Ecological Hydrogen Production and Water Sterilization: An Innovative Approach to the Trigeneration of Renewable Energy Sources for Water Desalination: A Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrogen Yield by System
2.2. Thermochemical Solar Cycles
2.3. Using Solar Steam to Improve Methane
2.4. Solar Methane Separation
2.5. Comparison of Hydrogen Getting Methods
3. Results
3.1. Installation Calculation
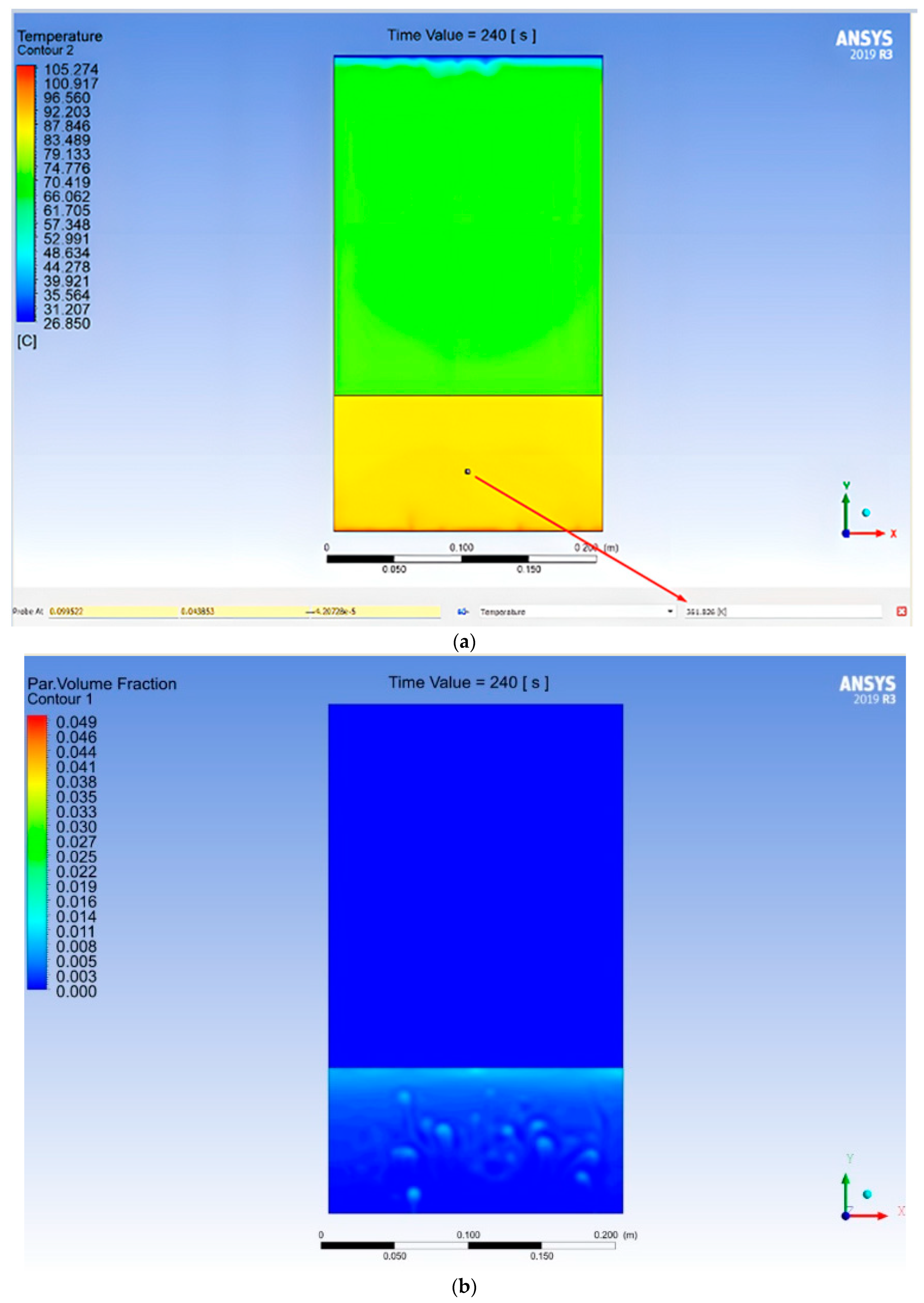
3.2. Modeling in ANSYS
- The emulsion flow is set from the condition of the appearance of a two-zone liquid flow at the beginning of boiling on the lower surface.
- The temperature of the liquid saturation container is set from the theoretical calculation condition.
- Based on the saturation temperature, the burdened degree of dryness of the vapor-liquid mixture in the transition state is established.
4. Grid
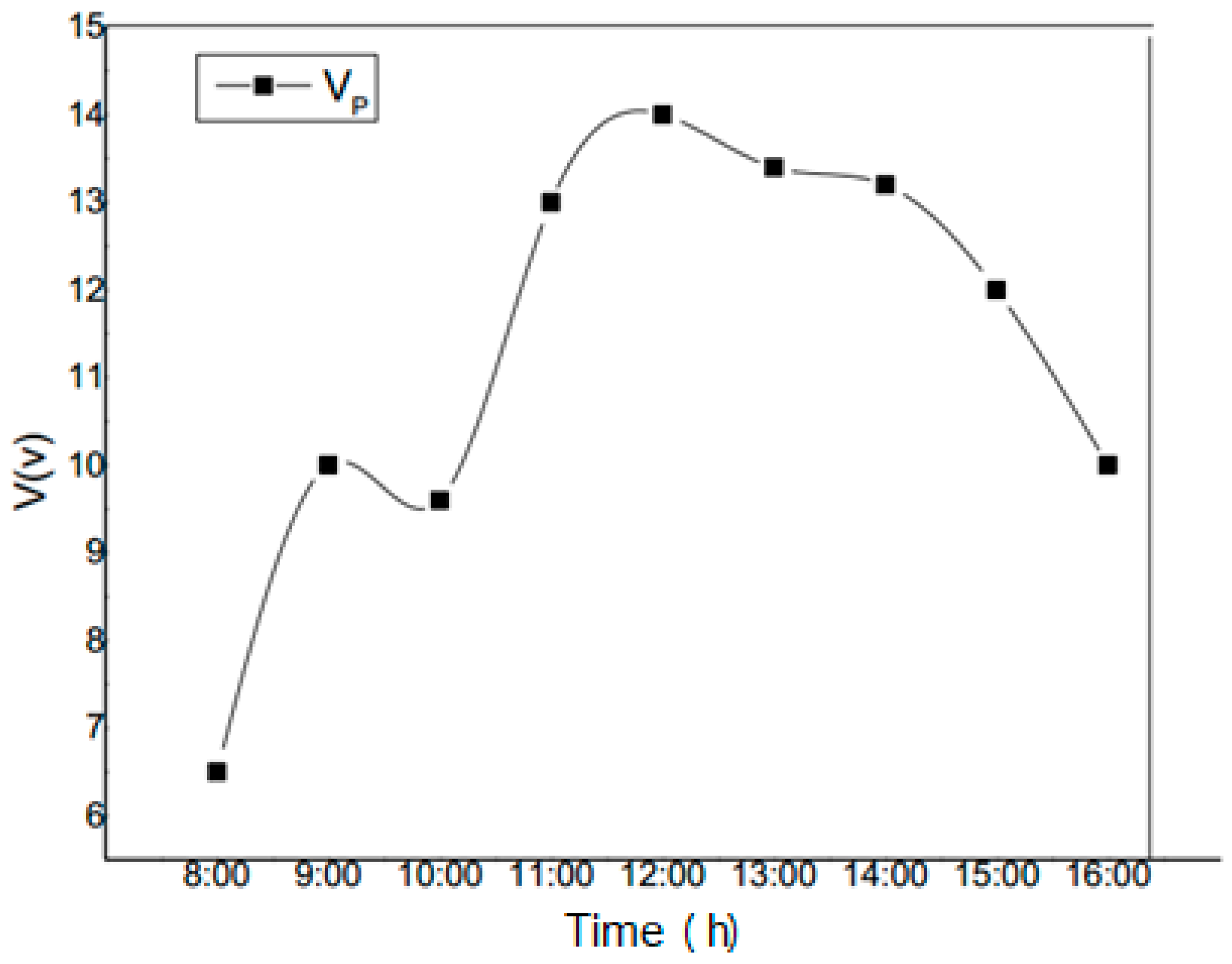
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| e | electron |
| t’hw | heating water temperature |
| tout.w | outboard water temperature |
| Whw | volume flow of heating water |
| dscale | scale layer thickness |
| hs | ship’s draft |
| average heating water temperature in the evaporator | |
| heating water inlet temperature | |
| heating water temperature at the evaporator outlet | |
| average cooling water temperature in the condenser | |
| condenser inlet cooling water temperature | |
| condenser outlet cooling water temperature | |
| Δtc | temperature difference in the condenser |
| Kc | heat transfer coefficients in the condenser |
| Kev | heat transfer coefficients in the evaporator |
| t2 | temperature of the secondary steam in the evaporator |
| evaporator heat load | |
| ρhw | heating water density in the evaporator |
| chw | heat capacity of heating water in the evaporator |
| Qc | condenser thermal load |
| η | heat utilization factor |
| δtout.w | amount of heating of the cooling water in the condenser |
| Wcool | cooling outboard water consumption |
| ρout.w | density of cooling water in the condenser |
| cout.w | heat capacity of the cooling water in the condenser |
| condenser outlet cooling water temperature | |
| °C | HEU purge ratio |
| Wb | amount of brine to be removed |
| W2 | evaporated water consumption |
| Sb | salinity of brine |
| S0 | outboard water salinity |
| δtb | temperature depression |
| g | acceleration of gravity |
| x | reduced boiling brine level |
| lev | evaporator tube length |
| ρb | brine density |
| pb | average design pressure of boiling brine |
| p2 | vapor pressure in the evaporator |
| Δph | mean hydrostatic pressure correction |
| δth | temperature difference taking into account the hydrostatic effect |
| saturation temperature | |
| tb | average design temperature of boiling brine |
| vhw | average speed of heating water in the annulus |
| Fse | open area for the passage of heating water |
| Re | Reynolds criterion |
| dout | outer diameter of evaporator tubes |
| kinematic viscosity | |
| Nu | Nusselt criterion |
| Prwall | wall Prandtl criterion |
| Prliquid | liquid Prandtl criterion |
| λhw | thermal conductivity of heating water |
| α1 | heat transfer coefficient from heating water to evaporator pipes |
| twall | average pipe wall temperature of the evaporator heating battery |
| δtev | average temperature difference between pipe wall and boiling brine |
| α2 | heat transfer coefficient from pipes to boiling brine |
| pp | average design pressure of boiling brine |
| δwall | pipe wall thickness and scale |
| din | inner diameter |
| λwall | coefficient of thermal conductivity of the pipe wall material |
| λscale | thermal conductivity coefficient of scale |
| Kev | heat transfer coefficient from heating water to brine |
| Δtev | temperature difference in the evaporator heating coil |
| Fev | evaporator surface |
| evaporator heat load | |
| design value of the thermal load of the condenser | |
| calculated evaporator heat load | |
| W2 | evaluation of HEU productivity by the amount of evaporated water |
| ρfw | feed water density |
| cfw | feed water heat capacity |
| tfw | feed water temperature |
| vout.w | cooling water speed |
| fev | number of capacitor strokes |
| zev | number of condenser tubes |
| design temperature difference in the condenser | |
| average calculated heating water temperature | |
| temperature of the secondary steam in the evaporator | |
| design value of the amount of heating of the cooling water in the condenser | |
| Δt | temperature difference between heating water and secondary steam |
| pc | vapor pressure |
| vapor pressure in the evaporator | |
| Δp | steam resistance of louvered separator and condenser tube bundle |
| M | condenser cooling rate |
| qt | specific heat load of the condenser |
| Fc | capacitor surface |
| qst | specific steam load of the condenser |
| ρg | distillate density |
| heating of cooling water in the condenser | |
| h″ | secondary steam enthalpy |
| h’ | enthalpy of distillate |
| H | pump head |
| ping | discharge pressure |
| psu | suction pressure |
| ηm | pump mechanical efficiency |
| Nout.w | pump power |
| Ne | drive motor power |
| ηem | motor efficiency |
| Pc | absolute pressure in the condenser |
| Q | pump delivery |
| qe | power consumption for distillate production. |
References
- Möller, S.; Kaucic, D.; Sattler, C. Hydrogen production by solar reforming of natural gas: A comparison study of two possible process configurations. J. Sol. Energy Eng. 2006, 128, 16–23. [Google Scholar] [CrossRef]
- Le Duigou, A.; Borgard, J.M.; Larousse, B.; Doizi, D.; Allen, R.; Ewan, B.; Hpriestman, G.; Elder, R.; Devonshire, R.; Ramos, V. HYTHEC: An EC funded search for a long-term massive hydrogen production route using solar and nuclear technologies. Int. J. Hydrogen Energy 2007, 32, 1516–1529. [Google Scholar] [CrossRef]
- Poitou, S.; Rodriguez, G.; Haquet, N.; Cachon, L.; Bucci, P.; Tochon, P.; Chaumat, V.; Gillia, O.; Gwinner, B.; Balbaud, F. Development program of the SO3 decomposer as a key component of the sulphur based thermochemical cycles: New steps towards feasibility demonstration. In Proceedings of the 17th World Hydrogen Energy Conference, Brisbane, Australia, 15–19 June 2008; Available online: https://www.researchgate.net/publication/228413536_Development_program_of_a_key_component_of_the_iodine_sulfur_thermochemical_cycle_the_SO_3_decomposer (accessed on 12 February 2023).
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Dash, S.K.; Chakraborty, S.; Elangovan, D. A Brief Review of Hydrogen Production Methods and Their Challenges. Energies 2023, 16, 1141. [Google Scholar] [CrossRef]
- Anaya, K.; Oni, A.O.; Kumar, A. Investigating the techno-economic and environmental performance of chemical looping technology for hydrogen production. Sustain. Energy Technol. Assess. 2023, 56, 103008. [Google Scholar] [CrossRef]
- Spath, P.L.; Amos, W.A. Using a concentrating solar reactor to produce hydrogen and carbon black via thermal decomposition of natural gas: Feasibility and economics. Trans. ASME 2003, 125, 159–164. [Google Scholar] [CrossRef]
- Hinkley, J.T.; Heenan, A.R.; Low, A.C.; Watson, M. Hydrogen as an export commodity—Capital expenditure and energy evaluation of hydrogen carriers. Int. J. Hydrogen Energy 2022, 47, 35959–35975. [Google Scholar] [CrossRef]
- Li, P.; Chen, L.; Xia, S.; Zhang, L.; Kong, R.; Ge, Y.; Feng, H. Entropy generation rate minimization for steam methane reforming reactor heated by molten salt. Energy Rep. 2020, 6, 685–697. [Google Scholar] [CrossRef]
- Vostrikov, S.V.; Samarov, A.A.; Turovtsev, V.V.; Wasserscheid, P.; Müller, K.; Verevkin, S.P. Thermodynamic Analysis of Chemical Hydrogen Storage: Energetics of Liquid Organic Hydrogen Carrier Systems Based on Methyl-Substituted Indoles. Materials 2023, 16, 2924. [Google Scholar] [CrossRef]
- Jensen, S.H.; Larsen, P.J.; Mogensen, M. Hydrogen and synthetic fuel production from renewable energy sources. Int. J. Hydrogen Energy 2007, 32, 3253–3257. [Google Scholar] [CrossRef]
- Ewan, B.; Graf, D.; Monnerie, N.; Sattler, C.; Le Naour, F.; Stein, W.; Hikley, J.l. Innovative High Temperature Routes for Hydrogen Production—Coordinated Action (INNOHYP CA); Final report INNOHYP CA-FR-CEA/07–05; 2007. [Google Scholar] [CrossRef]
- Padro, C.E.G.; Putsche, V. Survey of the Economics of Hydrogen Technologies; National Renewable Energy Laboratory: Golden, CO, USA, 1999. [Google Scholar]
- Well-to-Wheels Analysis of Future Automotive Fuels and Powertrains in the European Context; WELL-TO-TANK Report, EUCAR, CONCAWE, JRC, Version 2c; SAE International: Warrendale, PA, USA, 2007; Available online: http://ies.jrc.ec.europa.eu/WTW (accessed on 12 February 2023).
- World Energy Technology Outlook—2050—WETO H2. European Commission, Directorate-General for Research, Brussels 2006. Available online: https://espas.secure.europarl.europa.eu/orbis/sites/default/files/generated/document/en/WETO-H2.pdf (accessed on 25 February 2023).
- Prospects for Hydrogen and Fuel Cells. International Energy Agency (IEA), Paris. 2005. Available online: https://www.iea.org/reports/prospects-for-hydrogen-and-fuel-cells (accessed on 10 May 2023).
- Nitsch, J. Potenziale der Wasserstoffwirtschaft. Externe Expertise für das WBGU-Hauptgutachten 2003 “Welt im Wandel: Energiewende zur Nachhaltigkeit”; Springer: Berlin/Heidelberg, Germany, 2003; (In German). Available online: http://www.wbgu.de/wbgu_jg2003_ex06.pdf (accessed on 12 May 2023).
- Mueller-Langer, F.; Tzimas, E.; Kaltschmitt, M.; Peteves, S. Technoeconomic assessment of hydrogen production processes for the hydrogen economy for the short and medium term. Int. J. Hydrogen Energy 2007, 32, 3797–3810. [Google Scholar] [CrossRef]
- Fischedick, M.; Esken, A.; Pastowski, A.; Schüwer, D.; Supersberger, N.; Nitsch, J. RECCS: Strukturellökonomisch-ökologischer Vergleich regenerativer Energietechnologien (RE) mit Carbon Capture and Storage (CCS). Final report BMU, Feb. 2007. Int. J. Hydrogen Energy 2009, 34, 4256–4267. (In German) [Google Scholar]
- Energy Technology Perspectives 2008; International Energy Agency (IEA): Paris, France, 2008.
- IFA-International Fertilizer Industry Association, France: Ammonia Statistics 2006. Personal communication and e-mail, Ms Marcel-Monnier, IFA 29.05.2007. Available online: https://www.indexmundi.com/minerals/?country=fr&product=ammonia&graph=production (accessed on 25 February 2023).
- Hydrogen (H2)-Based Iron Making. 2022. Available online: https://worldsteel.org/publications/fact-sheets/ (accessed on 25 May 2023).
- Government, US. Energy Information Administration (EIA): Country Analysis Briefs. 2007. Available online: https://www.eia.doe.gov/emeu/cabs/index.html (accessed on 12 May 2023).
- Innovation Outlook. Renewable Methanol. 2021. Available online: https://www.methanol.org/wp-content/uploads/2020/04/IRENA_Innovation_Renewable_Methanol_2021.pdf (accessed on 25 May 2023).
- USGS—U.S. Geological Survey: Nitrogen Statistics and Information. Available online: http://minerals.usgs.gov/minerals/pubs/commodity/nitrogen/ (accessed on 3 May 2023).
- U.S. Government. Energy Information Administration (EIA): Refinery Capacity Report 2023. Available online: https://www.eia.gov/petroleum/refinerycapacity/ (accessed on 25 May 2023).
- BP Statistical Review of World Energy 2008—Refinery Capacities. Available online: http://www.bp.com (accessed on 19 May 2023).
- USGS—U.S. Geological Survey: International Minerals Statistics and Information. Available online: http://minerals.usgs.gov/minerals/pubs/country/ (accessed on 9 May 2023).
- Trieb, F. Trans-Mediterranean Interconnection for Concentrating Solar Power (TRANS CSP). Final Report, DLR-ITT, project funded by the German Ministry for Environment, Nature Conservation, and Nuclear Safety (BMU). Available online: http://www.dlr.de/tt/Portaldata/41/Resources/dokumente/institut/system/projects/TRANS-CSP_Full_Report_Final.pdf (accessed on 11 May 2023).
- Graf, D.; Monnerie, N.; Roeb, M.; Schmitz, M.; Sattler, C. Economic comparison of solar hydrogen generation by means of thermochemical cycles and electrolysis. Int. J. Hydrogen Energy 2008, 33, 4511–4519. [Google Scholar] [CrossRef]
- Couturier, K.; Joppich, F.; Wörner, A.; Tamme, R. Tank design for on-board hydrogen storage in metal hydrides. In Proceedings of the 2nd International Conference on Energy Sustainability, Jacksonville, FL, USA, 10–14 August 2008; ES 2008. ASME: Jacksonville, FL, USA, 2008. [Google Scholar]
- Schindler, J.; Wurster, R.; Zerta, M.; Blandow, V.; Zittel, W. Where will the energy for hydrogen production come from?—Status and alternatives. Ottobrunn: EHA (European Hydrogen Association), Ludwig–Bölkow–Systemtechnik GmbH (LBST). 2006. Available online: https://www.h2euro.org/wp-content/uploads/2009/01/eha_h2production_brochure_eng_0407.pdf (accessed on 7 March 2023).
- Knoche, K.F. Thermochemical H2-production with a solar driven sulphur–iodine-process. Sol. Therm. Energy Util. DLR 1988, 4, 441–498. [Google Scholar]
- Kolb, G.J.; Diver, R.B.; Siegel, N. Central-station solar hydrogen power plant. J. Sol. Energy Eng. 2007, 129, 179–183. [Google Scholar] [CrossRef]
- Liberatore, R. S-I solar evaluation costs and considerations on economic comparisons among thermochemical cycles. In Proceedings of the IEA/HIA Task 25 Meeting, Rome, Italy, 9–10 October 2008. [Google Scholar]
- Kolb, G.J.; Diver, R.B. Screening Analysis of Solar Thermochemical Hydrogen Concepts; Sandia Report SAND2008-1900; Sandia National Laboratories: Albuquerque, NM, USA, 2008. [Google Scholar]
- García-Chávez, R.J.; Chávez-Ramirez, A.U.; Villafán-Vidales, H.I.; Velázquez-Fernández, J.B.; Rosales, I.H. Thermal study of a solar distiller using computational fluid dynamics (CFD). Rev. Mex. De Ing. Química 2020, 2, 455–467. Available online: https://www.researchgate.net/publication/339201525_Thermal_study_of_a_solar_distiller_using_computational_fluid_dynamics_CFD (accessed on 7 March 2023). [CrossRef]
- Aly, N.H.; El-Figi, A.K. Thermal performance of seawater desalination plants. Desalination 2003, 158, 127–142. [Google Scholar] [CrossRef]
- Farahat, M.A.; Mousa, M.M.; Mahmoud, N.H. Solar Distiller with Flat Plate Collector and Thermal Storage. Int. Conf. Appl. Mech. Mech. Eng. 2016, 17, 1–11. [Google Scholar] [CrossRef]
- Sommariva, C.; Hogg, H.; Callister, K. Cost reduction and design lifetime increase in thermal desalination plants: Thermodynamic and corrosion resistance combined analysis for heat exchange tubes material selection. Desalination 2003, 158, 17–21. [Google Scholar] [CrossRef]
- Mahmoud, N.H.; Mousa, M.M.; Farahat, M.A. Performance Study of Solar Distiller with Thermal Storage System. In Proceedings of the 1st International Conference, Ninth Conference of Sustainable Environmental Development, Rome, Italy, 8 September 2021. [Google Scholar]
- Tanaka, H.; Park, C.-D. Experimental study of distiller with heat pipe utilizing waste heat from a portable electric generator. Desalination 2012, 302, 43–49. [Google Scholar] [CrossRef]
- Joo, H.-J.; Kwak, H.-Y. Performance evaluation of multi-effect distiller for optimized solar thermal desalination. Appl. Therm. Eng. 2013, 61, 491–499. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, H.; Zhao, Y.; Ma, X. Solar-driven natural vacuum desalination system with inner condenser. Appl. Therm. Eng. 2021, 196, 117320. [Google Scholar] [CrossRef]
- Khokhani, P.; Patel, K.; Jikadra, V.; Patel, A.; Patel, S.K. Development of Solar Thermal Desalination Plant Using Vacuum Tubes. Int. J. Emerg. Technol. Innov. Res. 2018, 5, 11. [Google Scholar]
- Kumar, R.S.; Mani, A.; Kumaraswamy, S. Analysis of a jet-pump-assisted vacuum desalination system using power plant waste heat. Desalination 2005, 179, 345–354. [Google Scholar] [CrossRef]
- Triki, Z.; Bouaziz, M.; Boumaza, M. Performance and cost evaluation of an autonomous solar vacuum membrane distillation desalination plant. Desalination Water Treat. 2017, 73, 107–120. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhang, X.C.; Fang, Y.Q.; Zhu, M.M. The Natural Vacuum Desalination Technology in Seawater Desalination. Appl. Mech. Mater. 2014, 675–677, 851–855. [Google Scholar] [CrossRef]
- Bilgil, A.; Hırlakoğlu, B. An experimental study on desalination at vacuum environment under low pressure and low condensation temperatures. Desalination 2017, 411, 9–18. [Google Scholar] [CrossRef]
- Ben Abdallah, S.; Frikha, N.; Gabsi, S. Design of an autonomous solar desalination plant using vacuum membrane distillation, the MEDINA project. Chem. Eng. Res. Des. 2013, 91, 2782–2788. [Google Scholar] [CrossRef]
- Balaji, D.; Abraham, R.; Murthy, M.R. Experimental study on the vacuum load of low-temperature thermal desalination plant. Desalination Water Treat. 2016, 57, 26830–26844. [Google Scholar] [CrossRef]
- Balaji, D.; Abraham, R.; Murthy, M.R. Process simulation of vacuum desalination systems for producing potable drinking water from saline sea water. Desalination Water Treat. 2017, 57, 26830–26844. [Google Scholar] [CrossRef]
- Lemeshko, M.A. Energy-Efficient Method of Sea Water Desalination; EDN TMLARX; Lemeshko, M.A., Umnov, I.V., Eds.; Bulletin of the Vladimir Dahl Lugansk State University: Moscow, Russia, 2021; Volume 11, pp. 221–224. [Google Scholar]
- Lemeshko, M.A. Desalination of Sea Water by Solar Energy in a Vacuum (In Russian) /M. A. Lemeshko, D. S. Bondarev, I. S. Bagnyuk//Advanced Technologies in Construction and Technosphere Safety: Collection of Scientific Papers, Shakhty, February 10, 2022 years—10 2023—Mines: ISOiP (branch) of DSTU in Shakhty, 2022—P. 9-14—EDN RBKPPT, Moscow. Available online: https://elibrary.ru/item.asp?id=50341381 (accessed on 7 March 2023).
- Lemeshko, M.A. Water Desalination System in a Solar Collector (In Russian) /M. A. Lemeshko, I. V. Umnov//Scientific spring—2022. Technical sciences: Collection of scientific papers: Scientific electronic edition, Shakhty, May 16–20, 2022/Institute of Service and Entrepreneurship (branch) DSTU in Shakhty—Shakhty: Institute of Service and Entrepreneurship (branch) of DSTU in Shakhty, Moscow, 2022—P. 196-202—EDN OMWKNH. Available online: https://elibrary.ru/item.asp?id=49183205&ysclid=llgq6hqem5597152454 (accessed on 7 March 2023).
- Bashurov, B.P.; Skiba, A.N.; Chebanov, V.S. Functional Reliability and Control of the Technical Condition of Ship Auxiliary Mechanisms (In Russian): Russia, 2009, 192p. Available online: https://elibrary.ru/qnwayt?ysclid=llgqmlku3u376734960 (accessed on 7 March 2023).
- Alnaimat, F.; Rashid, Y. Thermal Energy Storage in Solar Power Plants: A Review of the Materials, Associated Limitations, and Proposed Solutions. Energies 2019, 12, 4164. [Google Scholar] [CrossRef]
- Osintsev, K.; Aliukov, S.; Kuskarbekova, S.; Tarasova, T.; Karelin, A.; Konchakov, V.; Kornyakova, O. Increasing Thermal Efficiency: Methods, Case Studies, and Integration of Heat Exchangers with Renewable Energy Sources and Heat Pumps for Desalination. Energies 2023, 16, 4930. [Google Scholar] [CrossRef]
- Osintsev, K.; Aliukov, S.; Kovalev, A.; Bolkov, Y.; Kuskarbekova, S.; Olinichenko, A. Scientific Approaches to Solving the Problem of Joint Processes of Bubble Boiling of Refrigerant and Its Movement in a Heat Pump Heat Exchanger. Energies 2023, 16, 4405. [Google Scholar] [CrossRef]



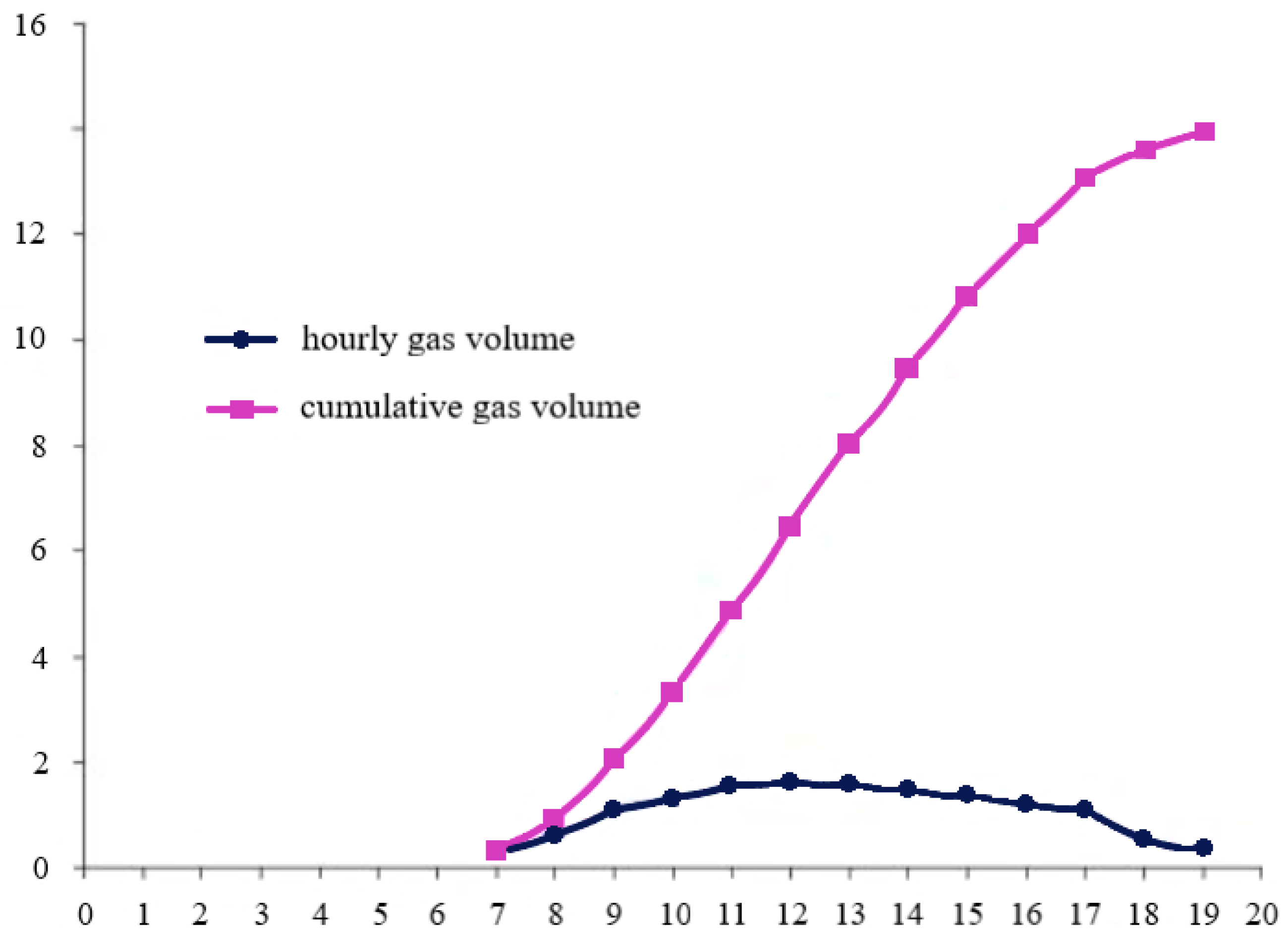
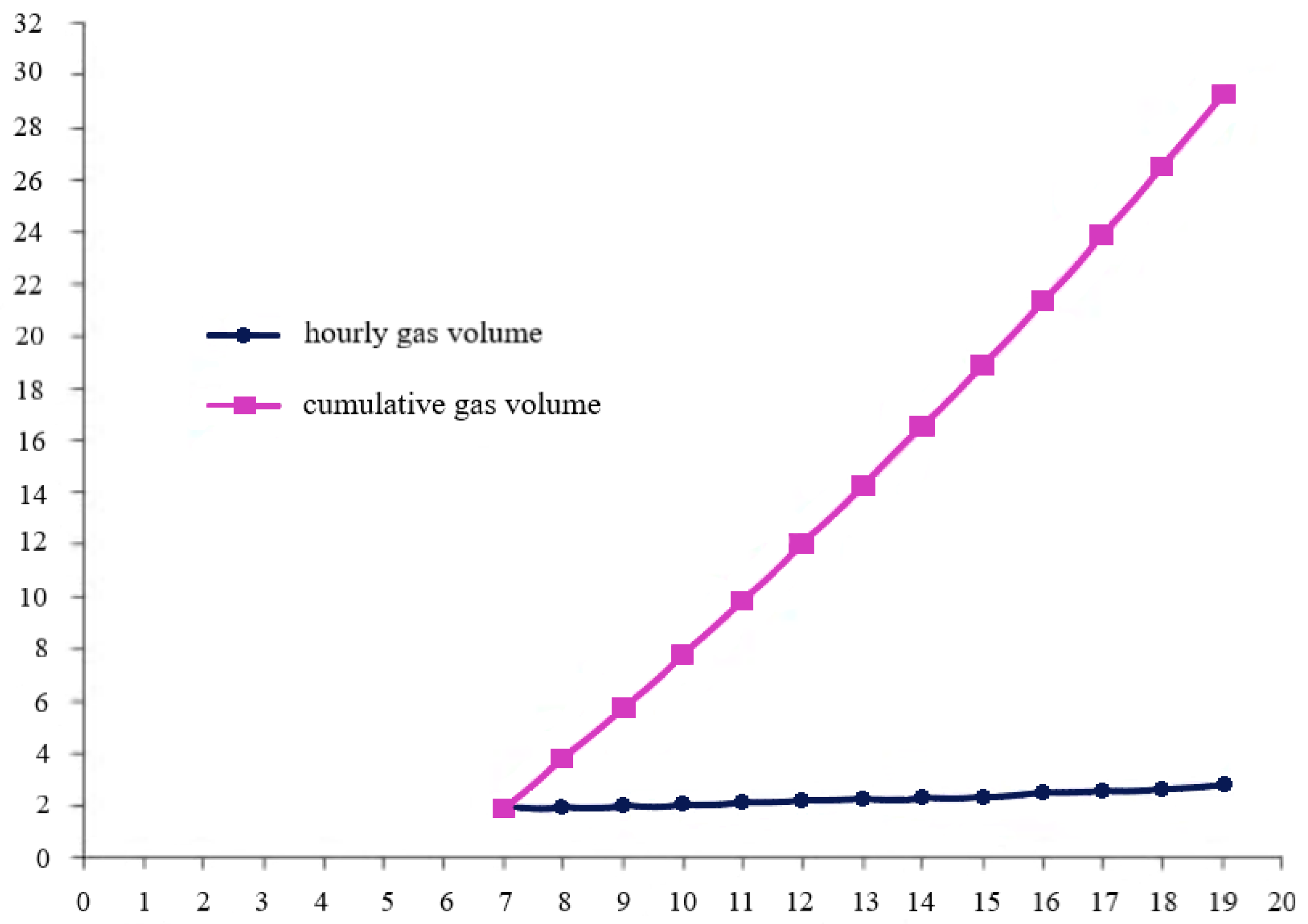


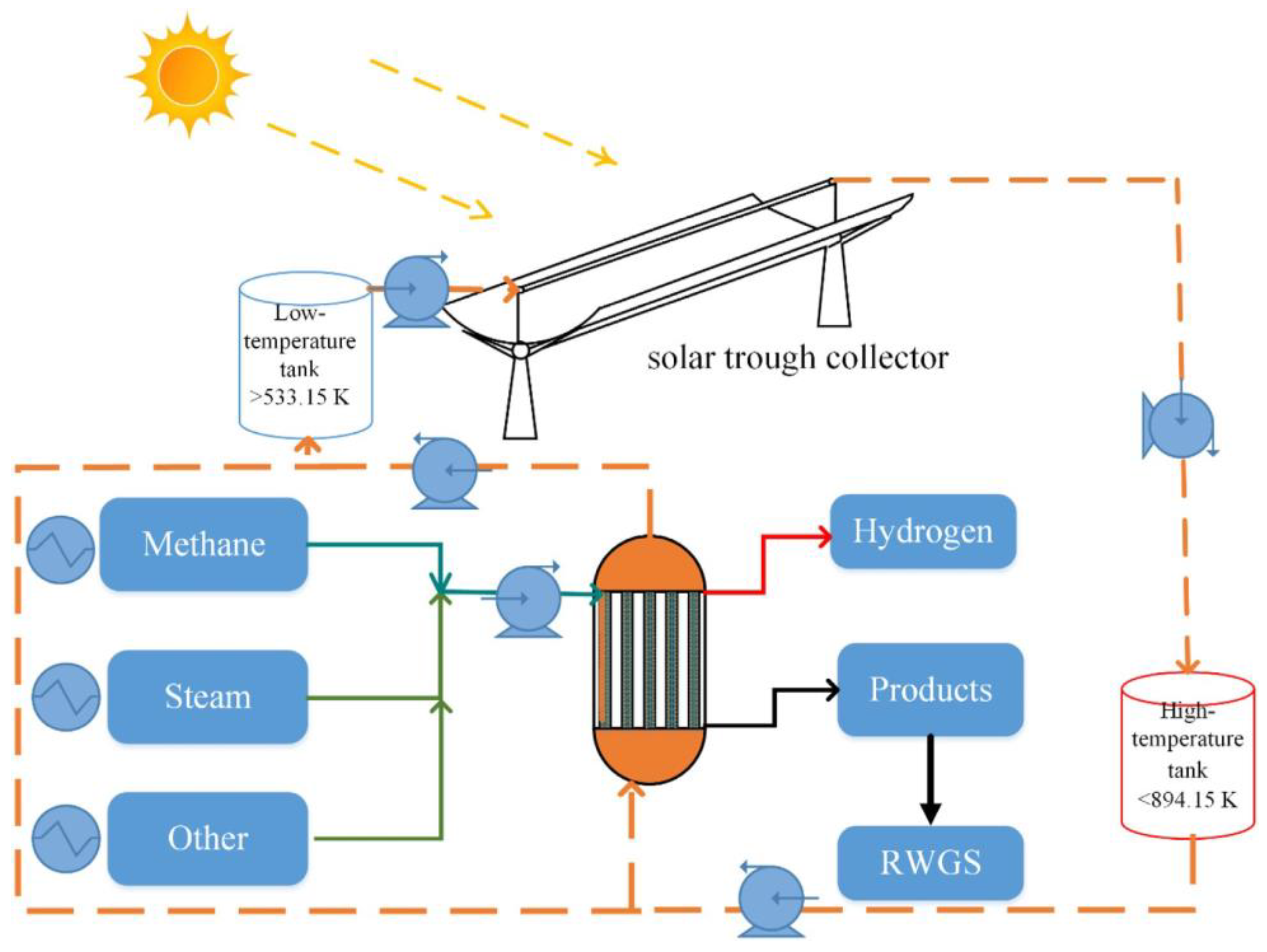
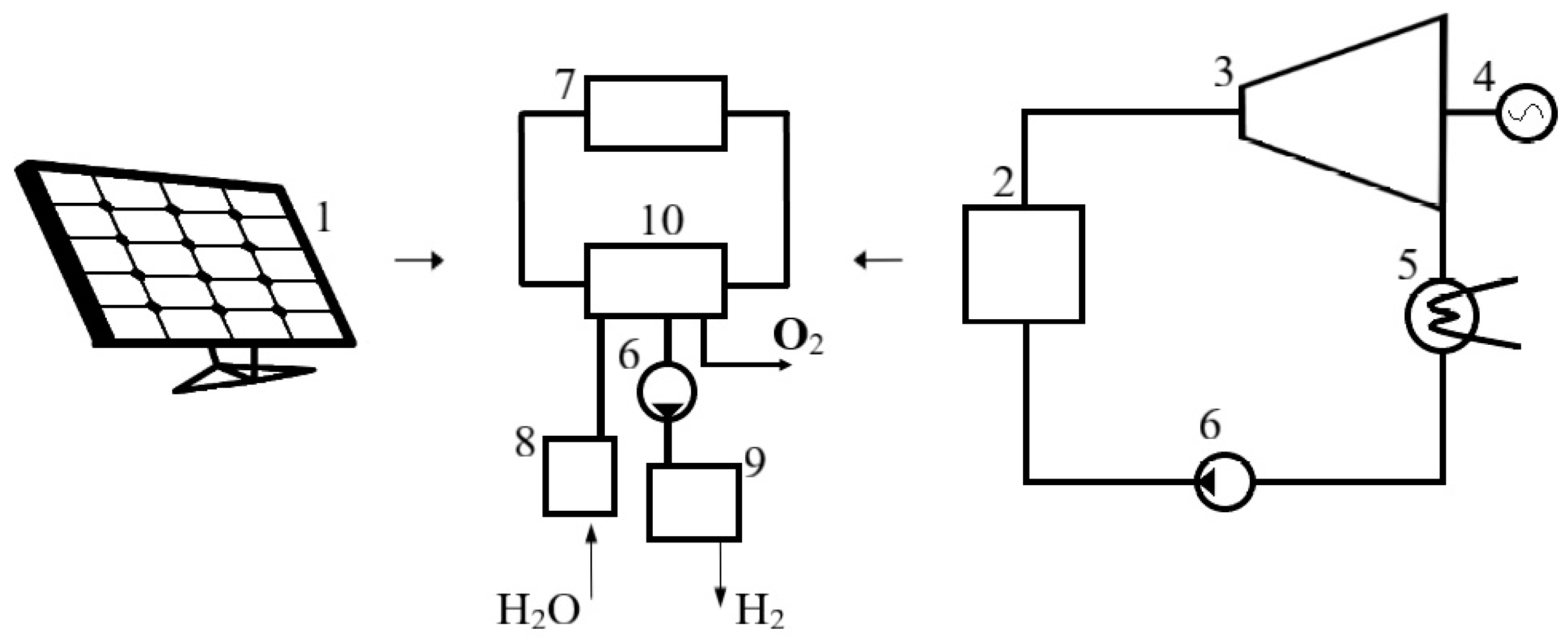
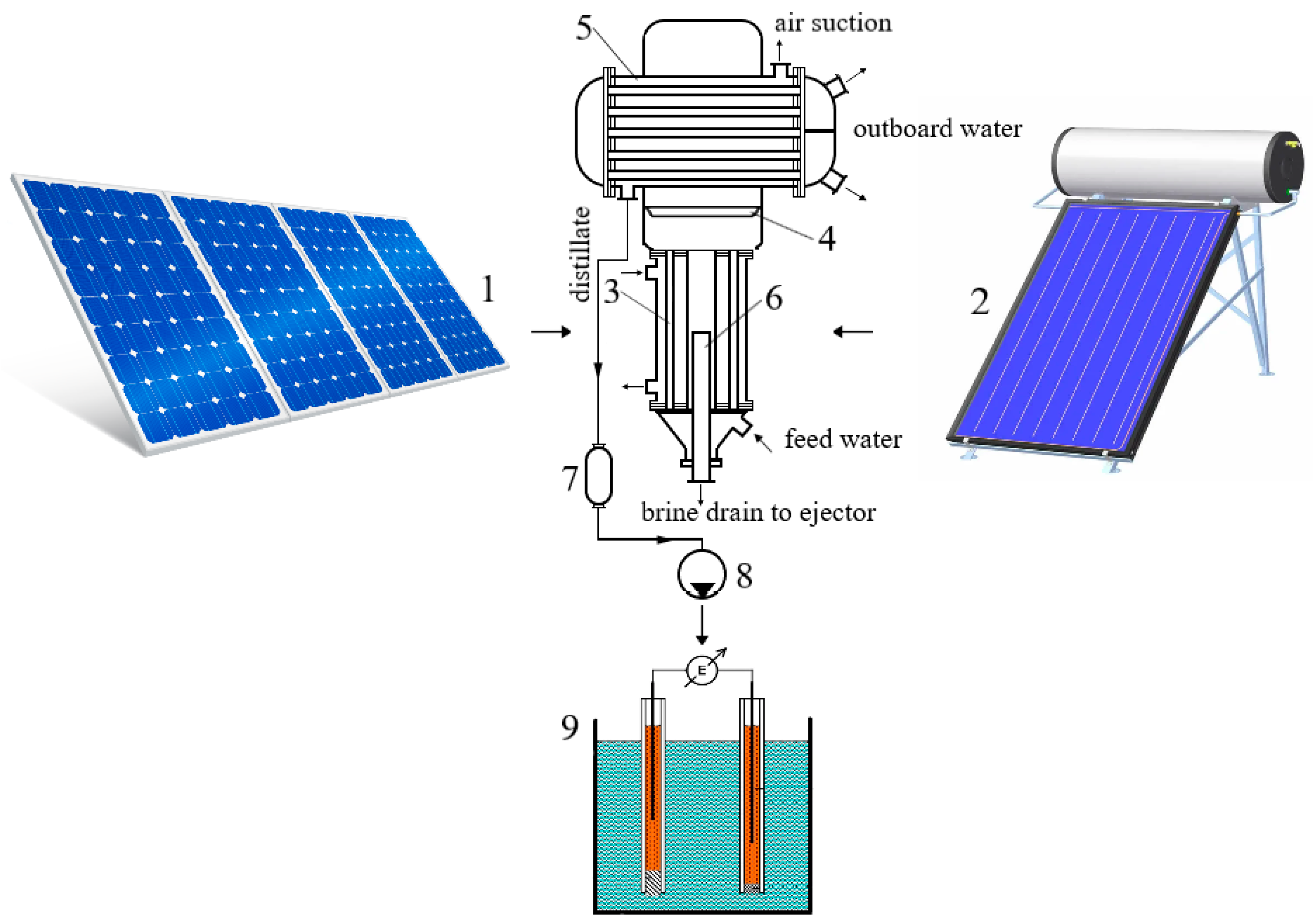





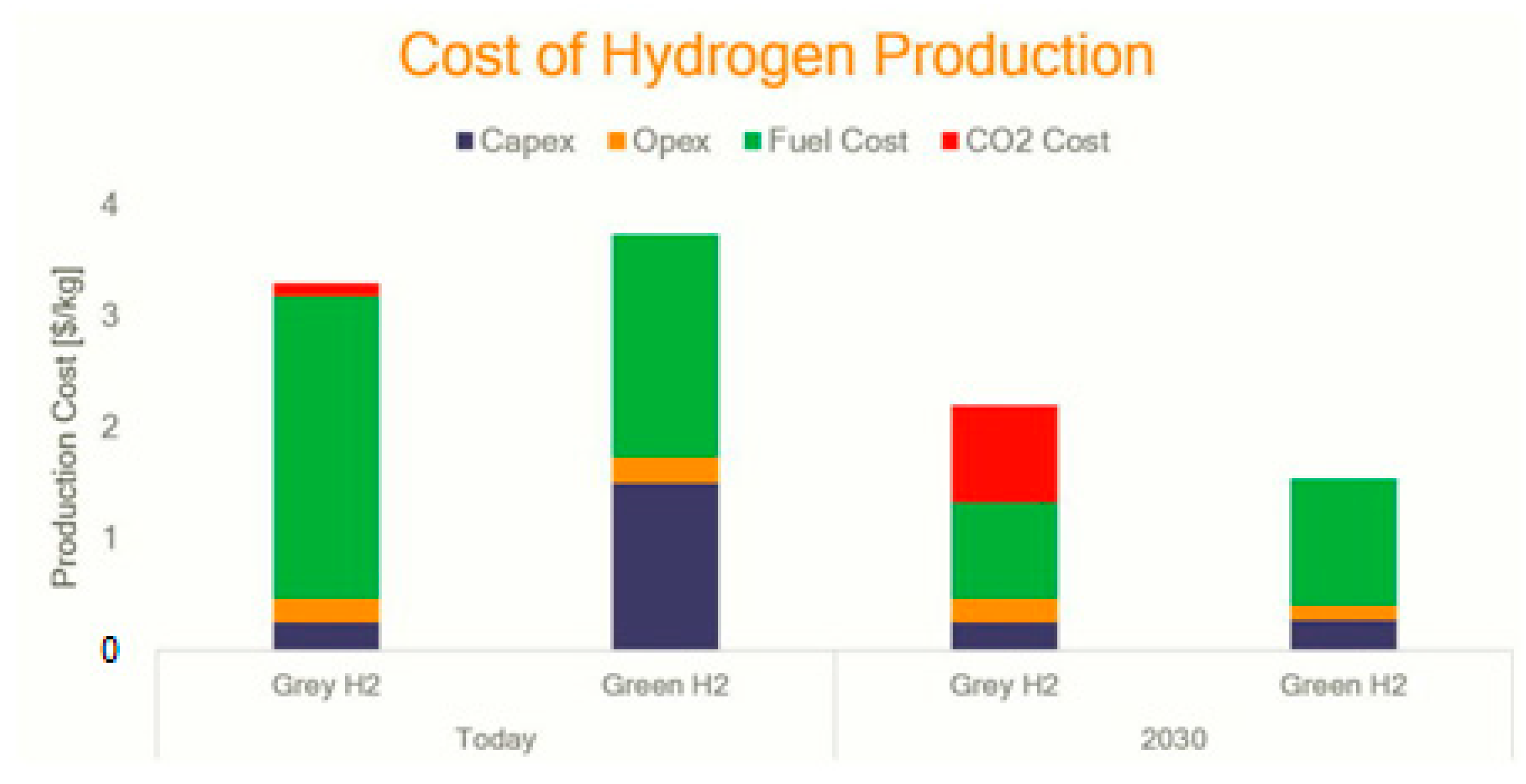



| Year of Construction of the Station | Production Hydrogen Plant Capacity, Tones/Day | Cost Price, $/kg | |
|---|---|---|---|
| 1 | Commissioned before 2022 | 607 | 3.66 |
| 2 | Put into operation in 2022 with an increase in production efficiency | 678 | 3.51 |
| Model type | Bsm 200 m-72 |
| Solar cell type | Mono 125 × 125 cell a grade |
| Pm 210 W, Vm 38 V, Im 5.5 A | Voc 44.6 V, Isc 5.8 A |
| Size | 1580 × 808 × 35mm |
| Weight | 16.5 kg |
| Output tolerance | 0–4% |
| Standard test condition | 999 W/m2, am 1.6, 25 °C |
| Operating temperature | −42 °C…+87 °C |
| Manufacture warranty | 11 years |
| Power performance warranty | >90% after 16 year, >85% after 26 year |
| Hydrogen Demand EJ | Theoretical Number of Plants with 50 MWs SSMR | Theoretical Number of Plants with 50 MWs Metal Oxide TC | |
|---|---|---|---|
| Refineries existing | 0.44 | 380 | 860 |
| Refineries additional | 0.8 | 700 | 1600 |
| Ammonia production | 0.51 | 440 | 1003 |
| DRI (pig iron) production | 0.12 | 98 | 220 |
| Methanol production | 0.2 | 166 | – |
| Total countries | 2.07 | 1784 | 1683 |
| Given | Unit of Measurement | Formula | Option 1 | Option 2 | Option 3 |
|---|---|---|---|---|---|
| t’hw | °C | – | 68 | 68 | 90 |
| tout.w | °C | – | 4 | 4 | 4 |
| Whw | m3/h | – | 80 | 150 | 80 |
| dscale | mm | – | 0.1 | 0.1 | 0.1 |
| hs. | m | – | 5 | 5 | 5 |
| Calculation | Unit of measurement | Formula | Option 1 | Option 2 | Option 3 |
| The average temperature of the heating water in the evaporator when cooling the heating water is 6 °C; 8 °C; 10 °C. | °C | 65 64 63 | 65 64 63 | 87 86 85 | |
| The average temperature of the cooling water in the condenser | °C | 7 | 7 | 7 | |
| Temperature pressure in the condenser | °C | 29.82 29.41 28.99 | 29.82 29.41 28.99 | 38.94 38.52 38.11 | |
| Secondary steam temperature in the evaporator | °C | 36.82338 36.40916 35.99495 | 36.82338 36.40916 35.99495 | 45.93607 45.52186 45.10765 | |
| Secondary steam pressure in the evaporator | kPa | – | 6.2162 6.0812 5.946 | 6.2162 6.0812 5.946 | 10.04 9.84 9.63 |
| Heat of vaporization of secondary steam | kJ/kg | – | 2568 2567 2566.7 | 2568 2567 2566.7 | 2392.3 2393.3 2394.3 |
| Heating water density | kg/m3 | – | 980.4 980.94 981.48 | 980.4 980.94 981.48 | 989 990.5 992 |
| Heat load of the evaporator | kJ/h | 1,971,310 2,629,861 3,289,136 | 3,696,206 4,930,989 6,167,130 | 1,988,602 2,655,491 3,324,390 | |
| Thermal load of the condenser | kJ/h | 1,892,457 2,524,666 3,157,570 | 3,548,358 4,733,750 5,920,444 | 1,909,058 2,549,271 3,191,415 | |
| The amount of heating of the cooling water in the condenser | °C | 4.85 6.47 8.08 | 9.09 12.13 15.16 | 4.85 6.47 8.08 | |
| The temperature of the cooling water at the outlet of the condenser | °C | 8.85 10.47 12.08 | 13.09 16.13 19.16 | 8.85 10.47 12.08 | |
| Salinity of seawater | g/L | – | 30 | 30 | 30 |
| HEU purge coefficient | – | 3 | 3 | 3 | |
| Brine salinity | g/L | 40 | 40 | 40 | |
| Temperature depression | °C | 0.5 | 0.267 | 0.5 | |
| Correction taking into account the average hydrostatic pressure | kPa | 1.784 | 1.784 | 1.784 | |
| Average design pressure of boiling brine | kPa | 7.999 7.865 7.729 | 7.999 7.865 7.729 | 11.824 11.624 11.414 | |
| Temperature difference taking into account the hydrostatic effect | °C | 4.70 4.77 4.83 | 4.70 4.77 4.83 | 3.16 3.28 3.39 | |
| Average design temperature of boiling brine | °C | 42.025 41.676 41.329 | 42.025 41.676 41.329 | 49.6 49.3 49 | |
| The area of the living section for the passage of heating water | m2 | – | 0.0154 | 0.0154 | 0.0154 |
| The average speed of heating water in the inter-tube space of the evaporator | m/s | 1.44 | 1.44 | 1.44 | |
| Kinematic viscosity of heating water | m2/s | – | 0.445 0.451 0.458 | 0.445 0.451 0.458 | 0.345 0.341 0.345 |
| Reynolds criterion for the flow of heating water | – | 45,397.8 44,793.84 44,109.21 | 85,120.87 83,988.44 82,704.78 | 58,556.58 59,243.46 58,556.58 | |
| The Nusselt Criterion | – | 349.339 346.543 343.355 | 509.387 505.310 500.662 | 406.979 409.837 406.979 | |
| Thermal conductivity of heating water | W/(m·°C) | – | 659 658 657 | 678 677 676 | 659 658 657 |
| Heat transfer coefficient from the heating water to the evaporator pipes | W/(m·°C) | 16,443,893 16,287,529 16,113,182 | 24,668,894 24,435,359 24,174,828 | 19,157,074 19,262,315 19,098,934 | |
| The average wall temperature of the pipes of the heating battery of the evaporator | °C | 53.512 52.838 52.164 | 53.512 52.838 52.164 | 68.3 67.65 67 | |
| The average temperature difference between the pipe wall and the boiling brine | °C | 11.48743 11.1619 10.8355 | 11.48743 11.1619 10.8355 | 18.7 18.35 18 | |
| Heat transfer coefficient from pipes to boiling brine | W/(m·°C) | 1740.405 1611.662 1488.931 | 1740.405 1611.662 1488.931 | 6794.061 6437.442 6090.232 | |
| Pipe wall thickness and scale | m | 0.001 | 0.001 | 0.001 | |
| Coefficient of thermal conductivity of the pipe wall material | W/(m·°C) | – | 28 | 28 | 28 |
| Thermal conductivity coefficient of scale | W/(m·°C) | – | 0.8 | 0.8 | 0.8 |
| Heat transfer coefficient from heating water to brine | W/(m·°C) | 796.112 795.821 795.498 | 796.112 795.822 795.498 | 798.830 798.778 798.722 | |
| Temperature pressure in the evaporator heating battery | °C | 36.085 34.268 32.428 | 34.545 32.240 29.928 | 54.471 52.692 50.904 | |
| Heat load of the evaporator | kJ/h | 2,585,560 2,454,476 2,321,748 | 2,475,208 2,309,198 2,142,746 | 3,916,221 3,788,064 3,659,278 | |
| The calculated value of the heat load of the condenser | kJ/h | 2,277,000 | 2,128,500 | 3,366,000 | |
| Evaluation of HEU performance by the amount of evaporated water | m3/h | 0.774 | 0.722 | 0.786 | |
| The speed of cooling water in the condenser pipes | m/s | 1.036 | 1.036 | 1.036 | |
| Heat transfer coefficient of the condenser | W/(m2·°C) | 2095.952 | 2091.838 | 2125.407 | |
| Calculated temperature pressure in the condenser | °C | 27.179 | 27.195 | 31.243 | |
| Secondary steam temperature in the evaporator | °C | 34.153 | 33.975 | 39.639 | |
| Secondary steam pressure in the evaporator | kPa | – | 5.4 | 5.29 | 5.6 |
| Temperature difference of heating water and secondary steam | °C | 30.046 | 30.224 | 35.560 | |
| Secondary steam pressure | kPa | 5.22 | 5.11 | 5.42 | |
| Distillate temperature | °C | – | 33.67 | 33.28 | 34.3 |
| Enthalpy of distillate | kJ/kg | – | 140.98 | 139.38 | 143.52 |
| Enthalpy of secondary steam | kJ/kg | – | 2562.2 | 2561.8 | 2563.6 |
| Distillate density | kg/m 3 | – | 993.24 | 994.6 | 994 |
| The multiplicity of condenser cooling | – | 122.684 | 131.520 | 120.790 | |
| Specific heat load of the condenser | kJ/(m2 h) | 87,576.92 | 81,865.38 | 129,461.5 | |
| Specific steam load of the condenser | kJ/(m2 h) | 29.581 | 27.631 | 30.067 | |
| Heating of the cooling water in the condenser | °C | 4.864 | 4.545 | 4.941 | |
| Intake water pump head | m | 26.767 | 26.767 | 26.767 | |
| Pump feed | m3/s | 0.0316 | 0.0316 | 0.0316 | |
| Pump power | kW | 10.647 | 10.647 | 10.647 | |
| Power of the drive motor | kW | 12.52696 | 12.52696 | 12.52696 | |
| The suction pressure of the distillate pump | kPa | −92.08 | −92.19 | −91.88 | |
| Discharge pressure | kPa | – | 264.7 | 264.7 | 264.7 |
| Pump head | m | 36.616 | 36.577 | 36.568 | |
| Pump feed | m/s | 0.00086 | 0.000803 | 0.000874 | |
| Pump power | kW | 0.341 | 0.318 | 0.346 | |
| Power of the drive motor | kW | 0.426 | 0.397 | 0.432 | |
| Electricity consumption for the production of 1 m3 of distillate | kW | 16.728 | 17.893 | 16.478 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solomin, E.; Salah, Z.; Osintsev, K.; Aliukov, S.; Kuskarbekova, S.; Konchakov, V.; Olinichenko, A.; Karelin, A.; Tarasova, T. Ecological Hydrogen Production and Water Sterilization: An Innovative Approach to the Trigeneration of Renewable Energy Sources for Water Desalination: A Review. Energies 2023, 16, 6118. https://doi.org/10.3390/en16176118
Solomin E, Salah Z, Osintsev K, Aliukov S, Kuskarbekova S, Konchakov V, Olinichenko A, Karelin A, Tarasova T. Ecological Hydrogen Production and Water Sterilization: An Innovative Approach to the Trigeneration of Renewable Energy Sources for Water Desalination: A Review. Energies. 2023; 16(17):6118. https://doi.org/10.3390/en16176118
Chicago/Turabian StyleSolomin, Evgeny, Zaid Salah, Konstantin Osintsev, Sergei Aliukov, Sulpan Kuskarbekova, Vladimir Konchakov, Alyona Olinichenko, Alexander Karelin, and Tatyana Tarasova. 2023. "Ecological Hydrogen Production and Water Sterilization: An Innovative Approach to the Trigeneration of Renewable Energy Sources for Water Desalination: A Review" Energies 16, no. 17: 6118. https://doi.org/10.3390/en16176118





