A Review on Dry Deposition Techniques: Pathways to Enhanced Perovskite Solar Cells
Abstract
:1. Introduction
2. Dry Processes for Perovskite Film Deposition
2.1. Evaporation
2.1.1. Single-Step Evaporation
2.1.2. Multi-Step Evaporation
2.2. Chemical Vapor Deposition
2.2.1. Double-Zone Furnace with Carrier Gas Flow
2.2.2. Single-Zone Furnace
2.3. Sputtering
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tian, C.; Wang, F.; Wang, Y.; Yang, Z.; Chen, X.; Mei, J.; Liu, H.; Zhao, D. Chemical vapor deposition method grown all-inorganic perovskite microcrystals for self-powered photodetectors. ACS Appl. Mater. Interfaces 2019, 11, 15804–15812. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tsai, W.-L.; Li, C.-S.; Hsu, B.-W.; Chen, C.-Y.; Wu, C.-I.; Lin, H.-W. High-quality conformal homogeneous all-vacuum deposited CsPbCl3 thin films and their UV photodiode applications. ACS Appl. Mater. Interfaces 2019, 11, 47054–47062. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, K.B.; Patel, J.B.; Rothmann, M.U.; Xia, C.Q.; Oliver, R.D.; Herz, L.M.; Snaith, H.J.; Johnston, M.B. Control over crystal size in vapor deposited metal-halide perovskite films. ACS Energy Lett. 2020, 5, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-M.; Lin, L.-J.; Yan, Y.-J.; Yu, S.-H.; Chou, M.M.; Hsieh, H.-F.; Ho, C.-J.; Chen, R.-S. The Extremely Enhanced Photocurrent Response in Topological Insulator Nanosheets with High Conductance. Nanoscale Res. Lett. 2018, 13, 371. [Google Scholar] [CrossRef] [PubMed]
- Kottokkaran, R.; Gaonkar, H.A.; Bagheri, B.; Dalal, V.L. Efficient pin inorganic CsPbI3 perovskite solar cell deposited using layer-by-layer vacuum deposition. J. Vac. Sci. Technol. A 2018, 36, 041201. [Google Scholar] [CrossRef]
- Bonomi, S.; Marongiu, D.; Sestu, N.; Saba, M.; Patrini, M.; Bongiovanni, G.; Malavasi, L. Novel physical vapor deposition approach to hybrid perovskites: Growth of MAPbI3 thin films by RF-magnetron sputtering. Sci. Rep. 2018, 8, 15388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyden, M.R.; Ono, L.K.; Raga, S.R.; Kato, Y.; Wang, S.; Qi, Y. High performance perovskite solar cells by hybrid chemical vapor deposition. J. Mater. Chem. A 2014, 2, 18742–18745. [Google Scholar] [CrossRef] [Green Version]
- Shahiduzzaman, M.; Yonezawa, K.; Yamamoto, K.; Ripolles, T.S.; Karakawa, M.; Kuwabara, T.; Takahashi, K.; Hayase, S.; Taima, T. Improved reproducibility and intercalation control of efficient planar inorganic perovskite solar cells by simple alternate vacuum deposition of PbI2 and CsI. ACS Omega 2017, 2, 4464–4469. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Shi, B.; Xu, Q.; Li, Y.; Chen, B.; Wang, Q.; Wang, P.; Zhao, Y.; Zhang, X. Crystalline quality control in sequential vapor deposited perovskite film toward high efficiency and large scale solar cells. Sol. Energy Mater. Sol. Cells 2021, 233, 111382. [Google Scholar] [CrossRef]
- Teuscher, J.; Ulianov, A.; Müntener, O.; Grätzel, M.; Tétreault, N. Control and study of the stoichiometry in evaporated perovskite solar cells. ChemSusChem 2015, 8, 3847–3852. [Google Scholar] [CrossRef]
- Zanoni, K.P.; Martínez-Goyeneche, L.; Dreessen, C.; Sessolo, M.; Bolink, H.J. Photovoltaic Devices Using Sublimed Methylammonium Lead Iodide Perovskites: Long-Term Reproducible Processing. Sol. RRL 2023, 7, 2201073. [Google Scholar] [CrossRef]
- Li, H.; Tan, L.; Jiang, C.; Li, M.; Zhou, J.; Ye, Y.; Liu, Y.; Yi, C. Molten salt strategy for reproducible evaporation of efficient perovskite solar cells. Adv. Funct. Mater. 2023, 33, 2211232. [Google Scholar] [CrossRef]
- Espinoza, C.; Barría-Cáceres, F.; Angel, F.A. Simple dual-QCM method to control CH3NH3I deposition for reproducible vacuum-processed halide perovskite photovoltaic devices. Mater. Lett. 2022, 321, 132459. [Google Scholar] [CrossRef]
- Kim, B.-S.; Kim, T.-M.; Choi, M.-S.; Shim, H.-S.; Kim, J.-J. Fully vacuum–processed perovskite solar cells with high open circuit voltage using MoO3/NPB as hole extraction layers. Org. Electron. 2015, 17, 102–106. [Google Scholar] [CrossRef]
- Du, P.; Li, J.; Wang, L.; Sun, L.; Wang, X.; Xu, X.; Yang, L.; Pang, J.; Liang, W.; Luo, J. Efficient and large-area all vacuum-deposited perovskite light-emitting diodes via spatial confinement. Nat. Commun. 2021, 12, 4751. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, Q.; Zhang, D.; Tang, Y.; Shu, L.; Zhu, Y.; Fan, Z. Scalable all-evaporation fabrication of efficient light-emitting diodes with hybrid 2D–3D perovskite nanostructures. Adv. Funct. Mater. 2020, 30, 2002913. [Google Scholar] [CrossRef]
- Leyden, M.R.; Meng, L.; Jiang, Y.; Ono, L.K.; Qiu, L.; Juarez-Perez, E.J.; Qin, C.; Adachi, C.; Qi, Y. Methylammonium lead bromide perovskite light-emitting diodes by chemical vapor deposition. J. Phys. Chem. Lett. 2017, 8, 3193–3198. [Google Scholar] [CrossRef] [Green Version]
- Higgins, M.; Reyes-Banda, M.G.; Martínez-Falomir, G.; El Bouanani, L.; Murillo, B.; Chavez-Urbiola, I.; Pintor-Monroy, M.; Ely, F.; Mathew, X.; Quevedo-Lopez, M. Solvent-free and large area compatible deposition of methylammonium lead bromide perovskite by close space sublimation and its application in PIN diodes. Thin Solid Film. 2019, 692, 137585. [Google Scholar] [CrossRef]
- Zhan, X.; Zhang, X.; Liu, Z.; Chen, C.; Kong, L.; Jiang, S.; Xi, S.; Liao, G.; Liu, X. Boosting the performance of self-powered CsPbCl3-based UV photodetectors by a sequential vapor-deposition strategy and heterojunction engineering. ACS Appl. Mater. Interfaces 2021, 13, 45744–45757. [Google Scholar] [CrossRef]
- Borchert, J.; Milot, R.L.; Patel, J.B.; Davies, C.L.; Wright, A.D.; Martínez Maestro, L.; Snaith, H.J.; Herz, L.M.; Johnston, M.B. Large-area, highly uniform evaporated formamidinium lead triiodide thin films for solar cells. ACS Energy Lett. 2017, 2, 2799–2804. [Google Scholar] [CrossRef]
- Forgács, D.; Gil-Escrig, L.; Pérez-Del-Rey, D.; Momblona, C.; Werner, J.; Niesen, B.; Ballif, C.; Sessolo, M.; Bolink, H.J. Efficient monolithic perovskite/perovskite tandem solar cells. Adv. Energy Mater 2017, 7, 1602121. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-W.; Bae, S.; Hwang, J.-K.; Lee, W.; Lee, S.; Hyun, J.Y.; Cho, K.; Kim, S.; Heinz, F.D.; Bin Choi, S. Perovskites fabricated on textured silicon surfaces for tandem solar cells. Commun. Chem. 2020, 3, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.-K.; Lee, S.-W.; Lee, W.; Bae, S.; Kang, D.; Jeong, S.H.; Lee, S.; Pyun, D.; Hwang, J.-S.; Cho, S. Sputtered PbI2 with Post Processing for Perovskite Solar Cells. Sol. RRL 2023, 7, 2300214. [Google Scholar] [CrossRef]
- Li, H.; Liu, M.; Li, M.; Park, H.; Mathews, N.; Qi, Y.; Zhang, X.; Bolink, H.J.; Leo, K.; Graetzel, M. Applications of vacuum vapor deposition for perovskite solar cells: A progress review. iEnergy 2022, 1, 434–452. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Wu, J.; Wen, W.; Qi, Y. Fabrication of efficient metal halide perovskite solar cells by vacuum thermal evaporation: A progress review. Curr. Opin. Electrochem. 2018, 11, 130–140. [Google Scholar] [CrossRef]
- Qiu, L.; He, S.; Jiang, Y.; Qi, Y. Metal halide perovskite solar cells by modified chemical vapor deposition. J. Mater. Chem. A 2021, 9, 22759–22780. [Google Scholar] [CrossRef]
- Jiang, Y.; He, S.; Qiu, L.; Zhao, Y.; Qi, Y. Perovskite solar cells by vapor deposition based and assisted methods. Appl. Phys. Rev. 2022, 9, 021305. [Google Scholar] [CrossRef]
- Tan, L.; Zhou, J.; Zhao, X.; Wang, S.; Li, M.; Jiang, C.; Li, H.; Zhang, Y.; Ye, Y.; Tress, W. Combined Vacuum Evaporation and Solution Process for High-Efficiency Large-Area Perovskite Solar Cells with Exceptional Reproducibility. Adv. Mater. 2023, 35, 2205027. [Google Scholar] [CrossRef]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef]
- Malinkiewicz, O.; Roldán-Carmona, C.; Soriano, A.; Bandiello, E.; Camacho, L.; Nazeeruddin, M.K.; Bolink, H.J. Metal-oxide-free methylammonium lead iodide perovskite-based solar cells: The influence of organic charge transport layers. Adv. Energy Mater. 2014, 4, 1400345. [Google Scholar] [CrossRef]
- Momblona Rincón, M.C.; Gil Escrig, L.; Bandiello, E.; Hutter, E.M.; Sessolo, M.; Lederer, K.; Blochwitz-Nimoth, J.; Bolink, H. Efficient vacuum deposited pin and nip perovskite solar cells employing doped charge transport layers. Energy Environ. Sci. 2016, 9, 3456–3463. [Google Scholar] [CrossRef]
- Gil-Escrig, L.; Momblona, C.; La-Placa, M.G.; Boix, P.P.; Sessolo, M.; Bolink, H.J. Vacuum deposited triple-cation mixed-halide perovskite solar cells. Adv. Energy Mater. 2018, 8, 1703506. [Google Scholar] [CrossRef]
- Chiang, Y.-H.; Anaya, M.; Stranks, S.D. Multisource vacuum deposition of methylammonium-free perovskite solar cells. ACS Energy Lett. 2020, 5, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, H.; Chin, X.Y.; Dewi, H.A.; Vergeer, K.; Goh, T.W.; Lim, J.W.M.; Lew, J.H.; Loh, K.P.; Soci, C. Highly efficient thermally co-evaporated perovskite solar cells and mini-modules. Joule 2020, 4, 1035–1053. [Google Scholar] [CrossRef]
- Roß, M.; Severin, S.; Stutz, M.B.; Wagner, P.; Köbler, H.; Favin-Lévêque, M.; Al-Ashouri, A.; Korb, P.; Tockhorn, P.; Abate, A. Co-evaporated formamidinium lead iodide based perovskites with 1000 h constant stability for fully textured monolithic perovskite/silicon tandem solar cells. Adv. Energy Mater. 2021, 11, 2101460. [Google Scholar] [CrossRef]
- Li, J.; Dewi, H.A.; Wang, H.; Zhao, J.; Tiwari, N.; Yantara, N.; Malinauskas, T.; Getautis, V.; Savenije, T.J.; Mathews, N. Co-Evaporated MAPbI3 with Graded Fermi Levels Enables Highly Performing, Scalable, and Flexible p-i-n Perovskite Solar Cells. Adv. Funct. Mater. 2021, 31, 2103252. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, R.; Kroll, M.; Hofstetter, Y.J.; Jia, X.; Becker-Koch, D.; Paulus, F.; Löffler, M.; Nehm, F.; Leo, K. Efficient Thermally Evaporated γ-CsPbI3 Perovskite Solar Cells. Adv. Energy Mater. 2021, 11, 2100299. [Google Scholar] [CrossRef]
- Erdenebileg, E.; Wang, H.; Li, J.; Singh, N.; Dewi, H.A.; Tiwari, N.; Mathews, N.; Mhaisalkar, S.; Bruno, A. Low-Temperature Atomic Layer Deposited Electron Transport Layers for Co-Evaporated Perovskite Solar Cells. Sol. RRL 2022, 6, 2100842. [Google Scholar] [CrossRef]
- Fan, P.; Gu, D.; Liang, G.-X.; Luo, J.-T.; Chen, J.-L.; Zheng, Z.-H.; Zhang, D.-P. High-performance perovskite CH3NH3PbI3 thin films for solar cells prepared by single-source physical vapour deposition. Sci. Rep. 2016, 6, 29910. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.W.; Kang, H.W.; Hsiao, S.Y.; Yang, P.F.; Chiang, K.M.; Lin, H.W. Efficient and uniform planar-type perovskite solar cells by simple sequential vacuum deposition. Adv. Mater. 2014, 26, 6647–6652. [Google Scholar] [CrossRef]
- Yang, D.; Yang, Z.; Qin, W.; Zhang, Y.; Liu, S.F.; Li, C. Alternating precursor layer deposition for highly stable perovskite films towards efficient solar cells using vacuum deposition. J. Mater. Chem. A 2015, 3, 9401–9405. [Google Scholar] [CrossRef]
- Choi, W.-G.; Na, S.; Park, C.-G.; Moon, T. Organic-cation-mixed (FA, MA) PbI3 through sequential vapor growth for planar perovskite solar cells. Sol. Energy 2019, 178, 56–60. [Google Scholar] [CrossRef]
- Kam, M.; Zhu, Y.; Zhang, D.; Gu, L.; Chen, J.; Fan, Z. Efficient mixed-cation mixed-halide perovskite solar cells by all-vacuum sequential deposition using metal oxide electron transport layer. Sol. RRL 2019, 3, 1900050. [Google Scholar] [CrossRef]
- Feng, J.; Jiao, Y.; Wang, H.; Zhu, X.; Sun, Y.; Du, M.; Cao, Y.; Yang, D.; Liu, S.F. High-throughput large-area vacuum deposition for high-performance formamidine-based perovskite solar cells. Energy Environ. Sci. 2021, 14, 3035–3043. [Google Scholar] [CrossRef]
- Li, H.; Zhou, J.; Tan, L.; Li, M.; Jiang, C.; Wang, S.; Zhao, X.; Liu, Y.; Zhang, Y.; Ye, Y. Sequential vacuum-evaporated perovskite solar cells with more than 24% efficiency. Sci. Adv. 2022, 8, eabo7422. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yang, D.; Yang, R.; Yang, B.; Yang, Z.; Ren, X.; Zhang, J.; Niu, J.; Feng, J.; Liu, S.F. Superior stability for perovskite solar cells with 20% efficiency using vacuum co-evaporation. Nanoscale 2017, 9, 12316–12323. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhang, Y.; Chai, N.; Deng, X.; Zhong, J.; Huang, F.; Peng, Y.; Ku, Z.; Cheng, Y.-B. Large-area perovskite solar cells with Cs x FA 1− x PbI 3− y Br y thin films deposited by a vapor–solid reaction method. J. Mater. Chem. A 2018, 6, 21143–21148. [Google Scholar] [CrossRef]
- Kim, B.-S.; Gil-Escrig, L.; Sessolo, M.; Bolink, H.J. Deposition kinetics and compositional control of vacuum-processed CH3NH3PbI3 perovskite. J. Phys. Chem. Lett. 2020, 11, 6852–6859. [Google Scholar] [CrossRef]
- Leyden, M.R.; Jiang, Y.; Qi, Y. Chemical vapor deposition grown formamidinium perovskite solar modules with high steady state power and thermal stability. J. Mater. Chem. A 2016, 4, 13125–13132. [Google Scholar] [CrossRef] [Green Version]
- Leyden, M.R.; Lee, M.V.; Raga, S.R.; Qi, Y. Large formamidinium lead trihalide perovskite solar cells using chemical vapor deposition with high reproducibility and tunable chlorine concentrations. J. Mater. Chem. A 2015, 3, 16097–16103. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Qu, H.; Cao, J.; Tai, H.; Li, J.; Zheng, N. Vapor-assisted crystallization control toward high performance perovskite photovoltaics with over 18% efficiency in the ambient atmosphere. J. Mater. Chem. A 2016, 4, 13203–13210. [Google Scholar] [CrossRef]
- Ng, A.; Ren, Z.; Shen, Q.; Cheung, S.H.; Gokkaya, H.C.; So, S.K.; Djurišić, A.B.; Wan, Y.; Wu, X.; Surya, C. Crystal engineering for low defect density and high efficiency hybrid chemical vapor deposition grown perovskite solar cells. ACS Appl. Mater. Interfaces 2016, 8, 32805–32814. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Leyden, M.R.; Qiu, L.; Wang, S.; Ono, L.K.; Wu, Z.; Juarez-Perez, E.J.; Qi, Y. Combination of hybrid CVD and cation exchange for upscaling Cs-substituted mixed cation perovskite solar cells with high efficiency and stability. Adv. Funct. Mater. 2018, 28, 1703835. [Google Scholar] [CrossRef]
- Shen, Q.; Ng, A.; Ren, Z.; Gokkaya, H.C.; Djurisic, A.B.; Zapien, J.A.; Surya, C. Characterization of low-frequency excess noise in CH3NH3PbI3-based solar cells grown by solution and hybrid chemical vapor deposition techniques. ACS Appl. Mater. Interfaces 2018, 10, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Remeika, M.; Hu, Z.; Juarez-Perez, E.J.; Qiu, L.; Liu, Z.; Kim, T.; Ono, L.K.; Son, D.Y.; Hawash, Z. Negligible-Pb-waste and upscalable perovskite deposition technology for high-operational-stability perovskite solar modules. Adv. Energy Mater. 2019, 9, 1803047. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.; He, S.; Liu, Z.; Ono, L.K.; Son, D.-Y.; Liu, Y.; Tong, G.; Qi, Y. Rapid hybrid chemical vapor deposition for efficient and hysteresis-free perovskite solar modules with an operation lifetime exceeding 800 hours. J. Mater. Chem. A 2020, 8, 23404–23412. [Google Scholar] [CrossRef]
- Tong, G.; Zhang, J.; Bu, T.; Ono, L.K.; Zhang, C.; Liu, Y.; Ding, C.; Wu, T.; Mariotti, S.; Kazaoui, S. Holistic Strategies Lead to Enhanced Efficiency and Stability of Hybrid Chemical Vapor Deposition Based Perovskite Solar Cells and Modules. Adv. Energy Mater. 2023, 13, 2300153. [Google Scholar] [CrossRef]
- Shen, P.S.; Chen, J.S.; Chiang, Y.H.; Li, M.H.; Guo, T.F.; Chen, P. Low-pressure hybrid chemical vapor growth for efficient perovskite solar cells and large-area module. Adv. Mater. Interfaces 2016, 3, 1500849. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Xiao, L.; Zhang, B.; Dai, S.; Yao, J. Mixed-Organic-Cation (FA) x (MA) 1–x PbI3 Planar Perovskite Solar Cells with 16.48% Efficiency via a Low-Pressure Vapor-Assisted Solution Process. ACS Appl. Mater. Interfaces 2017, 9, 2449–2458. [Google Scholar] [CrossRef]
- Li, M.H.; Yeh, H.H.; Chiang, Y.H.; Jeng, U.S.; Su, C.J.; Shiu, H.W.; Hsu, Y.J.; Kosugi, N.; Ohigashi, T.; Chen, Y.A. Highly efficient 2D/3D hybrid perovskite solar cells via low-pressure vapor-assisted solution process. Adv. Mater. 2018, 30, 1801401. [Google Scholar] [CrossRef]
- Tong, G.; Li, H.; Li, G.; Zhang, T.; Li, C.; Yu, L.; Xu, J.; Jiang, Y.; Shi, Y.; Chen, K. Mixed cation perovskite solar cells by stack-sequence chemical vapor deposition with self-passivation and gradient absorption layer. Nano Energy 2018, 48, 536–542. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Zhao, C.; Zhang, B.; Liu, X.; Dai, S.; Yao, J. Efficient Planar Heterojunction FA1–x Cs x PbI3 Perovskite Solar Cells with Suppressed Carrier Recombination and Enhanced Open Circuit Voltage via Anion-Exchange Process. ACS Appl. Mater. Interfaces 2019, 11, 4597–4606. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, D.; Chen, Z.; Li, Z.; Wang, J.; Chen, J.; Gong, L.; Xu, J.; Chen, K.; Liu, P. Structural regulation for highly efficient and stable perovskite solar cells via mixed-vapor deposition. Acs. Appl. Energ. Mater. 2020, 3, 6544–6551. [Google Scholar] [CrossRef]
- Luo, L.; Ku, Z.; Li, W.; Zheng, X.; Li, X.; Huang, F.; Peng, Y.; Ding, L.; Cheng, Y.-B. 19.59% Efficiency from Rb0. 04-Cs0. 14FA0. 86Pb (BryI1-y) 3 perovskite solar cells made by vapor-solid reaction technique. Sci. Bull. 2021, 66, 962–964. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Wang, C.; Zhang, G.; Zhao, Q.; Fang, C.; Li, W.; Huang, F.; Ku, Z.; Cheng, Y.-B. High-Performance Rb–Cs0. 14FA0. 86Pb (BrxI1−x) 3 Perovskite Solar Cells Achieved by Regulating the Halogen Exchange in Vapor–Solid Reaction Process. Sol. RRL 2021, 5, 2100102. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, M.; Liu, W.; Yue, X.; Cui, P.; Wei, D. CH3NH3PbI3 converted from reactive magnetron sputtered PbO for large area perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 163, 250–254. [Google Scholar] [CrossRef]
- Hwang, J.-K.; Lee, S.-W.; Lee, W.; Bae, S.; Cho, K.; Kim, S.; Lee, S.; Hyun, J.Y.; Kang, Y.; Lee, H.-S. Conformal perovskite films on 100 cm2 textured silicon surface using two-step vacuum process. Thin Solid Film. 2020, 693, 137694. [Google Scholar] [CrossRef]
- Gao, B.; Hu, J.; Zuo, Z.; Qi, Q.; Peng, Z.; Chen, H.; Yan, K.; Hou, S.; Zou, D. Doping mechanism of perovskite films with PbCl2 prepared by magnetron sputtering for enhanced efficiency of solar cells. ACS Appl. Mater. Interfaces 2022, 14, 40062–40071. [Google Scholar] [CrossRef]
- Hwang, J.-K.; Cho, S.; Lee, W.; Lee, S.; Jeong, S.-H.; Pyun, D.; Bae, S.; Gwak, J.; Kang, Y.; Kim, D. Nanoarchitectonics of wide-bandgap perovskite films using sputtered-PbI2 precursor and ion-exchange method. Appl. Phys. A 2023, 129, 260. [Google Scholar] [CrossRef]
- Ono, L.K.; Wang, S.; Kato, Y.; Raga, S.R.; Qi, Y. Fabrication of semi-transparent perovskite films with centimeter-scale superior uniformity by the hybrid deposition method. Energy Environ. Sci. 2014, 7, 3989–3993. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Ke, W.; Grice, C.R.; Cimaroli, A.J.; Tan, X.; Yang, M.; Collins, R.W.; Zhang, H.; Zhu, K.; Yan, Y. Annealing-free efficient vacuum-deposited planar perovskite solar cells with evaporated fullerenes as electron-selective layers. Nano Energy 2016, 19, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Longo, G.; Momblona, C.; La-Placa, M.-G.; Gil-Escrig, L.; Sessolo, M.; Bolink, H.J. Fully vacuum-processed wide band gap mixed-halide perovskite solar cells. ACS Energy Lett. 2017, 3, 214–219. [Google Scholar] [CrossRef] [Green Version]
- Park, C.-G.; Choi, W.-G.; Na, S.; Moon, T. All-inorganic perovskite CsPbl2Br through co-evaporation for planar heterojunction solar cells. Electron. Mater. Lett. 2019, 15, 56–60. [Google Scholar] [CrossRef]
- Igual-Munoz, A.M.; Navarro-Alapont, J.; Dreessen, C.; Palazon, F.; Sessolo, M.; Bolink, H.J. Room-temperature vacuum deposition of CsPbI2Br perovskite films from multiple sources and mixed halide precursors. Chem. Mater. 2020, 32, 8641–8652. [Google Scholar] [CrossRef]
- Gil-Escrig, L.; Dreessen, C.; Palazon, F.; Hawash, Z.; Moons, E.; Albrecht, S.; Sessolo, M.; Bolink, H.J. Efficient wide-bandgap mixed-cation and mixed-halide perovskite solar cells by vacuum deposition. ACS Energy Lett. 2021, 6, 827–836. [Google Scholar] [CrossRef]
- Duan, Y.; Zhao, G.; Liu, X.; Ma, J.; Chen, S.; Song, Y.; Pi, X.; Yu, X.; Yang, D.; Zhang, Y. Highly efficient and stable inorganic CsPbBr3 perovskite solar cells via vacuum co-evaporation. Appl. Surf. Sci. 2021, 562, 150153. [Google Scholar] [CrossRef]
- Peng, H.; Su, Z.; Zheng, Z.; Lan, H.; Luo, J.; Fan, P.; Liang, G. High-quality perovskite CH3NH3PbI3 thin films for solar cells prepared by single-source thermal evaporation combined with solvent treatment. Materials 2019, 12, 1237. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Gao, R.; Gao, F.; Lei, J.; Wang, H.; Wu, X.; Li, J.; Liu, H.; Hua, X.; Liu, S.F. Fabrication of efficient CsPbBr3 perovskite solar cells by single-source thermal evaporation. J. Alloys Compd. 2020, 818, 152903. [Google Scholar] [CrossRef]
- Yadav, R.; Roy, M.; Banappanavar, G.; Aslam, M. Growth of Hybrid Perovskite Films via Single-Source Perovskite Nanoparticle Evaporation. Chem. Asian J. 2022, 17, e202200087. [Google Scholar] [CrossRef]
- Liu, X.; Tan, X.; Liu, Z.; Sun, B.; Li, J.; Xi, S.; Shi, T.; Liao, G. Sequentially vacuum evaporated high-quality CsPbBr3 films for efficient carbon-based planar heterojunction perovskite solar cells. J. Power Sources 2019, 443, 227269. [Google Scholar] [CrossRef]
- Hua, J.; Deng, X.; Niu, C.; Huang, F.; Peng, Y.; Li, W.; Ku, Z.; Cheng, Y.-B. A pressure-assisted annealing method for high quality CsPbBr 3 film deposited by sequential thermal evaporation. RSC Adv. 2020, 10, 8905–8909. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Hua, J.; Huang, F.; Peng, Y.; Li, W.; Ku, Z.; Cheng, Y.-B. Improving the crystal growth of a Cs 0.24 FA 0.76 PbI 3− x Br x perovskite in a vapor–solid reaction process using strontium iodide. Sustain. Energy Fuels 2020, 4, 2491–2496. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Gu, L.; Gao, Y.; Reckmeier, C.; He, J.; Rogach, A.L.; Yao, Y.; Fan, Z. Fabrication of efficient planar perovskite solar cells using a one-step chemical vapor deposition method. Sci. Rep. 2015, 5, 14083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, P.; Liu, Z.; Xia, W.; Yuan, C.; Cheng, J.; Lu, Y. Uniform, stable, and efficient planar-heterojunction perovskite solar cells by facile low-pressure chemical vapor deposition under fully open-air conditions. ACS Appl. Mater. Interfaces 2015, 7, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Ioakeimidis, A.; Christodoulou, C.; Lux-Steiner, M.; Fostiropoulos, K. Effect of PbI2 deposition rate on two-step PVD/CVD all-vacuum prepared perovskite. J. Solid State Chem. 2016, 244, 20–24. [Google Scholar] [CrossRef]
- Luo, P.; Zhou, S.; Zhou, Y.; Xia, W.; Sun, L.; Cheng, J.; Xu, C.; Lu, Y. Fabrication of Cs x FA1–x PbI3 Mixed-Cation Perovskites via Gas-Phase-Assisted Compositional Modulation for Efficient and Stable Photovoltaic Devices. ACS Appl. Mater. Interfaces 2017, 9, 42708–42716. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; He, S.; Jiang, Y.; Son, D.-Y.; Ono, L.K.; Liu, Z.; Kim, T.; Bouloumis, T.; Kazaoui, S.; Qi, Y. Hybrid chemical vapor deposition enables scalable and stable Cs-FA mixed cation perovskite solar modules with a designated area of 91.8 cm2 approaching 10% efficiency. J. Mater. Chem. A 2019, 7, 6920–6929. [Google Scholar] [CrossRef]
- Sahli, F.; Miaz, N.; Salsi, N.; Bucher, C.; Schafflutzel, A.; Guesnay, Q.; Duchêne, L.; Niesen, B.; Ballif, C.; Jeangros, Q. Vapor transport deposition of methylammonium iodide for perovskite solar cells. ACS Appl. Energ. Mater. 2021, 4, 4333–4343. [Google Scholar] [CrossRef]
- Raifuku, I.; Ishikawa, Y.; Bourgeteau, T.; Bonnassieux, Y.; i Cabarrocas, P.R.; Uraoka, Y. Fabrication of perovskite solar cells using sputter-processed CH3NH3PbI3 films. Appl. Phys. Express 2017, 10, 094101. [Google Scholar] [CrossRef]
- Gao, B.; Hu, J.; Tang, S.; Xiao, X.; Chen, H.; Zuo, Z.; Qi, Q.; Peng, Z.; Wen, J.; Zou, D. Organic-Inorganic Perovskite Films and Efficient Planar Heterojunction Solar Cells by Magnetron Sputtering. Adv. Sci. 2021, 8, 2102081. [Google Scholar] [CrossRef]
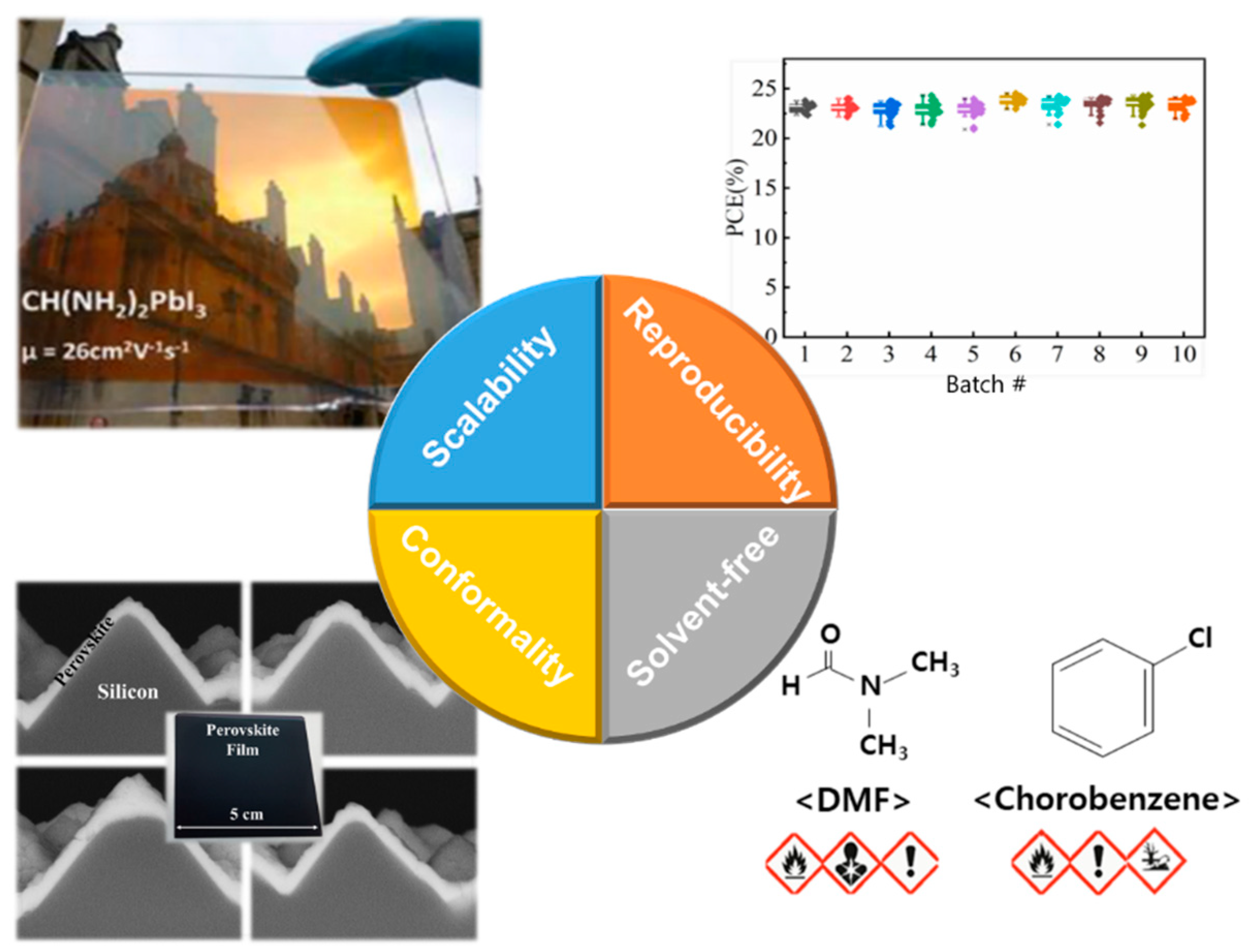
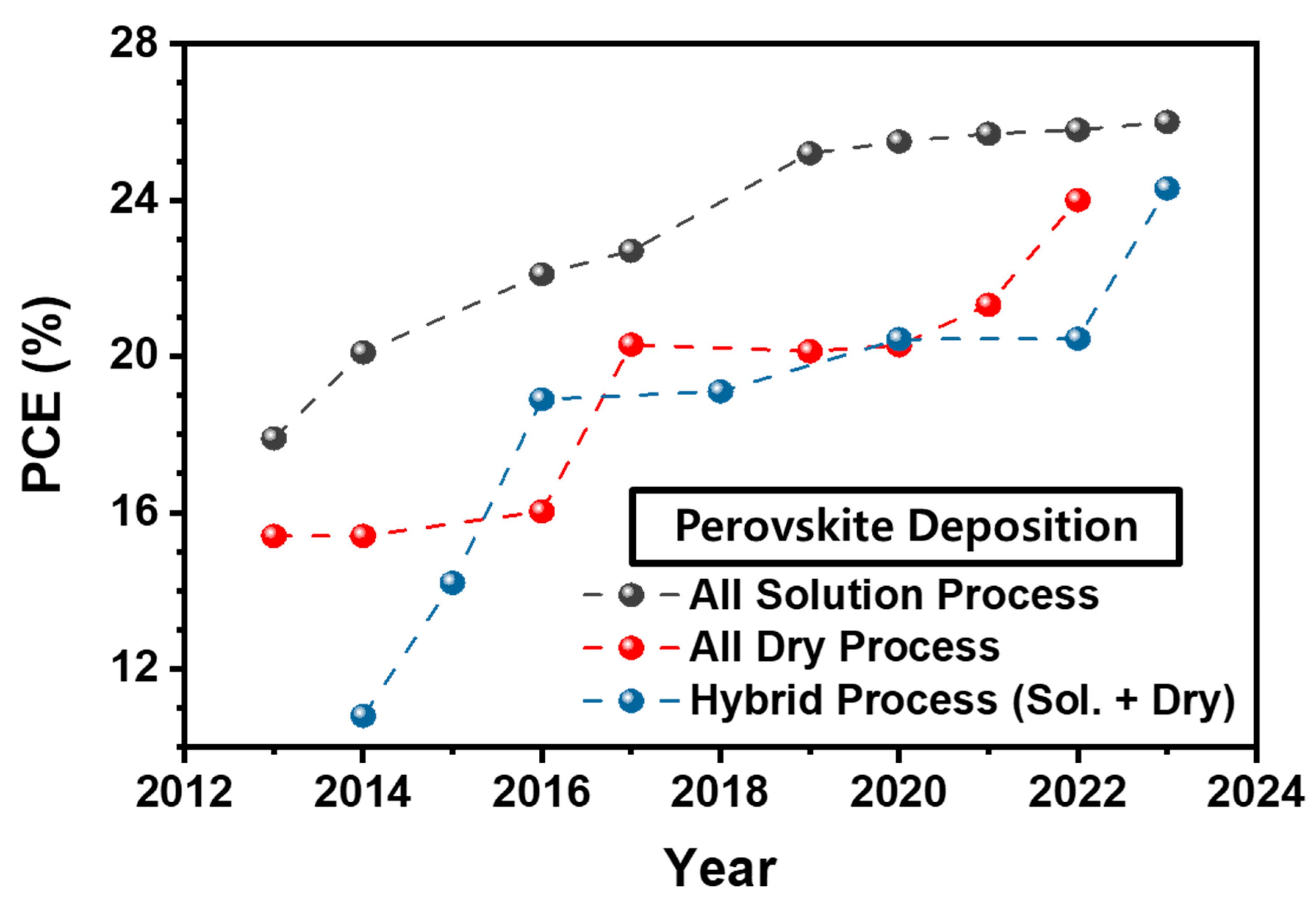
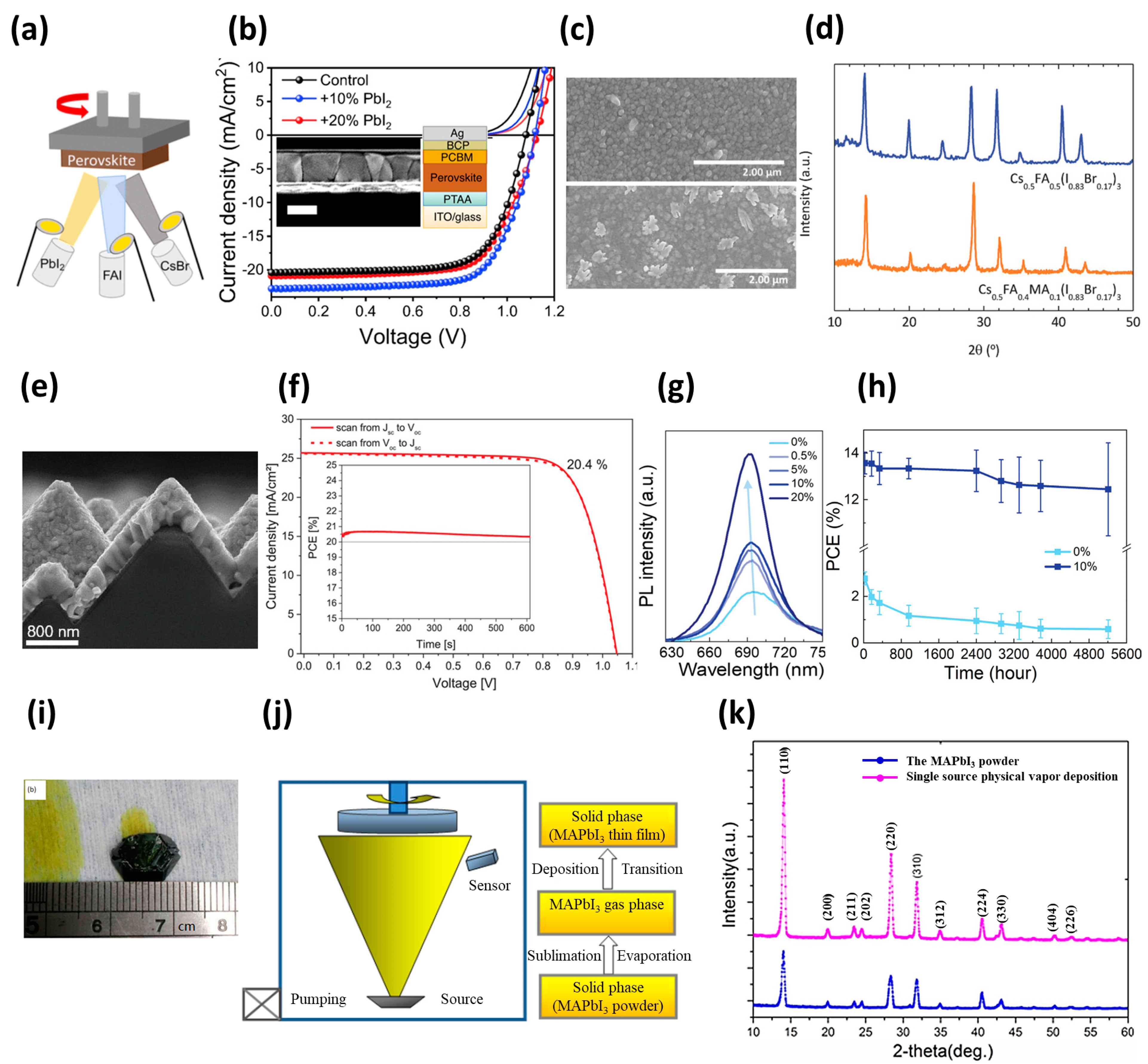

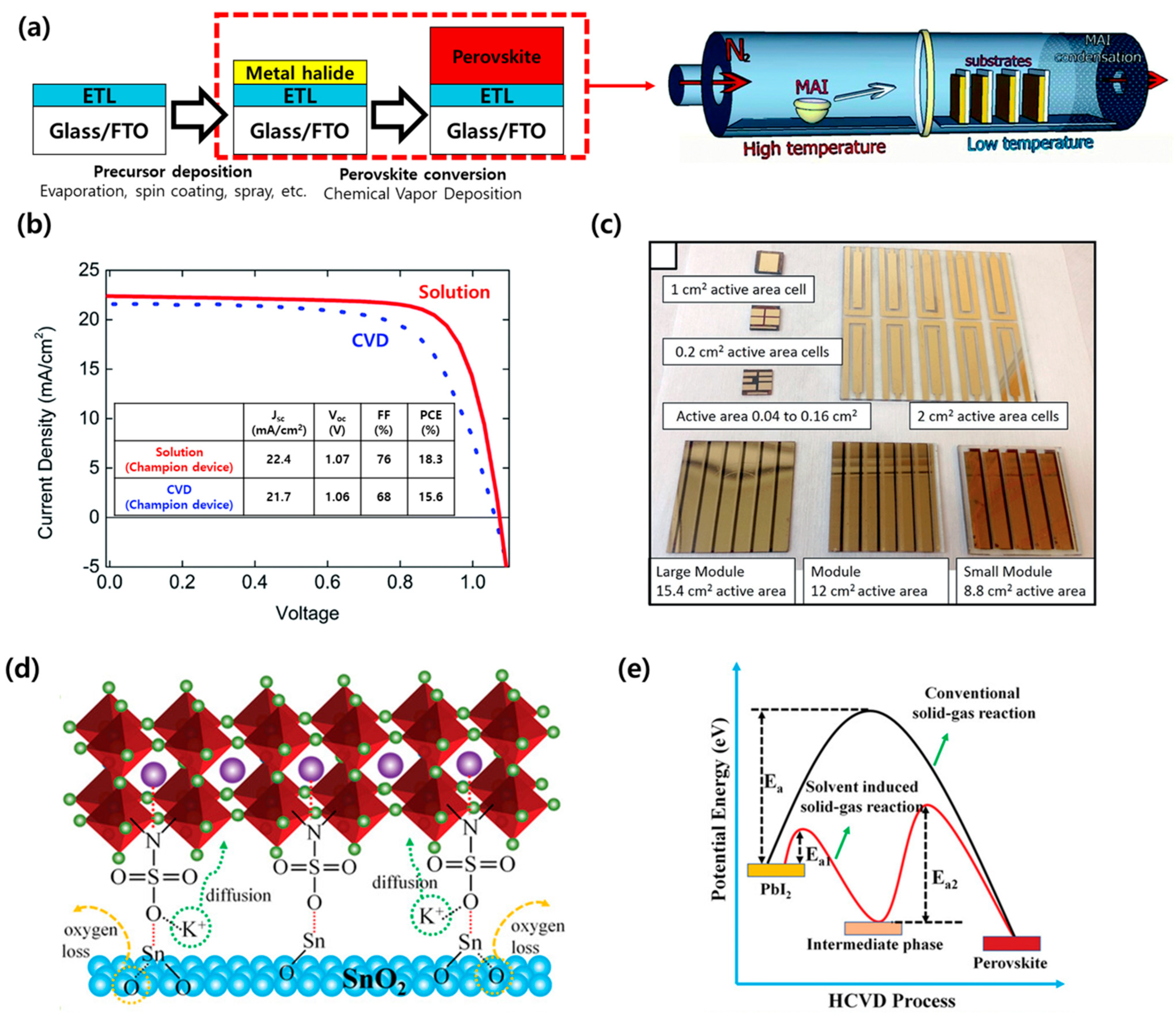
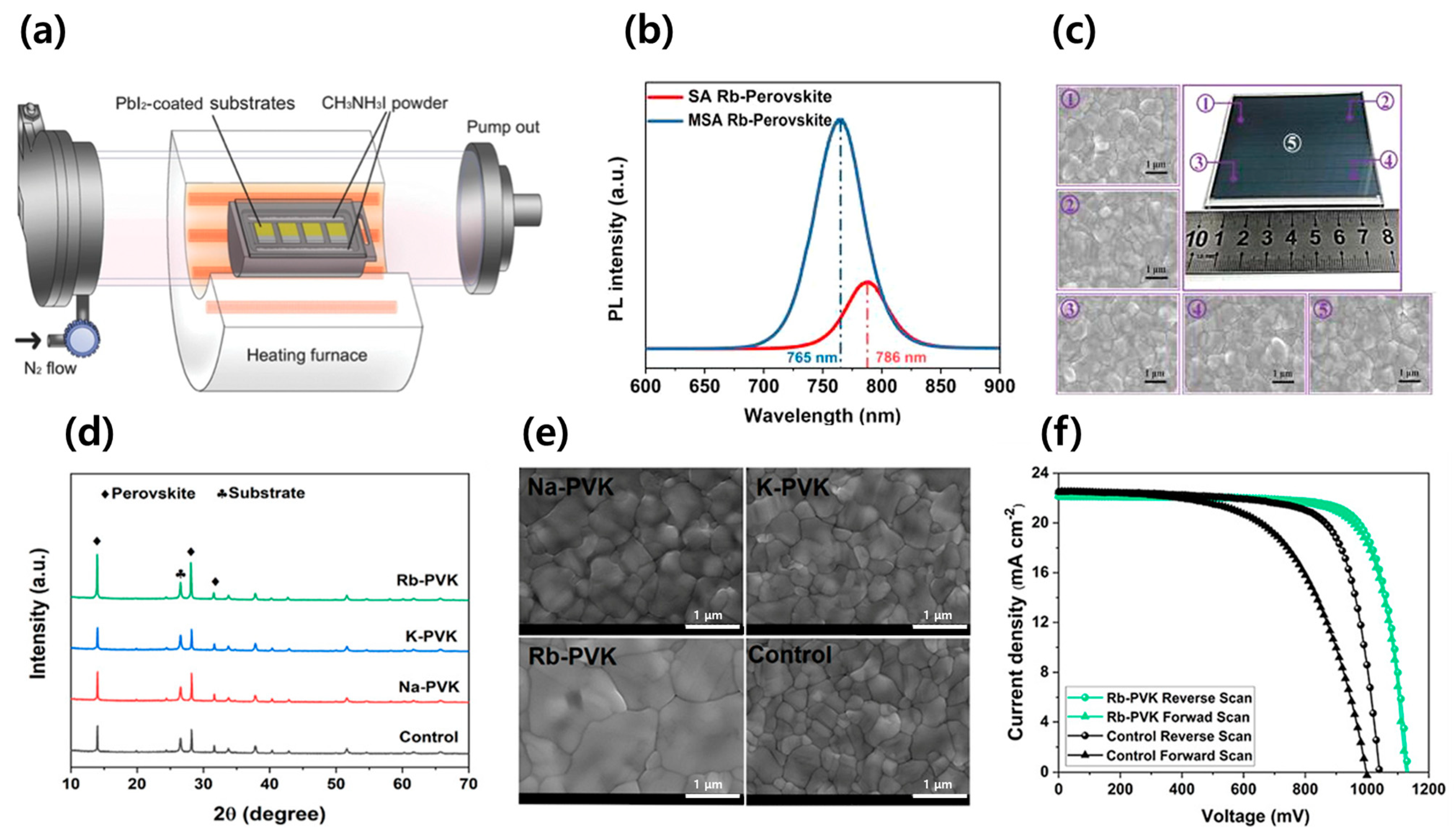
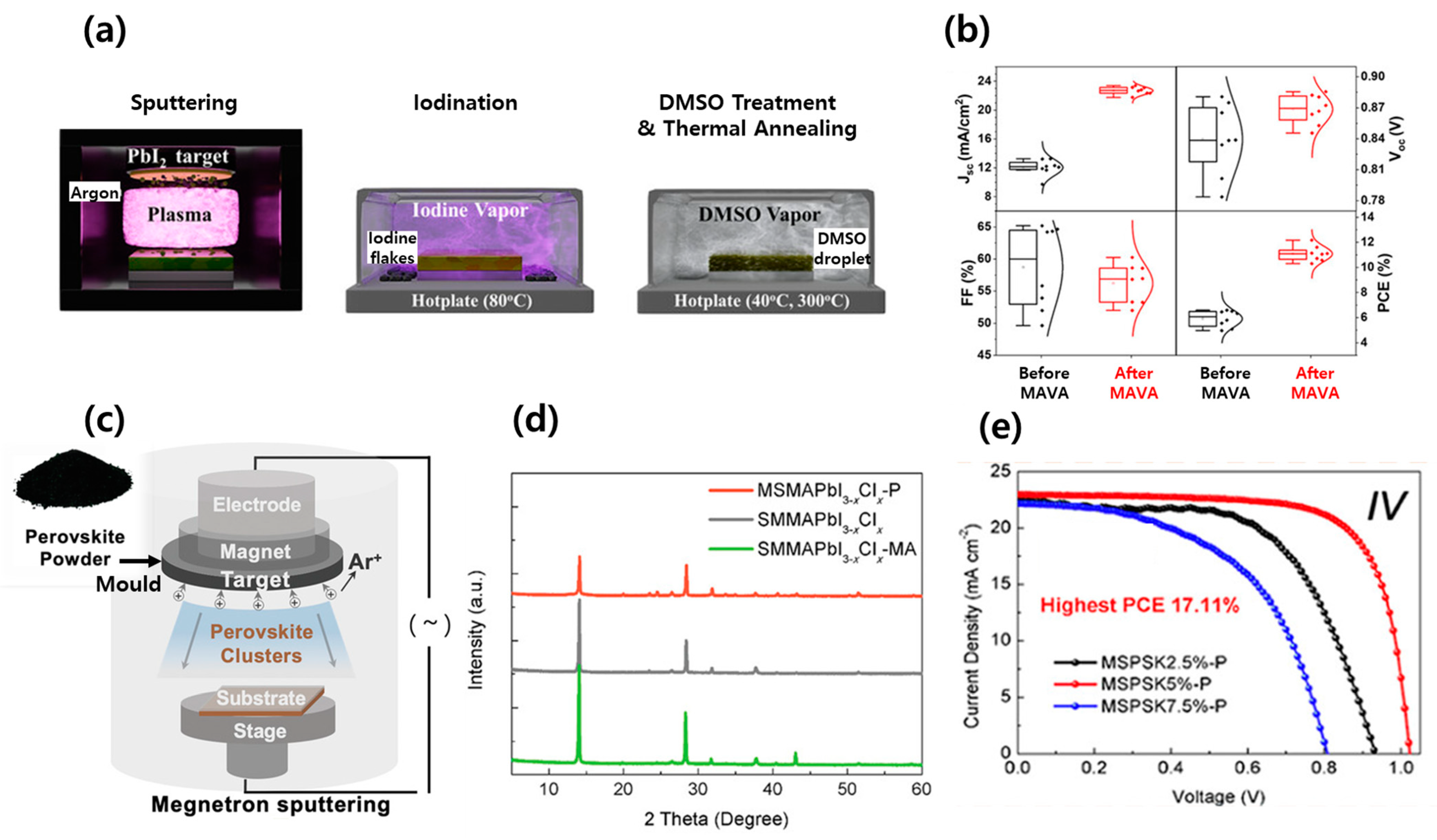
| Device Structure | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) | Area (cm2) | Year | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Evaporation (Co-deposition) | FTO/c-TiO2/Perovskite/Spiro-OMeTAD/Ag | 1.07 | 21.5 | 67 | 15.4 | 0.076 | 2013 | [29] |
| ITO/PEDOT:PSS/polyTPD/MAPbI3/PCBM/3TPYMB/Au | 1.09 | 18.2 | 75 | 14.8 | 0.065 | 2014 | [30] | |
| ITO/C60:Phlm/C60/MAPbI3/TaTm/TaTm:F6-TCNNQ/Ag | 1.14 | 22.08 | 80.5 | 20.3 | 0.1 | 2016 | [31] | |
| ITO/C60:Phlm/C60/Cs0.5FA0.4MA0.1Pb(I3Br0.17)3/TaTm/TaTm:F6-TCNNQ/Au | 1.146 | 17 | 82 | 16 | - | 2018 | [32] | |
| ITO/PTAA/FA0.7Cs0.3Pb(I0.9Br0.1)3/PCBM/BCP/Ag | 1.06 | 23 | 74.6 | 18.1 | 0.155 | 2020 | [33] | |
| FTO/TiO2/SnO2/PCBM/MAPbI3/Spiro-OMeTAD/Au | 1.12 | 23.3 | 77.7 | 20.28 | 0.1 | 2020 | [34] | |
| ITO/MeO-2PACz/FAxMA1–xPbI3/C60/BCP/Cu | 1.05 | 25.7 | 75.91 | 20.4 | 0.16 | 2021 | [35] | |
| ITO/Spiro-TTB/MAPbI3/PCBM/BCP/Ag/LiF | 1.13 | 22.1 | 81.3 | 20.3 | 0.086 | 2021 | [36] | |
| ITO/PTAA/CsPbI3/PCBM/BCP/Ag | 1.09 | 17.3 | 79.4 | 15 | 0.045 | 2021 | [37] | |
| FTO/SnO2/MAPbI3/Spiro-OMeTAD/Au | 1.08 | 22.7 | 78.8 | 19.3 | 0.09 | 2022 | [38] | |
| Evaporation (Sequential) | ITO/PEDOT:PSS/MAPbI3/PCBM/Ag | 0.93 | 19.47 | 60 | 10.9 | 0.09 | 2016 | [39] |
| ITO/PEDOT:PSS/MAPbI3xClx/C60/Bphen/Ca/Ag | 1.02 | 20.9 | 72.2 | 15.4 | 0.05 | 2014 | [40] | |
| FTO/c-TiO2/MAPbI3/Spiro-OMeTAD/Au | 1 | 22.27 | 72 | 16.03 | 0.071 | 2015 | [41] | |
| FTO/c-TiO2/FAxMA1–xPbI3/Spiro-OMeTAD/Au | 0.98 | 22.4 | 73 | 15.8 | 0.09 | 2019 | [42] | |
| FTO/c-TiO2/FAxMA1–xPbI3-yBry/CuPc/Au | 1.02 | 19.16 | 77.3 | 15.14 | - | 2019 | [43] | |
| FTO/c-TiO2/FAxCs1–xPbI3/Spiro-OMeTAD/Au | 1.11 | 24.88 | 77.2 | 21.32 | 0.09 | 2021 | [44] | |
| FTO/SnO2/Cs0.05FA0.95PbI3/Spiro-OMeTAD/Au | 1.15 | 25.9 | 81 | 24 | 0.1 | 2022 | [45] | |
| FTO/SnO2/CsxFA1-xPb(IyBr1-y)3/Spiro-OMeTAD/Au | 1.2 | 24.4 | 83.3 | 24.3 | 0.1 | 2023 | [28] | |
| FTO/TiO2/MAxCs1–xPbI3/Spiro-OMeTAD/Au | 1.1 | 23.17 | 79 | 20.13 | 0.09 | 2017 | [46] | |
| FTO/SnO2/Cs0.24FA0.76PbI3-yBry/Spiro-MeOTAD/Au | 1.065 | 22.88 | 71 | 17.29 | 0.16 | 2018 | [47] | |
| Chemical Vapor Deposition (Double-zone) | FTO/c-TiO2/MAPbI3–xClx/Spiro-MeOTAD/Au | 0.92 | 19.1 | 62 | 10.8 | 0.07–0.1 | 2014 | [7] |
| FTO/c-TiO2/FAPbI3–xClx/Spiro-MeOTAD/Au | 1.03 | 20.9 | 66 | 14.2 | 0.04 | 2015 | [48] | |
| FTO/TiO2/MAPbI3/Spiro-MeOTAD/Au | 0.97 | 18.0 | 64 | 11.1 | - | 2015 | [49] | |
| FTO/c-TiO2/MAPbI3–xClx/Spiro-MeOTAD/Au | 1.06 | 21.7 | 68 | 15.6 | 0.09 | 2016 | [50] | |
| FTO/c-TiO2/MAPbI3/Spiro-MeOTAD/Au | 1.06 | 22.08 | 80 | 18.9 | 0.11 | 2016 | [51] | |
| FTO/c-TiO2/m-TiO2/MAPbI3/Spiro-MeOTAD/Au | 1.00 | 23.0 | 77 | 17.6 | 0.06 | 2016 | [52] | |
| FTO/c-TiO2/CsxFA1-xPbI3/Spiro-MeOTAD/Au | 1.00 | 22.0 | 75.2 | 16.6 | 0.1 | 2017 | [53] | |
| FTO/c-TiO2/m-TiO2/MAPbI3/Spiro-MeOTAD/Au | 1.01 | 24.2 | 69 | 16.9 | 0.06 | 2018 | [54] | |
| FTO/c-TiO2/FAPbIxBr3–x/Spiro-MeOTAD/Au | 1.03 | 21.1 | 74 | 16.1 | 2 | 2019 | [55] | |
| FTO/c-TiO2/FA0.93Cs0.07PbI3/Spiro-MeOTAD/Au | 0.99 | 22.3 | 70.2 | 15.5 | 0.1 | 2020 | [56] | |
| ITO/SnO2/H2KNO3S/Cs0.05FA0.5MA0.45PbI3/Spiro-MeOTAD/Au | 1.15 | 23.93 | 80 | 21.98 | 0.09 | 2023 | [57] | |
| Chemical Vapor Deposition (Single-zone) | FTO/c-TiO2/MAPbI3/Spiro-MeOTAD/Au | 0.972 | 21.15 | 75 | 15.37 | 0.24 | 2016 | [58] |
| FTO/c-TiO2/C60/(FA)x(MA)1–xPbI3/Spiro-MeOTAD/Au | 1 | 22.51 | 73.56 | 16.48 | 0.09 | 2017 | [59] | |
| FTO/c-TiO2/m-TiO2/(PEA2MAn-1PbnI3n+1)/Spiro-MeOTAD/Au | 1.08 | 21.91 | 80.36 | 19.1 | 0.2 | 2018 | [60] | |
| FTO/c-TiO2/Cs0.15FA0.85PbI2.85Br0.15/Spiro-MeOTAD/Au | 1.06 | 22.82 | 75.4 | 18.22 | 0.09 | 2018 | [61] | |
| FTO/c-TiO2/Cs0.1FA0.9PbI3/Spiro-MeOTAD/Au | 0.99 | 22.87 | 74.82 | 16.39 | - | 2019 | [62] | |
| FTO/c-TiO2/C60/(PEA2MAn-1PbnI3n+1)/Spiro-MeOTAD/Au | 1.08 | 23.75 | 70.4 | 18.08 | - | 2020 | [63] | |
| FTO/SnO2/Rb0.04Cs0.14FA0.86Pb(BryI1-y)3/Spiro-MeOTAD/Au | 1.127 | 22.63 | 76.8 | 19.59 | 0.148 | 2021 | [64] | |
| FTO/SnO2/Rb0.04Cs0.14FA0.86Pb(Br0.03I0.97)3/Spiro-MeOTAD/Au | 1.13 | 22.2 | 78 | 19.6 | 0.16 | 2021 | [65] | |
| Sputtering | FTO/NRT/MAPbI3/Spiro-MeOTAD/Au | 1.04 | 20.2 | 66.9 | 14.1 | 0.09 | 2017 | [66] |
| FTO/TiO2/MAPbI3/Spiro-MeOTAD/Au | 0.856 | 22.1 | 53.8 | 10.2 | 0.075 | 2020 | [67] | |
| FTO/TiO2/MAPbI3/Spiro-MeOTAD/Au | 0.791 | 19.5 | 71.2 | 11.1 | 0.078 | 2020 | [22] | |
| FTO/c-TiO2/m-TiO2/MAPbI3-xClx/Spiro-MeOTAD/Au | 0.97 | 22.53 | 71 | 15.52 | 0.06 | 2021 | [23] | |
| FTO/TiO2/MAPbI3/Spiro-MeOTAD/Au | 0.98 | 22.96 | 76 | 17.1 | 0.08 | 2022 | [68] | |
| FTO/TiO2/MAPbI3/Spiro-MeOTAD/Au | 0.86 | 23.4 | 60.3 | 12.2 | 0.075 | 2023 | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.-K.; Jeong, S.-H.; Kim, D.; Lee, H.-S.; Kang, Y. A Review on Dry Deposition Techniques: Pathways to Enhanced Perovskite Solar Cells. Energies 2023, 16, 5977. https://doi.org/10.3390/en16165977
Hwang J-K, Jeong S-H, Kim D, Lee H-S, Kang Y. A Review on Dry Deposition Techniques: Pathways to Enhanced Perovskite Solar Cells. Energies. 2023; 16(16):5977. https://doi.org/10.3390/en16165977
Chicago/Turabian StyleHwang, Jae-Keun, Seok-Hyun Jeong, Donghwan Kim, Hae-Seok Lee, and Yoonmook Kang. 2023. "A Review on Dry Deposition Techniques: Pathways to Enhanced Perovskite Solar Cells" Energies 16, no. 16: 5977. https://doi.org/10.3390/en16165977






