Future Technologies for Recycling Spent Lithium-Ion Batteries (LIBs) from Electric Vehicles—Overview of Latest Trends and Challenges
Abstract
:1. Introduction
2. Lithium-Ion Batteries—Composition, Reactions, Advantages, and Disadvantages
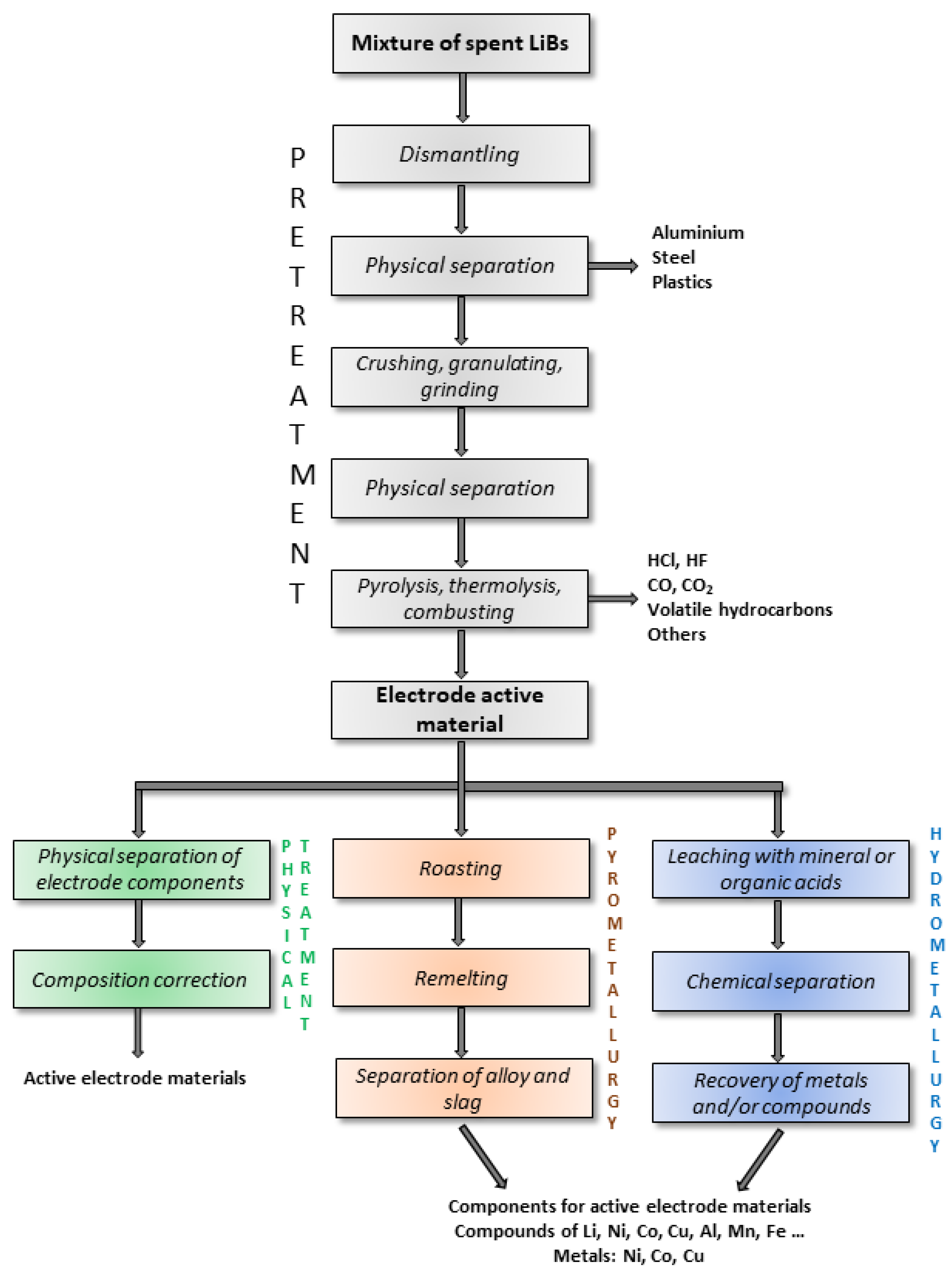
3. Characteristics of Current Industrial Technologies for Recycling LIBs
3.1. Pyrometallurgical Methods
3.2. Hydrometallurgical Methods
| Separation Method | PLS Components | Chemicals Used for Separation | Literature |
|---|---|---|---|
| Solvent extraction | Co, Mn, Ni, Li, Cu, Al, and Fe Ni, Co, Mn, Li, Fe Ni, Co, Li Co, Li, Al, Cu, Fe (Ni, Mn) Co, Mn, Li, Al, Fe Co, Li Co, Ni, Li | D2EHPA D2EHPA Cyanex 272 Cyanex 272 Cyanex 272 + PC-88A Cyphos IL-101 TBP, various ionic liquids | [82] [72] [83] [84] [85] [86] [87] |
| Ion exchange/adsorption | Co, Li Co, Ni, Li, Mn, Cu, Al, Fe Co, Ni, Mn, Li, Al, Fe Li, Fe | various zeolites Lewatit TP260, Purolite S-930, Finex CS12GC, Finex CA16GC Dowex M4195 IRC120, HPR1200, HPR2900 | [88] [65] [89] |
| Precipitation | Fe, Mn, Li Mn, Ni, Co, Li Co, Mn, Ni, Li, Cu, Al, Fe Ni, Co, Mn, Li, Fe | H3PO4/Na3PO4 NaOH/Na2CO3/Na2C2O4/Na3PO4 KMnO4 (NH4)2C2O4, Na2CO3 LiOH | [90] [91] [82] [72] |
| Electrochemical methods | Ni, Co, Li Li, Co Ni, Co, Mn, Li Ni, Co, Li Li, Co | ---- ---- ---- ---- ---- | [37,53,83,92] |
4. Environmental Impact—Challenges of LIB Recycling
5. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building safe lithium-ion batteries for electric vehicles: A review. Electrochem. Energy Rev. 2020, 3, 1–42. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical processes for recycling spent lithium-ion batteries: A critical review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Leal, V.M.; Ribeiro, J.S.; Coelho, E.L.D.; Freitas, M.B.J.G. Recycling of spent lithium-ion batteries as a sustainable solution to obtain raw materials for different applications. J. Energy Chem. 2023, 79, 118–134. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, H.; Tan, N.; Zhu, M.; Tan, W.; Daramola, D.; Gu, T. Advances in bioleaching of waste lithium batteries under metal ion stress. Bioresour. Bioprocess. 2023, 10, 19. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Z.; Wu, B.; Tang, Y.; Evans, S. Environmental life cycle assessment of recycling technologies for ternary lithium-ion batteries. J. Clean. Prod. 2023, 389, 136008. [Google Scholar] [CrossRef]
- Chagnes, A.; Pospiech, B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Pospiech, B. Selective recovery of cobalt(II) towards lithium(I) from chloride media by transport across polymer inclusion membrane with triisooctylamine. Pol. J. Chem. Technol. 2014, 16, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Pospiech, B. Separation of Co from Ni and Li from chloride media using polymer inclusion membrane system with thiosalicylate based ionic liquid. Physicochem. Probl. Miner. Process. 2022, 58, 152997. [Google Scholar] [CrossRef]
- Natarajan, S.; Krishnamoorthy, K.; Sathyaseelan, A.; Mariappan, V.K.; Pazhamalai, P.; Manoharan, S.; Kim, S.J. A new route for the recycling of spent lithium-ion batteries towards advanced energy storage, conversion, and harvesting systems. Nano Energy 2022, 101, 107595. [Google Scholar] [CrossRef]
- Barik, S.; Prabaharan, G.; Kumar, B. An innovative approach to recover the metal values from lithium-ion batteries. Waste Manag. 2016, 51, 222–226. [Google Scholar] [CrossRef]
- Tawonezvi, T.; Nomnqa, M.; Petrik, L.; Bladergroen, B.J. Recovery and recycling of valuable metals from spent lithium-ion batteries: A comprehensive review and analysis. Energies 2023, 16, 1365. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang Liu, L.J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; Li, B. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Karim Zaghib, K.; Henri Groult, H. Comparative issues of cathode materials for Li-ion batteries. Inorganics 2014, 2, 132–154. [Google Scholar] [CrossRef] [Green Version]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef] [Green Version]
- Kundu, S.; Ein-Eli, Y. A review on design considerations in polymer and polymer composite solid-state electrolytes for solid Li batteries. J. Power Sources 2023, 553, 232267. [Google Scholar] [CrossRef]
- Kushnir, D. Lithium Ion Battery Recycling Technology 2015. In Current State and Future Prospects; Environmental Systems Analysis; Chalmers University: Göteborg, Sweden, 2015; p. 18. Available online: http://publications.lib.chalmers.se/records/fulltext/230991/local_230991.pdf (accessed on 12 May 2023).
- Romare, M.; Dahllöfn, L. The Life Cycle Energy Consumption and Greenhouse Gas Emissions from Lithium-Ion Batteries. In A Study with Focus on Current Technology and Batteries for Light-Duty Vehicles; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2017; Available online: https://www.energimyndigheten.se/globalassets/forskning--innovation/transporter/c243-the-life-cycle-energy-consumption-and-co2-emissions-from-lithium-ion-batteries-.pdf (accessed on 12 May 2023).
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Xinkai Fu, X. Lithium-ion battery supply chain considerations: Analysis of potential bottlenecks in critical metals. Joule 2017, 1, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Wassiliadis, N.; Steinsträter, M.; Schreiber, M.; Rosner, P.; Nicoletti, L.; Schmid, F.; Ank, M.; Teichert, O.; Wildfeuer, L.; Schneider, J.; et al. Quantifying the state of the art of electric powertrains in battery electric vehicles: Range, efficiency, and lifetime from component to system level of the Volkswagen ID.3. Etransportation 2022, 12, 100167. [Google Scholar] [CrossRef]
- Arcus, C. Tesla Model 3 & Chevy Bolt Battery Packs Examined; CleanTechnica. 2018. Available online: https://cleantechnica.com/2018/07/08/tesla-model-3-chevy-bolt-battery-packs-examined/ (accessed on 12 May 2023).
- What Is Included in the Li-Ion Battery from an Electric Car? How Much Lithium, How Much Cobalt? Here’s the Answer. Available online: https://elektrowoz.pl/magazyny-energii/co-wchodzi-w-sklad-baterii-li-ion-z-samochodu-elektrycznego-ile-jest-litu-ile-kobaltu-oto-odpowiedz/ (accessed on 12 May 2023). (In Polish).
- Bloomberg Precious and Industrial Metals Markets. Available online: https://www.bloomberg.com/markets/commodities/futures/metals (accessed on 28 April 2023).
- Gaines, L.; Dai, Q.; Vaughey, J.T.; Gillard, S. Direct Recycling R&D at the ReCell Center. Recycling 2021, 6, 31. [Google Scholar] [CrossRef]
- Mrozik, W.; Rajaeifar, M.A.; Heidrich, O.; Christensen, P. Environmental impacts pollution sources and pathways of spent lithium-ion batteries. Energy Environ. Sci. 2021, 14, 6099–6121. [Google Scholar] [CrossRef]
- Wu, X.; Ma, J.; Wang, J.; Zhang, X.; Zhou, G.; Liang, Z. Progress, Key issues, and future prospects for Li-ion battery recycling. Glob. Chall. 2022, 6, 2200067. [Google Scholar] [CrossRef]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-ion battery recycling—Overview of techniques and trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Sobianowska-Turek, A. Hydrometallurgical recovery of metals: Ce, La, Co, Fe, Mn, Ni and Zn from the stream of used Ni-MH cells. Waste Manag. 2018, 77, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Mousa, E.; Hu, X.; Ye, G. Effect of graphite on the recovery of valuable metals from spent Li-ion batteries in baths of hot metal and steel. Recycling 2022, 7, 5. [Google Scholar] [CrossRef]
- Quintana, C.; Cybulski, O.; Mikulak-Lucznik, B.; Klucznik, T.; Grzybowski, B.A. One pot, three phase recycling of metals from Lit Ion Batteries in rotating, concentric liquid reactors. Adv. Mater. 2023, 35, 2211946. [Google Scholar] [CrossRef]
- Jung, J.C.-Y.; Sui, P.-C.; Zhang, J. A review of recycling spent lithium-ion battery cathode materials using hydrometallurgical treatments. J. Energy Storage 2021, 35, 102217. [Google Scholar] [CrossRef]
- Ordoñez, J.; Gago, E.J.; Girard, A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sust. Energ. Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Jiang, L.; Wen, J.; Wang, H.; Guan, R.; Zhang, J.; Zeng, G. Regeneration and reutilization of cathode materials from spent lithium-ion batteries. Chem. Eng. J. 2020, 383, 123089. [Google Scholar] [CrossRef]
- Armand, M.; Axmann, P.; Bresser, D.; Copley, M.; Edström, K.; Ekberg, C.; Guyomard, D.; Lestriez, B.; Novák, P.; Petranikova, M.; et al. Lithium-ion batteries—Current state of the art and anticipated developments. J. Power Sources 2020, 479, 228708. [Google Scholar] [CrossRef]
- Chen, X.; Xu, B.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Separation and recovery of metal values from leaching liquor of mixed-type of spent lithium-ion batteries. Sep. Purif. Technol. 2015, 144, 197–205. [Google Scholar] [CrossRef]
- Dutta, D.; Kumari, A.; Panda, R.; Jha, S.; Gupta, D.; Goel, S.; Jha, M.K. Close loop separation process for the recovery of Co, Cu, Mn, Fe and Li from spent lithium-ion batteries. Sep. Purif. Technol. 2018, 200, 327–334. [Google Scholar] [CrossRef]
- Gupta, S.; Pant, K.K.; Corder, G. An environmentally benign closed-loop process for the selective recovery of valuable metals from industrial end-of-life lithium-ion batteries. Chem. Eng. J. 2022, 446, 137397. [Google Scholar] [CrossRef]
- Liu, G.; Chen, Z.; Luo, F.; Liu, T.; Xi, X.; Wang, Z.; Gao, Z.; Shao, P.; Wu, D.; Luo, X.; et al. One-step nickel-cobalt alloy electrodeposition from spent lithium-ion battery via synergistic pH adjustment and Mn2+ supplementation. Sep. Purif. Technol. 2023, 314, 123581. [Google Scholar] [CrossRef]
- Mishra, G.; Jha, R.; Meshram, A.; Singh, K.K. A review on recycling of lithium-ion batteries to recover critical metals. J. Environ. Chem. Eng. 2022, 10, 108534. [Google Scholar] [CrossRef]
- Kim, S.; Bang, J.; Yoo, J.; Shin, Y.; Bae, J.; Jeong, J.; Kim, K.; Dong, P.; Kwon, K. A comprehensive review on the pretreatment process in lithium-ion battery recycling. J. Clean. Prod. 2021, 294, 126329. [Google Scholar] [CrossRef]
- Verdugo, L.; Zhang, L.; Saito, K.; Bruckard, W.; Menacho, J.; Hoadley, A. Flotation behaviour of the most common electrode materials in lithium ion batteries. Sep. Purif. Technol. 2022, 301, 121885. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, G.; Xu, S.; He, Y.; Liu, X. Thermal treatment process for the recovery of valuable metals from spent lithium-ion batteries. Hydrometallurgy 2016, 165, 390–396. [Google Scholar] [CrossRef]
- Yu, W.; Guo, Y.; Xu, S.; Yang, Y.; Zhao, Y.; Zhang, J. Comprehensive recycling of lithium-ion batteries: Fundamentals, pretreatment, and perspectives. Energy Storage Mater. 2023, 54, 172–220. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, B.; Lu, Z.; Liu, N.; Zhu, J. Challenges and recent progress in the development of Si anodes for lithium-ion battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef] [Green Version]
- Liao, Z.; Zhang, S.; Zhao, Y.; Qiu, Z.; Li, K.; Han, D.; Zhang, G.; Habetler, T.G. Experimental evaluation of thermolysis-driven gas emissions from LiPF6-carbonate electrolyte used in lithium-ion batteries. J. Energy Chem. 2020, 49, 124–135. [Google Scholar] [CrossRef]
- Jian, Y.; Zongliang, Z.; Gang, Z.; Liangxing, J.; Fangyang, L.; Ming, J.; Yanqing, L. Process study of chloride roasting and water leaching for the extraction of valuable metals from spent lithium-ion batteries. Hydrometallurgy 2021, 203, 105638. [Google Scholar] [CrossRef]
- Zhang, G.; Yuan, X.; Tay, C.Y.; He, Y.; Wang, H.; Duan, C. Selective recycling of lithium from spent lithium-ion batteries by carbothermal reduction combined with multistage leaching. Sep. Purif. Technol. 2023, 314, 123555. [Google Scholar] [CrossRef]
- Nan, J.; Han, D.; Zuo, X. Recovery of metal values from spent lithium-ion batteries with chemical deposition and solvent extraction. J. Power Sources 2005, 152, 278–284. [Google Scholar] [CrossRef]
- Swain, B.; Jeong, J.; Lee, J.-C.; Lee, G.-H.; Sohn, J.-S. Hydrometallurgical process for recovery of cobalt from waste cathodic active material generated during manufacturing of lithium ion batteries. J. Power Sources 2007, 167, 536–544. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, K. Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries. J. Hazard. Mater. 2011, 194, 378–384. [Google Scholar] [CrossRef]
- Takacova, Z.; Havlik, T.; Kukurugya, F.; Orac, D. Cobalt and lithium recovery from active mass of spent Li-ion batteries: Theoretical and experimental approach. Hydrometallurgy 2016, 163, 9–17. [Google Scholar] [CrossRef]
- Castillo, S.; Ansart, F.; Laberty-Robert, C.; Portal, J. Advances in the recovery of spent lithium battery compounds. J. Power Sources 2002, 112, 247–254. [Google Scholar] [CrossRef]
- Lee, C.K.; Rhee, K.I. Reductive leaching of cathodic active materials from lithium ion battery wastes. Hydrometallurgy 2003, 68, 5–10. [Google Scholar] [CrossRef]
- Li, L.; Chen, R.; Sun, F.; Wu, F.; Liu, J. Preparation of LiCoO2 films from spent lithium-ion batteries by a combined recycling process. Hydrometallurgy 2011, 108, 220–225. [Google Scholar] [CrossRef]
- de Oliveira Demarco, J.; Stefanello Cadore, J.; da Silveira de Oliveira, F.; Hiromitsu Tanabe, E.; Assumpção Bertuol, D. Recovery of metals from spent lithium-ion batteries using organic acids. Hydrometallurgy 2019, 190, 105169. [Google Scholar] [CrossRef]
- Musariri, B.; Akdogan, G.; Dorfling, C.; Bradshaw, S. Evaluating organic acids as alternative leaching reagents for metal recovery from lithium ion batteries. Miner. Eng. 2019, 137, 108–117. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Wang, T.-Y.; Wu, L.-J.; Min, X.-B.; Liu, H.; Wen, D.-Q.; Ke, Y.; Wang, Z.-B.; Tang, Y.-W.; Fu, H.-K. Recovery of valuable metals from spent ternary Li-ion batteries: Dissolution with amidosulfonic acid and d-glucose. Hydrometallurgy 2019, 190, 105162. [Google Scholar] [CrossRef]
- Chang, D.; Yang, S.; Shi, P.; Jie, Y.; Hu, F.; Fang, G.; Chen, Y. Selective recovery of lithium and efficient leaching of transition metals from spent LiNixCoyMnzO2 batteries based on a synergistic roasting process. Chem. Eng. J. 2022, 449, 137752. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Zeng, Z.; Gao, Y.; Liu, C.; Sun, X. Separation of lithium and transition metals from the leachate of spent lithium-ion battery by extraction-precipitation with p-tert-butylphenoxy acetic acid. Hydrometallurgy 2021, 206, 105768. [Google Scholar] [CrossRef]
- Huang, T.; Liu, L.; Zhang, S. Recovery of cobalt, lithium, and manganese from the cathode active materials of spent lithium-ion batteries in a bio-electro-hydrometallurgical process. Hydrometallurgy 2019, 188, 101–111. [Google Scholar] [CrossRef]
- Joo, S.-H.; Shin Dj Oh, C.; Wang, J.-P.; Senanayake, G.; Shin, S.M. Selective extraction and separation of nickel from cobalt, manganese and lithium in pre-treated leach liquors of ternary cathode material of spent lithium-ion batteries using synergism caused by Versatic 10 acid and LIX 84-I. Hydrometallurgy 2016, 159, 65–74. [Google Scholar] [CrossRef]
- Joo, S.-H.; Shin, S.M.; Shin, D.; Oh, C.; Wang, J.-P. Extractive separation studies of manganese from spent lithium battery leachate using mixture of PC88A and Versatic 10 acid in kerosene. Hydrometallurgy 2015, 156, 136–141. [Google Scholar] [CrossRef]
- Leite, D.d.S.; Carvalho, P.L.G.; de Lemos, L.R.; Mageste, A.B.; Rodrigues, G.D. Hydrometallurgical separation of copper and cobalt from lithium-ion batteries using aqueous two-phase systems. Hydrometallurgy 2017, 169, 245–252. [Google Scholar] [CrossRef]
- Li, B.; Wu, C.; Hu, D.; Xu, J.; Zhang, T.; Tong, J.; Fang, X. Copper extraction from the ammonia leach liquor of spent lithium ion batteries for regenerating LiNi0.5Co0.5O2 by co-precipitation. Hydrometallurgy 2020, 193, 105310. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Fu, Y.; Huang, H.; Zhong, Z.; Wang, Y. Recovery of Fe, Mn, Ni and Co in sulfuric acid leaching liquor of spent lithium ion batteries for synthesis of lithium ion-sieve and NixCoyMn1-x-y(OH)2. Hydrometallurgy 2019, 190, 105190. [Google Scholar] [CrossRef]
- Strauss, M.L.; Diaz, L.A.; McNally, J.; Klaehn, J.; Lister, T.E. Separation of cobalt, nickel, and manganese in leach solutions of waste lithium-ion batteries using Dowex M4195 ion exchange resin. Hydrometallurgy 2021, 206, 105757. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, M.; Yao, Y.; Wang, H.; Tong, B.; Zhao, Z. A novel approach for the selective extraction of Li+ from the leaching solution of spent lithium-ion batteries using benzo-15-crown-5 ether as extractant. Sep. Purif. Technol. 2020, 237, 116325. [Google Scholar] [CrossRef]
- Virolainen, S.; Fallah Fini, M.; Laitinen, A.; Sainio, T. Solvent extraction fractionation of Li-ion battery leachate containing Li, Ni, and Co. Sep. Purif. Technol. 2017, 179, 274–282. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, Z.; Xue, F.; Yang, B.; Guo, G.; Qiu, J. A more simple and efficient process for recovery of cobalt and lithium from spent lithium-ion batteries with citric acid. Sep. Purif. Technol. 2019, 215, 398–402. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Y.; Makuza, B.; Zhu, F.; Wang, H.; Hong, N.; Long, Z.; Deng, W.; Zou, G.; Hou, H.; et al. Selective lithium extraction and regeneration of LiCoO2 cathode materials from the spent lithium-ion battery. Chem. Eng. J. 2023, 452, 139258. [Google Scholar] [CrossRef]
- Zheng, Q.; Cao, Z.; Wu, S.; Li, Q.; Wang, M.; Guan, W.; Zhang, G. An efficient method for separation of Ni(II) and Co(II) with novel extractant NNPA: Synthesis, characterization, extraction behaviors, crystal structures and DFT computational studies. J. Environ. Chem. Eng. 2023, 11, 109815. [Google Scholar] [CrossRef]
- Barbieri, E.M.S.; Lima, E.P.C.; Cantarino, S.J.; Lelis, M.F.F.; Freitas, M.B.J.G. Recycling of spent ion-lithium batteries as cobalt hydroxide and cobalt oxide films formed under a conductive glass substrate, and their electrochemical properties. J. Power Sources 2014, 269, 158–163. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Manag. 2015, 38, 349–356. [Google Scholar] [CrossRef]
- Djoudi, N.; Mostefa, M.L.P.; Muhr, H. Precipitation of cobalt salts for recovery in leachates. Chem. Eng. Technol. 2019, 42, 1492–1499. [Google Scholar] [CrossRef]
- Liu, J.; Mak, T.-Y.; Meng, Z.; Wang, X.; Cao, Y.; Lu, Z.; Suen, D.W.-S.; Lu, X.-Y.; Tang, Y. Efficient recovery of lithium as Li2CO3 and cobalt as Co3O4 from spent lithium-ion batteries after leaching with p-toluene sulfonic acid. Hydrometallurgy 2023, 216, 106012. [Google Scholar] [CrossRef]
- Liu, T.; Chen, J.; Li, H.; Li, K. An integrated process for the separation and recovery of valuable metals from the spent LiNi0.5Co0.2Mn0.3O2 cathode materials. Sep. Purif. Technol. 2020, 245, 116869. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Z. Recovery of lithium from spent lithium-ion batteries using precipitation and electrodialysis techniques. Sep. Purif. Technol. 2018, 206, 335–342. [Google Scholar] [CrossRef]
- Verma, A.; Corbin, D.R.; Shiflett, M.B. Lithium and cobalt recovery for lithium-ion battery recycle using an improved oxalate process with hydrogen peroxide. Hydrometallurgy 2021, 203, 105694. [Google Scholar] [CrossRef]
- Xuan, W.; Chagnes, A.; Xiao, X.; Olsson, R.T.; Forsberg, K. Antisolvent Precipitation for metal recovery from citric acid solution in recycling of NMC cathode materials. Metals 2022, 12, 607. [Google Scholar] [CrossRef]
- Freitas, M.B.J.G.; Celante, V.G.; Pietre, M.K. Electrochemical recovery of cobalt and copper from spent Li-ion batteries as multilayer deposits. J. Power Sources 2010, 195, 3309–3315. [Google Scholar] [CrossRef]
- Garcia, E.M.; Santos, J.S.; Pereira, E.C.; Freitas, M.B.J.G. Electrodeposition of cobalt from spent Li-ion battery cathodes by the electrochemistry quartz crystal microbalance technique. J. Power Sources 2008, 185, 549–553. [Google Scholar] [CrossRef]
- Lupi, C.; Pasquali, M.; Dell’Era, A. Nickel and cobalt recycling from lithium-ion batteries by electrochemical processes. Waste Manag. 2005, 25, 215–220. [Google Scholar] [CrossRef]
- Vieceli, N.; Vonderstein, C.; Swiontekc, T.; Stopić, S.; Dertmann, C.; Sojka, R.; Reinhardt, N.; Ekberg, C.; Friedrich, B.; Petranikova, M. Recycling of Li-ion batteries from industrial processing: Upscaled hydrometallurgical treatment and recovery of high purity manganese by solvent extraction. Solvent Extr. Ion Exch. 2023, 41, 205–220. [Google Scholar] [CrossRef]
- Lupi, C.; Pasquali, M. Electrolytic nickel recovery from lithium-ion batteries. Miner. Eng. 2003, 16, 537–542. [Google Scholar] [CrossRef]
- Kang, J.; Senanayake, G.; Sohn, J.; Shin, S.M. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy 2010, 100, 168–171. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.M.; Shen, X.Y.; Deng, F.L.; Wang, F.C.; Wu, Y.; Liu, H.Z. Synergistic extraction and separation of valuable metals from waste cathodic material of lithium ion batteries using Cyanex272 and PC-88A. Sep. Purif. Technol. 2011, 78, 345–351. [Google Scholar] [CrossRef]
- Xu, L.; Chen, C.; Fu, M.-L. Separation of cobalt and lithium from spent lithium-ion battery leach liquors by ionic liquid extraction using Cyphos IL-101. Hydrometallurgy 2020, 197, 105439. [Google Scholar] [CrossRef]
- Zante, G.; Masmoudi, A.; Barillon, R.; Trébouet, D.; Boltoeva, M. Separation of lithium, cobalt and nickel from spent lithium-ion batteries using TBP and imidazolium-based ionic liquids. J. Ind. Eng. Chem. 2020, 82, 269–277. [Google Scholar] [CrossRef]
- Wang, J.; Huo, X.; Zhang, F.; Wang, L.; Wang, X.; Li, J.; Yang, J. Separation of cobalt and lithium from spent LiCoO2 batteries using zeolite NaA and the resulting ion exchange product for N2/O2 separation. Sep. Purif. Technol. 2023, 313, 123449. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Qu, D. Proof-of-Concept study of ion-exchange method for the recycling of LiFePO4 cathode. Waste Manag. 2023, 157, 1–7. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, M.; Yu, W.; Ma, X.; Lei, S.; Sun, W.; Song, S.; Hu, W. Recovering Fe, Mn and Li from LiMn1-xFexPO4 cathode material of spent lithium-ion battery by gradient precipitation. Sustain. Mater. Technol. 2023, 36, e00625. [Google Scholar] [CrossRef]
- Verma, V.; Joseph, J.R.; Chaudhary, R.; Srinivasan, M. Upcycling spent cathode materials from Li-ion batteries to precursors: Challenges and opportunities. J. Env. Chem. Eng. 2023, 11, 110216. [Google Scholar] [CrossRef]
- Jang, Y.; Hou, C.-H.; Kwon, K.; Kang, J.S.; Chung, E. Selective recovery of lithium and ammonium from spent lithium-ion batteries using intercalation electrodes. Chemosphere 2023, 317, 137865. [Google Scholar] [CrossRef]
- Zou, H.; Gratz, E.; Apelian, D.; Wang, Y. A novel method to recycle mixed cathode materials for lithium ion batteries. Green Chem. 2013, 15, 1183–1191. [Google Scholar] [CrossRef]
- Gu, S.; Zhang, L.; Fu, B.; Ahn, J.W.; Wang, X. Recycling of mixed lithium-ion battery cathode materials with spent lead-acid battery electrolyte with the assistance of thermodynamic simulations. J. Clean. Prod. 2020, 266, 121827. [Google Scholar] [CrossRef]
- Filomeno, G.; Feraco, S. Economic, Technical and environmental aspects of recycling lithium batteries: A literature review. Glob. J.Eng. B 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Drabik, E.; Rizos, V. Prospects for Electric Vehicle Batteries in a Circular Economy. CEPS Research Report No. 2018/05. Available online: https://www.ceps.eu/ceps-publications/prospects-end-life-electric-vehicle-batteries-circular-economy/ (accessed on 5 July 2023).
- Steward, D.M.; Mayyas, A.T.; Mann, M.K. Economics and challenges of Li-ion battery recycling from end-of-life vehicles. Procedia Manuf. 2019, 33, 272–279. [Google Scholar] [CrossRef]
- Pagliaro, M.; Meneguzzo, F. Lithium battery reusing and recycling: A circular economy insight. Heliyon 2019, 5, e01866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedjalkov, A.; Meyer, J.; Köhring, M.; Doering, A.; Angelmahr, M.; Dahle, S.; Sander, A.; Fischer, A.; Schade, W. Toxic gas emissions from damaged lithium ion batteries—Analysis and safety enhancement solution. Batteries 2016, 2, 5. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Sommerville, R.; Shaw-Stewart, J.; Goodship, V.; Rowson, N.; Kendrick, E. A review of physical processes used in the safe recycling of lithium ion batteries. Sustain. Mater. Technol. 2020, 25, e00197. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Wang, H.; Feng, Y.; Xie, W.; Zhu, X. Removal of Organics by Pyrolysis for Enhancing Liberation and Flotation Behavior of Electrode Materials Derived from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 2205–2214. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, W.; Han, J.; Jiao, F.; Qin, W.; Liu, T.; Zhao, C. Pyrolysis and physical separation for the recovery of spent LiFePO4 batteries. Waste Manag. 2019, 89, 83–93. [Google Scholar] [CrossRef]
- Xiao, S.; Ren, G.; Xie, M.; Pan, B.; Fan, Y.; Wang, F.; Xia, X. Recovery of valuable metals from spent lithium-ion batteries by smelting reduction process based on MnO–SiO2–Al2O3 slag system. J. Sustain. Metall. 2017, 3, 703–710. [Google Scholar] [CrossRef]
- Asadi Dalini, E.; Karimi, G.; Zandevakili, S.; Goodarzi, M. A review on environmental, economic and hydrometallurgical processes of recycling spent lithium-ion batteries. Miner. Process. Extr. Metall. Rev. 2021, 42, 451–472. [Google Scholar] [CrossRef]
- Sloop, S.E.; Crandon, L.; Allen, M.; Lerner, M.M.; Zhang, H.; Sirisaksoontorn, W.; Gaines, L.; Kim, J.; Lee, M. Cathode healing methods for recycling of lithium-ion batteries. Sustain. Mater. Technol. 2019, 22, e00113. [Google Scholar] [CrossRef]
- Shin, H.; Zhan, R.; Dhindsa, K.S.; Pan, L.; Han, T. Electrochemical performance of recycled cathode active materials using froth flotation-based separation process. J. Electrochem. Soc. 2020, 167, e00113. [Google Scholar] [CrossRef]
- Wagner-Wenz, R.; van Zuilichem, A.J.; Göllner-Völker, L.; Berberich, K.; Weidenkaff, A.; Schebek, L. Recycling routes of lithium-ion batteries: A critical review of the development status, the process performance, and life-cycle environmental impacts. MRS Energy Sustain. 2023, 10, 1–34. [Google Scholar] [CrossRef]
- Zhang, G.; Du, Z.; He, Y.; Wang, H.; Xie, W.; Zhang, T. A sustainable process for the recovery of anode and cathode materials derived from spent lithium-ion batteries. Sustainability 2019, 11, 2363. [Google Scholar] [CrossRef] [Green Version]
- Pinegar, H.; Smith, Y.R. Recycling of end-of-life lithium ion batteries, Part I: Commercial processes. J. Sustain. Metall. 2019, 5, 402–416. [Google Scholar] [CrossRef]
- Dang, H.; Wang, B.; Chang, Z.; Wu, X.; Feng, J.; Zhou, H.; Li, W.; Sun, C. Recycled lithium from simulated pyrometallurgical slag by chlorination roasting. ACS Sustain. Chem. Eng. 2018, 6, 13160–13167. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J. Hazard. Mater. 2010, 176, 288–293. [Google Scholar] [CrossRef]
- Kamińska, E.S.; Pawlak, P. The ecobalance analysis of the Retriev recycling technology of used car lithium-ion batteries. Mater. Econ. Logist. J. 2020, LXXII, 35–44. [Google Scholar] [CrossRef]


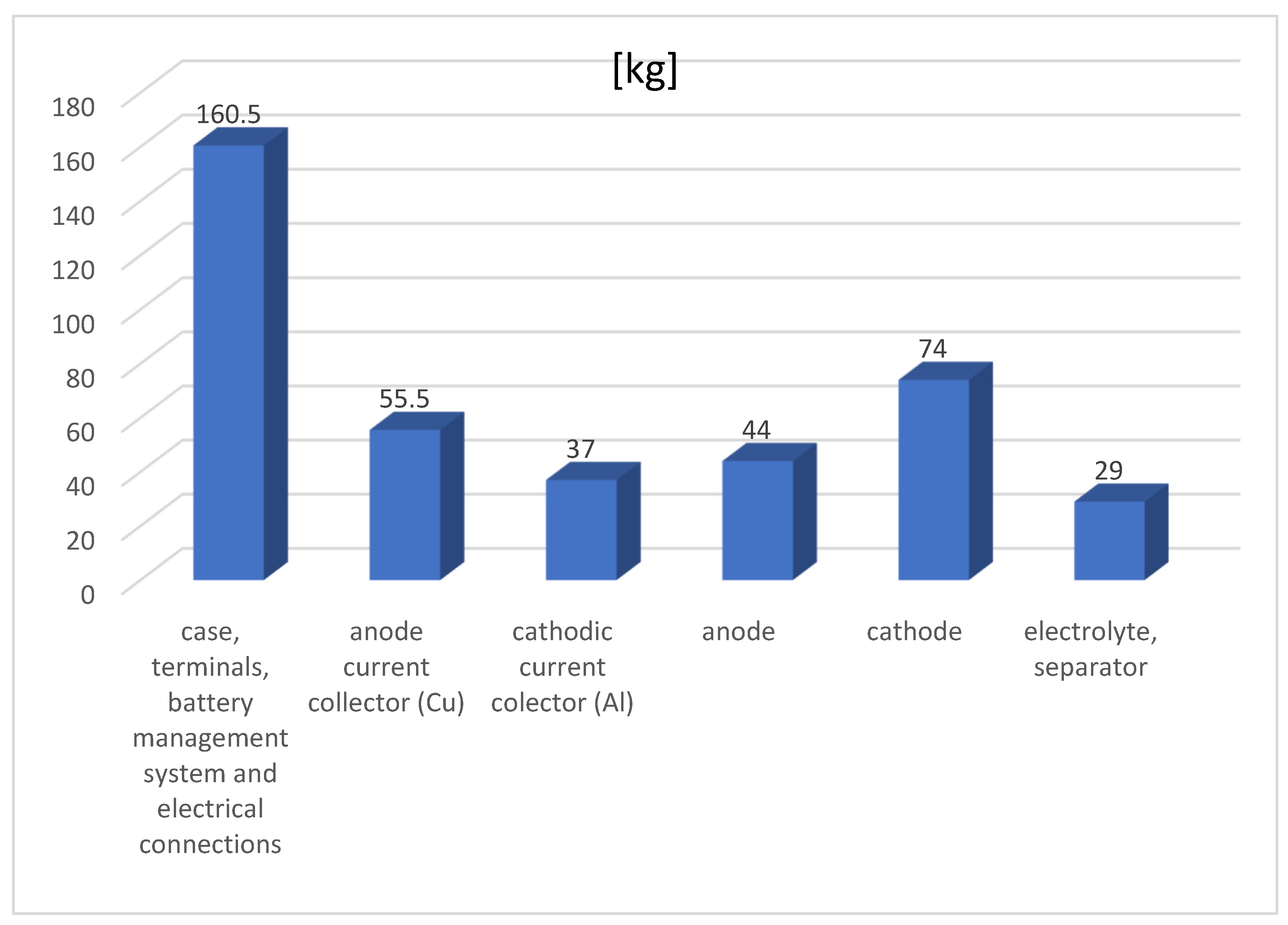

| Type of LIB | Estimated Cost of Cathode, USD/kg |
|---|---|
| LCO | 59.6 |
| NMC111 | 31.0 |
| NMC811 | 28.8 |
| NCA | 31.2 |
| LMO | 16.0 |
| LFP | 12.0 |
| Component | Mass Percentage, % |
|---|---|
| Cobalt (Co) | 5–20 |
| Nickel (Ni) | 5–10 |
| Lithium (Li) | 5–7 |
| Other metals: copper (Cu), aluminum (Al), iron (Fe), etc. | 5–10 |
| Organic compounds | ~15 |
| Plastic materials | ~7 |
| Pollution to Air | ||
|---|---|---|
| Method | Type of Process | Impurities |
| pretreatment | pyrolysis | CO, HF, C2H4, C2H5OH, CH3CHO, C6H6 |
| discharging with NaCl solution | flammable H2 and toxic Cl2 | |
| pyrometallurgy | roasting, smelting | CO, HF, dioxins, furans |
| hydrometallurgy | inorganic acid leaching | Cl2, SO3, and NOx |
| sodium metabisulphite leaching | emissions of sulphurous gases | |
| Wastewater | ||
| pretreatment | discharging in brine | composition of the brine |
| direct physical | flotation | waste effluent (there is no information on the characteristics of post-flotation wastewater) |
| wet crushing | HF, large amounts of CaF2 | |
| hydrometallurgy | acid leaching, Co, Ni, and Li recovery | acidic wastewater containing unrecovered cations (Li+, Ni2, Al3+, Fe3+), brine can contain electrolyte, graphite, and solvents |
| Solid Waste | ||
| pretreatment | pyrolysis | pyrolytic tar consists of aromatic long-chain alkenes and light alcohols |
| pyrometallurgy | smelting | slag (consists mainly of silica, calcium oxide, aluminum oxide, and signifcant amounts of lithium) fine dust |
| hydrometallurgy | filtration and washing with distilled water | insoluble impurities, residual graphite |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giza, K.; Pospiech, B.; Gęga, J. Future Technologies for Recycling Spent Lithium-Ion Batteries (LIBs) from Electric Vehicles—Overview of Latest Trends and Challenges. Energies 2023, 16, 5777. https://doi.org/10.3390/en16155777
Giza K, Pospiech B, Gęga J. Future Technologies for Recycling Spent Lithium-Ion Batteries (LIBs) from Electric Vehicles—Overview of Latest Trends and Challenges. Energies. 2023; 16(15):5777. https://doi.org/10.3390/en16155777
Chicago/Turabian StyleGiza, Krystyna, Beata Pospiech, and Jerzy Gęga. 2023. "Future Technologies for Recycling Spent Lithium-Ion Batteries (LIBs) from Electric Vehicles—Overview of Latest Trends and Challenges" Energies 16, no. 15: 5777. https://doi.org/10.3390/en16155777






