Evaluating Green Solvents for Bio-Oil Extraction: Advancements, Challenges, and Future Perspectives
Abstract
1. Introduction
2. Traditional Solvents vs. Green Solvents
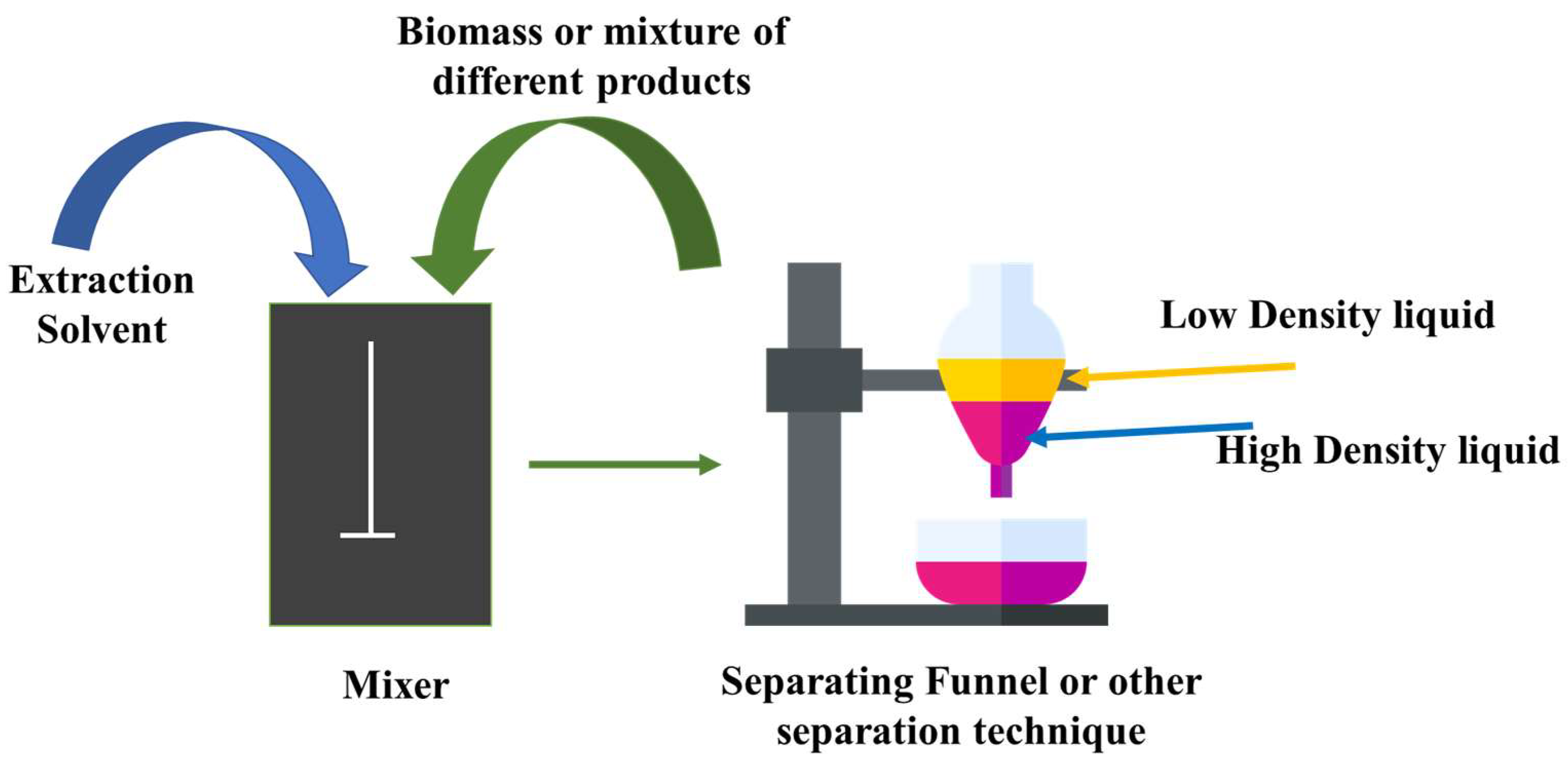
2.1. Solvent Extraction with Traditional Organic Solvents
2.2. Environmental and Health Concerns Associated with Traditional Solvents
“VOC means any compound of carbon, excluding carbon monoxide, carbon dioxide, carbonic acid, metallic carbides or carbonates, and ammonium carbonate, which participates in atmospheric photochemical reactions, except those designated by EPA as having negligible photochemical reactivity”.
“VOCs, are organic chemical compounds whose composition makes it possible for them to evaporate under normal indoor atmospheric conditions of temperature and pressure”[56].
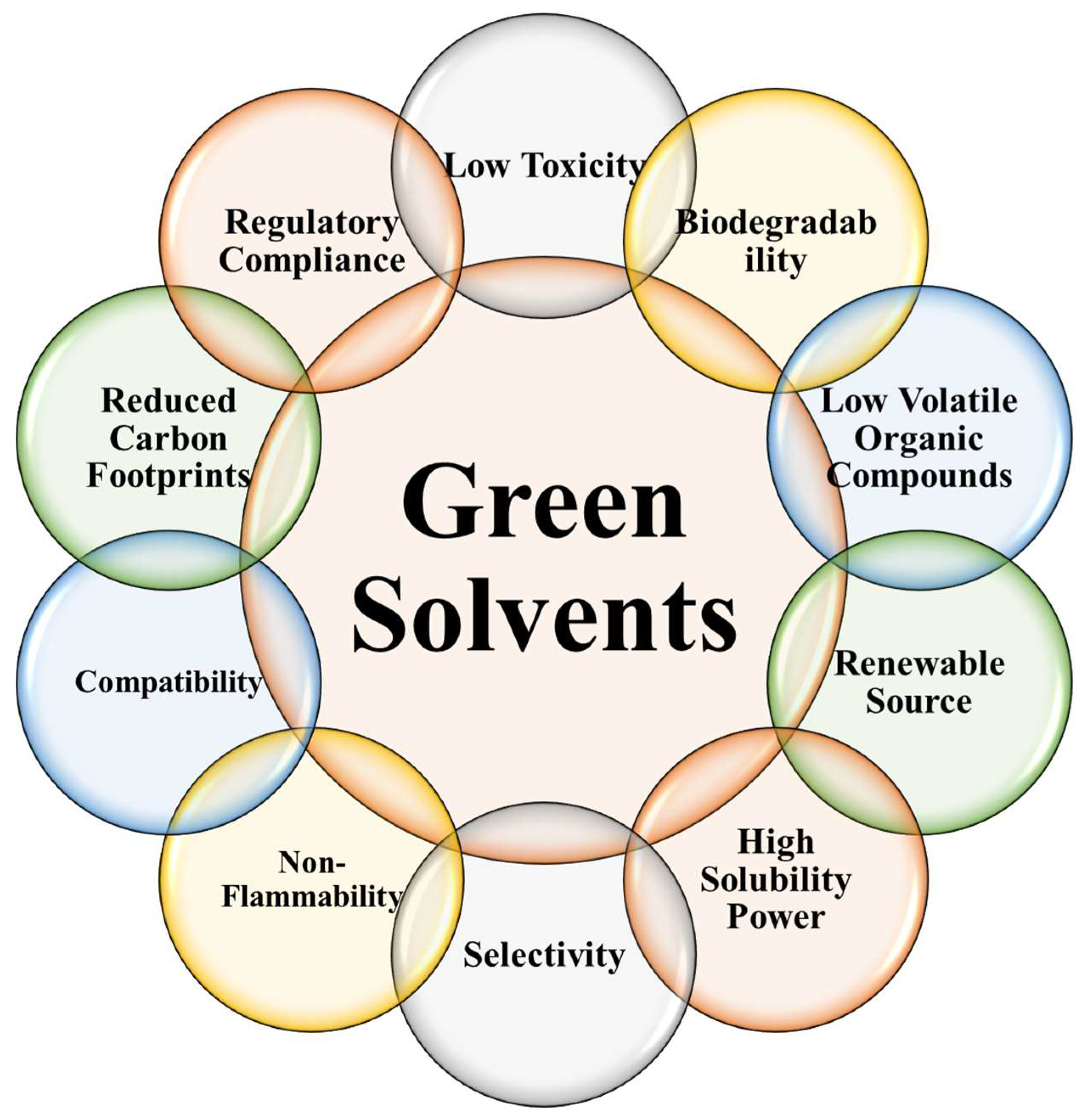
2.3. Introduction to Green Solvents and Their Advantages
3. Classification of Green Solvents
- Composition: Bio-based solvents are derived from renewable biomass sources, such as plants, algae, or microorganisms.
- Properties: Bio-based solvents are typically non-toxic and biodegradable, and have low VOC content. They offer favorable health and environmental profiles compared to petroleum-based solvents.
- Composition: Water is the primary component of water-based solvents, often supplemented with small amounts of other solvents or additives.
- Properties: Water-based solvents are non-toxic, non-flammable, and have low VOC emissions. They are readily available, inexpensive, and have high heat capacity.
- Composition: Supercritical fluids are typically gases or liquids that are above their critical temperature and pressure, resulting in a distinct supercritical state.
- Properties: Supercritical fluids are a hybrid between a gas and a liquid. Their solvating power is adjustable, they have low viscosity, and high diffusivity. The most popular choice for supercritical fluids is supercritical carbon dioxide (CO2).
- Composition: DES are liquid solvents composed of a eutectic mixture of two or more components, typically a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA) [117]. These components can include both bio-based and non-bio-based compounds.
- Properties: They often have low toxicity, low volatility, and high thermal stability. DES can be tailored to possess specific characteristics, such as tunable polarity, viscosity, and solubility, by selecting different combinations of HBD and HBA components [118].
- Applications: DES find applications across various industries and processes. They have been used as green solvents in extraction processes for natural products, such as extraction of bioactive compounds from plant materials. DES have also shown promise in catalysis and electrochemistry, and as reaction media for organic synthesis. In addition, DES have been explored for their potential in industrial applications such as metal processing, biomass conversion, and separation processes [119].
3.1. Bio-Based Solvents
3.1.1. Ethyl Lactate
3.1.2. Limonene
3.1.3. 2-Methyltetrahydrofuran (2-MeTHF)
3.1.4. γ-Valerolactone (GVL)
3.1.5. Dimethyl Carbonate (DMC)
3.1.6. Ionic Liquids (ILs)
3.1.7. Terpenes
3.2. Water-Based Solvents
3.2.1. Water
3.2.2. Aqueous Solution of Acids
3.2.3. Aqueous Solutions of Bases
3.2.4. Aqueous Alcohol Solutions
3.2.5. Aqueous Organic Solvents
3.3. Supercritical Fluids
3.3.1. Supercritical Carbon Dioxide (scCO2)
3.3.2. Supercritical Water (scH2O)
3.3.3. Supercritical Ethanol (scEtOH)
3.3.4. Supercritical Propane (scC3H8)
3.3.5. Supercritical Nitrogen (scN2)
3.4. Deep Eutectic Solvents
3.4.1. Choline Chloride–Urea
3.4.2. Choline Chloride–Glycerol
3.4.3. Choline Chloride–Ethylene Glycol
3.5. Properties of Green Solvents
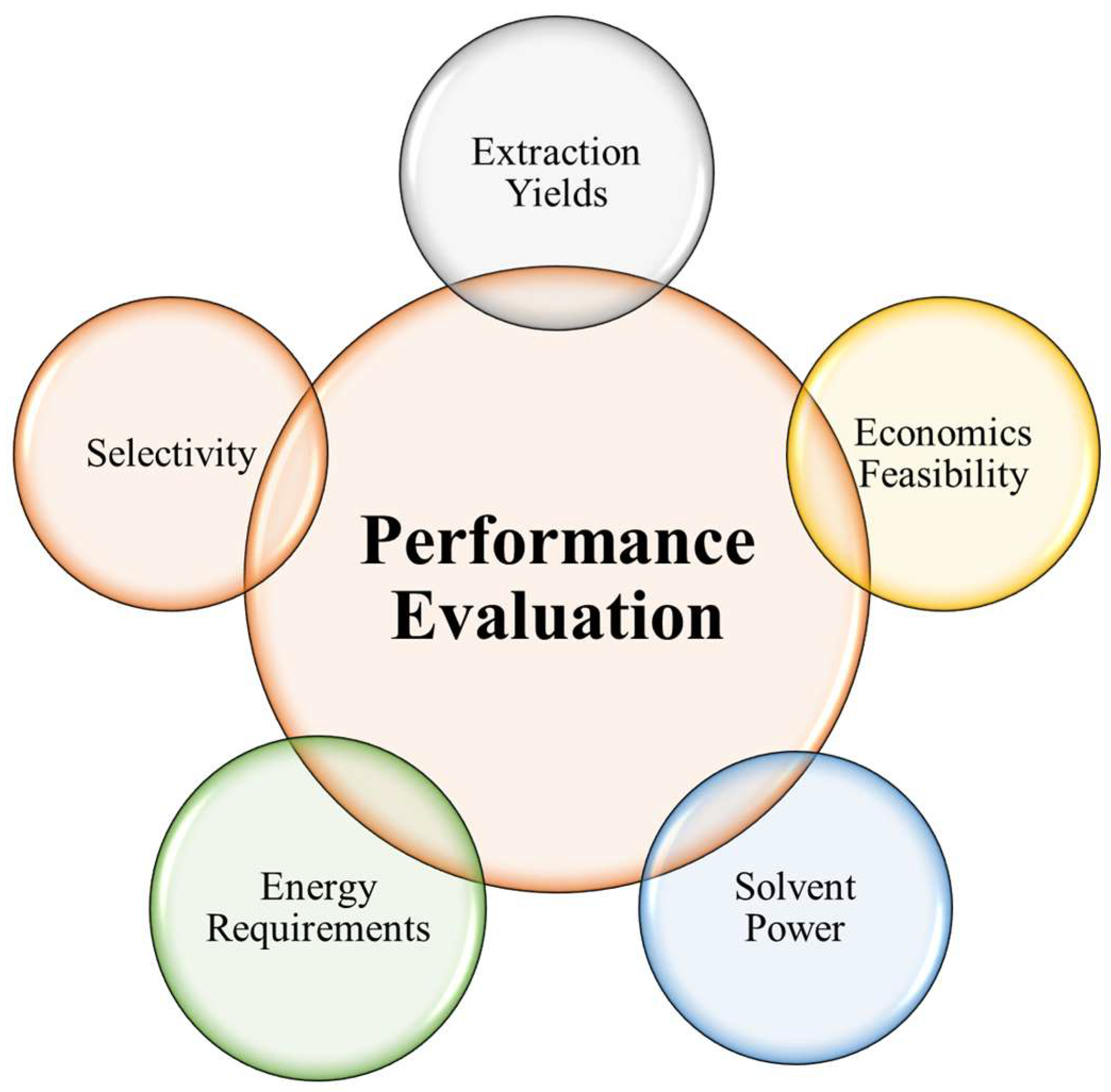
4. Performance Evaluation of Green Solvents
4.1. Solvent Power
4.1.1. Solubility Parameters
Interactions with Different Solute Classes
4.1.2. Bio-Oil Extraction Yields Using Green Solvents
4.1.3. Selectivity
4.2. Energy Requirements
4.2.1. Reduced Energy Consumption
4.2.2. Economics Feasibility and Scalability
5. Environmental Concerns: Challenges and Limitations
5.1. Safety, Health and Environmental Issues
| Solvents | Safety Score | Health Score | Environmental Score | Ranking by Default | Ranking by Discussion | Overall Health and Environmental Concerns | |
|---|---|---|---|---|---|---|---|
| Water- and Bio-Based solvents | Water | 1 | 1 | 1 | Recommended | Recommended | Water and bio-based green extraction solvents offer reduced toxicity and environmental impact. Concerns include water pollution, human health effects (inhalation and skin contact), ecological impacts on aquatic ecosystems, and sustainability of sourcing and production. Proper waste management, safety measures, and research are essential for safe and responsible use. |
| Ethanol | 4 | 3 | 3 | Problematic | Recommended | ||
| EA | 5 | 3 | 3 | Recommended | Recommended | ||
| Diethyl ether | 10 | 3 | 7 | Hazardous | Highly Hazardous | ||
| MTBE | 8 | 3 | 5 | Hazardous | Hazardous | ||
| MeTHF | 6 | 5 | 3 | Problematic | Problematic | ||
| DME | 7 | 10 | 3 | Hazardous | Hazardous | ||
| Cyclohexane | 6 | 3 | 7 | Problematic | Problematic | ||
| Benzene | 6 | 10 | 3 | Hazardous | Highly Hazardous | ||
| Carbon disulfide | 9 | 7 | 7 | Hazardous | Highly Hazardous | ||
| Glycerol | 1 | 1 | 7 | Problematic | Problematic | ||
| GVL | 1 | 5 | 7 | Problematic | Problematic | ||
| CPME | 7 | 2 | 5 | Problematic | Problematic | ||
| D-limonene | 4 | 2 | 7 | Problematic | Problematic | ||
| p-cymene | 4 | 5 | 5 | Problematic | Problematic | ||
| DMC | 4 | 1 | 3 | Recommended | Recommended | ||
| Ethyl lactate | 3 | 4 | 5 | Problematic | Problematic | Deep Eutectic Solvents (DES) extraction solvents present low toxicity and biodegradability. | |
| DES Solvents | ChCl (HBA) | - | - | - | Not a hazardous substance [235] | ||
| Gly (HBD1) | - | - | - | ||||
| ChCl/Gly | - | - | - | All DESs were toxic [236] | |||
5.2. Other Challenges and Limitations
- Sourcing and availability of biomass feedstock;
- Cost fluctuations and economic feasibility;
- Variability in solvent properties due to biomass composition;
- Limited compatibility with certain solute classes.
- Limited solubility for hydrophobic compounds;
- Potential formation of emulsions in biphasic systems;
- Difficulty in extracting polar compounds;
- Challenges in separating solvent from water after extraction.
- High energy requirements for maintaining supercritical conditions;
- Limited solubility for certain compounds;
- High capital costs associated with supercritical fluid extraction equipment;
- Challenges in recovering and recycling supercritical fluids.
- Limited understanding of long-term environmental impacts;
- Challenges in optimizing DES properties for specific applications;
- Potential for high viscosity and reduced mass transfer rates;
- Limited availability of DES with desired properties.
5.2.1. Bio-Based Solvents Challenges
5.2.2. Water-Based Solvents Challenges
5.2.3. Supercritical Fluid Challenges
5.2.4. DES Challenges
6. Future Research and Outlook
6.1. Bio-Based Solvents
6.2. Water-Based Solvents
6.3. Supercritical Fluids
- Exploration of alternative sources for supercritical fluids with high availability and sustainability.
- Development of innovative methods for extracting and producing supercritical fluids from renewable resources.
- Optimization of supercritical fluid extraction processes to enhance efficiency and reduce energy consumption.
- Development of cost-effective methods for supercritical fluid generation and recovery.
- Modification of supercritical fluid properties through the addition of modifiers or co-solvents to improve selectivity and extraction efficiency.
- Investigation of new solvents or solvent blends with tailored properties for specific extraction applications.
- Evaluation of the environmental impact and carbon footprint of supercritical fluid extraction processes.
- Implementation of sustainable practices in supercritical fluid production, such as using renewable energy sources and reducing waste generation.
- Investigation of scale-up strategies for supercritical fluid extraction processes to facilitate commercial production.
- Application of supercritical fluid extraction in various industries, such as pharmaceuticals, food processing, and natural product extraction.
- Integration of supercritical fluid extraction with other green technologies to enhance overall process efficiency and sustainability.
- Development of innovative extraction techniques and equipment to improve the performance and versatility of supercritical fluid extraction.
6.4. DES Solvents
- Exploration of novel DES compositions to expand the range of solvents available for different extraction applications.
- Investigation of the influence of DES composition and structure on extraction efficiency, selectivity, and solute compatibility.
- Development of environmentally friendly and sustainable methods for synthesizing DES.
- Assessment of the environmental impact and biodegradability of DES to ensure their safe and sustainable use.
- Optimization of DES extraction processes through the study of extraction parameters, such as temperature, pressure, and solvent-to-feed ratio.
- Development of innovative extraction techniques and equipment to improve the efficiency and scalability of DES-based extraction processes.
- Development of efficient and cost-effective methods for the recovery and recycling of DES to minimize waste generation.
- Exploration of techniques for regenerating and reusing DES to enhance the economic feasibility and sustainability of the extraction process.
- Investigation of the solute–solvent interactions in DES-based extraction processes to enhance selectivity and improve the extraction of target compounds.
- Understanding the influence of DES composition on solute compatibility and the extraction of different classes of compounds.
- Evaluation of the scalability of DES-based extraction processes for industrial applications.
- Demonstration of the feasibility and economic viability of DES extraction on a larger scale in various industries, such as pharmaceuticals, biotechnology, and natural product extraction.
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC Global Warming of 1.5 °C. Available online: https://www.ipcc.ch/sr15/ (accessed on 8 July 2023).
- BP Statistical Review of World Energy 2020. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2020-full-report.pdf (accessed on 8 July 2023).
- Wu, L.; Wei, W.; Chen, Z.; Chen, X.; Ni, B.-J. Long-Chain Alcohol Production in Open Culture Anaerobic Fermentation. Chem. Eng. J. 2023, 452, 139225. [Google Scholar] [CrossRef]
- Zhou, Y.-W.; Kumar, V.; Harirchi, S.; Vigneswaran, V.S.; Murphy, J.D.; Sharma, P.; Tong, Y.W.; Binod, P.; Sindhu, R.; Sarsaiya, S.; et al. Recovery of Value-Added Products from Biowaste: A Review. Bioresour. Technol. 2022, 360, 127565. [Google Scholar] [CrossRef]
- Akindolire, M.A.; Rama, H.; Roopnarain, A. Psychrophilic Anaerobic Digestion: A Critical Evaluation of Microorganisms and Enzymes to Drive the Process. Renew. Sustain. Energy Rev. 2022, 161, 112394. [Google Scholar] [CrossRef]
- Wang, K.; Yun, S.; Xing, T.; Li, B.; Abbas, Y.; Liu, X. Binary and Ternary Trace Elements to Enhance Anaerobic Digestion of Cattle Manure: Focusing on Kinetic Models for Biogas Production and Digestate Utilization. Bioresour. Technol. 2021, 323, 124571. [Google Scholar] [CrossRef]
- Manikandan, S.; Vickram, S.; Sirohi, R.; Subbaiya, R.; Krishnan, R.A.; Karmegam, N.; Sumathijones, C.; Rajagopal, R.; Chang, S.W.; Ravindran, B.; et al. Critical Review of Biochemical Pathways to Transformation of Waste and Biomass into Bioenergy. Bioresour. Technol. 2023, 372, 128679. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Cheng, S.; Cross, J.S. Biomass Feedstocks for Liquid Biofuels Production in Hawaii & Tropical Islands: A Review. Int. J. Renew. Energy Dev. 2021, 11, 111–132. [Google Scholar] [CrossRef]
- Alipour, Z.; Borugadda, V.B.; Wang, H.; Dalai, A.K. Syngas Production through Dry Reforming: A Review on Catalysts and Their Materials, Preparation Methods and Reactor Type. Chem. Eng. J. 2023, 452, 139416. [Google Scholar] [CrossRef]
- Haghighi, M.; Zare, L.; Ghiasi, M. Biodiesel Production from Spirulina Algae Oil over [Cu(H2PDC)(H2O)2] Complex Using Transesterification Reaction: Experimental Study and DFT Approach. Chem. Eng. J. 2022, 430, 132777. [Google Scholar] [CrossRef]
- Kwon, G.; Kim, K.-H.; Kwon, E.E.; Rinklebe, J.; Wang, H.; Kim, K.-H. Beneficial Use of Fe-Impregnated Bentonite as a Catalyst for Pyrolysis of Grass Cut into Syngas, Bio-Oil and Biochar. Chem. Eng. J. 2022, 448, 137502. [Google Scholar] [CrossRef]
- Leng, L.; Zhang, W.; Peng, H.; Li, H.; Jiang, S.; Huang, H. Nitrogen in Bio-Oil Produced from Hydrothermal Liquefaction of Biomass: A Review. Chem. Eng. J. 2020, 401, 126030. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Hu, X.; Gholizadeh, M. Progress in Application of the Pyrolytic Lignin from Pyrolysis of Biomass. Chem. Eng. J. 2021, 419, 129560. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Cross, J.S. Biodiesel Production from Wet Sewage Sludge and Reduced CO2 Emissions Compared to Incineration in Tokyo, Japan. Fuel 2023, 341, 127614. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. The Future of Aviation Soars with HTL-Based SAFs: Exploring Potential and Overcoming Challenges Using Organic Wet Feedstocks. Sustain. Energy Fuels 2023. [Google Scholar] [CrossRef]
- Moon, M.; Park, W.-K.; Lee, S.-Y.; Hwang, K.-R.; Lee, S.; Kim, M.-S.; Kim, B.; Oh, Y.-K.; Lee, J.Y. Utilization of Whole Microalgal Biomass for Advanced Biofuel and Biorefinery Applications. Renew. Sustain. Energy Rev. 2022, 160, 112269. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Vickram, S.; Dey, N.; Gulothungan, G.; Subbaiya, R.; Govarthanan, M.; Karmegam, N.; Kim, W. The Urge of Algal Biomass-Based Fuels for Environmental Sustainability against a Steady Tide of Biofuel Conflict Analysis: Is Third-Generation Algal Biorefinery a Boon? Fuel 2022, 317, 123494. [Google Scholar] [CrossRef]
- Zhu, Z.; Rosendahl, L.; Toor, S.; Yu, D.; Chen, G. Hydrothermal Liquefaction of Barley Straw to Bio-Crude Oil: Effects of Reaction Temperature and Aqueous Phase Recirculation. Appl. Energy 2015, 137, 183–192. [Google Scholar] [CrossRef]
- Rahman, T.; Jahromi, H.K.; Roy, P.; Adhikari, S.; Hassani, E.; Oh, T.-S. Hydrothermal Liquefaction of Municipal Sewage Sludge: Effect of Red Mud Catalyst in Ethylene and Inert Ambiences. Energy Convers. Manag. 2021, 245, 114615. [Google Scholar] [CrossRef]
- Thomsen, L.; Carvalho, P.; Passos, J.S.D.; Anastasakis, K.; Bester, K.; Biller, P. Hydrothermal Liquefaction of Sewage Sludge; Energy Considerations and Fate of Micropollutants during Pilot Scale Processing. Water Res. 2020, 183, 116101. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Y.; Zhang, J.; Schideman, L.; Yu, G.P.; Zhang, P.; Minarick, M. Co-Liquefaction of Swine Manure and Mixed-Culture Algal Biomass from a Wastewater Treatment System to Produce Bio-Crude Oil. Appl. Energy 2014, 128, 209–216. [Google Scholar] [CrossRef]
- Perrier, A.; Delsart, C.; Boussetta, N.; Grimi, N.; Citeau, M.; Vorobiev, E. Effect of Ultrasound and Green Solvents Addition on the Oil Extraction Efficiency from Rapeseed Flakes. Ultrason. Sonochem. 2017, 39, 58–65. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.P.; Chen, F.; Davis, M.M.; Davison, B.D.; Dixon, R.A.; Gilna, P.; Keller, M.B.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Clark, J.H.; Deswarte, F.E.I.; Farmer, T.W. The Integration of Green Chemistry into Future Biorefineries. Biofuels Bioprod. Biorefin. 2009, 3, 72–90. [Google Scholar] [CrossRef]
- De Jesus, S.S.; Filho, R.M. Recent Advances in Lipid Extraction Using Green Solvents. Renew. Sustain. Energy Rev. 2020, 133, 110289. [Google Scholar] [CrossRef]
- Janicka, P.; Płotka-Wasylka, J.; Szczepańska, N.; Chabowska, A.M.; Fares, M.Y.; Andruch, V.; Kaykhaii, M.; Gębicki, J. Trends in the New Generation of Green Solvents in Extraction Processes. Curr. Opin. Green Sustain. Chem. 2022, 37, 100670. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, S.; Banerjee, R.; Agarwal, D.C.; Kulkarni, K.S.; Ramesh, K.P. Green Solvents and Technologies for Oil Extraction from Oilseeds. Chem. Cent. J. 2017, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Af, T. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, E.; Ma, F.; Jiang, K. An Integrated Biorefinery Process for Co-Production of Xylose and Glucose Using Maleic Acid as Efficient Catalyst. Bioresour. Technol. 2021, 325, 124698. [Google Scholar] [CrossRef] [PubMed]
- Stephan, C.; Dicko, M.; Stringari, P.; Coquelet, C. Liquid-Liquid Equilibria of Water + Solutes (Acetic Acid/Acetol/Furfural/Guaiacol/Methanol/Phenol/Propanal) + Solvents (Isopropyl Acetate/Toluene) Ternary Systems for Pyrolysis Oil Fractionation. Fluid Phase Equilibria 2018, 468, 49–57. [Google Scholar] [CrossRef]
- Kanaujia, P.K.; Naik, D.V.; Tripathi, D.; Singh, R.; Poddar, M.K.; Konathala, L.N.S.; Sharma, Y. Pyrolysis of Jatropha Curcas Seed Cake Followed by Optimization of Liquid-liquid Extraction Procedure for the Obtained Bio-Oil. J. Anal. Appl. Pyrolysis 2016, 118, 202–224. [Google Scholar] [CrossRef]
- Kumar, S.; Lange, J.-P.; Van Rossum, G.; Kersten, S.R.A. Bio-Oil Fractionation by Temperature-Swing Extraction: Principle and Application. Biomass Bioenergy 2015, 83, 96–104. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X.; Jiang, X.; Liu, S.; Lin, J.; Lin, X.; Zhang, Y.; Shi, C.; Chaohua, Z.; Yang, J. Application of Polysaccharide-Rich Solution Derived from Waste Macroalgae Enteromorpha Prolifera in Cherry Tomato Preservation and Utilizing Post-Extraction Residue for Crude Bio-Oil Production. Food Chem. 2023, 409, 135301. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Xu, D.; Wang, Y.; Wang, Y.; Kapusta, K.; Guo, Y. Catalytic Hydrothermal Liquefaction of Municipal Sludge for Biocrude Production over Non-Noble Bimetallic Catalyst in Ethanol Solvent. Fuel 2023, 331, 125812. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, H.; Xu, S.; Qian, L.-X.; Li, H.; Gao, J.; Zhao, G.; Ray, M.B.; Xu, C. Influence of Extraction Solvents on the Recovery Yields and Properties of Bio-Oils from Woody Biomass Liquefaction in Sub-Critical Water, Ethanol or Water–Ethanol Mixed Solvent. Fuel 2022, 307, 121930. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Lu, X.; Kan, T.; Weldekidan, H.; He, J.; Dastjerdi, B.; Scott, J. Bio-Oil Upgrading with Catalytic Pyrolysis of Biomass Using Copper/Zeolite-Nickel/Zeolite and Copper-Nickel/Zeolite Catalysts. Bioresour. Technol. 2019, 279, 404–409. [Google Scholar] [CrossRef]
- Li, B.; Song, M.; Xie, X.; Wei, J.; Xu, D.; Ding, K.; Huang, Y.; Zhang, S.; Hu, X.; Zhang, S.; et al. Oxidative Fast Pyrolysis of Biomass in a Quartz Tube Fluidized Bed Reactor: Effect of Oxygen Equivalence Ratio. Energy 2023, 270, 126987. [Google Scholar] [CrossRef]
- Aravind, S.V.; Ahmed, G.; Kishore, N. Pyrolysis of Delonix Regia Using Metal Oxide Catalysts and Solvent Effect on Fuel Fraction of Bio-Oil. Results Eng. 2023, 17, 100876. [Google Scholar] [CrossRef]
- Iliopoulou, E.F.; Pachatouridou, E.; Marianou, A.A.; Michailof, C.; Kalogiannis, K.K.; Lappas, A.A. Catalytic Pyrolysis of Olive Mill Wastes towards Advanced Bio-Fuels and Bio-Chemicals Using Metal Oxide Catalysts. Catal. Today 2023, 420, 114151. [Google Scholar] [CrossRef]
- Andrade, Y.; Schneider, J.K.; Farrapeira, R.; Lucas, A.N.L.; Da Mota, I.D.P.; Bjerk, T.R.; Krause, L.C.; Caramão, E.B.; Hynek, R. Chromatographic Analysis of N-compounds from the Pyrolysis of Spent Coffee Grounds. SSC Plus 2022, 6, 2200057. [Google Scholar] [CrossRef]
- Li, H.; Xia, S.; Li, Y.; Ma, P.; Zhao, C. Stability Evaluation of Fast Pyrolysis Oil from Rice Straw. Chem. Eng. Sci. 2015, 135, 258–265. [Google Scholar] [CrossRef]
- Li, H.; Xia, S.; Ma, P. Upgrading Fast Pyrolysis Oil: Solvent–Anti-Solvent Extraction and Blending with Diesel. Energy Convers. Manag. 2016, 110, 378–385. [Google Scholar] [CrossRef]
- Tsirigka, A.; Ntoula, M.; Kontogiannopoulos, K.N.; Karabelas, A.J.; Patsios, S.I. Optimization of Solvent Extraction of Lipids from Yarrowia Lipolytica towards Industrial Applications. Fermentation 2022, 9, 35. [Google Scholar] [CrossRef]
- Shafi, A.; Farooq, U.; Akram, K.; Majeed, H.; Hakim, A.; Jayasinghe, M. Cucumis Melo Seed Oil: Agro-food By-product with Natural Anti-hyperlipidemic Potential. J. Sci. Food Agric. 2022, 103, 1644–1650. [Google Scholar] [CrossRef]
- Özcan, M.M.; Öztürk, Ö.; Lemiasheuski, V. Quality Properties, Fatty Acid Composition, and Mineral Contents of Some Citrus Seeds and Oils Extracted by Solvent Extraction. Erwerbs-Obstbau 2022, 65, 127–132. [Google Scholar] [CrossRef]
- Abreu-Jaureguí, C.; Reynel-Ávila, H.E.; Bonilla-Petriciolet, A. Biodiesel Production from Wastewater Scum of Dairy Industry: Lipid Extraction Studies and Reaction Routes. Fuel 2023, 342, 127868. [Google Scholar] [CrossRef]
- Usman, M.; Shou, C.; Cross, J.S. Biodiesel Production from Urban and Suburban Municipal Sewage Sludges in Tokyo, Japan. In Proceedings of the 2022 International Conference and Utility Exhibition on Energy, Environment and Climate Change (ICUE), Pattaya, Thailand, 26–28 October 2022. [Google Scholar] [CrossRef]
- Patiño, Y.; Mantecón, L.; Polo, S.M.T.; Faba, L.; Díaz, E.; Ordóñez, S. Effect of Sludge Features and Extraction-Esterification Technology on the Synthesis of Biodiesel from Secondary Wastewater Treatment Sludges. Bioresour. Technol. 2018, 247, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Dancs, G.; Kakucska, G.; Dobrányi, S.; Ecker, J.; Fülöp, L. Efficient Method for the Determination of the Neutral Lipid Content of Oil-Producing Microalgae Strains Required for Biodiesel. Fuel 2023, 331, 125831. [Google Scholar] [CrossRef]
- Eswaramoorthi, Y.; Pandian, S.; Sahadevan, R. Kinetic studies on the extraction of oil from a new feedstock (Chukrasia tabularis L. seed) for biodiesel production using a heterogeneous catalyst. Environ. Sci. Pollut. Res. 2022, 30, 14565–14579. [Google Scholar] [CrossRef]
- Ntalikwa, J.W. Solvent Extraction of Jatropha Oil for Biodiesel Production: Effects of Solvent-to-Solid Ratio, Particle Size, Type of Solvent, Extraction Time, and Temperature on Oil Yield. J. Renew. Energy 2021, 2021, 9221168. [Google Scholar] [CrossRef]
- Kesharvani, S.; Dwivedi, G.; Verma, T.N.; Verma, P. Sustainable Alternative Fuel Derived from Different Feedstocks and Its Comparative Life Cycle Assessment. Sustain. Energy Technol. Assess. 2023, 57, 103159. [Google Scholar] [CrossRef]
- HSE Solvents—Controlling Hazardous Substances in Construction. Available online: https://www.hse.gov.uk/construction/healthrisks/hazardous-substances/solvents.htm (accessed on 8 July 2023).
- European Commission Extraction Solvents. Available online: https://food.ec.europa.eu/safety/food-improvement-agents/extraction-solvents_en (accessed on 8 July 2023).
- Rury, M. The Hidden Dangers of Organic Solvents. Biotage 2023. Available online: https://www.biotage.com/blog/the-hidden-dangers-of-organic-solvents (accessed on 8 July 2023).
- EPA Indoor Air Quality (IAQ) | US EPA. Available online: https://www.epa.gov/indoor-air-quality-iaq (accessed on 8 July 2023).
- EPA What Are Volatile Organic Compounds (VOCs)? | US EPA. Available online: https://www.epa.gov/indoor-air-quality-iaq/what-are-volatile-organic-compounds-vocs (accessed on 8 July 2023).
- Bamai, Y.A. Semi-Volatile Organic Compounds (SVOCs): Phthalates and Phosphorous Frame Retardants and Health Risks. In Current Topics in Environmental Health and Preventive Medicine; Springer Nature: Berlin/Heidelberg, Germany, 2019; pp. 159–178. [Google Scholar]
- Salthammer, T. Very Volatile Organic Compounds: An Understudied Class of Indoor Air Pollutants. Indoor Air 2014, 26, 25–38. [Google Scholar] [CrossRef]
- EPA Methanol. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/methanol.pdf (accessed on 8 July 2023).
- ATSDR Methanol, Toxicological Profiles | ATSDR. Available online: https://www.atsdr.cdc.gov/toxprofiledocs/index.html (accessed on 8 July 2023).
- ECHA Substance Information—ECHA. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.028.773 (accessed on 8 July 2023).
- CDC CDC—NIOSH Pocket Guide to Chemical Hazards—Methyl Alcohol. Available online: https://www.cdc.gov/niosh/npg/npgd0397.html (accessed on 8 July 2023).
- DCCEEW Methanol. Available online: https://www.dcceew.gov.au/environment/protection/npi/substances/fact-sheets/methanol#:~:text=Methanol%20may%20affect%20animals%2C%20birds,affect%20their%20appearance%20or%20behaviour (accessed on 8 July 2023).
- Bell-Young, L. What Is Dichloromethane? ReAgent Chemical Services. 2021. Available online: https://www.chemicals.co.uk/blog/what-is-dichloromethane (accessed on 8 July 2023).
- CDC Methylene Chloride | Toxicological Profile | ATSDR. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=234&tid=42 (accessed on 8 July 2023).
- ECHA Dichloromethane: Substance Information—ECHA. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.000.391 (accessed on 8 July 2023).
- EPA Methylene Chloride (Dichloromethane). Available online: https://www.epa.gov/sites/default/files/2016-09/documents/methylene-chloride.pdf (accessed on 8 July 2023).
- DCCEEW Ethanol (Ethyl Alcohol)—DCCEEW. Available online: https://www.dcceew.gov.au/environment/protection/npi/substances/fact-sheets/ethanol-ethyl-alcohol (accessed on 8 July 2023).
- US EPA TLVs and BEIs: Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents & Biological Exposure Indices. Available online: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/7310932 (accessed on 8 July 2023).
- CDC CDC—NIOSH Pocket Guide to Chemical Hazards—Endrin. Available online: https://www.cdc.gov/niosh/npg/npgd0252.html (accessed on 8 July 2023).
- EPA Ethanol. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/ethanol.pdf (accessed on 8 July 2023).
- EPA Chloroform. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/chloroform.pdf (accessed on 8 July 2023).
- DCCEEW Chloroform (Trichloromethane). Available online: https://www.dcceew.gov.au/environment/protection/npi/substances/fact-sheets/chloroform-trichloromethane (accessed on 8 July 2023).
- CDC Chloroform | Toxicological Profile | ATSDR. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=53&tid=16 (accessed on 8 July 2023).
- IARC IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Available online: https://publications.iarc.fr/_publications/media/download/2931/d7a4e802483b1374482768a36a7c78e1b33aa1c8.pdf (accessed on 8 July 2023).
- EPA Hexane. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/hexane.pdf (accessed on 8 July 2023).
- National Academies Press (US) N-Hexane: Acute Exposure Guideline Levels. Available online: https://www.ncbi.nlm.nih.gov/books/NBK201488/#:~:text=In%20humans%2C%20n%2Dhexane%20is,n%2Dhexane%20or%20commercial%20hexane (accessed on 8 July 2023).
- CDC N-Hexane | Toxicological Profile | ATSDR. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=393&tid=68 (accessed on 8 July 2023).
- CDC NIOSH Pocket Guide to Chemical Hazards-Hexafluoroacetone. Available online: https://www.cdc.gov/niosh/npg/npgd0319.html (accessed on 8 July 2023).
- NJ Diethyl Ether. Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/0701.pdf (accessed on 8 July 2023).
- HS Toxicological Summary for: Ethyl Ether. Available online: https://www.health.state.mn.us/communities/environment/risk/docs/guidance/gw/ethylether.pdf (accessed on 8 July 2023).
- CDC—NIOSH Pocket Guide to Chemical Hazards—Dichlorvos. Available online: https://www.cdc.gov/niosh/npg/npgd0202.html (accessed on 8 July 2023).
- EPA Ethyl Ether. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/ethyl-ether.pdf (accessed on 8 July 2023).
- Acetone: General Information. Available online: https://www.gov.uk/government/publications/acetone-properties-and-incident-management/acetone-general-information (accessed on 8 July 2023).
- DCCEEW Acetone. Available online: https://www.dcceew.gov.au/environment/protection/npi/substances/fact-sheets/acetone#:~:text=It%20can%20move%20from%20the,can%20be%20degraded%20within%20days (accessed on 8 July 2023).
- CDC Acetone | Toxicological Profile | ATSDR. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=5&tid=1 (accessed on 8 July 2023).
- CDC NIOSH Pocket Guide to Chemical Hazards -Acetylene Tetrabromide. Available online: https://www.cdc.gov/niosh/npg/npgd0009.html (accessed on 8 July 2023).
- CDC Acetone. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/acetone.pdf (accessed on 8 July 2023).
- Benzene—DCCEEW. Available online: https://www.dcceew.gov.au/environment/protection/npi/resource/student/benzene#:~:text=What%20effect%20does%20benzene%20have,to%20contaminate%20water%20and%20soil (accessed on 8 July 2023).
- Duarte-Davidson, R.; Courage, C.; Rushton, L.; Levy, L. Benzene in the environment: An assessment of the potential risks to the health of the population. Occup. Environ. Med. 2001, 58, 2–13. [Google Scholar] [CrossRef]
- CDC Benzene | Toxicological Profile | ATSDR. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=40&tid=14 (accessed on 8 July 2023).
- EPA Toxicological Profile for Benzene | US EPA. Available online: https://www.epa.gov/foia/toxicological-profile-benzene (accessed on 8 July 2023).
- Iarc Benzene. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Benzene-2018 (accessed on 8 July 2023).
- EPA Benzene. Available online: https://www.epa.gov/sites/default/files/2020-07/documents/benzene.pdf (accessed on 8 July 2023).
- NJ Furfural-Hazardous Substance Fact Sheet. Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/0953.pdf (accessed on 8 July 2023).
- CDC Fluorides, Hydrogen Fluoride, and Fluorine | Toxicological Profile | ATSDR. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=212&tid=38 (accessed on 8 July 2023).
- CDC CDC—NIOSH Pocket Guide to Chemical Hazards—Grain Dust (Oat, Wheat, Barley). Available online: https://www.cdc.gov/niosh/npg/npgd0305.html (accessed on 8 July 2023).
- EPA Furfural (Furan-2-Carbaldehyde). Available online: https://www.epa.gov/sites/default/files/2016-09/documents/furfural.pdf (accessed on 8 July 2023).
- Morales-Gonzalez, O.M.; Zhang, C.; Li, S.; Hessel, V. Solvent Impact Assessment for the “One-Flow Functional Solvent Factory”. Chem. Eng. Sci. X 2019, 3, 100024. [Google Scholar] [CrossRef]
- CDC CDC—NIOSH Pocket Guide to Chemical Hazards—Acetylsalicylic Acid. Available online: https://www.cdc.gov/niosh/npg/npgd0010.html (accessed on 8 July 2023).
- EPA Acetic Acid. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/acetic-acid.pdf (accessed on 8 July 2023).
- Atsdr Toxicological Profile for Stoddard Solvent. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp79.pdf (accessed on 8 July 2023).
- CDC NIOSH Pocket Guide to Chemical Hazards-Phosgene. Available online: https://www.cdc.gov/niosh/npg/npgd0504.html (accessed on 8 July 2023).
- EPA Guidance for Hazardous Waste Combustion Units—Petroleum Refining Industry. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/petroleum-refining-industry.pdf (accessed on 8 July 2023).
- Sheldon, R.A. Green Solvents for Sustainable Organic Synthesis: State of the Art. Green Chem. 2005, 7, 267. [Google Scholar] [CrossRef]
- Conelli, D.; Margiotta, N.; Grisorio, R.; Suranna, G.P. Implementation of Sustainable Solvents in Green Polymerization Approaches. Macromol. Chem. Phys. 2020, 222, 2000382. [Google Scholar] [CrossRef]
- Marteel-Parrish, A.E.; Abraham, M.A. Green Chemistry and Engineering: A Pathway to Sustainability. 2013. Available online: https://www.perlego.com/book/997542/green-chemistry-and-engineering-a-pathway-to-sustainability-pdf (accessed on 8 July 2023).
- Imperato, G.; König, B.; Chiappe, C. Ionic Green Solvents from Renewable Resources. Eur. J. Org. Chem. 2007, 2007, 1049–1058. [Google Scholar] [CrossRef]
- Warner, P.T.A.J.C. Green Chemistry: Theory and Practice. CiNii Books. 1998. Available online: https://www.amazon.com/Green-Chemistry-Practice-Paul-Anastas/dp/0198506988 (accessed on 8 July 2023).
- Dicks, A.P.; Hent, A. Green Chemistry Metrics: A Guide to Determining and Evaluating Process Greenness; Springer: Berlin/Heidelberg, Germany, 2014; Available online: https://link.springer.com/book/10.1007/978-3-319-10500-0 (accessed on 8 July 2023).
- Sheldon, R.A. Metrics of Green Chemistry and Sustainability: Past, Present, and Future. ACS Sustain. Chem. Eng. 2017, 6, 32–48. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Zimmerman, J.B.; De Winter, T.M.; Petitjean, L.; Melnikov, F.; Lam, C.B.I.; Lounsbury, A.W.; Mellor, K.E.; Janković, N.; Tu, Q.; et al. The Green ChemisTREE: 20 Years after Taking Root with the 12 Principles. Green Chem. 2018, 20, 1929–1961. [Google Scholar] [CrossRef]
- Pereira, C.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl Lactate as a Solvent: Properties, Applications and Production Processes—A Review. Green Chem. 2011, 13, 2658. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Kim, K.-H. Green Synthesis of Metal–Organic Frameworks: A State-of-the-Art Review of Potential Environmental and Medical Applications. Coord. Chem. Rev. 2020, 420, 213407. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical Fluid Extraction and Fractionation of Natural Matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.B.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Smith, E.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.; Looi, C.Y. Applications of Deep Eutectic Solvents in Biotechnology and Bioengineering—Promises and Challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.Z.; Hussein, H.A.; Alshajrawi, O.M.S. Ethyl Lactate as a Green Solvent in the Pharmaceutical Industry. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 185–194. [Google Scholar] [CrossRef]
- Virot, M.; Tomao, V.; Ginies, C.; Chemat, F. Total Lipid Extraction of Food Using D-Limonene as an Alternative to n-Hexane. Chromatographia 2008, 68, 311–313. [Google Scholar] [CrossRef]
- Soszka, E.; Jȩdrzejczyk, M.; Keller, N.; Ruppert, A.M. High Yield Production of 2-Methyltetrahydrofuran Biofuel with Reusable Ni-Co Catalysts. Fuel 2023, 332, 126118. [Google Scholar] [CrossRef]
- Raj, T.; Chandrasekhar, K.; Banu, J.R.; Rene, E.R.; Aslam, M.; Kim, S.H. Synthesis of γ-Valerolactone (GVL) and Their Applications for Lignocellulosic Deconstruction for Sustainable Green Biorefineries. Fuel 2021, 303, 121333. [Google Scholar] [CrossRef]
- Pyo, S.-H.; Park, J.-W.; Chang, T.-S.; Hatti-Kaul, R. Dimethyl Carbonate as a Green Chemical. Curr. Opin. Green Sustain. Chem. 2017, 5, 61–66. [Google Scholar] [CrossRef]
- Lim, J.H.; Chua, L.S.; Mustaffa, A.A. Ionic Liquids as Green Solvent and Their Applications in Bioactive Compounds Extraction from Plants. Process Biochem. 2022, 122, 292–306. [Google Scholar] [CrossRef]
- Da Silva, R.V.; Rocha-Santos, T.; Duarte, A.C. Supercritical Fluid Extraction of Bioactive Compounds. Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Talibi, M.; Hellier, P.; Ladommatos, N. Investigating the Combustion and Emissions Characteristics of Biomass-Derived Platform Fuels as Gasoline Extenders in a Single Cylinder Spark-Ignition Engine; SAE International: Beijing, China, 2017. [Google Scholar]
- Teoh, K.-S.; Melchiorre, M.; Kreth, F.A.; Bothe, A.; Köps, L.; Ruffo, F.; Balducci, A. Γ-Valerolactone as Sustainable and Low-Toxic Solvent for Electrical Double Layer Capacitors. ChemSusChem 2022, 16, e202201845. [Google Scholar] [CrossRef]
- Fiorani, G.; Perosa, A.; Selva, M. Dimethyl Carbonate: A Versatile Reagent for a Sustainable Valorization of Renewables. Green Chem. 2018, 20, 288–322. [Google Scholar] [CrossRef]
- O’Neill, M.; Sankar, M.; Hintermair, U. Sustainable Synthesis of Dimethyl- and Diethyl Carbonate from CO2 in Batch and Continuous Flow—Lessons from Thermodynamics and the Importance of Catalyst Stability. ACS Sustain. Chem. Eng. 2022, 10, 5243–5257. [Google Scholar] [CrossRef]
- Choi, Y.D.; Verpoorte, R. Green Solvents for the Extraction of Bioactive Compounds from Natural Products Using Ionic Liquids and Deep Eutectic Solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, S.; Ma, P. Use of Ionic Liquids as ‘Green’ Solvents for Extractions. J. Chem. Technol. Biotechnol. 2005, 80, 1089–1096. [Google Scholar] [CrossRef]
- Kong, X.-J.; Li, J.-R. An Overview of Metal–Organic Frameworks for Green Chemical Engineering. Engineering 2021, 7, 1115–1139. [Google Scholar] [CrossRef]
- Souza, M.M.V.M.; Santos, M.; Sumere, B.R.; Da Silva, L.C.; Cunha, D.; Martínez, J.; Barbero, G.F. Isolation of Gallic Acid, Caffeine and Flavonols from Black Tea by on-Line Coupling of Pressurized Liquid Extraction with an Adsorbent for the Production of Functional Bakery Products. Lebensm.-Wiss. Technol. 2020, 117, 108661. [Google Scholar] [CrossRef]
- Patra, A.; Abdullah, S.; Pradhan, R.C. Review on the Extraction of Bioactive Compounds and Characterization of Fruit Industry By-Products. Bioresour. Bioprocess. 2022, 9, 14. [Google Scholar] [CrossRef]
- Mahindrakar, K.V.; Rathod, V.K. Ultrasonic Assisted Aqueous Extraction of Catechin and Gallic Acid from Syzygium Cumini Seed Kernel and Evaluation of Total Phenolic, Flavonoid Contents and Antioxidant Activity. Chem. Eng. Process. 2020, 149, 107841. [Google Scholar] [CrossRef]
- Da Silva, L.C.; Souza, M.M.V.M.; Sumere, B.R.; Silva, L.F.; Da Cunha, D.T.; Barbero, G.F.; Bezerra, R.M.N.; Martínez, J. Simultaneous Extraction and Separation of Bioactive Compounds from Apple Pomace Using Pressurized Liquids Coupled On-Line with Solid-Phase Extraction. Food Chem. 2020, 318, 126450. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, H.; Chua, B.L.; Mah, S.H.; Chow, Y.H. An Insight into Extraction, Isolation, Identification and Quantification of Bioactive Compounds from Crataegus monogyna Plant Extract. Rev. Agric. Sci. J.-STAGE 2022, 10, 304–327. [Google Scholar] [CrossRef]
- Muhamad, I.I.; Hassan, N.A.; Mamat, S.S.; Nawi, N.M.; Rashid, W.; Tan, N.A.H. Extraction Technologies and Solvents of Phytocompounds from Plant Materials: Physicochemical Characterization and Identification of Ingredients and Bioactive Compounds from Plant Extract Using Various Instrumentations. In Ingredients Extraction by Physicochemical Methods in Food; Academic Press: London, UK, 2017; pp. 523–560. [Google Scholar]
- Alternative Solvents for Natural Products Extraction; Springer Nature: Berlin/Heidelberg, Germany, 2014; Available online: https://link.springer.com/book/10.1007/978-3-662-43628-8 (accessed on 8 July 2023).
- Chemat, F.; Strube, J. Green Extraction of Natural Products: Theory and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2016; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9783527676828 (accessed on 8 July 2023).
- Zhou, F.; Hearne, Z.; Li, C.-J. Water—the Greenest Solvent Overall. Curr. Opin. Green Sustain. Chem. 2019, 18, 118–123. [Google Scholar] [CrossRef]
- McHugh, M.; Krukonis, V. Supercritical Fluid Extraction: Principles and Practice; Elsevier: Amsterdam, The Netherlands, 2013; Available online: https://www.sciencedirect.com/book/9780080518176/supercritical-fluid-extraction (accessed on 8 July 2023).
- Ray, A.; Dubey, K.; Marathe, S.J.; Singhal, R. Supercritical Fluid Extraction of Bioactives from Fruit Waste and Its Therapeutic Potential. Food Biosci. 2023, 52, 102418. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, H.; Xu, Y.; Hu, M.; Hu, Z.-T.; Wang, J.; Pan, Z. Reactor for Biomass Conversion and Waste Treatment in Supercritical Water: A Review. Renew. Sustain. Energy Rev. 2023, 171, 113031. [Google Scholar] [CrossRef]
- Koschinsky, A.; Garbe-Schönberg, D.; Sander, S.G.; Schmidt, K.; Gennerich, H.-H.; Strauss, H. Hydrothermal Venting at Pressure-Temperature Conditions above the Critical Point of Seawater, 5 °S on the Mid-Atlantic Ridge. Geology 2008, 36, 615. [Google Scholar] [CrossRef]
- Al-Muntaser, A.A.; Varfolomeev, M.A.; Suwaid, M.A.; Feoktistov, D.A.; Yuan, C.; Klimovitskii, A.E.; Gareev, B.I.; Djimasbe, R.; Nurgaliev, D.K.; Kudryashov, S.I.; et al. Hydrogen Donating Capacity of Water in Catalytic and Non-Catalytic Aquathermolysis of Extra-Heavy Oil: Deuterium Tracing Study. Fuel 2021, 283, 118957. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, D.; Hao, B.; Guo, S.; Guo, Y.; Wang, S. Chemical Reactions of Organic Compounds in Supercritical Water Gasification and Oxidation. Water Res. 2021, 190, 116634. [Google Scholar] [CrossRef]
- Subramani, V.B.; Atanda, L.; Doherty, W.O.S.; Rackemann, D.W.; Moghaddam, L. Co-Liquefaction of Cotton Gin Trash and Low-Density Polyethylene Wastes via Supercritical Ethanolysis for Hydrocarbon-Rich Oil. Energy Convers. Manag. 2023, 290, 117216. [Google Scholar] [CrossRef]
- Wang, X.; Xu, W.; Zhang, D.; Li, X.; Shi, J. Structural Characteristics–Reactivity Relationships for Catalytic Depolymerization of Lignin into Aromatic Compounds: A Review. Int. J. Mol. Sci. 2023, 24, 8330. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.; Hardi, F.; Kim, J.; Suh, D.I. Effect of Heating Rate on Biomass Liquefaction: Differences between Subcritical Water and Supercritical Ethanol. Energy 2014, 68, 420–427. [Google Scholar] [CrossRef]
- Furusjö, E.; Akalin, M.; Karagöz, S. Experimental Design for Extraction of Bio-Oils from Flax Seeds under Supercritical Ethanol Conditions. Clean Technol. Environ. Policy 2015, 18, 461–471. [Google Scholar] [CrossRef]
- Zeb, H.; Choi, J.; Kim, Y.; Kim, J. A New Role of Supercritical Ethanol in Macroalgae Liquefaction (Saccharina japonica): Understanding Ethanol Participation, Yield, and Energy Efficiency. Energy 2017, 118, 116–126. [Google Scholar] [CrossRef]
- Kuo, T.M.; Gardner, H. Lipid Biotechnology; CRC Press: Boca Raton, FL, USA, 2002; Available online: https://www.amazon.com/Lipid-Biotechnology-Tsung-Min-Kuo/dp/0824706196 (accessed on 8 July 2023).
- King, J.W. Supercritical Fluid Technology for Lipid Extraction, Fractionation, and Reactions. In Lipid Biotechnology; Taylor and Francis: Abingdon, UK, 2002; pp. 656–680. [Google Scholar] [CrossRef]
- Sun, X.; Li, X.; Tan, X.; Zheng, W.; Zhu, G.; Cai, J.; Zhang, Y.-Y. Pyrolysis of Heavy Oil in Supercritical Multi-Thermal Fluid: An Effective Recovery Agent for Heavy Oils. J. Pet. Sci. Eng. 2021, 196, 107784. [Google Scholar] [CrossRef]
- Ma, C. Injection Production Process of Fluids Produced by Supercritical Water. Oxidation. Patent CN102322248A, 18 January 2021. [Google Scholar]
- Prabhune, A.; Dey, R. Green and Sustainable Solvents of the Future: Deep Eutectic Solvents. J. Mol. Liq. 2023, 379, 121676. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.-L.; Chen, C.-T.A.; Sun, P.; Patel, A.K.; Singhania, R.R.; Nargotra, P.; Dong, C. Deep Eutectic Solvents as Promising Pretreatment Agents for Sustainable Lignocellulosic Biorefineries: A Review. Bioresour. Technol. 2022, 360, 127631. [Google Scholar] [CrossRef] [PubMed]
- Atilhan, M.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep Eutectic Solvents: Physicochemical Properties and Gas Separation Applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- El-Deen, A.K.; Shimizu, K. Deep Eutectic Solvents as Promising Green Solvents in Dispersive Liquid–Liquid Microextraction Based on Solidification of Floating Organic Droplet: Recent Applications, Challenges and Future Perspectives. Molecules 2021, 26, 7406. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Welton, T. Solvents and Sustainable Chemistry. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471, 20150502. [Google Scholar] [CrossRef]
- Oklu, N.K.; Matsinha, L.C.; Makhubela, B.C.E. Bio-Solvents: Synthesis, Industrial Production and Applications. In Solvents, Ionic Liquids and Solvent Effects; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- PubChem Water. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/962 (accessed on 8 July 2023).
- The Drive to Make Things Happen. Available online: https://vrchemistry.chem.ox.ac.uk/potential/text/solutions3.htm (accessed on 8 July 2023).
- PubChem Hazardous Substances Data Bank (HSDB): 82. Available online: https://pubchem.ncbi.nlm.nih.gov/source/hsdb/82#section=FDA-Requirements-(Complete) (accessed on 8 July 2023).
- Sierra-Amor, R.I. CRC Handbook of Laboratory Safety, 5th ed.; Keith Furr, A., Ed.; CRC Press LCC: Boca Raton, FL, USA, 2000; p. 774. ISBN 0-8493-2523-4. [Google Scholar] [CrossRef]
- Vedantu Ethyl Acetate. VEDANTU. 2022. Available online: https://www.vedantu.com/chemistry/ethyl-acetate (accessed on 8 July 2023).
- LSU Ethyl Acetate Solvent Properties. Available online: https://macro.lsu.edu/howto/solvents/ethylacetate.htm (accessed on 8 July 2023).
- LSU Ethyl Ether Solvent Properties. Available online: https://macro.lsu.edu/howto/solvents/ether.htm (accessed on 8 July 2023).
- PubChem Diethyl Ether. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Diethyl-Ether#section=Computed-Descriptors (accessed on 8 July 2023).
- ICSC 1164—Methyl Tert-Butyl Ether. Available online: https://inchem.org/documents/icsc/icsc/eics1164.htm (accessed on 8 July 2023).
- GESTIS-Stoffdatenbank. Available online: https://gestis.dguv.de/data?name=510639&lang=en (accessed on 8 July 2023).
- Pace, V.; Holzer, W.; Hoyos, P.; Hernáiz, M.J.; Alcántara, A.R. 2-Methyltetrahydrofuran. In Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 1–6. [Google Scholar] [CrossRef]
- GESTIS-Stoffdatenbank. Available online: https://gestis.dguv.de/data?name=025460&lang=en (accessed on 8 July 2023).
- Dimethylether. Available online: https://web.archive.org/web/20211106032850/https://encyclopedia.airliquide.com/dimethylether (accessed on 8 July 2023).
- PubChem Cyclohexane. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cyclohexane#section=Structures (accessed on 8 July 2023).
- DCCEEW Cyclohexane. Available online: https://www.dcceew.gov.au/environment/protection/npi/substances/fact-sheets/cyclohexane (accessed on 8 July 2023).
- Admin Benzene (C6H6)—Definition, Discovery, Structure, Resonance, Aromaticity & Uses of Benzene (C6H6). BYJUS 2023. Available online: https://byjus.com/chemistry/benzene/ (accessed on 8 July 2023).
- DCCEEW Carbon Disulfide. Available online: https://www.dcceew.gov.au/environment/protection/npi/substances/fact-sheets/carbon-disulfide#:~:text=Pure%20carbon%20disulfide%20is%20a,at%200.016%20to%200.42%20ppm (accessed on 8 July 2023).
- Worldofchemicals. Glycerol: Properties, Production and Uses. 2017. Available online: worldofchemicals.com (accessed on 8 July 2023).
- Wikipedia. Wikipedia Contributors National Institute for Occupational Safety and Health. 2023. Available online: https://en.wikipedia.org/wiki/National_Institute_for_Occupational_Safety_and_Health (accessed on 8 July 2023).
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A Sustainable Liquid for Energy and Carbon-Based Chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Baird, Z.S.; Uusi-Kyyny, P.; Pokki, J.-P.; Pedegert, E.; Alopaeus, V. Vapor Pressures, Densities, and PC-SAFT Parameters for 11 Bio-Compounds. Int. J. Thermophys. 2019, 40, 102. [Google Scholar] [CrossRef]
- 5614-37-9 CAS | Cyclopentyl Methyl Ether (CPME) | High Purity Solvents | Article No. 03139. Available online: https://www.lobachemie.com/High-Purity-Solvents-03139/CYCLOPENTYL-METHYL-ETHER-CPME-CASNO-5614-37-9.aspx (accessed on 8 July 2023).
- Cyclopentyl Methyl Ether (CPME) | Specialty Solvents | Zeon Corporation. Available online: https://www.zeon.co.jp/en/business/enterprise/special/solvent-cpme/ (accessed on 8 July 2023).
- Gov, N.O. of R. and R.U. D-LIMONENE | CAMEO Chemicals | NOAA. Available online: https://cameochemicals.noaa.gov/chemical/20568 (accessed on 8 July 2023).
- Sonu, K.S.; Mann, B.; Sharma, R.; Kumar, R.; Singh, R. Physico-Chemical and Antimicrobial Properties of d-Limonene Oil Nanoemulsion Stabilized by Whey Protein–Maltodextrin Conjugates. J. Food Sci. Technol. 2018, 55, 2749–2757. [Google Scholar] [CrossRef]
- P-Cymene | 99-87-6. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB9262508.htm (accessed on 8 July 2023).
- Dimethyl Carbonate | 616-38-6. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB8853983.htm (accessed on 8 July 2023).
- Ethyl L(-)-Lactate. Available online: https://www.chembk.com/en/chem/Ethyl%20L(-)-lactate (accessed on 8 July 2023).
- Hansen, C. Hansen Solubility Parameters; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Hildebrand, B.J.H. The Solubility of Non-Electrolytes. CiNii Books 1936. Available online: http://ci.nii.ac.jp/ncid/BA2379215X (accessed on 8 July 2023).
- Panayiotou, C.; Zuburtikudis, I.; Khalifeh, H.A.; Hatzimanikatis, V. Linear Solvation–Energy Relationships (LSER) and Equation-of-State Thermodynamics: On the Extraction of Thermodynamic Information from the LSER Database. Liquids 2023, 3, 66–89. [Google Scholar] [CrossRef]
- Reichardt, C.L.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2010. [Google Scholar] [CrossRef]
- Sicaire, A.-G.; Vian, M.A.; Fine, F.; Joffre, F.; Carré, P.; Tostain, S.; Chemat, F. Alternative Bio-Based Solvents for Extraction of Fat and Oils: Solubility Prediction, Global Yield, Extraction Kinetics, Chemical Composition and Cost of Manufacturing. Int. J. Mol. Sci. 2015, 16, 8430–8453. [Google Scholar] [CrossRef]
- Breil, C.; Meullemiestre, A.; Vian, M.A.; Chemat, F. Bio-Based Solvents for Green Extraction of Lipids from Oleaginous Yeast Biomass for Sustainable Aviation Biofuel. Molecules 2016, 21, 196. [Google Scholar] [CrossRef] [PubMed]
- Mamidipally, P.K.; Liu, S.X. First Approach on Rice Bran Oil Extraction Using Limonene. Eur. J. Lipid Sci. Technol. 2004, 106, 122–125. [Google Scholar] [CrossRef]
- Rebey, I.B.; Bourgou, S.; Detry, P.; Wannes, W.A.; Kenny, T.; Ksouri, R.; Sellami, I.H.; Fauconnier, M.-L. Green Extraction of Fennel and Anise Edible Oils Using Bio-Based Solvent and Supercritical Fluid: Assessment of Chemical Composition, Antioxidant Property, and Oxidative Stability. Food Bioprocess Technol. 2019, 12, 1798–1807. [Google Scholar] [CrossRef]
- Bourgou, S.; Rebey, I.B.; Dakhlaoui, S.; Msaada, K.; Tounsi, M.S.; Ksouri, R.; Fauconnier, M.-L.; Hamrouni-Sellami, I. Green Extraction of Oil FromCarum Carviseeds Using Bio-based Solvent and Supercritical Fluid: Evaluation of Its Antioxidant and Anti-inflammatory Activities. Phytochem. Anal. 2019, 31, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, C.D.; Vian, M.A.; Ginies, C.; Elmaataoui, M.; Chemat, F. Terpenes as Green Solvents for Extraction of Oil from Microalgae. Molecules 2012, 17, 8196–8205. [Google Scholar] [CrossRef]
- Mahmood, W.M.F.W.; Theodoropoulos, C.; Gonzalez-Miquel, M. Enhanced Microalgal Lipid Extraction Using Bio-Based Solvents for Sustainable Biofuel Production. Green Chem. 2017, 19, 5723–5733. [Google Scholar] [CrossRef]
- Li, P.; Kanda, H.; Makino, H. Simultaneous Production of Bio-Solid Fuel and Bio-Crude from Vegetal Biomass Using Liquefied Dimethyl Ether. Fuel 2014, 116, 370–376. [Google Scholar] [CrossRef]
- Li, P.; Makino, H. Liquefied Dimethyl Ether: An Energy-Saving, Green Extraction Solvent. In Alternative Solvents for Natural Products Extraction; Springer: Berlin/Heidelberg, Germany, 2014; pp. 91–106. [Google Scholar] [CrossRef]
- De Jesus, S.S.; Ferreira, G.K.; Moreira, L.S.; Maciel, M.R.W.; Filho, R.M. Comparison of Several Methods for Effective Lipid Extraction from Wet Microalgae Using Green Solvents. Renew. Energy 2019, 143, 130–141. [Google Scholar] [CrossRef]
- Akretche-K, S.; Ferhat, Z.; Amiali, M. Vegetable and Nut Oils Extraction by D-Limonene as Alternative Solvent. Int. J. Agric. Res. 2017, 12, 82–87. [Google Scholar] [CrossRef]
- Ben-Youssef, S.; Fakhfakh, J.; Breil, C.; Vian, M.A.; Chemat, F.; Allouche, N. Green Extraction Procedures of Lipids from Tunisian Date Palm Seeds. Ind. Crops Prod. 2017, 108, 520–525. [Google Scholar] [CrossRef]
- Xiao, Z.; Qingdan, W.; Zheng, X.; Zhang, L.; Zou, D.; Chen, B.; Wang, B.; Liu, F. Thermochemical Liquefaction of Brassica Napus Straw: Effect of Liquefaction Parameters on Biocrude. Ind. Crops Prod. 2022, 188, 115564. [Google Scholar] [CrossRef]
- Yan, W.; Duan, P.; Wang, F.; Xu, Y. Composition of the Bio-Oil from the Hydrothermal Liquefaction of Duckweed and the Influence of the Extraction Solvents. Fuel 2016, 185, 229–235. [Google Scholar] [CrossRef]
- Jiang, J.; Savage, P.E. Using Solvents to Reduce the Metal Content in Crude Bio-Oil from Hydrothermal Liquefaction of Microalgae. Ind. Eng. Chem. Res. 2019, 58, 22488–22496. [Google Scholar] [CrossRef]
- Patil, P.D.; Dandamudi, K.P.R.; Wang, J.; Deng, Q.; Deng, S. Extraction of Bio-Oils from Algae with Supercritical Carbon Dioxide and Co-Solvents. J. Supercrit. Fluids 2018, 135, 60–68. [Google Scholar] [CrossRef]
- Babiker, E.E.; Al-Juhaimi, F.; Choi, Y. Supercritical Fluid Extraction of Phenolic Compounds and Antioxidants from Grape (Vitis labrusca B). Seeds. Plant Foods Hum. Nutr. 2012, 67, 407–414. [Google Scholar] [CrossRef]
- Abdelmoez, W.; Abdelfatah, R.M.; Tayeb, A.M.; Yoshida, H. Extraction of Cottonseed Oil Using Subcritical Water Technology. AIChE J. 2010, 57, 2353–2359. [Google Scholar] [CrossRef]
- Abdelmoez, W.; Abdelhamid, M.I.; Yoshida, H. Extraction of Jojoba Oil Using Subcritical Water Technology. Recent Pat. Chem. Eng. 2012, 5, 63–70. [Google Scholar] [CrossRef]
- Halim, N.D.A.; Abidin, Z.Z.; Izhar, S.; Hean, C.G.; Harun, M. Optimization Studies and Compositional Analysis of Subcritical Water Extraction of Essential Oil from Citrus hystrix DC. leaves. J. Supercrit. Fluids 2021, 178, 105384. [Google Scholar] [CrossRef]
- Alhassan, Y.; Kumar, R.; Bugaje, I.M. Hydrothermal Liquefaction of De-Oiled Jatropha Curcas Cake Using Deep Eutectic Solvents (DESs) as Catalysts and Co-Solvents. Bioresour. Technol. 2016, 199, 375–381. [Google Scholar] [CrossRef]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Aziz, M.F.A.; Sazili, A.Q. Green Extraction of Bioactive Compounds from Plant Biomass and Their Application in Meat as Natural Antioxidant. Antioxidants 2021, 10, 1465. [Google Scholar] [CrossRef]
- Lainez-Cerón, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T.; Palou, E.; López-Malo, A. Extraction of Bioactive Compounds from Plants by Means of New Environmentally Friendly Solvents. In Research and Technological Advances in Food Science; Academic Press: Cambridge, MA, USA, 2022; pp. 301–332. [Google Scholar] [CrossRef]
- Endara, M.J.; Soule, A.J.; Forrister, D.L.; Dexter, K.G.; Pennington, R.T.; Nicholls, J.A.; Loiseau, O.; Kursar, T.A.; Coley, P.D. The Role of Plant Secondary Metabolites in Shaping Regional and Local Plant Community Assembly. J. Ecol. 2021, 110, 34–45. [Google Scholar] [CrossRef]
- Ozturk, B.; Esteban, J.; Gonzalez-Miquel, M. Deterpenation of Citrus Essential Oils Using Glycerol-Based Deep Eutectic Solvents. J. Chem. Eng. Data 2018, 63, 2384–2393. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Hayyan, M.; AlSaadi, M.A.; Akib, S.; Hayyan, A.; Hashim, M.A. Glycerol-Based Deep Eutectic Solvents: Physical Properties. J. Mol. Liq. 2016, 215, 98–103. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, P.; Zheng, W.; Yu, G.-W.; Li, Z.; She, Y.; Lee, M.-R. Three-Stage Microwave Extraction of Cumin (Cuminum cyminum L.) Seed Essential Oil with Natural Deep Eutectic Solvents. Ind. Crops Prod. 2019, 140, 111660. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, Z.; Chen, J.; Yang, G.; He, M. Facile Sulfation of Cellulose via Recyclable Ternary Deep Eutectic Solvents for Low-Cost Cellulose Nanofibril Preparation. Nanoscale Adv. 2023, 5, 356–360. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.-J. Lignocellulosic Biomass Pretreatment by Deep Eutectic Solvents on Lignin Extraction and Saccharification Enhancement: A Review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ragauskas, A.J.; Wan, C. Lignin Extraction and Upgrading Using Deep Eutectic Solvents. Ind. Crops Prod. 2020, 147, 112241. [Google Scholar] [CrossRef]
- Lomba, L.; Giner, B.; Bandrés, I.; Lafuente, C.; Pino, M.R. Physicochemical Properties of Green Solvents Derived from Biomass. Green Chem. 2011, 13, 2062. [Google Scholar] [CrossRef]
- Nanda, B.; Sailaja, M.; Mohapatra, P.K.; Pradhan, R.; Nanda, B.B. Green Solvents: A Suitable Alternative for Sustainable Chemistry. Mater. Today Proc. 2021, 47, 1234–1240. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology Development for the Production of Biobased Products from Biorefinery Carbohydrates—The US Department of Energy’s “Top 10” Revisited. Green Chem. 2010, 12, 539. [Google Scholar] [CrossRef]
- Schuur, B. Selection and Design of Ionic Liquids as Solvents in Extractive Distillation and Extraction Processes. Chem. Pap. 2015, 69, 245–253. [Google Scholar] [CrossRef]
- Zhou, H.; Shaoyuan, B.; Zhang, Y.-N.; Xu, D.; Wang, M.L. Recent Advances in Ionic Liquids and Ionic Liquid-Functionalized Graphene: Catalytic Application and Environmental Remediation. Int. J. Environ. Res. Public Health 2022, 19, 7584. [Google Scholar] [CrossRef]
- CHEM21 Solvent Selection Guide: CHEM21. Available online: http://www.chem21.eu/project/chem21-solvent-selection-guide/ (accessed on 8 July 2023).
- Prat, D.; Wells, A.; Hayler, J.D.; Sneddon, H.F.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.O. CHEM21 Selection Guide of Classical- and Less Classical-Solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- Regulation (EC) No 1907/2006—Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) | Safety and Health at Work EU-OSHA. Available online: https://osha.europa.eu/en/legislation/directives/regulation-ec-no-1907-2006-of-the-european-parliament-and-of-the-council (accessed on 8 July 2023).
- Nejrotti, S.; Antenucci, A.; Pontremoli, C.; Gontrani, L.; Barbero, N.; Carbone, M.; Dini, D. Critical Assessment of the Sustainability of Deep Eutectic Solvents: A Case Study on Six Choline Chloride-Based Mixtures. ACS Omega 2022, 7, 47449–47461. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.; Hayyan, A.; Al-Saadi, M.; AlNashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are Deep Eutectic Solvents Benign or Toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef] [PubMed]
- Lomba, L.; Ribate, M.P.; Sangüesa, E.; Concha, J.; Garralaga, M.A.; Errazquin, D.; García, C.; Giner, B. Deep Eutectic Solvents: Are They Safe? Appl. Sci. 2021, 11, 10061. [Google Scholar] [CrossRef]
- Winterton, N. The Green Solvent: A Critical Perspective. Clean Technol. Environ. Policy 2021, 23, 2499–2522. [Google Scholar] [CrossRef] [PubMed]
- Meshksar, M.; Afshariani, F.; Rahimpour, M.R. Industrial Applications of Green Solvents for Sustainable Development of Technologies in Organic Synthesis. In Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2020; pp. 435–455. [Google Scholar]
- Occhiuzzi, J.; Politano, G.G.; D’Olimpio, G.; Politano, A. The Quest for Green Solvents for the Sustainable Production of Nanosheets of Two-Dimensional (2D) Materials, a Key Issue in the Roadmap for the Ecology Transition in the Flatland. Molecules 2023, 28, 1484. [Google Scholar] [CrossRef] [PubMed]
- Häckl, K.; Kunz, W. Some Aspects of Green Solvents. Comptes Rendus Chim. 2018, 21, 572–580. [Google Scholar] [CrossRef]
- Iqbal, M.; Tao, Y.; Xie, S.; Zhu, Y.; Chen, D.; Wang, X.; Huang, L.; Peng, D.; Sattar, A.; Shabbir, M.A.B.; et al. Aqueous Two-Phase System (ATPS): An Overview and Advances in Its Applications. Biol. Proced. Online 2016, 18, 18. [Google Scholar] [CrossRef]
- Singla, M.; Sit, N. Theoretical Aspects and Applications of Aqueous Two-Phase Systems. ChemBioEng 2022, 10, 65–80. [Google Scholar] [CrossRef]
- Teixeira, A.G.; Agarwal, R.; Ko, K.R.; Grant-Burt, J.; Leung, B.M.; Frampton, J. Emerging Biotechnology Applications of Aqueous Two-Phase Systems. Adv. Healthc. Mater. 2017, 7, 1701036. [Google Scholar] [CrossRef]
- Unido Extraction Technologies for Medicinal and Aromatic Plants. Available online: https://www.unido.org/sites/default/files/2009-10/Extraction_technologies_for_medicinal_and_aromatic_plants_0.pdf (accessed on 8 July 2023).
- Anastas, P.T.; Kirchhoff, M.M. Origins, Current Status, and Future Challenges of Green Chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef]
- Brunner, G. Supercritical Fluids: Technology and Application to Food Processing. J. Food Eng. 2005, 67, 21–33. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Girotra, P.; Singh, S.; Nagpal, K. Supercritical Fluid Technology: A Promising Approach in Pharmaceutical Research. Pharm. Dev. Technol. 2012, 18, 22–38. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, H.; Row, K.H. Application of Deep Eutectic Solvents in the Extraction and Separation of Target Compounds from Various Samples. J. Sep. Sci. 2015, 38, 1053–1064. [Google Scholar] [CrossRef]
- Farooq, M.; Abbasi, N.M.; Anderson, J.L. Deep Eutectic Solvents in Separations: Methods of Preparation, Polarity, and Applications in Extractions and Capillary Electrochromatography. J. Chromatogr. A 2020, 1633, 461613. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, W.; Zhang, N.; Soladoye, O.P.; Zhang, Y.; Fu, Y. Deep Eutectic Solvents as New Media for Green Extraction of Food Proteins: Opportunity and Challenges. Food Res. Int. 2022, 161, 111842. [Google Scholar] [CrossRef] [PubMed]
- Scelsi, E.; Angelini, A.; Frison, N. Deep Eutectic Solvents for the Valorisation of Lignocellulosic Biomasses towards Fine Chemicals. Biomass 2021, 1, 29–59. [Google Scholar] [CrossRef]





| Bio-Oil | Temperature (°C) | Extraction Solvents | Extraction Yield (wt.%) | Ref. | |
|---|---|---|---|---|---|
| Feedstocks | Production Process | ||||
| Biomass | Thermal Liquefaction | 25–70 | Alcohols (i.e., methanol) | 24–65 | [32] |
| Alkanes (i.e., n-hexane) | |||||
| Acetic acids | |||||
| Furfural | |||||
| Diethyl ether | |||||
| Toluene | |||||
| Ethylbenzene | |||||
| Water | |||||
| Water/methanol mixture | |||||
| Microalgae | Hydrothermal Liquefaction (HTL) | 250–310 | Dichloromethane (DCM) | 23 | [33] |
| Sewage sludge (SS) | HTL | 350 | DCM | 9.34 | [34] |
| SS | Catalytic-HTL (catalyst and ethanol as solvent) | 350 | DCM | 61.51 | |
| Rubberwood sawdust | Liquefaction in subcritical water | 280–320 | Acetone | 30.41 | [35] |
| Ethanol | 23.45 | ||||
| DCM | 28.14 | ||||
| Ethyl acetate (EA) | 27.66 | ||||
| Rubberwood sawdust | Liquefaction in subcritical ethanol | 280–320 | Acetone | 30.29 | |
| Ethanol | 25.15 | ||||
| DCM | 29.76 | ||||
| EA | 31.69 | ||||
| Pine wood | Catalytic-Pyrolysis | 500 with heating rate (100/min) | DCM | 61.1–72 | [36] |
| Pine wood | Non-catalytic Pyrolysis | 500 with heating rate (100/min) | DCM | 71.6 | |
| Pine sawdust | Fast Pyrolysis | 500 | Acetone | ~36 * | [37] |
| Pine sawdust | Catalytic-Pyrolysis | 550 | Acetone | 53.4 | |
| Delonix regia (Lignocellulosic biomass) | Catalytic-Pyrolysis | 600 | DCM | 26.48 | [38] |
| Delonix regia | Non-catalytic Pyrolysis | 600 | DCM | 27.18 | |
| Olive mill waste water sludge | Fast Pyrolysis | 500 | DCM | 60 | [39] |
| Spent coffee grounds traditional | Pyrolysis | 700 | DCM | 12.64 | [40] |
| Spent coffee grounds decaffeinated | Pyrolysis | 700 | DCM | 9.90 | |
| Jatropha Curcas seed cake | Slow Pyrolysis | 550 with heating rate (5/min) | EA | 3.79 | [31] |
| DCM | 3.63 | ||||
| Carbon tetrachloride | 1.02 | ||||
| Diethyl ether | 2.50 | ||||
| Benzene | 2.02 | ||||
| hexane | 2.34 | ||||
| Rice straw | Pyrolysis | 500 | DCM | 41–47 | [41,42] |
| Yarrowia lipolytica (oleaginous yeast) | SE + | Room Temperature (RT) | Methanol/Hexane | 10–28 | [43] |
| Sweet melon seeds | SE + | RT | n-Hexane | 0.5–1 | [44] |
| Citrus seeds (mandarin) | SE (Soxhlet) + | 60 | n-Hexane | 27.61 | [45] |
| Citrus seeds (bitter orange) | SE (Soxhlet) + | 60 | n-Hexane | 36.42 | |
| Wastewater scum of dairy industry | SE | 25–50 | Hexane | 48–54 | [46] |
| Wet SS | SE | RT | Hexane | 38–41 | [14] |
| Dewatered SS | SE | RT | Hexane | 30 | [47] |
| Dried SS | SE (Soxhlet) + | n-Hexane | 0.94–1.75 | [48] | |
| Lyophilized algae | SE (Soxhlet) + | n-Hexane | 13.41 | [49] | |
| Chukrasia tabularis L. (C. tabularis) seed | SE (Soxhlet) + | n-Hexane | 27.9 | [50] | |
| Petroleum ether | 27.7 | ||||
| Chloroform | 24 | ||||
| Acetone | 15 | ||||
| Chukrasia tabularis L. (C. tabularis) seed | AUH = + SE (Soxhlet) + | n-Hexane | 32 | ||
| Petroleum ether | 30 | ||||
| Chloroform | 26 | ||||
| Acetone | 15.2 | ||||
| Seeds of Jatropha curcas | SE+ | 25–80 | n-Hexane | 41–46 | [51] |
| Petroleum ether | 39–44 | ||||
| Ethanol | 37–41 | ||||
| Karanja Seed | SE (Soxhlet) + | 50 | n-Hexane | 22–34 | [52] |
| Algae | 40–50 | ||||
| Solvents | Classification | Boiling Range (°C) | Environmental and Human Health Hazards | Ref. | |
|---|---|---|---|---|---|
| Environmental Concerns | Human Health Concerns | ||||
| Methanol | VVOCs | <0 to 50–100 °C | Water Pollution: Methanol contaminates water, harms aquatic ecosystems. Groundwater Contamination: Leaked methanol endangers groundwater quality. Atmospheric Pollution: Methanol worsens smog and formaldehyde formation. | Toxicity: Highly toxic if ingested or absorbed. Inhalation: Respiratory irritation and CNS depression. Fire Hazard: Flammable and explosive. Handle with care. | [55,56,59,60,61,62,63,64] |
| DCM | Improper disposal contaminates water, threatens aquatic life. DCM contributes to ozone depletion, damaging the ozone layer. DCM pollutes air, contributes to ozone and smog formation. | DCM is a potential human carcinogen, linked to liver and lung cancer. Inhalation causes dizziness, headaches, confusion, and potential neurological damage. Metabolized in the liver, DCM can cause liver toxicity and affect kidney function. | [65,66,67,68] | ||
| Ethanol | VOC emissions contribute to ozone and smog formation. Energy-intensive production and crop cultivation impact land and water, and cause deforestation. Improper handling contaminates water and soil, harming ecosystems. | Flammability and Fire Hazard: Highly flammable, requires proper storage and fire safety. Inhalation Risks: Respiratory irritation, CNS depression, potential organ damage. Impaired Judgement and Addiction: Excessive use leads to intoxication, impaired coordination, addiction. | [69,70,71,72] | ||
| Chloroform | Persistence and Bioaccumulation: Chloroform harms ecosystems and aquatic life by persisting and bioaccumulating. Water Contamination: Improper disposal pollutes water, threatens drinking water supplies. Ozone Depletion: Chloroform contributes to upper atmosphere ozone depletion. | Carcinogenic Potential: Probable human carcinogen linked to liver, kidney, and bladder cancers. Central Nervous System Effects: Inhalation causes dizziness, confusion, potential unconsciousness. Liver and Kidney Damage: Chronic exposure leads to toxicity in liver and kidneys. | [73,74,75,76] | ||
| n-Hexane | VOCs | 50–100 to 240–260 | Air Pollution: Volatile emissions contribute to ozone and smog formation in VOC-rich areas. Soil and Water Contamination: Improper disposal harms soil, water, and aquatic life. Hazardous Waste Generation: Hexane use creates hazardous waste, needs proper disposal to prevent pollution. | Inhalation Hazards: Headaches, dizziness, respiratory irritation. Prolonged exposure leads to neuropathy. Flammability: Highly flammable, fire and explosion hazards. Follow proper handling. Occupational Health Risks: Prolonged exposure causes neurological disorders. Implement safety measures. | [55,77,78,79,80] |
| Ethyl ether | VOC Emissions: Contributes to ground-level ozone and smog formation, degrading air quality. Flammability: Highly flammable, forms explosive mixtures. Improper handling poses fire and explosion risks. | Inhalation Hazards: Respiratory irritation, dizziness, CNS depression. Narcotic Effects: Drowsiness, loss of consciousness. Environmental Persistence: Short lifespan, but improper disposal causes contamination. | [81,82,83,84] | ||
| Acetone | VOC Emissions: Degrades air quality, contributes to ozone and smog formation. Water Contamination: Improper disposal harms aquatic ecosystems. Effects on Ozone Layer: Negligible ozone depletion potential, not regulated. | Inhalation Hazards: Respiratory irritation, coughing, CNS depression with excessive exposure. Skin and Eye Irritation: Contact causes dryness, redness, eye irritation. Use protective measures. Flammability: Highly flammable, low flash point. Follow proper storage, fire safety. | [85,86,87,88,89] | ||
| Benzene | Air Pollution: VOC contributes to ozone and smog formation. Water and Soil Contamination: Improper disposal pollutes water and soil, risks ecosystems. Toxic Effects on Plants and Animals: Impairs growth, reduces crop yields, harms aquatic organisms. | Carcinogenicity: Human carcinogen linked to blood-related cancers. Blood Disorders: Affects cell production, causes anemia, impaired immune function. Neurological and Developmental Effects: Headaches, cognitive impairment, potential developmental abnormalities. | [90,91,92,93,94,95] | ||
| Furfural | Air Pollution: Contributes to ozone, smog, degrading air quality. Water Contamination: Improper disposal harms water, risks aquatic organisms. Soil and Ecosystem Impacts: Potential effects on soil, microbial activity. Further study needed. | Irritation and Sensitization: Causes skin, eye, respiratory irritation; risk for individuals with respiratory conditions. Occupational Hazards: Workers at risk; implement safety measures. Carcinogenic Potential: Further research needed on genotoxic and carcinogenic effects. | [96,97,98,99] | ||
| Acetic acid | Water Contamination: Improper disposal contaminates water, harms aquatic organisms. Acidification: Disrupts ecosystems, impacts aquatic life. Hazardous Waste Generation: Proper disposal prevents environmental pollution. | Inhalation Hazards: Irritates respiratory system, worsens pre-existing conditions. Skin and Eye Irritation: Causes burns, eye damage on contact. Corrosive Properties: Damages tissues with prolonged contact. | [100,101,102] | ||
| Petroleum ether | Air Pollution: VOC emissions contribute to ozone, smog formation. Water and Soil Contamination: Improper handling contaminates, harms ecosystems. Persistence: Petroleum ether can persist, contaminate soil, water. | Flammability and Explosion Hazard: Highly flammable, forms explosive mixtures. Inhalation Hazards: Respiratory irritation, CNS effects with prolonged exposure. Skin and Eye Irritation: Causes dryness, redness, temporary vision disturbances. | [103,104,105] | ||
| Solvents | Molar Mass (g.mol−1) | Solubility in Water | Boiling Point (°C) | Melting Point (°C) | Density (g/mL) | Vapor Pressure | Viscosity | Appearance | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Water | 18 | 100 | 0 | 1.000 at 3.98 °C | 23.8 mmHg at 25 °C | 0.8949 mPa·s at 25 °C | colorless | [165,166] | |
| Ethanol | 46.07 | 106 mg/mL at 25 °C | 78.24 | −114.4 | 0.7893 at 20 °C | 10 kPa at 29.2 °C | 1.074 mPa·s at 25 °C | colorless | [167,168] |
| EA | 88.1 | 8.3 g/100 mL at 20 °C | 77.1 | −83.6 | 0.9006 at 20 °C 0.8945 at 25 °C | 73.0 Torr at 20 °C | 0.45 cP at 20 °C | colorless | [169,170] |
| Diethyl ether | 74.12 | 6.05 g/100 mL at 25 °C | 34.55 | −116.3 | 0.7133 at 20 °C 0.7076 at 25 °C | 442 Torr at 20 °C | 0.24 cP at 20 °C | colorless | [171,172] |
| MTBE ** | 88.2 | 4.2 g/100 mL at 20 °C | 55 | −109 | 0.74 at 25 °C | 27 kPa at 20 °C | 0.36 cP at 25 °C | colorless | [173] |
| MeTHF * | 86.13 | 15 g/100 mL at 25 °C | 80.2 | −136 | 0.854 at 25 °C | 3.6 kPa at 20 °C 34.5 kPa at 50 °C | 4 mPa·s at 25 °C | colorless | [174,175] |
| DME ++ | 46.07 | 71 g/L at 20 °C | −24 | −141 | 0.735 (liquid, at −25 °C) | 592.8 kPa at 25 °C | colorless gas | [176,177] | |
| Cyclohexane | 84.161 | immiscible | 80.7 | 6.47 | 0.7739, liquid at 25 °C | 78 mmHg at 20 °C | 1.02 cP at 17 °C | colorless | [178,179] |
| Benzene | 78.114 | immiscible | 80.5 | 5.5 | 0.8765 at 20 °C | 12.7 kPa at 25 °C | 0.6076 cP at 25 °C | colorless | [180] |
| Carbon disulfide | 76.13 | slightly soluble | 46.5 | −111.5 | 1.266 at 25 °C | 48.1 kPa at 25 °C | 0.363 cP at 20 °C | colorless | [181] |
| Glycerol | 92.094 | miscible | 290 | 17.9 | 1.26 at 25 °C | 0.40 Pa at 50 °C | 1.412 Pa·s at 20 °C | colorless | [182,183] |
| GVL | 100.116 | 100 mg/mL | 205 | −31 | 1.0546 at 20 °C | 3.5 kPa at 80 °C | colorless | [184,185] | |
| CPME + | 1.1 g/100 g at 23 °C | 106 | −140 | 0.86 at 25 °C | 59.9 hPa at 25 °C | 0.57 cP at 20 °C | colorless | [186,187] | |
| D-limonene | 136.238 | insoluble | 176 | −74.35 | 0.844 at 25 °C | 190 Pa at 20 °C | 0.8462 mPa·s at 25 °C | colorless | [188,189] |
| p-cymene | 134.222 | insoluble | 177 | −68 | 0.86 at 25 °C | 1.5 mmHg at 20 °C | 0.81–7.1 mm2/s | colorless | [190] |
| DMC | 90.078 | 13.9 g/100 mL at 25 °C | 90 | 2–4 | 1.069 at 25 °C | 18 mmHg at 21.1 °C | 0.625 cP at 20 °C | colorless | [191] |
| Ethyl lactate | 118.131 | miscible | 154.2 | −25 | 1.0328 at 20 °C | 1.16 mmHg at 25 °C | 0.0261 cP at 20 °C | colorless | [192] |
| Feedstocks | Process | Extraction | Green Solvents | Extraction Yield (wt.%) | Conventional Solvents | Extraction Yield (wt.%) | Ref. |
|---|---|---|---|---|---|---|---|
| Rapeseed oil | SE | Fatty acids | MeTHF | 45.96 | n-Hexane | 46.34 | [197] |
| Oleaginous Yeast Biomass | SE | Lipids | MeTHF | 14.56 | n-Hexane | 14.04 | [198] |
| CPME | 15.33 | ||||||
| IPA - | 15.48 | ||||||
| ethanol | 15.02 | ||||||
| ethyl acetate | 14.05 | ||||||
| ethyl lactate | 15.23 | ||||||
| DMC | 14.94 | ||||||
| p-cymene | 15.57 | ||||||
| d-Limonene | 13.67 | ||||||
| α-pinene | 13.97 | ||||||
| Rice bran | SE | Oil | d-limonene | 15.8 | n-Hexane | 18.9 | [199] |
| Fennel seed | SE | Oil | MeTHF | ~20 | n-Hexane | ~15.3 | [200] |
| Anise seed | SE | Oil | MeTHF | ~23 | n-Hexane | ~15.1 | |
| Carum carvi seeds | SE | Oil | MeTHF | ~16 | n-Hexane | ~12 | [201] |
| Microalgae (C. vulgaris) | SE | Oil | d-limonene | 1.29 | n-Hexane | 0.88 | [202] |
| α-pinene | 0.91 | ||||||
| ρ-cymene | 1.52 | ||||||
| Microalgae (Chlorella vulgaris) | SE | Oil | Ethyl acetate | 11.7 | n-Hexane | 7 | [203] |
| Ethyl lactate | 20.8 | ||||||
| CPME+ | 9.4 | ||||||
| MeTHF | 14.8 | ||||||
| Microalgae (Nannochloropsis sp.) | Ethyl acetate | 13.6 | n-Hexane | 9.8 | |||
| Ethyl lactate | 31.1 | ||||||
| CPME | 11.4 | ||||||
| MeTHF | 19.1 | ||||||
| Spent coffee ground | SE | DME Bio-crude | Liquified DME | 16.8 | [204] | ||
| Tea leaf waste | 16.2 | ||||||
| Orange peel | 6.2 | ||||||
| Gramineous weed | 5.8 | ||||||
| Spent coffee ground | SE | DME Bio-crude | Liquified DME | 16.8 | n-Hexane | 17.2 | [205] |
| Tea leaf waste | 16.2 | 1.9 | |||||
| Orange peel | 6.2 | 0.9 | |||||
| Wet microalgae (Chlorella pyrenoidosa) | SE | Lipids | MeTHF | 69.8 | [206] | ||
| CPME | 68.0 | ||||||
| Dried rapeseed (Brassica napus) | SE | FFA | MeTHF | 99.2 | [197] | ||
| Sterols | 91.9 | ||||||
| Almond (dry) | SE | Lipids | D-limonene | 95.7 | [207] | ||
| Corn (dry) | 95.3 | ||||||
| Olive (dry) | 93.2 | ||||||
| Dried palm seeds (Phoenix dactylifera L.) | SE | Lipids | MeTHF | 82.5 | [208] | ||
| Brassica napus straw | SE | HTL Bio-crude | Ethyl acetate + Ethanol | 22.11 | [209] | ||
| Duckweed Lemna minor (L. minor) | SE | HTL Bio-crude | Ethyl acetate | 20 | DCM | 24 | [210] |
| Carbon disulfide | 17 | n-Hexane | 3 | ||||
| Cyclohexane | 9 | Petroleum ether | 4 | ||||
| Benzene | 18 | ||||||
| IPA | 26 | ||||||
| Diethyl ether | 17 | ||||||
| Dichloroethane | 19 | ||||||
| Microalgae (Nannochloropsis sp.) | SE | HTL Bio-crude | MTBE | 29–37 | DCM | 26–35 | [211] |
| Ethyl acetate | 19–45 | n-Hexane | 13–28 | ||||
| Acetone | 29–32 | ||||||
| Chloroform | 32–40 | ||||||
| Fennel seed | SFE -- | Oil | Sc-CO2 | ~15 | n-Hexane | ~15.3 | [200] |
| Anise seed | SFE | Oil | SC-CO2 | ~17 | n-Hexane | ~15.1 | |
| Carum carvi seeds | SFE | Oil | SC-CO2 | ~12 | n-Hexane | ~12 | [201] |
| Algae (Nannochloropsis salina sp.) | SFE | Oil | SC-CO2 | 31.37 | [212] | ||
| Grape Seed | SFE | Phenolic Compounds | SC-CO2 | 12.32 | [213] | ||
| Cotton Seed | SFE | Oil | SC-H2O | 21.7–56 | [214] | ||
| Jojoba Seed | SFE | Oil | SC-H2O | 94 | [215] | ||
| Citrus hystrix DC. leaves | SFE | Oil | SC-H2O | 2.14 | [216] | ||
| De-oiled Jatropha curcas cake | SE | HTL Bio-crude | ChCl–KOH DES (1:4) ~ | 43.53 | [217] | ||
| ChCl–p-TsOH DES (1:4) ~ | 38.31 | ||||||
| ChCl–Gly (1:3) ~ | 26.63 | ||||||
| ChCl–FeCl3 (1:3) ~ | 30.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. Evaluating Green Solvents for Bio-Oil Extraction: Advancements, Challenges, and Future Perspectives. Energies 2023, 16, 5852. https://doi.org/10.3390/en16155852
Usman M, Cheng S, Boonyubol S, Cross JS. Evaluating Green Solvents for Bio-Oil Extraction: Advancements, Challenges, and Future Perspectives. Energies. 2023; 16(15):5852. https://doi.org/10.3390/en16155852
Chicago/Turabian StyleUsman, Muhammad, Shuo Cheng, Sasipa Boonyubol, and Jeffrey S. Cross. 2023. "Evaluating Green Solvents for Bio-Oil Extraction: Advancements, Challenges, and Future Perspectives" Energies 16, no. 15: 5852. https://doi.org/10.3390/en16155852
APA StyleUsman, M., Cheng, S., Boonyubol, S., & Cross, J. S. (2023). Evaluating Green Solvents for Bio-Oil Extraction: Advancements, Challenges, and Future Perspectives. Energies, 16(15), 5852. https://doi.org/10.3390/en16155852