Removal of Organic Micro-Pollutants from Wastewater in Electrochemical Processes—Review
Abstract
:1. Introduction
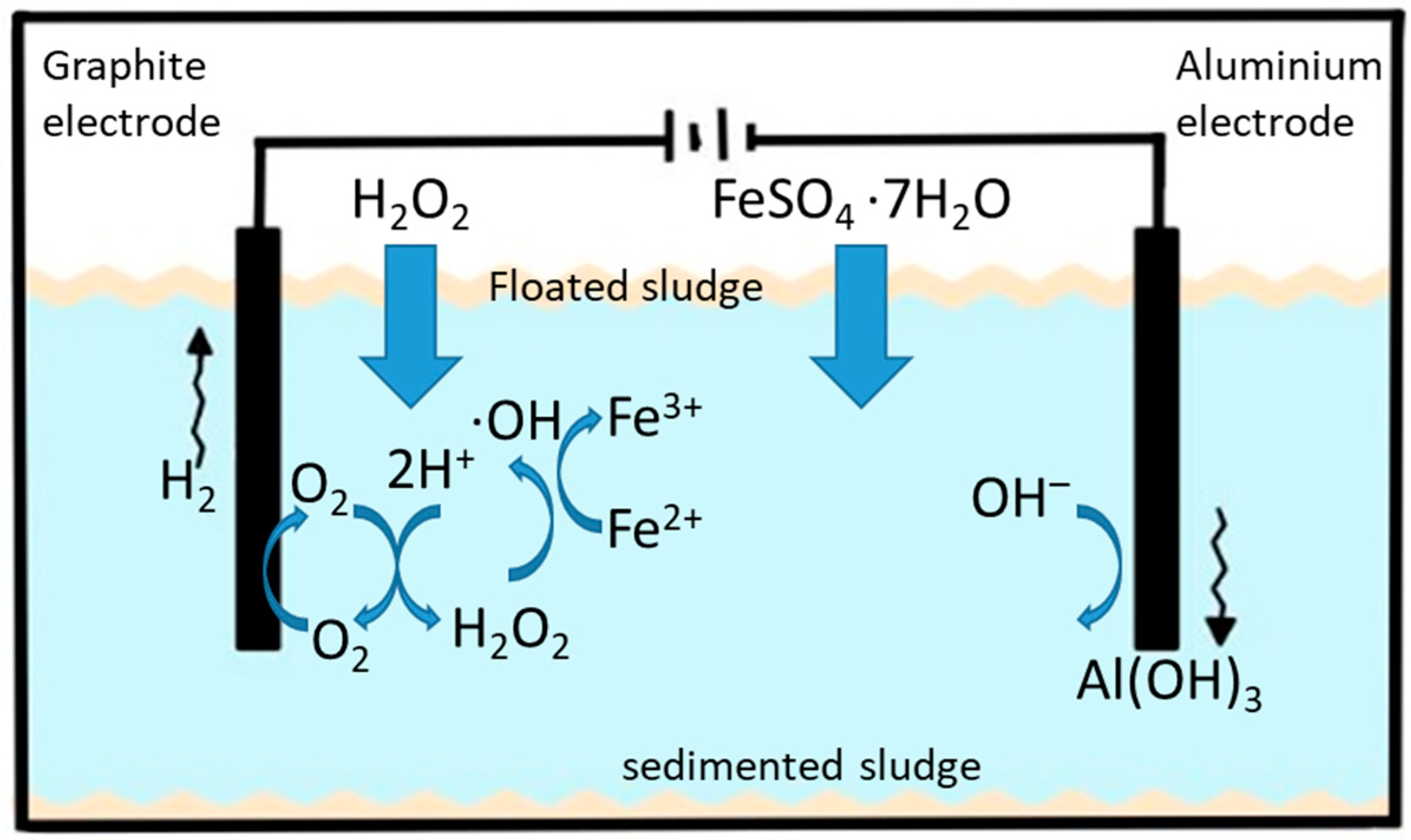
2. Electrocoagulation
- I the current intensity (A);
- t the retention time (s);
- V the volume of the treated wastewater (m3);
- F the Faraday’s constant (96.487 C/mol);
- M the mass of aluminium (26.98 g/mol) and mass of iron (55.847 g/mol);
- z the number of electron transfer (e.g., zAl = zFe = 3).
- E the specific energy consumption (kWh/kg of COD removed);
- U the applied voltage (V);
- I the current intensity (A);
- t the retention time (h);
- COD0 the chemical oxygen demand before treatment (g/L);
- CODt the chemical oxygen demand after treatment (g/L);
- V the volume of the treated wastewater (L).
3. Electroflotation
4. Electrochemical Advanced Oxidation Processes
4.1. Electro-Fenton Process
4.2. Integrated Sono-Electro-Fenton and Photo-Electro-Fenton Processes
5. Modifications of AOPs
6. Conclusions
- -
- highly efficient method of POPs degradation (above 90%);
- -
- easy control of the technical parameters of the process: current density, solution pH, catalyst concentration;
- -
- minimization of consumption of chemical reagents;
- -
- possible reduction of process time;
- -
- anode materials with high overpotential allowing the generation of additional hydroxyl radicals;
- -
- cathode materials with high overpotential increasing H2O2 production and allowing the regeneration of iron ions.
- -
- release of inorganic ions during mineralization of POPs containing heteroatoms;
- -
- use of a supporting electrolyte;
- -
- use of an acidic solution for pH correction;
- -
- formation of refractory by-products as intermediates (this is specific to all oxidation processes).
- -
- use of hybrid processes;
- -
- integrated biological and electrochemical processes for the removal of organic xenobiotics from water and wastewater;
- -
- use of renewable energy sources to enhance electrochemical processes;
- -
- development of new electrode materials.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abdel-Shafy, J.H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiemb, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Samer, M. (Ed.) Biological and Chemical Wastewater Treatment Processes; IntechOpenLimited: London, UK, 2015; ISBN 978-953-51-6390-92014. [Google Scholar] [CrossRef] [Green Version]
- European Community. European Community Regulation No. 850/2004, Persistent Organic Pollutants in the Environment, Information Materials; European Community: Warsaw, Poland, 2008. [Google Scholar]
- Włodarczyk-Makuła, M. Physical and Chemical Fates of Organic Micropollutants; Scholar Press, OmniScriptum GmbH & Co. KG: Saarbrucken, Germany, 2015; ISBN 978-3-639-85930-0. [Google Scholar]
- Płuciennik-Koropczuk, E.; Myszograj, M.; Myszograj, S. The influence of the Poles’ lifestyle on the quantity and quality of municipal wastewater. Civ. Environ. Eng. Rep. 2021, 31, 265–275. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Polychlorinated and Polybrominated Biphenyls, 107, Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2015; ISBN 978-92-832-0173-1.
- International Agency for Research on Cancer. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures, 92, Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2010; ISBN 978-92-832-1292-8.
- Petrie, B.; Barden, R.; Kacprzyk-Hordern, A. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Gracia-Lor, E.; Sancho, J.V.; Serrano, R.; Hernández, F. Occurence and removal of pharmaceutical in wastewater treatment plants AT the Spanish Mediterranean area of Valencia. Chemosphere 2012, 87, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Gerrity, D.; Snyder, S. Wastewater and drinking water treatment technologies. In Human Pharmaceuticals in the Environment: Current and Future Perspectives, Emerging Topics in Ecotoxicology; Brooks, B.W., Huggett, D.B., Eds.; Springer: Berlin, Germany, 2012. [Google Scholar]
- Giannakis, S.; Vives, F.A.G.; Grandjean, D.; Magnet, A.; De Alencastro, L.F.; Pulgarin, C. Effect of advanced oxidation processes on the micropollutants and the effluent organic matter contained in municipal wastewater previously treated by three different secondary methods. Water Res. 2015, 84, 295–306. [Google Scholar] [CrossRef]
- Köck-Schulmeyer, M.; Villagrasa, M.; López de Alda, M.; Céspedes-Sánchez, R.; Ventura, F.; Barceló, D. Occurrence and behavior of pesticides in wastewater treatment plants and their environmental impact. Sci. Total Environ. 2013, 458–460, 466–476. [Google Scholar] [CrossRef]
- Włodarczyk-Makuła, M.; Wiśniowska, E.; Turek, A.; Obstój, A. Removal of PAHs from coking wastewater during photo degradation process. Desalination Water Treat. 2016, 57, 1262–1272. [Google Scholar] [CrossRef]
- Bodzek, M. Membrane technologies for the removal of micropollutants in water treatment. In Advances in Membrane Technologies for Water Treatment: Materials, Processes and Applications; Basile, A., Cassano, A., Rastogi, N., Eds.; Elsevier Science: Amsterdam, The Netherlands; Woodhead Publishing Ltd.: Cambridge, UK, 2015; pp. 465–515. [Google Scholar]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Santos-Juanes Jorda, L.; Ballesteros Martın, M.M.; Ortega Gomez, E.; Cabrera Reina, A.; Roman Sanchez, I.M.; Casas Lopez, J.L.; Sanches Perez, J.A. Economic evaluation of the photo-Fenton process. Mineralization level and reaction time: The keys for increasing plant efficiency. J. Hazard. Mater. 2011, 186, 1924–1929. [Google Scholar] [CrossRef]
- Gopinath, A.; Pisharody, L.; Popat, A.; Nidheesh, P.V. Supported catalysts for heterogeneous electro-Fenton processes: Recent trends and future directions. Curr. Opin. Solid State Mater. Sci. 2022, 26, 100981. [Google Scholar] [CrossRef]
- Cheng, M.; Guangming, Z.; Huang, D.; Lai, C. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds-a review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Gzar, H.A.; Jasim, N.A.; Kseer, K.M. Electrocoagulation and chemical coagulation for treatment of Al-Kut textile wastewater: A comparative study. Period. Eng. Nat. Sci. 2020, 8, 1580–1590. [Google Scholar]
- Rafique, L.; Adnan, A.; Taha, A.; Bano, S.; Vambol, S.; Mushtaq, T.; Ilyas, N.; Hussain, S.; Borysova, L.; Kovalov, O. Application of copper and aluminium electrode in electro coagulation process for municipal wastewater treatment: A case study at Karachi. Ecol. Quest. 2023, 34. [Google Scholar] [CrossRef]
- Nassar, S.O.A.; Yusoff, M.S.; Halim, H.; Kamal, N.H.M.; Bashir, M.J.K.; Manan, T.S.B.A.; Aziz, H.A.M.; Mojiri, A. Ultrasonic (US)-Assisted electrocoagulation (EC) process for oil and grease (O&G) removal from restaurant wastewater. Separations 2022, 10, 61. [Google Scholar] [CrossRef]
- Mores, R.; Mello, P.D.A.; Zakrzevski, C.A.; Treichel, H.; Kunz, A.; Steffens, J.; Dallago, R.M. Reduction of soluble organic carbon and removal of total phosphorus and metals from swine wastewater by electrocoagulation. Braz. J. Chem. Eng. 2018, 35, 1231–1240. [Google Scholar] [CrossRef] [Green Version]
- Can, O.T. COD removal from fruit-juice production wastewater by electrooxidation electrocoagulation and electro-Fenton processes. Desalination Water Treat. 2014, 52, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Marlina, E.; Purwanto, P. Electro-Fenton for industrial wastewater treatment: A review. In Proceedings of the 4th International Conference on Energy, Environment, Epidemiology and Information System (ICENIS 2019), Semarang, Indonesia, 7–8 August 2019; Volume 125, p. 03003. [Google Scholar] [CrossRef] [Green Version]
- McGinnis, B.D.; Adams, V.D.; Middlebrooks, E.J. Degradation of ethylene glycol in photo Fenton systems. Water Res. 2000, 34, 2346–2354. [Google Scholar] [CrossRef]
- Posavcic, H.; Halkijevic, I.; Vouk, D. Oily wastewater treatment by hybrid ultrasound and electrocoagulation batch process. Desalination Water Treat. 2021, 235, 127–134. [Google Scholar] [CrossRef]
- Özyonar, F.; Gökkuş, Ö.; Sabuni, M. Removal of disperse and reactive dyes from aqueous solutions using ultrasound-assisted electrocoagulation. Chemosphere 2020, 258, 127325. [Google Scholar] [CrossRef]
- Khaldi, S.; Lounici, H.; Drouiche, M.; Drouiche, N. Treatment of ointment pharmaceutical wastewater by electrocoagulation process. Desalination Water Treat. 2017, 71, 152–158. [Google Scholar] [CrossRef]
- Smoczyński, L.; Muńska, K.M.; Pierożyński, B.; Kosobucka, M. Electrocoagulation of model wastewater on iron electrodes. Proc. ECOpole 2012. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Comninellis, C.; Kapalka, A.; Malato, S.; Parsons, S.A.; Poulios, I.; Mantzavinos, D. Advanced oxidation processes for water treatment: Advances and trends for R&D. J. Chem. Technol. Biotechnol. 2008, 83, 769–776. [Google Scholar] [CrossRef]
- Abdulredha, M.; Al-Samarrai, S.Y.; Hussein, A.H.; Samaka, I.; Al-Ansari, N.; Aldhaibani, O.A. Electrochemical defluorination of water: An experimental and morphological study. J. Water Sanit. Hyg. Dev. 2022, 12, 394. [Google Scholar] [CrossRef]
- Medel, A.; Lugo, F.; Meas, Y. Application of electrochemical processes for treating effluents from hydrocarbon industries. In Electrochemical Water and Wastewater Treatment; Butterworth-Heinemann: Oxford, UK, 2018; pp. 365–392. [Google Scholar] [CrossRef]
- Ai, Z.; Lu, L.; Li, J.; Zhang, L.; Qiu, J.; Wu, M. Fe2O3 core–shell nanowires as iron reagent. Efficient degradation of Rhodamine B by a noval sono-Fenton process. J. Phys. Chem. C 2007, 111, 4087–4093. [Google Scholar] [CrossRef]
- Molina, R.; Martinez, F.; Melero, J.A.; Bremner, D.H.; Chakinala, A.G. Mineralization of phenol by a heterogeneous ultrasound/Fe-SBA-15/H2O2 process: Multivariate study by factorial design of experiments. Appl. Catal. B Environ. 2006, 66, 198–207. [Google Scholar] [CrossRef]
- Sirtori, C.; Zapata, A.; Gernjaka, W.; Malato, S.; Lopez, A.; Agüera, A. Solar photo-Fenton degradation of nalidixic acid in waters and wastewaters of different composition. Analytical assessment by LC–TOF-MS. Water Res. 2011, 45, 1736–1744. [Google Scholar] [CrossRef]
- Guivarch, E.; Trevin, S.; Lahitte, C.; Oturan, M.A. Degradation of azo dyes in water by electro-Fenton process. Environ. Chem. Lett. 2003, 1, 38–44. [Google Scholar] [CrossRef]
- Da Rocha, O.R.S.; Dantas, R.F.; Bezerra, M.M.M.; Lima, M.M.; Lins, V. Solar photo-Fenton treatment of petroleum extraction wastewater. Desalination Water Treat. 2013, 51, 5785–5791. [Google Scholar] [CrossRef]
- Oliveira da Mota, I.; Castroa, J.A.; Casqueira, R.G.; Oliveira Junior, A.G. Study of electroflotation method for treatment of wastewater from washing soil contaminated by heavy metals. J. Mater. Res. Technol. 2015, 4, 109–113. [Google Scholar] [CrossRef]
- Zaidi, S.; Chaabane, T.; Sivasankar, V.; Darchen, A.; Maachi, R.; Msagati, T.A.M. Electro-coagulation coupled electro-flotation process: Feasible choice in doxycycline removal from pharmaceutical effluents. Arab. J. Chem. 2019, 12, 2798–2809. [Google Scholar] [CrossRef]
- Zayas, T.; de Gante, A.; Guadalupe Tenorio Arvide, M.; Vega Hernández, M.; Soriano-Moro, G.; Salgado, L. Treatment of nixtamalization wastewater (nejayote) using electrocoagulation and combined chemical coagulation/electrocoagulation processes. Desalination Water Treat. 2022, 280, 44–51. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Y.; Almatrafi, E.; Hu, T.; Zhou Ch Song, B.; Zeng, Z.; Zeng, G. Efficient removal of microplastics from wastewater by an electrocoagulation process. Chem. Eng. J. 2022, 428, 131161. [Google Scholar] [CrossRef]
- Sharma, S.; Ruparelia, J.P.; Patel, M.L. A general review on Advanced Oxidation Processes for waste water treatment. In Proceedings of the International Conference on Current Trends In Technology, Dubai, United Arab Emirates, 26–27 October 2011. [Google Scholar]
- Babuponnusami, A.; Muthukuma, M. A review on Fenton and improvements to the Fenton. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Matavos-Aramyan, S.; Moussavi, M. Advances in Fenton and Fenton based oxidation processes for industrial effluent contaminants control-a review. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 555–594. [Google Scholar] [CrossRef] [Green Version]
- Stathoulopoulos, A.; Mantzavinos, D.; Frontistis, Z. Coupling persulfate-based AOPs: A novel approach for piroxicam degradation in aqueous matrices. Water 2020, 12, 1530. [Google Scholar] [CrossRef]
- Kajitvichyanukul, P.; Suntronvi, N. Evaluation of biodegradability and oxidation degree of hospital wastewater using photo-Fenton process as the pretreatment method. J. Hazard. Mater. 2006, 16, 384–391. [Google Scholar] [CrossRef]
- Barbusiński, K. Advanced Oxidation in the Treatment of Selected Industrial Wastewater; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2013. [Google Scholar]
- Ziembowicz, S.; Kida, M. Imitations and future directions of application of the Fenton-like process in micropollutants degradation in water and wastewater treatment: A critical review. Chemosphere 2022, 296, 134041. [Google Scholar] [CrossRef]
- Kozak, J.; Włodarczyk-Makuła, M. Comparison of the PAHs degradation effectiveness using CaO2 or H2O2 under the photo-Fenton reaction. Desalination Water Treat. 2018, 134, 57–64. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Z.; Li, K.; Huang, T.; Ma, J.; Wen, G. Degradation of micropollutants and formation of oxidation by-products during the ozone/peroxymonosulfate system: A critical review. Water 2021, 13, 3126. [Google Scholar] [CrossRef]
- Xia, X.; Zhu, F.; Li, J.; Yang, H.; Wei, L.; Li, Q.; Jiang, J.; Zhang, G.; Zhao, Q. A Review Study on Sulfate-Radical-Based AdvancedOxidation Processes for Domestic/Industrial Wastewater Treatment: Degradation, Efficiency, and Mechanism. Front. Chem. 2020, 8, 592056. [Google Scholar] [CrossRef]
- Wacławek, S.; Antoš, V.; Hrabák, P.; Černík, M.; Elliott, D. Remediation of hexachlorocyclohexanes by electrochemically activated persulfates. Environ. Sci. Pollut. Res. 2016, 23, 765–773. [Google Scholar] [CrossRef]
- Miralles-Cuevas, S.; Darowna, D.; Wanag, A.; Mozia, S.; Malato, S.; Oller, I. Comparison of UV/H2O2, UV/S2O82−, solar/Fe(II)/H2O2 and solar/Fe(II)/S2O82− at pilot plant scale for the elimination of micro-contaminants in natural water: An economic assessment. Chem. Eng. J. 2017, 310, 514–524. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Olvera-Vargas, H.; Oturan, N.; Oturan, M.A. Heterogeneous Electro-Fenton Process: Principles and Applications. In The Handbook of Environmental Chemistry; Zhou, M., Oturan, M., Sires, I., Eds.; Electro-Fenton Process: Singapore, 2017; pp. 85–110. [Google Scholar]
- Brillas, E.; Calpe, J.; Casado, J. Mineralization of 2,4-D by advanced electrochemical oxidation processes. Water Res. 2000, 34, 2253. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Morselli, R.; Garcia-Gomez, J.; Michaud, P.A.; Rodrigo, M.A.; Comninellis, C. Electro-generation of hydroxyl radicals on boron-doped dimond electrodes. J. Electrochem. Soc. 2003, 150, 79–83. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Application of diamond electrodes to electrochemical processes. Electrochim. Acta 2005, 51, 191–199. [Google Scholar] [CrossRef]
- Ting, W.P.; Lu, M.C.; Huang, Y.H. The reactor design and comparison of Fenton, electro-Fenton and photoelectron-Fenton processes for mineralization of benzene sulfonic acid (BSA). J. Hazard. Mater. 2008, 156, 421–427. [Google Scholar] [CrossRef]
- Nayebi, B.; Ayati, B. Degradation of emerging amoxicillin compound from water using the electro-Fenton process with an aluminum anode. Water Conserv. Sci. Eng. 2021, 6, 45–54. [Google Scholar] [CrossRef]
- Oturan, N.; Oturan, M.A. Electro-Fenton process: Background, new developments, and applications in electrochemical. In Water and Wastewater Treatment; Martínez-Huitle, C.A., Rodrigo, M.A., Scialdone, O., Eds.; Butterworth-Heinemann: Oxford, UK, 2018. [Google Scholar]
- Brillas, E.; Casado, J. Aniline degradation by electro-Fenton and peroxicoagulation processes using a flow reactor for waste water treatment. Chemosphere 2002, 47, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Uribe, B.A.F.; Zamora, R.M.R. Electro-Fenton as a feasible advanced treatment process to produce reclaimed water. Water Sci. Technol. 2004, 50, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, D.; Zhou, J. Removal of COD from landfill leachate by electro-Fenton method. J. Hazard. Mater. 2006, 135, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.S.; Sung, C.F. Investigation of carbofuran decomposition by a combination of ultrasound and Fenton process. Sustain. Environ. Res. 2010, 20, 213–219. [Google Scholar]
- Behfar, R.; Davarnejad, R. Pharmaceutical wastewater treatment using UV-enhanced electro-Fenton process: Comparative study. Water Environ. Res. 2019, 95, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Cisternas, C.; Salazar, R. Application of electrochemical processes for treating effluents from landfill leachate as well as the agro and food industries. In Electrochemical Water and Wastewater Treatment; Martínez-Huitle, C.A., Rodrigo, M.A., Scialdone, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 393–419. [Google Scholar]
- Palmas, S.; Mascia, M.; Vacca, A.; Mais, L.; Corgidu, S.; Petrucci, E. Practical aspects on electrochemical disinfection of urban and domestic wastewater. In Electrochemical Water and Wastewater Treatment; Martínez-Huitle, C.A., Rodrigo, M.A., Scialdone, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 421–447. [Google Scholar]
- Ebrahiem, E.E.; Al-Maghrabi, M.N.; Mobarki, A.R. Removal of organic pollutants from industrial wastewater by applying photo-Fenton oxidation technology. Arab. J. Chem. 2017, 10, 1674–1679. [Google Scholar] [CrossRef]
- Pieczykolan, B.; Płonka, I.; Barbusiński, K. Discoloration of dye wastewater by modified UV-Fenton process with sodium percarbonate. Archit. Civ. Eng. Environ. 2016, 4, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Kozak, J.; Włodarczyk-Makuła, M. The use of sodium percarbonate in the Fenton reaction for the PAHs Oxidation. Civ. Environ. Eng. Rep. 2018, 28, 124–139. [Google Scholar] [CrossRef] [Green Version]
- Kozak, J.; Włodarczyk-Makuła, M. Application of sodium carbonate—Hydrogen peroxide for PAHs degradation from real wastewater and evaluation of their toxicity TEQ value. Desalination Water Treat. 2020, 199, 362–370. [Google Scholar] [CrossRef]
- Liang, J.; Komarov, S.; Hayashi, N.; Eiki, E. Recent trends in the decomposition of chlorinated aromatic hydrocarbons by ultrasound irradiation and Fenton’s reagent. J. Mater. Cycles Waste Manag. 2007, 9, 47–55. [Google Scholar] [CrossRef]
- Gou, Z.; Zheng, Z.; Zheng, S.; Hu, W.; Feng, R. Effect of various sono-oxidation parameters on the removal of aqueous 2,4-dinitrophenol. Ultrason. Sonochem. 2005, 12, 461–465. [Google Scholar] [CrossRef]
- Pereira, M.C.; Oliveira, L.C.A.; Murad, E. Iron oxide catalysts: Fenton and Fenton like reactions—A review. Clay Miner. 2012, 47, 285–302. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Heckert, E.G.; Seal, S.; Self, W.T. Fenton-like reaction catalyzed by the rare earth inner transition metal cerium. Environ. Sci. Technol. 2008, 42, 5014–5019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrido-Ramírez, E.G.; Theng, B.K.G.; Mora, M.L. Clays and oxide minerals as catalysts and nano catalysts in Fenton-like reactions—A review. Appl. Clay Sci. 2010, 47, 182–192. [Google Scholar] [CrossRef]
- Olvera-Vargas, H.; Trellu, C.; Oturan, N.; Oturan, M. Bio-electro-Fenton: A new combined processes. Principle and applications. In Electro-Fenton Process: New Trends and Scale-Up; Zhou, M., Ed.; Springer Nature: Singapore, 2017. [Google Scholar]
- Gao, Y.; Yang, Y.; Lin, X.; Fu, M.; Hu, W.; Tong, H.; Tao, Z. Investigation and study of three different composite cathodes for proton-conducting solid oxide fuel cells. Sep. Purif. Technol. 2022, 300, 121890. [Google Scholar] [CrossRef]
- Fu, M.; Li, K.; Yang, Y.; Zeng, Q.; Zeng, L.; Tao, Z. Fabrication and study of LaNi0.6Fe0.4O3-δ and Sm0.5Sr0.5CoO3-δ composite cathode for proton-conducting solid oxide fuel cells. Sep. Purif. Technol. 2022, 287, 120581. [Google Scholar] [CrossRef]
- Tao, Z.; Fu, M.; Liu, Y.; Gao, Y.; Tong, H.; Hu, W.; Libin, L.; Bi, L. High-performing proton-conducting solid oxide fuel cells with triple-conducting cathode of Pr0.5Ba0.5(Co0.7Fe0.3)O3-δ tailored with W. Int. J. Hydrogen Energy 2022, 47, 1947–1953. [Google Scholar] [CrossRef]

| Process | Electrode | Distance of Electrodes (cm) | Current Density (mA/cm2) | pH | Power Consumption | Pollutant/ Initial Concentration (mg/L) | Removal Efficiency (%) | References |
|---|---|---|---|---|---|---|---|---|
| Electrocoagulation | Aluminium electrode | 5–15 | 1.5–3.5 | 4–10 | 5.03 kWh/m3 | Fluoride/20 mg/L | 93% | [33] |
| Aluminium anode/ iron anode | 5–25 | 20 | 4–12 | 0.054–0.221 kWh/kg CODremoval | Fruit juice wastewater COD 960–48,500 mg/L | COD 59–84% | [24] | |
| Aluminium anode/ Fe cathode | 2 | - | Wastewater from production of corn flour/COD 3164 mg/L, turbidity 1 NTU | COD 84% turbidity 98% | [42] | |||
| Aluminium electrodes | 6.2–8.3 | 2.0–45.0 kWh/m3 | Textile wastewater/turbidity 22–28 NTU | Turbidity 75.4% | [20] | |||
| Fe/Al, Fe/Fe, Al/Al, Al/Fe electrode | 2 | 15.56 | 7.89 | 0.48 kWh/kg COD | Pharmaceutical wastewater/COD 5000 mg/L, turbidity 3280 NTU | COD 95% turbidity 98% | [29] | |
| Spiral rod anode made from aluminium, cylindrical stainless-steel cathode | 0.15 | 4–26 | 3–10 | 1.54–48.16 kWh/kg COD | Petroleum wastewater/COD-955 mg/L | COD 73.36% | [22] | |
| Iron electrodes | 1 | 7.2–9.1 | Synthetic wastewater/ COD 14,000 mg/L, turbidity 100 NTU, phosphorus 124 mg/L | COD 43% turbidity 62% phosphorus 51–58% | [30] | |||
| Fe and Al anodes and Cu cathode | 2 | 10 | 7.2 | Microplastic | 93.2% for PE, 91.7% for PMMA, 98.2% for CA, and 98.4% for PP | [43] | ||
| Electroflotation | Stainless-steel wire | 1 | 35 | 10 | 14 kWh/m3 | Heavy metal/15 mg/L | Pb, Ba, Zn 89–97% | [40] |
| Electrocoagulation/ Electroflotation | Aluminium electrodes/graphite cathode/stainless anode | 1.0–2.5 | 3.59–14.39 | 6.03–8.02 | 1.505–3.675 kWh/m3 | Doxycycline hyclate/60–180 mg/L | 90–96% | [41] |
| Electrocoagulation and ultrasound (20 kHz) | Iron electrodes; aluminium electrodes | 9.1–36.4 16.7–66.8 | 7.6–8.8 | - | Oil wastewater, heavy metal/COD 288–310 mg/L, Cr 71 mg/L, Ni 86 mg/L, Pb 118 mg/L | COD 27–35% Cr, Ni, Pb 91–99% | [27] |
| Electrical Energy | |
|---|---|
| Sono-Fenton: | Electro-Fenton: |
| Photo-Fenton: | Sono-electro-Fenton: |
| Sono-photo-Fenton: | Photo-electro-Fenton: |
| Ozonation/oxidation with hydrogen peroxide with exposure to UV radiation or ultrasound | Electrochemical oxidation Anodic oxidation |
| Process | Basic Chemical Reaction | Advantages | |
|---|---|---|---|
| Fenton reaction | (17) | It only requires the participation of Fenton’s reagent. The process works at ambient temperature. | |
| Photo-Fenton (UV radiation or sunlight) | (36) | Minimises sewage sludge formation. Generates additional hydroxyl radicals. | |
| (17) | |||
| (37) | |||
| Electro-Fenton | (38) | In situ generation of H2O2 and Fe2+ without the need to add substrate. The source of iron may be ions, Fe3+, or a cast iron anode. | |
| (17) | |||
| (39) | |||
| Process | Electrodes, Fe:H2O2 Ratio/Catalyst Doses (mg/L) | Reaction Time (min) | Current Density (mA/cm2) | pH | Pollutant/Initial Concentration | Removal Efficiency (%) | References |
|---|---|---|---|---|---|---|---|
| Electro-Fenton | Iron electrodes/3.78/ | 10–70 | 20–80 | 3.5–5 | Pharmaceutical wastewater | COD: 83–87 | [68] |
| Photo-electro-Fenton UVA lamps 3–9 W | Iron electrodes/4.29/ | 10–70 | 20–80 | 2–5 | COD: 92–93 | ||
| Electro-Fenton | /3.41–11.37 | 25 | 20 | 3.5 | Fruit juice wastewater | COD: 84 | [24] |
| Electro-Fenton anode oxidation | Activated carbon fibre (ACF) cathode; RuO2/TiO2 anode | 120 360 480 270 | 6.67 | 3.0 | Antibiotics: levofloxacin and cefalexin | 100 COD: 68 TOC: 72 TOC: 47 | [81] |
| Electro-Fenton | Graphite electrode modified activated carbon and polytetrafluoroethylene (PTFE) | 35 | 5.0 | 3.0 | Methyl orange/50 mg/L | 100 | [25] |
| Anode Ti/RuO2, graphite cathode/ Fe2+: 0.3 mM | 180 | 200 mA | 3.0 | Anionic surfactants LAS/50 mg/L | 100 | ||
| Platinum anode, graphite cathode/ Fe3+: 0.2 mM | 50 | 50 mA | Ibuprofen | 100 | |||
| Titanium anode Ti/IrO2-RuO2, continuous system, cathode = gas diffusion electrode | 200 mA | 3.0 | Tartrazine | 80 | |||
| Iron electrodes/H2O2 = 37.2 mM Stainless steel cathode/nickel anode, Fe2+: 5 mg/L | 5 90 | 0.8 900 mA | 5.2 | Phenol/250 mg/L Phenol | 100 95 | ||
| Electro-Fenton-like process and iron catalyst | 1000 mg/L 2000 mg/L | 480 | 300 mA | 3.0 | Levofloxacin/0.23 mM sulfamethazine/ 0.2 mM | 95 | [18] |
| 800 mg/L | 180 | 31.84 | 7.0 | Diclofenac/50 mg/L | 85 | ||
| 1000 mg/L | 480 | 3.0 | Tetracycline/ 0.2 mM | 99 | |||
| Electro-Fenton Supported catalyst | Iron catalyst: 50 mg/L; activated carbon | 250 | 12 mA | 3.0 | Phenol/100 mg/L | 100 | |
| Fe0 catalyst: 0.1 mg/L | 120 | 60 mA | 3.0 | Phenol/50 mg/L | 91 | ||
| Fe-C catalyst; 6 g/L | 360 | 100 mA | 6.7 | 2,4-dichlorophenol (2,4-DCP) | 70 | ||
| Cu-doped Fe@Fe2 O3 (50 wt%Cu) | 120 | 40 | 3.0 | Tetracycline/20 mg/L | 98 | ||
| FeOCl dose: 0.25% | 240 | 2500 mV | 6.5 | Tetracycline/0.4 mM | >95 | ||
| Electo-Fenton | Graphite felt GF cathode, Pt anode Fe2+: 0.2 mM | 0–7 | 2.08–20.83 | 3.0 | Sulphamethazine/0.2 mM | 100 | [32] |
| Anodic oxidation | 0–90 | 4.16–20.83 | 3.0 | 90–95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodarczyk-Makuła, M.; Myszograj, S.; Włodarczyk, M. Removal of Organic Micro-Pollutants from Wastewater in Electrochemical Processes—Review. Energies 2023, 16, 5591. https://doi.org/10.3390/en16155591
Włodarczyk-Makuła M, Myszograj S, Włodarczyk M. Removal of Organic Micro-Pollutants from Wastewater in Electrochemical Processes—Review. Energies. 2023; 16(15):5591. https://doi.org/10.3390/en16155591
Chicago/Turabian StyleWłodarczyk-Makuła, Maria, Sylwia Myszograj, and Maciej Włodarczyk. 2023. "Removal of Organic Micro-Pollutants from Wastewater in Electrochemical Processes—Review" Energies 16, no. 15: 5591. https://doi.org/10.3390/en16155591
APA StyleWłodarczyk-Makuła, M., Myszograj, S., & Włodarczyk, M. (2023). Removal of Organic Micro-Pollutants from Wastewater in Electrochemical Processes—Review. Energies, 16(15), 5591. https://doi.org/10.3390/en16155591








