Tailoring NiMo-Based Catalysts for Production of Low-Viscosity Sustainable Hydrocarbon Bases for Drilling Muds from Secondary Gas Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation
2.2. Characterization of the Catalyst
2.3. Catalytic Performance
2.4. Physico-Chemical Studies of the Hydrocarbon Bases for Drilling Muds
3. Results and Discussion
3.1. Investigation of Catalyst Characteristics
3.2. Catalytic Tests
3.3. Production of Sustainable Hydrocarbon Bases for Drilling Muds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Apaleke, A.S.; Al-Majed, A.A.; Hossain, M.E. Drilling fluid: State of the art and future trend. In Proceedings of the North Africa Technical Conference and Exhibition, Cairo, Egypt, 20–22 February 2012; OnePetro: Mumbai, India, 2012. [Google Scholar] [CrossRef] [Green Version]
- Caenn, R.; Chillingar, G.V. Drilling fluids: State of the art. J. Pet. Sci. Eng. 1996, 14, 221–230. [Google Scholar] [CrossRef]
- Jones, T.G.J.; Hughes, T.L. Drilling fluid suspensions. In Suspensions: Fundamentals and Applications in the Petroleum Industry; Schramm, L.L., Ed.; American Chemical Society: Washington, DC, USA, 1996; pp. 463–564. [Google Scholar] [CrossRef]
- Ukeles, S.D.; Grinbaum, B. Drilling fluids. In Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed.; Kirk-Othmer, R.E., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2000; pp. 1–42. [Google Scholar]
- OGP (International Association of Oil & Gas Producers)/IPIECA(Environmental Conservation Association). Drilling Fluids and Health Risk Management: A Guide for Drilling Personnel, Managers and Health Professionals in the Oil and Gas Industry; OGP Report Number 396; OGP/IPIECA: London, UK, 2009. [Google Scholar]
- Barbosa, M.I.R. Bentonites Treated with Polymeric Additives for Application in Drilling Fluids. Doctoral Dissertation, Federal University of Campina Grande, Campina Grande, Brazil, 2006. [Google Scholar]
- Oliveira, F.F.; Sodré, C.H.; Marinho, J.L.G. Numerical Investigation of Non-Newtonian Drilling Fluids During the Occurrence of a Gas Kick in a Petroleum Reservoir. Braz. J. Chem. Eng. 2016, 33, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Caenn, R.; Darley, H.C.; Gray, G.R. Composition and Properties of Drilling and Completion Fluids; Gulf Professional Publishing: Houston, TX, USA, 2011. [Google Scholar]
- IOGP (International Association of Oil & Gas Producers). Environmental Fate and Effects of Ocean Discharge of Drill Cuttings and Associated Drilling Fluids from Offshore Oil and Gas Operations; IOGP Report Number 543, Version 1; IOGP: London, UK, 2016. [Google Scholar]
- IOGP (International Association of Oil & Gas Producers). Environmental Performance Indicators—2021 Data; IOGP Report Number 2021e, Version 1; IOGP: London, UK, 2022. [Google Scholar]
- The Business Research Company. Drilling Fluids Global Market Report 2023—By Product (Oil-Based, Synthetic-Based, Water-Based, Other Products), by Application (Onshore, Offshore), by End-User (Crude Petroleum Companies, Natural Gas Extraction Companies)—Market Size, Trends, and Global Forecast 2023–2032. 2023. Available online: https://www.thebusinessresearchcompany.com/report/drilling-fluids-global-market-report (accessed on 26 June 2023).
- Verified Market Research. Global Drilling Fluids Market Size by Product, by Application, by Geographic Scope and Forecast. 2021. Available online: https://www.verifiedmarketresearch.com/product/drilling-fluids-market/ (accessed on 26 June 2023).
- Fortune Business Insights. Drilling Fluids Market Size, Share & COVID-19 Impact Analysis, by Type (Water-Based, Oil-Based, Synthetic-Based and Others), by Application (Onshore and Offshore), and Regional Forecast, 2021–2028. 2021. Available online: https://www.fortunebusinessinsights.com/industry-reports/drilling-fluid-market-100401 (accessed on 26 June 2023).
- Straits Research. Drilling Fluids Market: Information by Application (Onshore, Offshore), Fluid System (Water-Based System, Oil-Based System, Synthetic-Based System), Well Type (Conventional Wells), and Region—Forecast Till 2030. 2021. Available online: https://straitsresearch.com/report/drilling-fluids-market (accessed on 26 June 2023).
- Data Bridge Market Research. Global Drilling Fluids Market–Industry Trends and Forecast to 2028. 2021. Available online: https://www.databridgemarketresearch.com/reports/global-drilling-fluids-market (accessed on 26 June 2023).
- Guyomar, P.Y.; Theyskens, A.A. Process for the Production of Hydrocarbon Fluids. U.S. Patent No. 7,311,814, 25 December 2007. [Google Scholar]
- Aubry, C.; Grasso, G.; Dath, J.P. Method for Obtaining Hydrocarbon Solvents with Boiling Point above 300 °C and Pour Point Lower than or Equal to −25 °C. U.S. Patent No. 10,836,968, 17 November 2020. [Google Scholar]
- Aubry, C.; Nokerman, J. Process for the Production of Hydrocarbon Fluids Having a Low Aromatic Content. U.S. Patent No. 9,315,742, 19 April 2016. [Google Scholar]
- Ferreira, C.; Caudrelier, F.; Benghalem, A. Process for the Production of Fluids. Patent No. 2022/029234 A1, 2022. [Google Scholar]
- Zelenskij, K.V.; Dubrovskij, D.A.; Lejmeter, T.D.; Kuzora, I.E.; Semenov, I.A.; Stadnik, A.V.; Marushchenko, I.Y.; Sergeev, V.A. Method for Obtaining the Hydrocarbon Base of Drilling Fluids. Patent No. RU 2762672 C1, 21 December 2021. [Google Scholar]
- Karpov, N.V.; Vakhromov, N.N.; Dutlov, E.V.; Gudkevich, I.V.; Bubnov, M.A.; Borisanov, D.V. Method of Obtaining a Component for Drilling Fluids. Patent No RU 2668612 C1, 2 October 2018. [Google Scholar]
- Karpov, N.V.; Vakhromov, N.N.; Dutlov, E.V.; Gudkevich, I.V.; Bubnov, M.A.; Borisanov, D.V. Method of Obtaining a Component for Drilling Fluids. Patent No RU 2774182 C1, 15 June 2022. [Google Scholar]
- Karpov, N.V.; Vakhromov, N.N.; Dutlov, E.V.; Gudkevich, I.V.; Bubnov, M.A.; Borisanov, D.V. Method of Obtaining a Component for Drilling Fluids. Patent No RU 2775650 C1, 6 July 2022. [Google Scholar]
- Karpov, N.V.; Vakhromov, N.N.; Dutlov, E.V.; Gudkevich, I.V.; Bubnov, M.A.; Borisanov, D.V. Method of Obtaining a Component for Drilling Fluids. Patent No RU 2775651 C1, 6 July 2022. [Google Scholar]
- Karpov, N.V.; Vakhromov, N.N.; Dutlov, E.V.; Bubnov, M.A.; Gudkevich, I.V.; Maksimov, A.L.; Gritsenko, A.I.; Ratkin, L.S.; Sharin, E.A.; Borisanov, D.V. Analysis of Properties of Diesel Fuels of Different Origins for Their Use as Feedstock for Producing Hydrocarbon Bases for Drilling Fluids. Chem. Technol. Fuels Oils 2022, 58, 247–251. [Google Scholar] [CrossRef]
- Litvinenko, V.; Bowbrick, I.; Naumov, I.; Zaitseva, Z. Global guidelines and requirements for professional competencies of natural resource extraction engineers: Implications for ESG principles and sustainable development goals. J. Clean. Prod. 2022, 338, 130530. [Google Scholar] [CrossRef]
- Fadairo, A.; Adeyemi, G.; Ogunkunle, T.; Ling, K.; Rasouli, V.; Effiong, E.; Ayoo, J. Study the suitability of neem seed oil for formulation of eco-friendly oil based drilling fluid. Pet. Res. 2021, 6, 283–290. [Google Scholar] [CrossRef]
- Adesina, F.; Anthony, A.; Gbadegesin, A.; Eseoghene, O.; Oyakhire, A. Environmental impact evaluation of a safe drilling mud. In SPE Middle East Health, Safety, Security, and Environment Conference and Exhibition; OnePetro: Mumbai, India, 2012. [Google Scholar] [CrossRef]
- Paul, A.A.L.; Adewale, F.J. Novel Synthetic-Based Drilling Fluid through Enzymatic Interesterification of Canola Oil. Int. J. Chem. Eng. 2018, 2018, 6418090. [Google Scholar] [CrossRef] [Green Version]
- Adewale, D.; Ogunrinde, J.O. Development of environmentally friendly oil based mud using palm-oil and groundnut-oil. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Tinapa-Calabar, Nigeria, 31 July–7 August 2010; p. SPE-140720. [Google Scholar] [CrossRef]
- Kania, D.; Yunus, R.; Omar, R.; Rashid, S.A.; Jan, B.M. A review of biolubricants in drilling fluids: Recent research, performance, and applications. J. Pet. Sci. Eng. 2015, 135, 177–184. [Google Scholar] [CrossRef]
- Aboulrous, A.A.; Haddad, A.S.; Rafati, R.; Boyou, N.V.; Alsabagh, A.M. Review of synthesis, characteristics and technical challenges of biodiesel based drilling fluids. J. Clean Prod. 2022, 336, 130344. [Google Scholar] [CrossRef]
- Bisht, D.; Petri, J. Considerations for Upgrading Light Cycle Oil with Hydroprocessing Technologies. Indian Chem. Eng. 2014, 56, 321–335. [Google Scholar] [CrossRef]
- Kosolapova, S.M.; Smal, M.S.; Rudko, V.A.; Pyagay, I.N. A New Approach for Synthesizing Fatty Acid Esters from Linoleic-Type Vegetable Oil. Processes 2023, 11, 1534. [Google Scholar] [CrossRef]
- Peng, C.; Fang, X.-C.; Zeng, R.-H.; Guo, R.; Hao, W.-Y. Commercial analysis of catalytic hydroprocessing technologies in producing diesel and gasoline by light cycle oil. Catal. Today 2016, 276, 11–18. [Google Scholar] [CrossRef]
- Yin, C.; Wu, T.; Dong, C.; Li, F.; Liu, D.; Liu, C. Preparation of highly active unsupported Ni–Si–Mo catalyst for the deep hydrogenation of aromatics. J. Alloys Compd. 2020, 834, 155076. [Google Scholar] [CrossRef]
- Oh, Y.; Shin, J.; Noh, H.; Kim, C.; Kim, Y.-S.; Lee, Y.-K.; Lee, J.K. Selective hydrotreating and hydrocracking of FCC light cycle oil into high-value light aromatic hydrocarbons. Appl. Catal. A Gen. 2019, 577, 86–98. [Google Scholar] [CrossRef]
- Laredo, G.C.; Merino, P.M.V.; Hernández, P.S. Light Cycle Oil Upgrading to High Quality Fuels and Petrochemicals: A Review. Ind. Eng. Chem. Res. 2018, 57, 7315–7321. [Google Scholar] [CrossRef]
- Palos, R.; Gutiérrez, A.; Arandes, J.M.; Bilbao, J. Catalyst used in fluid catalytic cracking (FCC) unit as a support of NiMoP catalyst for light cycle oil hydroprocessing. Fuel 2018, 216, 142–152. [Google Scholar] [CrossRef]
- Guo, R.; Cao, Z.; Fang, X. The development of catalysts and their stacking technology for diesel ultra-deep hydrosulfurization. Catal. Today 2018, 316, 21–25. [Google Scholar] [CrossRef]
- Calemma, V.; Giardino, R.; Ferrari, M. Upgrading of LCO by partial hydrogenation of aromatics and ring opening of naphthenes over bi-functional catalysts. Fuel Process Technol. 2010, 91, 770–776. [Google Scholar] [CrossRef]
- Solmanov, P.S.; Maximov, N.M.; Eremina, Y.V.; Zhilkina, E.O.; Dryaglin, Y.Y.; Tomina, N.N. Hydrotreating of mixtures of diesel fractions with gasoline and light coker gas oil. Pet. Chem. 2013, 53, 177–180. [Google Scholar] [CrossRef]
- Wei, Q.; Wen, S.; Tao, X.; Zhang, T.; Zhou, Y.; Chung, K.; Xu, C. Hydrodenitrogenation of basic and non-basic nitrogen-containing compounds in coker gas oil. Fuel Process Technol. 2015, 129, 76–84. [Google Scholar] [CrossRef]
- Anabtawi, J.A. Effects of hydrotreating coker light gas oil on fuel quality. Fuel Sci. Technol. Int. 1993, 11, 1409–1424. [Google Scholar] [CrossRef]
- Boahene, P.; Soni, K.; Dalai, A.; Adjaye, J. Hydrotreating of coker light gas oil on Ti-modified HMS supports using Ni/HPMo catalysts. Appl. Catal. B Environ. 2011, 101, 294–305. [Google Scholar] [CrossRef]
- Tomina, N.N.; Maksimov, N.M.; Solmanov, P.S.; Samsonov, M.V.; Moiseev, A.V.; Pimerzin, A.A. Hydrotreating of Mixtures of Straight-Run Diesel Fraction with Coker Gas Oil over Modified Co(Ni)-Mo/Al2O3Catalysts. Russ. J. Gen. Chem. 2018, 88, 1970–1975. [Google Scholar] [CrossRef]
- Al-Barood, A.; Stanislaus, A. Ultra-deep desulfurization of coker and straight-run gas oils: Effect of lowering feedstock 95% boiling point. Fuel Process Technol. 2007, 88, 309–315. [Google Scholar] [CrossRef]
- Budukva, S.V.; Eletskii, P.M.; Zaikina, O.O.; Sosnin, G.A.; Yakovlev, V.A. Secondary Middle Distillates and Their Processing (Review). Pet. Chem. 2019, 59, 941–955. [Google Scholar] [CrossRef]
- Marafi, M.; Stanislaus, A.; Furimsky, E. Handbook of Spent Hydroprocessing Catalysts; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Tanimu, A.; Alhooshani, K. Advanced hydrodesulfurization catalysts: A review of design and synthesis. Energy Fuels 2019, 33, 2810–2838. [Google Scholar] [CrossRef]
- Topsoe, H.; Clausen, B.S.; Massoth, F.E. Hydro Treating Catalysis: Science and Technology; Taylor and Francis: Abingdon, UK, 1996. [Google Scholar]
- Eijsbouts, S. On the flexibility of the active phase in hydrotreating catalysts. Appl. Catal. A Gen. 1997, 158, 53–92. [Google Scholar] [CrossRef]
- Hensen, E.; Kooyman, P.; van der Meer, Y.; van der Kraan, A.; de Beer, V.; van Veen, J.; van Santen, R. The Relation between Morphology and Hydrotreating Activity for Supported MoS2 Particles. J. Catal. 2001, 199, 224–235. [Google Scholar] [CrossRef]
- Hensen, E.; de Beer, V.; van Veen, J.; van Santen, R. A Refinement on the Notion of Type I and II (Co)MoS Phases in Hydrotreating Catalysts. Catal. Lett. 2002, 84, 59–67. [Google Scholar] [CrossRef]
- Breysse, M.; Geantet, C.; Afanasiev, P.; Blanchard, J.; Vrinat, M. Recent studies on the preparation, activation and design of active phases and supports of hydrotreating catalysts. Catal. Today 2008, 130, 3–13. [Google Scholar] [CrossRef]
- Griboval, A.; Blanchard, P.; Payen, E.; Fournier, M.; Dubois, J. Alumina supported HDS catalysts prepared by impregnation with new heteropolycompounds. Comparison with catalysts prepared by conventional Co–Mo–P coimpregnation. Catal. Today 1998, 45, 277–283. [Google Scholar] [CrossRef]
- Cabello, C.I.; Cabrerizo, F.M.; Alvarez, A.; Thomas, H.J. Decamolybdodicobaltate(III) heteropolyanion: Structural, spectroscopical, thermal and hydrotreating catalytic properties. J. Mol. Catal. A Chem. 2002, 186, 89–100. [Google Scholar] [CrossRef]
- Mazurelle, J.; Lamonier, C.; Lancelot, C.; Payen, E.; Pichon, C.; Guillaume, D. Use of the cobalt salt of the heteropolyanion [Co2Mo10O38H4]6−for the preparation of CoMo HDS catalysts supported on Al2O3, TiO2 and ZrO2. Catal. Today 2008, 130, 41–49. [Google Scholar] [CrossRef]
- Lamonier, C.; Martin, C.; Mazurelle, J.; Harlé, V.; Guillaume, D.; Payen, E. Molybdocobaltate cobalt salts: New starting materials for hydrotreating catalysts. Appl. Catal. B Environ. 2007, 70, 548–556. [Google Scholar] [CrossRef]
- Escalona, E.E.; Pereira-Almao, P.R.; Castillo, J.; Hung, J.; Bolívar, C.; Scott, C.E. Nanometric bimetallic sulfides prepared via thermal decomposition of Ni and Fe heteropolymolybdate emulsions. Catal. Lett. 2006, 112, 227–230. [Google Scholar] [CrossRef]
- Cattaneo, R.; Rota, F.; Prins, R. An XAFS Study of the Different Influence of Chelating Ligands on the HDN and HDS of γ-Al2O3-Supported NiMo Catalysts. J. Catal. 2001, 199, 318–327. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, H.; Kang, H.; Zhao, L.; Liu, X.; Huang, W.; Zhou, Y.; Wei, Q. Effect of organic phosphorus addition on the state of active metal species and catalytic performance of NiW/Al2O3 hydrodesulfurization catalyst. Fuel 2023, 340, 127547. [Google Scholar] [CrossRef]
- Escobar, J.; Barrera, M.C.; Toledo, J.A.; Cortés-Jácome, M.A.; Angeles-Chávez, C.; Núñez, S.; Santes, V.; Gómez, E.; Díaz, L.; Romero, E.; et al. Effect of ethyleneglycol addition on the properties of P-doped NiMo/Al2O3 HDS catalysts: Part I. Materials preparation and characterization. Appl. Catal. B Environ. 2009, 88, 564–575. [Google Scholar] [CrossRef]
- Kwak, C.; Lee, J.J.; Bae, J.S.; Choi, K.; Moon, S.H. Hydrodesulfurization of DBT, 4-MDBT, and 4,6-DMDBT on fluorinated CoMoS/Al2O3 catalysts. Appl. Catal. A Gen. 2000, 200, 233–242. [Google Scholar] [CrossRef]
- Oliviero, L.; Maugé, F.; Afanasiev, P.; Pedraza-Parra, C.; Geantet, C. Organic additives for hydrotreating catalysts: A review of main families and action mechanisms. Catal. Today 2020, 377, 3–16. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Matsubayashi, N.; Sato, T.; Shimada, H.; Nishijima, A. Molybdate catalysts prepared by a novel impregnation method: Effect of citric acid as a ligand on the catalytic activities. Appl. Catal. A Gen. 1991, 79, 145–159. [Google Scholar] [CrossRef]
- Ramírez, J.; Gutiérrez-Alejandre, A.; Sánchez-Minero, F.; Macías-Alcántara, V.; Castillo-Villalón, P.; Oliviero, L.; Maugé, F. HDS of 4,6-DMDBT over NiMoP/(x)Ti-SBA-15 catalysts prepared with H3PMo12O40. Energy Fuels 2012, 26, 773–782. [Google Scholar] [CrossRef]
- Pashigreva, A.; Klimov, O.; Bukhtiyarova, G.; Fedotov, M.; Kochubey, D.; Chesalov, Y.; Zaikovskii, V.; Prosvirin, I.; Noskov, A. The superior activity of the CoMo hydrotreating catalysts, prepared using citric acid: What’s the reason? Prep. Catal. V-Sci. Bases Prep. Heterog. Catal. Proc. Fifth Int. Symp. 2010, 175, 109–116. [Google Scholar] [CrossRef]
- Nuzhdin, A.L.; Bukhtiyarova, G.A.; Plyusnin, P.E.; Porsin, A.A.; Bukhtiyarov, V.I. Effect of Mono-, Di-, and Triethylene Glycol on the Sulfidation Behavior of NiMo(P)/Al2O3 Hydrotreating Catalysts. Catal. Lett. 2019, 149, 3304–3311. [Google Scholar] [CrossRef]
- Sukandar, D.; Badriyah, L.; Rustyawan, W.; Adawiah, A. Effect of Additional Polyethylene Glycol and Citric Acid on Characteristics of NiMo/g-Al2O3 Catalyst in Light Cycle Gas Oil Hydrodesulfurisation. Bull. Chem. React. Eng. Catal. 2023, 18, 162–172. [Google Scholar] [CrossRef]
- Iwamoto, R.; Kagami, N.; Sakoda, Y.; Iino, A. Effect of Polyethylene Glycol Addition on NiO-MoO3/Al2O3 and NiO-MoO3-P2O5/Al2O3 Hydrodesulfurization Catalyst. J. Jpn. Pet. Inst. 2005, 48, 351–357. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, R.; Weber, T.; Shido, T.; Prins, R. A Quick EXAFS Study of the Sulfidation of NiMo/SiO2 Hydrotreating Catalysts Prepared with Chelating Ligands. J. Catal. 2000, 191, 225–236. [Google Scholar] [CrossRef]
- Rana, M.S.; Ramírez, J.; Gutierrezalejandre, A.; Ancheyta, J.; Cedeño, L.; Maity, S. Support effects in CoMo hydrodesulfurization catalysts prepared with EDTA as a chelating agent. J. Catal. 2007, 246, 100–108. [Google Scholar] [CrossRef]
- Lélias, M.; Le Guludec, E.; Mariey, L.; van Gestel, J.; Travert, A.; Oliviero, L.; Maugé, F. Effect of EDTA addition on the structure and activity of the active phase of cobalt–molybdenum sulfide hydrotreatment catalysts. Catal. Today 2010, 150, 179–185. [Google Scholar] [CrossRef]
- Spetsov, E.A.; Artyushevskiy, D.I.; Konoplin, R.R.; Sizyakov, V.M. Phase composition of aluminium hydroxides and its calculation based on thermal analysis data. Tsvetnye Met. 2023, 5, 37–44. [Google Scholar] [CrossRef]
- Ancheyta, J.; Rana, M.S.; Furimsky, E. Hydroprocessing of heavy petroleum feeds: Tutorial. Catal. Today 2005, 109, 3–15. [Google Scholar] [CrossRef]
- Chen, W.; Nie, H.; Long, X.; Li, M.; Zhang, L.; Li, D. Role of pore structure on the activity and stability of sulfide catalyst. Catal. Today 2020, 377, 69–81. [Google Scholar] [CrossRef]
- Iusovskii, A.; Boldushevskii, R.; Mozhaev, A.; Shmelkova, O.; Guseva, A.; Chernysheva, E.; Kapustin, V.; Pronchenkov, I.; Nikulshin, P. New NiMo/Al2O3 Catalysts for Hydrodearomatization of Secondary Middle Distillates. Chem. Technol. Fuels Oils 2022, 58, 502–510. [Google Scholar] [CrossRef]
- Mozhaev, A.; Nikulshin, P.; Pimerzin, A.; Maslakov, K. Investigation of co-promotion effect in NiCoMoS/Al2O3 catalysts based on Co2Mo10-heteropolyacid and nickel citrate. Catal. Today 2015, 271, 80–90. [Google Scholar] [CrossRef]
- Kasztelan, S.; Toulhoat, H.; Grimblot, J.; Bonnelle, J. A geometrical model of the active phase of hydrotreating catalysts. Appl. Catal. 1984, 13, 127–159. [Google Scholar] [CrossRef]
- Marchand, K.; Legens, C.; Guillaume, D.; Raybaud, P. A Rational Comparison of the Optimal Promoter Edge Decoration of HDT NiMoS vs CoMoS Catalysts. Oil Gas Sci. Technol. Rev. d’IFP Energies Nouv. 2009, 64, 719–730. [Google Scholar] [CrossRef]
- Beccat, P.; Da Silva, P.; Huiban, Y.; Kasztelan, S. Quantitative Surface Analysis by Xps (X-ray Photoelectron Spectroscopy): Application to Hydrotreating Catalysts. Oil Gas Sci. Technol. Rev. d’IFP Energies Nouv. 1999, 54, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Gandubert, A.D.; Legens, C.; Guillaume, D.; Rebours, S.; Payen, E. X-ray Photoelectron Spectroscopy Surface Quantification of Sulfided CoMoP Catalysts—Relation Between Activity and Promoted Sites—Part I: Influence of the Co/Mo Ratio. Oil Gas Sci. Technol. Rev. d’IFP Energies Nouv. 2007, 62, 79–89. [Google Scholar] [CrossRef]
- Qiu, L.; Xu, G. Peak overlaps and corresponding solutions in the X-ray photoelectron spectroscopic study of hydrodesulfurization catalysts. Appl. Surf. Sci. 2010, 256, 3413–3417. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, Y.; Tang, S.; Wang, B.; Wang, Y.; Song, Y.; Xin, X.; Zhang, Y.; Li, X. Tuning interlayer spacing of MoS2 for enhanced hydrogen evolution reaction. J. Alloys Compd. 2021, 864, 158581. [Google Scholar] [CrossRef]
- Zhang, M.-H.; Fan, J.-Y.; Chi, K.; Duan, A.-J.; Zhao, Z.; Meng, X.-L.; Zhang, H.-L. Synthesis, characterization, and catalytic performance of NiMo catalysts supported on different crystal alumina materials in the hydrodesulfurization of diesel. Fuel Process Technol. 2017, 156, 446–453. [Google Scholar] [CrossRef]
- Ninh, T.; Massin, L.; Laurenti, D.; Vrinat, M. A new approach in the evaluation of the support effect for NiMo hydrodesulfurization catalysts. Appl. Catal. A Gen. 2011, 407, 29–39. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z.; Zheng, P.; Chen, Z.; Duan, A.; Xu, C.; Jiao, J.; Zhang, H.; Cao, Z.; Ge, B. Synthesis of NiMo catalysts supported on mesoporous Al2O3 with different crystal forms and superior catalytic performance for the hydrodesulfurization of dibenzothiophene and 4,6-dimethyldibenzothiophene. J. Catal. 2016, 344, 680–691. [Google Scholar] [CrossRef]
- Taromi, A.A.; Kaliaguine, S. Green diesel production via continuous hydrotreatment of triglycerides over mesostructured γ-alumina supported NiMo/CoMo catalysts. Fuel Process Technol. 2018, 171, 20–30. [Google Scholar] [CrossRef]
- Houssenbay, S.; Kasztelan, S.; Toulhoat, H.; Bonnelle, J.P.; Grimblot, J. Nature of the different nickel species in sulfided bulk and alumina-supported nickel-molybdenum hydrotreating catalysts. J. Phys. Chem. 1989, 93, 7176–7180. [Google Scholar] [CrossRef]
- Calsia Drilling Fluids. Available online: https://calumet.com/products/solvents/calsia/ (accessed on 26 June 2023).




| Properties | LCO | LCGO | LCO (Major Proportion) + LCGO |
|---|---|---|---|
| Density at 15 °C (kg/m3) | 950.6 | 914.2 | 945.1 |
| Total sulfur content (wt%) | 1.108 | 1.008 | 1.093 |
| Distillation Temperature (°C): | |||
| IBP | 189 | 218 | 194 |
| 10 vol% | 210 | 263 | 215 |
| 50 vol% | 254 | 308 | 262 |
| 90 vol% | 323 | 352 | 326 |
| 96 vol% | 338 | 364 | 342 |
| yield at 340 °C | 98 | - | - |
| yield at 360 °C | - | 94 | - |
| Total aromatics content (wt%): | 77.7 | 44.3 | 75.1 |
| Mono-aromatics | 26.0 | 14.7 | 25.1 |
| Bi-aromatics | 44.0 | 17.7 | 41.5 |
| Tri-aromatics+ | 7.7 | 11.9 | 8.5 |
| Iodine number (I2 g/100 g) | 17.0 | 36.5 | 21.2 |
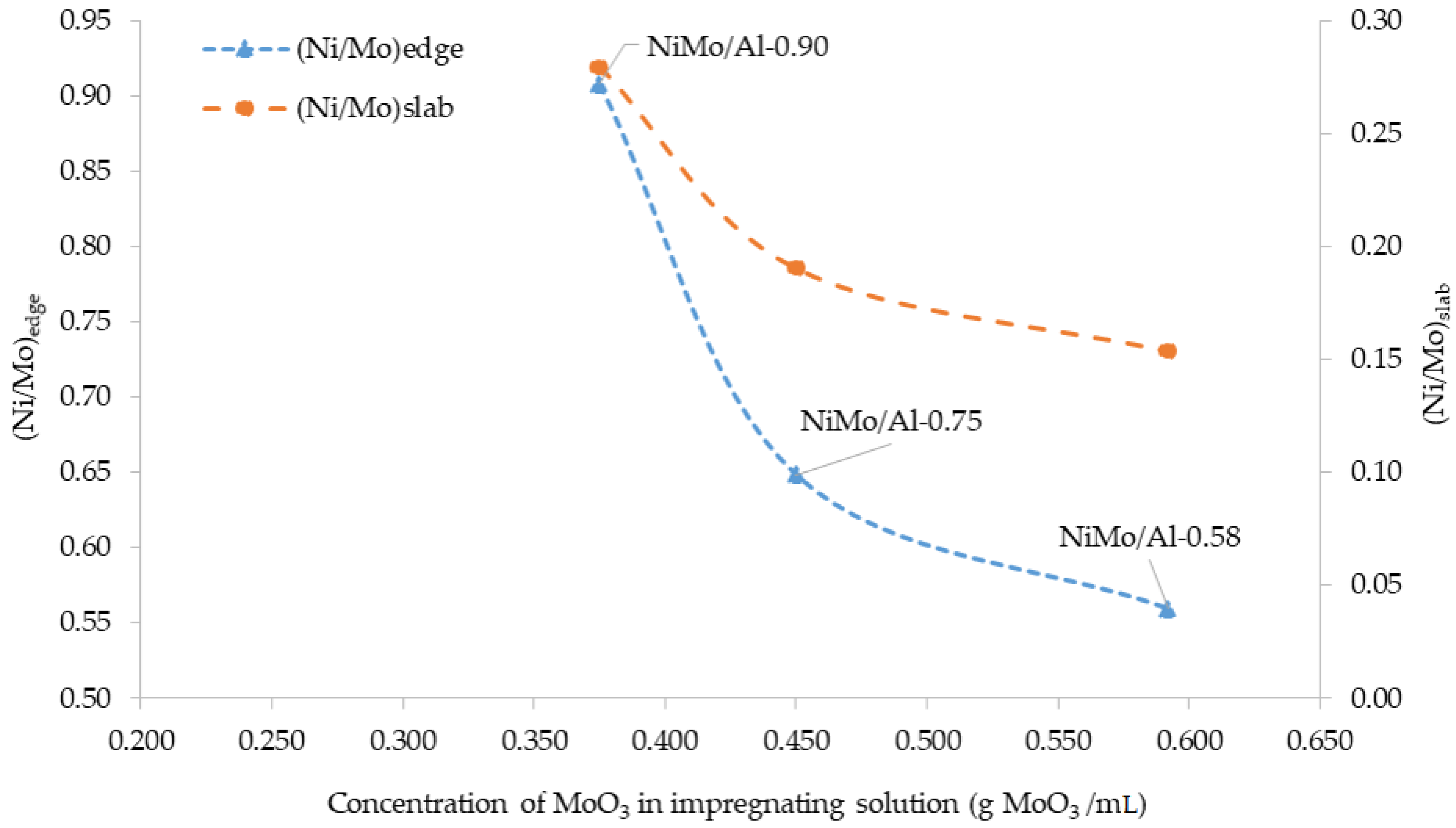
| Properties | Al-0.90 | NiMo/Al-0.90 * | Al-0.75 | NiMo/Al-0.75 * | Al-0.58 | NiMo/Al-0.58 * |
|---|---|---|---|---|---|---|
| Concentration of MoO3 in impregnating solutions (g/mL) | - | 0.37 | - | 0.45 | - | 0.59 |
| Textural characteristics: | ||||||
| S (m2/g) | 309 | 208 | 254 | 175 | 271 | 187 |
| Vp (cm3/g) | 0.90 | 0.61 | 0.75 | 0.50 | 0.58 | 0.39 |
| Def (nm) | 7.0 (bimodal max at 7.0 and 9.5) | 5.0 | 13.0 | 9.4 | 6.0 | 5.0 |
| Properties | NiMo/Al-0.90 | NiMo/Al-0.75 | NiMo/Al-0.58 |
|---|---|---|---|
| 3.8 | 4.0 | 4.3 | |
| 2.9 | 2.6 | 1.9 | |
| D | 0.31 | 0.29 | 0.28 |
| (fe/fc)NiMo | 4.0 | 3.1 | 2.9 |
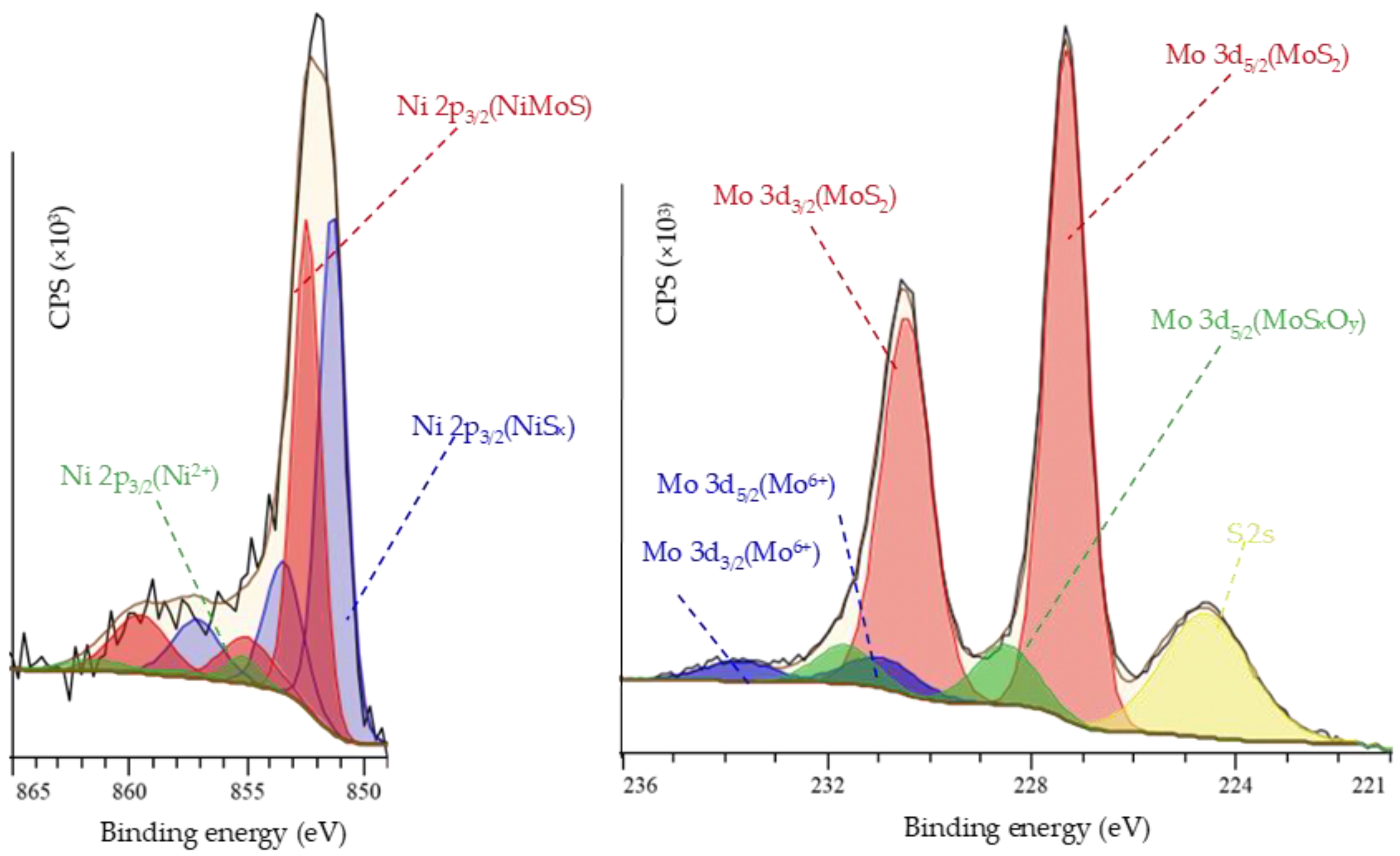
| Catalyst | Mo Content, % Rel | Ni Content, % Rel. | (Ni/Mo)slab | (Ni/Mo)edge | ||||
|---|---|---|---|---|---|---|---|---|
| MoS2 | MoSxOy | Mo6+ | NiMoS | NiSx | Ni2+ | |||
| NiMo/Al-0.90 | 81 | 12 | 7 | 39 | 55 | 6 | 0.28 | 0.91 |
| NiMo/Al-0.75 | 83 | 11 | 6 | 45 | 51 | 4 | 0.19 | 0.65 |
| NiMo/Al-0.58 | 83 | 12 | 5 | 31 | 60 | 9 | 0.15 | 0.56 |
| Properties | Feedstock LCO (Major Proportion) + LCGO | Liquid Product | ||
|---|---|---|---|---|
| NiMo/Al-0.90 | NiMo/Al-0.75 | NiMo/Al-0.58 | ||
| Density at 15 °C (kg/m3) | 945.1 | 857.0 | 855.0 | 869.1 |
| Total sulfur content (wt%) | 1.093 | 0.0007 | 0.0007 | 0.0009 |
| Total aromatic content (wt%): | 75.1 | 26.1 | 21.6 | 38.9 |
| Mono-aromatics | 25.1 | 25.9 | 21.1 | 37.9 |
| Bi-aromatics | 41.5 | 0.2 | 0.5 | 0.9 |
| Tri-aromatics+ | 8.5 | 0.0 | 0.0 | 0.1 |
| HYD (%) | - | 65.3 | 71.3 | 48.2 |
| Specific HYD (%/mmol Mo) | - | 2.09 | 2.31 | 1.54 |
| Properties | Values for Total Hydrogenated Liquid Product |
|---|---|
| Density at 20 °C (kg/m3) | 833.8 |
| Total sulfur content (wt%) | <0.001 |
| Distillation yield (vol%), at a temperature: | |
| IBP | 59 |
| 10 | 176 |
| 50 | 217 |
| 90 | 288 |
| 95 | 318 |
| Total aromatic content (wt%): | 1.9 |
| Mono-aromatics | 1.8 |
| Bi-aromatics | 0.1 |
| Tri-aromatics+ | 0.0 |
| Pour Point (°C) | –55 |
| Properties | Distillate 200–270 °C (Yield 66.7 wt%) | Commonly Used Commercial NAF Base Fluids [5,20,91] |
|---|---|---|
| Color | clear | clear |
| Viscosity at 40 °C (mm2/s) | 2.45 | 1.6 ÷ 3.2 |
| Flash point (°C) | 84 | >70 |
| Pour point (°C) | −52 | –18 ÷ –50 |
| Total aromatic content (wt%) | 1.4 | <0.2 ÷ 3.0 |
| Total sulfur content (wt%) | 0.0001 | |
| Density (kg/m3) at 15 °C | 846.4 | 804 ÷ 827 |
| Aniline point (°C) | 82 | >72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iusovskii, A.; Boldushevskii, R.; Mozhaev, A.; Shmelkova, O.; Pavlycheva, E.; Koklyukhin, A.; Nikulshin, P. Tailoring NiMo-Based Catalysts for Production of Low-Viscosity Sustainable Hydrocarbon Bases for Drilling Muds from Secondary Gas Oils. Energies 2023, 16, 5859. https://doi.org/10.3390/en16165859
Iusovskii A, Boldushevskii R, Mozhaev A, Shmelkova O, Pavlycheva E, Koklyukhin A, Nikulshin P. Tailoring NiMo-Based Catalysts for Production of Low-Viscosity Sustainable Hydrocarbon Bases for Drilling Muds from Secondary Gas Oils. Energies. 2023; 16(16):5859. https://doi.org/10.3390/en16165859
Chicago/Turabian StyleIusovskii, Aleksei, Roman Boldushevskii, Aleksandr Mozhaev, Olga Shmelkova, Elizaveta Pavlycheva, Aleksandr Koklyukhin, and Pavel Nikulshin. 2023. "Tailoring NiMo-Based Catalysts for Production of Low-Viscosity Sustainable Hydrocarbon Bases for Drilling Muds from Secondary Gas Oils" Energies 16, no. 16: 5859. https://doi.org/10.3390/en16165859
APA StyleIusovskii, A., Boldushevskii, R., Mozhaev, A., Shmelkova, O., Pavlycheva, E., Koklyukhin, A., & Nikulshin, P. (2023). Tailoring NiMo-Based Catalysts for Production of Low-Viscosity Sustainable Hydrocarbon Bases for Drilling Muds from Secondary Gas Oils. Energies, 16(16), 5859. https://doi.org/10.3390/en16165859







