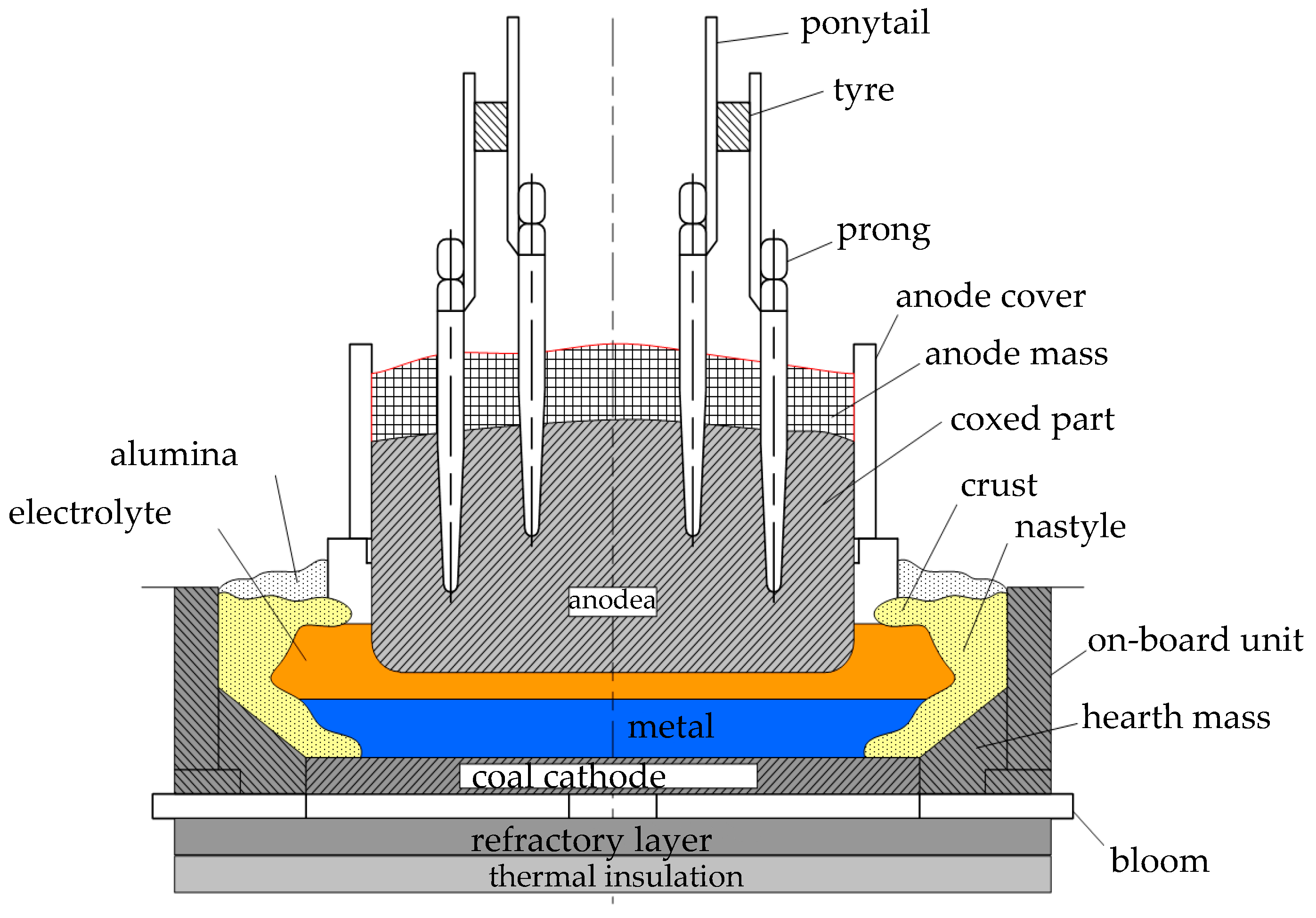
The relationship between the parameters of the object requires a new approach in modelling, since this process is complex and multi-connected, and many previous developments broke the system into sub-processes, for which mathematical models were developed and then linked; therefore, the synthesised mathematical model should generally reflect all the relationships occurring in the electrolyser. In order to obtain a mathematical model of the system and its further implementation based on technical means, a conceptual model of the process of aluminium production in the Soderberg electrolyser is developed.
Correlation analysis is used to build a conceptual model and to determine the relationship between the variables and their significance. The assessments are individual and based on the opinion of individual experts.
The experts are expected to respond with a completed worksheet and/or comments on the work completed. When assessing the degree of importance of the dimensions, the assessor assigns them a ranking number on a scale from 1 to 10, where the scores have the following meaning:
The specified significance level for the number of samples up to 100 units is 0.05.
Construction of Conceptual and Mathematical Model
Any system design or programme writing starts with the stage of building a conceptual model of the object. A conceptual model is a set of concepts describing the area under study. An important factor in its construction is the analysis of the scientific literature, which gives a fairly clear idea of the structure of the object and the elements that are in the relationship.
The use of a graphical representation of the conceptual model is more preferable than in the form of text, because the information expressed by a diagram or scheme clearly demonstrates the direction of the main relationships [
37].
All the concepts included in the conceptual model are concretised and interpreted so that the conceptual model constructed can serve as a basis for the further development of data collection techniques, etc. Some terms are used in mathematical concepts, making it possible to describe them using equations and formulae.
The construction of the conceptual model is based on the data obtained at the stages of expert assessment and the formation of significant parameters.
Table 8 is the result, and it is from its conclusions that the conceptual model will be built. The “central” object of the model is the process under consideration: aluminium electrolysis taking place in the Soderberg electrolyser.
The most important parameters are the parameters with the highest weight, shown in
Table 8, and belonging to the group of input and output parameters. The constructed conceptual model showing the significant technological parameters is presented in
Figure 7.
Based on the above study on identifying the most significant parameters of the technological process, it is possible to establish functional relationships between them.
It should be noted that most of the parameters have a non-linear relationship, so improvement of one of them may lead to the deterioration of another. There is no formula that includes all TP variables simultaneously, but a system of equations describing individual parameters can reflect the relationship between the parameters. Many parameters can be described using the existing laws and mathematical formulae, but there is a category of parameters that require qualitative descriptions.
The state of technology largely depends on the quality of the metal produced and compliance with technological regulations. Primary aluminium containing 99.7–99.8% of the main component has the maximum quotation on the London Metal Exchange. In order to maintain the grade level of metal not lower than A7 at the production facility after the overhaul of the electrolysers, its low grade primary metal (A0–A6) is diluted in the foundry department with A8 grade to A7 grade during the first two weeks.
The bauxite raw material base in Russia is mainly low- and medium-grade, which reduces the quality of the extracted alumina; therefore, nepheline ores also have to be used as aluminium raw material. In order to meet the aluminium smelters’ demand for alumina, raw materials are imported from abroad.
The calculation of raw material import costs is based on production and economic indicators. In general terms, in order to calculate the cost of raw materials purchased from abroad, it is necessary to summarise a number of indicators, which vary depending on the circumstances.
A formula that can be used to calculate the cost of raw materials is
where
Hp—material consumption rate for products, kg.;
Цм—material price, RUB/tonne;
Ктз—coefficient that takes into account transport and procurement costs.
The material balance is the ratio between the materials produced in the electrolysis process and those loaded into the electrolyser. According to the data obtained from the material balance, the change in the composition of the electrolyte and the required expenditure of raw materials, materials and electricity are determined [
10].
The concentration of alumina in the electrolyte is expressed from the material balance by the dissolved alumina and is strictly regulated by stabilisation within the permissible range of variation, because insufficient concentration is the cause of the anodic effect, and excessive concentration reduces the solubility of alumina in the electrolyte melt:
where
is the mass of the electrolyte melt, kg;
is the concentration of alumina in the electrolyte, wt. fraction;
is the flow rate of loaded alumina, kg/s;
is the stoichiometric coefficient of the alumina decomposition reaction, which allows us to determine the quantitative and qualitative relations between the used components of raw materials.
The amount of aluminium in the electrolyser is the aluminium formed during the electrolysis process and is represented as the following equation:
The capacity of the electrolyser
(kg/h) is the amount of aluminium released per unit time:
where
is the current on the electrolyser, A;
is the electrochemical equivalent of aluminium, kg/А·с;
is the current yield, mass fraction.
The material input to the electrolysis bath is calculated from the capacity of the electrolyser:
where
is the specific consumption of alumina, anode mass and fluoride salts, respectively, kg/kgAl;
is the consumption of alumina, anode mass and fluoride salts, respectively, kg.
The aluminium fluoride content in the cryolite is necessary to maintain the chemical composition of the electrolyte; the quality of aluminium fluoride has the following requirements:
- -
Contains at least 30% aluminium and 61% fluorine;
- -
Contains impurities: Na ≤ 0.5%, SiO2 + Fe2O3 ≤ 0.38%, SO4 ≤ 0.5%, P2O5 ≤ 0.04%.
In order to control the cryolite ratio, the concentration of the aluminium fluoride content in the electrolyte must be maintained. The aluminium fluoride bath is fed with aluminium fluoride by means of an automatic control system that monitors the electrolyte level. The control is based on daily discrete measurements and qualitative analyses updated at least three times a week. The automatic control systems of cryolite ratio “TROLL” and “Stella” calculate the optimal additives by the sum of three independent variable components dynamically reacting to the perturbation each on its own channel for deviation from the nominal value [
14]:
where
is the total (calculated) addition of fluorosols for the correction of KO and the technological state of the electrolyser, kg;
is the current KO additive (laboratory data), kg;
is the temperature additive (measured value), kg;
is the additive taking into account the normative consumption of fluorosols—the amount of fluorides required for obtaining a certain mass of metal and maintaining KO in the absence of other factors.
The additive for the current KO is equal to:
where
is the mass of electrolyte in the electrolyser, kg;
is the basic KO (set KO for the given bath);
is the current DO (data from the laboratory);
is the constant characterising the raw material used.
Correction by KO occurs only when both are not equal to each other.
The temperature additive allows correction and is calculated by the following formula:
where
is the temperature of the electrolyser, K;
is the temperature coefficient;
is the electrolysis temperature, K.
The system also contains a formula for calculating the variable component M
norm:
where
is the electrolyte level in the electrolyser;
is the basic electrolyte level in the electrolyser;
is the standard consumption of fluorosols.
The height of the molten aluminium layer at the bottom of the electrolyser is measured by various methods, such as using a steel rod by hand, but the efficiency of such methods is low, so currently, a method based on the interface trace on steel rods is used. When a steel rod is vertically immersed in an electrolyte solution and molten aluminium, a trace called the interface trace is formed. This method measures the distance between the reference point on the steel rod and the trace formed. The height of the molten aluminium layer is calculated according to the expression [
38]:
where
is the distance from the fixation point to the electrolyser bed, cm, which is determined once during rod calibration and is further corrected as the electrolyser bed wears out at least twice a year;
is the distance from the fixation point to the reference point, cm, determined once during rod calibration;
is the distance from the reference point to the trace of the interfacial boundary between the electrolyte and the molten aluminium, cm, determined at each measurement of the height of the molten aluminium layer.
The amount of heat in the electrolyser is described by the energy balance equation:
where
is the amount of heat in the electrolyser, including the heat of the electrolyte melting, J;
is the electric power consumption (electric power), W;
, is the heat of the input and output material flows (alumina, anode, carbon, aluminium, exhaust gases), W;
is the flow of the heat losses into the environment, W;
is the energy effect of all chemical reactions in the electrolyser (at reaction temperature), W.
Electricity consumption:
where
is the voltage on the electrolyser, V;
is the electrolyte resistance, Ohm;
is the reversible part of the energy effect of the chemical reaction (change in internal energy of the reacting substances), W.
Heat loss flux to the environment:
where
is the heat loss flux through the electrolyte melting boundary, W;
is the heat loss fluxes through the boundary of the electrolyser bottom and anodes, W.
Heat loss flux through the electrolyte melting boundary:
where
is the heat transfer coefficient in the melting zone, W/(m
2·°C);
is the area of contact between the electrolyte and anodes, m2;
is the temperature in the melting zone, °C;
is the ambient temperature, °C.
Heat loss flux through the electrolyte melting boundary under the electrolyser and anodes:
where
is the heat transfer coefficient from the electrolyte through the electrolyser bottom and anodes to the atmosphere, W/(m
2·°C);
is the total area of the electrolyser base and anodes, m2.
is the electrolyte temperature, °C.
Energy effect of all chemical reactions occurring in the electrolyser:
is the irreversible part of the energy effect of the chemical reaction (Peltier heat), W;
In the process of electrolysis, the mass of the liquid electrolyte is not constant, as there is an inflow and outflow of substances, so the interfacial heat balance equation determines the change in mass over time:
where
is the specific heat of melting for the electrolyte, J/kg;
is the mass of the alumina melt, kg;
is the heat transfer coefficient from the electrolyte to the alumina melting zone. W/(m2·°C).
Substituting Equations (8)–(13) into (7), we obtain the energy balance equation for determining the temperature of the electrolyte in the electrolyser:
where
is the heat capacity of the electrolyte, W/(kg·°C), calculated from the heat capacity of the substances that make up the electrolyte.
The magnetic field in the electrolyser appears near the current carrying elements of the electrolyser. The electromagnetic force acting on the melt results from the interaction of the magnetic field with the current flowing through the electrolyser.
where
,
,
are the components of current density, А/cm
2;
, , are the components of magnetic field strength, A/m.
The operating voltage on the electrolyser is taken from the anode and cathode ties and characterises the stationary state of the operating mode:
where
R is the resistance of the electrolyser, Ohm;
is the reverse ЭДС on the electrolyser, V.
To control and calculate the pole-to-pole distance, the following equation is used:
where
is the anode burn rate, m/s;
is the rate of change in the aluminium level, m/s.
Since the resistance of the electrolyte is proportional to the resistance of the interpole distance, the value of the interpole distance can be expressed using the indirect method:
where
is the cross-sectional area of the electrolyte, m
2;
is the resistance of the electrolyte in the interpole distance, Ohm;
is the specific resistance of the electrolyte, Оhm·m.
The electric balance is based on the calculation of the current values and voltage drop in the structural elements of the electrolyser. The voltage drop in various nodes and areas depends on the resistance of these elements and the variation of the series current:
where
is the polarisation voltage, V.
The empirical equation of M.A. Korobov reflects the dependence of the voltage drop on the design parameters of the anode and conducting pins, as well as the specific resistance of the anode and current density [
6]. For electrolysers with top current supply, the voltage drop in the self-firing anodes is calculated from the following expression:
where
is the number of current-carrying pins;
is the average distance from all current-carrying pins to the anode bottom, m;
is the average specific resistance of anode Ohm·m;
is the anode current density, A/m2;
is the anode area, m2.
The voltage drop in the cathode device is also determined by M.A. Korobov’s equation:
where
is the length of the current path along the block, m;
is the specific electrical resistance of the cathode block, Ohm·m;
is half of the width of the bath shaft, m.
is the cross-sectional area of the cathode rod, taking into account the cast iron casting, m2.
G.V. Forsblom and V.P. Mashovets proposed a formula for calculating the voltage drop in the electrolyte:
where
is the anode perimeter, m.
The voltage drop in the electrolyte accounts for a large share of the bath voltage; therefore, it is important to control this indicator. Reducing the MPR to permissible values helps to reduce the amount of heat generated in the electrolyte during the flow of the electric current, as well as to stabilise the electric balance.
The voltage drop in the harness is determined by the sum of the voltage drop at the anode and cathode, as well as in the electrolyser contacts:
where
is the voltage drop on the anode harness, V;
is the voltage drop on the cathode harness.
The anodic current density, current density in the pins and in the electrolyte are equal to:
where
—total area of pins, mm
2;
—area of the metal mirror, cm2.
The critical current density
can be estimated by the Pionte equation:
The limiting current density
is equal to
The greatest influence on the current yield is the shape of the metal–electrolyte interface and the circulation of the electrolyte and aluminium; therefore, the current yield is determined by Korobov’s empirical formula, which reflects the relationship with various characteristics of the electrolysis bath:
The current redistribution on the electrolyser elements occurs continuously as the anode pins are repositioned, anode blocks are replaced, the electrolyser is treated, and other technological operations are performed. To ensure a uniform energy input into electrolysis and its corresponding consumption, it is necessary to maintain the energy balance of the electrolyser, depending on the state of the production technology, which determines the optimal technical and economic indicators of the process [
7]. It is accepted to estimate the change in the current distribution over the electrolyser nodes by the value of the current distribution non-uniformity coefficient
:
where
is the average current value;
is the current value of current on the i-th current conduit;
is the number of branches.
It is worth noting that the measurement of the current value by individual elements of the electrolyser is hindered by powerful magnetic fields and high current values, since the current reaches several thousand Amer.
The electricity costs are determined by the specific energy consumption W (kW·h/kg) for aluminium production, which is equal to the ratio of energy input to the amount of metal produced:
where
is the total electrical work, J;
is the average voltage, V.
Reducing the cost of electricity is a consequence of reducing the power consumption, which is one of the main tasks for energy systems. Electricity consumption is not simultaneous, as some electricity consumers work only at certain time intervals; most often the peak of consumption is observed during morning and evening working hours, as most of the maintenance machinery, lighting equipment, etc., do not use electricity during night hours. The uniform distribution of electricity is a production problem. In order to solve this problem, single-rate and two-rate tariffs have been introduced in Russia [
39,
40,
41].
The single-rate tariff is designed for small capacity, which does not exceed 100 kW. In this tariff, payment is based on the results of electricity meter readings.
The two-rate tariff implies two rates—basic and additional. This payment system was in effect for a long time in the USSR [
42,
43,
44,
45]., but it was not profitable for aluminium producers, because the basic rate was paid for the maximum load controlled by special meters during the month during peak hours and amounted to 1 MW, and the additional rate was paid for the electricity consumption recorded by the meters. Generating companies do not take into account the fact that the power consumption of the aluminium electrolyser series is stable. Rates for declared capacity are determined by the company on a monthly basis (RUB/MW∙h), and for consumed electricity—on an hourly basis (RUB/MW∙h) [
46].
where
is the number of days in the month (Nday = 28, 29, 30, 31);
is the price for electricity fixed by meters, rub./MW∙h;
is the price for the declared capacity, rub./MW∙h.
It should be noted that the cost of electricity produced by hydroelectric power plants is lower than that of thermal power plants. The transport of purchased electricity is also paid according to tariffs, depending on the network—federal or interregional, through which it is delivered to the consumer. Thus, in case of interregional transport, the share of electricity transport can be up to 70% and federal transport up to 20%.
The mathematical model is a system of equations that allows us to estimate and determine the parameters of the technological process that influence the main performance indicators of the electrolyser, as well as to establish internal and external relations for the object.
The title of the formulae included in the mathematical model (26) are given in
Table 9. The results obtained in the chapter can be used as a basis for the development of algorithms for optimal electrolyser control.