Abstract
In this article, typomorphic associations of microelements in carbonaceous caustobioliths and oils are defined as indicators of naphthogenesis based on the analytical survey of the literature and our own research. Various approaches to the studying of crude oil genesis from the position of abiotic and complex approaches (polygenesis) are considered. Such approaches are relevant to the prospect and development of offshore oil deposits, localized at ultra-deep levels. For deep deposits, the most prominent hypothesis of oil origin is the abiogenious theory. In the foundation of that approach lays the assumption that hydrocarbons are formed mainly due to reactions of the formed mantle gases and the host rock. Key factors for these reactions to proceed are specific conditions concerning temperature, pressure, and specific catalysts. The article presents the results of thermodynamic and quantum-chemical modeling of the possibility of the organic and organometallic compound formation using the software package HSC Chemistry 6.0. Pointed out the possibility of a low molecular weight hydrocarbon formation due to the contact of ascending flows of mantle gases with cooling natural barriers. The primary synthesis proceeds with the interaction of fluid forms of H2, CO2, and H2S. The estimation of the bonding energy depending on the structure of organometallic compounds was performed using the Avogadro software package. The program used the method of bond potential energy minimization to find the most stable structure of molecules. The metals nickel and vanadium, as the main companion metals of oil, are of the most interest from the position of analysis of their form of existence in the possible formation of hydrocarbons. Vanadium’s and nickel’s accumulation in asphaltene fractions of oils, probably, is caused by complex compounds of metalloporphyrin’s formation. In addition, the high sulfur content is probably associated with polymerization of organic molecules due to the formation of di-sulphide bonds. The method of cavitation extraction of organometallic compounds from oil and complex mineral–oil raw materials has been developed for experimental confirmation of microelements the extraction capability from oil raw materials.
1. Introduction
Clean energy issues, which require various raw materials, including hydrocarbons, to create and develop, are now becoming increasingly important [1,2,3]. However, at the same time, the mineral sector is faced with the problem of sources depletion for the easily beneficiated minerals containing carbon of different degrees of metamorphism [4,5,6]. For example, the limited oil reserves, combined with the projected growth of world energy consumption, will lead to significant structural, technological, and other changes in the world fuel and energy industry in the next 20–30 years [7,8,9,10,11,12,13].
Superdeep deposits have great potential for expanding the hydrocarbon resource base. The main growth of the explored mineral resource base reserves is provided by such fields [14,15]. A wide range of research is focused on studying the oil’s properties from such fields, as well as the conditions peculiarities of their development and prospecting [12,16,17,18,19]. Many works show the accumulation of hydrocarbons in collector rocks, which are presumably the results of biodegradation. At the same time, a serious influence of host rocks and the proximity of the Earth’s mantle on the processes of hydrocarbon transformation is noted [20]. From this point of view, the peculiarity of the composition of oils of deep deposits is interesting due to the presence of products of processes occurring in the mantle and the host rocks at the high temperatures and pressures. Thus, a study of the geochemistry of 38 ultra-deep gas wells (depth of more than 6000 m) in the Sichuan Basin was carried out [21]. The largest mass fraction in the gas mixture is methane, the value of which can reach 99.56% with an average of 86.6%. The mass fraction of ethane is 0.13% on average; no presence of higher alkanes was detected. There is also a high content of H2S, the highest value is 25.21%, and on average it is 5.45%. It is assumed that the presence of hydrogen sulfide is caused by two mechanisms: thermochemical reduction of sulfates and thermal decomposition of sulfides. Additionally, for deep oil deposits, the distinctive feature is the high content of heterocyclic compounds [22,23], which have a high capacity to form complex compounds with many transition metals [24,25,26].
Deep oil deposits are characterized by a significant geological age, which is largely confirmed by the high content of various metals (including vanadium and nickel) [27,28]. Vanadium and nickel are closely associated with the heavy oil fraction, and in many cases, the bitumen of these fractions is considered as strategic metals’ potential sources [24]. The metals are predominantly associated with asphaltenes and are found in complex metalloporphyrin compounds [29,30,31].
The oil basin’s occurrence depth of 6000 m and below determines the high cost of exploration and prospecting works [32,33,34]. Reducing capital costs for these types of work is possible with new technological solutions to identify markers of hydrocarbon deposits. In these areas, it is possible to apply search methods based on the analysis of the host rock properties. This is because the host rocks affect the composition and characteristics of natural hydrocarbon deposits and are potential geological markers. However, to substantiate the relevance of such geological markers for prospecting works, it is necessary to understand the genesis of deep crude oil deposits.
Certain aspects of the genesis theory of deep hydrocarbon deposits cannot be fully explained by the classical biogenic theory of oil formation. Special properties of deep oil, such as high content of methane, presence of nickel and vanadium in significant quantities, geological age, and high sulfur content in oil can be explained from the position of the abiogenous theory of hydrocarbon formation. This theory assumes that hydrocarbons are formed mainly due to the reactions of mantle gases and host rock [35,36]. Studies have shown that oxygen and nitrogen are part of the metalloporphyrins’ molecular structure. The presence of metals in the oil samples is due to their presence in the fluids. Moreover, such metals as V, Ni, Fe, Mo, etc. can be considered mantle marks. These metals (especially V and Ni) show high catalytic activity in relation to the carbonaceous substance and can (as well as S, N, and O) participate in the formation of oils by entering their structure in the form of V- and Ni-porphyrins [37].
In works [38,39], special classification was used to identify geochemical indicators depending on the concentration of microelements (ME) in carbon containing minerals of different degrees of metamorphism (peat, coal, oil shale, and oil). Approximate estimation of the average major ME concentrations (Cic, g/t) and the degree of the initial substances’ concentration in ashes in relation to clays rock clarks (QicA) are shown in Table 1. To estimate the degree of concentration the following formula was used:
where A—ash-content (10, 20, 50, and 0.1 for peat, coal, oil shale, and crude oil, respectively); Ki—Clarke ME in argillaceous rock; 1.13—correction factor related to the loss of mass during calcination.
QicA = CicA/1.13Ki

Table 1.
Content of microelements in various carbonaceous rocks and oil.
The presence of metalloporphyrins (vanadium and nickel and their ratio) in crude oils is additional proof of their abiogenous nature.
The possibility and mechanism of abiogenic short-chain gaseous alkanes formation were discussed in [40]. According to the assumption, the main component formed by the Fischer–Tropsch synthesis is methane. Isotopic composition studies have shown that hydrocarbons from the Qinsheng gas field in the Songliao Basin include alkanes formed by the Fischer–Tropsch reaction and captured by the reservoir during the release of gas from the mantle.
One of the key factors for the possibility of these reactions is the specific conditions in terms of temperature, pressure, and catalysts. Experimental reproduction of possible mantle formation conditions of oils is a rather laborious task.
The difficulty of confirming these hypotheses in laboratory conditions is due to the potentially low yields of the target products and extreme conditions for temperature and pressure [41]. A possible solution is the application of various chemical process models [42,43]. These models relate the structural features of the molecules to the physical properties of interest. The basic hypothesis of QSPR is that molecules similar in structure will have similar physical properties [44], and for QSAR models, perhaps chemical or biological similarity [45]. Therefore, it is possible to train a model defining a specific relationship between structure and properties/activity on a training dataset and apply it to similar molecules to predict their properties and activity [46].
Thus, the exhaustibility and low quality of mineral resources makes scientists around the world search for alternative mineral’s sources of different genesis (including liquid and solid) and create new methods of their search to increase active reserves of fossil hydrocarbons against the unstable demand and supply in the world markets. Involvement in the deep oil deposits development is associated with significant costs of exploration work.
This paper addresses the necessity of the oil deposits exploration cost abatement by an attempt to substantiate the geological markers of such deposit’s occurrence based on thermodynamic and quantum-chemical molecular modeling. Applied modeling techniques address the specific peculiarities of the deep oil from the possible abiogenic nature of the hydrocarbon’s formation.
2. Materials and Methods
The research was conducted in two stages, which included theoretical and experimental research.
The first stage. For modeling of possible processes of oil genesis in the work the module of calculation of Gibbs free energy of program HSC Chemistry 6.0 was used. The main purpose of the module is to determine the change in thermodynamic functions during a chemical reaction. The essence of the approach is to estimate the total probability of the potential reaction of hydrocarbon formation based on the value of the change in Gibbs energy (2).
where ΔG—Gibbs energy change value, kJ/mol; ΔH—the value of enthalpy change, kJ/mol, ΔS—entropy change value, kJ/(mol·K); and T—absolute temperature, K.
A negative value of the change in Gibbs energy means a high probability that the reaction will proceed in the forward direction, while a positive value means an extremely low probability that the reaction will occur. The lower the value of the Gibbs energy change, the more probable the reaction is. The thermodynamic functions in Equation (2) were calculated based on the database of standard values of enthalpy and entropy (Table 2).

Table 2.
Example of a program calculation in the HSC Chemistry 6.0.
The stability of the molecular structure of compounds was assessed using the free software package Avogadro. The Avogadro package is a molecular editor and visualizer whose main purpose is cross-platform use in computational chemistry, molecular modeling, bioinformatics, materials science, and related fields. The program used the UFF bond potential energy minimization method to find the most stable structure of a molecule. The UFF method belongs to the class of molecular mechanics methods. These methods are developed for obtaining the optimal geometric characteristics and energies of multi-atomic systems, based on the equations of mechanics. The total energy of the molecule is the sum of the following energies: chemical interaction energy, valence angle energy, torsional interaction energy, van der Waals interaction energy, and electrostatic interaction energy. Description of changes in these energies is made based on atoms’ interaction interpretation inside molecules as absolutely solid bodies connected by strings (Figure 1), according to the following equations [47]:
where Ub—binding energy potential (kJ·mol−1); Kr = 12.5 kJ·mol−1/Å2—force field constant for calculating the binding energy potential; lp = 4.7 Å—assumed equilibrium distance between particles; and l—distance between particles, Å;
where Ua—bond angle potential energy (kJ·mol−1); Ka = 25 kJ·mol−1/rad2—force field constant for calculating bond angle potential; Qp = 120 rad—accepted equilibrium bond angle; and Q—bond angle, rad;
where Uc—interaction potential of charged particles (kJ·mol−1); qi, qj—relative particle charge (C/mol); ε0—vacuum permittivity (F/m); and εl—relative permittivity of the medium;
where UW—van der Waals interaction potential, through the Lennard-Jones potential; εW—the value of the minimum energy barrier (potential well); and σ—distance at which interaction is minimal.
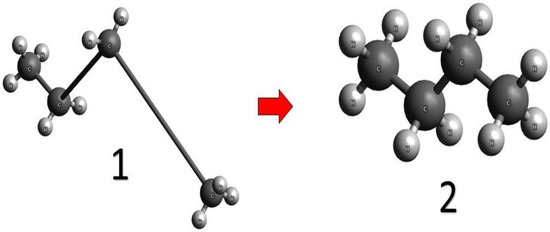
Figure 1.
Example of molecular structure optimization based on the UFF method in the Avogadro software package [48].
The second stage was the experimental confirmation of the possibility of formation and extraction of microelements from oil raw materials. Experimental studies were carried out on the facilities of the Saint Petersburg Mining University. Crushing to a size of-2 mm was carried out in a jaw crusher, grinding to a grade of −0.071 microns in a ball mill. Flotation experiments were carried out on the Laarmann flotation bench test machine with the chamber volume of 1.5 L. As the result carbonaceous concentrate and carbonaceous flotation tailings were obtained. Ultrasonic treatment was carried out using INLAB I100-6/1-1 unit (Ultrasonic Technologies-INLAB LLC, St. Petersburg, Russia). Working frequency of the ultrasound unit varied from 22 to 44 kHz, which made it possible to achieve the intensity from 5 to 25 W/cm2. Cavitation treatment was carried out in a chamber (V = 0.5 L) with an agitator. The speed of the stirrer was varied from 1000 to 5000 rpm. The conditions and methodology of ultrasonic and cavitation treatment are presented in [49]. The samples were examined using a Vega 3 LMH scanning electron microscope with an Oxford Instruments INCA Energy 250/X-max 20 X-ray energy dispersive microanalysis system.
3. Results
3.1. Modeling of Presumptive Genesis Processes of Deep Oil Deposits
Initially, the possibility of the formation of low molecular weight hydrocarbons at contact with ascending flows of mantle gases with cooling rock barriers was assessed. Due to a sharp cooling of the gas’s mass and the formation of condensate. The primary synthesis probably proceeds from the interaction of fluid compounds H2, CO2, and H2S [50]. The mechanism of the supposed interaction reaction is as follows:
(3n + 1)H2S + nCO2 = CnH2n+2 + 2nH2O + (3n + 1)S
The set of conditions used to calculate the Gibbs-specific energy changes in the HSC chemistry software package is summarized in Table 3. The graphical interpretation of the results is presented in Figure 2.

Table 3.
Reaction conditions for calculating thermodynamic functions.
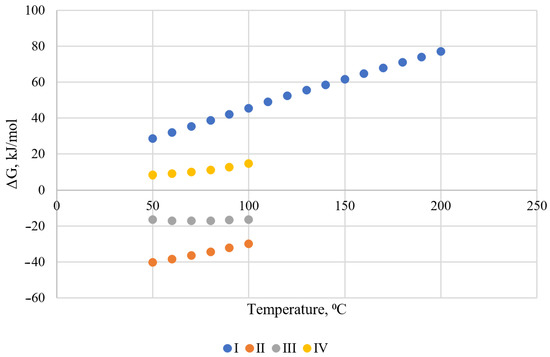
Figure 2.
Graphical interpretation of calculations of the values of the specific change in the Gibbs energy of the reaction of the interaction of fluid compounds.
Because of reaction thermodynamic modeling under the set of conditions I, it was found that the reaction cannot proceed under normal conditions, where all reactants are in gaseous form. Such reactions are possible only in interaction with condensed hydrogen sulfide and high pressures [50]. The critical temperature of hydrogen sulfide condensation is 100.4 °C. Accordingly, the cooling of a gas mixture by host rocks should occur at temperatures below this. Based on the obtained results of modeling the reaction at the set of conditions II, it was found that the formation reaction is possible (change of Gibbs energy < 0) in the temperature range from 50 °C to 100 °C. The formation reactions are exothermic, and their intensity increases with decreasing temperature. However, the propane formation reaction according to this mechanism (set of conditions IV), under these conditions according to calculations, is no longer possible. The possibility of the formation of methane and ethane is consistent with the high content of associated natural gas from deep oil fields, but this mechanism does not fully describe the process of formation of high-molecular alkanes.
The Fischer–Tropsch reaction can serve as an alternative mechanism of hydrocarbon formation. In this reaction, the interaction of carbon monoxide and hydrogen on metal catalysts leads to the synthesis of the following saturated hydrocarbons:
nCO + (2n + 1)H2 = CnH2n+2 + nH2O
This reaction has been used in industry to produce synthetic hydrocarbons. This reaction proceeds mainly on metallic catalysts (Fe, Co), at high pressures (Fe—10–15 MPa; Co—3 MPa). Under industrial conditions, the course of Fischer–Tropsch synthesis and its products are determined by a combination of catalyst types and technological conditions. These facts can serve as an explanation of variations in the chemical composition of deposits of deep oils and the presence of a fraction of solid sulfide minerals.
Graphical interpretation of results of thermodynamic modeling of the Fischer–Tropsch reaction for the formation of propane, hexane, and decane at a pressure of 100 bar in the temperature range from 300 °C to 450 °C (industrial synthesis conditions on iron catalysts) resulted in Figure 3.
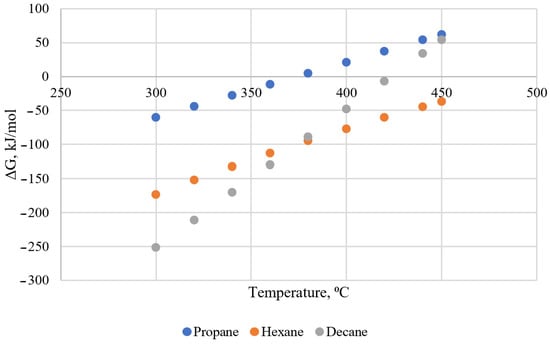
Figure 3.
Graphical interpretation of the calculated values of the Gibbs change duration of the reaction of fluid compounds.
Based on the results obtained, it was found that for the synthesis conditions on iron catalyst the process can proceed with the synthesis of hydrocarbons. The flow of the reaction is more likely the lower the temperature, at the same time the reaction is only possible if the pressure in the reaction mixture will fall during the reaction. Additionally, specific conditions for the reaction are the presence of metallic catalysts. In addition to iron, transition metals of the d-subgroup (Ni, Co, V) can serve as catalysts. Metals, such as nickel and vanadium, as the main companion metals of heavy oils are of the greatest interest from the position of the analysis of their form of existence at the possible formation of hydrocarbons.
For a temperature of 500 degrees Celsius, the conventional ceiling of the Fischer–Tropsch process, Eh-pH diagrams were made (Figure 4 and Figure 5).
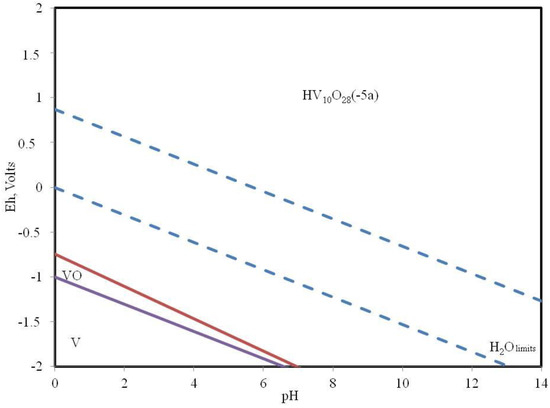
Figure 4.
Pourbaix diagram of vanadium-carbon-sulfur-nitrogen elements at 500 °C.
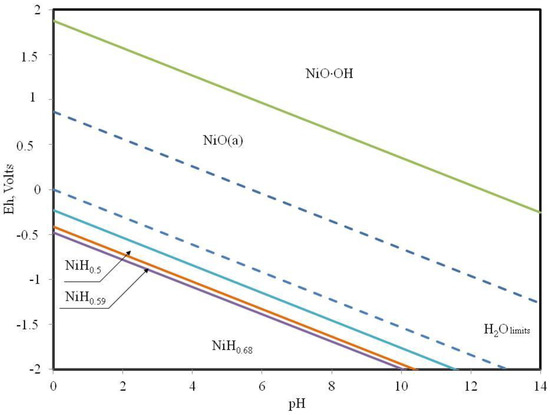
Figure 5.
Pourbaix diagram of nickel-carbon-sulfur-nitrogen elements at 500 degrees Celsius.
It was found that pure vanadium and vanadium oxide can exist at a temperature of 500 degrees Celsius at negative values of the redox potential and pH values from 0 to 6.9. These forms of vanadium compounds have the greatest theoretical catalytic activity with respect to the processes of dehydrogenation of alkanes and their isomerization.
It was found that nickel oxide can exist at a temperature of 500 degrees Celsius in a wide range of values of the redox potential. In an acidic environment, nickel oxide predominantly exists at positive values of the redox potential. Nickel oxide has the greatest theoretical catalytic activity with respect to alkane dehydrogenation and isomerization processes.
The wider zone of the existence of the nickel compound capable of catalysis can be an indication of its higher priority in the deep oils from the position of the abiogenous theory of the origin of oils. The correlation between the composition of oils and the ratio of nickel and vanadium content is probably also related to their conditions of existence. Collector rocks, which were formed in acid environments, should contain vanadium for the successful course of processes of transformation of paraffins into various derivatives. For the successful course of processes of accumulation of hydrocarbons in the presence of nickel oxides in sour environments, mineral compounds possessing an oxidative potential, and in the case of a basic environment, a reducing potential are necessary.
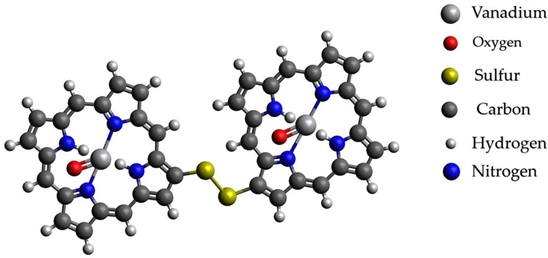
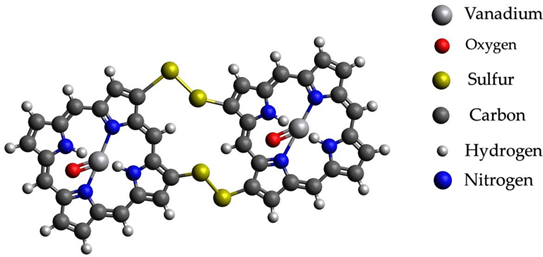
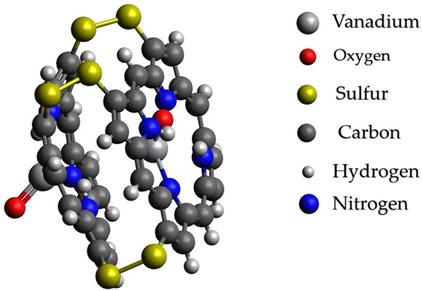
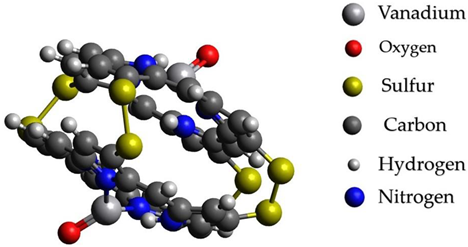
After processes of transformation of paraffins into unsaturated hydrocarbons and nitrogen- and sulfur-containing compounds, changing conditions probably should promote the capture of catalytically active metals in complex compounds with basic carrier compounds of nickel and vanadium in heavy fractions of oils-porphins. At the same time, probably, a more stable existence of molecules of a large fraction of oil occurs with the formation of sulfide bridges from elementary sulfur present as products of the reaction of hydrogen sulfide from mantle gases. The formation of sulfide bridges unites molecules and allows them to considerably reduce the energy of connections. Graphical representations of the investigated structures are summarized in Table 4.

Table 4.
Graphical representations of vanadium and nickel porphin carrier molecules and possible structures with sulfide bridges.
The results of the calculation of the potential bonding energy by the UFF method are shown in Figure 6.
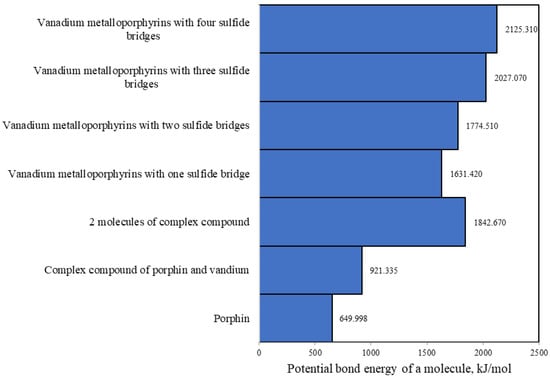
Figure 6.
Potential bonding energy of porphin molecules during condensation due to sulfide bridges.
Based on the analysis of the obtained data, it was found that the potential energy of the two molecules of the metalloporphyrin complex is 68.16 kJ/mol greater than that of the condensed form with two sulfide bridges. Consequently, the existence of the form with two sulfide bridges and one sulfide bridge is more likely than the existence of two separate complexes.
3.2. Cavitation Extraction of Organometallic Compounds of Trace Elements from Oil and Complex Mineral Oil Raw Materials
If we consider a solid carbon-containing raw material (for example, bituminous shale), then first it is necessary to prepare the raw material before cavitation extraction (Figure 7).
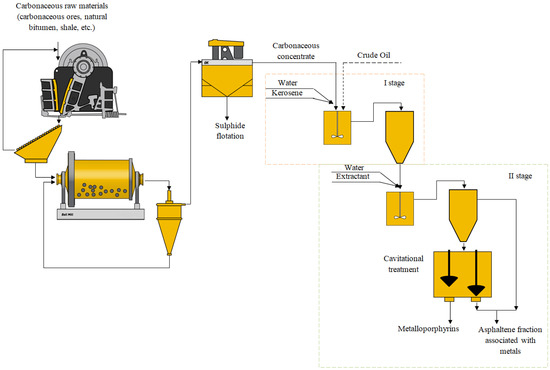
Figure 7.
Scheme of extraction of organometallic compounds from oil and complex mineral oil raw materials.
The initial raw material is crushed (to a size of 2 mm) in a closed cycle with a screen, then the sub-slice product goes to the ball mill, where the crushing to the class of 71 microns takes place. The mill discharge goes to a hydrocyclone, the sands of which are returned for regrinding, and the discharge goes to carbon flotation to isolate the carbonaceous matter associated with vanadium and nickel. Flotation was carried out using the following reagents: soda ash was used as a medium regulator at a rate of 2000 g/t, carbonaceous collector–kerosene, and foaming agent–Flotanol 7196. After flotation the carbonaceous concentrate went to cavitational treatment stage. Description and results of research on cavitation extraction are presented in [49].
The proposed method was tested on a sample of bituminous shale of the Yareg oil field. The asphaltene fraction obtained as a result contains a large part of vanadium and nickel. The results of the study of samples using a scanning electron microscope are shown in Figure 8 and Table 5.

Figure 8.
Micrographs of the asphaltene fraction associated with metals.

Table 5.
Elemental composition of spectra of asphaltene fraction associated with metals.
The conducted studies confirm the possibility of extraction of carbonaceous matter associated with metals from the matrix. Additionally, the analysis of the obtained asphaltene fraction by vanadium and nickel allows us to substantiate geochemical indicators in the search for oil deposits and assessment of their prospects.
4. Conclusions
The current trend of mineral resources exploitation and industrial development dictates the necessity of crude oil production increase. The application of various approaches that could lower the capital costs in the stage of oil deposit exploration would significantly affect the cost of the final product. This becomes essential in the case of deep oil deposits. This paper presents the results on the substantiation of search geological markers of the occurrence of such deposits because of thermodynamic and quantum-chemical molecular modeling.
According to the results of thermodynamic modeling, it was found that the formation of short-chain alkanes is possible by the Fischer–Tropsch mechanism. Compounds of vanadium and nickel can serve as catalysts. Their accumulation in asphaltene fractions of oils, probably, is caused by the formation of complex compounds of metalloporphines. In addition, the high sulfur content is likely connected with the polymerization of organic molecules due to formation of sulfide bridges.
Based on the experimental and theoretical studies, the method of cavitation extraction of organometallic compounds from oil and complex mineral oil raw materials using light hydrocarbon solvents and chemical extractants has been proposed. The main feature of this method—using ultrasonic impact in cavitation mode from the carbonaceous rocks is extracted carbonaceous substance associated with vanadium and nickel. By application of the vanadium and nickel proposed extraction method of obtained compounds can be studied further on the subject of oil deposits properties. The most important of them is the age of the deposit which correlates with the vanadium-to-nickel ratio. This information can be used in the prediction of the oil reservoir volume and extraction peculiarities for the exploitation costs assessment. Further, obtained compounds of vanadium and nickel can serve as possible catalysts in the studies of crude oil genesis.
Author Contributions
T.A.—conceived, designed the experiments, and analyzed the data; N.N.—implementation and processing of the analysis results; V.K.—performed the experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bobylev, Y.N. Global Oil Market: Main Trends 2018; Russian Economic Developments; IEP: Moscow, Russia, 2019; Volume 26, pp. 10–13. [Google Scholar]
- Dvoynikov, M.V.; Leusheva, E.L. Modern trends in hydrocarbon resources development. J. Min. Inst. 2022, 258, 879–880. [Google Scholar]
- Grigoryev, L.M.; Kheifets, E.A. Oil Market: Conflict Between Recovery And Energy Transition. Vopr. Ekon. 2022, 9, 5–33. [Google Scholar] [CrossRef]
- Litvinenko, V.S.; Petrov, E.I.; Vasilevskaya, D.V.; Yakovenko, A.V.; Naumov, I.A.; Ratnikov, M.A. Assessment of the role of the state in the management of mineral resources. J. Min. Inst. 2022, 259, 95–111. [Google Scholar] [CrossRef]
- Golubev, I.A.; Golubev, A.V.; Laptev, A.B. Practice of using the magnetic treatment devices to intensify the processes of primary oil treating. J. Min. Inst. 2020, 245, 554–560. [Google Scholar] [CrossRef]
- Sukhanov, A.A.; Yakutseni, V.P.; Petrov, Y.E. Petroleum geology. Theory Pract. 2012, 7, 23. [Google Scholar]
- Litvinenko, V.S.; Tsvetkov, P.S.; Dvoynikov, M.V.; Buslaev, G.V. Barriers to implementation of hydrogen initiatives in the context of global energy sustainable development. J. Min. Inst. 2020, 244, 428–438. [Google Scholar] [CrossRef]
- Carayannis, E.G.; Ilinova, A.; Cherepovitsyn, A. The Future of Energy and the Case of the Arctic Offshore: The Role of Strategic Management. J. Mar. Sci. Eng. 2021, 9, 134. [Google Scholar] [CrossRef]
- Tang, T.; Zhang, L.; Guo, Z.; Gu, X. Development of Cathode and Anode Materials in Lithium Sulfur Batteries. Chin. J. Rare Met. 2022, 46, 954–964. [Google Scholar]
- Wan, C.; Zhou, L.; Xu, S.; Jin, B.; Ge, X.; Qian, X.; Xu, L.; Chen, F.; Zhan, X.; Yang, Y.; et al. Defect engineered mesoporous graphitic carbon nitride modified with AgPd nanoparticles for enhanced photocatalytic hydrogen evolution from formic acid. Chem. Eng. J. 2022, 429, 132388. [Google Scholar] [CrossRef]
- Pang, D.; Li, W.; Zhang, N.; He, H.; Mao, S.; Chen, Y.; Cao, L.; Li, C.; Li, A.; Han, X. Direct observation of oxygen vacancy formation and migration over ceria surface by in situ environmental transmission electron microscopy. J. Rare Earths, 2023; in press. [Google Scholar] [CrossRef]
- Dvoynikov, M.V.; Sidorkin, D.I.; Yurtaev, S.L.; Grokhotov, E.I.; Ulyanov, D.S. Drilling of deep and ultra-deep wells for prospecting and exploration of new raw mineral fields. J. Min. Inst. 2022, 258, 945–955. [Google Scholar] [CrossRef]
- Galkin, V.I.; Martyushev, D.A.; Ponomareva, I.N.; Chernykh, I.A. Developing features of the near-bottomhole zones in productive formations at fields with high gas saturation of formation oil. J. Min. Inst. 2021, 249, 386–392. [Google Scholar] [CrossRef]
- Kozlov, S.V.; Kopylov, I.S. Regularities of Occurrence of Unique and Large Oil and Gas Deposits in The Earth Crust. Deep Zones of Hydrocarbons Generation and Primary Asthenosphere Earthquakes As A Uniform Planetary Process. Bull. Perm Univ. Geol. 2019, 18, 64–72. [Google Scholar] [CrossRef]
- Ivanov, K.S. About Possible Maximum Depth of Oil Deposits. Izv. Ural. Gos. Gorn. Univ. 2018, 41–49. [Google Scholar] [CrossRef]
- Prischepa, O.; Nefedov, Y.; Nikiforova, V. Arctic Shelf Oil and Gas Prospects from Lower-Middle Paleozoic Sediments of the Timan–Pechora Oil and Gas Province Based on the Results of a Regional Study. Resources 2022, 11, 3. [Google Scholar] [CrossRef]
- Leusheva, E.; Alikhanov, N.; Morenov, V. Barite-Free Muds for Drilling-in the Formations with Abnormally High Pressure. Fluids 2022, 7, 268. [Google Scholar] [CrossRef]
- Gendler, S.G.; Fayzylov, I.R. Application Efficiency of Closed Gathering System Toward Microclimate Normalization In Operating Galleries In Oil Mines. Min. Inf. Anal. Bull. 2021, 65–78. [Google Scholar] [CrossRef]
- Grishchenko, A.I.; Semenov, A.S.; Melnikov, B.E. Modeling the processes of deformation and destruction of the rock sample during its extraction from great depths. J. Min. Inst. 2021, 248, 243–252. [Google Scholar] [CrossRef]
- Xu, C.; Zou, W.; Yang, Y.; Duan, Y.; Shen, Y.; Luo, B.; Ni, C.; Fu, X.; Zhang, J. Status and Prospects of Deep Oil and Gas Resources Exploration and Development Onshore China. J. Nat. Gas Geosci. 2018, 3, 11–24. [Google Scholar] [CrossRef]
- Dai, J.; Ni, Y.; Qin, S.; Huang, S.; Peng, W.; Han, W. Geochemical Characteristics of Ultra-Deep Natural Gas in the Sichuan Basin, SW China. Pet. Explor. Dev. 2018, 45, 619–628. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Wei, G.; Yang, W.; Xie, Z.; Li, Z.; Guo, J.; Wang, Y.; Ma, W.; Li, J.; et al. New Progresses in Basic Geological Theories and Future Exploration Domains of Natural Gas in China. Nat. Gas Ind. B 2018, 5, 434–443. [Google Scholar] [CrossRef]
- Boyko, Y.I.; Korobkov, V.F. Criteria for Deep Oil Formation in the Sakmara Underthrust Zone of the Kazakh Urals. Ural. Geol. J. 2018, 4, 19–30. [Google Scholar]
- Abdolahnezhad, M.; Lindsay, M.B.J. Geochemical Conditions Influence Vanadium, Nickel, and Molybdenum Release from Oil Sands Fluid Petroleum Coke. J. Contam. Hydrol. 2022, 245, 103955. [Google Scholar] [CrossRef]
- Sanz-Robinson, J.; Sugiyama, I.; Williams-Jones, A.E. The Solubility of Palladium (Pd) in Crude Oil at 150, 200 and 250 °C and Its Application to Ore Genesis. Chem. Geol. 2020, 531, 119320. [Google Scholar] [CrossRef]
- Li, J.; Kong, L.; Wu, K.; Ma, J.; Liu, F.; Liu, M. Genesis of H2S in Jurassic Associated Gas in Pengyang Area, Ordos Basin, NW China. J. Nat. Gas Geosci. 2022, 7, 159–170. [Google Scholar] [CrossRef]
- López, L.; Mónaco, S.L. Vanadium, nickel and sulfur in crude oils and source rocks and their relationship with biomarkers: Implications for the origin of crude oils in Venezuelan basins. Org. Geochem. 2017, 104, 53–68. [Google Scholar] [CrossRef]
- Lu, P.; Zhuo, Z.; Zhang, W.; Sun, T.; Sun, W.; Lu, J. Quantitative Analysis of Trace Elements (Vanadium, Sodium, and Calcium) in Petroleum Coke Using Laser-Induced Breakdown Spectroscopy with Binder. Spectrochim. Acta Part B At. Spectrosc. 2022, 190, 106388. [Google Scholar] [CrossRef]
- Minikaeva, S.N.; Harlampidi, H.E.; Yakubov, M.R.; Romanov, G.V.; Milordov, D.V.; Yakubova, S.G. Features of Concentration and Extraction of Natural Porphyrins from Resins and Asphaltenes of Heavy Oil. Bull. Kazan Technol. Univ. 2010, 9, 568–578. [Google Scholar]
- Yakubova, S.G.; Abilova, G.R.; Tazeeva, E.G. A Comparative Analysis of Vanadyl Porphyrins Isolated from Heavy Oil Asphaltenes with High and Low Vanadium Content. Pet. Chem. 2022, 62, 83–93. [Google Scholar] [CrossRef]
- Aleksandrova, T.N.; Aleksandrov, A.V.; Nikolaeva, N.V.; Romashev, A.O. Processing of heavy oils and natural bitumens taking into account their rheological properties; Mediapapir LLC: St. Petersburg, Russia, 2017; p. 146. [Google Scholar]
- Romanyuk, V.B.; Boyarko, G.Y. On the capitalization of the costs of geological exploration. Miner. Resour. Russ. Econ. Manag. 2014, 5, 31–36. [Google Scholar]
- Ways of development of oil and gas resources in the russian sector of the arctic. Report of academician A.E. Kontorovich. Bull. Russ. Acad. Sci. 2015, 85, 420–430. [CrossRef]
- Postnova, E.V.; Zhidovinov, S.N. Recent Tendencies of Resource Base Development of Hidrocarbon Raw Material and Ways of Improving Efficiency of Exploration Activity in Ural-Povolje Region. Oil Gas Geol. 2008, 5, 2–10. [Google Scholar]
- Letnikov, F.A. Synergetic Aspects of Formation of Deep Oil. Deep Oil. 2013, 1, 790–810. [Google Scholar]
- Timurziev, A.I. State of The Art of The Theory of An Origin And Prac-Tice For Searches of Oil: Theses To Creation of The Scientific Theory of Forecasting And Searches of Deep Oil. Deep Oil. 2013, 1, 18–44. [Google Scholar]
- Lur’e, M.A. Sources of hydrocarbons, heterocomponents, and trace elements of abiogenic oil: Properties and composition of deep fluids. Geol. Neft. I Gaza 2020, 3, 43–49. [Google Scholar] [CrossRef]
- Shpirt, M.Y.; Sadowski, V.V. Microelements of Combustible Minerals; Kuchkovo Field: Moscow, Russia, 2010; p. 384. [Google Scholar]
- Alexandrova, T.N. Key directions in processing carbonaceous rocks. J. Min. Inst. 2016, 220, 568–572. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, X.; Zhu, D.; Meng, Q.; Xu, H.; Peng, W.; Huang, X.; Liu, J. Generation and Resource Potential of Abiogenic Alkane Gas under Organic–Inorganic Interactions in Petroliferous Basins. J. Nat. Gas Geosci. 2021, 6, 79–87. [Google Scholar] [CrossRef]
- Kalz, K.F.; Kraehnert, R.; Dvoyashkin, M.; Dittmeyer, R.; Gläser, R.; Krewer, U.; Reuter, K.; Grunwaldt, J.-D. Future Challenges in Heterogeneous Catalysis: Understanding Catalysts under Dynamic Reaction Conditions. ChemCatChem 2017, 9, 17–29. [Google Scholar] [CrossRef]
- Zaera, F. Molecular Approaches to Heterogeneous Catalysis. Coord. Chem. Rev. 2021, 448, 214179. [Google Scholar] [CrossRef]
- Filippov, E.V.; Zakharov, L.A.; Martyushev, D.A.; Ponomareva, I.N. Reproduction of reservoir pressure by machine learning methods and study of its influence on the cracks formation process in hydraulic fracturing. J. Min. Inst. 2022, 258, 924–932. [Google Scholar] [CrossRef]
- Shchelokov, A.; Palko, N.; Potemkin, V.; Grishina, M.; Morozov, R.; Korina, E.; Uchaev, D.; Krivtsov, I.; Bol’shakov, O. Adsorption of Native Amino Acids on Nanocrystalline TiO2: Physical Chemistry, QSPR, and Theoretical Modeling. Langmuir 2019, 35, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Muratov, E.N.; Bajorath, J.; Sheridan, R.P.; Tetko, I.V.; Filimonov, D.; Poroikov, V.; Oprea, T.I.; Baskin, I.I.; Varnek, A.; Roitberg, A.; et al. QSAR without Borders. Chem. Soc. Rev. 2020, 49, 3525–3564. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Fang, D.; Ito, S.; Okamoto, Y.; Ovchinnikov, V.; Cui, Q. QM/MM free energy simulations: Recent progress and challenges. Mol. Simul. 2016, 42, 1056–1078. Available online: https://www.tandfonline.com/doi/abs/10.1080/08927022.2015.1132317 (accessed on 27 December 2022). [CrossRef] [PubMed]
- Leach, A.R. Molecular Modelling: Principles and Applications; Pearson Education: London, UK, 2001. [Google Scholar]
- Team, T.A. Auto Optimize Tool. Avogadro. Available online: https://avogadro.cc/docs/tools/auto-optimize-tool/ (accessed on 25 January 2023).
- Romashev, A.; He, D.; Aleksandrova, T.; Nikolaeva, N. Technological Typomorphic Associations in Caustobiolites and Methods of Their Extraction. Metals 2021, 11, 121. [Google Scholar] [CrossRef]
- Lurie, M.A.; Schmidt, F.K. Genetic Aspects of Oil and Gas Formation, Sulfur and Metal Content of Oils; Reports of the Academy of Sciences; Springer: Berlin/Heidelberg, Germany, 2009; p. 424. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).