Experimental Study on the Influence of Wettability Alteration on Gas–Water Two-Phase Flow and Coalbed Methane Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Contact Angle Measurement
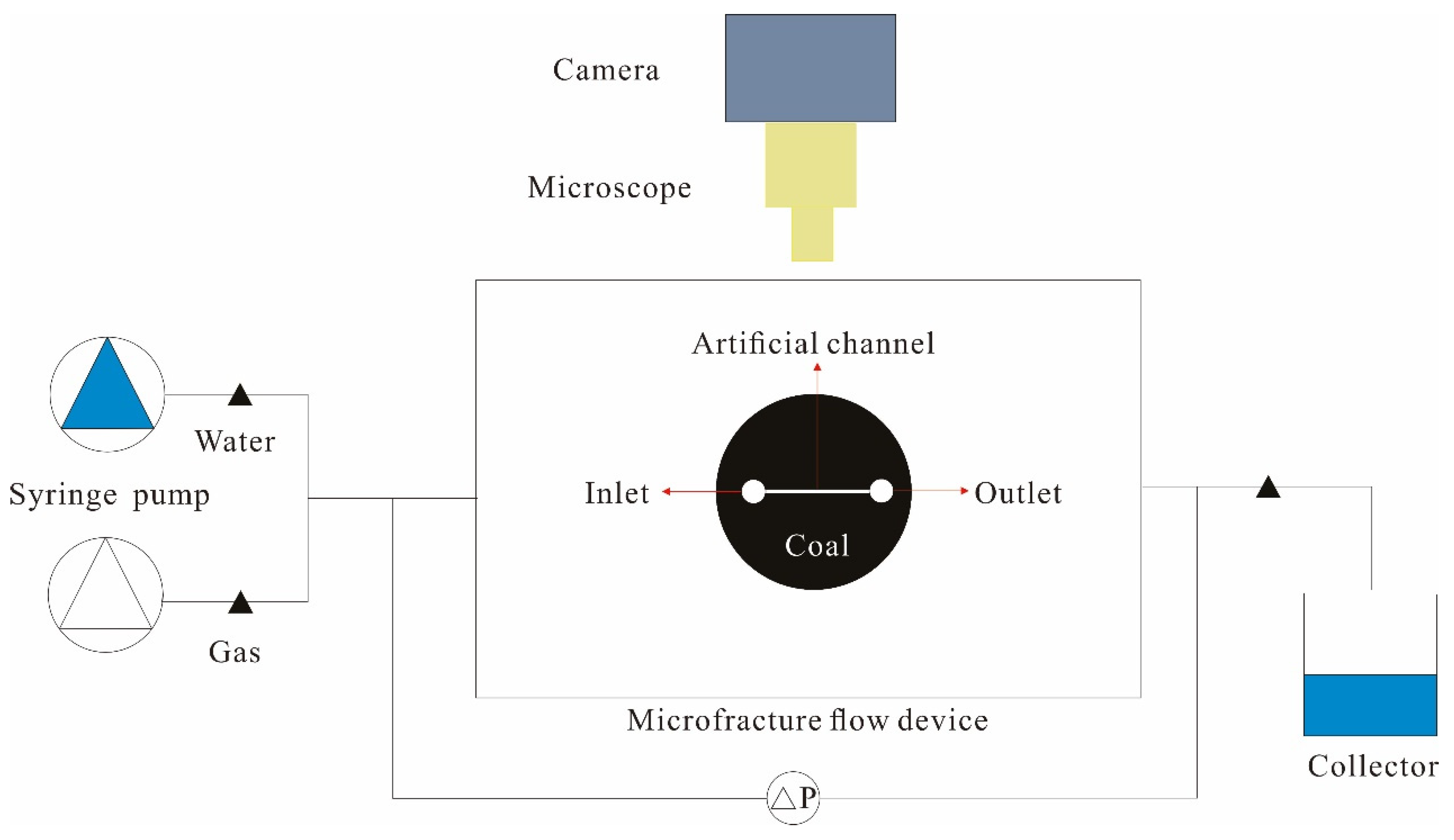
2.3. Gas–Water Flow Experiment
2.4. Chemical Treatment of Samples
3. Results and Discussion
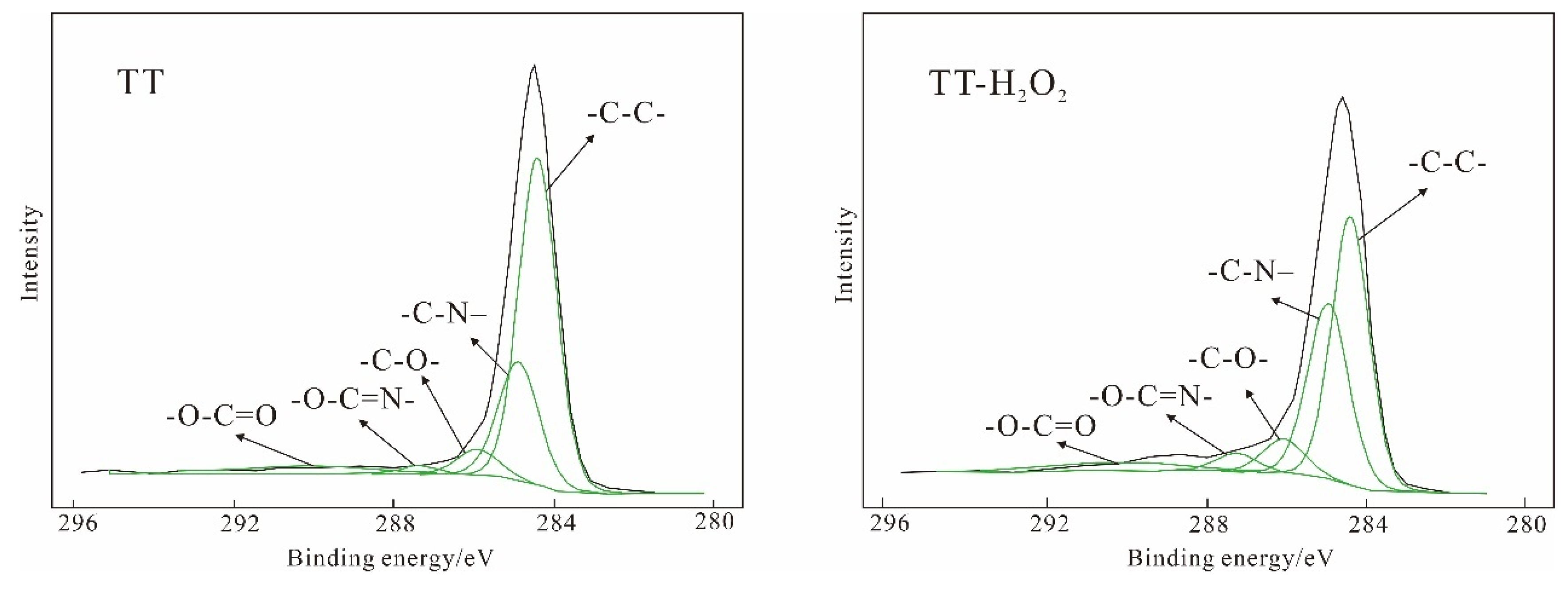
3.1. Characteristics of Coal Surface after Chemical Treatment
3.1.1. Characteristics of Coal Surface after SiO2 Treatment
3.1.2. Characteristics of Coal Surface after H2O2 Treatment
3.2. Alteration of Wettability after Chemical Treatment
3.3. The Influence of Coal Wettability on the Gas–Water Flow
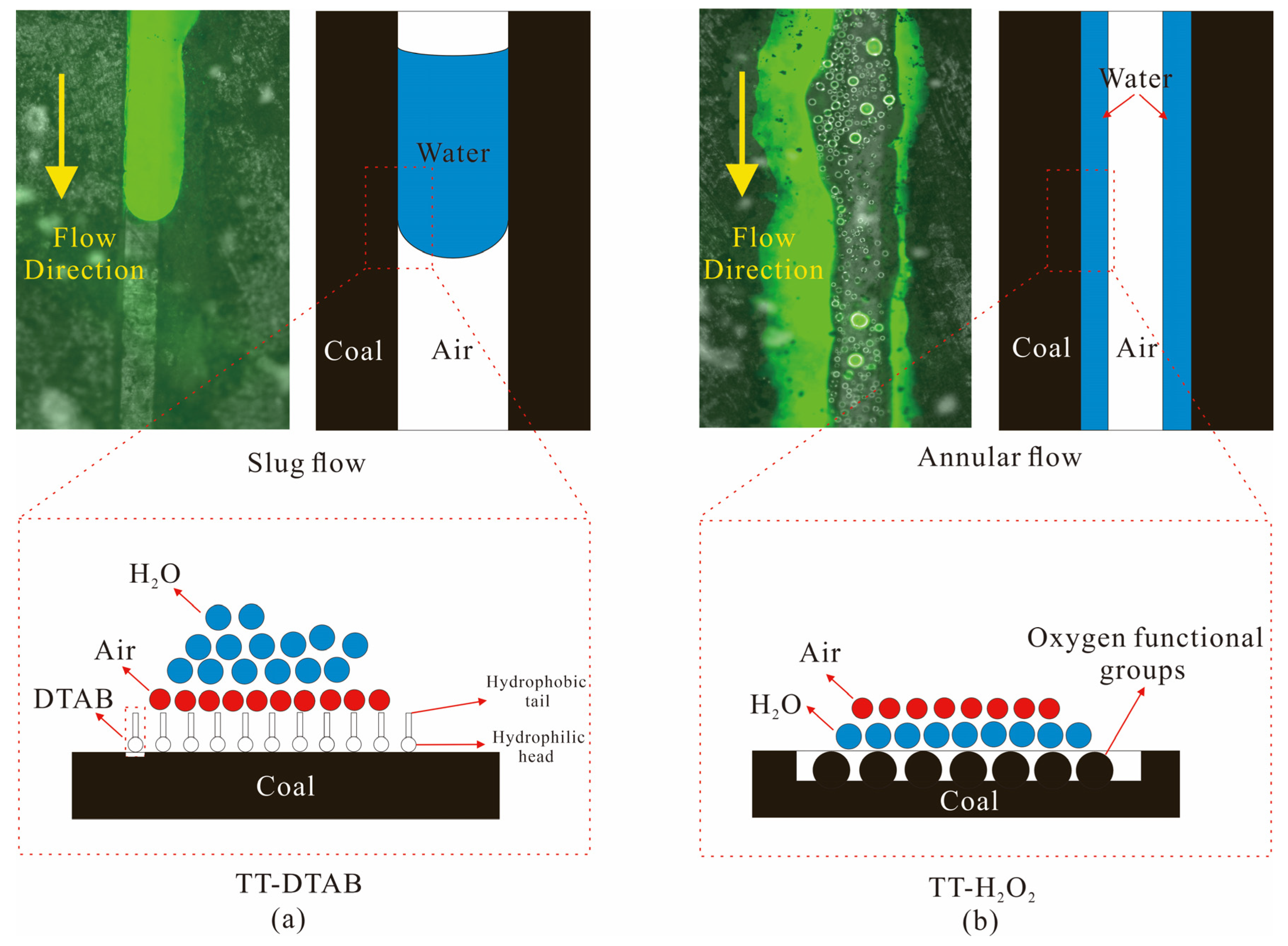
3.3.1. The Influence of Wettability on the Gas–Water Interface
3.3.2. The Influence of Wettability on Gas–Water Flow State
3.4. Relationship between Coal Wettability and Water States
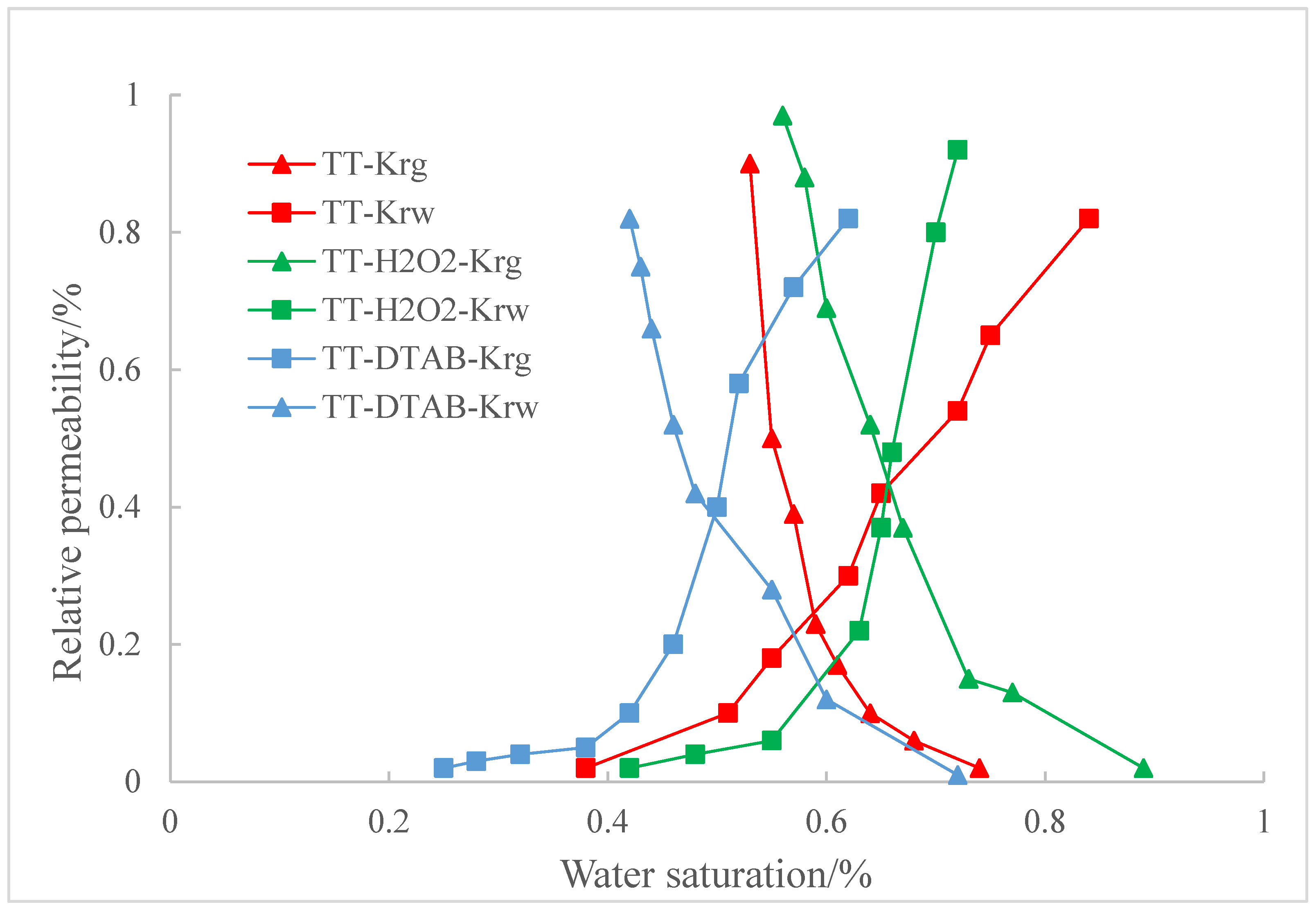
3.5. The Influence of Coal Wettability on Relative Permeability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| CBM | Coalbed methane |
| FJT | Code of coal samples from Feng Jia Ta mine |
| TT | Code of coal samples from Tai Tou mine |
| QC | Code of coal samples from Qin Cheng |
| SiO2 | Silicon dioxide |
| H2O2 | Hydrogen peroxide |
| DTAB | Dodecyl trimethyl ammonium bromide |
| FJT-SiO2 | Sample FJT treated with silicon dioxide |
| TT-SiO2 | Sample TT treated with silicon dioxide |
| QC-SiO2 | Sample QC treated with silicon dioxide |
| FJT-H2O2 | Sample FJT treated with hydrogen peroxide |
| TT-H2O2 | Sample TT treated with hydrogen peroxide |
| QC-H2O2 | Sample QC treated with hydrogen peroxide |
| FJT-DTAB | Sample FJT treated with dodecyl trimethyl ammonium bromide |
| TT-DTAB | Sample TT treated with dodecyl trimethyl ammonium bromide |
| QC-DTAB | Sample QC treated with dodecyl trimethyl ammonium bromide |
| Krg | Relative permeability of the gas |
| Krw | Relative permeability of the water |
| P | Capillary force |
| vol. | Volume fraction |
| wt. | Mass fraction |
| Ro, max | Maximum reflectance of vitrinite |
References
- Xin, F.D.; Xu, H.; Tang, D.Z.; Cao, C. Differences in Accumulation Patterns of Low-Rank Coalbed Methane in China under the Control of the First Coalification Jump. Fuel 2022, 324, 46–57. [Google Scholar] [CrossRef]
- Liu, D.M.; Jia, Q.F.; Cai, Y.D.; Gao, C.J.; Qiu, F.; Zhao, Z.; Chen, S.Y. A New Insight into Coalbed Methane Occurrence and Accumulation in the Qinshui Basin, China. Gondwana Res. 2022, 111, 280–297. [Google Scholar] [CrossRef]
- Shuxun, S.; Sijie, H.; Shiqi, L.; Xiaozhi, Z.; Mengxi, L.; Qiujia, H.; Cong, Z. Comprehensive Study on the Enrichment Mechanism of Coalbed Methane in High Rank Coal Reservoirs. J. China Coal Soc. 2022, 47, 388–403. [Google Scholar]
- Tao, S.; Pan, Z.J.; Tang, S.L.; Chen, S.D. Current Status and Geological Conditions for the Applicability of CBM Drilling Technologies in China: A Review. Int. J. Coal Geol. 2019, 202, 95–108. [Google Scholar] [CrossRef]
- Wu, Y.N.; Tao, S.; Tian, W.G.; Chen, H.; Chen, S.D. Advantageous Seepage Channel in Coal Seam and Its Effects on the Distribution of High-Yield Areas in the Fanzhuang CBM Block, Southern Qinshui Basin, China. Nat. Resour. Res. 2021, 30, 2361–2376. [Google Scholar] [CrossRef]
- Dutka, B. Effect of Depth on the Sorption Capacity of Coals Affected by Outburst Hazard. Fuel 2021, 306, 121611. [Google Scholar] [CrossRef]
- Shu, L.Y.; Wang, K.; Liu, Z.S.; Zhao, W.; Zhu, N.N.; Lei, Y. A Novel Physical Model of Coal and Gas Outbursts Mechanism: Insights into the Process and Initiation Criterion of Outbursts. Fuel 2022, 323, 124305. [Google Scholar] [CrossRef]
- Crow, D.J.G.; Giarola, S.; Hawkes, A.D. A Dynamic Model of Global Natural Gas Supply. Appl. Energy 2018, 218, 452–469. [Google Scholar] [CrossRef]
- Qin, Y.; Moore, T.A.; Shen, J.; Yang, Z.B.; Shen, Y.L.; Wang, G. Resources and Geology of Coalbed Methane in China: A Review. Int. Geol. Rev. 2018, 60, 777–812. [Google Scholar] [CrossRef]
- Wang, Z.W.; Liu, S.M.; Qin, Y. Coal Wettability in Coalbed Methane Production: A Critical Review. Fuel 2021, 303, 121277. [Google Scholar] [CrossRef]
- Shen, J.; Qin, Y.; Wang, G.X.; Fu, X.H.; Wei, C.T.; Lei, B. Relative Permeabilities of Gas and Water for Different Rank Coals. Int. J. Coal Geol. 2011, 86, 266–275. [Google Scholar] [CrossRef]
- Clarkson, C.R. Production Data Analysis of Unconventional Gas Wells: Review of Theory and Best Practices. Int. J. Coal Geol. 2013, 109, 101–146. [Google Scholar] [CrossRef]
- Ofori, P.; Firth, B.; O’Brien, G.; McNally, C.; Nguyen, A.V. Assessing the Hydrophobicity of Petrographically Heterogeneous Coal Surfaces. Energy Fuels 2010, 24, 5965–5971. [Google Scholar] [CrossRef]
- Gosiewska, A.; Drelich, J.; Laskowski, J.S.; Pawlik, M. Mineral Matter Distribution on Coal Surface and Its Effect on Coal Wettability. J. Colloid Interface Sci. 2002, 247, 107–116. [Google Scholar] [CrossRef]
- Susana, L.; Campaci, F.; Santomaso, A.C. Wettability of Mineral and Metallic Powders: Applicability and Limitations of Sessile Drop Method and Washburn’s Technique. Powder Technol. 2012, 226, 68–77. [Google Scholar] [CrossRef]
- Li, Q.Z.; Lin, B.Q.; Zhao, S.; Dai, H.M. Surface Physical Properties and Its Effects on the Wetting Behaviors of Respirable Coal Mine Dust. Powder Technol. 2013, 233, 137–145. [Google Scholar] [CrossRef]
- Ibrahim, A.F.; Nasr-El-Din, H.A. Effects of Formation-Water Salinity, Formation Pressure, Gas Composition, and Gas-Flow Rate on Carbon Dioxide Sequestration in Coal Formations. SPE J. 2017, 22, 1530–1541. [Google Scholar] [CrossRef]
- Wang, X.; Qu, Z.; Ren, G. Collective Enhancement in Hydrophobicity and Electrical Conductivity of Gas Diffusion Layer and the Electrochemical Performance of PEMFCs. J. Power Sources 2023, 575, 233077. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Feng, Q.H.; Zhang, X.M.; Wen, S.M.; Zhai, Y.Y. Relative Permeability of Coal: A Review. Transp. Porous Media 2015, 106, 563–594. [Google Scholar] [CrossRef]
- Mahoney, S.A.; Rufford, T.E.; Johnson, D.; Dmyterko, A.S.K.; Rodrigues, S.; Esterle, J.; Rudolph, V.; Steel, K.M. The Effect of Rank, Lithotype and Roughness on Contact Angle Measurements in Coal Cleats. Int. J. Coal Geol. 2017, 179, 302–315. [Google Scholar] [CrossRef]
- Chen, Y.X.; Liu, D.M.; Yao, Y.B.; Cai, Y.D.; Chen, L.W. Dynamic Permeability Change during Coalbed Methane Production and Its Controlling Factors. J. Nat. Gas Sci. Eng. 2015, 25, 335–346. [Google Scholar] [CrossRef]
- Mchirgui, W.; Millet, O.; Amiri, O. Theoretical Study and Simulation of Moisture Transport in Unsaturated Porous Media by Periodic Homogenization. Int. J. Numer. Anal. Methods Geomech. 2015, 39, 409–435. [Google Scholar] [CrossRef]
- Chen, D.; Pan, Z.J.; Liu, J.S.; Connell, L.D. Modeling and Simulation of Moisture Effect on Gas Storage and Transport in Coal Seams. Energy Fuels 2012, 26, 1695–1706. [Google Scholar] [CrossRef]
- Teng, T.; Wang, J.G.; Gao, F.; Ju, Y.; Xia, T.Q. Impact of Water Film Evaporation on Gas Transport Property in Fractured Wet Coal Seams. Transp. Porous Media 2016, 113, 357–382. [Google Scholar] [CrossRef]
- Karimi, A.; Fakhroueian, Z.; Bahramian, A.; Khiabani, N.P.; Darabad, J.B.; Azin, R.; Arya, S. Wettability Alteration in Carbonates Using Zirconium Oxide Nanofluids: EOR Implications. Energy Fuels 2012, 26, 1028–1036. [Google Scholar] [CrossRef]
- Kilau, H.W.; Pahlman, J.E. Coal wetting ability of surfactant solutions and the effect of multivalent anion additions. Colloids Surf. 1987, 26, 217–242. [Google Scholar] [CrossRef]
- Naik, S.; You, Z.; Bedrikovetsky, P. Effect of Wettability Alteration on Productivity Enhancement in Unconventional Gas Reservoirs: Application of Nanotechnology. In Proceedings of the Society of Petroleum Engineers—SPE Asia Pacific Unconventional Resources Conference and Exhibition, Brisbane, Australia, 9–11 November 2015. [Google Scholar] [CrossRef]
- Wang, G.D.; Ren, T.; Wang, K.; Zhou, A.T. Improved Apparent Permeability Models of Gas Flow in Coal with Klinkenberg Effect. Fuel 2014, 128, 53–61. [Google Scholar] [CrossRef]
- Si, L.L.; Li, Z.H.; Yang, Y.L. Evolution Characteristics of Gas Permeability Under Multiple Factors. Transp. Porous Media 2019, 127, 415–432. [Google Scholar] [CrossRef]
- Edalatpour, M.; Liu, L.; Jacobi, A.M.; Eid, K.F.; Sommer, A.D. Managing Water on Heat Transfer Surfaces: A Critical Review of Techniques to Modify Surface Wettability for Applications with Condensation or Evaporation. Appl. Energy 2018, 222, 967–992. [Google Scholar] [CrossRef]
- Shen, J.; Qin, Y.; Li, Y.P.; Wang, G. Experimental Investigation into the Relative Permeability of Gas and Water in Low-Rank Coal. J. Pet. Sci. Eng. 2019, 175, 303–316. [Google Scholar] [CrossRef]
- Liu, Z.X.; Liu, Z.C.; Zong, Z.M.; Wei, X.Y.; Wang, J.; Lee, C.W. GC/MS Analysis of Water-Soluble Products from the Mild Oxidation of Longkou Brown Coal with H2O2. Energy Fuels 2003, 17, 424–426. [Google Scholar] [CrossRef]
- Liu, F.J.; Wei, X.Y.; Zhu, Y.; Wang, Y.G.; Li, P.; Fan, X.; Zhao, Y.P.; Zong, Z.M.; Zhao, W.; Wei, Y.B. Oxidation of Shengli Lignite with Aqueous Sodium Hypochlorite Promoted by Pretreatment with Aqueous Hydrogen Peroxide. Fuel 2013, 111, 211–215. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Diao, J.; Williams, M.C. Characterization of the Wettability of Solid Particles by Film Flotation 1. Experimental investigation. Colloids Surf. 1991, 60, 127–144. [Google Scholar] [CrossRef]
- Polat, M.; Polat, H.; Chander, S. Physical and Chemical Interactions in Coal Flotation. Int. J. Miner. Process. 2003, 72, 199–213. [Google Scholar] [CrossRef]
- Sadler, L.Y.; Chang, C. Chemical enhancement of coal seam permeability. In Situ 1988, 12, 103375. [Google Scholar]
- Bayat, A.E.; Junin, R.; Samsuri, A.; Piroozian, A.; Hokmabadi, M. Impact of Metal Oxide Nanoparticles on Enhanced Oil Recovery from Limestone Media at Several Temperatures. Energy Fuels 2014, 28, 6255–6266. [Google Scholar] [CrossRef]
- Cubaud, T.; Ulmanella, U.; Ho, C.M. Two-Phase Flow in Microchannels with Surface Modifications. Fluid Dyn. Res. 2006, 38, 772–786. [Google Scholar] [CrossRef]
- Gautam, S.; Liu, T.T.; Cole, D. Sorption, Structure and Dynamics of CO2 and Ethane in Silicalite at High Pressure: A Combined Monte Carlo and Molecular Dynamics Simulation Study. Molecules 2019, 24, 99. [Google Scholar] [CrossRef]
- Li, P.; Ma, D.M.; Zhang, J.C.; Tang, X.; Huo, Z.P.; Li, Z.; Liu, J.L. Effect of Wettability on Adsorption and Desorption of Coalbed Methane: A Case Study from Low-Rank Coals in the Southwestern Ordos Basin, China. Ind. Eng. Chem. Res. 2018, 57, 12003–12015. [Google Scholar] [CrossRef]
- Zhou, Y.A.; Sun, W.J.; Chu, W.; Liu, X.Q.; Jing, F.L.; Xue, Y. Theoretical Insight into the Enhanced CH4 Desorption via H2O Adsorption on Different Rank Coal Surfaces. J. Energy Chem. 2016, 25, 677–682. [Google Scholar] [CrossRef]




| Sample | Ro, Max (%) | Maceral Composition (vol, %) | Proximate Analysis (wt, %) | ||||
|---|---|---|---|---|---|---|---|
| Vitrinite | Inertinite | Minerals | Moisture | Ash | Volatile | ||
| FJT | 0.68 | 47.2 | 38.2 | 9.7 | 4.8 | 12.4 | 30.6 |
| TT | 1.51 | 70.9 | 21.9 | 0 | 0.8 | 9.8 | 19.6 |
| QC | 3.02 | 57.1 | 35.1 | 0 | 3.1 | 13.9 | 8.2 |
| Sample | Dry Particle Size/nm | Surface Area/m2/g | Mean Particle Size/nm |
|---|---|---|---|
| SiO2 | 12 | 190–220 | 450 |
| Sample Code | Atomic Composition (%) | |||||
|---|---|---|---|---|---|---|
| C | O | N | Al | Si | Ca | |
| FJT | 81.41 | 10.59 | 1.44 | 1.73 | 3.86 | 0.97 |
| FJT-H2O2 | 76.15 | 15.93 | 2.21 | 1.25 | 3.57 | 0.89 |
| TT | 88.75 | 4.21 | 1.72 | 1.36 | 3.52 | 0.44 |
| TT-H2O2 | 81.46 | 11.24 | 2.06 | 1.16 | 3.32 | 0.76 |
| QC | 91.33 | 3.12 | 1.51 | 1.15 | 1.55 | 1.34 |
| QC-H2O2 | 86.17 | 8.34 | 2.11 | 1.36 | 1.26 | 0.76 |
| Sample | Contact Angle/° | Standard Deviations | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Average | ||
| FJT | 44.3 | 38.1 | 49.5 | 49.9 | 46.9 | 45.74 | 4.84 |
| FJT-SiO2 | 36.8 | 46.3 | 34.2 | 37.4 | 47.5 | 40.44 | 6.03 |
| FJT-H2O2 | 36.3 | 30.9 | 33.7 | 32.5 | 34.6 | 33.60 | 2.05 |
| FJT-DTAB | 96.3 | 97.6 | 103.2 | 98.7 | 101.5 | 99.46 | 2.84 |
| TT | 71.2 | 71 | 70.3 | 59.05 | 59 | 66.09 | 6.46 |
| TT-SiO2 | 47.4 | 55.2 | 45.3 | 51.6 | 60.1 | 51.92 | 5.42 |
| TT-H2O2 | 36.5 | 37.1 | 32.3 | 35.5 | 37.6 | 35.80 | 2.11 |
| TT-DTAB | 95.4 | 100.5 | 103.2 | 99.4 | 97.8 | 99.26 | 2.92 |
| QC | 93.5 | 96.6 | 97.1 | 106 | 100.5 | 98.74 | 4.76 |
| QC-SiO2 | 71.2 | 65.4 | 66.3 | 75.4 | 78.4 | 71.34 | 5.64 |
| QC-H2O2 | 60.8 | 60.2 | 56.4 | 57.4 | 56.2 | 58.20 | 2.16 |
| QC-DTAB | 99.5 | 103.2 | 104.6 | 103.5 | 98.4 | 101.84 | 2.72 |
| Sample | Pressure Drop/kPa | Injection Rate/µL/min | Contact Angle/° | ||
|---|---|---|---|---|---|
| Left | Right | Average | |||
| TT | 3.3 | 10 | 122.1 | 124.1 | 123.1 |
| TT-SiO2 | 1.8 | 10 | 123.6 | 87.8 | 105.7 |
| TT-H2O2 | 0 | 10 | 87.7 | 77.3 | 82.5 |
| TT-DTAB | 4.6 | 10 | 136.2 | 146.5 | 141.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, A.; Shu, L.; Huo, Z. Experimental Study on the Influence of Wettability Alteration on Gas–Water Two-Phase Flow and Coalbed Methane Production. Energies 2023, 16, 5756. https://doi.org/10.3390/en16155756
Zhang A, Shu L, Huo Z. Experimental Study on the Influence of Wettability Alteration on Gas–Water Two-Phase Flow and Coalbed Methane Production. Energies. 2023; 16(15):5756. https://doi.org/10.3390/en16155756
Chicago/Turabian StyleZhang, Aoxiang, Longyong Shu, and Zhonggang Huo. 2023. "Experimental Study on the Influence of Wettability Alteration on Gas–Water Two-Phase Flow and Coalbed Methane Production" Energies 16, no. 15: 5756. https://doi.org/10.3390/en16155756
APA StyleZhang, A., Shu, L., & Huo, Z. (2023). Experimental Study on the Influence of Wettability Alteration on Gas–Water Two-Phase Flow and Coalbed Methane Production. Energies, 16(15), 5756. https://doi.org/10.3390/en16155756









