, F.; Farooq, A.; Roberts, W.L. Experimental and kinetic modeling study of laminar flame speed of dimethoxymethane and ammonia blends. Energy Fuels 2020, 34, 14726–14740. [Google Scholar] [CrossRef]
- Lubrano Lavadera, M.; Han, X.; Konnov, A.A. Comparative effect of ammonia addition on the laminar burning velocities of methane, n-heptane, and iso-octane. Energy Fuels 2020, 35, 7156–7168. [Google Scholar] [CrossRef]
- Cellek, M.S. The decreasing effect of ammonia enrichment on the combustion emission of hydrogen, methane, and propane fuels. Int. J. Hydrogen Energy 2022, 47, 19916–19934. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, C.; Wang, D.; Zhang, T.; Zhai, Y.; Wang, S. Experimental and numerical study on laminar burning velocity and premixed combustion characteristics of NH3/C3H8/air mixtures. Fuel 2023, 331, 125936. [Google Scholar] [CrossRef]
- Berwal, P.; Kumar, S. Laminar burning velocity measurement of CH4/H2/NH3-air premixed flames at high mixture temperatures. Fuel 2023, 331, 125809. [Google Scholar] [CrossRef]
- Wang, S.; Elbaz, A.M.; Wang, G.; Wang, Z.; Roberts, W.L. Turbulent flame speed of NH3/CH4/H2/H2O/air-mixtures: Effects of elevated pressure and Lewis number. Combust. Flame 2023, 247, 112488. [Google Scholar] [CrossRef]
- Stocks, M.; Fazeli, R.; Hughes, L.; Beck, F.J. Global emissions implications from co-combusting ammonia in coal fired power stations: An analysis of the Japan-Australia supply chain. J. Clean. Prod. 2022, 336, 130092. [Google Scholar] [CrossRef]
- Trencher, G.; Downie, C.; Hasegawa, K.; Asuka, J. Divestment trends in Japan’s international coal businesses. Renew. Sustain. Energy Rev. 2020, 124, 109779. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.; Liu, X.; Zhu, J.; Xu, J.; Xu, M. Mitigating CO2 emission in pulverized coal-fired power plant via co-firing ammonia: A simulation study of flue gas streams and exergy efficiency. Energy Convers. Manag. 2022, 256, 115328. [Google Scholar] [CrossRef]
- Wang, X.; Fan, W.; Chen, J.; Feng, G.; Zhang, X. Experimental study on effects of air-staged strategy and NH3 co-firing ratios on NO formation characteristics in ammonia/coal co-firing process. Fuel 2023, 332, 126217. [Google Scholar] [CrossRef]
- Cardoso, J.S.; Silva, V.; Chavando, J.A.M.; Eusébio, D.; Hall, M.J. Numerical modelling of the coal phase-out through ammonia and biomass co-firing in a pilot-scale fluidized bed reactor. Fuel Commun. 2022, 10, 100055. [Google Scholar] [CrossRef]
- Chen, P.; Jiang, B.; Wang, H.; Gu, M.; Fang, Y.; Wang, P. Experimental and theoretical calculations study on heterogeneous reduction of NO by char/NH3 in the reduction zone of ammonia co-firing with pulverized coal: Influence of mineral Fe. Fuel 2022, 310, 122374. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Zhu, R.; Tan, J.; He, Y.; Cen, K. Co-firing characteristics and fuel-N transformation of ammonia/pulverized coal binary fuel. Fuel 2023, 337, 126857. [Google Scholar] [CrossRef]
- Ma, P.; Huang, Q.; Wu, Z.; Lyu, J.; Li, S. Optical diagnostics on coal ignition and gas-phase combustion in co-firing ammonia with pulverized coal on a two-stage flat flame burner. Proc. Combust. Inst. 2022, 39, 3457–3466. [Google Scholar] [CrossRef]
- Lim, H.; Park, Y.; Lee, Y.; Lee, Y.; Chae, T.; Lee, J.; Yang, W.; Kim, J. Impact of ammonium sulfate and kaolin on ash deposition during co-firing of straw pellets and pulverized coal. Korean J. Chem. Eng. 2022, 39, 2089–2098. [Google Scholar] [CrossRef]
- Chen, L.; Wang, C.; Wang, W. Effect of ammonia co-firing on heat transfer, safety, and economy of coal-fired boilers. Fuel 2023, 334, 126649. [Google Scholar] [CrossRef]
- Korberg, A.D.; Brynolf, S.; Grahn, M.; Skov, I.R. Techno-economic assessment of advanced fuels and propulsion systems in future fossil-free ships. Renew. Sustain. Energy Rev. 2021, 142, 110861. [Google Scholar] [CrossRef]
- Mallouppas, G.; Ioannou, C.; Yfantis, E.A. A Review of the Latest Trends in the Use of Green Ammonia as an Energy Carrier in Maritime Industry. Energies 2022, 15, 1453. [Google Scholar] [CrossRef]
- Tsang, S.; Ayvali, T.; Van Vrijaldenhoven, T. The Position of Ammonia in Decarbonising Maritime Industry: An Overview and Perspectives: Part I: Technological advantages and the momentum towards ammonia-propelled shipping. Johns. Matthey Technol. Rev. 2021, 65, 275–290. [Google Scholar]
- Cardoso, J.S.; Silva, V.; Rocha, R.C.; Hall, M.J.; Costa, M.; Eusébio, D. Ammonia as an energy vector: Current and future prospects for low-carbon fuel applications in internal combustion engines. J. Clean. Prod. 2021, 296, 126562. [Google Scholar] [CrossRef]
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrogen Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Yapicioglu, A.; Dincer, I. Performance assesment of hydrogen and ammonia combustion with various fuels for power generators. Int. J. Hydrogen Energy 2018, 43, 21037–21048. [Google Scholar] [CrossRef]
- Chiong, M.-C.; Chong, C.T.; Ng, J.-H.; Mashruk, S.; Chong, W.W.F.; Samiran, N.A.; Mong, G.R.; Valera-Medina, A. Advancements of combustion technologies in the ammonia-fuelled engines. Energy Convers. Manag. 2021, 244, 114460. [Google Scholar] [CrossRef]
- Kurien, C.; Varma, P.S.; Mittal, M. Effect of ammonia energy fractions on combustion stability and engine characteristics of gaseous (ammonia/methane) fuelled spark ignition engine. Int. J. Hydrogen Energy 2023, 48, 1391–1400. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Liu, L. Feasibility study of hydrogen jet flame ignition of ammonia fuel in marine low speed engine. Int. J. Hydrogen Energy 2023, 48, 327–336. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, L.; Zhong, L.; Liu, P.; Wei, H. Experimental Investigation on the Combustion Characteristics of NH3/H2/air by the Spark Ignition and Turbulent Jet Ignition. Combust. Sci. Technol. 2022, 1–22. [Google Scholar] [CrossRef]
- Mercier, A.; Mounaïm-Rousselle, C.; Brequigny, P.; Bouriot, J.; Dumand, C. Improvement of SI engine combustion with ammonia as fuel: Effect of ammonia dissociation prior to combustion. Fuel Commun. 2022, 11, 100058. [Google Scholar] [CrossRef]
- Ezzat, M.; Dincer, I. Development and assessment of a new hybrid vehicle with ammonia and hydrogen. Appl. Energy 2018, 219, 226–239. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C. Experimental investigation on ammonia combustion behavior in a spark-ignition engine by means of laminar and turbulent expanding flames. Proc. Combust. Inst. 2021, 38, 5859–5868. [Google Scholar] [CrossRef]
- Liu, S.; Lin, Z.; Zhang, H.; Lei, N.; Qi, Y.; Wang, Z. Impact of ammonia addition on knock resistance and combustion performance in a gasoline engine with high compression ratio. Energy 2023, 262, 125458. [Google Scholar] [CrossRef]
- Dinesh, M.; Pandey, J.K.; Kumar, G. Study of performance, combustion, and NOx emission behavior of an SI engine fuelled with ammonia/hydrogen blends at various compression ratio. Int. J. Hydrogen Energy 2022, 47, 25391–25403. [Google Scholar] [CrossRef]
- Zhu, R.; Fang, X.; Xu, C.; Zhao, M.; Zhang, H.; Davy, M. Pulsating one-dimensional detonation in ammonia-hydrogen–air mixtures. Int. J. Hydrogen Energy 2022, 47, 21517–21536. [Google Scholar] [CrossRef]
- Mounaïm-Rousselle, C.; Bréquigny, P.; Dumand, C.; Houillé, S. Operating limits for ammonia fuel spark-ignition engine. Energies 2021, 14, 4141. [Google Scholar] [CrossRef]
- Mounaïm-Rousselle, C.; Brequigny, P. Ammonia as fuel for low-carbon spark-ignition engines of tomorrow’s passenger cars. Front. Mech. Eng. 2020, 6, 70. [Google Scholar] [CrossRef]
- Zhang, H.; Li, G.; Long, Y.; Zhang, Z.; Wei, W.; Zhou, M.; Belal, B.Y. Numerical study on combustion and emission characteristics of a spark-ignition ammonia engine added with hydrogen-rich gas from exhaust-fuel reforming. Fuel 2023, 332, 125939. [Google Scholar] [CrossRef]
- Yang, W.; Dinesh, K.R.; Luo, K.; Thévenin, D. Direct numerical simulations of auto-igniting mixing layers in ammonia and ammonia-hydrogen combustion under engine-relevant conditions. Int. J. Hydrogen Energy 2022, 47, 38055–38074. [Google Scholar] [CrossRef]
- Xin, G.; Ji, C.; Wang, S.; Meng, H.; Chang, K.; Yang, J. Effect of different volume fractions of ammonia on the combustion and emission characteristics of the hydrogen-fueled engine. Int. J. Hydrogen Energy 2022, 47, 16297–16308. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, L.; Zhong, L.; Wei, H. Enhanced combustion of ammonia engine based on novel air-assisted pre-chamber turbulent jet ignition. Energy Convers. Manag. 2023, 276, 116526. [Google Scholar] [CrossRef]
- Oh, S.; Park, C.; Ahn, M.; Jang, H.; Kim, S. Experimental approach for reducing nitrogen oxides emissions from ammonia–natural gas dual-fuel spark-ignition engine. Fuel 2023, 332, 126065. [Google Scholar] [CrossRef]
- Salek, F.; Babaie, M.; Shakeri, A.; Hosseini, S.V.; Bodisco, T.; Zare, A. Numerical study of engine performance and emissions for port injection of ammonia into a gasoline\ethanol dual-fuel spark ignition engine. Appl. Sci. 2021, 11, 1441. [Google Scholar] [CrossRef]
- Zheng, K.; Ning, X.; Huo, X.; Chen, M.; Wang, X.; Wang, J. Explosion Characteristics of NH3/CH3OH/Air Mixtures. Energy Fuels 2022, 36, 12737–12749. [Google Scholar] [CrossRef]
- Rehbein, M.; Meier, C.; Eilts, P.; Scholl, S. Mixtures of ammonia and organic solvents as alternative fuel for internal combustion engines. Energy Fuels 2019, 33, 10331–10342. [Google Scholar] [CrossRef]
- Yousefi, A.; Guo, H.; Dev, S.; Lafrance, S.; Liko, B. A study on split diesel injection on thermal efficiency and emissions of an ammonia/diesel dual-fuel engine. Fuel 2022, 316, 123412. [Google Scholar] [CrossRef]
- Kane, S.P.; Northrop, W.F. Thermochemical recuperation to enable efficient ammonia-diesel dual-fuel combustion in a compression ignition engine. Energies 2021, 14, 7540. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, W.; Liang, Y.; Yu, L.; Lu, X. An experimental and detailed kinetic modeling study of the auto-ignition of NH3/diesel mixtures: Part 1-NH3 substitution ratio from 20% to 90%. Combust. Flame 2023, 251, 112391. [Google Scholar] [CrossRef]
- Li, T.; Zhou, X.; Wang, N.; Wang, X.; Chen, R.; Li, S.; Yi, P. A comparison between low-and high-pressure injection dual-fuel modes of diesel-pilot-ignition ammonia combustion engines. J. Energy Inst. 2022, 102, 362–373. [Google Scholar] [CrossRef]
- Zhou, X.; Li, T.; Wang, N.; Wang, X.; Chen, R.; Li, S. Pilot diesel-ignited ammonia dual fuel low-speed marine engines: A comparative analysis of ammonia premixed and high-pressure spray combustion modes with CFD simulation. Renew. Sustain. Energy Rev. 2023, 173, 113108. [Google Scholar] [CrossRef]
- Nadimi, E.; Przybyła, G.; Lewandowski, M.T.; Adamczyk, W. Effects of ammonia on combustion, emissions, and performance of the ammonia/diesel dual-fuel compression ignition engine. J. Energy Inst. 2023, 107, 101158. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, D.; Yang, W.; Qian, Y.; Mao, Y.; Lu, X. Investigation on the potential of using carbon-free ammonia in large two-stroke marine engines by dual-fuel combustion strategy. Energy 2023, 263, 125748. [Google Scholar] [CrossRef]
- Zhang, Z.; Long, W.; Dong, P.; Tian, H.; Tian, J.; Li, B.; Wang, Y. Performance characteristics of a two-stroke low speed engine applying ammonia/diesel dual direct injection strategy. Fuel 2023, 332, 126086. [Google Scholar] [CrossRef]
- Kuta, K.; Przybyła, G.; Kurzydym, D.; Żmudka, Z. Experimental and numerical investigation of dual-fuel CI ammonia engine emissions and after-treatment with V2O5/SiO2–TiO2 SCR. Fuel 2023, 334, 126523. [Google Scholar] [CrossRef]
- Rodríguez, C.G.; Lamas, M.I.; Rodríguez, J.d.D.; Abbas, A. Possibilities of ammonia as both fuel and NOx reductant in marine engines: A numerical study. J. Mar. Sci. Eng. 2022, 10, 43. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Y.; Wang, Y.; Wu, J.; Fu, S. Exploration of environmentally friendly marine power technology-ammonia/diesel stratified injection. J. Clean. Prod. 2022, 380, 135014. [Google Scholar] [CrossRef]
- Frost, J.; Tall, A.; Sheriff, A.M.; Schönborn, A.; Hellier, P. An experimental and modelling study of dual fuel aqueous ammonia and diesel combustion in a single cylinder compression ignition engine. Int. J. Hydrogen Energy 2021, 46, 35495–35510. [Google Scholar] [CrossRef]
- Kurien, C.; Mittal, M. Review on the production and utilization of green ammonia as an alternate fuel in dual-fuel compression ignition engines. Energy Convers. Manag. 2022, 251, 114990. [Google Scholar] [CrossRef]
- Sivasubramanian, R.; Sajin, J.; Omanakuttan Pillai, G. Effect of ammonia to reduce emission from biodiesel fuelled diesel engine. Int. J. Ambient Energy 2022, 43, 661–665. [Google Scholar] [CrossRef]
- Zhou, D.; Tay, K.L.; Tu, Y.; Li, J.; Yang, W.; Zhao, D. A numerical investigation on the injection timing of boot injection rate-shapes in a kerosene-diesel engine with a clustered dynamic adaptive chemistry method. Appl. Energy 2018, 220, 117–126. [Google Scholar] [CrossRef]
- Pham, Q.; Park, S.; Agarwal, A.K.; Park, S. Review of dual-fuel combustion in the compression-ignition engine: Spray, combustion, and emission. Energy 2022, 250, 123778. [Google Scholar] [CrossRef]
- Xu, L.; Chang, Y.; Treacy, M.; Zhou, Y.; Jia, M.; Bai, X.-S. A skeletal chemical kinetic mechanism for ammonia/n-heptane combustion. Fuel 2023, 331, 125830. [Google Scholar] [CrossRef]
- Førby, N.; Thomsen, T.B.; Cordtz, R.F.; Bræstrup, F.; Schramm, J. Ignition and combustion study of premixed ammonia using GDI pilot injection in CI engine. Fuel 2023, 331, 125768. [Google Scholar] [CrossRef]
- Dong, S.; Wang, B.; Jiang, Z.; Li, Y.; Gao, W.; Wang, Z.; Cheng, X.; Curran, H.J. An experimental and kinetic modeling study of ammonia/n-heptane blends. Combust. Flame 2022, 246, 112428. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, W.; Feng, Y.; Wang, W.; Zhu, J.; Qian, Y.; Lu, X. The effect of ammonia addition on the low-temperature autoignition of n-heptane: An experimental and modeling study. Combust. Flame 2020, 217, 4–11. [Google Scholar] [CrossRef]
- Xu, S.; Li, G.; Zhou, M.; Yu, W.; Zhang, Z.; Hou, D.; Yu, F. Experimental and kinetic studies of extinction limits of counterflow cool and hot diffusion flames of ammonia/n-dodecane. Combust. Flame 2022, 245, 112316. [Google Scholar] [CrossRef]
- Wüthrich, S.; Cartier, P.; Süess, P.; Schneider, B.; Obrecht, P.; Herrmann, K. Optical investigation and thermodynamic analysis of premixed ammonia dual-fuel combustion initiated by dodecane pilot fuel. Fuel Commun. 2022, 12, 100074. [Google Scholar] [CrossRef]
- Wang, C.; Feng, Y.; Liu, Z.; Wang, Y.; Fang, J.; Qin, J.; Shao, J.; Huang, H. Assessment of thermodynamic performance and CO2 emission reduction for a supersonic precooled turbine engine cycle fueled with a new green fuel of ammonia. Energy 2022, 261, 125272. [Google Scholar] [CrossRef]
- Li, S.; Li, T.; Wang, N.; Zhou, X.; Chen, R.; Yi, P. An investigation on near-field and far-field characteristics of superheated ammonia spray. Fuel 2022, 324, 124683. [Google Scholar] [CrossRef]
- Gross, C.W.; Kong, S.-C. Performance characteristics of a compression-ignition engine using direct-injection ammonia–DME mixtures. Fuel 2013, 103, 1069–1079. [Google Scholar] [CrossRef]
- İnci, M.; Büyük, M.; Demir, M.H.; İlbey, G. A review and research on fuel cell electric vehicles: Topologies, power electronic converters, energy management methods, technical challenges, marketing and future aspects. Renew. Sustain. Energy Rev. 2021, 137, 110648. [Google Scholar] [CrossRef]
- Wang, G.; Yu, Y.; Liu, H.; Gong, C.; Wen, S.; Wang, X.; Tu, Z. Progress on design and development of polymer electrolyte membrane fuel cell systems for vehicle applications: A review. Fuel Process. Technol. 2018, 179, 203–228. [Google Scholar] [CrossRef]
- Ogungbemi, E.; Wilberforce, T.; Ijaodola, O.; Thompson, J.; Olabi, A. Selection of proton exchange membrane fuel cell for transportation. Int. J. Hydrogen Energy 2021, 46, 30625–30640. [Google Scholar] [CrossRef]
- Jeerh, G.; Zhang, M.; Tao, S. Recent progress in ammonia fuel cells and their potential applications. J. Mater. Chem. A 2021, 9, 727–752. [Google Scholar] [CrossRef]
- Mukelabai, M.D.; Gillard, J.M.; Patchigolla, K. A novel integration of a green power-to-ammonia to power system: Reversible solid oxide fuel cell for hydrogen and power production coupled with an ammonia synthesis unit. Int. J. Hydrogen Energy 2021, 46, 18546–18556. [Google Scholar] [CrossRef]
- Wen, D.; Aziz, M. Techno-economic analyses of power-to-ammonia-to-power and biomass-to-ammonia-to-power pathways for carbon neutrality scenario. Appl. Energy 2022, 319, 119272. [Google Scholar] [CrossRef]
- Wang, B.; Li, T.; Gong, F.; Othman, M.H.D.; Xiao, R. Ammonia as a green energy carrier: Electrochemical synthesis and direct ammonia fuel cell-a comprehensive review. Fuel Process. Technol. 2022, 235, 107380. [Google Scholar] [CrossRef]
- Al-Hamed, K.H.; Dincer, I. A novel ammonia solid oxide fuel cell-based powering system with on-board hydrogen production for clean locomotives. Energy 2021, 220, 119771. [Google Scholar] [CrossRef]
- Hauch, A.; Küngas, R.; Blennow, P.; Hansen, A.B.; Hansen, J.B.; Mathiesen, B.V.; Mogensen, M.B. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, eaba6118. [Google Scholar] [CrossRef] [PubMed]
- Jantakananuruk, N.; Page, J.R.; Armstrong, C.D.; Persky, J.; Datta, R.; Teixeira, A.R. Integrated thermal reforming and electro-oxidation in ammonia-fueled tubular solid oxide fuel cells toward autothermal operation. J. Power Sources 2022, 548, 231999. [Google Scholar] [CrossRef]
- Mao, X.; Sang, J.; Xi, C.; Liu, Z.; Yang, J.; Guan, W.; Wang, J.; Xia, C.; Singhal, S.C. Performance evaluation of ammonia-fueled flat-tube solid oxide fuel cells with different build-in catalysts. Int. J. Hydrogen Energy 2022, 47, 23324–23334. [Google Scholar] [CrossRef]
- Xu, K.; Zhu, F.; Hou, M.; Li, C.; Zhang, H.; Chen, Y. Activating and stabilizing the surface of anode for high-performing direct-ammonia solid oxide fuel cells. Nano Res. 2023, 16, 2454–2462. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, H.; Xu, K.; Zhou, Y.; Zhao, B.; Yuan, W.; Sasaki, K.; Choi, Y.; Chen, Y.; Liu, M. A high-performance and durable direct NH3 tubular protonic ceramic fuel cell integrated with an internal catalyst layer. Appl. Catal. B Environ. 2022, 306, 121071. [Google Scholar] [CrossRef]
- Asmare, M.; İlbaş, M. Direct ammonia fueled solid oxide fuel cells: A comprehensive review on challenges, opportunities and future outlooks. Int. J. Energy Technol. 2020, 2, 70–91. [Google Scholar] [CrossRef]
- Welander, M.M.; Hu, B.; Belko, S.; Lee, K.X.; Dubey, P.K.; Robinson, I.; Singh, P.; Tucker, M.C. Direct utilization of gaseous fuels in metal supported solid oxide fuel cells. Int. J. Hydrogen Energy 2023, 48, 1533–1539. [Google Scholar] [CrossRef]
- Tan, W.C.; Iwai, H.; Kishimoto, M.; Brus, G.; Szmyd, J.S.; Yoshida, H. Numerical analysis on effect of aspect ratio of planar solid oxide fuel cell fueled with decomposed ammonia. J. Power Sources 2018, 384, 367–378. [Google Scholar] [CrossRef]
- Kishimoto, M.; Muroyama, H.; Suzuki, S.; Saito, M.; Koide, T.; Takahashi, Y.; Horiuchi, T.; Yamasaki, H.; Matsumoto, S.; Kubo, H. Development of 1 kW-class Ammonia-fueled Solid Oxide Fuel Cell Stack. Fuel Cells 2020, 20, 80–88. [Google Scholar] [CrossRef]
- Siddiqui, O.; Dincer, I. A review and comparative assessment of direct ammonia fuel cells. Therm. Sci. Eng. Prog. 2018, 5, 568–578. [Google Scholar] [CrossRef]
- Rathore, S.S.; Biswas, S.; Fini, D.; Kulkarni, A.P.; Giddey, S. Direct ammonia solid-oxide fuel cells: A review of progress and prospects. Int. J. Hydrogen Energy 2021, 46, 35365–35384. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, S.; Wang, X.; Lin, B.; Chen, C.; Jiang, L. Facile Synthesis and High-Value Utilization of Ammonia. Chin. J. Chem. 2022, 40, 953–964. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, P.; Jeerh, G.; Chen, S.; Shields, J.; Wang, H.; Tao, S. Electricity generation from ammonia in landfill leachate by an alkaline membrane fuel cell based on precious-metal-free electrodes. ACS Sustain. Chem. Eng. 2020, 8, 12817–12824. [Google Scholar] [CrossRef]
- Dekel, D.R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Hren, M.; Božič, M.; Fakin, D.; Kleinschek, K.S.; Gorgieva, S. Alkaline membrane fuel cells: Anion exchange membranes and fuels. Sustain. Energy Fuels 2021, 5, 604–637. [Google Scholar] [CrossRef]
- Medvedev, J.J.; Tobolovskaya, Y.; Medvedeva, X.V.; Tatarchuk, S.W.; Li, F.; Klinkova, A. Pathways of ammonia electrooxidation on nickel hydroxide anodes and an alternative route towards recycled fertilizers. Green Chem. 2022, 24, 1578–1589. [Google Scholar] [CrossRef]
- Elishav, O.; Mosevitzky Lis, B.; Miller, E.M.; Arent, D.J.; Valera-Medina, A.; Grinberg Dana, A.; Shter, G.E.; Grader, G.S. Progress and prospective of nitrogen-based alternative fuels. Chem. Rev. 2020, 120, 5352–5436. [Google Scholar] [CrossRef]
- Nhiem, L.T. An alternative approach for utilizing urea as fuel source. Braz. J. Chem. Eng. 2022, 1–6. [Google Scholar] [CrossRef]
- Wang, D.; Chen, C.; Wang, S. Defect engineering for advanced electrocatalytic conversion of nitrogen-containing molecules. Sci. China Chem. 2023, 66, 1052–1072. [Google Scholar] [CrossRef]
- Liu, X.; Qin, H.; Ye, Z.; Yao, D.; Miao, W.; Mao, S. Interconnected Mn-doped Ni (OH) 2 nanosheet layer for bifunctional urea oxidation and hydrogen evolution: The relation between current drop and urea concentration during the long-term operation. ACS EST Eng. 2022, 2, 853–862. [Google Scholar] [CrossRef]
- Song, M.; Zhang, Z.; Li, Q.; Jin, W.; Wu, Z.; Fu, G.; Liu, X. Ni-foam supported Co(OH)F and Co–P nanoarrays for energy-efficient hydrogen production via urea electrolysis. J. Mater. Chem. A 2019, 7, 3697–3703. [Google Scholar] [CrossRef]
- Gnana kumar, G.; Farithkhan, A.; Manthiram, A. Direct urea fuel cells: Recent progress and critical challenges of urea oxidation electrocatalysis. Adv. Energy Sustain. Res. 2020, 1, 2000015. [Google Scholar] [CrossRef]
- Nangan, S.; Ding, Y.; Alhakemy, A.Z.; Liu, Y.; Wen, Z. Hybrid alkali-acid urea-nitrate fuel cell for degrading nitrogen-rich wastewater. Appl. Catal. B Environ. 2021, 286, 119892. [Google Scholar] [CrossRef]
- Zhang, Q.; Kazim, F.M.; Ma, S.; Qu, K.; Li, M.; Wang, Y.; Hu, H.; Cai, W.; Yang, Z. Nitrogen dopants in nickel nanoparticles embedded carbon nanotubes promote overall urea oxidation. Appl. Catal. B Environ. 2021, 280, 119436. [Google Scholar] [CrossRef]
- Sayed, E.T.; Abdelkareem, M.A.; Bahaa, A.; Eisa, T.; Alawadhi, H.; Al-Asheh, S.; Chae, K.-J.; Olabi, A. Synthesis and performance evaluation of various metal chalcogenides as active anodes for direct urea fuel cells. Renew. Sustain. Energy Rev. 2021, 150, 111470. [Google Scholar] [CrossRef]
- Putri, Y.M.T.A.; Gunlazuardi, J.; Yulizar, Y.; Wibowo, R.; Einaga, Y.; Ivandini, T.A. Recent progress in direct urea fuel cell. Open Chem. 2021, 19, 1116–1133. [Google Scholar] [CrossRef]
- Li, B.; Song, C.; Yan, J.; Ye, K.; Cheng, K.; Cao, D.; Wang, G. Effect of graphene on the performance of nickel foam-based CoNi nanosheet anode catalyzed direct urea-hydrogen peroxide fuel cell. Int. J. Hydrogen Energy 2020, 45, 10569–10579. [Google Scholar] [CrossRef]
- Wen, X. NiFe-LDH/MWCNTs/NF nanohybrids as a high-performance bifunctional electrocatalyst for overall urea electrolysis. Int. J. Hydrogen Energy 2020, 45, 14660–14668. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, W.; Ni, B.-J. Transition metal chalcogenides as emerging electrocatalysts for urea electrolysis. Curr. Opin. Electrochem. 2022, 31, 100888. [Google Scholar] [CrossRef]
- Singh, R.K.; Rajavelu, K.; Montag, M.; Schechter, A. Advances in catalytic electrooxidation of urea: A review. Energy Technol. 2021, 9, 2100017. [Google Scholar] [CrossRef]
- Wu, T.-H.; Lin, Y.-C.; Hou, B.-W.; Liang, W.-Y. Nanostructured β– NiS catalyst for enhanced and stable electro–oxidation of urea. Catalysts 2020, 10, 1280. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, J.; Li, J.; Wu, Q. Urea Electrooxidation: Current Development and Understanding of Ni-Based Catalysts. ChemElectroChem 2020, 7, 3211–3228. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, L.; Li, J.; Tian, X.; Wu, X.; Feng, L. High valence state of Ni and Mo synergism in NiS2-MoS2 hetero-nanorods catalyst with layered surface structure for urea electrocatalysis. J. Energy Chem. 2022, 66, 483–492. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, C.; Li, J.; Liu, H.; Su, G.; Shi, Z.; Huang, M. Redistributing interfacial charge density of Ni12P5/Ni3P via Fe doping for ultrafast urea oxidation catalysis at large current densities. Chem. Eng. J. 2023, 452, 139362. [Google Scholar] [CrossRef]
- Mehregan, M.; Moghiman, M. Experimental investigation of the distinct effects of nanoparticles addition and urea-SCR after-treatment system on NOx emissions in a blended-biodiesel fueled internal combustion engine. Fuel 2020, 262, 116609. [Google Scholar] [CrossRef]
- Demir, U.; Kozan, A.; Özer, S. Experimental investigation of the effect of urea addition to fuel on engine performance and emissions in diesel engines. Fuel 2022, 311, 122578. [Google Scholar] [CrossRef]
- Yang, B.-M. Effect of Exhaust Gas Flow and Back Pressure on Urea Dosing Unit and Pipe of SCR in Industrial Diesel Engine. Int. J. Mech. Eng. Robot. Res. 2022, 11, 106–113. [Google Scholar] [CrossRef]
- Elishav, O.; Lewin, D.R.; Shter, G.E.; Grader, G.S. The nitrogen economy: Economic feasibility analysis of nitrogen-based fuels as energy carriers. Appl. Energy 2017, 185, 183–188. [Google Scholar] [CrossRef]
- Kopchikov, V. Alternative fuels and assessment of their applicability in internal combustion engines. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; p. 062015. [Google Scholar]
- Kim, J.D.; Choi, M.Y.; Choi, H.C. Graphene-oxide-supported Pt nanoparticles with high activity and stability for hydrazine electro-oxidation in a strong acidic solution. Appl. Surf. Sci. 2017, 420, 700–706. [Google Scholar] [CrossRef]
- Akbar, K.; Kim, J.H.; Lee, Z.; Kim, M.; Yi, Y.; Chun, S.-H. Superaerophobic graphene nano-hills for direct hydrazine fuel cells. NPG Asia Mater. 2017, 9, e378. [Google Scholar] [CrossRef]
- Serov, A.; Kwak, C. Direct hydrazine fuel cells: A review. Appl. Catal. B Environ. 2010, 98, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Guan, X.; Li, H.; Zeng, S.; Li, R.; Yao, Q.; Chen, H.; Zheng, Y.; Qu, K. Robust Ru-N metal-support interaction to promote self-powered H2 production assisted by hydrazine oxidation. Nano Energy 2022, 100, 107467. [Google Scholar] [CrossRef]
- Deng, J.; Li, X.; Imhanria, S.; Chen, K.; Deng, X.; Wang, W. Molybdenum carbide-nitrogen doped carbon composites as effective non-precious electrocatalyst for direct hydrazine fuel cell. Electrochim. Acta 2021, 384, 138417. [Google Scholar] [CrossRef]
- de Oliveira, D.C.; Silva, W.O.; Chatenet, M.; Lima, F.H. NiOx-Pt/C nanocomposites: Highly active electrocatalysts for the electrochemical oxidation of hydrazine. Appl. Catal. B Environ. 2017, 201, 22–28. [Google Scholar] [CrossRef]
- Ding, J.; Kannan, P.; Wang, P.; Ji, S.; Wang, H.; Liu, Q.; Gai, H.; Liu, F.; Wang, R. Synthesis of nitrogen-doped MnO/carbon network as an advanced catalyst for direct hydrazine fuel cells. J. Power Sources 2019, 413, 209–215. [Google Scholar] [CrossRef]
- Liu, X.; He, J.; Zhao, S.; Liu, Y.; Zhao, Z.; Luo, J.; Hu, G.; Sun, X.; Ding, Y. Self-powered H2 production with bifunctional hydrazine as sole consumable. Nat. Commun. 2018, 9, 4365. [Google Scholar] [CrossRef] [Green Version]
- Serov, A.; Padilla, M.; Roy, A.J.; Atanassov, P.; Sakamoto, T.; Asazawa, K.; Tanaka, H. Anode catalysts for direct hydrazine fuel cells: From laboratory test to an electric vehicle. Angew. Chem. 2014, 126, 10504–10507. [Google Scholar] [CrossRef]
- Khalafallah, D.; Zhi, M.; Hong, Z. Development trends on nickel-based electrocatalysts for direct hydrazine fuel cells. ChemCatChem 2021, 13, 81–110. [Google Scholar] [CrossRef]
- Tang, P.; Wen, H.; Wang, P. Hierarchically nanostructured Ni2Fe2N as an efficient electrocatalyst for hydrazine oxidation reaction. Chem. Eng. J. 2022, 431, 134123. [Google Scholar] [CrossRef]
- Jeong, J.; Choun, M.; Lee, J. Tree-Bark-Shaped N-Doped Porous Carbon Anode for Hydrazine Fuel Cells. Angew. Chem. Int. Ed. 2017, 56, 13513–13516. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, M.; Shen, Y.; Wang, L.; Ma, Y.; Li, Y.; Bo, X.; Wang, Z.; Zhao, C. Hierarchical nanoporous Ni(Cu) alloy anchored on amorphous NiFeP as efficient bifunctional electrocatalysts for hydrogen evolution and hydrazine oxidation. J. Catal. 2019, 373, 180–189. [Google Scholar] [CrossRef]
- Munde, A.; Sharma, P.; Dhawale, S.; Kadam, R.G.; Kumar, S.; Kale, H.B.; Filip, J.; Zboril, R.; Sathe, B.R.; Gawande, M.B. Interface Engineering of SRu-mC3N4 Heterostructures for Enhanced Electrochemical Hydrazine Oxidation Reactions. Catalysts 2022, 12, 1560. [Google Scholar] [CrossRef]
- Yu, Y.; Lee, S.J.; Theerthagiri, J.; Lee, Y.; Choi, M.Y. Architecting the AuPt alloys for hydrazine oxidation as an anolyte in fuel cell: Comparative analysis of hydrazine splitting and water splitting for energy-saving H2 generation. Appl. Catal. B Environ. 2022, 316, 121603. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Wan, L.-y.; Han, Y.; Hong, Y.; Huang, L.; Yang, X.; Wang, Y.; Zaghib, K.; Zhou, Z. KOH-doped polybenzimidazole membrane for direct hydrazine fuel cell. J. Colloid Interface Sci. 2020, 563, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.W.; Rondiya, S.R.; Dzade, N.Y. First-principles DFT insights into the adsorption of hydrazine on bimetallic β1-NiZn catalyst: Implications for direct hydrazine fuel cells. Appl. Surf. Sci. 2021, 536, 147648. [Google Scholar] [CrossRef]
- Sun, H.; Gao, L.; Kumar, A.; Cao, Z.; Chang, Z.; Liu, W.; Sun, X. Superaerophobic CoP Nanowire Arrays as a Highly Effective Anode Electrocatalyst for Direct Hydrazine Fuel Cells. ACS Appl. Energy Mater. 2022, 5, 9455–9462. [Google Scholar] [CrossRef]
- Crisafulli, R.; de Paula, D.F.; Zignani, S.C.; Spadaro, L.; Palella, A.; Boninelli, S.; Dias, J.A.; Linares, J.J. Promoting Effect of Cu on Pd Applied to the Hydrazine Electro-Oxidation and Direct Hydrazine Fuel Cells. Catalysts 2022, 12, 1639. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Daneshvari-Esfahlan, V.; Aghajani, H.; Wolf, S.; Hacker, V. Palladium-nickel electrocatalysts on nitrogen-doped reduced graphene oxide nanosheets for direct hydrazine/hydrogen peroxide fuel cells. Catalysts 2021, 11, 1372. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Daneshvari-Esfahlan, V.; Wolf, S.; Hacker, V. Cobalt-modified palladium nanocatalyst on nitrogen-doped reduced graphene oxide for direct hydrazine fuel cell. RSC Adv. 2021, 11, 39223–39232. [Google Scholar] [CrossRef] [PubMed]
- Grinberg Dana, A.; Shter, G.E.; Grader, G.S. Nitrogen-Based Alternative Fuels: Progress and Future Prospects. Energy Technol. 2016, 4, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Goodman, S.M. The Impact of EU Anti-Dumping Duties on Urea Ammonium Nitrate Solution; Office of Industries, US International Trade Commission: Washington, DC, USA, 2020.
- Dana, A.G.; Shter, G.E.; Grader, G.S. Nitrogen-based alternative fuel: An environmentally friendly combustion approach. RSC Adv. 2014, 4, 10051–10059. [Google Scholar] [CrossRef]
- Grinberg Dana, A.; Shter, G.E.; Grader, G.S. Nitrogen-Based Alternative Fuel: Safety Considerations. Energy Technol. 2015, 3, 976–981. [Google Scholar] [CrossRef]
- Mosevitzky, B.; Shter, G.E.; Grader, G.S. Auto-ignition of a carbon-free aqueous ammonia/ammonium nitrate monofuel: A thermal and barometric analysis. Fuel Process. Technol. 2017, 159, 363–368. [Google Scholar] [CrossRef]
- Grinberg Dana, A.; Elishav, O.; Bardow, A.; Shter, G.E.; Grader, G.S. Nitrogen-based fuels: A power-to-fuel-to-power analysis. Angew. Chem. Int. Ed. 2016, 55, 8798–8805. [Google Scholar] [CrossRef] [Green Version]
- Mosevitzky, B.; Shter, G.E.; Grader, G.S. Effect of equivalence ratio on the thermal autoignition of aqueous ammonia ammonium nitrate monofuel. Combust. Flame 2018, 188, 142–149. [Google Scholar] [CrossRef]
- Mosevitzky, B.; Azoulay, R.; Naamat, L.; Shter, G.E.; Grader, G.S. Effects of water content and diluent pressure on the ignition of aqueous ammonia/ammonium nitrate and urea/ammonium nitrate fuels. Appl. Energy 2018, 224, 300–308. [Google Scholar] [CrossRef]
- Grinberg Dana, A.; Mosevitzky, B.; Tvil, G.; Epstein, M.; Shter, G.E.; Grader, G.S. Flow reactor combustion of aqueous urea ammonium nitrate fuel. Energy Fuels 2016, 30, 2474–2477. [Google Scholar] [CrossRef]
- Oluwoye, I.; Altarawneh, M.; Gore, J.; Dlugogorski, B.Z. Burning properties of redox crystals of ammonium nitrate and saccharides. Combust. Flame 2020, 213, 132–139. [Google Scholar] [CrossRef]
- Oluwoye, I.; Mosallanejad, S.; Soubans, G.; Altarawneh, M.; Gore, J.; Dlugogorski, B.Z. Thermal decomposition of ammonium nitrate on rust surface: Risk of low-temperature fire. Fire Saf. J. 2021, 120, 103063. [Google Scholar] [CrossRef]
- Hossain, F. Adaptation measures (AMs) and mitigation policies (MPs) to climate change and sustainable blue economy: A global perspective. J. Water Clim. Change 2021, 12, 1344–1369. [Google Scholar] [CrossRef]
- Schiffer, Z.J.; Biswas, S.; Manthiram, K. Ammonium Formate as a Safe, Energy-Dense Electrochemical Fuel Ionic Liquid. ACS Energy Lett. 2022, 7, 3260–3267. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Chen, R. Direct formate fuel cells: A review. J. Power Sources 2016, 320, 127–139. [Google Scholar] [CrossRef]
- Su, J.; Yang, L.; Lu, M.; Lin, H. Highly efficient hydrogen storage system based on ammonium bicarbonate/formate redox equilibrium over palladium nanocatalysts. ChemSusChem 2015, 8, 813–816. [Google Scholar] [CrossRef]
- Nakajima, K.; Tominaga, M.; Waseda, M.; Miura, H.; Shishido, T. Highly efficient supported palladium–gold alloy catalysts for hydrogen storage based on ammonium bicarbonate/formate redox cycle. ACS Sustain. Chem. Eng. 2019, 7, 6522–6530. [Google Scholar] [CrossRef]
- Bahuguna, A.; Sasson, Y. Formate-Bicarbonate Cycle as a Vehicle for Hydrogen and Energy Storage. ChemSusChem 2021, 14, 1258–1283. [Google Scholar] [CrossRef]
- Russo, D.; Calabrese, M.; Marotta, R.; Andreozzi, R.; Di Benedetto, A. Thermodynamics of the cyclic formate/bicarbonate interconversion for hydrogen storage. Int. J. Hydrogen Energy 2022, 47, 31370–31380. [Google Scholar] [CrossRef]
- Dong, Z.; Mukhtar, A.; Ludwig, T.; Akhade, S.A.; Kang, S.; Wood, B.; Grubel, K.; Engelhard, M.; Autrey, T.; Lin, H. Efficient Pd on carbon catalyst for ammonium formate dehydrogenation: Effect of surface oxygen functional groups. Appl. Catal. B Environ. 2023, 321, 122015. [Google Scholar] [CrossRef]
- Lan, R.; Irvine, J.T.; Tao, S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Int. J. Hydrogen Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- Lee, K.R.; Song, D.; Park, S.B.; Han, J.-I. A direct ammonium carbonate fuel cell with an anion exchange membrane. Rsc Adv. 2014, 4, 5638–5641. [Google Scholar] [CrossRef]
- Lakshmi, K.S.; Ji, X.; Chen, T.-Y.; Vedhanarayanan, B.; Lin, T.-W. Pseudocapacitive and battery-type organic polymer electrodes for a 1.9 V hybrid supercapacitor with a record concentration of ammonium acetate. J. Power Sources 2021, 511, 230434. [Google Scholar] [CrossRef]
- Bashirnezhad, K.; Mehregan, M.; Kebriyaee, S.A. Experimental analysis of the influence of urea injection upon NOx emissions in internal combustion engines fueled with biodiesels. J. Energy Inst. 2016, 89, 115–120. [Google Scholar] [CrossRef]
- Mehregan, M.; Moghiman, M. Effects of nano-additives on pollutants emission and engine performance in a urea-SCR equipped diesel engine fueled with blended-biodiesel. Fuel 2018, 222, 402–406. [Google Scholar] [CrossRef]
- An, H.; Yang, W.; Li, J.; Zhou, D. Modeling analysis of urea direct injection on the NOx emission reduction of biodiesel fueled diesel engines. Energy Convers. Manag. 2015, 101, 442–449. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Zhong, Y.; Lei, G.; Li, Z.; Zhang, J.; Zhang, T. Transition metal complexes based on hypergolic anions for catalysis of ammonium perchlorate thermal decomposition. Energy Fuels 2020, 34, 14667–14675. [Google Scholar] [CrossRef]
- Li, R.; Li, X.; Tang, P.; Yang, B.; Xie, L.; Yang, G. Defect-engineered sp2 carbon as highly active catalyst and reactive fuel for combustion of ammonium perchlorate. Chem. Eng. J. 2021, 426, 131918. [Google Scholar] [CrossRef]
- Nagendra, K.; Vijay, C.; Ingole, M.; Ramakrishna, P. Combustion of ammonium perchlorate monopropellant: Role of heat loss. Combust. Flame 2019, 209, 363–375. [Google Scholar] [CrossRef]
- Li, R.; Li, X.; Yang, G.; Guo, C.; Zhang, L.; Hao, C.; Zhu, W. Defective-activated-carbon-encapsulated Co as a super reactive catalyst for combustion of ammonium perchlorate. Appl. Surf. Sci. 2023, 615, 156349. [Google Scholar] [CrossRef]
- Yang, L.; Xu, R.; Mi, Z.; Wan, Y.; Fu, X.; Jiang, L.; Jian, Y.; Li, J.; Zhang, G. Enhanced anti-migration performance of carbon nanotubes confined ferrocenyl compounds and their synergistic catalytic activity on the thermal decomposition of ammonium perchlorate. Mater. Today Chem. 2022, 26, 101168. [Google Scholar] [CrossRef]
- Demirci, U.B. Ammonia borane: An extensively studied, though not yet implemented, hydrogen carrier. Energies 2020, 13, 3071. [Google Scholar] [CrossRef]
- Baier, M.J.; Ramachandran, P.V.; Son, S.F. Characterization of the hypergolic ignition delay of ammonia borane. J. Propuls. Power 2019, 35, 182–189. [Google Scholar] [CrossRef]
- Navlani-García, M.; Salinas-Torres, D.; Cazorla-Amorós, D. Hydrolytic dehydrogenation of ammonia borane attained by Ru-based catalysts: An auspicious option to produce hydrogen from a solid hydrogen carrier molecule. Energies 2021, 14, 2199. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, L.; Luo, X.; Wan, C.; Xu, L. Recent advances in noble metal catalysts for hydrogen production from ammonia borane. Catalysts 2020, 10, 788. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Navlani-García, M.; Kuwahara, Y.; Mori, K.; Yamashita, H. Non-noble metal doped perovskite as a promising catalyst for ammonia borane dehydrogenation. Catal. Today 2020, 351, 6–11. [Google Scholar] [CrossRef]
- Cai, H.-K.; Jiang, Z.-Y.; Xu, S.; Xu, Y.; Lu, P.; Dong, J. Polymer Hydrogel Supported Ni/Pd Alloys for Hydrogen Gas Production from Hydrolysis of Dimethylamine Borane with a Long Recyclable Lifetime. Polymers 2022, 14, 4647. [Google Scholar] [CrossRef] [PubMed]
- Karaboğa, S. Tungsten (VI) oxide supported rhodium (0) nanoparticles; highly efficient catalyst for H2 production from dimethylamine borane. Int. J. Hydrogen Energy 2021, 46, 17763–17775. [Google Scholar] [CrossRef]
- Shiota, K.; Izato, Y.-I.; Habu, H.; Miyake, A. Reactivity analysis of ammonium dinitramide binary mixtures based on ab initio calculations and thermal analysis. J. Therm. Anal. Calorim. 2019, 138, 2615–2622. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Zhang, T.; Liu, Y.; Yang, R.; Chen, Y. Catalytic bed slenderness ratio and ADN/methanol ratio for decomposition and combustion characteristics within ammonium dinitramide (ADN)-based green aerospace thruster. Chin. J. Chem. Eng. 2019, 27, 1159–1165. [Google Scholar] [CrossRef]
- Li, H.-M.; Li, G.-X.; Li, L. Comparative investigation on combustion characteristics of ADN-based liquid propellants in inert gas and oxidizing gas atmospheres with resistive ignition method. Fuel 2023, 334, 126742. [Google Scholar] [CrossRef]
- Shen, J.; Yu, Y.; Liu, X.; Cao, J. Experimental research on microwave ignition and combustion characteristics of ADN-based liquid propellant. Micromachines 2022, 13, 510. [Google Scholar] [CrossRef]
- Klerke, A.; Christensen, C.H.; Nørskov, J.K.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mater. Chem. 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Assumpção, M.H.; da Silva, S.G.; de Souza, R.F.; Buzzo, G.S.; Spinacé, E.V.; Neto, A.O.; Silva, J.C.M. Direct ammonia fuel cell performance using PtIr/C as anode electrocatalysts. Int. J. Hydrogen Energy 2014, 39, 5148–5152. [Google Scholar] [CrossRef]
- Ghavam, S.; Vahdati, M.; Wilson, I.G.; Styring, P. Sustainable ammonia production processes. Front. Energy Res. 2021, 9, 580808. [Google Scholar] [CrossRef]
- Saygin, D.; Blanco, H.; Boshell, F.; Cordonnier, J.; Rouwenhorst, K.; Lathwal, P.; Gielen, D. Ammonia Production from Clean Hydrogen and the Implications for Global Natural Gas Demand. Sustainability 2023, 15, 1623. [Google Scholar] [CrossRef]
- Liu, X.; Elgowainy, A.; Wang, M. Life cycle energy use and greenhouse gas emissions of ammonia production from renewable resources and industrial by-products. Green Chem. 2020, 22, 5751–5761. [Google Scholar] [CrossRef]
- Chisalita, D.-A.; Petrescu, L.; Cormos, C.-C. Environmental evaluation of european ammonia production considering various hydrogen supply chains. Renew. Sustain. Energy Rev. 2020, 130, 109964. [Google Scholar] [CrossRef]
- Hochman, G.; Goldman, A.S.; Felder, F.A.; Mayer, J.M.; Miller, A.J.; Holland, P.L.; Goldman, L.A.; Manocha, P.; Song, Z.; Aleti, S. Potential economic feasibility of direct electrochemical nitrogen reduction as a route to ammonia. ACS Sustain. Chem. Eng. 2020, 8, 8938–8948. [Google Scholar] [CrossRef]
- Xu, H.; Ithisuphalap, K.; Li, Y.; Mukherjee, S.; Lattimer, J.; Soloveichik, G.; Wu, G. Electrochemical ammonia synthesis through N2 and H2O under ambient conditions: Theory, practices, and challenges for catalysts and electrolytes. Nano Energy 2020, 69, 104469. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, T.; Luo, Y.; Kong, Q.; Li, T.; Lu, S.; Alshehri, A.A.; Alzahrani, K.A.; Sun, X. Recent advances in strategies for highly selective electrocatalytic N2 reduction toward ambient NH3 synthesis. Curr. Opin. Electrochem. 2021, 29, 100766. [Google Scholar] [CrossRef]
- Garagounis, I.; Vourros, A.; Stoukides, D.; Dasopoulos, D.; Stoukides, M. Electrochemical synthesis of ammonia: Recent efforts and future outlook. Membranes 2019, 9, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
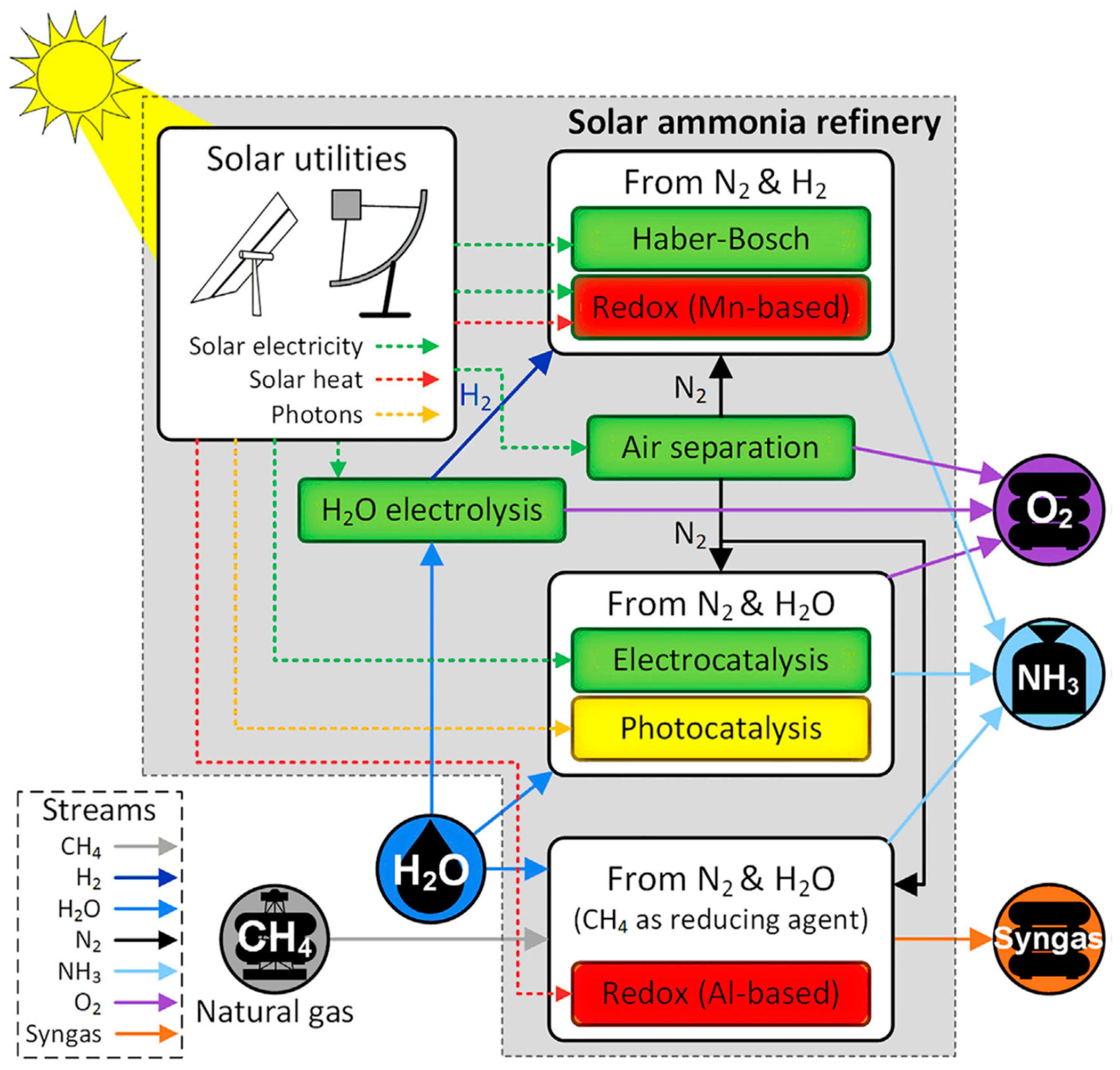
- Wang, L.; Xia, M.; Wang, H.; Huang, K.; Qian, C.; Maravelias, C.T.; Ozin, G.A. Greening ammonia toward the solar ammonia refinery. Joule 2018, 2, 1055–1074. [Google Scholar] [CrossRef] [Green Version]
- Service, R.F. Liquid sunshine. Science 2018, 361, 120–123. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Choi, J.; Suryanto, B.H.; Jalili, R.; Chatti, M.; Azofra, L.M.; Simonov, A.N. Liquefied sunshine: Transforming renewables into fertilizers and energy carriers with electromaterials. Adv. Mater. 2020, 32, 1904804. [Google Scholar] [CrossRef]
- Makhloufi, C.; Kezibri, N. Large-scale decomposition of green ammonia for pure hydrogen production. Int. J. Hydrogen Energy 2021, 46, 34777–34787. [Google Scholar] [CrossRef]
- Huang, C.; Yu, Y.; Tang, X.; Liu, Z.; Zhang, J.; Ye, C.; Ye, Y.; Zhang, R. Hydrogen generation by ammonia decomposition over Co/CeO2 catalyst: Influence of support morphologies. Appl. Surf. Sci. 2020, 532, 147335. [Google Scholar] [CrossRef]
- Pashchenko, D.; Mustafin, R. Ammonia decomposition in the thermochemical waste-heat recuperation systems: A view from low and high heating value. Energy Convers. Manag. 2022, 251, 114959. [Google Scholar] [CrossRef]
- Pashchenko, D.; Mustafin, R.; Karpilov, I. Ammonia-fired chemically recuperated gas turbine: Thermodynamic analysis of cycle and recuperation system. Energy 2022, 252, 124081. [Google Scholar] [CrossRef]
- Pashchenko, D. Thermochemical waste-heat recuperation as on-board hydrogen production technology. Int. J. Hydrogen Energy 2021, 46, 28961–28968. [Google Scholar] [CrossRef]
- Itoh, N.; Kikuchi, Y.; Furusawa, T.; Sato, T. Tube-wall catalytic membrane reactor for hydrogen production by low-temperature ammonia decomposition. Int. J. Hydrogen Energy 2021, 46, 20257–20265. [Google Scholar] [CrossRef]
- Tang, H.; Wang, Y.; Zhang, W.; Liu, Z.; Li, L.; Han, W.; Li, Y. Catalytic activity of Ru supported on SmCeOx for ammonia decomposition: The effect of Sm doping. J. Solid State Chem. 2021, 295, 121946. [Google Scholar] [CrossRef]
- Kirste, K.G.; McAulay, K.; Bell, T.E.; Stoian, D.; Laassiri, S.; Daisley, A.; Hargreaves, J.S.; Mathisen, K.; Torrente-Murciano, L. COx-free hydrogen production from ammonia–mimicking the activity of Ru catalysts with unsupported Co-Re alloys. Appl. Catal. B Environ. 2021, 280, 119405. [Google Scholar] [CrossRef]
- El-Shafie, M.; Kambara, S.; Hayakawa, Y. Energy and exergy analysis of hydrogen production from ammonia decomposition systems using non-thermal plasma. Int. J. Hydrogen Energy 2021, 46, 29361–29375. [Google Scholar] [CrossRef]
- Sittichompoo, S.; Nozari, H.; Herreros, J.; Serhan, N.; da Silva, J.; York, A.; Millington, P.; Tsolakis, A. Exhaust energy recovery via catalytic ammonia decomposition to hydrogen for low carbon clean vehicles. Fuel 2021, 285, 119111. [Google Scholar] [CrossRef]
- Khan, W.U.; Alasiri, H.S.; Ali, S.A.; Hossain, M.M. Recent Advances in Bimetallic Catalysts for Hydrogen Production from Ammonia. Chem. Rec. 2022, 22, e202200030. [Google Scholar] [CrossRef]
- Rothan, Y.A.; Ali, F.F.; Issakhov, A.; Selim, M.M.; Li, Z. Optimization analysis of hydrogen production using ammonia decomposition. J. Mol. Liq. 2021, 335, 116190. [Google Scholar] [CrossRef]
- Fang, H.; Liu, D.; Luo, Y.; Zhou, Y.; Liang, S.; Wang, X.; Lin, B.; Jiang, L. Challenges and opportunities of Ru-based catalysts toward the synthesis and utilization of ammonia. ACS Catal. 2022, 12, 3938–3954. [Google Scholar] [CrossRef]
- Chang, F.; Gao, W.; Guo, J.; Chen, P. Emerging Materials and Methods toward Ammonia-Based Energy Storage and Conversion. Adv. Mater. 2021, 33, 2005721. [Google Scholar] [CrossRef]










| Properties | Compressed Hydrogen | Liquid Hydrogen | Methanol | Liquid Ammonia |
|---|---|---|---|---|
| Storage method | Compression | Liquefaction | Ambient | Liquefaction |
| Temperature (°C) | 25 | −252.9 | 25 | 25 |
| Storage pressure (MPa) | 69 | 0.1 | 0.1 | 0.99 |
| Density (kg/m3) | 39 | 70.8 | 792 | 600 |
| Explosive limit in air (% Vol) | 4–75 | 4–75 | 6.7–36 | 15–28 |
| Gravimetric energy density (MJ/kg) | 120 | 120 | 20.1 | 18.6 |
| Volumetric energy density (MJ/L) | 4.5 | 8.49 | 15.8 | 12.7 |
| Gravimetric hydrogen content (wt.%) | 100 | 100 | 12.5 | 17.8 |
| Volumetric hydrogen content (kg-H2/m3) | 42.2 | 70.8 | 99 | 121 |
| Hydrogen release | Pressure release | Evaporation | Catalytic decomposition T > 200 °C | Catalytic decomposition T > 400 °C |
| Energy to extract hydrogen (kJ/mol-H2) | --- | 0.907 | 16.3 | 30.6 |
| NH3 | H2 | CH4 | Diesel | Biodiesel | Gasoline | Ethanol | |
|---|---|---|---|---|---|---|---|
| Lower Heating Value (MJ/kg) | 18.8 | 120 | 50 | 45 | 37 | 42.5 | 27 |
| Density (kg/m3) | 730 | 80 | 660 | 850 | 860 | 700 | 790 |
| Autoignition temperature (°C) | 651 | 520 | 630 | 254 | 363 | 370 | 423 |
| Flammability limit (Equivalence ratio) | 0.63–1.4 | 0.1–7.1 | 0.5–1.7 | 0.8–6.5 | – | 0.55–4.24 | – |
| Maximum laminar flame speed (cm/s) | 7 | 291 | 37 | 90 | 90 | 43 | 48 |
| Organic Solvent | Functional Group | Carbon Atoms Number |
|---|---|---|
| Alkanes | non | 5–8 |
| Alcohols | –OH | 1–8 |
| Aldehydes | R–C=O | 3–8 |
| Ketones | R–C(=O)–R′ | 3–8 |
| Carboxylic acids | R–COOH | 1–8 |
| Ethers | R–C–O–C–R | 4–6, 8 |
| Esters | R–C(=O)OC–R | 2–8 |
| Carbonates | R–O–C(=O)–O–R | 3–5 |
| Primary amines | R–NH2 | 3–8 |
| Secondary amines | R–N(–H)–R | 3, 4, 6, 8 |
| Fuel | H2 (a) | NH3 (b) | NH3 (c) | NH3 (d) | (e) N2H4 | (f) UAN | (g) AAN |
|---|---|---|---|---|---|---|---|
| Phase | gas | liq. | liq. | liq. | liq. | ||
| Energy density ρ (kg/m3) | 39.2 | 639 | 606 | 682 | 1004 | 1330 | 1106 |
| Viscosity μ (mPa·s) | 0.011 | 0.17 | 0.13 | 0.26 | 0.90 | N/A | N/A |
| Specific energy (MJ/kg) | 143 | --- | --- | 22.5 | 19.4 | 3.35 | 3.66 |
| Vol. Energy Density (GJ/m3) | 5.60 | 14.4 | 13.7 | 15.4 | 19.5 | 4.46 | 4.05 |
| Octane Number (RON) | >130 | 130 | 130 | N/A | N/A | N/A | |
| Flash point (°K) | N/A | N/A | N/A | 325 | N/A | N/A | |
| Autoignition temp. (AIT) (°K) | 810 | 924 | 924 | 438 | 604 | 586 | |
| Adiabatic flame temp. (Tad) °K | 2519 | 2107 | 2107 | 2433 | 1349 | 1334 | |
| Flame speed (SL) (m/s) | 2.2 | 0.07 | 0.07 | N/A | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estevez, R.; López-Tenllado, F.J.; Aguado-Deblas, L.; Bautista, F.M.; Romero, A.A.; Luna, D. Current Research on Green Ammonia (NH3) as a Potential Vector Energy for Power Storage and Engine Fuels: A Review. Energies 2023, 16, 5451. https://doi.org/10.3390/en16145451
Estevez R, López-Tenllado FJ, Aguado-Deblas L, Bautista FM, Romero AA, Luna D. Current Research on Green Ammonia (NH3) as a Potential Vector Energy for Power Storage and Engine Fuels: A Review. Energies. 2023; 16(14):5451. https://doi.org/10.3390/en16145451
Chicago/Turabian StyleEstevez, Rafael, Francisco J. López-Tenllado, Laura Aguado-Deblas, Felipa M. Bautista, Antonio A. Romero, and Diego Luna. 2023. "Current Research on Green Ammonia (NH3) as a Potential Vector Energy for Power Storage and Engine Fuels: A Review" Energies 16, no. 14: 5451. https://doi.org/10.3390/en16145451
APA StyleEstevez, R., López-Tenllado, F. J., Aguado-Deblas, L., Bautista, F. M., Romero, A. A., & Luna, D. (2023). Current Research on Green Ammonia (NH3) as a Potential Vector Energy for Power Storage and Engine Fuels: A Review. Energies, 16(14), 5451. https://doi.org/10.3390/en16145451









