Effect of Electro-Oil Acclimation of an Indigenous Strain on the Performance of Sediment Microbial Fuel Cells (SMFC)
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.1.1. Chemical Materials
2.1.2. Other Materials
2.2. Experimental Methods
2.2.1. Isolation and Identification of Indigenous Bacteria
2.2.2. Electric-Oil-Induced Domestication
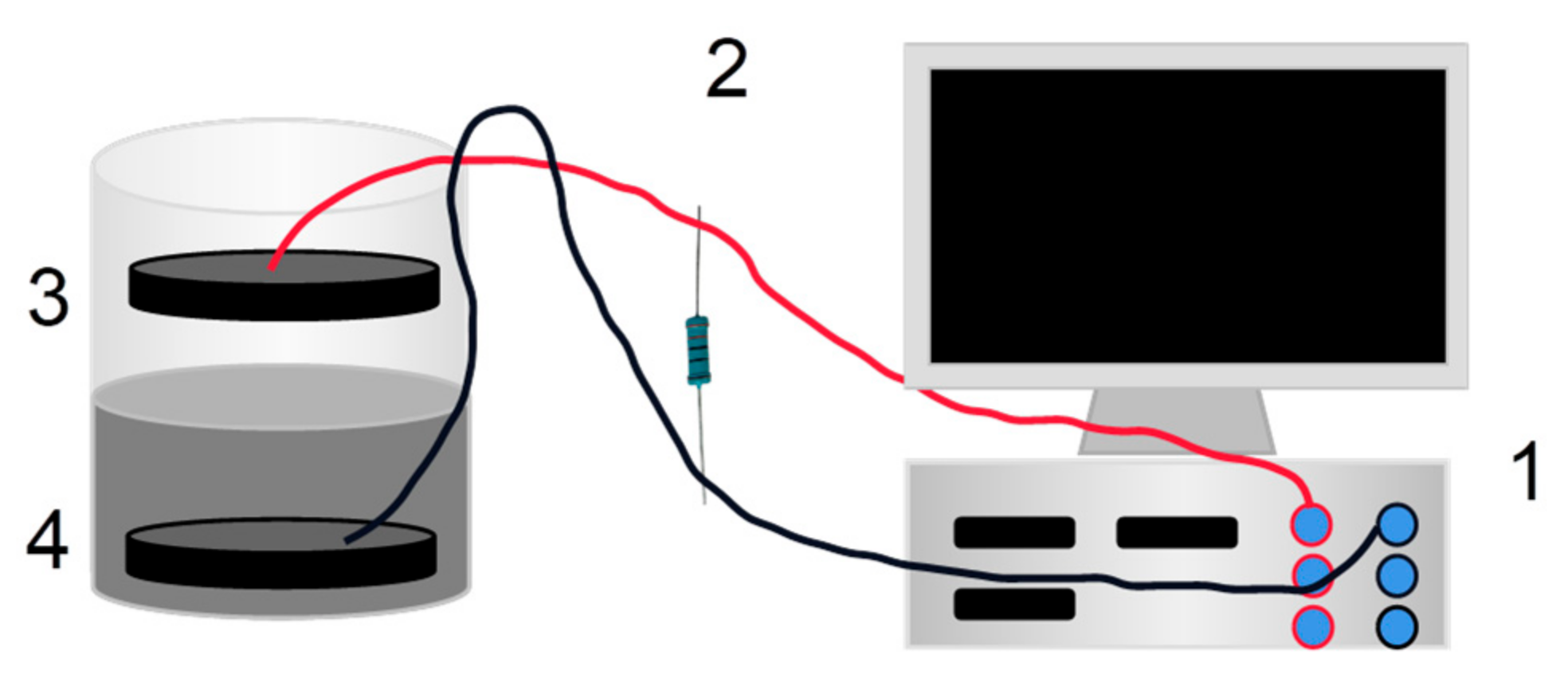
2.2.3. Construction of SMFC
2.2.4. SMFC Electricity Generation and Oil Removal Test
2.2.5. Gas Chromatography-Mass Spectrometry n-Alkanes Component Test
2.2.6. Cyclic Voltammetry and Chronoamperometry Test
3. Results and Discussion
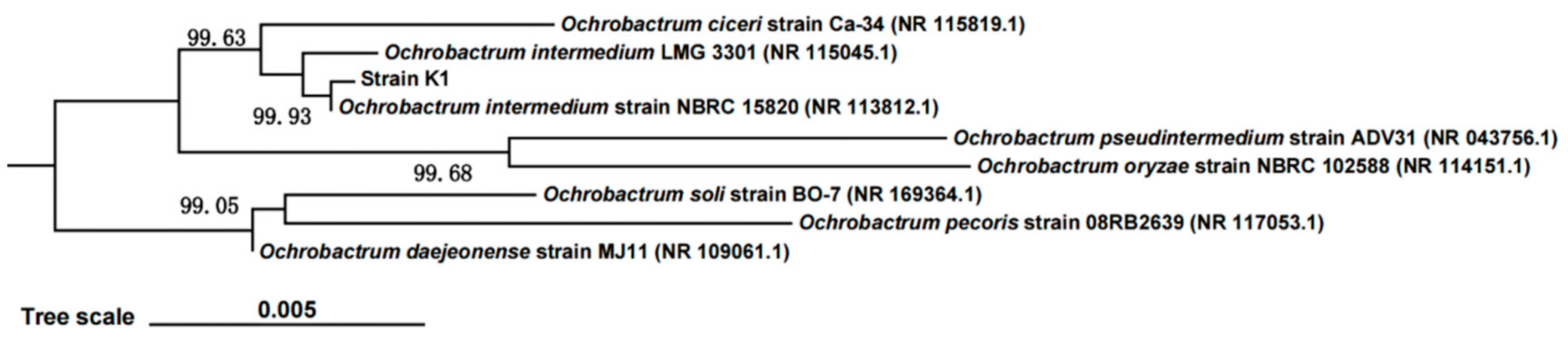
3.1. Strain Identification
3.2. Electric-Oil-Induced Domestication
3.3. Analysis of the Effect of Strain K1 on SMFC
3.4. Electrochemical Activity Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ge, C.; Lei, D.; Liu, S.; Shen, Y.; Liu, Z.; Shan, H. Pilot Studies on Treatment of Oily Solid Waste with Ex Situ Smoldering Remediation Technology. Chin. J. Environ. Eng. 2022, 16, 601–611. [Google Scholar] [CrossRef]
- Liu, F.; Liu, H.; Zhu, H.; Xie, Y.; Zhang, D.; Cheng, Y.; Zhang, J.; Feng, R.; Yang, S. Remediation of petroleum hydrocarbon contaminated groundwater by biochar immobilized bacterial consortia. Environ. Chem. 2022, 41, 3436–3447. [Google Scholar] [CrossRef]
- Pinedo, J.; Ibáñez, R.; Lijzen, J.P.A.; Irabien, A. Assessment of soil pollution based on total petroleum hydrocarbons and individual oil substances. J. Environ. Manag. 2013, 130, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ding, Y.; Hu, Y.; Cao, B.; Rice, S.A.; Kjelleberg, S.; Song, H. Enhancing bidirectional electron transfer of Shewanella oneidensis by a synthetic flavin pathway. ACS Synth. Biol. 2015, 4, 815–823. [Google Scholar] [CrossRef]
- Song, X.; Wang, W.; Cao, X.; Wang, Y.; Zou, L.; Ge, X.; Zhao, Y.; Si, Z.; Wang, Y. Chlorella vulgaris on the cathode promoted the performance of sediment microbial fuel cells for electrogenesis and pollutant removal. Sci. Total Environ. 2020, 728, 138011. [Google Scholar] [CrossRef]
- Xia, C.; Xu, M.; Liu, J.; Guo, J.; Yang, Y. Sediment microbial fuel cell prefers to degrade organic chemicals with higher polarity. Bioresour. Technol. 2015, 190, 420–423. [Google Scholar] [CrossRef]
- Wang, A.; Cheng, H.; Ren, N.; Cui, D.; Lin, N.; Wu, W. Sediment microbial fuel cell with floating biocathode for organic removal and energy recovery. Front. Environ. Sci. Eng. 2012, 6, 569–574. [Google Scholar] [CrossRef]
- Song, T.; Tan, W.; Wu, X.; Zhou, C.C. Effect of graphite felt and activated carbon fiber felt on performance of freshwater sediment microbial fuel cell. J. Chem. Technol. Biotechnol. 2012, 87, 1436–1440. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Q.; Wu, M.; Ding, J.; Zhang, W. Biodegradation of organic matter and anodic microbial communities analysis in sediment microbial fuel cells with/without Fe (III) oxide addition. Bioresour. Technol. 2017, 225, 402–408. [Google Scholar] [CrossRef]
- Guo, H.; Xie, S.; Deng, H.; Geng, X.; Wang, P.; Huang, C.; Tang, S. Electricity production and bacterial communities of microbial fuel cell supplied with oily sludge. Environ. Prog. Sustain. Energy 2020, 39, e13409. [Google Scholar] [CrossRef]
- Cai, T.; Zhang, Y.; Wang, N.; Zhang, Z.; Lu, X.; Zhen, G. Electrochemically active microorganisms sense charge transfer resistance for regulating biofilm electroactivity spatio-temporal distribution, and catabolic pathway. Chem. Eng. J. 2022, 442, 136248. [Google Scholar] [CrossRef]
- Rui, Z.; Yun, W.; Lutian, W.; Qiang, W.; Hongwei, Z. Cathode Denitrification of Microbial Fuel Cells. Prog. Chem. 2020, 32, 2013–2021. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, J.; Zhang, Z.; Song, B.; Liu, F.; Zhang, J. Effect of carbon cloth-loaded Co3O4 nanosheet array anode on the power generation performance of microbial fuel cell. Chin. J. Environ. Eng. 2022, 16, 2447–2456. [Google Scholar] [CrossRef]
- Qian, Z.; Yang, L.; Xie, B.; Liu, H.; Liu, H. Isolation and identification of a simultaneous electricity production and denitrification strain in a microbial fuel cell with biocathode and its characteristics. Chin. J. Environ. Eng. 2019, 13, 1986–1994. [Google Scholar] [CrossRef]
- You, Z.; Li, F.; Hao, S. Design and construction of electroactive cells by synthetic biology strategies. Synth. Biol. J. 2022, 3, 1031. [Google Scholar] [CrossRef]
- Lin, T.; Ding, W.; Sun, L.; Wang, L.; Liu, C.-G.; Song, H. Engineered Shewanella oneidensis-reduced graphene oxide biohybrid with enhanced biosynthesis and transport of flavins enabled a highest bioelectricity output in microbial fuel cells. Nano Energy 2018, 50, 639–648. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, M.; Guo, J.; Sun, G. Bacterial extracellular electron transfer in bioelectrochemical systems. Process Biochem. 2012, 47, 1707–1714. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, Y.; Wu, Y.; Chen, B.; Zhao, F. Electron transfer mediators in microbial electrochemical systems. Prog. Chem. 2014, 26, 1859. [Google Scholar] [CrossRef]
- Shao, Y.-Z.; Che, J.; Cheng, C.; Jiang, Z.-Y.; Xue, C. Advances in Molecular Biological Methods to Improve Extracellular Electron Transport Efficiency of Electroactive Microorganisms. China Biotechnol. 2021, 41, 50–59. [Google Scholar] [CrossRef]
- Huang, Y.; He, Z.; Xu, L.; Yang, B.; Hou, Y.; Lei, L.; Li, Z. Alternating current enhanced bioremediation of petroleum hydrocarbon-contaminated soils. Environ. Sci. Pollut. Res. 2021, 28, 47562–47573. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhong, W.; Mu, H. Refinery residual sludge reduction technologies by using multi-function microbe. J. Petrochem. Univ. 2010, 23, 23. [Google Scholar]
- Cerqueira, V.S.; Hollenbach, E.B.; Maboni, F.; Vainstein, M.H.; Camargo, F.A.O.; Maria do Carmo, R.P.; Bento, F.M. Biodegradation potential of oily sludge by pure and mixed bacterial cultures. Bioresour. Technol. 2011, 102, 11003–11010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, L.J.A.; Alves, F.C.; de França, F.P. A review of the technological solutions for the treatment of oily sludges from petroleum refineries. Waste Manag. Res. 2012, 30, 1016–1030. [Google Scholar] [CrossRef] [PubMed]
- ElMekawy, A.; Hegab, H.M.; Dominguez-Benetton, X.; Pant, D. Internal resistance of microfluidic microbial fuel cell: Challenges and potential opportunities. Bioresour. Technol. 2013, 142, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Jiang, Z.-H.; Lay, C.-H.; Zhou, D. Electricity generation from swine wastewater in microbial fuel cell: Hydraulic reaction time effect. Int. J. Hydrog. Energy 2016, 41, 21820–21826. [Google Scholar] [CrossRef]
- Hirose, A.; Kouzuma, A.; Watanabe, K. Towards development of electrogenetics using electrochemically active bacteria. Biotechnol. Adv. 2019, 37, 107351. [Google Scholar] [CrossRef]
- Modestra, J.A.; Mohan, S.V. Bio-electrocatalyzed electron efflux in Gram positive and Gram negative bacteria: An insight into disparity in electron transfer kinetics. RSC Adv. 2014, 4, 34045–34055. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Y.; Cao, B.; Gao, Y.-G.; Yan, X. Adjustable bidirectional extracellular electron transfer between Comamonas testosteroni biofilms and electrode via distinct electron mediators. Electrochem. Commun. 2015, 59, 43–47. [Google Scholar] [CrossRef]
- Chen, L.; Xiao, C.; Luo, X.; Sun, W. Study on biological degradation and transform characteristics of different components in petroleum hydrocarbon used by bacterial consortium. Environ. Earth Sci. 2016, 75, 816. [Google Scholar] [CrossRef]
- Hobday, A.J.; Smith, A.D.M.; Stobutzki, I.C.; Bulman, C.; Daley, R.; Dambacher, J.M.; Deng, R.A.; Dowdney, J.; Fuller, M.; Furlani, D.; et al. Ecological risk assessment for the effects of fishing. Fish. Res. 2011, 108, 372–384. [Google Scholar] [CrossRef]
- Velho-Pereira, S.; Kamat, N. Antimicrobial screening of actinobacteria using a modified cross-streak method. Indian J. Pharm. Sci. 2011, 73, 223. [Google Scholar] [CrossRef] [Green Version]
- Wolcott, R.D.; Hanson, J.D.; Rees, E.J.; Koenig, C.D.; Wolcott, R.A.; Cox, S.B.; White, J.S. Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair Regen. 2016, 24, 163–174. [Google Scholar] [CrossRef]
- Narisetty, V.; Prabhu, A.A.; Bommareddy, R.R.; Cox, R.; Agrawal, D.; Misra, A.; Haider, M.A.; Bhatnagar, A.; Pandey, A.; Kumar, V. Development of Hypertolerant Strain of Yarrowia lipolytica Accumulating Succinic Acid Using High Levels of Acetate. ACS Sustain. Chem. Eng. 2022, 10, 10858–10869. [Google Scholar] [CrossRef]
- Carmona-Martinez, A.A.; Harnisch, F.; Fitzgerald, L.A.; Schröder, U. Cyclic voltammetric analysis of the electron transfer of Shewanella oneidensis MR-1 and nanofilament and cytochrome knock-out mutants. Bioelectrochemistry 2011, 81, 74–80. [Google Scholar] [CrossRef]
- Lemos, J.G.; Stefanello, A.; Garcia, M.V.; Furian, A.F.; Cichoski, A.J.; Copetti, M.V. Potential of electrolyzed water to inactivate bread and cheese spoilage fungi. Food Res. Int. 2022, 162, 111931. [Google Scholar] [CrossRef]
- Light, S.H.; Su, L.; Rivera-Lugo, R.; Cornejo, J.A.; Louie, A.; Iavarone, A.T.; Ajo-Franklin, C.M.; Portnoy, D.A. A flavin-based extracellular electron transfer mechanism in diverse Gram-positive bacteria. Nature 2018, 562, 140–144. [Google Scholar] [CrossRef]
- Kokko, M.E.; Mäkinen, A.E.; Sulonen, M.L.K.; Puhakka, J.A. Effects of anode potentials on bioelectrogenic conversion of xylose and microbial community compositions. Biochem. Eng. J. 2015, 101, 248–252. [Google Scholar] [CrossRef]
- Kang, M.; Ren, L.; Ren, H.; Zhao, Y.; Kawamura, K.; Zhang, H.; Wei, L.; Sun, Y.; Wang, Z.; Fu, P. Primary biogenic and anthropogenic sources of organic aerosols in Beijing, China: Insights from saccharides and n-alkanes. Environ. Pollut. 2018, 243 Pt B, 1579–1587. [Google Scholar] [CrossRef]
- Fan, J.; Okyay, T.O.; Rodrigues, D.F. The synergism of temperature, pH and growth phases on heavy metal biosorption by two environmental isolates. J. Hazard. Mater. 2014, 279, 236–243. [Google Scholar] [CrossRef]
- Zhai, Q.; Gong, S.; Wang, Y.; Lyu, Q.; Liu, Y.; Ling, Y.; Wang, J.; Simon, G.P.; Cheng, W. Enokitake mushroom-like standing gold nanowires toward wearable noninvasive bimodal glucose and strain sensing. ACS Appl. Mater. Interfaces 2019, 11, 9724–9729. [Google Scholar] [CrossRef]
- Wang, W.; He, Y.; Wang, B.; Dong, M.; Zhang, H.; Shen, C. Experimental study on wax removal and viscosity reduction of waxy crude oil by Ochrobactrum intermedium. J. Pet. Sci. Eng. 2022, 213, 110445. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, H.; Feng, Y.; Dong, B.; Wang, Y.; Ge, C. Iron and carbon granules added to anode enhanced the sludge decrement and electrical performance of sludge microbial fuel cell. Chem. Eng. J. 2019, 372, 572–580. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Tang, L.; Ren, R.; You, W.; Farooq, R.; Wang, Z.; Zhang, Y. Changes in bacterial community structure and humic acid composition in response to enhanced extracellular electron transfer process in coastal sediment. Arch. Microbiol. 2019, 201, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, T.Y.; Chung, Y.C. A comparison of bioelectricity in microbial fuel cells with aerobic and anaerobic anodes. Environ. Technol. 2014, 35, 286–293. [Google Scholar] [CrossRef]
- Wu, M.S.; Xu, X.; Zhao, Q.; Wang, Z.Y. Simultaneous removal of heavy metals and biodegradation of organic matter with sediment microbial fuel cells. RSC Adv. 2017, 7, 53433–53438. [Google Scholar] [CrossRef] [Green Version]
- Wingfors, H.; Hägglund, L.; Magnusson, R. Characterization of the size-distribution of aerosols and particle-bound content of oxygenated PAHs, PAHs, and n-alkanes in urban environments in Afghanistan. Atmos. Environ. 2011, 45, 4360–4369. [Google Scholar] [CrossRef]





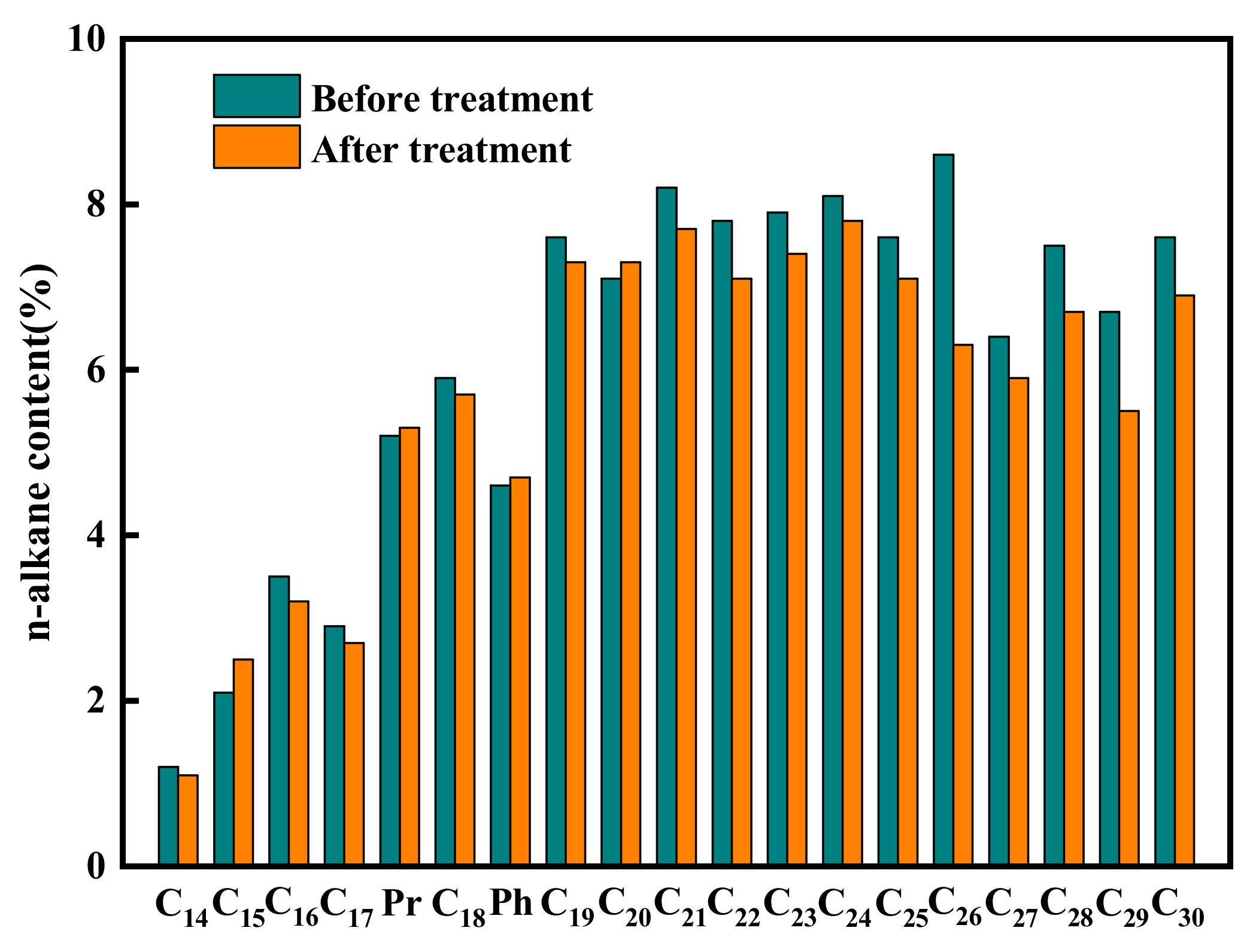
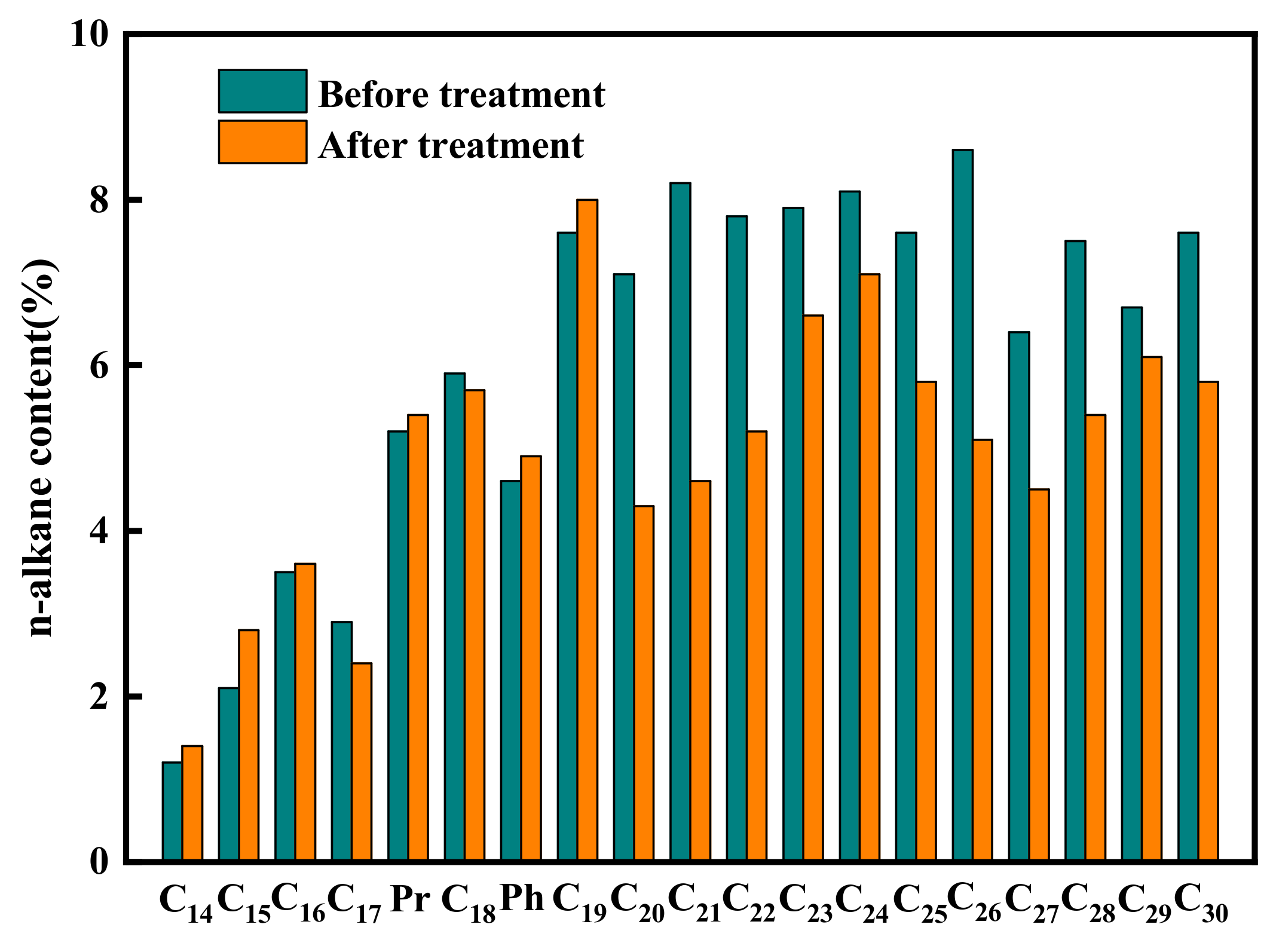





| Parameter | Main Peak Carbon Number | OEP | W∑(C21−)/W∑(C22+) | W(Pr)/W(W17) | W(Ph)/W(C18) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | |
| Group 1 | 26 | 24 | 1.38 | 1.37 | 0.72 | 0.79 | 1.79 | 1.96 | 0.78 | 0.82 |
| Group 2 | 26 | 19 | 1.38 | 1.68 | 0.72 | 0.95 | 1.79 | 2.25 | 0.78 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Tang, S.; Ren, W.; Cheng, Y.; Gao, J.; Huang, C.; Fu, K. Effect of Electro-Oil Acclimation of an Indigenous Strain on the Performance of Sediment Microbial Fuel Cells (SMFC). Energies 2023, 16, 5582. https://doi.org/10.3390/en16145582
Pan Y, Tang S, Ren W, Cheng Y, Gao J, Huang C, Fu K. Effect of Electro-Oil Acclimation of an Indigenous Strain on the Performance of Sediment Microbial Fuel Cells (SMFC). Energies. 2023; 16(14):5582. https://doi.org/10.3390/en16145582
Chicago/Turabian StylePan, Yao, Shanfa Tang, Wen Ren, Yuanpeng Cheng, Jie Gao, Chunfeng Huang, and Ke Fu. 2023. "Effect of Electro-Oil Acclimation of an Indigenous Strain on the Performance of Sediment Microbial Fuel Cells (SMFC)" Energies 16, no. 14: 5582. https://doi.org/10.3390/en16145582





