Abstract
This study investigated the impact of dual-fuel operation using ethanol-blended diesel fuel enriched with hydroxy gas on CRDI engine performance, combustion, and emission characteristics. Neat diesel fuel was used to run the engine, along with a 20% volume fraction of an ethanol-diesel mixture that had been enhanced with three distinct streams of hydroxy gas, namely 1, 1.5, and 2 LPM. Hydroxy gas was generated by an electrolysis technique using a plate-type dry cell electrolyser (316 L stainless steel) in the presence of a NaOH catalyst. Compared to E20 (Ethanol 20%) fuel, HHO gas enrichment with lower proportions of ethanol blend E20 + 2LPM had a 2.74% increase of BTE and a 5.89% decrease of BSEC at a 5.02 bar BMEP condition. Similarly, HC, CO, and smoke emissions decreased by 4.61%, 5.19%, and 3.1%, while NOx emissions and EGT increased by 3.22% and 3.06% compared to E20. With the addition of HHO gas, combustion characteristics such as HRR, CP, and ignition delay improve while the combustion duration increases. At maximum BMEP, cylinder pressure and heat release rate increase by 3.18% and 6.58% for E20 + 2LPM HHO, respectively. It was found that the 20% volume of the ethanol-diesel blend, with 2 LPM of hydroxy gas, positively affects engine characteristics.
1. Introduction
Modern living standards are considerably supported by energy across the globe. From 7.3 billion (as of now) to 9.1 billion (in 2040), the global population is expected to grow quickly. India will overtake China as the world’s most populous country in 2025. By 2040, the world’s energy demand is expected to rise by roughly 25%, which will increase to 100% by 2060. Sport utility vehicles (SUVs), light trucks, heavy trucks, off-road vehicles, and passenger cars will travel over 14 trillion miles in 2040, up about 60% from today [1]. In 2017–2018, diesel accounted for the greater part of consumption (40%), and the next in line is kerosene (4%), naphtha (6%), LPG (11%), petroleum coke (13%), and gasoline/motor spirit (13%) [2]. The other large problem with using more fossil fuels is increasing emissions. The ecology is impacted, and there are serious health risks when NOx, UHC, CO2, and CO emissions are at their maximum. Of 180 countries, India is the fourth most polluting country globally [3]. Using an alternate fuel can help minimize carbon emissions. The combustion of fossil fuels exposes previously accumulated carbon into the environment [4]. Biofuels, on the other hand, emit carbon that was removed from the environment by the crops used to make them. Therefore, biofuels are considered net-CO2 free fuels [5]. In this context, most studies focus on biodiesel blends and supplemental gaseous fuels for compression-ignition engines with increased efficiency, low fuel consumption, and low emissions. To augment the increasing depletion of fossil-based fuel, hydrogen is gaining ground because of its quick burn velocity, low ignition energy, and wide combustibility range, which results in higher brake thermal efficiency and lower levels of HC, CO2, CO, and smoke emissions, except NOx. One sensible approach for starting combustion with hydrogen in compression-ignition engines is the dual-fuel mode. Diesel is an ignition source for hydrogen combustion because hydrogen gas has a higher auto-ignition temperature and needs an ignition source to start burning [6]. In the dual-fuel operation of diesel and hydrogen fuel using an electronically controlled intake manifold on a four-stroke single-cylinder diesel engine, and conventional diesel fuel was injected at an angle of 23° BDC (pilot injection), and hydrogen gas was injected using the timed manifold injection method. The test result shows that the BTE was raised to 39.53% at 60% of the engine load, and the BSEC was lowered to 15.24% at 40% of the engine load. The dual-fuel operation reduced NOx emissions at 20% of the engine load [7]. On the other hand, hydrogen gas must be stored in huge reservoirs because of its lower density. Therefore, researchers concentrate on producing hydroxy gas by an onboard technique using the electrolysis method in the presence of NaOH, NaCl, or KOH, etc., that separates one oxygen atom (O) and two hydrogen atoms utilising an electronic control unit (ECU) to analyse three different catalysts, KOH, NaOH, and NaCl, in the electrolyser. The test’s results show that the sodium hydroxide (NaOH) catalyst has more efficiency than the other two catalysts. Additionally, engine torque and specific fuel consumption improved by an average of 19.1% and 14%, while engine emissions of CO and HC fell by 13.5% and 5%, respectively [8]. The stationary commercial single-cylinder diesel engine with on-board HHO gas generation uses 20–30 g/L of KOH electrolyte catalyst. The results from this study conclude that the cell’s performance was highest at six to ten amps of current. The engine torque, engine power, and BSFC were enhanced by an average of 3.81%, 2.91%, and 2.78%, respectively [9]. The rate of HHO gas production using a plate-type, cylindrical-type, and wire-type electrolyser along with three distinct electrolyte catalysts such as NaOH, KOH, and NaCl is higher when a plate-type electrode is combined with a NaOH electrolyte solution, and it has also been discovered that HHO gas enriched with B10 biodiesel improves the effectiveness of diesel engines. The experimental results revealed that it increased 8.31% of brake power, 7.1% of brake torque, and 20% of NOx emissions [10]. Another researcher delved into the exploration of three distinct variants of dry cells: the alpha cell consisting of two stacks, 11 plates, and five cells; the beta cell comprising two stacks, 13 plates, and six cells; and the omega cell housing four stacks, 21 plates, and six cells. These cells were studied alongside the utilization of HHO gas with an NaOH electrolyte catalyst in a 1500 cc gasoline engine. Among these, the beta-cell emerged as the most suitable for integration into a vehicle engine. This integration resulted in a remarkable reduction of 17% in SFC, 27% in HC, and 17% in CO emissions [11]. Even so, several studies have focused on increasing the use of biodiesel in CI engines along with a hydroxy gas as the ignition source to improve engine performance and emissions. Aside from carrying out investigations involving the utilization of HHO gas enriched with a 20% volume of palm biodiesel blended into diesel fuel, various parameters were fine-tuned with respect to time. These parameters encompassed the quantity of electrolytes (KOH), the count of electrodes, the spacing between electrodes, the rate of current flow, and the generation of HHO gas. According to the test results, the Specific Fuel Consumption (SFC), brake power, and CO and HC Emissions improved by 5%, 2%, and 20%, respectively [12]. In another study, the performance of a 3907 cc engine was examined. This study investigated the use of low-sulphur diesel combined with microalgae-derived biodiesel (MB20), which was further enhanced with the incorporation of hydroxy and hydrogen gas. The outcomes of the testing indicated that the introduction of hydroxyl gas showed improvements in terms of brake power and engine torque. Simultaneously, this addition led to elevated NOx emissions while effectively reducing CO and CO2 emissions levels [13]. In a 10% sunflower oil blend, 90% of diesel fuel is mixed with HHO gas and pure hydrogen gas. The outcomes indicated that, with the exception of NOx emissions, the inclusion of HHO gas in the B10 sunflower biodiesel blend resulted in favourable enhancements across various parameters, including brake power, torque, specific fuel consumption, and the reduction of CO, HC, and CO2 emissions [14]. The impact of hydrogen and HHO gas augmentation was studied in a 3.6 L four-cylinder engine using a consistent flow rate of 10 LPM; the study focused on a 20% blend of castor methyl ester (CME) with diesel fuel. The findings revealed that the amalgamation of HHO with 20% CME exhibited superior performance compared to the combination of pure hydrogen (H2) with 20% CME. However, it is noteworthy that both fuel mixtures led to an increase in NOx emissions, with incremental rises of 4.3%, 9%, and 27%, respectively [15]. The main drawback to using hydrogen is the increased NOx emissions caused by the fuel’s higher heating value. This can be reduced by using higher proportions of ethanol. The vaporization’s increased enthalpy lowers the combustion chamber’s peak temperature. Ethanol’s cooling impact raises the oxygen level, encouraging a longer and smoother combustion process [16]. The influence of an innovative combination involving hydrogen-enriched ethanol and tamanu methyl ester was remarkable, leading to significant reductions in NOx emissions. Specifically, the reduction percentages were 8.88% for E10TME90, 17.03% for E20TME80, and 14.81% for E30TME70, as indicated in a study [17]. In another research instance, comparisons were made among ternary fuel mixtures, namely E10 D30 NCO60, E20 D30 NCO50, and E30 D30 NCO40, which encompassed castor oil, ethanol, and diesel components. The results showed that adding 30% ethanol to the ternary fuel blend reduced NOx emission by 7.7% [18].
To avoid using a large, pressurized storage tank, this study evaluates the onboard production of hydroxy gas (HHO) using a plate-type dry cell electrolyser (316 L stainless steel) in the presence of a NaOH catalyst powered by an engine battery and coupled to the engine alternator. This research delves into the study of performance, combustion, and emission traits exhibited by three different flows of HHO gas: 1 LPM, 1.5 LPM, and 2 LPM, when combined with a 20% ethanol-diesel mixture in common rail direct injection engines operating in dual-fuel mode. The culmination of this study is presented in the results and discussion section of the research article, where the findings are elaborated upon and analysed.
2. Materials and Methods
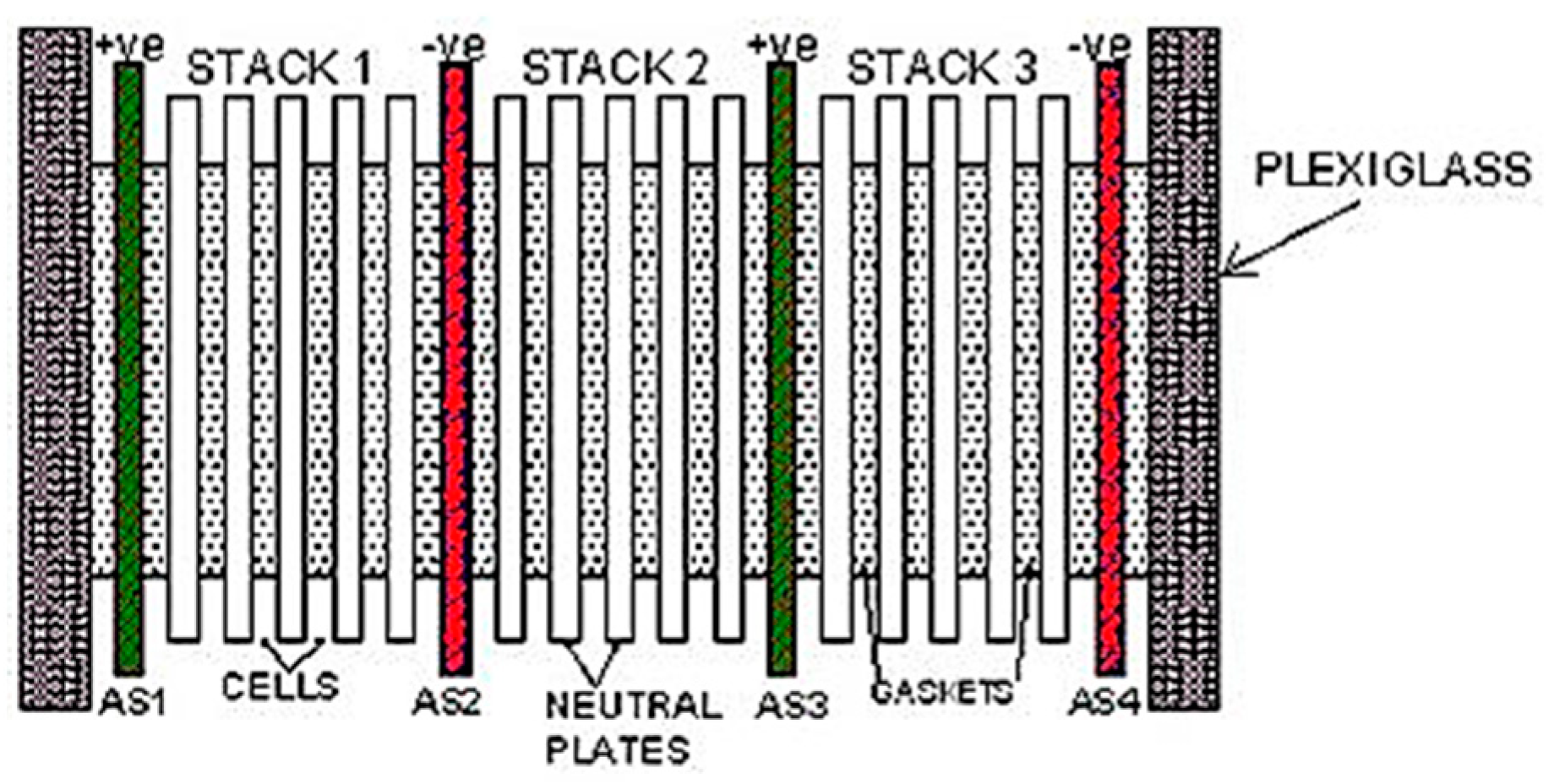
The plate-type dry cell electrolyser was constructed using 316 L stainless steel and comprised nineteen plates, along with twenty rubber square gaskets, two transparent plexiglass sections, hoses, steel nails, steel nuts, a bubbler tank, and an electrolyte tank. This cell was designed with a 1 L capacity for the electrolyte solution, featuring a 2 mm electrode gap. The configuration encompassed three stacks, eighteen cells, fifteen neutral plates, and four active plates divided into two negative poles and two positive poles, arranged as (+ve nnnn −ve nnnn + ve nnnn −ve). A configuration of the plate-type dry cell electrolyser and detailed specifications can be found in Figure 1 and Table 1.
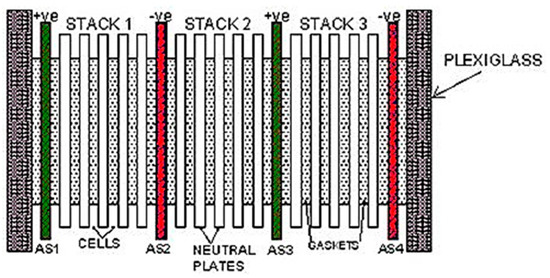
Figure 1.
Configuration of the plate-type dry cell electrolyser.

Table 1.
Specification of the plate-type dry cell electrolyser.
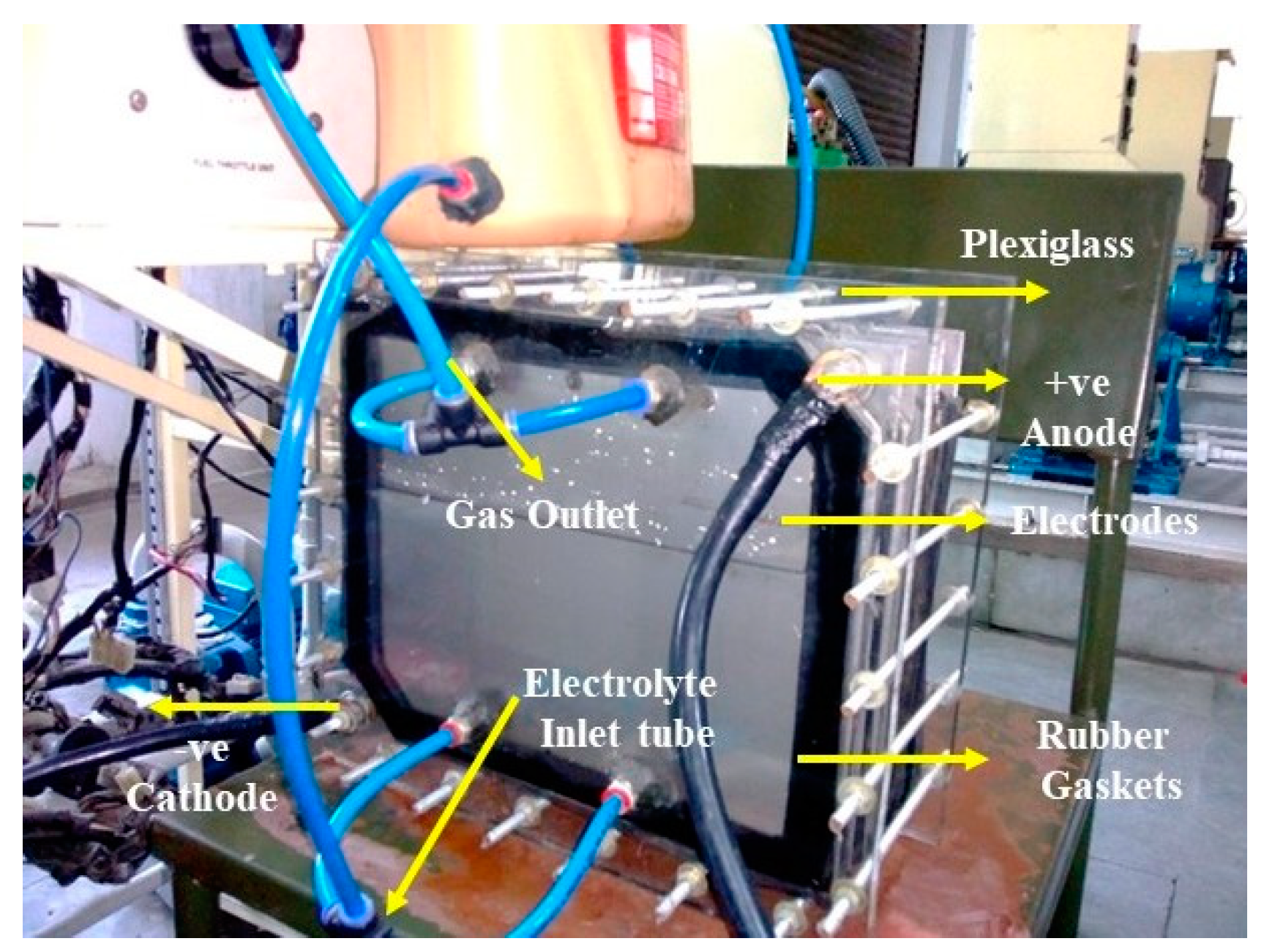
Several factors influence the rate of HHO gas generation, including the composition of the electrode material, the geometric attributes of the electrode, the gap maintained between electrodes, the nature of the electrolyte, its concentration, and the extent of current conveyed. In order to make HHO gas, water is electrolyzed with a 13.5-volt DC power source from an engine alternator, 20 g/L of NaOH electrolytic catalyst solution, and Figure 2 shows a photographic image of a plate-type dry cell electrolyser. The two positive poles of the electrolyser facilitate the production of hydrogen gas at the anode, while the cathode, which corresponds to the two negative poles, forms oxygen gas during this electrolysis process [19]. The overall anode and cathode reactions are as follows:
2H2O(l) → 2H2(g) + O2(g) (overall reaction)
4OH−(aq) → O2(g) + 2H2O(l) + 4e− (anode)
2H2O(l) + 2e− → H2(g) + 2OH−(aq) (cathode)
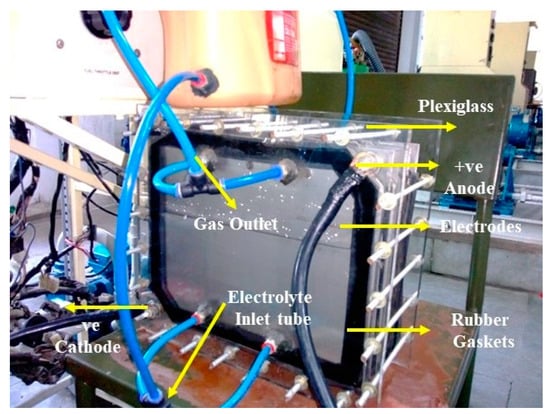
Figure 2.
Photographic image of a plate-type dry cell electrolyser.
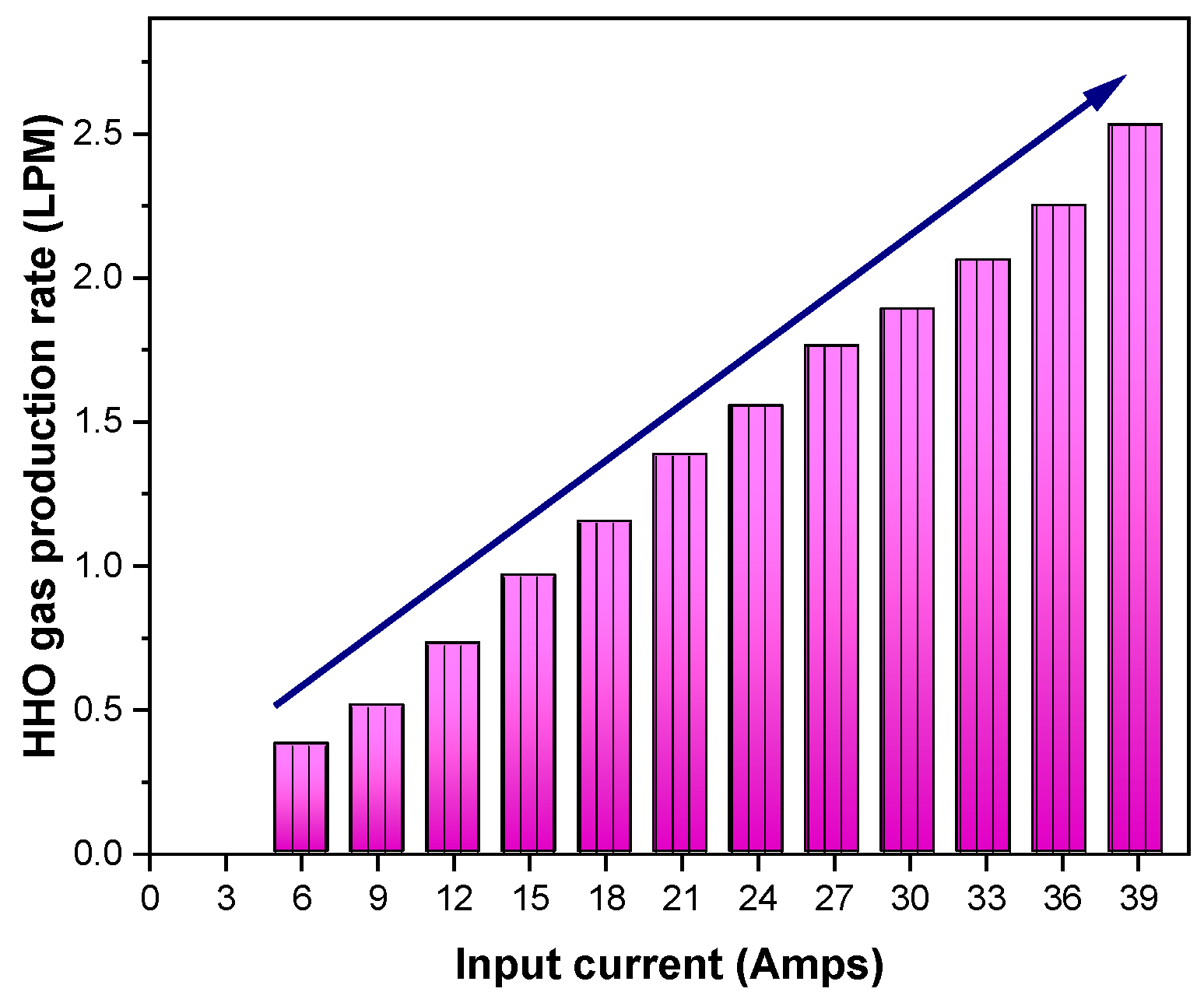
As shown in Figure 3, HHO gas was made in this experiment by changing the flow of current, which was denoted by the letter I and ranged from 6 to 39 amps. Elevating the current I (in amperes) corresponds to an augmentation in gas production due to the direct relationship between the quantity of produced HHO gas and the current density. The volume of HHO gas generated by an electrolyser is governed by the magnitude of the current traversing the plate’s surface area and the efficiency of water penetration. The current traverses each cell’s area, transitioning from one plate to the subsequent one. In accordance with Faraday’s theory, it is established that 0.54 amps of current are required per square inch of plate area [20]. The following relationships are used by Faraday’s law to compute gas production:
HHO production = 28.3 × 0.0003689 CFM × operating amps across each stack (A) × count of stacks × count of cells in each stack
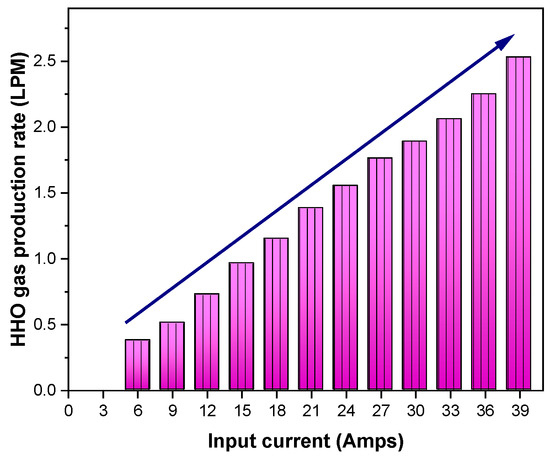
Figure 3.
Variation of HHO gas production with respect to input current.
3. Experimental Procedure
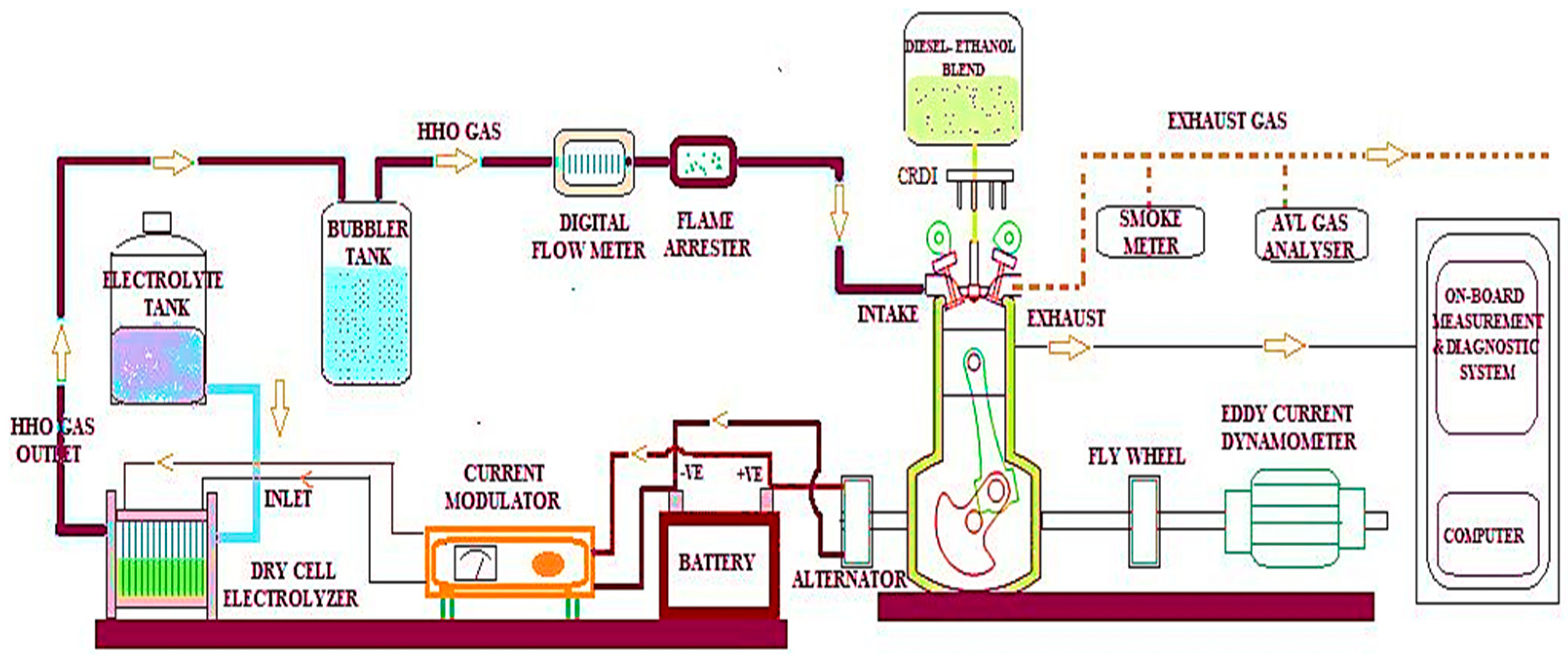
The dry cell electrolyser used in this experiment produced HHO gas, which was then delivered through the air inlet manifold to the Kirloskar TV1 four-stroke single-cylinder common rail direct injection diesel engine under dual-fuel operation at different load conditions (0 to 100%). The Kirloskar TV1, CRDI single cylinder four-stroke CRDI diesel engine was used in this research and the engine’s technical specifications are 110 mm stroke, 87.5 mm bore, 17.5:1 compression ratio, 1500 rom with 5.2 kW rated power, 661 cc displacement, 23⁰ bTDC injection timing, and 800 bars injection pressure. The battery and current modulator supply the dry cell electrolyser with 13.5 volts of DC from the engine alternator (amps). The engine has been water-cooled and connected to a dynamometer to generate brake power. A digital gas flow metre is used to record HHO gas flows with an accuracy of ±0.001 LPM. To avert engine backfire, a setup involving a bubbler tank and flame arrestor was employed. Enhancing the flow of current was achieved by feeding one litre of electrolytic catalyst solution containing 20 g of concentrated NaOH into a dry cell electrolyser using gravity. The tests were performed with three different streams of HHO gas, such as 1, 1.5, and 2 LPM, a 20% mix of lab-grade ethanol and an 80% blend of straight diesel, and the properties listed in Table 2. Engine exhaust emissions are measured using the AVL gas analyser with an accuracy of ±1 ppm and ±0.01% and smoke metre with an accuracy of ±0.05%. Figure 4 represents the schematic diagram of the test engine.

Table 2.
Properties of diesel, ethanol and HHO gas.
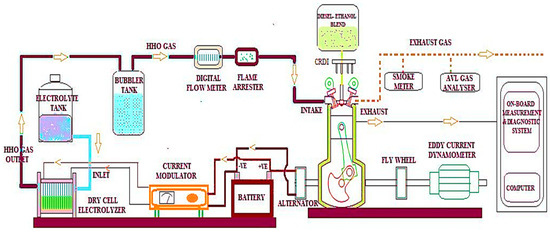
Figure 4.
Schematic diagram of the experimental configuration.
Uncertainty Interpretation
The evaluation of uncertainty in the experimental measurements is a vital necessity since it reveals the degree to which the gathered outcome is correct and within the acceptable range [25]. Several researchers have proposed techniques; however, the strategy that investigates the evaluation of any independent component’s uncertainties based on the uncertainty of the independent parameter is adopted in this investigation [26]. If y1, y2, y3, and yn are independent variables and X is the dependent variable, the uncertainty of the X variable (WX) may be calculated as follows [27]:
The Equation (5) was used to evaluate the uncertainty values of the experimental measurements. The calculated uncertainty ranges for BTE, and BSEC are ±0.4219 and ±130.26, respectively. The estimated uncertainty estimates are consistent with existing investigations.
4. Result and Discussion
4.1. Brake Thermal Efficiency
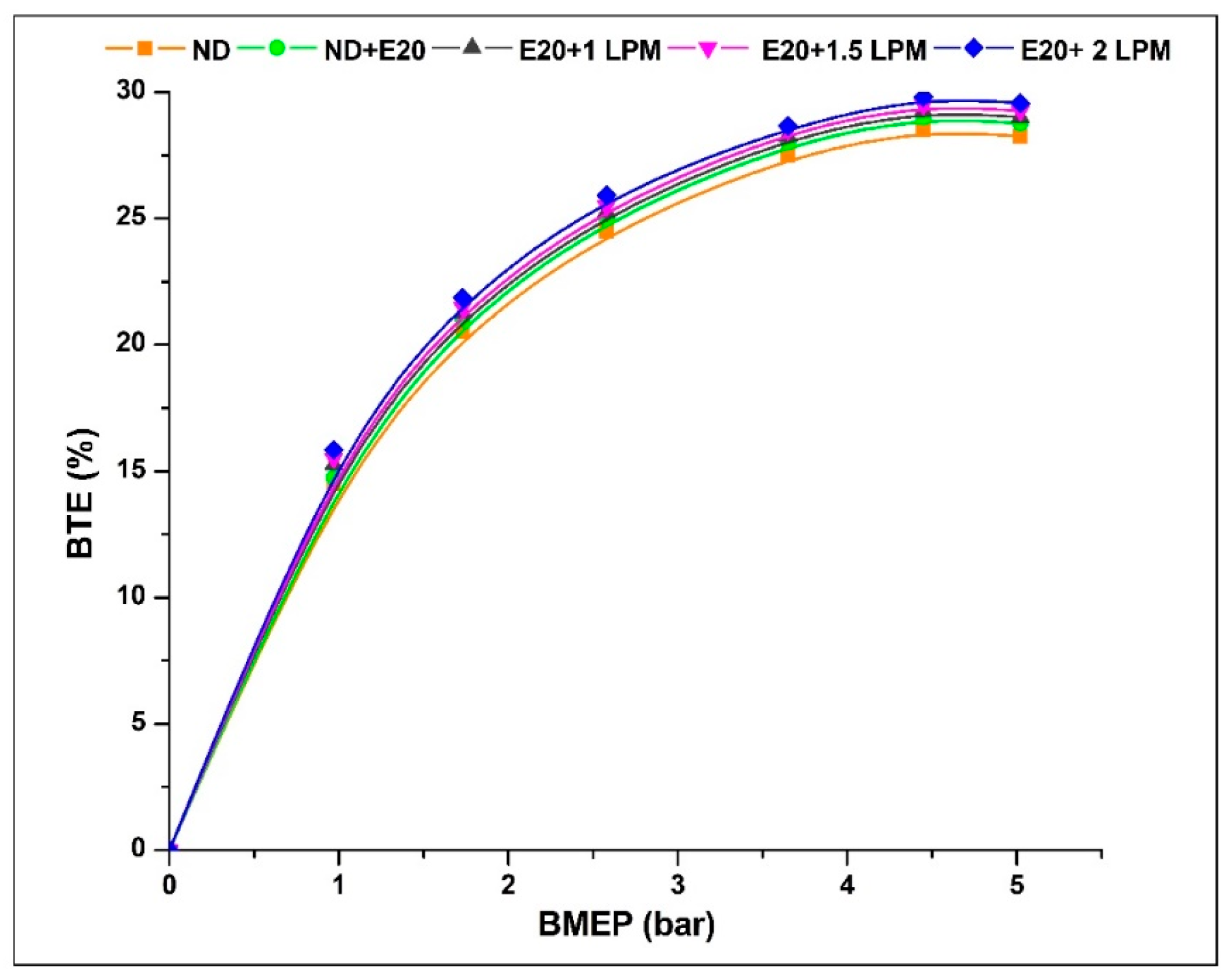
Figure 5 depicts the enhancement in engine BTE for the HHO-enriched diesel-ethanol blend under high BMEP circumstances. In addition, the BTE percentage rises with the addition of HHO-LPM. The BTE is commonly performed to represent the engine’s economic effectiveness. The BTE percentage at 0.97 bar BMEP is 14.48, 14.75, 15.22, 15.31, and 15.42% for ND, ND + E20, E20 + 1 LPM, E20 + 1.5 LPM, and E20 + 2LPM, respectively. At 5.02 bar BMEP, the BTE percentages for ND, ND + E20, E20 + 1, E20 + 1.5, and E20 + 2 LPM are 28.25, 28.75, 29.05, 29.25, and 29.56%. Compared to neat diesel with a 20% ethanol blend, at maximum BMEP, the E20 + 1.5LPM and E20 + 2 LPM enhance the BTE by 1.70 and 2.74%, respectively. Because of the inclusion of atomic oxygen and hydrogen within the gas blend, the heat produced during the premixed-phase combustion was increased. This rise in heat stemmed from augmented homogeneity and hydrogen’s properties, such as rapid flame propagation and diminished quenching distance, extending the ignition range [28]. Additionally, the incorporation of a lesser proportion of ethanol within the blend was responsible for increased volatility and improved spray attributes [29].
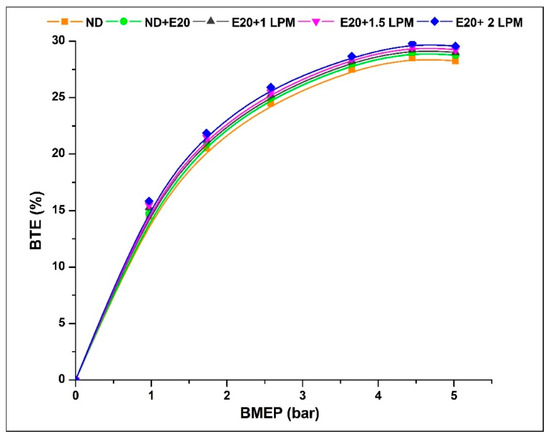
Figure 5.
Variations of BTE for 20% ethanol blend with the effect of hydroxy gas enrichment at three different LPM levels.
4.2. Brake-Specific Energy Consumption
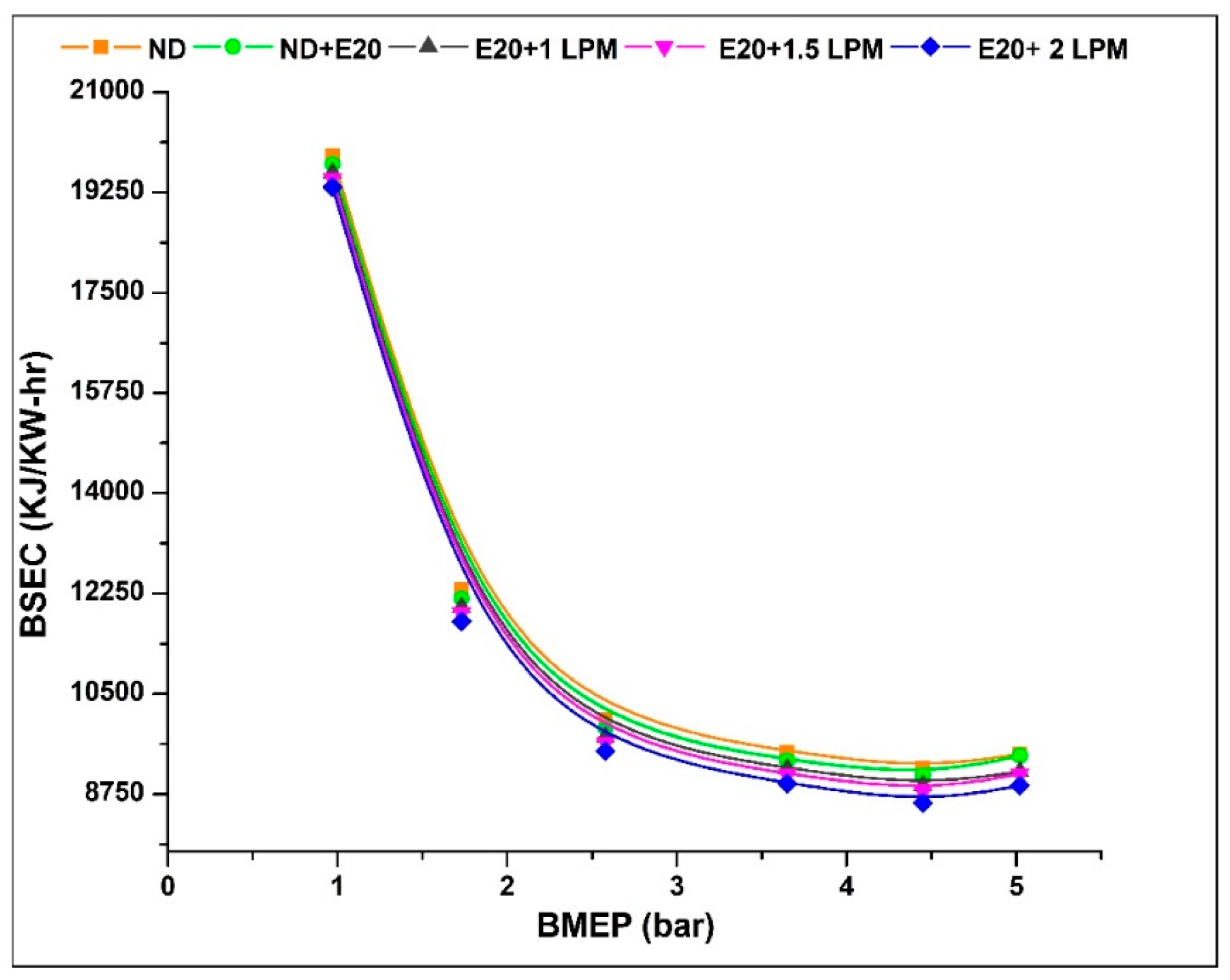
Figure 6 depicts the fluctuation of BSEC with engine BMEP. The BSEC value at 0.97 bar BMEP is 19,890, 19,740, 19,590, 19,490 and 19,340 kJ/kW-h for ND, ND + E20, E20 + 1 LPM, E20 + 1.5 LPM, and E20 + 2LPM, respectively. At 5.02 bar BMEP, the BSEC values for ND, ND + E20, E20 + 1 LPM, E20 + 1.5 LPM, and E20 + 2LPM are 9450, 9425, 9150, 9100, and 8900 kJ/kW-h. Using the HHO gas in diesel-ethanol blend fuel decreases BSEC by 3.58, and 5.89% for E20 + 1.5 LPM and E20 + 2LPM. The decrease in BSEC was attributed to the homogeneous mixing of HHO with air (strong hydroxyl diffusion coefficient) and the oxygen factor of HHO gas with ethanol, which aids fuel throughout the burning process and results in improved combustion. Because HHO has a high flame speed and flammability, adding hydrogen to the fuel would allow it to combust quicker and more completely under high-pressure conditions. Furthermore, the low ignition energy of the HHO gas combination allows the diesel-ethanol fuel blend to be safely burnt under leaner circumstances.
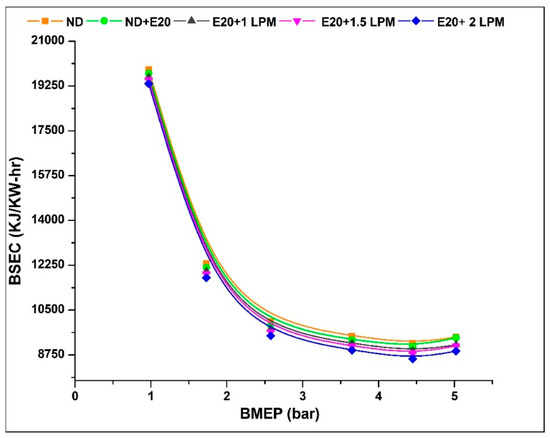
Figure 6.
Variations of BSEC for 20% ethanol blend with the effect of hydroxy gas enrichment at three different LPM levels.
4.3. Oxides of Nitrogen Emission (NOx)
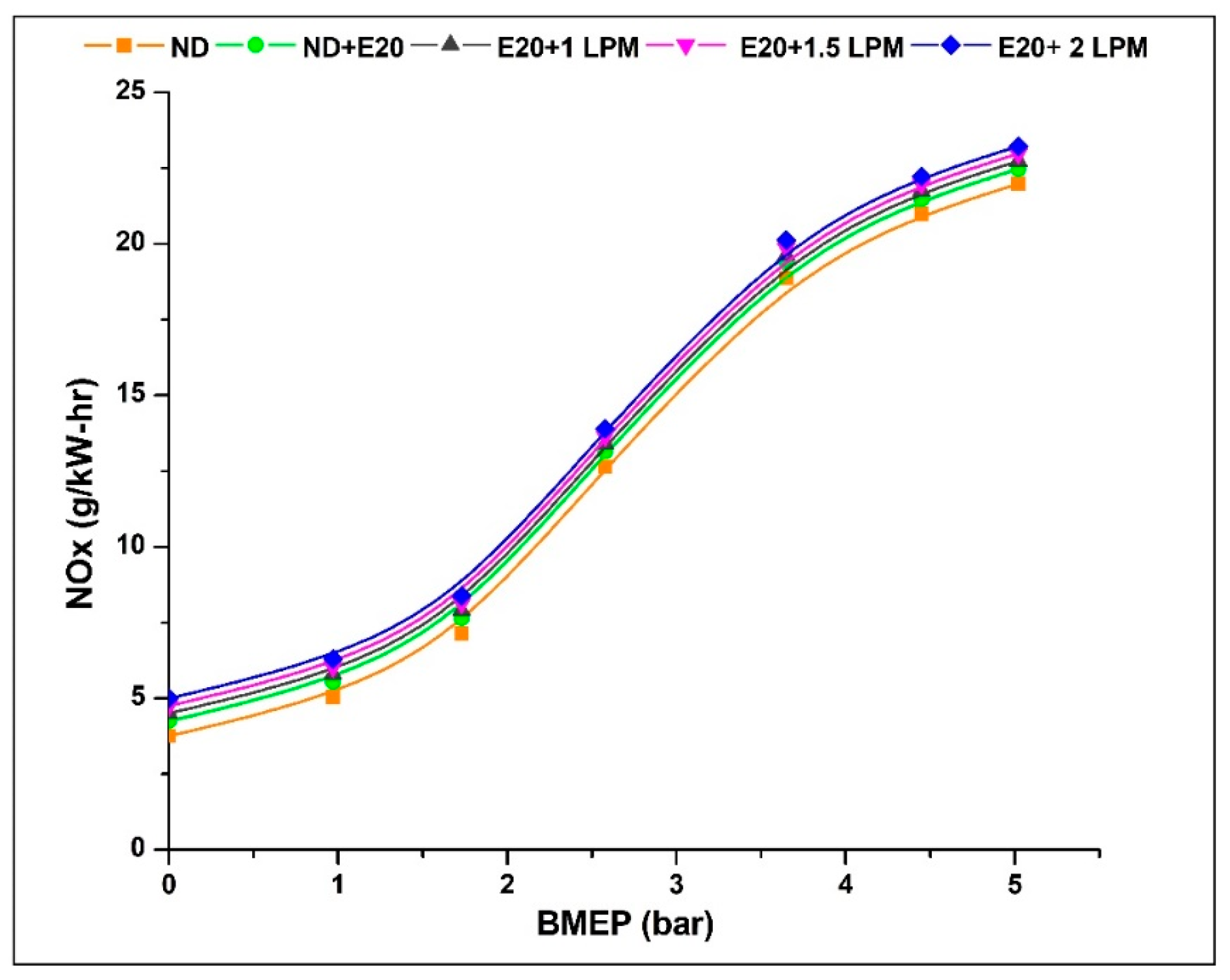
NOx emissions, or nitrogen oxides, primarily arise from the elevated temperatures within the combustion chamber, encompassing both NO and NO2 components. This emission is contingent on temperature, given that the fusion of nitrogen and oxygen molecules is improbable at lower temperatures. Figure 7 shows the difference in Nox emissions between HHO enrichment and not enrichment at different BMEP values. Notably, Nox emissions exhibit a steep escalation with increased BMEP for all conditions, with diesel yielding the lowest Nox levels. Upon the introduction of HHO gas, there is a noticeable rise in NOx emissions across all HHO enrichment levels. Temperature, oxygen concentration, and residence time all have a significant impact on this emission [30]. HHO gas has a considerable impact on these parameters because it adds more oxygen, raises the combustion temperature because hydrogen has a higher heating value, and slightly lengthens the residence time because hydrogen speeds up the flame, making it easier for fuel mixtures to burn quickly. Additionally, ethanol’s lower cetane number compared to diesel can lead to prolonged ignition delays and higher peak combustion temperatures. These elevated combustion temperatures encourage the combination of more nitrogen and oxygen molecules, thereby resulting in the formation of NOx compounds.
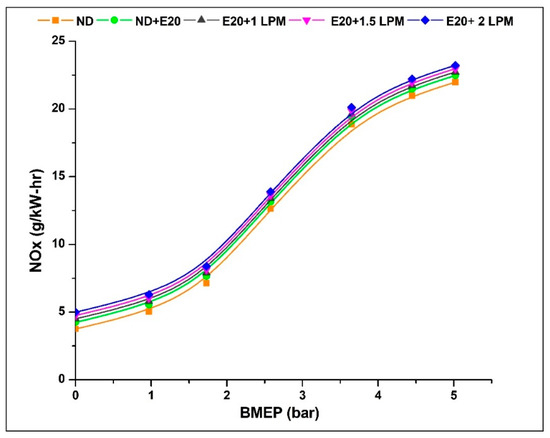
Figure 7.
Variations of NOx for 20% ethanol blend with the effect of hydroxy gas enrichment at three different LPM levels.
The NOx emission at 0.97 bar BMEP was 5.04, 5.54, 5.66, 5.78, and 5.90 g/kW-h for ND, ND + E20, E20 + 1 LPM, E20 + 1.5 LPM, and E20 + 2LPM, respectively. At 5.02 bar BMEP, the NOx emissions for ND, ND + E20, E20 + 1 LPM, E20 + 1.5 LPM, and E20 + 2LPM are 21.98, 22.48, 22.73, 22.98, and 23.23 g/kW-h, respectively. Thus, NOx emissions were 3.22% higher than E20 for E20 + 2 LPM at maximum BMEP conditions. These challenges can be addressed through the efficient implementation of exhaust gas recirculation [31].
4.4. Carbon Monoxide Emission (CO)
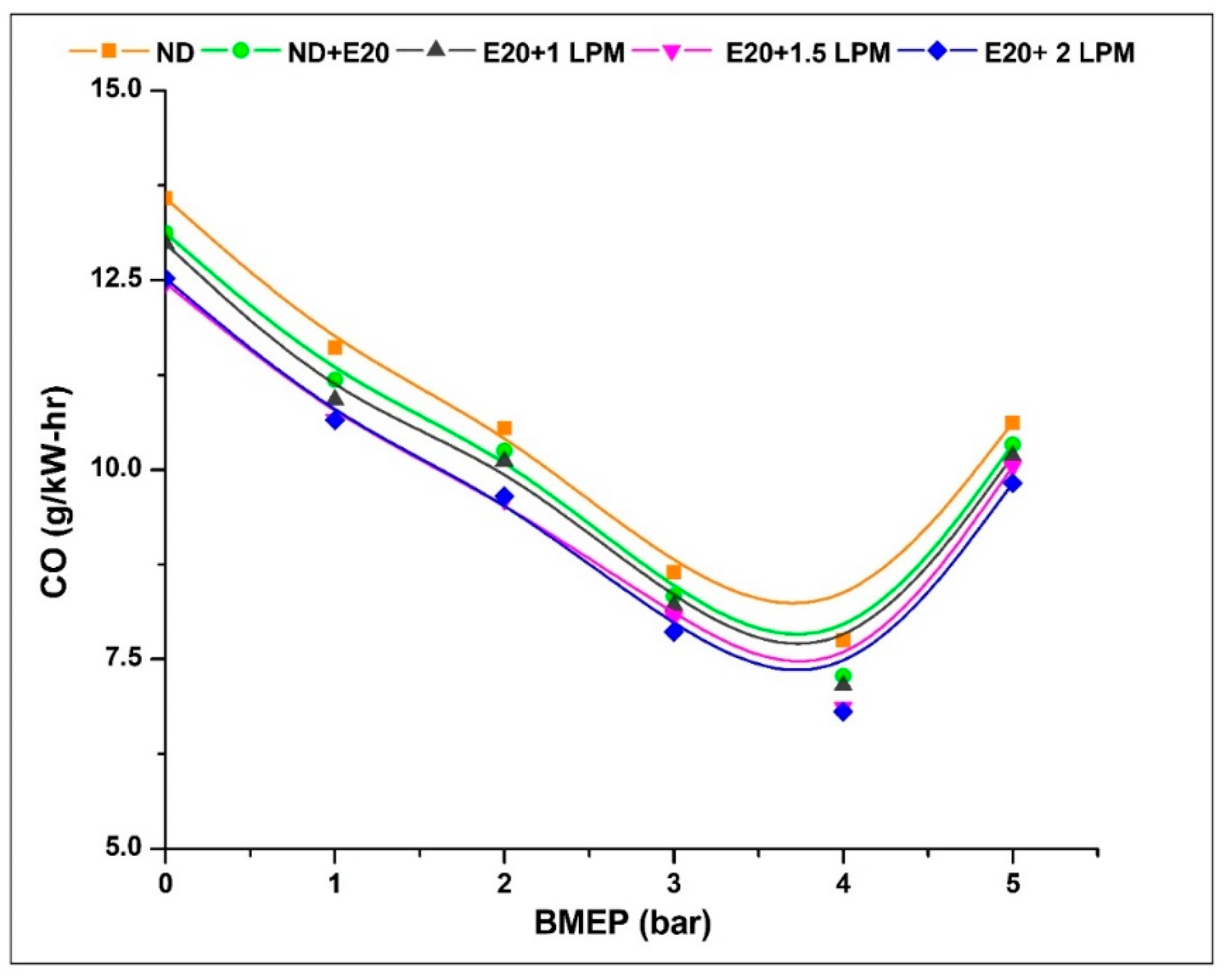
Illustrated in Figure 8 is the depiction of CO emissions originating from the diesel engine under varied BMEP conditions, both with and without HHO enrichment. CO emissions represent an intermediary byproduct resulting from the incomplete oxidation of carbon molecules, arising from limited oxygen availability and the creation of fuel-rich regions within the combustion chamber [32]. The CO emission at 0.97 bar BMEP was 13.58, 13.12, 12.98, 12.46, and 12.52 g/kW-h for ND, ND + E20, E20 + 1 LPM, E20 + 1.5 LPM, and E20 + 2 LPM, respectively. At 5.02 bar BMEP, the CO emission for ND, ND + E20, E20 + 1 LPM, E20 + 1.5 LPM, and E20 + 2 LPM is 10.62, 10.33, 10.18, 10.04, and 9.82 g/kW-h, respectively. Incorporating HHO gas at a lower level into the ethanol blend resulted in a reduction of CO emissions. This decrease can be attributed to ethanol’s elevated oxygen content, which enhances the combustion process and promotes thorough fuel combustion. Additionally, the absence of carbon in HHO gas played a pivotal role in diminishing CO emissions. Moreover, hydrogen’s heightened combustibility enhanced the flammability limit, thereby contributing further to the reduction of CO emissions [33]. Thus, CO emissions were reduced at 5.02 bar BMEP conditions by 2.88 and 5.19% for E20 + 1.5 LPM and E20 + 2 LPM, respectively, compared to diesel fuel without HHO enrichment.
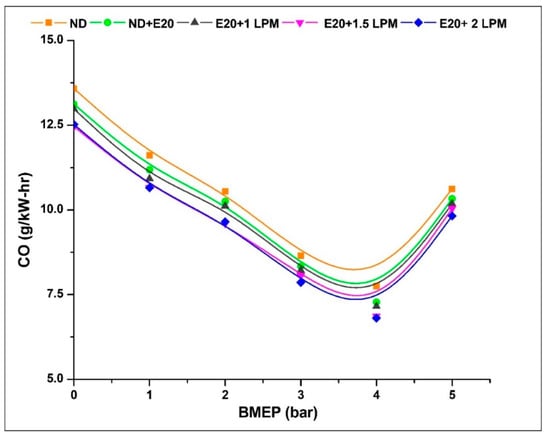
Figure 8.
Variations of CO for 20% ethanol blend with the effect of hydroxy gas enrichment at three different LPM levels.
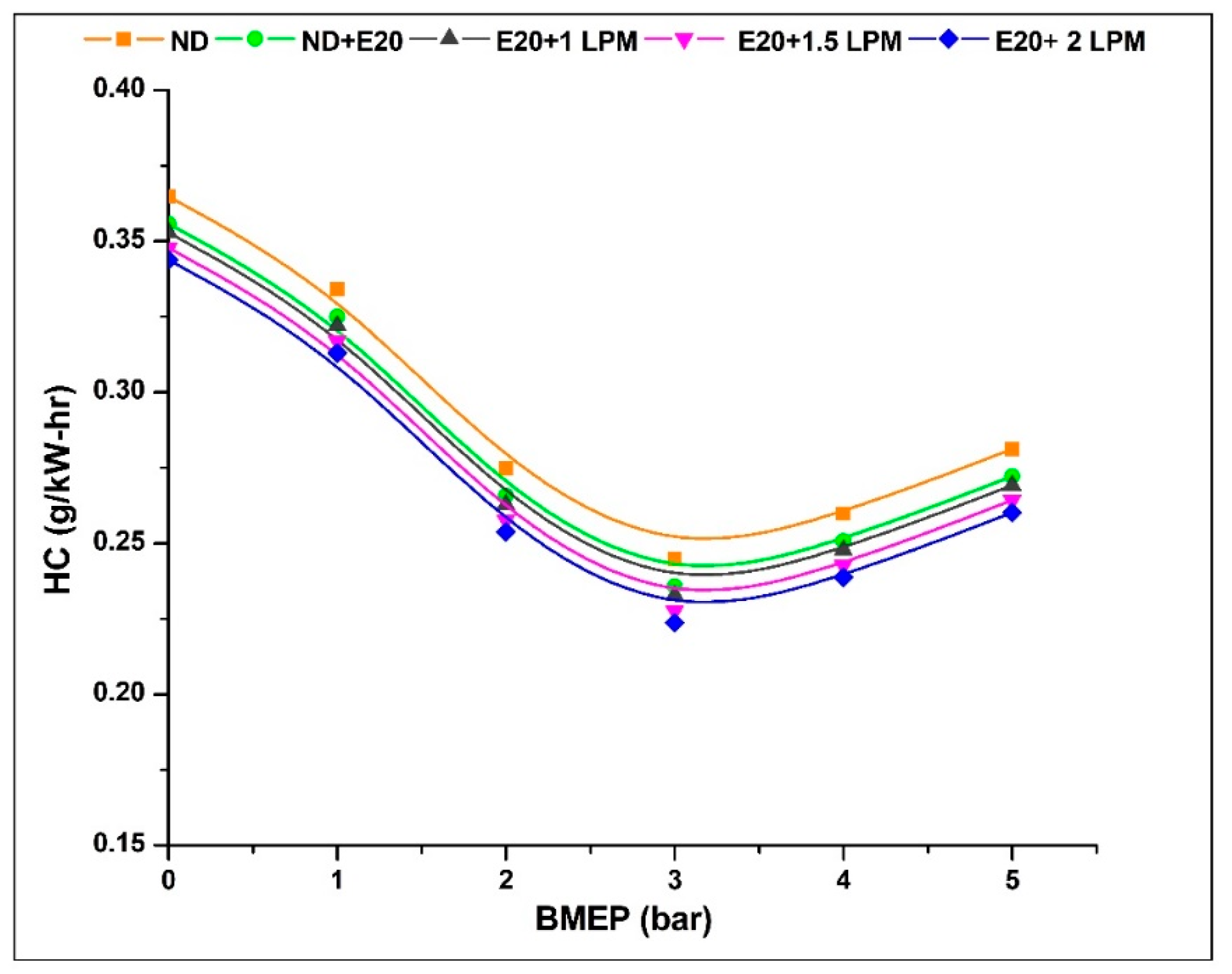
4.5. Hydrocarbon Emission (HC)
Fuel molecules that evade complete or partial combustion and reach the exit valve are referred to as unburned hydrocarbons (UBHC) or hydrocarbon emissions. The rise in HC emissions can be attributed to diverse factors, including a rich fuel mixture, scavenging processes, servicing issues, flame quenching, oxygen inadequacy, engine wear, valve overlap, and more [34]. As demonstrated in Figure 9, the HC emission patterns are depicted for the engine operating on diesel oil both with ethanol and without HHO enrichment under varying BMEP conditions. The HC emission at 0.97 bar BMEP was 0.364, 0.355, 0.352, 0.347, and 0.343 g/kW-h for ND, ND + E20, E20 + 1 LPM, E20 + 1.5 LPM, and E20 + 2 LPM, respectively. At 5.02 bar BMEP, the HC emission for ND, ND + E20, E20 + 1 LPM, E20 + 1.5 LPM, and E20 + 2 LPM is 0.281, 0.272, 0.269, 0.264, and 0.260 g/kW-h, respectively. Emission reduction of HC was attained for E20 + 1.5 LPM and E20 + 2 LPM, respectively, in contrast to E20 without HHO enrichment. The accelerated flame velocity of hydrogen facilitates the combustion of a greater number of fuel molecules, and the inherently shorter quenching distance aids in evading the flame-quenching phenomenon [35].
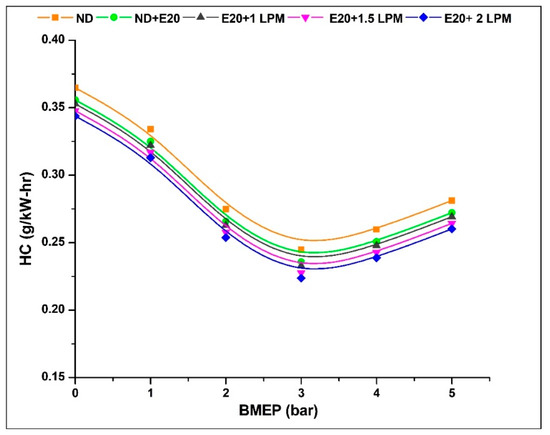
Figure 9.
Variations of HC for 20% ethanol blend with the effect of hydroxy gas enrichment at three different LPM levels.
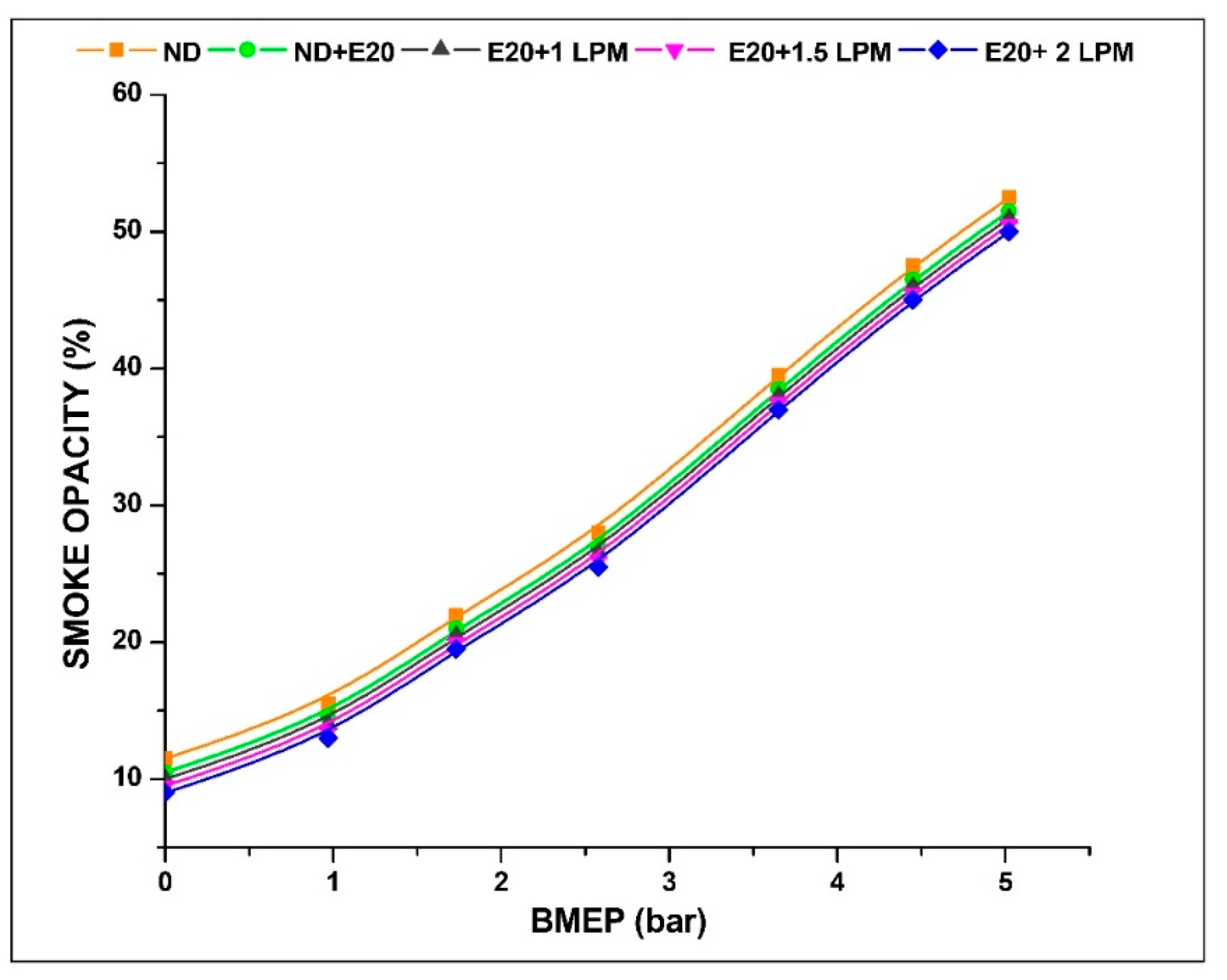
4.6. Smoke Emission
Smoke emissions, a characteristic commonly observed in the majority of diesel-powered engines, comprise a mixture of soot particles, liquid vapours, and minute solid particles present in small quantities [36]. Illustrated in Figure 10 is the smoke opacity level recorded from the diesel engine’s operation under varying BMEP conditions, both with and without the addition of HHO enrichment. The smoke emission at 0.97 bar BMEP is 15.5, 14.5, 14, 13.5, and 13% ND, ND + E20, E20 + 1 LPM, E20 + 1.5 LPM, and E20 + 2 LPM, respectively. At 5.02 bar BMEP, the smoke emission for ND, ND + E20, E20 + 1 LPM, E20 + 1.5 LPM, and E20 + 2 LPM are 52.5, 51.5, 51, 50.5, and 50%. The average reduction of smoke emissions for ND + E20, E20 + 1 LPM, E20 + 1.5 LPM, and E20 + 2 LPM are 4.26, 6.39, 8.52, and 10.64% compared to diesel fuel without HHO at maximum pressure conditions. At 5.02 bar BMEP, a 1.9 and 3.1% reduction of smoke emission was achieved for E20 + 1.5 LPM and E20 + 2 LPM, respectively, compared to E20 without HHO enrichment. Because hydrogen has a higher diffusion coefficient than many other fuels, it moves quickly through fuel blends, reducing the number of fuel-rich areas in the combustion chamber. This process fosters the creation of a uniform mixture, thereby contributing to the reduction of smoke emissions [37]. Notably, the actual amount of smoke reduction caused by soot oxidation by HHO gas could be greater than what has been seen. These differences could be caused by the presence of water vapours in the exhaust gases that come from burning hydrogen [38].
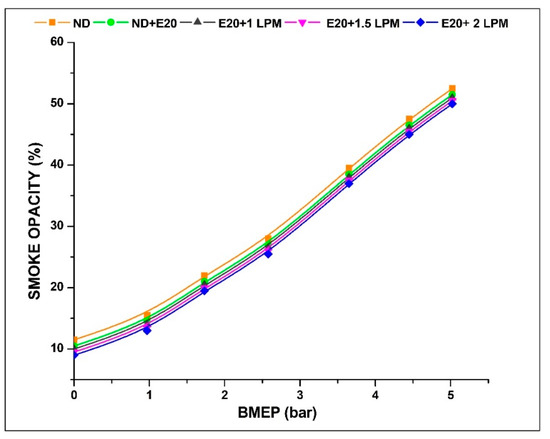
Figure 10.
Variations of smoke for 20% ethanol blend with the effect of hydroxy gas enrichment at three different LPM levels.
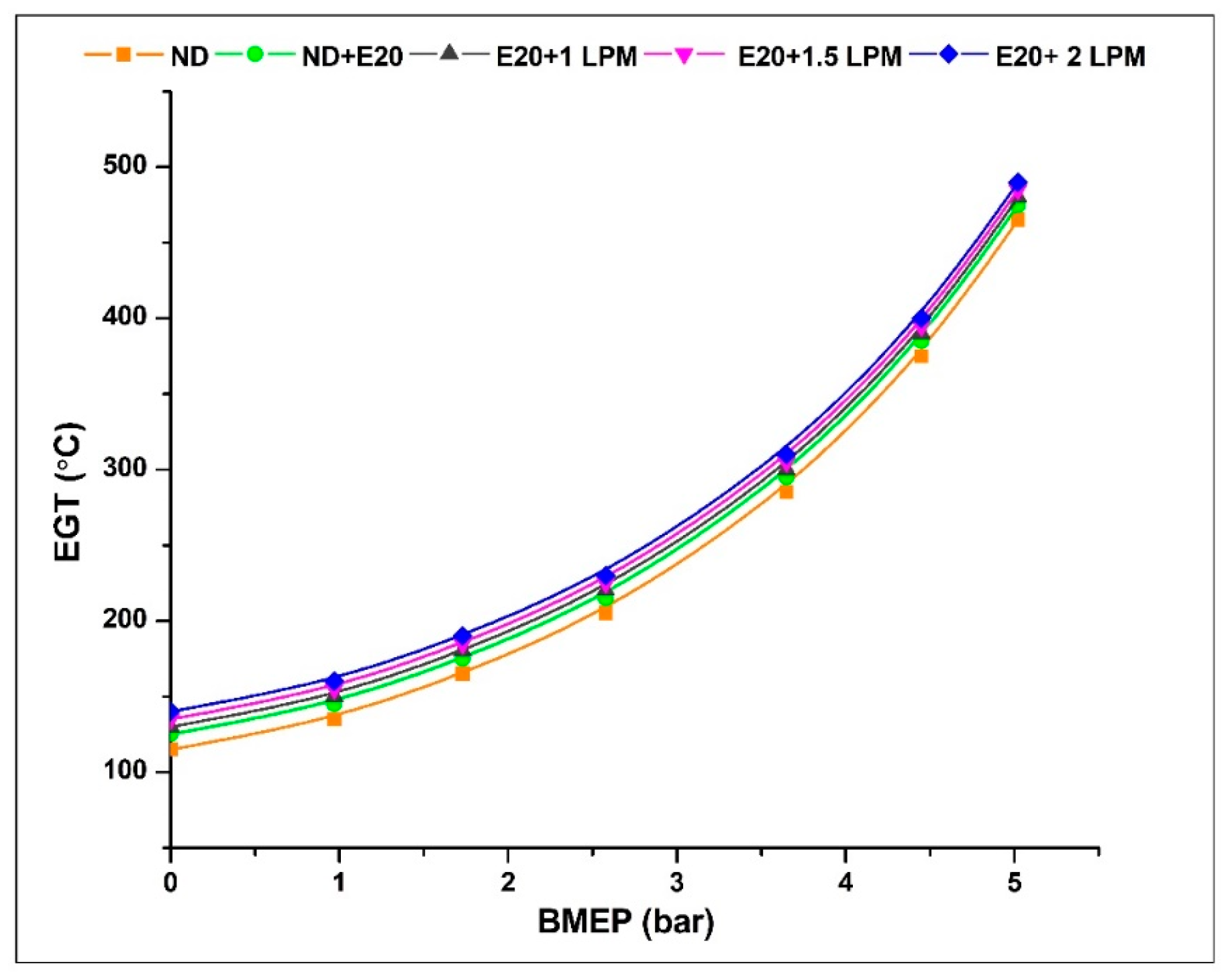
4.7. Exhaust Gas Temperature
Figure 11 depicts the variability of exhaust gas temperature about BMEP. Compared to diesel at identical pressure levels, HHO inductions are increasing. This is due to the additional hydrogen in HHO gas, which aids in enhancing burning effectiveness and increasing combustion temperature, resulting in better air-fuel integration. At 0.97 bar BMEP, the exhaust gas temperature was 135, 145, 150, 155, and 160 °C for ND, ND + E20, E20 + 1 LPM, E20 + 1.5 LPM, and E20 + 2 LPM, respectively. The exhaust gas temperatures for ND, ND + E20, E20 + 1 LPM, E20 + 1.5 LPM, and E20 + 2 LPM are 465, 475, 480, 485, and 490 °C at 5.02 bar BMEP. From all the flow rates, E20 + 1.5 LPM and E20 + 2 LPM result in a 2.06 and 3.06% increase in exhaust gas temperatures compared to E20 without adding HHO gas. This may be addressed by the high flame velocity of hydrogen in the gas mixture that led to the quick burning of the air-fuel mixture.
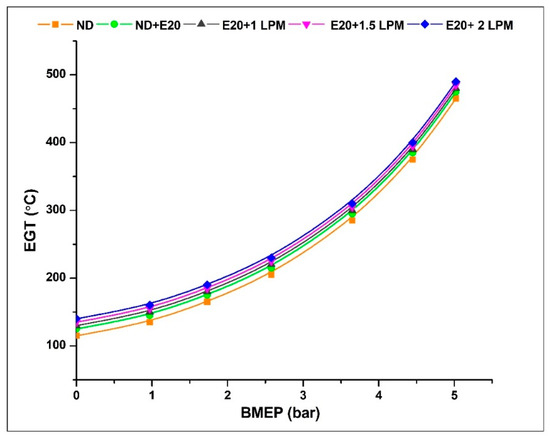
Figure 11.
Variations of EGT for 20% ethanol blend with the effect of hydroxy gas enrichment at three different LPM levels.
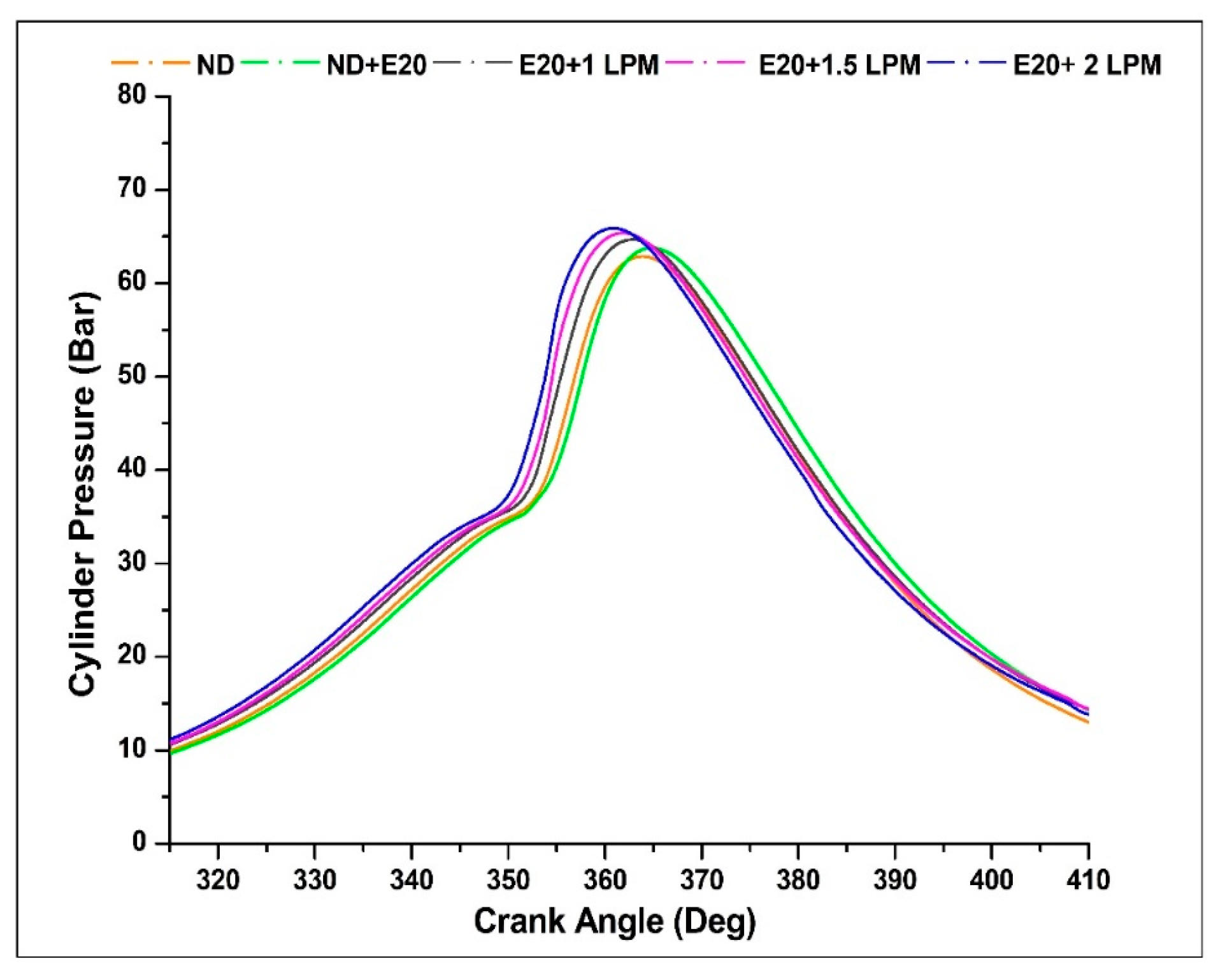
4.8. Cylinder Pressure
Depicted in Figure 12 are the cylinder pressure profiles for the engine operating on diesel oil with and without HHO enrichment across various crank angles, all under the rated engine speed of 1500 rpm. Introducing HHO gas results in a substantial augmentation of cylinder pressure values in contrast to non-HHO conditions. The peak pressure for diesel fuel without HHO stands at 62.93 bar, occurring at a crank angle of 365 °CA. Notably, the peak pressures for ND + E20, E20 + 1 LPM, E20 + 1.5 LPM, and E20 + 2 LPM registered as 63.83, 64.49, 65.43, and 65.93 bar, respectively, emerging within the crank angle range of 361 to 364 °CA. The integration of 2 LPM HHO gas enrichment led to amplified cylinder pressure when compared to the diesel-ethanol blend. This enhancement was progressive with the increase in HHO LPM levels. This enrichment strategy involves introducing hydrogen and additional oxygen gas into ethanol to participate in the combustion process. Given that the heating value of HHO gas is nearly 3.5-times greater than that of conventional diesel and biodiesel fuels, the combustion process with HHO-enriched blends releases more heat. This heightened heat release subsequently elevates the pressure generated within the combustion chamber. Furthermore, a minor advancement in the peak cylinder pressure timing was evident in compositions featuring HHO gas, potentially attributable to the swifter burning rate of hydrogen gas within the mixture [39].
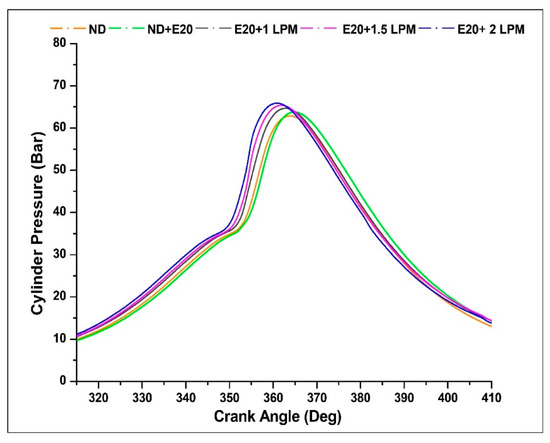
Figure 12.
Variations of CP for 20% ethanol blend with the effect of hydroxy gas enrichment at three different LPM levels.
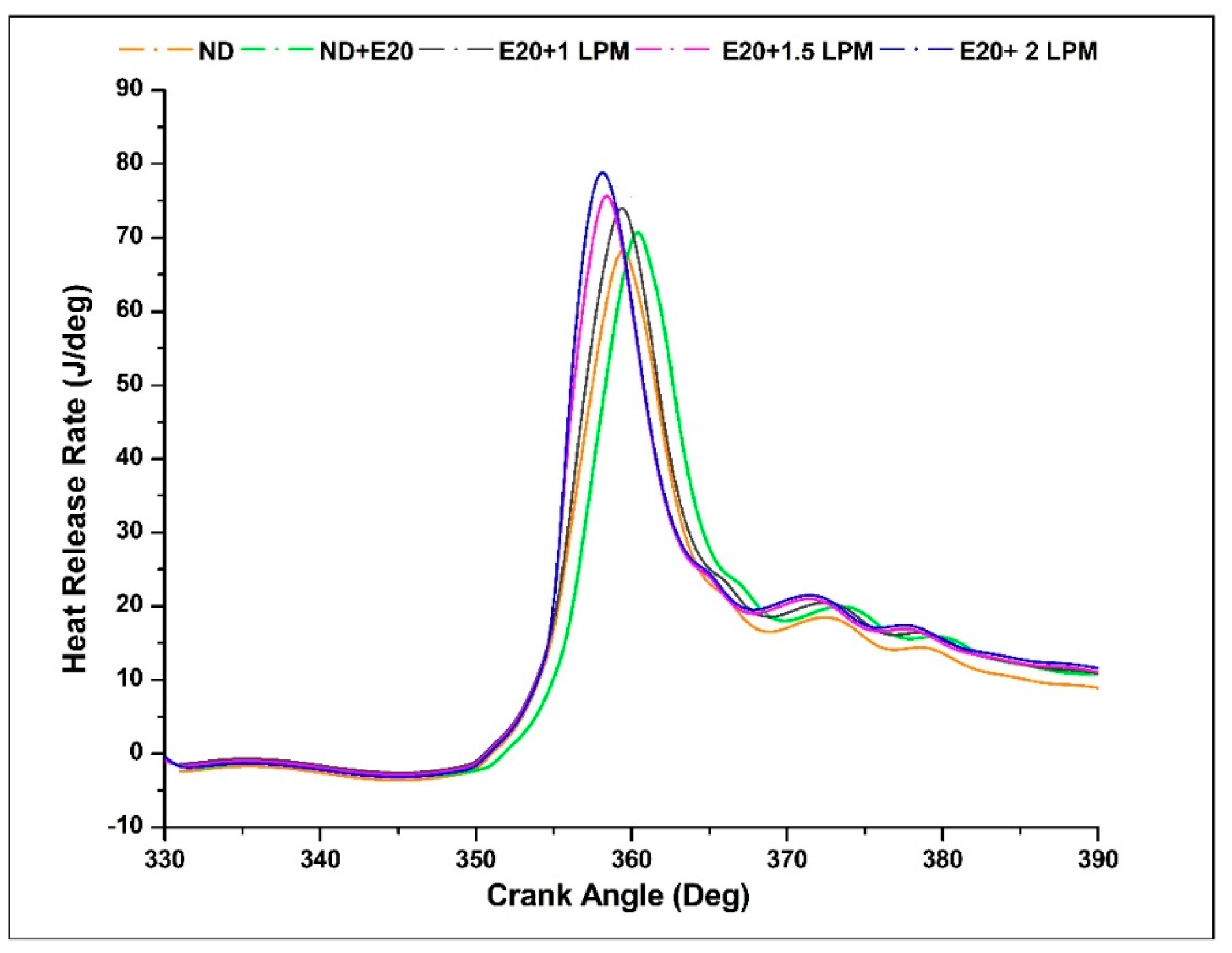
4.9. Heat Release Rate (HRR)
The Heat Release Rate (HRR) signifies the thermal energy liberated during the combustion of fuel per degree of crank angle. Displayed in Figure 13 is the depiction of HRR for the engine operating on diesel oil, both with and without HHO enrichment, across various crank angles. This was conducted at a BMEP of 5.05 bar and an engine speed of 1500 rpm. The integration of HHO gas led to an elevation in the peak HRR within the diesel-ethanol blend. The ignition of HHO facilitated a swifter combustion process owing to the heightened flame velocity of HHO gas. This phenomenon contributed to an accelerated constant-volume combustion, consequently resulting in a greater premixed HRR when compared to pure diesel. The remarkable combustion rate and rapid flame velocity inherent to hydrogen play a pivotal role in enhancing the overall combustion process and expediting the attainment of peak HRR values. The peak HRR values for ND, ND + E20, E20 + 1 LPM, E20 + 1.5 LPM, and E20 + 2 LPM are 72.23, 75.53, 76.83, 79.03, and 81.23 J/deg were achieved between 358 to 360 °CA. It was observed that more HHO gas was delivered during the injection stage, resulting in more fuel accessible for combustion and higher HRR due to higher exhaust gas temperature. Furthermore, hydrogen’s notably elevated Calorific Value (CV) characteristic translates into the release of heightened thermal energy during the combustion process. As a result, the incorporation of HHO gas leads to an increase in the peak Heat Release Rate (HRR) value. The maximum heat release rate is achieved with a flow rate of 2 LPM in conjunction with a 20% ethanol blend. In dual-fuel operation, a two-phase combustion pattern emerges. The initial phase involves the combustion of the diesel-ethanol mixture, while the subsequent phase encompasses the combustion of HHO gas via the ignition facilitated by the diesel-ethanol mixture. The presence of HHO gas within the intake air extends the ignition duration of diesel-ethanol blends, subsequently influencing the vaporisation of the diesel-ethanol blend within the cylinder. Consequently, the heat generation rate for HHO gas exhibits a more pronounced level during the premixed combustion phase and a comparatively lower rate during the diffusion combustion phase [40].
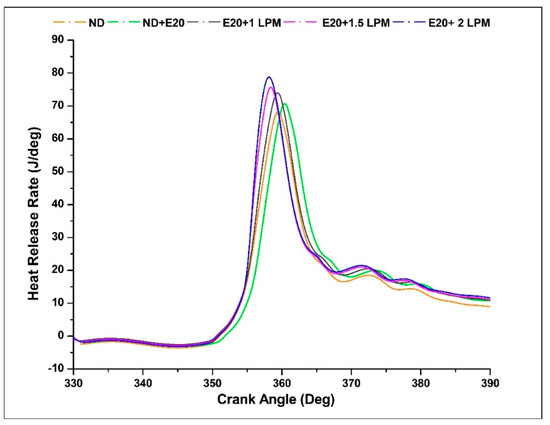
Figure 13.
Variations of HRR for 20% ethanol blend with the effect of hydroxy gas enrichment at three different LPM levels.
5. Conclusions
An experimental study employed a single-cylinder common rail direct injection diesel engine to assess the impacts of dual-fuel operation on combustion, emissions, and engine performance. Within this dual-fuel operational mode, the diesel engine was supplied with a blend of 20% volume ethanol-diesel, enriched with three separate flows of HHO gas at 1, 1.5, and 2 LPM. These experiments encompassed diverse load conditions ranging from 0 to 100%. The earlier-described findings stem from this experimental investigation.
- The SS316L plate-type dry cell electrolyser has been demonstrated as an effective device for producing HHO gas by on-board technique from a 20 g/L NaOH electrolytic catalyst solution.
- The incorporation of HHO gas led to noteworthy increases in both cylinder pressure and HRR values when compared to their respective counterparts. Under maximum BMEP conditions, the thermal brake efficiency experienced a notable enhancement of 2.74%. This was achieved using a 20% ethanol blend in conjunction with a consistent flow rate of 2 LPM of HHO gas, as compared to using only the 20% ethanol blend. Furthermore, there was a simultaneous decrease of 5.89% in Brake Specific Energy Consumption (BSEC), reducing it from 9245 to 8900 kJ/kW-h.
- At the peak BMEP condition, substantial reductions of 4.61%, 5.19%, and 3.1% were achieved in engine exhaust emissions, namely HC, CO, and smoke, through the utilization of E20 + 2 LPM HHO.
- The NOx emissions and EGT increased by 3.22% and 3.06% for E20 + 2 LPM compared to E20 at maximum BMEP condition.
- HHO gas induction demonstrated a successful way of enhancing performance and lowering diesel engine emissions. When combined with lower ethanol blends, this impact helped to reduce emission values further, and the experimental results indicate that adding 2 LPM of HHO gas to a 20% volume of ethanol-diesel blend improves engine performance, combustion, and emission characteristics.
- Future research will focus on optimizing electrolyser variables for efficient HHO gas production using plate-type dry cell electrolysers and optimizing engine process parameters for efficient dual-fuel operation with different combustion bowl geometries using machine learning techniques.
Author Contributions
Conceptualization, K.V.; Methodology, K.V.; Validation, D.I., M.I.T. and W.W.; Formal analysis, D.I. and M.I.T.; Data curation, D.S.; Writing—original draft, D.S.; Writing—review & editing, D.I.; Supervision, K.V. and W.W.; Project administration, K.V. and W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| BMEP | Brake Mean Effective Pressure (bar) |
| BSEC | Brake Specific Energy Consumption (kJ/kW-h) |
| BTE | Brake Thermal Efficiency (%) |
| CFM | Cubic Feet Per Minute |
| CO2 | Carbon dioxide |
| CO | Carbon monoxide (g/kW-h) |
| CP | Cylinder Pressure (bar) |
| CRDI | Common Rail Direct Injection |
| CI | Compression-Ignition |
| DC | Direct current |
| EGT | Exhaust Gas Temperature (°C) |
| E20 | Ethanol 20% with 80% of Neat Diesel |
| HC | Hydrocarbons (g/kW-h) |
| HHO | Hydroxy |
| HRR | Heat Release Rate (J/deg) |
| I.C. | Internal Combustion |
| LPM | Litre per minute |
| ND | Neat Diesel |
| NOx | Nitrogen oxides (g/kW-h) |
References
- The Outlook for Energy. A View to 2040. 2017. Available online: https://cdn.exxonmobil.com/media/global/files/outlook-for-energy/2017/2017-outlook-for-energy.pdf (accessed on 15 August 2019).
- Indian Natural Gas Petroleum & Natural Gas Statistics 2017–2018. 2019. Available online: https://petroleum.nic.in/more/Indi-an-png-statistics (accessed on 28 July 2019).
- Environmental Performance Index (EPI) 2018. Available online: https://www.google.com.hk/url?sa=i&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=0CAIQw7AJahcKEwjYwcvl_oiBAxUAAAAAHQAAAAAQAg&url=https%3A%2F%2Fepi.yale.edu%2Fdownloads%2Fepi2018policymakerssummaryv01.pdf&psig=AOvVaw3vNgFBT8zNdP0MobOfFlTW&ust=1693643179091482&opi=89978449 (accessed on 25 July 2019).
- Viswanathan, K.; Taipabu, M.I.; Wu, W. Novel Petit grain bitter orange waste peel oil biofuel investigation in diesel engine with modified fuel injection pressure and bowl geometry. Fuel 2022, 319, 123660. [Google Scholar] [CrossRef]
- Ganesan, N.; Viswanathan, K.; Karthic, S.; Ekambaram, P.; Wu, W.; Vo, D.-V.N. Split injection strategies based RCCI combustion analysis with waste cooking oil biofuel and methanol in an open ECU assisted CRDI engine. Fuel 2022, 319, 123710. [Google Scholar] [CrossRef]
- Viswanathan, K.; Paulraj, A. A comprehensive study on the performance and emission characteristics of a diesel engine with the blends of diesel, jojoba oil biodiesel, and butylated hydroxyl anisole as an alternative fuel. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 3216–3230. [Google Scholar] [CrossRef]
- Madhujit, D.; Abhishek, P.; Durbadal, D.; Sastry, G.R.K.; Panua, R.S.; Bose, P.K. An experimental investigation of performance-emission trade of characteristics of a CI engine using hydrogen as dual fuel. Energy 2015, 85, 569–585. [Google Scholar] [CrossRef]
- Yilmaz, A.C.; Uludamar, E.; Aydin, K. Effect of hydroxy (HHO) gas addition on performance and exhaust emissions in compression ignition engines. Int. J. Hydrogen Energy 2010, 35, 11366–11372. [Google Scholar] [CrossRef]
- Matienzo, J.M.R. Influence of addition of hydrogen produced on board in the performance of a stationary diesel engine. Int. J. Hydrogen Energy 2018, 43, 17889–17897. [Google Scholar] [CrossRef]
- Aydin, K.; Kenanoğlu, R. Effects of hydrogenation of fossil fuels with hydrogen and hydroxy gas on performance and emissions of internal combustion engines. Int. J. Hydrogen Energy 2018, 43, 14047–14058. [Google Scholar] [CrossRef]
- Ismail, T.M.; Ramzy, K.; Abelwhab, M.N.; Elnaghi, B.E.; Abd El-Salam, M.; Ismail, M.I. Performance of hybrid compression ignition engine using hydroxy (HHO) from dry cell. Energy Convers. Manag. 2018, 155, 287–300. [Google Scholar] [CrossRef]
- Masjuki, H.; Ruhul, A.; Mustafi, N.N.; Kalam, M.; Arbab, M.; Fattah, I.R. Study of production optimization and effect of hydroxyl gas on a CI engine performance and emission fueled with biodiesel blends. Int. J. Hydrogen Energy 2016, 41, 14519–14528. [Google Scholar] [CrossRef]
- Uludamar, E. Effect of hydroxy and hydrogen gas addition on diesel engine fuelled with microalgae biodiesel. Int. J. Hydrogen Energy 2018, 43, 18028–18036. [Google Scholar] [CrossRef]
- Baltacioglu, M.K.; Arat, H.T.; Özcanli, M.; Aydin, K. Experimental comparison of pure hydrogen and HHO (hydroxy) enriched biodiesel (B10) fuel in a commercial diesel engine. Int. J. Hydrogen Energy 2016, 41, 8347–8353. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Varuvel, E.; Karthickeyan, V.; Sonthalia, A.; Kumar, G.; Saravanan, C.; Dhinesh, B.; Pugazhendhi, A. Effect of hydrogen on compression-ignition (CI) engine fueled with vegetable oil/biodiesel from various feedstocks: A review. Int. J. Hydrogen Energy 2022, 47, 37648–37667. [Google Scholar] [CrossRef]
- Yilmaz, N.; Vigil, F.M.; Donaldson, A.B.; Darabseh, T. Investigation of CI engine emissions in biodiesel–ethanol–diesel blends as a function of ethanol concentration. Fuel 2014, 115, 790–793. [Google Scholar] [CrossRef]
- Parthasarathy, M.; Lalvani, J.I.J.; Dhinesh, B.; Annamalai, K. Effect of hydrogen on ethanol–biodiesel blend on performance and emission characteristics of a direct injection diesel engine. Ecotoxicol. Environ. Saf. 2016, 134, 433–439. [Google Scholar] [CrossRef]
- Prakash, T.; Geo, V.E.; Martin, L.J.; Nagalingam, B. Effect of ternary blends of bio-ethanol, diesel and castor oil on performance, emission and combustion in a CI engine. Renew. Energy 2018, 122, 301–309. [Google Scholar] [CrossRef]
- Taipabu, M.I.; Viswanathan, K.; Wu, W. Process design and optimization of green processes for the production of hydrogen and urea from glycerol. Int. J. Hydrogen Energy 2023, 48, 24212–24241. [Google Scholar] [CrossRef]
- Nabil, T.; Khairat Dawood, M.M. Enabling efficient use of oxy-hydrogen gas (HHO) in selected engineering applications; transportation and sustainable power generation. J. Clean. Prod. 2019, 237, 117798. [Google Scholar] [CrossRef]
- Hansen, A.C.; Zhang, Q.; Lyne, P.W. Ethanol–diesel fuel blends––A review. Bioresour. Technol. 2004, 96, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hong, G.; Huang, R. Numerical investigation to the dual-fuel spray combustion process in an ethanol direct injection plus gasoline port injection (EDI + GPI) engine. Energy Convers. Manag. 2015, 92, 275–286. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Boretti, A. Advances in hydrogen compression ignition internal combustion engines. Int. J. Hydrogen Energy 2011, 36, 12601–12606. [Google Scholar] [CrossRef]
- Madhankumar, S.; Viswanathan, K.; Wu, W. Energy, exergy and environmental impact analysis on the novel indirect solar dryer with fins inserted phase change material. Renew. Energy 2021, 176, 280–294. [Google Scholar] [CrossRef]
- Madhankumar, S.; Viswanathan, K. Computational and experimental study of a novel corrugated-type absorber plate solar collector with thermal energy storage moisture removal device. Appl. Energy 2022, 324, 119746. [Google Scholar] [CrossRef]
- Madhankumar, S.; Viswanathan, K.; Wu, W.; Taipabu, M.I. Analysis of indirect solar dryer with PCM energy storage material: Energy, economic, drying and optimization. Sol. Energy 2023, 249, 667–683. [Google Scholar] [CrossRef]
- Abdelaal, H.; Sadik, M.; Bassyouni, M.; Shalabi, M. A new approach to utilize Hydrogen as a safe fuel. Int. J. Hydrogen Energy 2005, 30, 1511–1514. [Google Scholar] [CrossRef]
- Geng, L.; Bi, L.; Li, Q.; Chen, H.; Xie, Y. Experimental study on spray characteristics, combustion stability, and emission performance of a CRDI diesel engine operated with biodiesel–ethanol blends. Energy Rep. 2021, 7, 904–915. [Google Scholar] [CrossRef]
- Verhelst, S.; Wallner, T. Hydrogen-fueled internal combustion engines. Prog. Energy Combust. Sci. 2009, 35, 490–527. [Google Scholar] [CrossRef]
- Samuel Panithasan, M.; Venkadesan, G. Evaluation of the Influence of 1, 4-Dioxane and Exhaust Gas Recirculation on the Performance and Emission Values of a Diesel Engine Fuelled with Low Viscous Biofuel Blend. In Proceedings of the ASME 2021 15th International Conference on Energy Sustainability Collocated with the ASME 2021 Heat Transfer Summer Conference, Virtual, 16–18 June 2021. ASME Digital Collection. [Google Scholar]
- Sharma, P.K.; Sharma, D.; Soni, S.L.; Jhalani, A.; Singh, D.; Sharma, S. Energy, exergy, and emission analysis of a hydroxyl fueled compression ignition engine under dual fuel mode. Fuel 2020, 265, 116923. [Google Scholar] [CrossRef]
- Gnanamoorthi, V.; Vimalananth, V. Effect of hydrogen fuel at higher flow rate under dual fuel mode in CRDI diesel engine. Int. J. Hydrogen Energy 2020, 45, 16874–16889. [Google Scholar] [CrossRef]
- Panithasan, M.S.; Gopalakichenin, D.; Venkadesan, G.; Malairajan, M. Evaluating the working characters of a diesel engine fueled with biodiesel blends added with rice husk Nano particles. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 1–19. [Google Scholar] [CrossRef]
- Jeyaseelan, T.; Chacko, N.; Pushyanth, N.; Alexander, J.; Porpatham, E. Partial hydrogenation and hydrogen induction: A comparative study with B20 operation in a turbocharged CRDI diesel engine. Int. J. Hydrogen Energy 2021, 46, 22659–22669. [Google Scholar] [CrossRef]
- Reşitoğlu, İ.A.; Altinişik, K.; Keskin, A. The pollutant emissions from diesel-engine vehicles and exhaust aftertreatment systems. Clean Technol. Environ. Policy 2015, 17, 15–27. [Google Scholar] [CrossRef]
- Sandalci, T.; Karagöz, Y. Experimental investigation of the combustion characteristics, emissions and performance of hydrogen port fuel injection in a diesel engine. Int. J. Hydrogen Energy 2014, 39, 18480–18489. [Google Scholar] [CrossRef]
- Rimkus, A.; Matijošius, J.; Bogdevičius, M.; Bereczky, Á.; Török, Á. An investigation of the efficiency of using O2 and H2 (hydrooxile gas-HHO) gas additives in a CI engine operating on diesel fuel and biodiesel. Energy 2018, 152, 640–651. [Google Scholar] [CrossRef]
- Li, G.; Long, Y.; Zhang, Z.; Liang, J.; Zhang, X.; Zhang, X.; Wang, Z. Performance and emissions characteristics of a lean-burn marine natural gas engine with the addition of hydrogen-rich reformate. Int. J. Hydrogen Energy 2019, 44, 31544–31556. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, H.; Liao, Y.; Nasif, O.; Alharbi, S.A.; Shanmugam, S.; Chi, N.T.L.; Shanmuganathan, R. Effects of hydrocarbon liquid and HHO as the alternate fuel for unmodified compression ignition engines. Fuel 2022, 324, 124726. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).