Abstract
2,3-butanediol (2,3-BDO) is an important biomass-derived platform chemical with various applications. Currently, the biological conversion of renewable carbon sources with bacteria or yeasts is a sustainable way to produce 2,3-BDO. Various carbon sources including glucose, glycerol, molasses and lignocellulose hydrolysate have been used for 2,3-BDO production, and the 2,3-BDO concentration in the fermentation broth can be higher than 150 g/L by optimizing the operating parameters with fed-batch operations. Various derivatives can be produced from 2,3-BDO, including isobutyraldehyde, 1,3-butadiene, methyl ethyl ketone (MEK), diacetyl, etc.; among these, there is a large market demand for MEK and 1,3-butadiene each year. Some of the derivatives can be used as fuel additives or to produce biofuels. Generally, there are three ways to produce hydrocarbon fuels from 2,3-BDO, which are via the steps of dehydration, carbon chain extension, and hydrogenation (or hydrodeoxygenation), with MEK or 1,3-butadiene as the intermediates. C8–C16 alkanes can be produced by these routes, which can be potentially used as bio-jet fuels. This review article focuses on the microbial production of 2,3-BDO, the biomass feedstock used for fermentation, the recovery of 2,3-BDO from the fermentation broth as well as the downstream derivative products and their potential application in bio-jet fuel production. It was concluded that 2,3-BDO is a promising biomass-derived product, but its production and application in the biofuel field is still facing the problem of high production cost. Future work is recommended to develop more efficient processes to increase the 2,3-BDO yield and more advanced technologies to produce hydrocarbon fuels.
1. Introduction
Due to the depletion of fossil resources, ever-growing energy demands, and the growing concern over carbon dioxide emissions, attempts to convert petroleum-based chemical manufacturing into carbon-neutral production from renewable resources are now in the limelight [1,2,3]. Biorefining with biomass as feedstocks has been actively pursued worldwide as a sustainable strategy not only to produce fuels but also to obtain various valuable chemicals [4] which. Most chemical compounds, which previously could only be produced by a chemical approach, can now be generated by biological processes from renewable resources by using enzymes or organisms.
2,3-butanediol (2,3-BDO) is a representative C4 platform chemical which can be produced from various biomass resources by biological routes. 2,3-BDO is also known as dimethylene glycol, 2,3-butylene glycol, and butane-2,3-diol (IUPAC name). At room temperature, 2,3-BDO is an odorless, colorless, transparent liquid. Three stereoisomers exist: a D-(−)- isomer and an L-(+)-isomer, which are optically active, and meso-2,3-BDO. The boiling points of 2,3-BDO range from 177 °C to 182 °C, being slightly different among the three stereoisomers [5].
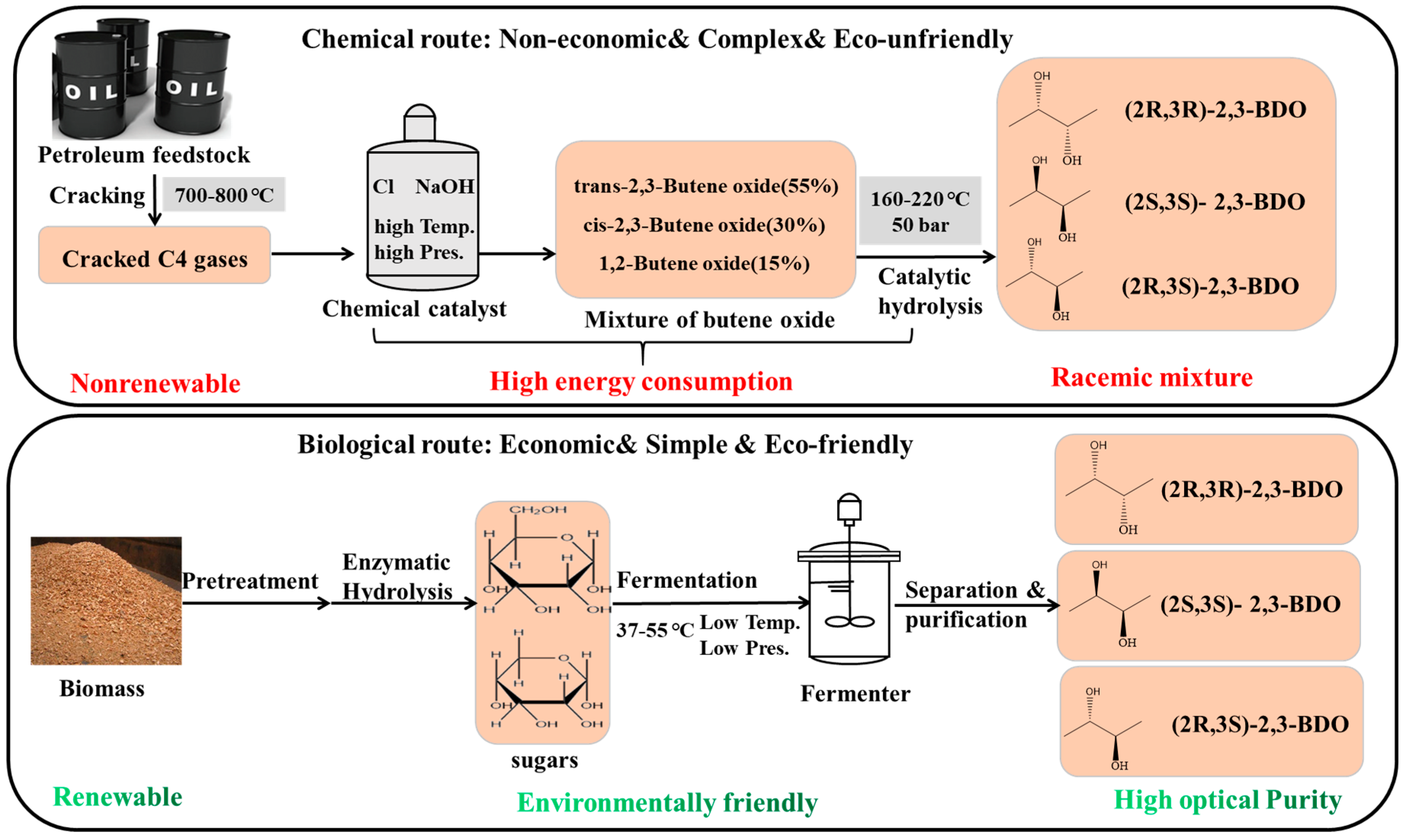
Currently, 2,3-BDO is manufactured in very limited quantities via petrochemical routes (Figure 1). The petrochemical route for BDO synthesis is primarily the catalytic hydrolysis of butene oxide at a high temperature (160–220 °C) under high pressure (50 bar) and with a high energy consumption [6]. Furthermore, upon chemical production, the selective production of the pure stereoisomer is difficult to control due to the complicated reactions and extremely expensive separation and purification process. Optically active 2,3-BDO with two chiral groups provides particular applications in the asymmetric synthesis. Recently, the biological production of 2,3-BDO has attracted more attention because it has wide applications in the manufacture of printing inks, perfumes, fumigants, moisteners, foods, and pharmaceuticals [7]. 2,3-BDO had a high market price of 1600 USD/ton with the production of 74 kt/year in 2018 [8]. The market for 2,3-BDO is predicted to reach 300 million dollars by the end of 2030 [9]. Thus, biorefining of biomass to produce 2,3-BDO is very attractive and sustainable, since using renewable biomass is a promising route for developing a carbon-neutral economy and a more sustainable future [10]. Therefore, this review summarizes the recent research progress on 2,3-BDO production, focusing on the microorganisms, feedstocks, separation and purification processes as well as its downstream derivative products and their potential use in biofuel production. To achieve a comprehensive review on the progress of 2,3-BDO production and its application, research and review articles were searched and selected using Web of Science TM as the main bibliographic database covering the years from 2009 to 2023 using the key words “2,3-butanediol”, “2,3-BD”, “2,3-BDO”, or “BDO”, from which 1945 articles were retrieved. Then, by considering the titles, relevance, and subject areas, 156 articles were selected for summarization and analysis.
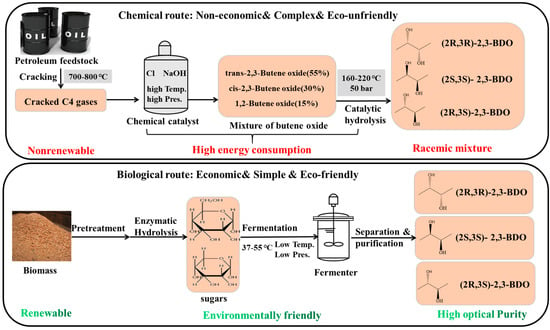
Figure 1.
Chemical and biological routes of 2,3-butanediol production.
2. Metabolic Pathways for Microbial Production of 2,3-BDO
The chemical production of 2,3-BDO has some drawbacks, and thus the biological process using microorganisms has been extensively investigated to obtain a high yield under mild conditions. The microbial production of 2,3-BDO has been investigated for over 100 years from the time Harden et al. first reported 2,3-BDO production by using Klebsiella pneumoniae in 1906 [11]. After that, Fulmer et al. reported the first industrial-scale production in 1933. Thereafter, numerous studies have been conducted to produce 2,3-BDO from various feedstock such as glucose, fructose, mannose, and galactose by several types of bacteria, yeasts or even algae [12]. However, the chemical production of 2,3-BDO using petroleum resources has gained more attention due to its easy availability and low manufacturing costs. Nonetheless, the biological production of 2,3-BDO from biomass resources has obvious environmental benefits and sustainability. Rehman et al. analyzed the footprint of bio-based 2,3-BDO production and found that CO2 emissions were 36% lower than that of petroleum-based processes [13]. Thus, instead of a conventional fossil-based 2,3-BDO product, large industries have tried to produce 2,3-BDO by utilizing sugar or a direct biomass, which is a future solution to the depletion of fossil fuels. As an example, GS Galtex, a South Korean company, produces 300 tons of 2,3-BDO by the biorefinery of sugarcane and cassava [14]. Many research studies are still underway to improve the efficiency of 2,3-BDO production as well as to widen the carbon sources.
2.1. Biological Function of 2,3-BDO
Biosynthesis of 2,3-BDO is considered ea mixed-acid fermentation pathway, which is accompanied by the formation of various end-products depending on the fermentation strategies and microorganisms [15]. Until now, the physiological function of the metabolic regulation of 2,3-BDO biosynthesis has not been completely elucidated yet. However, several physiological significances have been elucidated, including intracellular acidification prevention, energy and carbon reservation, and regulation of the NAD+/NADH ratio [8,16,17]. Since the production of 2,3-BDO in bacteria is accompanied by the formation of acids as co-products [18], the biosynthesis of 2,3-BDO would be triggered to prevent this acid formation as the pH decreases [19]. It has been proven that an extra supplementation of acid promotes the metabolic pathway of 2,3-BDO, suggesting that 2,3-BDO is a neutral metabolite to counteract too high intracellular acidification [20]. Moreover, many investigations have been conducted by using acetic acid, which is already known to be involved in the 2,3-BDO metabolic pathway [21] as acid supplementation to analyze the influence on 2,3-BDO biosynthesis [20]. The glycolysis during 2,3-BDO production is accompanied by the oxidation of NADH, which can contribute to the maintenance of the NAD+/NADH ratio in the cell [22]. Finally, 2,3-BDO can be reutilized when carbon and energy sources are depleted; hence, the metabolism of 2,3-BDO can be treated as a strategy for carbon and energy storage [23].
2.2. Metabolic Pathway of 2,3-BDO Biosynthesis
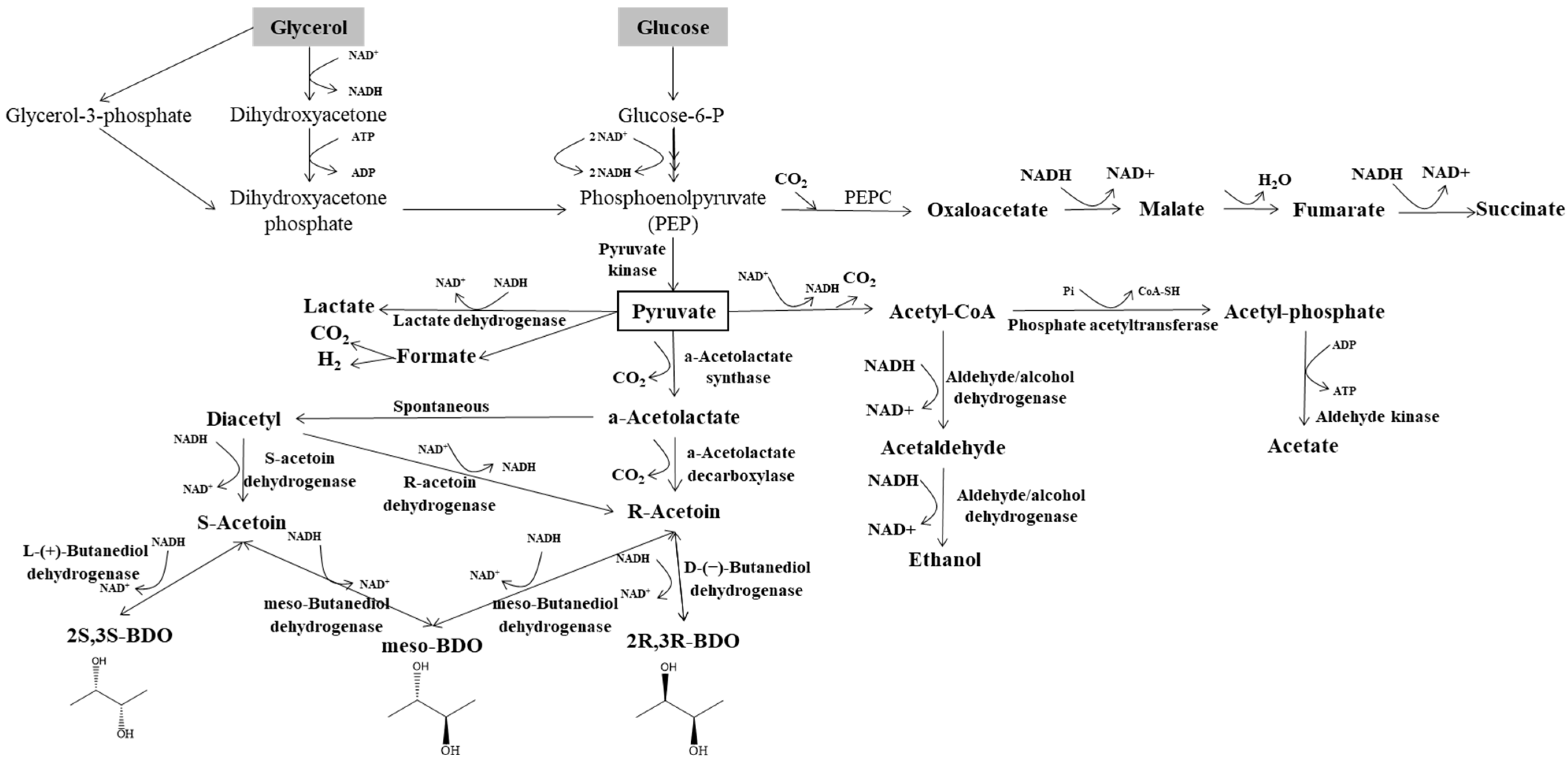
In bacterial metabolism, assorted monosaccharides (glucose, hexoses, pentoses) need to be converted to pyruvate in the first step. From glucose, pyruvate can be formed by the glycolysis pathway, while pentose needs an extra step involving pentose phosphate. The metabolic pathway of 2,3-BDO biosynthesis from glucose and glycerol is shown in Figure 2. Three key enzymes are required for the 2,3-BDO production from pyruvate, namely α-acetolactate synthase (ALS), α-acetolactate decarboxylase (α-ALD), and 2,3-BDO dehydrogenase or acetoin reductase (BDH, AR) [10]. Pyruvate is the key intermediate, which undergoes reduction or oxidation to produce several organic acids (lactate, formate, acetate, succinate), ethanol, and acetoin, depending on the pH value and oxygen content. To enhance the accumulation of 2,3-BDO and eliminate by-product formation during fermentation, genetic engineering and optimization of the fermentation parameters have been investigated [24]. Most microorganisms produce 2,3-BDO by three consecutive metabolic conversion steps: from pyruvate to α-acetolactate, from α-acetolactate to acetoin, and from acetoin to 2,3-BDO. First, pyruvate can be converted either into lactate, by employing L-/D-lactate dehydrogenase (LDH) and NADH, or into α-acetolactate via decarboxylation by ALS. Furthermore, α-acetolactate can spontaneously transform into diacetyl by decarboxylation with the existence of oxygen. The diacetyl can subsequently be converted into acetoin by employing the reducing power of NADH and diacetyl reductase (DAR). Otherwise, α-acetolactate can be converted into acetoin by α-ALD without the presence of oxygen. Finally, acetoin can be converted into 2,3-BDO by BDH. It is noteworthy that, as an intermediate compound prior to the formation of 2,3-BDO, acetoin would be obtained by the reversible metabolic conversion from 2,3-BDO. It has been reported that low pH is an effective inducer for all the vital enzymes, α-ALD, ALS, and BDH, resulting in the enhanced biosynthesis of 2,3-BDO [21,25].
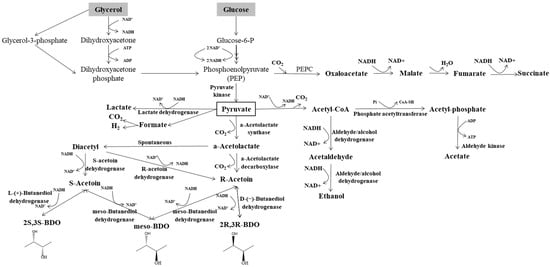
Figure 2.
Metabolism of 2,3-butanediol biosynthesis from glycerol and glucose (adapted from Maina et al. [7]).
2.3. Mechanisms of Biosynthesis of the 2,3-BDO Stereoisomer
Butanediol dehydrogenase (BDH) is a tetrameric enzyme that needs NADH as a coenzyme. Three stereoisomers of 2,3-BDO can be formed from two acetoin isomers due to the activity of BDHs with variable stereospecificities, or by a cyclic pathway. The exact mechanism of the stereoisomer formation of 2,3-BDO has not been clearly understood. Over the past few years, several investigations have been conducted to understand the formation of 2,3-BDO stereoisomers; in particular, various mechanisms of stereoisomers have been elaborated in K. pneumonia. In 1960, the first series of preliminary experiments were conducted based on the assumption that acetoin racemase, L-(+)-BDH and D-(−)-BDH were involved in the production of stereoisomers of 2,3-BDO. A possible hypothesis mechanism was deduced by Taylor and Juni, but the existence of racemase was not proven at that time [26]. Then, Voloch et al. developed a method that used liquid chromatography to determine the racemic acetoin, meso-2,3-BDO, (2R,3R)-2,3-BD, and (2S,3S)-2,3-BD and proposed a model that assumed that D-(−)-BDH, L-(+)-BDH, and acetoin racemase had an effect on the formation of stereoisomers. It was proved that only R-acetoin was produced from pyruvate via α-acetolactate in K. pneumoniae; thus, the formation of (2S,3S)-2,3-BDO was dependent on acetoin racemase. Only a slight amount of acetoin racemase was detected [27]. After that, Ui et al. established a method to measure all the types of stereoisomers of 2,3-BDO and acetoin [28]. Based on this finding, Ui et al. proposed a new model involving NADPH-link diacetyl reductase (S-acetoin forming) and D-(−)-BDH for the formation mechanism of 2,3-BDO isomers in P. polymyxa. Blue Sepharose CL-6B was used to separate and identify the two enzymes with standard pure isomers of 2,3-BDO and acetoin, respectively. In addition, acetoin and butanediol racemases were not detected. Therefore, the author concluded that R-isomers of acetoin were formed in the absence of NADH, while the racemate form of acetoin was formed in the presence of NADH. The D-isomer of 2,3-BDO was predominantly produced, together with a small amount of meso-form in P. polymyxa [29]. In the meantime, Ui et al. also proposed a model in K. pneumoniae considering the existing of three BDHs: meso-BDH, L-(+)-BDH, and D-(−)-BDH. The three types of BDH were separated individually, and their stereo specificities were verified by the utilization of pure isomers, including (2R,3R)-2,3-BDO, (2S,3S)-2,3-BDO, meso-2,3-BDO, R-acetoin, and S-acetoin. Also, the acetoin or 2,3-BDO racemase was not detected. Therefore, the author concluded that the R-acetoin was formed in the absence of NADH, while S-acetoin was formed in the presence of NADH [30]. In addition to that, Ui et al. further cloned and expressed the gene encoding D-(−)-BDH and L-(+)-BDH in E. coli to obtain insights into the mechanism of stereoisomer formation. The S form of acetoin can be converted to (2S,3S)-2,3-BDO by using diacetyl as a substrate [31]. Also, Ui et al. encoded ALS, ALD, and a single meso-hydrogenase into E. coli to produce pure meso-2,3-BDO with no L-form. These investigations revealed that the formation of 2,3-BDO isomers was primarily due to the different hydrogenases [32]. Later, Yan et al. reported, for the first time, the enantioselective biosynthesis of a pure (2R,3R)-2,3-BDO isomer in E. coli by utilizing the secondary alcohol dehydrogenase, which was characterized as specifically forming the R-configuration [33]. During aerobic fermentation in microbes, R-acetoin can be spontaneously generated from diacetyl, resulting in the formation of (2R,3R)-2,3-BDO and meso-2,3-BDO. In reverse, (2R,3R)-2,3-BDO could be converted to R-acetoin by employing the glycerol dehydrogenase (GDH) enzymes and NAD+. K. pneumoniae was found to produce meso-2,3-BDO and (2S,3S)-2,3-BDO by using glucose as a carbon source, whereas (2R,3R)-2,3-BDO isomers were primarily obtained by using glycerol as a carbon resource. Hence, the enzymes involved in glycerol metabolism were more likely related to the formation of (2R,3R)-2,3-BDO. Through gene-encoding methods, Chen et al. verified that K. pneumoniae dhaD was crucial for the (2R,3R)-2,3-BDO synthesis from R-acetoin by glycerol dehydrogenase with a stereospecificity [34]. Also, this enzyme can convert S-acetoin to meso-2,3-BDO. Until now, the acetoin racemase has never been proved experimentally, but the model proposed by Ui et al. is still considered a probable mechanism for the formation of 2,3-BDO stereoisomers.
2.4. Microorganisms to Produce 2,3-BDO
In nature, there are numerous microbial species which can produce 2,3-BDO from the pyruvate metabolic pathway. Besides bacteria, several yeasts and even algae have been reported to produce 2,3-BDO. However, considering the industrialization of 2,3-BDO production, bacteria appear to be the only alternative at present because of the very low productivity of other species. The 2,3-BDO yield and conversion efficiencies of sugars by some representative bacteria are summarized in Table 1. The representative species are Klebsiella pneumoniae, Klebsiella oxytoca, Enterobacter aerogenes, Paenibacillus polymyxa and Serratia marcescens, which are considered promising producers for scaled-up 2,3-BDO production. Species of Klebsiella were firstly investigated for 2,3-BDO production due to their ability to be cultivated rapidly in a simple medium on various carbon resources; in particular, K. pneumoniae can use almost all carbon resources, such as lignocellulosic biomass, agriculture waste, and glycerol. However, ethanol, lactate, and acetate are also produced as by-products, which increases the costs of downstream processing. Therefore, many research studies have been conducted on how to modify the metabolic pathways. When genes related to the formation of co-products, such as lactic acid, ethanol, and acetic acid were knocked out from K. pneumoniae, a higher titer of 2,3-BDO in batch fermentation could be achieved [35]. Guo et al. constructed K. pneumoniae mutants with the deletion of lactate dehydrogenase (LDH) and acetaldehyde dehydrogenase (ADH). Knockout of the adhE and pta genes could improve 2,3-BDO production, and deletion of ldhA could accelerate cell growth and shorten the cultivation time [36]. By overexpressing als, aldC, and ar/bdh genes individually and in combination in K. pneumoniae, BDO production was also improved. A high yield 2,3-BDO of 0.38 g/g was obtained by the recombinant strain [37].
Similarly, ethanol was one of the major by-products during 2,3-BDO production when K. Oxytoca ME-UD-3 was used. A homologous recombination technique was employed to inactivate ADH, which exhibited an obvious augmentation of 2,3-BDO production with a yield of 0.48 g/g, and no ethanol was detected [38]. After that, Cho et al. reported an enhancement of 2,3-BDO production by K. oxytoca M1 through the overexpression of budC. 2,3-BDO production was remarkably increased to 142.5 g/L, while the production of acetoin decreased. As a result of overexpression of budC, acetoin reductase presented an evidently high catalytic activity [39]. Jantama et al. eliminated alcohol dehydrogenase E, acetate kinase A-phosphotransacetylase, and lactate dehydrogenase A in K. oxytoca KMS005. The production of 117.4 g/L of 2,3-BDO was obtained, with a yield of 0.49 g/g and productivity of 1.20 g/L/h. Only trace amounts of acetate and ethanol were detected as by-products [40].
E. aerogenes is a competitive candidate for 2,3-BDO production, but its by-products, such as lactic acid, impede the target production efficiency. Kim et al. eliminated the lactate dehydrogenase gene to improve 2,3-BDO production. The ldhA deletion mutant resulted in a 97% decrease in lactate production (0.34 g/L), while the productivity and yield of 2,3-BDO increased by 16.3% and 11.1%, respectively.
Engineered Saccharomyces cerevisiae is a potential host strain to produce 2,3-BDO, as substantial amounts of pyruvate could be shunted to 2,3-BDO production rather than to ethanol synthesis. By optimizing the expression levels of Pdc in S. cerevisiae, the maximum 2,3-BDO titer was 154.3 g/L which was the highest among the reported microbial production studies [41].
Bacillus amyloliquefaciens B10-127 was also considered a possible candidate for the scaled-up production of 2,3-BDO, but its industrialization was impeded by the high yield of acetoin, lactate, and succinate as by-products. During 2,3-BDO production, BDH catalyzes the conversion from acetoin to 2,3-BDO with the concomitant oxidation of NADH to NAD+. Yang et al. overexpressed bdh and gapA and showed that this could effectively enhance 2,3-BDO production and inhibit the formation of by-products [42].
Also, S. marcescens can be used to produce 2,3-BDO; however, few studies on this are available. Bai et al. identified the slaC gene for meso-2,3-BD and (2S,3S)-2,3-BDO production. The bdhA gene from Bacillus subtilis 168 was introduced into S. marcescens MG1 to replace the slaC gene, leading to (2R,3R)-2,3-BDO accumulation. The excess (2R,3R)-2,3-BDO dehydrogenase expression could enhance the production of (2R,3R)-2,3-BDO to 89.81 g/L, with only 2.11 g/L meso-2,3-BDO formation [43].
Moreover, pure meso-2,3-BDO or (2R,3R)-2,3-BDO can be synthesized by Bacillus licheniformis MW3 (ΔbudC). Ge et al. developed a process that included the screening, analysis, mutation, and evaluation of chiral 2,3-BDO production [44]. Li et al. attempted to synthesize pure (2R,3R)-2,3-BDO by E. cloacae via deletion of the bdh, ldh, and frdA genes to block by-production formation. The engineered strain produced 152 g/L (2R,3R)-2,3-BDO with 3.5 g/L/h productivity [45]. Obliteration of the dudA gene in P. polymyxa ZJ-9 resulted in the production of (2R,3R)-2,3-BDO at 25.88 g/L with a 99% purity in fed-batch cultivation [46]. Then, the enhancement of (2R,3R)-2,3-BDO production to 111 g/L by P. polymyxa DSM 365 was achieved [47]. Thus, P. polymyxa is an efficient producer for (2R,3R)-2,3-BDO production.

Table 1.
Comparison of 2,3-BDO production by some microorganisms.
Table 1.
Comparison of 2,3-BDO production by some microorganisms.
| Microorganism | Biosynthesis Genes | Isomer | Carbon Source | Fermentation Mode | Temp. °C | pH | Titer g/L | BDO Yield g/g | Productivity g/L/h | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| K. pneumoniae | deletion of dhA, adhE, and pta | meso-, L-(+)- | glucose | fed-batch | 35 | 6.5 | 116 | 0.49 | - | [36] |
| K. pneumoniae KCTC12133BP | deletion of ldhA, adhE, and pta-ackA | mixture | glucose | fed-batch | 37 | 6.8 | 91 | 0.45 | 1.62 | [35] |
| K. pneumoniae SDM | - | mixture | glucose | fed-batch | 37 | 6 | 150 | 4.21 | - | [48] |
| K. oxytoca M1 | overexpression of budC | mixture | glucose | fed-batch | 30 | 6 | 142.5 | 1.47 | 0.42 | [39] |
| K. oxytoca ME-UD-3 | deletion of aldA | mixture | glucose | fed-batch | 37 | 6.5 | 130 | 1.63 | 0.48 | [38] |
| K. oxytoca KNIS005 | deletion of dhE, ackA-pta, ldhA | mixture | glucose | fed-batch | 37 | 6 | 117 | 1.2 | 0.49 | [40] |
| Bacillus amyloliquefaciens | overexpression of Bdh, gapA | mixture | glucose | fed-batch | 37 | 6.5 | 132.9 | 2.95 | - | [42] |
| Bacillus licheniformis MW3 | deletion of Gdh | D- (99%) | glucose | fed-batch | 50 | 7 | 123.7 | 2.95 | 0.508 | [44] |
| Bacillus licheniformis | deletion of budC | meso (99%) | glucose | fed-batch | 50 | 7 | 90.1 | 2.81 | 0.49 | [44] |
| Saccharomyces cerevisiae | CtPDC1 | mixture | glucose | fed-batch | 30 | 5.5 | 154.3 | 1.98 | 0.404 | [41] |
| E. aerogenes KCTC 2190 | deletion of ldhA | mixture | glucose | fed-batch and batch | 37 | 6.0 | 118.05 | - | - | [49] |
| E. cloacae SDM | deletion of ldh, frdA, and adh | D (97.5%) | glucose xylose | fed-batch | 30 | 7.4 | 152 | 3.5 | 97.7% | [45] |
| Paenibacillus polymyxa | - | D (98%) | glucose | fed-batch | 37 | 6 | 111 | - | - | [47] |
| S. marcescens | bdhA replacement of slaC | D | sucrose | fed-batch | 30 | 6 | 89.81 | 1.91 | 0.35 | [43] |
3. Feedstocks for 2,3-BDO Production
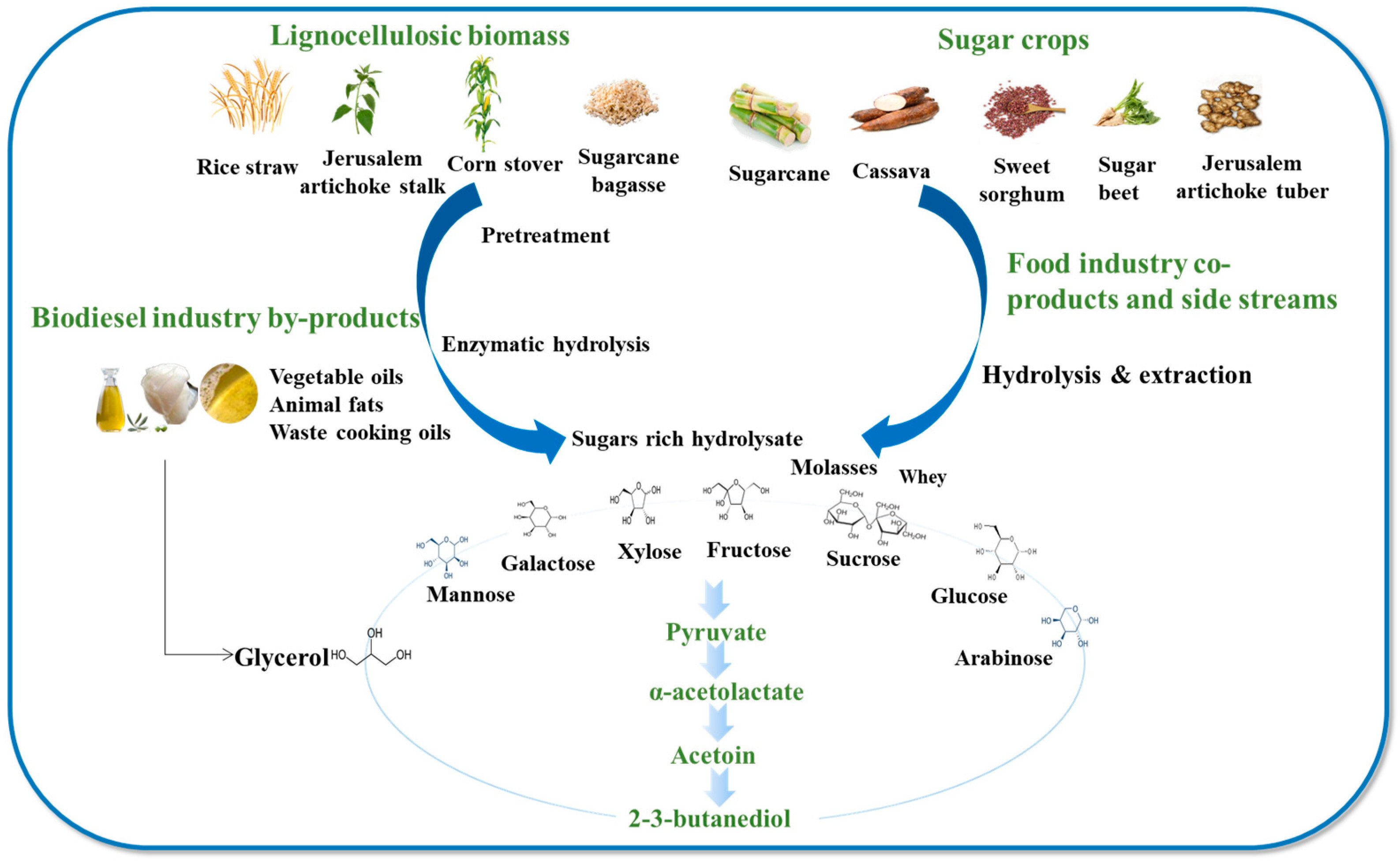
Although massive progress in metabolic engineering has been made to increase the efficiency of 2,3-BDO production, its microbial production from refined sugars has not been economically feasible [50]. The cost of commercial refined sugars exceeded 30% of the total cost [51]. Cheaply available and abundant substrates such as hydrolysates of starch, corn cob, Jerusalem artichoke, whey permeate, molasses, and crude glycerol derived from the biodiesel industry have attracted great interest for 2,3-BDO production (Figure 3) [52]. Generally, these feedstocks can be classified as lignocellulose biomass, food industry side streams, and by-products from the biofuel industry. Normally, the production of 2,3-BDO from lignocellulosic biomass includes three essential processes: (1) pretreatment of the biomass to break up interactions between the polymeric components and cellulose to make them more accessible for the upcoming step, commonly known as enzymatic hydrolysis or saccharification; (2) employing hydrolases like cellulases, xylanases, and other carbohydrases to hydrolyze the pre-treated biomass; and (3) fermentation of monosaccharides to produce 2,3-BDO. It is generally agreed that the effectiveness of pretreatment and enzymatic hydrolysis are the decisive factors determining the efficiency of 2,3-BDO production.
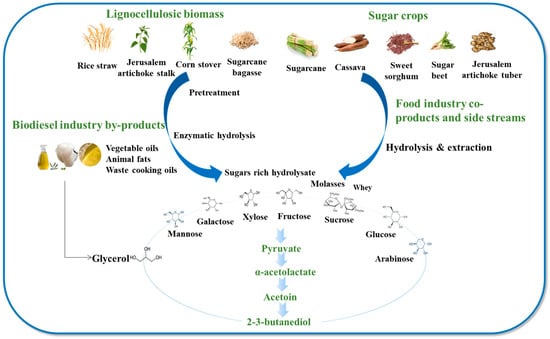
Figure 3.
Various carbon resources potentially used for 2,3-butanediol production.
3.1. Lignocellulosic Biomass
The recalcitrant structure of lignocellulosic biomass impedes hydrolases; in particular, affecting enzyme accessibility and activity and resulting in a low yield of sugars, which ultimately affects the production of 2,3-BDO. Cellulose consists of almost 35–55% lignocellulosic biomass, which is a linear polymer of ß-D-glucopyranose molecules held together by ß-(1,4) glycosidic linkage. The tight packing of the polymeric chain and the crystalline structure of native cellulose greatly hinder the enzyme digestion process. Different from the linear stack of cellulose, hemicelluloses are a group of branched polymers with short lateral chains which comprises 15–35% biomass. Their amorphous structure makes them much easier to degrade. Xylose, arabinose, glucose, galactose, and mannose can be obtained by hydrolysis of hemicelluloses. Lignin is a heterogeneous polymer of phenylpropanoid units and confers mechanical strength to plant cell walls, which makes lignocellulose water-resistant and recalcitrant to degradation [53]. Delignification can eliminate the physical blocking of lignin to cellulose, increase the cell wall porosity, and thus increase the accessibility of cellulose to enzymatic hydrolysis [54].
3.2. Pretreatment of Lignocellulosic Biomass
Since almost all kinds of biomass are recalcitrant to enzymic hydrolysis, a pretreatment process needs to be used to transform the biomass from its original form to a less compact form, thereby increasing the enzyme hydrolysis efficiency. Therefore, the aims of pretreatment are to break the hydrogen bonds in the cellulose crystalline structure, increase the surface accessibility of cellulose, and cleave the lignin and hemicellulose cross-links. Physical, chemical, physiochemical, biological and hybrid strategies have been explored to strengthen the pretreatment effect [55].
Milling and grinding reduce the particle size of biomass by mechanical force so that the surface/volume ratio increases. After milling or grinding, the size of biomass usually can be reduced to 0.2–2 mm [56]. Sitotaw et al. verified that the balling technique coupled with chemical and physicochemical pretreatments well-facilitated lignin removal, reduced crystallinity, and increased the specific surface area, which ultimately improved the hydrolyzed sugar yield for biofuel production [57].
Chemical pretreatment utilizes acids, alkali or organic solvents to remove lignin or to de-crystallize cellulose. By employing acids, lignin and hemicelluloses can be solubilized, and cellulose becomes more accessible for the subsequent hydrolysis process. Alkaline pretreatment with sodium, potassium, calcium or ammonium hydroxides results in the degradation of ester and glycosidic side chains, causing partial de-crystallization of cellulose and modification of the lignin structure [55]. Generally, a substantial amount of the hemicellulose fraction can be removed by acid pretreatment, while lignin can be dissolved by alkali. By using organosolv pretreatment, most of the cellulose fraction can be retained in the solid substrate for enzymatic hydrolysis, and a high purity of lignin and hemicellulosic sugars can be coproduced. Many organic solvents including ethanol, methanol, acetone, ethylene glycol, triethylene glycol, and tetrahydrofurfuryl alcohol were investigated and used in biomass pretreatment. Some of the ethanol organosolv processes have been developed toward industrial application [58,59].
Physicochemical pretreatment of lignocellulosic biomass outperforms physical, chemical, and biological pretreatments in terms of the reduction efficiency of cellulose crystallinity and lignin removal [60]. To maximize the utilization efficiency of lignocellulosic biomass, limit the use of harsh chemicals, and minimize the generation of by-products, various physicochemical pretreatments, including hydrothermal [61], ultrasound, microwave [62], supercritical carbon dioxide [63], radiation, plasma-based pretreatment and pulsed-electric field pretreatments, have been attempted [64]. In addition, multi-step and combined physicochemical processes have also been found to effectively fractionate the lignocellulose biomass polymeric components.
By employing fungi that produce lignin-degrading enzymes, biological pretreatment is also employed to increase cellulose digestibility. Enzymes in fungi can digest lignin and hemicellulose selectively without any significant degradation of cellulose. White, soft rot, brown, and stain fungi can be used for biological pretreatments in which lignin-degrading enzymes such as peroxidase and laccases are produced [65]. However, the predominant drawback of fungal pretreatment is that it is time consuming, even though the conditions are milder and the cost is lower. Shirkavand et al. reviewed the combinations of fungal pretreatment with other pretreatment methods, and indicated that these combinations resulted in a reduction in the operation time for the whole process. Therefore, future research could focus on the time reduction of the biological pretreatment process [66].
Remarkably, ionic liquid (IL) and ionic liquid-based solvent systems are emerging as a green and potential technology for biomass pretreatment to improve the subsequent enzymatic hydrolysis because of their non-toxic nature and adjustable dissolving ability [67]. The IL 1-ethyl-3-methylimidazolium acetate (EMIMAc) was found to remove lignin from wood flour and straw, leading to an increase in enzymatic efficiency [68]. Haykir et al. conducted a pretreatment with EMIMAc for the conversion of cotton stalks to glucose, and the efficiency was increased to 82–85% due to the capability of disrupting the crystalline structure of cellulose [69]. Parushi et al. reported a combination pretreatment of alkali and the ionic liquid 1-butyl-3-methyl imidazolium chloride for sunflower stalks, resulting in easier enzymatic saccharification and an increase in the sugar yield (163.42 mg/g biomass) [70].
3.3. Enzymatic Hydrolysis of Pretreated Biomass
In general, the formation of inhibitors during intensive pretreatment of 2,3-BDO affects the successive enzymatic hydrolysis and the upcoming fermentation process adversely. [71]. Enzymatic hydrolysis is one of key steps confining the efficiency of biomass-to-2,3-BDO conversion. Two major issues that need to be considered are (1) the screening for highly efficient cellulases and carbohydrases that have better access and cellulolytic hydrolysis kinetically; and (2) improving the stability of cellulases to sustain high catalytic activity toward variations in the biomass structure and harsh enzymatic hydrolysis conditions. Cellulases, hemicellulases, ligninase, and auxiliary enzymes can be applied to the hydrolysis of lignocellulose [72]. The factors influencing enzymatic hydrolysis efficiency, including lignin content, hemicellulose content, cellulose crystallinity, accessibility, pore size of particles, and degree of polymerization of cellulose have been studied to enhance the hydrolysis performance [73]. Many research studies on improving hydrolysis efficiency have been reported [74].
Two process configurations have been presented for the conversion of pretreated biomass to 2,3-BDO: separate hydrolysis and fermentation (SHF) and simultaneous saccharification fermentation (SSF). Wong et al. reported the BDO production from a variety of agricultural waste biomass by the SHF process [75]. The distinct advantage of SHF is the ability to operate the enzymatic hydrolysis and fermentation at optimal conditions separately; however, long processing time, need for additional reactor vessels, and the hazard of contaminants risk the economic sustainability of the process [76]. The advantage of SSF is that the enzymatic hydrolysis and fermentation take place in the same reactor, alleviating end-product inhibition, thus improving hydrolysis efficiency and reducing cost [77]. As expected, it is quite difficult to adjust the optimal operation parameters for either step. Adjustment of the operation conditions of the two processes is challenged by the need for a thorough understanding of enzyme–substrate interactions. Therefore, development of more robust enzymes and interpretation of the mechanisms are still needed in order to improve the efficiency [78].
3.4. Various Feedstocks for 2,3-BDO Production
In first-generation sugarcane biorefinery plants, ethanol, sugar, and electricity are the main products and molasses and vinasse are the by-products from sugar and ethanol production, respectively. The production of 2,3-BDO from cheap renewable feedstocks by various fermentation strategies are summarized in Table 2. Sugarcane molasses are a prospective cheap feedstock for 2,3-BDO production owing to their high sucrose content and low price. Jung et al. reported a 2,3-BDO production of 140 g/L in 54 h by E. aerogenes using sugarcane molasses as a carbon resource [79]. Deshmukh et al. reported that a genetically modified Bacillus subtilis can be utilized to produce 2,3-BDO using sugarcane molasses as feedstock [80]. Olga et al. reported 2,3-BDO production from sugarcane molasses by Enterobacter ludwigii with a concentration, yield, and productivity of 50.6 g/L, 0.31 g/g, and 2.66 g/L/h, respectively. The market price of sugarcane molasses is 0.05–0.2 USD/kg, and the minimum selling price of 2,3-BDO is 2.25–3.23 USD/kg, based on techno-economical assessment results [81]. Besides molasses, sugarcane bagasse is a widely available agriculture residue with a yield of 540 million metric tons per year worldwide [82]. Sugarcane bagasse contains 20–30% lignin, 30–35% hemicellulose, and 40–45% cellulose. Um et al. attempted to utilize sugarcane bagasse hydrolysate to produce 2,3-BDO by Enterobacter aerogenes. After a 72 h fermentation, the yield reached 0.395 g/g.
Typically, corn stover contains 30–60% cellulose, 20–40% hemicellulose, and 15–25% lignin [83]. In China, the annual production of corn stover reaches approximately 200 million tons, but over 50% of this is directly discarded on farmland for open-field burning [84]. It was predicted that 170–256 million dry tons of corn stover would be produced by 2030 in the United States [85]. Li et al. found that the Bacillus licheniformis strain X10 could use corn stover hydrolysate as feedstock to produce 2,3-BDO with a yield of 94% in a fed-batch fermentation since it could utilize glucose and xylose at the same time and show a high tolerance to vanillin, acetic acid, formic acid, and xylose [86]. Focusing on corn stover hydrolysate as a feedstock, Ma et al. compared three fermentation modes by using P. polymyxa to improve the production of (2R,3R)-,2,3-BDO, obtaining a concentration, productivity, and yield of 18.8 g/L, 1.13 g/L/h, and 0.313 g/g, respectively [87].
The stalk of the Jerusalem artichoke comprises cellulose and hemicellulose, and the tuber contains inulin, which can be hydrolyzed into glucose and fructose by inulinase. 2,3-BDO production at 91.63 g/L and 70.11 g/L was attained from Jerusalem artichoke tuber hydrolysate by K. pneumoniae by SSF and SHF, respectively, in fed-batch fermentation [88].
Apple pomace is a by-product generated from the apple juice industry. The original apple pomace contains cellulose (43.2%), hemicellulose (20.3%), lignin (20.3%), and soluble reducing sugar (5%). The B. amyloliquefaciens TUL 308 strain could produce more 2,3-BDO from apple pomace hydrolysates than from pure sugars, with a concentration of 34.24 g/L [89]. Also, by using the nonpathogenic bacterium Bacillus licheniformis NCIMB 8059, 77.6 g/L of 2,3-BDO was produced from the enzymatic hydrolysis of depeptidized apple pomace. The hydrolysate of apple pomace contains not only sugars but also essential minerals that may facilitate 2,3-BDO production [90].
Fruit and vegetable waste from open markets have also been selected and tested for 2,3-BDO production. Fed-batch fermentation of Enterobacter ludwigii FMCC 204 using fruit waste hydrolysates as feedstock resulted in a 2,3-BDO concentration, yield, and productivity of 50 g/L, 0.4 g/g, and 0.41 g/L/h, respectively. 2,3-BDO production from vegetable waste hydrolysate was 17.6 g/L in fed-batch fermentation [91]. Serratia marcescens H30 used sweet sorghum juice to produce 2,3-BDO, attaining a production of 109.44 g/L in fed-batch fermentation [92].
Cheese whey is a by-product from a waste stream of the cheese industry. Cheese whey is an attractive alternative feedstock to use as a medium in 2,3-BDO production [93,94]. Guo et al. evaluated several strains as 2,3-BDO producers by using cheese whey powder, and a two-stage pH control strategy was developed in pulsed fed-batch fermentation. The maximal 2,3-BDO concentration reached was 57.63 g/L by Klebsiella pneumoniae CICC 10781 [95]. Whey and permeate whey were fermented to produce 2,3-BDO by Escherichia coli K12 MG1655 with a yield of 0.43 g/g at 72 h. This study showed that permeate whey could be a good carbon source for 2,3-BDO production, reaching near 80% of the theoretical yield after 24 h [96].
Glycerol is the main by-product from the biodiesel industry, with a yield of 0.1 ton of glycerol per ton of biodiesel. It is a potential carbon source for 2,3-BDO production due to the incremental yield of biodiesel. Among the natural 2,3-BDO producers, K. pneumoniae and K. oxytoca tend to produce 2,3-BDO as the primary product and 1,3-propanediol (1,3-PDO) as a by-product under aerobic conditions. To date, using glycerol as the feedstock, the highest production of 2,3-BDO (131.5 g/L) has been obtained by K. oxytoca. Deletion of the pduC and ldhA genes increased 2,3-BDO production and reduced the formation of by-products such as 1,3-PDO and lactic acid [97]. Besides the Klebsiella species, Raoultella planticola, Raoultella terrigena, and B. amyloliquefaciens also have been reported to produce 2,3-BDO using glycerol without forming 1,3-PDO. B. amyloliquefaciens achieved 2,3-BDO production from crude glycerol at a concentration of 43.1 g/L without 1,3-PDO production. Moreover, when beet molasses was supplied as a co-substrate, the 2,3-BDO production increased to 83.3 g/L [98]. Yang et al. developed a two-stage pH control strategy and a three-stage dissolved-oxygen control strategy to enhance 2,3-BDO production by B. amyloliquefaciens. A higher titer, yield, and productivity of 102.3 g/L, 0.44 g/g, 1.16 g/L/h, respectively, was obtained [24]. Kim et al. reported 2,3-BDO production (78.1 g/L) from crude glycerol without the formation of 1,3-PDO by employing a budABC overexpression mutant of Raoultella ornithinolytica [99]. Even some 2,3-BDO producers lacking 1,3-PDO-forming ability can provide advantages in the further purification process of 2,3-BDO; however, the 2,3-BDO titers obtained by K. oxytoca and K. pneumoniae are still higher than from other species. In addition, Daniel et al. screened different bacteria for 2,3-BDO production from glycerol. 2,3-BDO production was increased to 19 g/L by the P. polymyxa PM 3605 strain when 10 g/L of sugarcane molasses was added as a co-substrate. The selectivity of (2S,3S)-2,3-BDO was high, and meso-2,3-BDO was not detected, indicating that P. polymyxa PM 3605 is a promising producer for pure (2S,3S)-2,3-BDO from glycerol [100]. Therefore, the combined utilization of two or more carbon resources may offer extra benefits for 2,3-BDO biosynthesis.

Table 2.
A summary of 2,3-BDO production from renewable feedstocks by various fermentation strategies.
Table 2.
A summary of 2,3-BDO production from renewable feedstocks by various fermentation strategies.
| Feedstock | Microorganism | Fermentation Type | Isomer | BDO g/L | Productivity (g/L/h) | Yield (g/g) | Ref. |
|---|---|---|---|---|---|---|---|
| Corn stover hydrolysate | E. cloacae SDM | SHF | D- | 119.4 | 2.3 | 95% | [45] |
| Corn stover hydrolysate | B. liqueniforms X10 | SHF | - | 74.0 | 2.1 | 94.6% | [86] |
| Corn stover hydrolysate | P. polymyxa ATCC | Batch | D- | 18.8 | 1.13 | 0.313 | [87] |
| Corncob residue | E. cloacae UV4 | Fed-batch SSF | - | 43.16 | 0.55 | 42.4% | [101] |
| Corncob hydrolysate (non-detoxifed acid) | E. cloacae M22 | SHF | - | 24.32 | - | - | [102] |
| Corncob hydrolysate | E. cloacae CICC 10011 | Fed-batch SHF | - | 55.7 | 0.93 | 0.227 | [103] |
| Sugarcane bagasse hydrolysate | E. aerogenes KCTC 2190 | SHF | - | 17.91 | - | 0.45 | [104] |
| Sugarcane bagasse hydrolysate | E. aerogenes for EMY-22_M1Gb | Fed-batch SHF | - | 114 | 1.49 | 0.44 | [105] |
| Rice-waste biomass hydrolysate | K. pneumoniae KMK-05 | SHF | - | 11.4 | 0.476 | 0.381 | [106] |
| Rice straw hydrolysate | Klebsiella sp. Zmd 30 | SHF | - | 24.6 | 2.41 | 62% | [75] |
| Oil palm front | E. cloacae SG1 | SSF | - | 30.74 | 0.32 | - | [107] |
| Kenaf core powder hydrolysates | K. pneumoniae KMK05 | SHF | - | 10.42 | 0.385 | [108] | |
| Soybean hull hydrolysate | K. pneumoniae BLh-1 | SHF | - | 21.9 | 0.28 | 0.51 | [109] |
| Apple pomace hydrolysate | B. subtilis LOCK 1086 | Batch- SHF | - | 51.53 | 0.43 | 0.29 | [110] |
| Apple pomace hydrolysate | B. licheniformis NCIMB 8059 | Batch- SHF | - | 77.6 | 0.42 | 0.32 | [90] |
| Sweet sorghum Juice | Serratia marcescens H30 | Batch- SHF | - | 109.44 | 1.4 | 0.42 | [92] |
| Cassava powder | E. cloacae subsp. dissolvens SDM | Fed-batch SSF | meso-, D-, L- | 93.9 | 3.3 | 0.42 | [111] |
| Jerusalem artichoke tuber | K. pneumoniae CICC 10011 | Fed-batch SHF | - | 70.11 | 1.25 | 0.23 | [88] |
| Fed-batch SSF | - | 91.63 | 2.29 | 0.32 | |||
| Jerusalem artichoke tuber extract and inulin | K. pneumoniae H3 | Fed-batch | - | 80.4 | 2.23 | 0.43 | [112] |
| Fruit and vegetable hydrolysate | E. ludwigii FMCC 204 | Fed-batch | - | 50 | 0.41 | 0.4 | [91] |
| Cheese whey powder | K. pneumoniae CICC 10781 | Fed-batch | - | 57.63 | 0.96 | 0.4 | [95] |
| Whey and permeate whey | Escherichia coli K12 MG1655 | Batch | - | - | 0.43 | [96] | |
| Bakery waste hydrolysate | B. amyloliquefaciens | Batch | D- | 55.2 | 1.19 | 0.42 | [113] |
| Crude glycerol | K. oxytoca | Fed-batch | meso, L- | 131.5 | 0.84 | 0.44 | [97] |
| Crude glycerol | B. amyloliquefaciens | Batch | - | 102.3 | 1.16 g | 0.44 | [24] |
| Glycerol supplemented with molasses | Polymyxa PM 3605 | L- | 19 | 0.13 | 0.35 | [100] |
4. 2,3-Butanediol Recovery and Purification Strategies
Downstream processing to separate and purify 2,3-BDO from the fermentation broth is also an important step. Generally, the recovery of 2,3-BDO needs three steps. First, microbial cells and proteins are removed by membrane filtration or high-speed centrifugation after a pretreatment process that includes pH adjustment and flocculation. Second, some impurities are removed and separated from the fermentation broth, which can be implemented by evaporation, electrodialysis, alcohol precipitation, solvent extraction, etc. Depending on the requirement of product purity, distillation or preparative liquid chromatography can be used as the last step.
The foremost economic obstacle to the scale-up of 2,3-BDO production may not be the fermentation efficiency but, rather, the recovery costs from the fermentation broth. The separation step normally accounts for more than half of the overall cost of the fermented product, since current technology can obtain a fermentation broth containing approximately 10 wt% 2,3-BDO [114]. Therefore, developing an efficient and cheap downstream process to recover 2,3-BDO from the fermentation broth is one of the major challenges to enable the scale-up of 2,3-BDO production from renewable feedstocks by microorganisms. The high boiling point (180–184 °C), high affinity to water due to the high hydrophilic properties of 2,3-BDO, and the dissolved and solid components in the fermentation broth lead to particular difficulties in the recovery of 2,3-BDO [8,115]. In particular, the utilization of inexpensive biomass raw materials such as hemicellulose and cellulose-derived carbohydrates results in many suspended solid particles in the fermentation broth, which then possesses high density and viscosity [116]. So far, diverse separation methods such as distillation [117], steam stripping [118], reverse osmosis [119], vacuum membrane distillation [120], pervaporation [121], solvent extraction [119], and salting-out [122] have been reported for 2,3-BDO recovery. Some of these methods attained a relatively high recovery efficiency of 2,3-BDO on a pilot scale, but they still consume tremendous amounts of energy. These methods have some limitations and drawbacks for commercial industry. Solvent extraction uses large amounts of hydrophilic solvents for the separation of 2,3-BDO. 2,3-BDO can be separated from the media by salting out, utilizing water-free potassium carbonate with good results. The separation efficiency was about 94–96% of 2,3-BDO by using 53–56 wt% potassium carbonate, but it remains a problem to recycle the salt. In addition, the precleaning process lowers the entire efficiency [122]. Normally, steam stripping and distillation are considered high energy consumption processes. Solvent extraction requires the utilization of large amounts of solvent, and the recovery efficiency is relatively low. The characteristics and limitations of representative separation and purification process are summarized in Table 3.
Until now, no single process has been reported that simultaneously achieves recovery and purification to the required concentration. Recently, the combined process was investigated to find a promising solution. The combination of distillation and liquid–liquid extraction has been reported to be a promising process for safe and cost-effective recovery [123]. Haider et al. proposed an extraction-assisted distillation process with iso-butanol and 1-butanol for the production of 2,3-BDO (99% purity), which would reduce the total annual cost by up to 10.42% and 18.9%, respectively. This combined process can support an increase in profitability and efficiency by significantly lessening the operational costs and only minimally increasing the capital investment, leading to the feasible commercialization of 2,3-BDO production [124]. Thus, the implementation of two or more methods may improve the recovery efficiency and economics by combining the advantages of each process. Combined processes such as extraction and pervaporation [125], extraction and salting out, vacuum distillation and alcohol precipitation [126], and hybrid extraction–distillation (HED) [127] have been explored as ways to improve the recovery efficiency and reduce the energy consumption. The extraction and salting-out combination has been utilized for 2,3-BDO separation by many researchers. The salting-out process can decrease the quantity of solvent needed for the extraction as well as increase the extraction efficiency, since the presence of salt can undermine the interaction between 2,3-BDO and water. Ethanol/ammonium sulfate [128], ethanol/dipotassium hydrogen phosphate [129], ethanol/potassium triphosphate [130], polyethylene glycol/potassium phosphate [131], and n-butanol/dipotassium hydrogen phosphate [132] systems have been evaluated for 2,3-BDO recovery, and the obtained efficiency was 91.7%, 98.13%, 99.5% (2,3-BDO purity of 91.0%), 94.5%, and 99%, respectively. This combined method provides a new possibility for the separation and purification of 2,3-BDO.
Another separation technique named fermentation–derivatization–recovery, or reaction extraction, has been developed. This method relies on the derivatization of 2,3-BDO into other compounds that can be easily subsequently separated and purified. In 2006, Hao et al. recovered 2,3-BDO from the fermentation broth via a reaction with butyraldehyde. 2-propl-4,5-dimental-1,3-dioxolane was formed from the acetalization of 2,3-BDO and was easily separated from the aqueous phase [133]. Acetaldehyde was also an efficient reactant and extractant for recovering 2,3-BDO from the fermentation broth under the catalysis of the ion-exchange resin HZ732 [134]. Recently, Li et al. used n-butanal as a reactant and extractant to derivatize 2,3-BDO to dioxolane, which could be easily separated from the aqueous phase. A 95% conversion of 2,3-BDO to dioxolane was achieved by using the resin HZ-732; subsequently 99% of 2,3-BD could be recovered from dioxolane [135]. The reactive extraction method needs less operating time and a small amount of reactant, but the process becomes more complicated because more reaction units are involved.
In addition, the combined process of alcohol precipitation and vacuum distillation can be applied to 2,3-BDO separation. Isopropanol showed the highest removal efficiency of organic acids and inorganic salts of 92.5% and 99.8%, respectively. After alcohol precipitation, vacuum distillation recovered 76.2% of the treated 2,3-BDO with a purity of 96.1%. Since vacuum distillation is an energy-intensive technique, the recovery efficiency needs to be improved together with a lower energy consumption for industrialization [126]. Compared with conventional distillation process, HED using oleyl alcohol as a solvent can reduce the total energy consumption and, as a result, improve the overall process economics, even though the capital cost of HED configuration is 9.5% higher than that of conventional distillation [127].
Although various separation and purification techniques for 2,3-BDO have been developed, the separation costs still remain high. Miana et al. evaluated the process by using 2,3-BDO as a platform chemical for the direct production of methyl ethyl ketone in the fermentation broth without the separation and purification of 2,3-BDO. The results demonstrated that the process is economically feasible since the bio-based MEK minimum selling price was close to the market price of petroleum-derived MEK (1.8 USD/kg) [136].

Table 3.
Methods for the separation and purification of 2,3-BDO from the fermentation broth.
Table 3.
Methods for the separation and purification of 2,3-BDO from the fermentation broth.
| Separation Method | Recovery Efficiency (w/w) | 2,3-BDO Purity (w/w) | Characteristics | Limitations | Ref. |
|---|---|---|---|---|---|
| Membrane filtration | Not Applicable | Not Applicable | Cells and debris can be removed | Fouling would decline the flux | [137] |
| Solvent extraction | Less than 80% | Not Applicable | Many solvents can be used to extract 2,3-BDO from the broth; the target product would be partitioned into the solvent phase. | A large amount of solvent needs to be used; the efficiency is low. | [119] |
| Pervaporation/vacuum membrane distillation | Concentrated from 40 g/L to 650 g/L | Not Applicable | Polytetrafluoro-ethylene membrane can be used to recover 2,3-BDO from the broth. | The recovery efficiency is relatively low. | [120,121] |
| Salting out | 94–96% | 97% | 2,3-BDO can be salted out from pretreated broth with a large amount of salt. | A precleaning process is necessary, and a large amount of salt needs to be recovered. | [122] |
| Extraction and salting out | 91% | 99.5% | Directly extract from fermentation broth without pretreatment and promising to scale up. It can handle high-density and high-viscosity broth. | A relatively large amount of solvent and salt is used (but less than that used in an individual process). | [119,130] |
| Reactive extraction | 90% | 99% | High purity of 2,3-BDO can be obtained by consuming less energy than solvent extraction plus distillation. | Extra solvent and catalyst need to be used. | [134] |
| Alcohol precipitation and vacuum distillation | 76.2 | 96.1% | The addition of alcohol can remove organic acids and inorganic salts from the broth without pH adjustment | Distillation is an energy-intensive process | [126] |
5. Derivatives of 2,3-Butanediol and Their Applications
5.1. Applications of 2,3-Butanediol
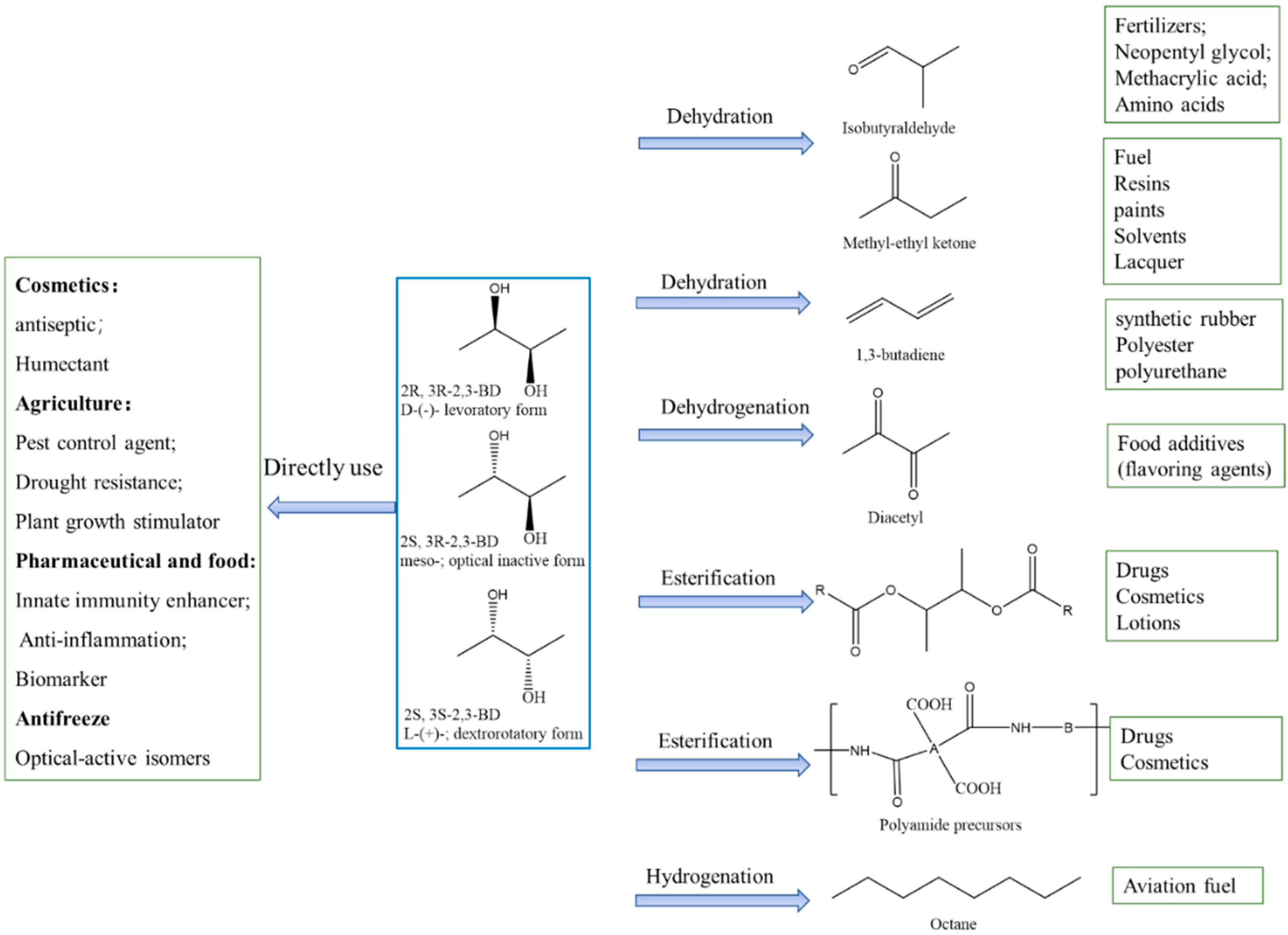
2,3-BDO has wide applications, including direct use or as a platform chemical by further conversion to value-added products (Figure 4). As a short-chain alcohol, 2,3-BDO is used as an organic solvent in industry because various substances such as fatty acid, fats, cellulose derivatives, and synthetic rubber can be well dissolved. Also, 2,3-BDO can be used as an antioxidant or UV absorber in cosmetic industries, and as an antifreeze in the low-temperature preservation of organs [138]; the L-form of 2,3-BDO, especially, has an extremely low freezing point (−60 °C), giving it great potential as an antifreeze agent. Moreover, it has potent applications in agriculture due to its antibacterial properties and growth-promoting effects on plants. Due to the biodegradability of 2,3-BDO, its agricultural potency as a pesticide is worth exploring. 2,3-BDO has been found to induce plant resistance to fungal pathogens. By using 2,3-BDO together with chemical fungicides, the efficacy of fungal disease control can be increased by 9%, thereby diminishing the use of synthetic fungicides [139]. Meso-2,3-BDO can trigger plant immunity to control bacterial wilt in tomato [140]. Moreover, Romano et al. reported that adding 2,3-BDO as a flavor additive to Baijiu improved its taste and led to a more lasting and richer flavor [141]. It also can be applied to fumigants, humidifiers, softeners, plasticizers, inks, coatings, cleaning lotions for electronics, and drug carriers [7]. Polyamide precursors of 2,3-BDO are widely utilized in drugs, cosmetics, and lotions owing to its lack of toxicity or skin irritation. Based on the supporting features of 2,3-BDO as a feasible substance in personal care products and cosmetics, it has been approved by the International Nomenclature of Cosmetic Ingredients [142].
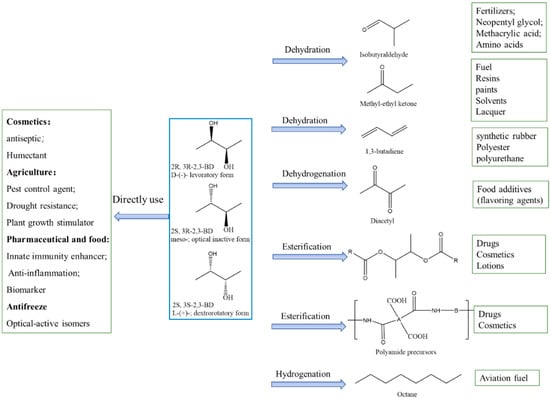
Figure 4.
Applications of 2,3-butanediol and its derivatives.
1,3-butadiene, which is an important precursor for synthetic rubber and plastic manufacturing, can be derived from the dehydration of 2,3-BDO. Acetoin obtained from 2,3-BDO oxidation is a commonly used food flavoring in dairy products, as is the diacetyl that is generated by further dehydrogenation. Diacetyl finds broad applications in the food industry as a flavoring agent due to its characteristic buttery aroma [143]. 2,3-BDO can be used as a precursor for the synthesis of polybutylene, terephthalate, γ-butyrolactone, and polyurethane. Polyurethane, especially, has broad applications because of its excellent properties. Butane-2,3-diyl diacetate, which can be synthesized from 2,3-BDO and acetic acid, is the key aroma component in common fruit such as banana and casaba, and it can be added as an additive to improve the creamy flavor in many kinds of food.
5.2. Applications in Biofuel Production and Fuel Additives
The issues of global warming and fossil resource depletion have undoubtedly led to renewed interest in biofuels, which refer to liquid, gas, and solid fuels that are predominantly derived from biomass. As a biomass-derived chemical, 2,3-BDO also shows great promise for the production of biofuels or fuel additives. The heat value of 2,3-BDO is up to 27,198 J/g, being close to that of ethanol (29,005 J/g) and significantly higher than that of methanol (22,081 J/g), raising the possibility for it to be used directly as a fuel additive [22]. The combined heating value of an equimolar mixture of 2,3-BDO and ethanol is 27,660 J/g. In addition, 2,3-BDO can serve as an “octane booster” for petrol owing to its high anti-knock index [8]. It is noteworthy that a 99% purity of 2,3-BDO needs to be achieved for its use as a fuel.
Methyl ethyl ketone (MEK) is an important derivative from the 2,3-BDO dehydration reaction, which is conventionally produced using C4 raffinates. Currently, MEK is produced by a two-step process that includes the hydration of butylene to produce secondary butyl alcohol and the dehydration of secondary butanol to MEK in succession, which requires high energy consumption and poses the risk of serious equipment corrosion. MEK is a valuable industrial solvent, especially in paintings, coatings, resins, and lacquers. Also, MEK is identified as a more potent fuel additive compared with ethanol due to its higher heat combustion (2444.3 kJ/mol and 1366.9 kJ/mol, respectively) [144], lower hydrocarbon emissions, lower oil dilution, and better cold-start properties [145].
Recently, Bai et al. reported a more comprehensive reaction mechanism for the dehydration of 2,3-BDO using sulfuric acid as a catalyst [146]. A newly identified by-product, 2-isopropyl-4,5-dimethyl-1,3-dioxolane, which is considered a next-generation fuel additive, is formed by the acetalization of 2,3-BDO with isobutyraldehyde. Similarly, 2-ethyl-2,4,5-trimethyl-1,3-dioxolane, formed by the ketalization of 2,3-BDO with MEK, is also a possible fuel additive. Harvey et al. conducted the selective dehydration of 2,3-BDO in a solvent-free process by using the solid acid catalyst Amberlyst-15. A complex mixture of 2-ethyl-2,4,5-trimethyl-1,3-dioxolane and 2-isopropyl-4,5-dimethyl-1,3-dioxolane was obtained. After purification, the anti-knock index of the dioxolane mixture was tested as being 90.5, which was comparable to high-octane gasoline. The volumetric net heat of combustion of dioxolanes was 34% higher than ethanol. In addition, the solubility of dioxolanes in water was tested to be 0.08 g/L, which is approximately an order of magnitude lower than that of the common gasoline oxygenate methyl tert-butyl ether (MTBE). Therefore, dioxolanes, which are easily derived from 2,3-BDO, might be potentially used as a sustainable gasoline blending component, diesel oxygenate, and industrial solvent [147]. Stapes explored a series of bio-derived C4–C8 aldehydes for their reaction with 2,3-BDO. As a result, 1,3-dioxolanes were produced by acetalization, which exhibited advantages over traditional diesel; thus, they could potentially augment petroleum-derived fuels. The methods exhibited prominent carbon yields (93%), atom economy (89%), and spontaneous phase separation, eliminating an extra purification process [148]. Based on that, Rajale et al. employed bio-derived aldehydes to separate and recover 2,3-BDO from the fermentation broth [149]. The above dioxolanes exhibited excellent characteristics for use in diesel fuels, giving 2,3-BDO derivatives great opportunity for future application as biofuels [150].
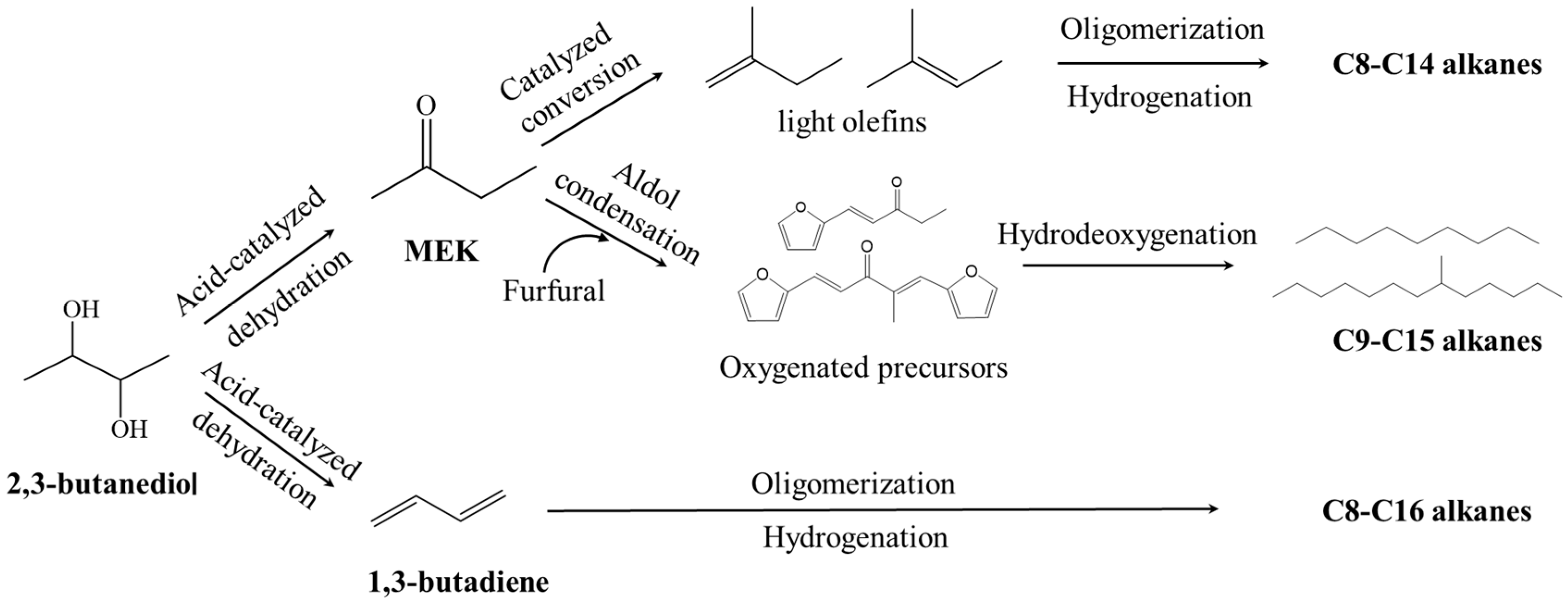
The production of biofuels such as hydrocarbon fuels (bio-jet fuels) from 2,3-BDO is also possible, although only a few relevant research studies have been reported. Typically, there are three routes for converting 2,3-BDO into bio-jet fuels, as shown in the following mechanisms in Figure 5. 2,3-BDO can be converted to MEK by acid-catalyzed dehydration, followed by catalyzed conversion to form light olefins that are further converted to alkanes by oligomerization and hydrogenation [151]. Affandy et al. [152] developed a new technique for producing a bio-jet fuel blendstock candidate directly from the 2,3-BDO fermentation broth. The catalytic steps included (1) MEK derivation from the dehydration of 2,3-BDO over AlPO4; (2) the derivation of olefins from MEK over Zn1Zr10Ox; (3) the oligomerization of olefins over a zeolite beta; and (4) hydrogenation over platinum/carbon catalysts. The final product mainly comprises isoalkanes, n-alkanes, and cycloalkanes at 31.7 wt%, 24.5 wt%, and 29.6 wt%, respectively. MEK also can be converted to oxygenated precursors followed by hydrodeoxygenation to obtain hydrocarbon bio-jet fuels. As reported by Cui et al. [153], a novel process was developed for the production of bio-jet fuel from biomass-derived furfural and MEK by an aldol reaction under alkaline conditions to extend the carbon chain. By changing the reaction conditions, including the type of solvent, the molar ratio between furfural and MEK, the loading of alkali, and temperature, the selectivity of the C9 and C15 oxygenated precursors could be easily adjusted. Hydrocarbon fuels were produced from hydrodeoxygenation of the precursors, with C1–C4, C5–C8, and C9–C14 alkane product yields of 15.4%, 7.9%, and 73%, respectively.
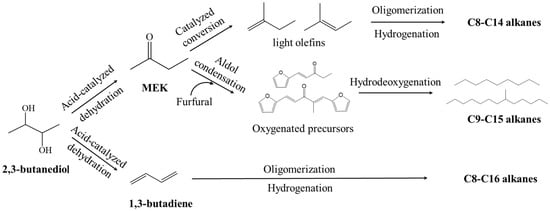
Figure 5.
Possible technical routes for the production of bio-jet fuels from 2,3-BDO.
Another way to convert 2,3-BDO into hydrocarbon fuels is via 1,3-butadiene, which is usually achieved by the vapor-phase catalytic dehydration of 2,3-BDO over metal oxide catalysts such as Sc2O3, γ-Al2O3, SiWO, and Y2Zr2O7 [154]. Liu et al. reported that 2,3-BDO was directly dehydrated to produce 1,3-butadiene by using alumina as the catalyst. Using only trace amounts of catalyst with a high flow rate in a fixed-bed reactor, the conversion of 2,3-BDO could be achieved at 12% with 60% 1,3-butadiene selectivity at 380 °C [155]. Oligomerization of 1,3-butadiene could efficiently extend the carbon-chain length required for liquid biofuels. Then, the oligomerization of butadiene could be catalyzed by palladium, obtaining mixtures of E-1-acetoxy-2,7-octadiene, 3-acetoxy-1,7-octadiene, E-1,3,7-octadiene, E-1-acetoxy-2-butene, and 3-acetoxybutene at 100 °C [156]. Further hydrogenation of the oligomerization products resulted in the formation of C8–C16 alkanes. However, it should be noted that these processes are still too expensive to be economically feasible. This is because platform chemicals, for instance, MEK, furfural and 1,3-butadiene, are even more expensive than jet fuels. Moreover, a large amount of H2 would be consumed in the hydrogenation or hydrodeoxygenation process, and the price of H2 is still too high for it to be used in the production of biofuels. However, these processes can provide future technologies for hydrocarbon fuel production from renewable biomass.
Different routes for the conversion of 2,3-BDO into biofuels and fuel additives are summarized in Table 4. 2,3-BDO and its oxygenated derivatives can be directly used as fuel additives, or further converted into bio-jet fuels and alkanes by several steps. However, currently, the main limitation of these additives and fuels are their relatively high cost, which must be a major consideration for their industrial application.

Table 4.
Comparison of 2,3-BDO as a feedstock for the production of biofuels and fuel additives.
6. Conclusions and Future Prospects
2,3-BDO is a promising biomass-derived platform chemical with many applications. Although 2,3-BDO can be produced by chemical conversion from fossil resources, its biosynthesis from various renewable biomass sources seems to be more sustainable. Some bacteria and yeasts have been screened and modified to produce good amounts of 2,3-BDO. By genetic engineering, pure optical isomers of 2,3-BDO can be selectively produced that show different biological activities and may be used in pharmaceuticals. Carbon sources including glucose, glycerol, molasses and lignocellulose hydrolysate have been used for 2,3-BDO production. Generally, hexoses are good carbon sources for microorganisms to produce 2,3-BDO, while the uptake rate of pentoses such as xylose is relatively lower. By using different fermentation processes, 2,3-BDO concentration in the fermentation broth can be higher than 150 g/L. Lignocellulosic biomass is a promising non-food feedstock, but it needs a relatively complicated process to convert the biomass to a fermentable sugars prior to fermentation. In particular, pretreatment to deconstruct the lignocellulose cell wall structure and increase cellulose digestibility is a prerequisite step. Different methods and their combinations have been developed to recover and purify 2,3-BDO from the fermentation broth, including membrane filtration, solvent extraction, pervaporation, salting out, extraction, vacuum distillation, etc. The obtained purity of 2,3-BDO can reach 99%. Various derivatives can be produced from 2,3-BDO, including isobutyraldehyde, 1,3-butadiene, MEK, diacetyl etc.; among these, there is a large market demand for MEK and 1,3-butadiene each year. Some of the derivatives can be used as fuel additives or to produce biofuels. Generally, there are three ways to produce hydrocarbon fuels from 2,3-BDO, i.e., through the steps of dehydration, carbon-chain extension, and hydrogenation (or hydrodeoxygenation), with MEK or 1,3-butadiene as the intermediates. C8–C16 alkanes can be produced by these routes, which can be potentially used as bio-jet fuels. Generally, as more and more attention has been paid to the reduction of carbon emission, biofuels definitely have attracted great interest in recent years. It is anticipated that the proportion of biofuels contributing to renewable energy will continually increase. As estimated by the International Energy Agency (IEA), biofuels will provide 27% of world transportation fuels by 2050 “https://www.iea.org/ (accessed on 3 August 2023)”. However, the production of 2,3-BDO and its application in the biofuel field are still facing some problems that need to be solved. In particular, the production cost of 2,3-BDO is still high, and the market for these products still needs to be developed. The following aspects are recommended for future research:
(1) Cheaper carbon sources should be used in order to reduce the material cost. The theoretical yield of 2,3-BDO from glucose by microbial fermentation is about 0.5 g/g. Carbon sources contribute a large part of the total materials cost. Therefore, a cheap but efficient carbon source would be an important way to reduce the production cost. From this point of view, some industrial wastes such as molasses and vinasse seem to be promising.
(2) When lignocellulosic biomass is used as a feedstock, cheap but efficient pretreatment technology should be developed. The efficiency of cellulose enzymatic hydrolysis also must be improved, which can be achieved by improving the specific activity of cellulases or the stability of the enzymes during hydrolysis. Increasing the fermentable sugar concentration in the hydrolysate must be considered, which requires the development of technology and reactors for high-solid enzymatic hydrolysis.
(3) Modification of the strains to reduce the formation of by-products and increasing the metabolic flux of 2,3-BDO is important to increase the process efficiency. Meanwhile, screening and modification of strains to produce pure stereoisomers benefit the economic feasibility due to their particular applications in organic synthesis.
(4) Process integration and techno-economic assessment should be performed with integrated consideration of the fermentation and 2,3-BDO purification. Efficient downstream processing should be developed to reduce energy consumption and to increase the 2,3-BDO recovery yield. A combination of several unit operations including concentration, desalination, distillation, etc. is necessary to guarantee product purity.
(5) The derivative products and their applications should be investigated in-depth. Currently, the market demand for 2,3-BDO is limited. However, conversion of 2,3-BDO into downstream products would increase the complexity of the process. In particular, the conversion of 2,3-BDO into bio-jet fuels needs more reaction units, and it consumes a large amount of H2, making the process not economically feasible yet. Therefore, how to reduce the production cost should still be a major consideration.
Author Contributions
Conceptualization, Y.B. and X.Z.; methodology, Y.B.; validation, H.F. and N.L.; resources, Y.B. and H.F.; writing—original draft preparation, Y.B.; writing—review and editing, X.Z.; visualization, N.L.; supervision, Y.B. and X.Z.; project administration, Y.B. and X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the R&D Program of Beijing Municipal Education Commission (No. KM202310011008) and the National Natural Science Foundation of China (No. 22178197).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 2,3-BDO | 2,3-butanediol |
| ALS | α-acetolactate synthase |
| α-ALD | α-acetolactate decarboxylase |
| BDH | 2,3-BDO dehydrogenase |
| AR | Acetoin reductase |
| LDH | L-/D-lactate dehydrogenase |
| ALS | α-acetolactate synthase |
| DAR | diacetyl reductase |
| α-ALD | α-acetolactate decarboxylase |
| GDH | Glycerol dehydrogenases |
| LDH | Lactate dehydrogenase |
| ADH | Acetaldehyde dehydrogenase |
| EMIMAc | Ethyl-3-methylimidazolium acetate |
| SHF | Separate hydrolysis and fermentation |
| SSF | Simultaneous saccharification and fermentation |
| 1,3-PDO | 1,3-propanediol |
| HED | Hybrid extraction–distillation |
| PTFE | Polytetrafluoro-ethylene |
| INCI | International Nomenclature of Cosmetic Ingredients |
| MTBE | Methyl tert-butyl ether |
| MEK | Methyl ethyl ketone |
References
- Sohn, Y.J.; Son, J.; Jo, S.Y.; Park, S.Y.; Yoo, J.I.; Baritugo, K.A.; Na, J.G.; Choi, J.I.; Kim, H.T.; Joo, J.C. Chemoautotroph Cupriavidus necator as a potential game-changer for global warming and plastic waste problem: A review. Bioresour. Technol. 2021, 340, 125693. [Google Scholar] [CrossRef] [PubMed]
- Sohn, Y.J.; Son, J.; Lim, H.J.; Lim, S.H.; Park, S.J. Valorization of lignocellulosic biomass for polyhydroxyalkanoate production: Status and perspectives. Bioresour. Technol. 2022, 360, 127575. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Joo, J.C.; Baritugo, K.A.; Jeong, S.; Lee, J.Y.; Lim, H.J.; Lim, S.H.; Yoo, J.I.; Park, S.J. Consolidated microbial production of four-, five-, and six-carbon organic acids from crop residues: Current status and perspectives. Bioresour. Technol. 2022, 351, 127001. [Google Scholar] [CrossRef]
- Lee, H.; Jung Sohn, Y.; Jeon, S.; Yang, H.; Son, J.; Jin Kim, Y.; Jae Park, S. Sugarcane wastes as microbial feedstocks: A review of the biorefinery framework from resource recovery to production of value-added products. Bioresour. Technol. 2023, 376, 128879. [Google Scholar] [CrossRef]
- Syu, M.J. Biological production of 2,3-butanediol. Appl. Microbiol. Biotechnol. 2001, 55, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, J.W.; Lee, Y.G.; Park, Y.C.; Seo, J.H. Metabolic engineering of Saccharomyces cerevisiae for 2,3-butanediol production. Appl. Microbiol. Biotechnol. 2017, 101, 2241–2250. [Google Scholar] [CrossRef]
- Maina, S.; Prabhu, A.A.; Vivek, N.; Vlysidis, A.; Koutinas, A.; Kumar, V. Prospects on bio-based 2,3-butanediol and acetoin production: Recent progress and advances. Biotechnol. Adv. 2022, 54, 107783. [Google Scholar] [CrossRef]
- Bialkowska, A.M. Strategies for efficient and economical 2,3-butanediol production: New trends in this field. World J. Microbiol. Biotechnol. 2016, 32, 200. [Google Scholar] [CrossRef]
- Chemicals & Materials, Market Research Report, 2019. 2,3-Butanediol Market (Application: Intermediate Chemicals, Plastics, Food Additives, Cosmetics and others)-Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2019–2027. Available online: https://www.transparencymarketresearch.com/2-3-butanediol-market.html (accessed on 26 July 2023).
- Nigam, P.S.; Singh, A. Production of liquid biofuels from renewable resources. Prog. Energy Combust. 2011, 37, 52–68. [Google Scholar] [CrossRef]
- Harden, A.; Walpole, G.S. Chemical action of Bacillus lactis aerogenes (Escherich) on glucose and mannitol: Production of 2 3-butyleneglycol and acetylmethylcarbinol. Proc. R. Soc. London. Ser. B Contain. Pap. A Biol. Character 1997, 77, 399–405. [Google Scholar]
- Sabra, W.; Groeger, C.; Zeng, A.P. Microbial Cell Factories for Diol Production. In Bioreactor Engineering Research and Industrial Applications i: Cell Factories; Ye, Q., Bao, J., Zhong, J.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 155, pp. 165–197. [Google Scholar]
- Rehman, S.; Islam, M.K.; Khanzada, N.K.; Zhuang, H.; Wang, H.; Chaiprapat, S.; Leu, S.-Y. Sustainability index accounting food and carbon benefits on circular 2,3-butanediol biorefinery with oil palm empty fruit bunches. Appl. Energy 2021, 303, 117667. [Google Scholar] [CrossRef]
- Tinôco, D.; Borschiver, S.; Coutinho, P.L.; Freire, D.M.G. Technological development of the bio-based 2,3-butanediol process. Biofuel. Bioprod. Bior. 2020, 15, 357–376. [Google Scholar] [CrossRef]
- Shi, L.; Gao, S.; Yu, Y.; Yang, H. Microbial production of 2,3-butanediol by a newly-isolated strain of Serratia marcescens. Biotechnol. Lett. 2014, 36, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, R.; Aertsen, A.; Michiels, C.W. Quorum-sensing-dependent switch to butanediol fermentation prevents lethal medium acidification in Aeromonas hydrophila AH-1N. Res. Microbiol. 2007, 158, 379–385. [Google Scholar] [CrossRef]
- Ji, X.J.; Huang, H.; Ouyang, P.K. Microbial 2,3-butanediol production: A state-of-the-art review. Biotechnol. Adv. 2011, 29, 351–364. [Google Scholar] [CrossRef]
- Ji, X.J.; Huang, H.; Li, S.; Du, J.; Lian, M. Enhanced 2,3-butanediol production by altering the mixed acid fermentation pathway in Klebsiella oxytoca. Biotechnol. Lett. 2008, 30, 731–734. [Google Scholar] [CrossRef]
- Blomqvist, K.; Nikkola, M.; Lehtovaara, P.; Suihko, M.L.; Airaksinen, U.; Straby, K.B.; Knowles, J.K.; Penttila, M.E. Characterization of the genes of the 2,3-butanediol operons from Klebsiella terrigena and Enterobacter aerogenes. J. Bacteriol. 1993, 175, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Nakashimada, Y.; Marwoto, B.; Kashiwamura, T.; Kakizono, T.; Nishio, N. Enhanced 2,3-butanediol production by addition of acetic acid in Paenibacillus polymyxa. J. Biosci. Bioeng. 2000, 90, 661–664. [Google Scholar] [CrossRef]
- Bryn, K.; Ulstrup, J.C.; Størmer, F.C. Effect of Acetate upon the Formation of Acetoin in Klebsiella and Enterobacter and Its Possible Practical Application in a Rapid Voges-Proskauer Test. Appl. Microbiol. 1973, 25, 511–512. [Google Scholar] [CrossRef]
- Celinska, E.; Grajek, W. Biotechnological production of 2,3-butanediol-current state and prospects. Biotechnol. Adv. 2009, 27, 715–725. [Google Scholar] [CrossRef]
- Xiao, Z.; Xu, P. Acetoin metabolism in bacteria. Crit. Rev. Microbiol. 2007, 33, 127–140. [Google Scholar] [CrossRef]
- Yang, T.W.; Rao, Z.M.; Zhang, X.; Xu, M.J.; Xu, Z.H.; Yang, S.T. Enhanced 2,3-butanediol production from biodiesel-derived glycerol by engineering of cofactor regeneration and manipulating carbon flux in Bacillus amyloliquefaciens. Microb. Cell Fact. 2015, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Stormer, F.C. Evidence for induction of the 2,3-butanediol-forming enzymes in Aerobacter aerogenes. FEBS. Lett. 1968, 2, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.B.; Juni, E. Stereoisomeric specificities of 2,3-butanediol dehydrogenases. Biochim. Biophys. Acta 1960, 39, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Voloch, M.; Ladisch, M.R.; Rodwell, V.W.; Tsao, G.T. Reduction of acetoin to 2,3-butanediol in Klebsiella pneumoniae: A new model. Biotechnol. Bioeng. 1983, 25, 173–183. [Google Scholar] [CrossRef]
- Ui, S.; Masuda, H.; Muraki, H. Separation and quantitation of acetoin isomers (D(-) and L(+)) by a combined use of enzyme and gas-chromatography. Agric. Biol. Chem. 1984, 48, 2835–2836. [Google Scholar]
- Ui, S.; Masuda, T.; Masuda, H.; Muraki, H. Mechanism for the formation of 2,3-butanediol stereoisomers in Bacillus polymyxa. J. Ferment. Bioeng. 1986, 64, 481–486. [Google Scholar] [CrossRef]
- Ui, S.; Matsuyama, N.; Masuda, H.; Muraki, H. Mechanism for the formation of 2,3-butanediol stereoisomers in klebsiella-pneumoniae. J. Ferment. Bioeng. 1984, 62, 551–559. [Google Scholar]
- Ui, S.; Mimura, A.; Ohkuma, M.; Kudo, T. Formation of a chiral acetoinic compound from diacetyl by Escherichia coli expressing meso-2,3-butanediol dehydrogenase. Lett. Appl. Microbiol. 1999, 28, 457–460. [Google Scholar] [CrossRef]
- Ui, S.; Okajima, Y.; Mimura, A.; Kanai, H.; Kudo, T. Molecular generation of an Escherichia coli strain producing only the meso-isomer of 2,3-butanediol. J. Ferment. Bioeng. 1997, 84, 185–189. [Google Scholar] [CrossRef]
- Yan, Y.J.; Lee, C.C.; Liao, J.C. Enantioselective synthesis of pure (R,R)-2,3-butanediol in Escherichia coli with stereospecific secondary alcohol dehydrogenases. Org. Biomol. Chem. 2009, 7, 3914–3917. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wei, D.; Shi, J.P.; Wang, M.; Hao, J. Mechanism of 2,3-Butanediol Stereoisomer Formation in Klebsiella Pneumoniae. Appl. Microbiol. Biotechnol. 2014, 98, 4603–4613. [Google Scholar] [CrossRef] [PubMed]
- Rathnasingh, C.; Park, J.M.; Kim, D.K.; Song, H.; Chang, Y.K. Metabolic engineering of Klebsiella pneumoniae and in silico investigation for enhanced 2,3-butanediol production. Biotechnol. Lett. 2016, 38, 975–982. [Google Scholar] [CrossRef]
- Guo, X.; Cao, C.; Wang, Y.; Li, C.; Wu, M.; Chen, Y.; Zhang, C.; Pei, H.; Xiao, D. Effect of the inactivation of lactate dehydrogenase, ethanol dehydrogenase, and phosphotransacetylase on 2,3-butanediol production in Klebsiella pneumoniae strain. Biotechnol. Biofuels 2014, 7, 44. [Google Scholar] [CrossRef]
- Guo, X.W.; Zhang, Y.H.; Cao, C.H.; Shen, T.; Wu, M.Y.; Chen, Y.F.; Zhang, C.Y.; Xiao, D.G. Enhanced production of 2,3-butanediol by overexpressing acetolactate synthase and acetoin reductase in Klebsiella pneumoniae. Biotechnol. Appl. Biochem. 2014, 61, 707–715. [Google Scholar] [CrossRef]
- Ji, X.J.; Huang, H.; Zhu, J.G.; Ren, L.J.; Nie, Z.K.; Du, J.; Li, S. Engineering Klebsiella oxytoca for efficient 2, 3-butanediol production through insertional inactivation of acetaldehyde dehydrogenase gene. Appl. Microbiol. Biotechnol. 2010, 85, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, T.; Woo, H.M.; Lee, J.; Kim, Y.; Um, Y. Enhanced 2,3-Butanediol Production by Optimizing Fermentation Conditions and Engineering Klebsiella oxytoca M1 through Overexpression of Acetoin Reductase. PLoS ONE 2015, 10, e0138109. [Google Scholar] [CrossRef]
- Jantama, K.; Polyiam, P.; Khunnonkwao, P.; Chan, S.; Sangproo, M.; Khor, K.; Jantama, S.S.; Kanchanatawee, S. Efficient reduction of the formation of by-products and improvement of production yield of 2,3-butanediol by a combined deletion of alcohol dehydrogenase, acetate kinase-phosphotransacetylase, and lactate dehydrogenase genes in metabolically engineered Klebsiella oxytoca in mineral salts medium. Metab. Eng. 2015, 30, 16–26. [Google Scholar]
- Kim, J.W.; Kim, J.; Seo, S.O.; Kim, K.H.; Jin, Y.S.; Seo, J.H. Enhanced production of 2,3-butanediol by engineered Saccharomyces cerevisiae through fine-tuning of pyruvate decarboxylase and NADH oxidase activities. Biotechnol. Biofuels 2016, 9, 265. [Google Scholar] [CrossRef]
- Yang, T.W.; Rao, Z.M.; Zhang, X.; Xu, M.J.; Xu, Z.H.; Yang, S.T. Improved Production of 2,3-Butanediol in Bacillus amyloliquefaciens by Over-Expression of Glyceraldehyde-3-Phosphate Dehydrogenase and 2,3-butanediol Dehydrogenase. PLoS ONE 2013, 8, e76149. [Google Scholar] [CrossRef]
- Bai, F.; Dai, L.; Fan, J.; Truong, N.; Rao, B.; Zhang, L.; Shen, Y. Engineered Serratia marcescens for efficient (3R)-acetoin and (2R,3R)-2,3-butanediol production. J. Ind. Microbiol. Biotechnol. 2015, 42, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Li, K.; Li, L.; Gao, C.; Zhang, L.; Ma, C.; Xu, P. Contracted but effective: Production of enantiopure 2,3-butanediol by thermophilic and GRAS Bacillus licheniformis. Green Chem. 2016, 18, 4693–4703. [Google Scholar] [CrossRef]
- Li, L.; Li, K.; Wang, Y.; Chen, C.; Xu, Y.; Zhang, L.; Han, B.; Gao, C.; Tao, F.; Ma, C.; et al. Metabolic engineering of Enterobacter cloacae for high-yield production of enantiopure (2R,3R)-2,3-butanediol from lignocellulose-derived sugars. Metab. Eng. 2015, 28, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, C.; Jiang, R.; Xu, H.; Xue, F.; Huang, W.; Ni, H.; Gao, J. Production of R,R-2,3-butanediol of ultra-high optical purity from Paenibacillus polymyxa ZJ-9 using homologous recombination. Bioresour. Technol. 2018, 261, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Hassler, T.; Schieder, D.; Pfaller, R.; Faulstich, M.; Sieber, V. Enhanced fed-batch fermentation of 2,3-butanediol by Paenibacillus polymyxa DSM 365. Bioresour. Technol. 2012, 124, 237–244. [Google Scholar] [CrossRef]
- Ma, C.; Wang, A.; Qin, J.; Li, L.; Ai, X.; Jiang, T.; Tang, H.; Xu, P. Enhanced 2,3-butanediol production by Klebsiella pneumoniae SDM. Appl. Microbiol. Biotechnol. 2009, 82, 49–57. [Google Scholar] [CrossRef]
- Jung, M.Y.; Ng, C.Y.; Song, H.; Lee, J.; Oh, M.K. Deletion of lactate dehydrogenase in Enterobacter aerogenes to enhance 2,3-butanediol production. Appl. Microbiol. Biotechnol. 2012, 95, 461–469. [Google Scholar] [CrossRef]
- Jang, M.-O.; Choi, G. Techno-economic analysis of butanol production from lignocellulosic biomass by concentrated acid pretreatment and hydrolysis plus continuous fermentation. Biochem. Eng. J. 2018, 134, 30–43. [Google Scholar] [CrossRef]
- Cha, J.W.; Jang, S.H.; Kim, Y.J.; Chang, Y.K.; Jeong, K.J. Engineering of Klebsiella oxytoca for production of 2,3-butanediol using mixed sugars derived from lignocellulosic hydrolysates. GCB Bioenergy 2020, 12, 275–286. [Google Scholar] [CrossRef]
- Salakkam, A.; Webb, C. Production of poly(3-hydroxybutyrate) from a complete feedstock derived from biodiesel by-products (crude glycerol and rapeseed meal). Biochem. Eng. J. 2018, 137, 358–364. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Wi, S.G.; Cho, E.J.; Lee, D.S.; Lee, S.J.; Lee, Y.J.; Bae, H.J. Lignocellulose conversion for biofuel: A new pretreatment greatly improves downstream biocatalytic hydrolysis of various lignocellulosic materials. Biotechnol. Biofuels 2015, 8, 228. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Maurya, D.P.; Singla, A.; Negi, S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 2015, 5, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Sitotaw, Y.W.; Habtu, N.G.; Gebreyohannes, A.Y.; Nunes, S.P.; Van Gerven, T. Ball milling as an important pretreatment technique in lignocellulose biorefineries: A review. Biomass Convers. Bior. 2021, 1–24. [Google Scholar]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lei, F.; Li, P.; Jiang, J. Lignocellulosic biomass to biofuels and biochemicals: A comprehensive review with a focus on ethanol organosolv pretreatment technology. Biotechnol. Bioeng. 2018, 115, 2683–2702. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.F.; Zhang, C.C.; Nan, J.; Ren, N.Q.; Lee, D.J.; Chen, C. Advances in pretreatment of lignocellulosic biomass for bioenergy production: Challenges and perspectives. Bioresour. Technol. 2022, 343, 126123. [Google Scholar] [CrossRef]
- Phuttaro, C.; Sawatdeenarunat, C.; Surendra, K.C.; Boonsawang, P.; Chaiprapat, S.; Khanal, S.K. Anaerobic digestion of hydrothermally-pretreated lignocellulosic biomass: Influence of pretreatment temperatures, inhibitors and soluble organics on methane yield. Bioresour. Technol. 2019, 284, 128–138. [Google Scholar] [CrossRef]
- Dai, L.; He, C.; Wang, Y.; Liu, Y.; Yu, Z.; Zhou, Y.; Fan, L.; Duan, D.; Ruan, R. Comparative study on microwave and conventional hydrothermal pretreatment of bamboo sawdust: Hydrochar properties and its pyrolysis behaviors. Energy Convers. Manag. 2017, 146, 1–7. [Google Scholar] [CrossRef]
- Escobar, E.L.N.; da Silva, T.A.; Pirich, C.L.; Corazza, M.L.; Pereira Ramos, L. Supercritical Fluids: A Promising Technique for Biomass Pretreatment and Fractionation. Front. Bioeng. Biotechnol. 2020, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Basak, B.; Kumar, R.; Bharadwaj, A.; Kim, T.H.; Kim, J.R.; Jang, M.; Oh, S.E.; Roh, H.S.; Jeon, B.H. Advances in physicochemical pretreatment strategies for lignocellulose biomass and their effectiveness in bioconversion for biofuel production. Bioresour. Technol. 2023, 369, 128413. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, R.; Parra Cruz, R.A.; Pakalapati, H.; Show, P.L.; Ling, T.C.; Chen, W.H.; Tao, Y. Recent advances in the pretreatment of microalgal and lignocellulosic biomass: A comprehensive review. Bioresour. Technol. 2020, 298, 122476. [Google Scholar] [CrossRef] [PubMed]
- Shirkavand, E.; Baroutian, S.; Gapes, D.J.; Young, B.R. Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment—A review. Renew. Sustain. Energy Rev. 2016, 54, 217–234. [Google Scholar] [CrossRef]
- Hou, Q.; Ju, M.; Li, W.; Liu, L.; Chen, Y.; Yang, Q. Pretreatment of Lignocellulosic Biomass with Ionic Liquids and Ionic Liquid-Based Solvent Systems. Molecules 2017, 22, 490. [Google Scholar] [CrossRef]
- Lee, S.H.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis. Biotechnol. Bioeng. 2009, 102, 1368–1376. [Google Scholar] [CrossRef]
- Haykir, N.I.; Bakir, U. Ionic liquid pretreatment allows utilization of high substrate loadings in enzymatic hydrolysis of biomass to produce ethanol from cotton stalks. Ind. Crop. Prod. 2013, 51, 408–414. [Google Scholar] [CrossRef]
- Nargotra, P.; Sharma, V.; Gupta, M.; Kour, S.; Bajaj, B.K. Application of ionic liquid and alkali pretreatment for enhancing saccharification of sunflower stalk biomass for potential biofuel-ethanol production. Bioresour. Technol. 2018, 267, 560–568. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, Y.; Chang, J.S.; Lee, D.J. Enzymes and enzymatic mechanisms in enzymatic degradation of lignocellulosic biomass: A mini-review. Bioresour. Technol. 2023, 367, 128252. [Google Scholar] [CrossRef]
- Guo, H.; He, T.; Lee, D.J. Contemporary proteomic research on lignocellulosic enzymes and enzymolysis: A review. Bioresour. Technol. 2022, 344, 126263. [Google Scholar] [CrossRef]
- Shaikh, H.M.; Anis, A.; Poulose, A.M.; Al-Zahrani, S.M.; Madhar, N.A.; Alhamidi, A.; Alam, M.A. Isolation and Characterization of Alpha and Nanocrystalline Cellulose from Date Palm (Phoenix dactylifera L.) Trunk Mesh. Polymers 2021, 13, 1893. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wu, X.; Huang, C.; Ling, Z.; Lai, C.; Yong, Q. Natural surfactant-aided dilute sulfuric acid pretreatment of waste wheat straw to enhance enzymatic hydrolysis efficiency. Bioresour. Technol. 2021, 324, 124651. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-L.; Huang, C.-C.; Lu, W.-B.; Chen, W.-M.; Chang, J.-S. Producing 2,3-butanediol from agricultural waste using an indigenous Klebsiella sp. Zmd30 strain. Biochem. Eng. J. 2012, 69, 32–40. [Google Scholar] [CrossRef]
- Singh, R.S.; Singh, T.; Hassan, M.; Larroche, C. Biofuels from inulin-rich feedstocks: A comprehensive review. Bioresour. Technol. 2022, 346, 126606. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, Y.; Gao, H.; Zhang, W.; Jiang, Y.; Xin, F.; Jiang, M. Challenges and Future Perspectives of Promising Biotechnologies for Lignocellulosic Biorefinery. Molecules 2021, 26, 5411. [Google Scholar] [CrossRef]
- Paul, P.K.; Al Azad, S.; Rahman, M.H.; Farjana, M.; Uddin, M.R.; Dey, D.; Mahmud, S.; Ema, T.I.; Biswas, P.; Anjum, M.; et al. Catabolic profiling of selective enzymes in the saccharification of non-food lignocellulose parts of biomass into functional edible sugars and bioenergy: An in silico bioprospecting. J. Adv. Vet. Anim. Res. 2022, 9, 19–32. [Google Scholar] [CrossRef]
- Jung, M.Y.; Jung, H.M.; Lee, J.; Oh, M.K. Alleviation of carbon catabolite repression in Enterobacter aerogenes for efficient utilization of sugarcane molasses for 2,3-butanediol production. Biotechnol. Biofuels 2015, 8, 106. [Google Scholar] [CrossRef]
- Deshmukh, A.N.; Nipanikar-Gokhale, P.; Jain, R. Engineering of Bacillus subtilis for the Production of 2,3-Butanediol from Sugarcane Molasses. Appl. Biochem. Biotechnol. 2016, 179, 321–331. [Google Scholar] [CrossRef]
- Psaki, O.; Maina, S.; Vlysidis, A.; Papanikolaou, S.; de Castro, A.M.; Freire, D.M.G.; Dheskali, E.; Kookos, I.; Koutinas, A. Optimisation of 2,3-butanediol production by Enterobacter ludwigii using sugarcane molasses. Biochem. Eng. J. 2019, 152, 107370. [Google Scholar] [CrossRef]
- Bezerra, T.L.; Ragauskas, A.J. A review of sugarcane bagasse for second-generation bioethanol and biopower production. Biofuels Bioprod. Bior. 2016, 10, 634–647. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 1398–1415. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, S.; Tong, Q.; Zhang, X.; Zhao, H.; Ma, S.; Xiu, A.; He, Y. Regional Characteristics and Causes of Haze Events in Northeast China. Chin. Geogr. Sci. 2018, 28, 836–850. [Google Scholar] [CrossRef]
- Aghaei, S.; Karimi Alavijeh, M.; Shafiei, M.; Karimi, K. A comprehensive review on bioethanol production from corn stover: Worldwide potential, environmental importance, and perspectives. Biomass BioEnergy 2022, 161, 106447. [Google Scholar] [CrossRef]
- Li, L.; Li, K.; Wang, K.; Chen, C.; Gao, C.; Ma, C.; Xu, P. Efficient production of 2,3-butanediol from corn stover hydrolysate by using a thermophilic Bacillus licheniformis strain. Bioresour. Technol. 2014, 170, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; He, M.; You, H.; Pan, L.; Wang, Z.; Wang, Y.; Hu, G.; Cui, Y.; Maeda, T. Improvement of (R,R)-2,3-butanediol production from corn stover hydrolysate by cell recycling continuous fermentation. Chem. Eng. J. 2018, 332, 361–369. [Google Scholar] [CrossRef]
- Sun, L.H.; Wang, X.D.; Dai, J.Y.; Xiu, Z.L. Microbial production of 2,3-butanediol from Jerusalem artichoke tubers by Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 2009, 82, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Sikora, B.; Kubik, C.; Kalinowska, H.; Gromek, E.; Białkowska, A.; Jędrzejczak-Krzepkowska, M.; Schüett, F.; Turkiewicz, M. Application of byproducts from food processing for production of 2,3-butanediol using Bacillus amyloliquefaciens TUL 308. Prep. Biochem. Biotechnol. 2016, 46, 610–619. [Google Scholar] [CrossRef]
- Bialkowska, A.M.; Gromek, E.; Krysiak, J.; Sikora, B.; Kalinowska, H.; Jedrzejczak-Krzepkowska, M.; Kubik, C.; Lang, S.; Schutt, F.; Turkiewicz, M. Application of enzymatic apple pomace hydrolysate to production of 2,3-butanediol by alkaliphilic Bacillus licheniformis NCIMB 8059. J. Ind. Microbiol Biotechnol. 2015, 42, 1609–1621. [Google Scholar] [CrossRef]
- Liakou, V.; Pateraki, C.; Palaiogeorgou, A.M.; Kopsahelis, N.; de Castro, A.M.; Freire, D.M.G.; Nychas, G.J.E.; Papanikolaou, S.; Koutinas, A. Valorisation of fruit and vegetable waste from open markets for the production of 2,3-butanediol. Food Bioprod. Process. 2018, 108, 27–36. [Google Scholar] [CrossRef]
- Yuan, J.; He, Y.-Z.; Guo, Z.-W.; Gao, H.-F.; Chen, F.-B.; Li, L.-Z.; Li, Y.-Y.; Zhang, L.-Y. Utilization of Sweet Sorghum Juice for Efficient 2,3-Butanediol Production by Serratia marcescens H30. BioResources 2017, 12, 4926–4942. [Google Scholar] [CrossRef]
- Lee, H.K.; Maddox, I.S. Microbial production of 2,3-butanediol from whey permeate. Biotechnol. Lett. 1984, 6, 815–818. [Google Scholar] [CrossRef]
- Speckman, R.A.; Collins, E.B. Microbial production of 2,3-butylene glycol from cheese whey. Appl. Environ. Microbiol. 1982, 43, 1216–1218. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Y.; Guo, J.; Wang, Q.; Zhang, Y.; Chen, Y.; Zhang, C.; Xiao, D. Efficient production of 2,3-butanediol from cheese whey powder (CWP) solution by Klebsiella pneumoniae through integrating pulsed fed-batch fermentation with a two-stage pH control strategy. Fuel 2017, 203, 469–477. [Google Scholar] [CrossRef]
- Sasmal, S.; Sarkar, S.; Aliaga-Alcalde, N.; Mohanta, S. Syntheses, structures, and magnetic properties of three one-dimensional end-to-end azide/cyanate-bridged copper(II) compounds exhibiting ferromagnetic interaction: New type of solid state isomerism. Inorg. Chem. 2011, 50, 5687–5695. [Google Scholar] [CrossRef]
- Cho, S.; Kim, T.; Woo, H.M.; Kim, Y.; Lee, J.; Um, Y. High production of 2,3-butanediol from biodiesel-derived crude glycerol by metabolically engineered Klebsiella oxytoca M1. Biotechnol. Biofuels 2015, 8, 146. [Google Scholar] [CrossRef]
- Yang, T.W.; Rao, Z.M.; Zhang, X.; Xu, M.J.; Xu, Z.H.; Yang, S.T. Fermentation of biodiesel-derived glycerol by Bacillus amyloliquefaciens: Effects of co-substrates on 2,3-butanediol production. Appl. Microbiol. Biotechnol. 2013, 97, 7651–7658. [Google Scholar] [CrossRef]
- Kim, T.; Cho, S.; Woo, H.M.; Lee, S.M.; Lee, J.; Um, Y.; Seo, J.H. High production of 2,3-butanediol from glycerol without 1,3-propanediol formation by Raoultella ornithinolytica B6. Appl. Microbiol. Biotechnol. 2017, 101, 2821–2830. [Google Scholar] [CrossRef]
- Tinôco, D.; de Castro, A.M.; Seldin, L.; Freire, D.M.G. Production of (2R,3R)-butanediol by Paenibacillus polymyxa PM 3605 from crude glycerol supplemented with sugarcane molasses. Process Biochem. 2021, 106, 88–95. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Peng, X.P.; Li, W.; Guo, X.W.; Xiao, D.G. Optimization of 2,3-butanediol production by Enterobacter cloacae in simultaneous saccharification and fermentation of corncob residue. Biotechnol. Appl. Bioc. 2014, 61, 501–509. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Y.J.; Zhang, W.; Cheng, K.K.; Liu, H.J.; Zhang, J.A. Screening of a highly inhibitor-tolerant bacterial strain for 2,3-BDO and organic acid production from non-detoxified corncob acid hydrolysate. AMB Express 2019, 9, 153. [Google Scholar] [CrossRef]
- Ling, H.; Cheng, K.; Ge, J.; Ping, W. Corncob Mild Alkaline Pretreatment for High 2,3-Butanediol Production by Spent Liquor Recycle Process. BioEnergy Res. 2017, 10, 566–574. [Google Scholar] [CrossRef]
- Um, J.; Kim, D.G.; Jung, M.Y.; Saratale, G.D.; Oh, M.K. Metabolic engineering of Enterobacter aerogenes for 2,3-butanediol production from sugarcane bagasse hydrolysate. Bioresour. Technol. 2017, 245, 1567–1574. [Google Scholar] [CrossRef]
- Kim, D.G.; Yoo, S.W.; Kim, M.; Ko, J.K.; Um, Y.; Oh, M.K. Improved 2,3-butanediol yield and productivity from lignocellulose biomass hydrolysate in metabolically engineered Enterobacter aerogenes. Bioresour. Technol. 2020, 309, 123386. [Google Scholar] [CrossRef]
- Saratale, G.D.; Jung, M.Y.; Oh, M.K. Reutilization of green liquor chemicals for pretreatment of whole rice waste biomass and its application to 2,3-butanediol production. Bioresour. Technol. 2016, 205, 90–96. [Google Scholar] [CrossRef]
- Hazeena, S.H.; Nair Salini, C.; Sindhu, R.; Pandey, A.; Binod, P. Simultaneous saccharification and fermentation of oil palm front for the production of 2,3-butanediol. Bioresour. Technol. 2019, 278, 145–149. [Google Scholar] [CrossRef]
- Saratale, R.G.; Shin, H.S.; Ghodake, G.S.; Kumar, G.; Oh, M.K.; Saratale, G.D. Combined effect of inorganic salts with calcium peroxide pretreatment for kenaf core biomass and their utilization for 2,3-butanediol production. Bioresour. Technol. 2018, 258, 26–32. [Google Scholar] [CrossRef]
- Cortivo, P.R.D.; Machado, J.; Hickert, L.R.; Rossi, D.M.; Ayub, M.A.Z. Production of 2,3-butanediol by Klebsiella pneumoniae BLh-1 and Pantoea agglomerans BL1 cultivated in acid and enzymatic hydrolysates of soybean hull. Biotechnol. Prog. 2019, 35, e2793. [Google Scholar] [CrossRef]
- Białkowska, A.M.; Jędrzejczak-Krzepkowska, M.; Gromek, E.; Krysiak, J.; Sikora, B.; Kalinowska, H.; Kubik, C.; Schütt, F.; Turkiewicz, M. Effects of genetic modifications and fermentation conditions on 2,3-butanediol production by alkaliphilic Bacillus subtilis. Appl. Microbiol. Biotechnol. 2016, 100, 2663–2676. [Google Scholar] [CrossRef]
- Wang, A.; Xu, Y.; Ma, C.; Gao, C.; Li, L.; Wang, Y.; Tao, F.; Xu, P. Efficient 2,3-butanediol production from cassava powder by a crop-biomass-utilizer, Enterobacter cloacae subsp. dissolvens SDM. PLoS ONE 2012, 7, e40442. [Google Scholar] [CrossRef]
- Dai, J.-Y.; Guan, W.-T.; Xiu, Z.-L. Bioconversion of inulin to 2,3-butanediol by a newly isolated Klebsiella pneumoniae producing inulinase. Process Biochem. 2020, 98, 247–253. [Google Scholar] [CrossRef]
- Maina, S.; Schneider, R.; Alexandri, M.; Papapostolou, H.; Nychas, G.J.; Koutinas, A.; Venus, J. Volumetric oxygen transfer coefficient as fermentation control parameter to manipulate the production of either acetoin or D-2,3-butanediol using bakery waste. Bioresour. Technol. 2021, 335, 125155. [Google Scholar] [CrossRef]
- Kubic, W.L.; Tan, E.C.D. Reactive Extraction Process for Separating 2,3-Butanediol from Fermentation Broth. Ind. Eng. Chem. Res. 2023, 62, 5241–5251. [Google Scholar] [CrossRef]
- Xiao, Z.; Lu, J.R. Strategies for enhancing fermentative production of acetoin: A review. Biotechnol. Adv. 2014, 32, 492–503. [Google Scholar] [CrossRef]
- Sabra, W.; Quitmann, H.; Zeng, A.P.; Dai, J.Y.; Xiu, Z.L. Microbial Production of 2,3-Butanediol. Compr. Biotechnol. 2011, 3, 87–97. [Google Scholar]
- Hong, J.; Van Duc Long, N.; Harvianto, G.R.; Haider, J.; Lee, M. Design and optimization of multi-effect-evaporation-assisted distillation configuration for recovery of 2,3-butanediol from fermentation broth. Chem. Eng. Process. 2019, 136, 107–115. [Google Scholar] [CrossRef]
- Garg, S.K.; Jain, A. Fermentative production of 2,3-butanediol: A review. Bioresour. Technol. 1995, 51, 103–109. [Google Scholar] [CrossRef]
- Xiu, Z.L.; Zeng, A.P. Present state and perspective of downstream processing of biologically produced 1,3-propanediol and 2,3-butanediol. Appl. Microbiol. Biotechnol. 2008, 78, 917–926. [Google Scholar] [CrossRef]
- Qureshi, N.; Meagher, M.M.; Hutkins, R.W. Recovery of 2,3-Butanediol by Vacuum Membrane Distillation∗. Sep. Sci. Technol. 1994, 29, 1733–1748. [Google Scholar] [CrossRef]
- Shao, P.; Kumar, A. Process energy efficiency in pervaporative and vacuum membrane distillation separation of 2,3-butanediol. Can. J. Chem. Eng. 2011, 89, 1255–1265. [Google Scholar] [CrossRef]
- Afschar, A.S.; Vaz Rossell, C.E.; Jonas, R.; Quesada Chanto, A.; Schaller, K. Microbial production and downstream processing of 2,3-butanediol. J. Biotechnol. 1993, 27, 317–329. [Google Scholar] [CrossRef]
- Harvianto, G.R.; Kang, K.J.; Lee, M. Process Design and Optimization of an Acetic Acid Recovery System in Terephthalic Acid Production via Hybrid Extraction–Distillation Using a Novel Mixed Solvent. Ind. Eng. Chem. Res. 2017, 56, 2168–2176. [Google Scholar] [CrossRef]
- Haider, J.; Harvianto, G.R.; Qyyum, M.A.; Lee, M. Cost- and Energy-Efficient Butanol-Based Extraction-Assisted Distillation Designs for Purification of 2,3-Butanediol for Use as a Drop-in Fuel. ACS Sustain. Chem. Eng. 2018, 6, 14901–14910. [Google Scholar] [CrossRef]
- Shao, P.; Kumar, A. Recovery of 2,3-butanediol from water by a solvent extraction and pervaporation separation scheme. J. Membr. Sci. 2009, 329, 160–168. [Google Scholar] [CrossRef]
- Jeon, S.; Kim, D.K.; Song, H.; Lee, H.J.; Park, S.; Seung, D.; Chang, Y.K. 2,3-Butanediol recovery from fermentation broth by alcohol precipitation and vacuum distillation. J. Biosci. Bioeng. 2014, 117, 464–470. [Google Scholar] [CrossRef]
- Harvianto, G.R.; Haider, J.; Hong, J.; Van Duc Long, N.; Shim, J.J.; Cho, M.H.; Kim, W.K.; Lee, M. Purification of 2,3-butanediol from fermentation broth: Process development and techno-economic analysis. Biotechnol. Biofuels 2018, 11, 18. [Google Scholar] [CrossRef]
- Li, Z.; Teng, H.; Xiu, Z. Aqueous two-phase extraction of 2,3-butanediol from fermentation broths using an ethanol/ammonium sulfate system. Process Biochem. 2010, 45, 731–737. [Google Scholar] [CrossRef]
- Jiang, B.; Li, Z.-G.; Dai, J.-Y.; Zhang, D.-J.; Xiu, Z.-L. Aqueous two-phase extraction of 2,3-butanediol from fermentation broths using an ethanol/phosphate system. Process Biochem. 2009, 44, 112–117. [Google Scholar] [CrossRef]
- Wan, F.; Kang, T.; Liu, A.; Zhou, C.; Liu, S.; Xu, Y.; Si, S. Salt induced phase separation extraction of 2,3-Butanediol from aqueous solutions: Recovery and recycling of potassium triphosphate. Process Biochem. 2023, 125, 222–231. [Google Scholar] [CrossRef]
- Silva, F.L.; Pinheiro, J.C.; Leite, M.J.L.; Proner, M.C.; da Silva, A.F.V.; Freire, D.M.G.; Treichel, H.; Ambrosi, A.; Di Luccio, M. Influence of different PEG/salt aqueous two-phase system on the extraction of 2,3-butanediol. Prep. Biochem. Biotechnol. 2022, 52, 1051–1059. [Google Scholar] [CrossRef]
- Birajdar, S.D.; Rajagopalan, S.; Sawant, J.S.; Padmanabhan, S. Continuous countercurrent liquid–liquid extraction method for the separation of 2,3-butanediol from fermentation broth using n-butanol and phosphate salt. Process Biochem. 2015, 50, 1449–1458. [Google Scholar] [CrossRef]
- Hao, J.; Xu, F.; Liu, H.; Liu, D. Downstream processing of 1,3-propanediol fermentation broth. J. Chem. Technol. Biotechnol. 2006, 81, 102–108. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Zhu, J.; Liu, J. Separation of 2,3-butanediol from fermentation broth by reactive-extraction using acetaldehyde-cyclohexane system. Biotechnol. Bioproc. E 2012, 17, 337–345. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Wu, Y.; Liu, J. Reactive extraction of 2,3-butanediol from fermentation broth. Korean J. Chem. Eng. 2013, 30, 154–159. [Google Scholar] [CrossRef]
- Maina, S.; Dheskali, E.; Papapostolou, H.; Castro, A.M.d.; Guimaraes Freire, D.M.; Nychas, G.J.E.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A. Bioprocess Development for 2,3-Butanediol Production from Crude Glycerol and Conceptual Process Design for Aqueous Conversion into Methyl Ethyl Ketone. ACS Sustain. Chem. Eng. 2021, 9, 8692–8705. [Google Scholar] [CrossRef]
- Sen Gupta, B.; Hashim, M.A.; Ramachandran, K.B.; Sen Gupta, I.; Cui, Z.F. The Effect of Gas Sparging in Cross-Flow Microfiltration of 2,3-Butanediol Fermentation Broth. Eng. Life Sci. 2005, 5, 54–57. [Google Scholar] [CrossRef]
- Soltys, K.A.; Batta, A.K.; Koneru, B. Successful nonfreezing, subzero preservation of rat liver with 2,3-butanediol and type I antifreeze protein. J. Surg. Res. 2001, 96, 30–34. [Google Scholar] [CrossRef]
- Duraisamy, K.; Ha, A.; Kim, J.; Park, A.R.; Kim, B.; Song, C.W.; Song, H.; Kim, J.C. Enhancement of Disease Control Efficacy of Chemical Fungicides Combined with Plant Resistance Inducer 2,3-Butanediol against Turfgrass Fungal Diseases. Plant Pathol. J. 2022, 38, 182–193. [Google Scholar] [CrossRef]
- Kim, B.; Park, A.R.; Song, C.W.; Song, H.; Kim, J.C. Biological Control Efficacy and Action Mechanism of Klebsiella pneumoniae JCK-2201 Producing Meso-2,3-Butanediol against Tomato Bacterial Wilt. Front. Microbiol. 2022, 13, 914589. [Google Scholar] [CrossRef]
- Romano, P.; Granchi, L.; Caruso, M.; Borra, G.; Palla, G.; Fiore, C.; Ganucci, D.; Caligiani, A.; Brandolini, V. The species-specific ratios of 2,3-butanediol and acetoin isomers as a tool to evaluate wine yeast performance. Int. J. Food Microbiol. 2003, 86, 163–168. [Google Scholar] [CrossRef]
- Song, C.W.; Park, J.M.; Chung, S.C.; Lee, S.Y.; Song, H. Microbial production of 2,3-butanediol for industrial applications. J. Ind. Microbiol. Biotechnol. 2019, 46, 1583–1601. [Google Scholar] [CrossRef]
- Allen, J.G.; Flanigan, S.S.; LeBlanc, M.; Vallarino, J.; MacNaughton, P.; Stewart, J.H.; Christiani, D.C. Flavoring Chemicals in E-Cigarettes: Diacetyl, 2,3-Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit-, Candy-, and Cocktail-Flavored E-Cigarettes. Environ. Health Perspect 2016, 124, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Song, H.; Lee, H.J.; Seung, D. In silico aided metabolic engineering of Klebsiella oxytoca and fermentation optimization for enhanced 2,3-butanediol production. J. Ind. Microbiol. Biotechnol. 2013, 40, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, F.; Heuser, B.; Thewes, M.; Kremer, F.; Pischinger, S.; Dahmen, M.; Hechinger, M.; Marquardt, W. Tailor-made fuels for future engine concepts. Int. J. Engine Res. 2015, 17, 16–27. [Google Scholar] [CrossRef]
- Bai, Y.; Page, S.J.; Zhang, J.; Liu, D.; Zhao, X. Kinetic modelling of acid-catalyzed liquid-phase dehydration of bio-based 2, 3-butanediol considering a newly identified by-product and an updated reaction network. Chem. Eng. J. 2020, 389, 124451. [Google Scholar] [CrossRef]
- Harvey, B.G.; Merriman, W.W.; Quintana, R.L. Renewable Gasoline, Solvents, and Fuel Additives from 2,3-Butanediol. ChemSusChem 2016, 9, 1814–1819. [Google Scholar] [CrossRef]
- Staples, O.; Moore, C.M.; Leal, J.H.; Semelsberger, T.A.; McEnally, C.S.; Pfefferle, L.D.; Sutton, A.D. A simple, solvent free method for transforming bio-derived aldehydes into cyclic acetals for renewable diesel fuels. Sustain. Energy Fuels 2018, 2, 2742–2746. [Google Scholar] [CrossRef]
- Rajale, T.; Yang, X.; Judge, E.J.; Moore, C.M.; Martinez, A.; Guo, M.F.; Ramasamy, K.K.; Elander, R.; Sutton, A.D. Separation, recovery and upgrading of 2,3-butanediol from fermentation broth. Biofuels Bioprod. Biorefining 2023, 17, 1003–1011. [Google Scholar] [CrossRef]
- Gaspar, D.J.; Mueller, C.J.; McCormick, R.L.; Martin, J.; Som, S.; Magnotti, G.M.; Burton, J.; Vardon, D.; Dagle, V.; Alleman, T.L.; et al. Top 13 Blendstocks Derived from Biomass for Mixing-Controlled Compression-Ignition (Diesel) Engines: Bioblendstocks with Potential for Decreased Emissions and Improved Operability; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2021. [Google Scholar]
- Dagle, V.L.; Dagle, R.A.; Kovarik, L.; Baddour, F.; Habas, S.E.; Elander, R. Single-step Conversion of Methyl Ethyl Ketone to Olefins over ZnxZryOz Catalysts in Water. Chemcatchem 2019, 11, 3393–3400. [Google Scholar] [CrossRef]
- Affandy, M.; Zhu, C.; Swita, M.; Hofstad, B.; Cronin, D.; Elander, R.; Lebarbier Dagle, V. Production and catalytic upgrading of 2,3-butanediol fermentation broth into sustainable aviation fuel blendstock and fuel properties measurement. Fuel 2023, 333, 126328. [Google Scholar] [CrossRef]
- Cui, X.K.; Zhao, X.B.; Liu, D.H. A novel route for the flexible preparation of hydrocarbon jet fuels from biomass-based platform chemicals: A case of using furfural and 2,3-butanediol as feedstocks. Green Chem. 2018, 20, 2018–2026. [Google Scholar] [CrossRef]
- Nakazono, K.; Takahashi, R.; Yamada, Y.; Sato, S. Dehydration of 2,3-butanediol to produce 1,3-butadiene over Sc2O3 catalyst prepared through hydrothermal aging. Mol. Catal. 2021, 516, 111996. [Google Scholar] [CrossRef]
- Liu, X.; Fabos, V.; Taylor, S.; Knight, D.W.; Whiston, K.; Hutchings, G.J. One-Step Production of 1,3-Butadiene from 2,3-Butanediol Dehydration. Chem.-Eur. J. 2016, 22, 12290–12294. [Google Scholar] [CrossRef] [PubMed]
- Pittman, C.U.; Wuu, S.K.; Jacobson, S.E. 1,3-Butadiene oligomerization catalyzed by polymer-attached palladium complexes—comparison with homogeneous catalysis. J. Catal. 1976, 44, 87–100. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).