Characteristics of Explosion Hazards in Methane–Air Mixtures Diluted by Hydrogen
Abstract
:1. Introduction
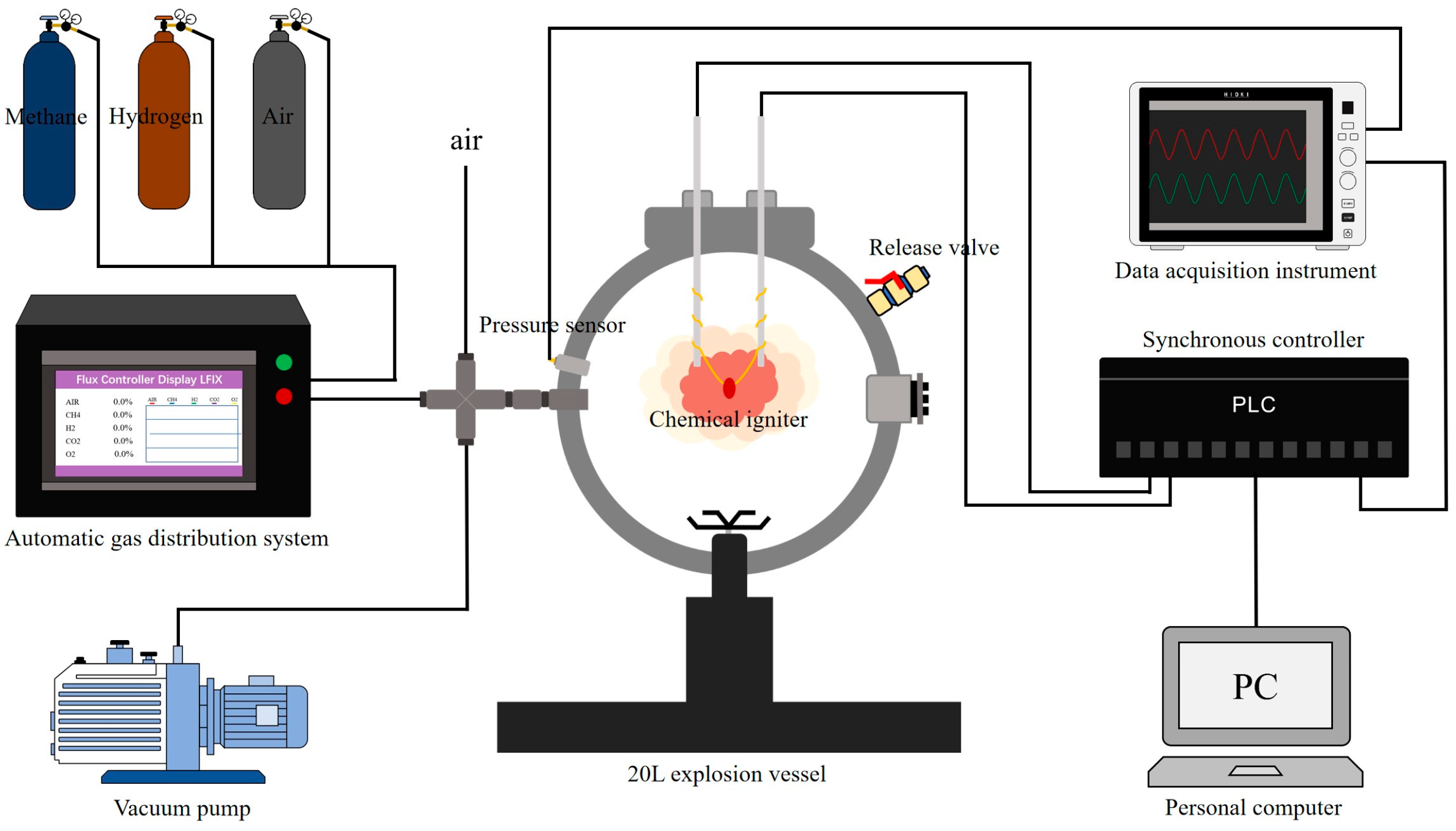
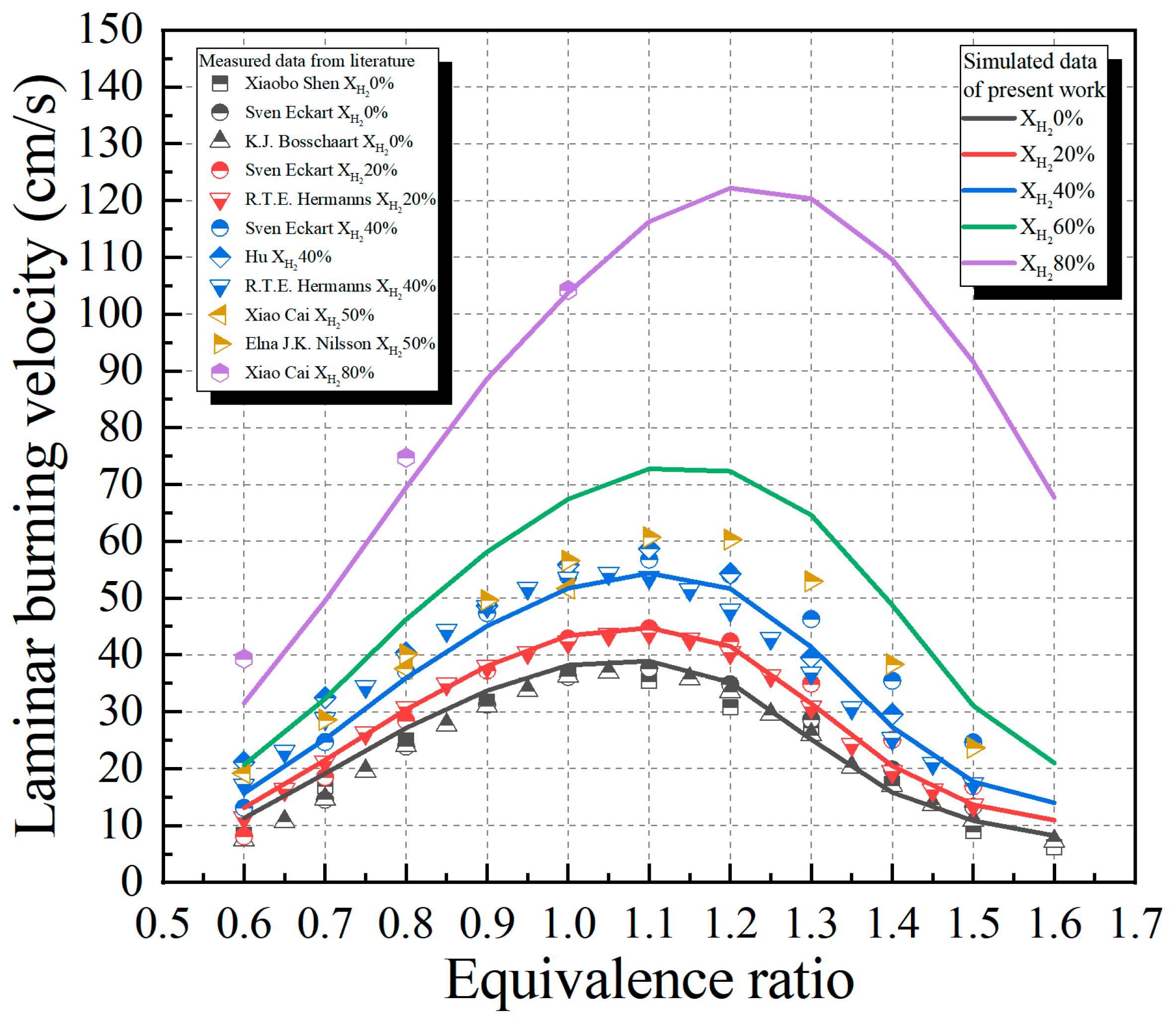
2. Experimental and Numerical Methods
3. Results and Discussion
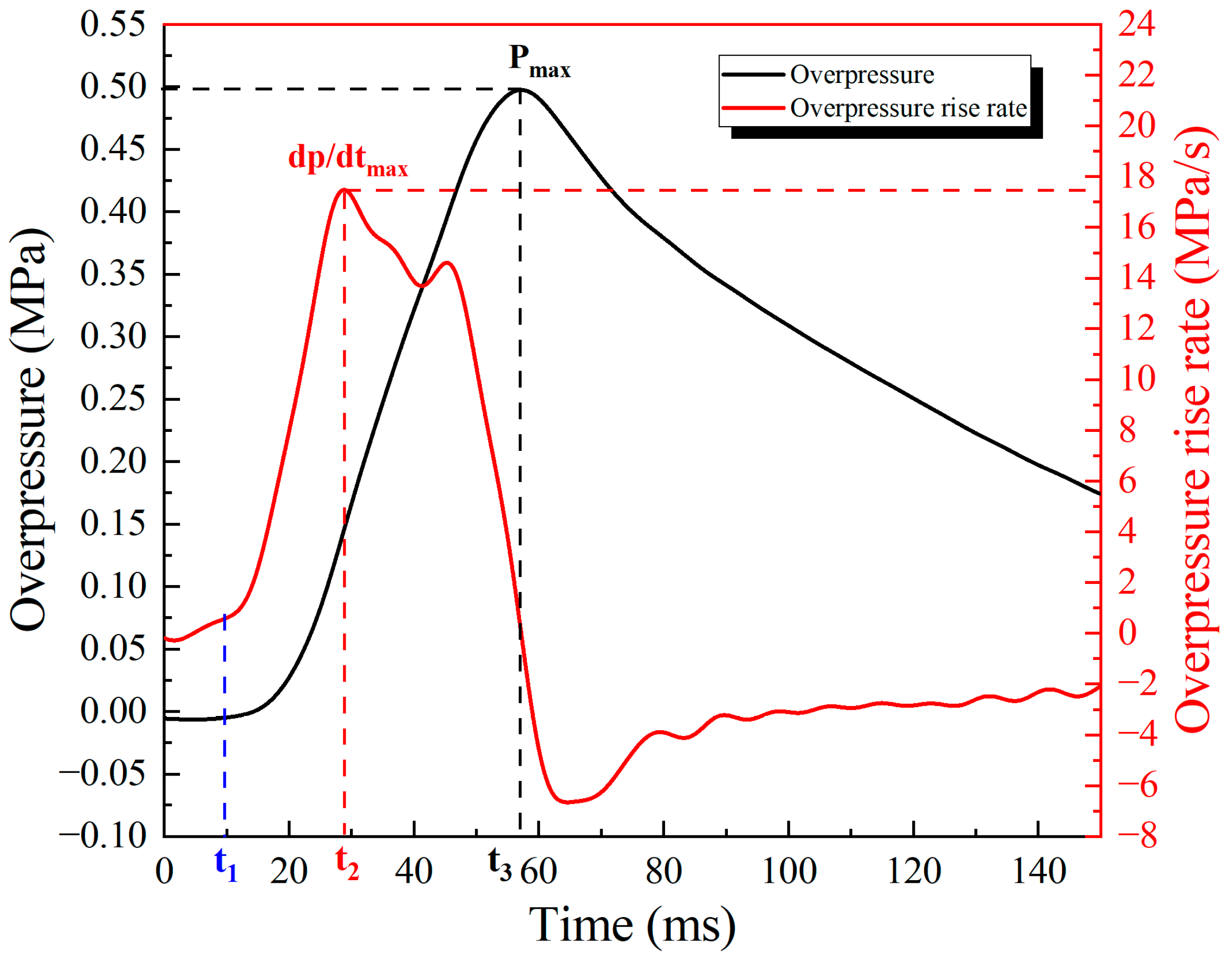
3.1. Experimental Results
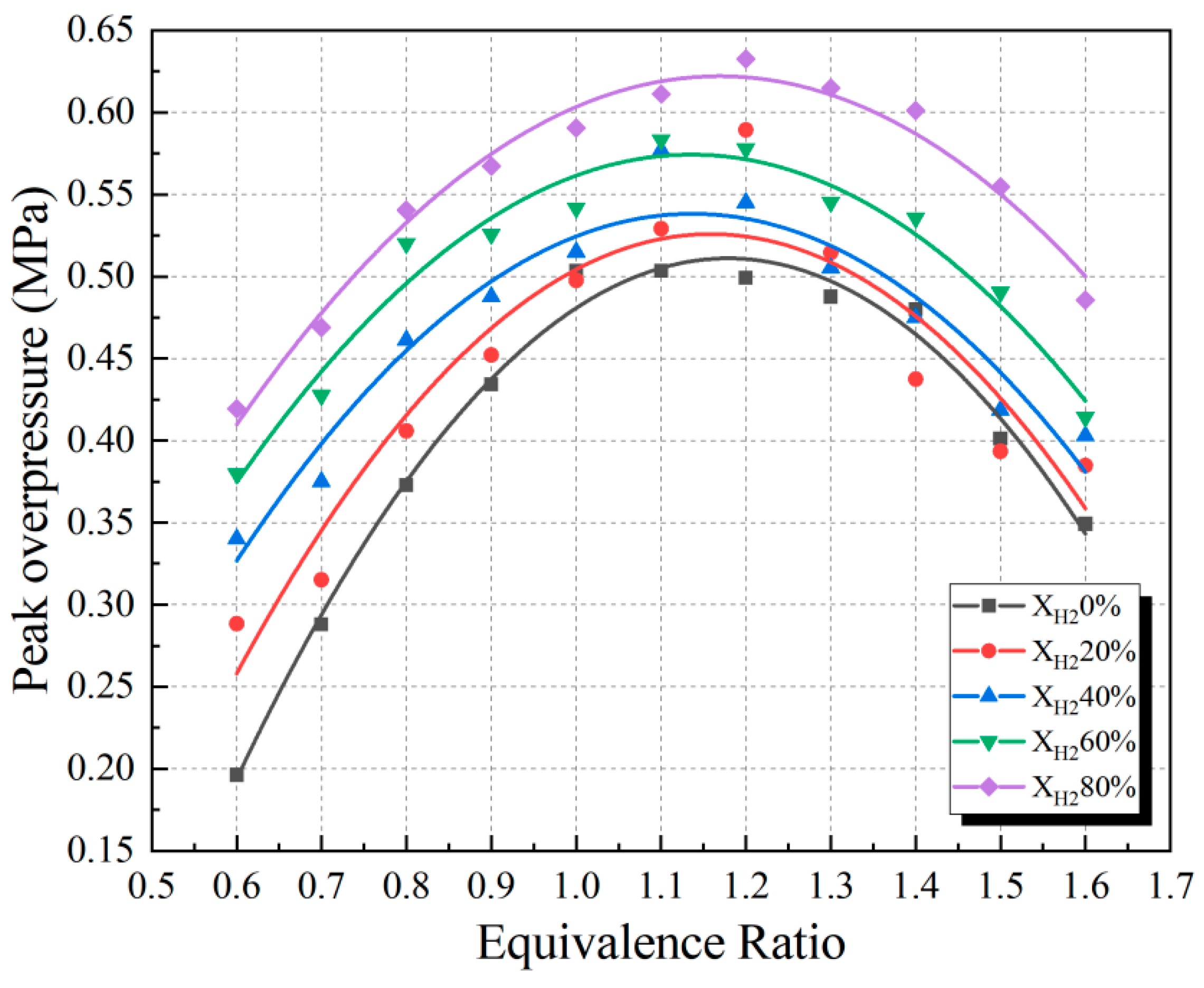
3.2. Effect of Hydrogen on the Peak Overpressure and Arrival Time of the Dangerous Moment of Premixed CH4/Air Combustion
3.3. Effect of Hydrogen on Explosion Hazards under Lean and Rich Combustion Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, T.; Chen, X.; Cheng, F.; Lu, K.; Shi, X.; Yu, W. Study on the synergistic inhibition mechanism of multicomponent powders on methane explosions. Powder Technol. 2023, 418, 118326. [Google Scholar] [CrossRef]
- Mitu, M.; Razus, D.; Schroeder, V. Laminar Burning Velocities of Hydrogen-Blended Methane–Air and Natural Gas–Air Mixtures, Calculated from the Early Stage of p(t) Records in a Spherical Vessel. Energies 2021, 14, 7556. [Google Scholar] [CrossRef]
- Liu, A.; Lu, X.; Zhou, X.; Xu, C.; Liang, X.; Xiong, K. Experimental investigation on suppression of methane explosion using KHCO3/zeolite composite powder. Powder Technol. 2023, 415, 118157. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, X.; Luo, Z.; Cheng, F.; Lu, K.; Shi, X.; Yu, W. Effect of N2 inerting on the inhibition of methane explosions by a multicomponent powder. Fuel 2023, 337, 127203. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Dou, K.; Luo, Z.-M.; Cheng, F.-M.; Xue, H.-L. Kinetic model of methane explosion in a spheroidal explosion tank. IOP Conf. Ser. Earth Environ. Sci. 2021, 647, 012039. [Google Scholar] [CrossRef]
- Dong, W.; Hu, J.; Xiang, L.; Chu, H.; Li, Z. Numerical Investigation on Combustion Characteristics of Laminar Premixed n-Heptane/Hydrogen/Air Flames at Elevated Pressure. Energy Fuels 2020, 34, 14768–14775. [Google Scholar] [CrossRef]
- Sun, Z.-Y.; Li, G.-X. Turbulence influence on explosion characteristics of stoichiometric and rich hydrogen/air mixtures in a spherical closed vessel. Energy Convers. Manag. 2017, 149, 526–535. [Google Scholar] [CrossRef]
- Wang, D.; Ji, C.; Wang, S.; Yang, J.; Tang, C. Experimental investigation on near wall ignited lean methane/hydrogen/air flame. Energy 2019, 168, 1094–1103. [Google Scholar] [CrossRef]
- Chu, H.; Ren, F.; Xiang, L.; Dong, S.; Qiao, F.; Xu, G. Numerical investigation on combustion characteristics of laminar premixed n-heptane/air flames at elevated initial temperature and pressure. J. Energy Inst. 2019, 92, 1821–1830. [Google Scholar] [CrossRef]
- Shen, X.; Xu, J.; Wen, J.X. Phenomenological characteristics of hydrogen/air premixed flame propagation in closed rectangular channels. Renew. Energy 2021, 174, 606–615. [Google Scholar] [CrossRef]
- Dai, T.; Zhang, B.; Liu, H. On the explosion characteristics for central and end-wall ignition in hydrogen-air mixtures: A comparative study. Int. J. Hydrogen Energy 2021, 46, 30861–30869. [Google Scholar] [CrossRef]
- Li, Y.; Bi, M.; Gao, W.; Cong, H.; Li, B. Self-Acceleration and Self-Similarity of Hydrogen–Methane–Air Flame at Elevated Pressure. Combust. Sci. Technol. 2019, 193, 1005–1021. [Google Scholar] [CrossRef]
- Shahi, R.R.; Tiwari, A.P.; Shaz, M.A.; Srivastava, O.N. Studies on de/rehydrogenation characteristics of nanocrystalline MgH2 co-catalyzed with Ti, Fe and Ni. Int. J. Hydrogen Energy 2013, 38, 2778–2784. [Google Scholar] [CrossRef]
- Okolie, J.A.; Patra, B.R.; Mukherjee, A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Futuristic applications of hydrogen in energy, biorefining, aerospace, pharmaceuticals and metallurgy. Int. J. Hydrogen Energy 2021, 46, 8885–8905. [Google Scholar] [CrossRef]
- Zheng, L.; Zhu, X.; Wang, Y.; Li, G.; Yu, S.; Pei, B.; Wang, Y.; Wang, W. Combined effect of ignition position and equivalence ratio on the characteristics of premixed hydrogen/air deflagrations. Int. J. Hydrogen Energy 2018, 43, 16430–16441. [Google Scholar] [CrossRef]
- Cao, W.; Li, W.; Yu, S.; Zhang, Y.; Shu, C.-M.; Liu, Y.; Luo, J.; Bu, L.; Tan, Y. Explosion venting hazards of temperature effects and pressure characteristics for premixed hydrogen-air mixtures in a spherical container. Fuel 2021, 290, 120034. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, X.; Wen, J.X.; Xiu, G. The behavior of methane/hydrogen/air premixed flame in a closed channel with inhibition. Fuel 2020, 265, 116810. [Google Scholar] [CrossRef]
- Xiao, H.; Mao, Z.; An, W.; Sun, J. Experimental and LES investigation of flame propagation in a hydrogen/air mixture in a combustion vessel. Chin. Sci. Bull. 2014, 59, 2496–2504. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, T.; Li, Y.; Zhang, T.; Li, X.; Zhang, Z.; Shang, S.; Zhou, Y.; Zhang, C.; Chen, X.; et al. Effect of ignition, initial pressure and temperature on the lower flammability limit of hydrogen/air mixture. Int. J. Hydrogen Energy 2022, 47, 15107–15119. [Google Scholar] [CrossRef]
- Hao, Q.; Luo, Z.; Wang, T.; Xie, C.; Zhang, S.; Bi, M.; Deng, J. The flammability limits and explosion behaviours of hydrogen-enriched methane-air mixtures. Exp. Therm. Fluid Sci. 2021, 126, 110395. [Google Scholar] [CrossRef]
- Emami, S.D.; Rajabi, M.; Hassan, C.R.C.; Hamid, M.D.A.; Kasmani, R.M.; Mazangi, M. Experimental study on premixed hydrogen/air and hydrogen–methane/air mixtures explosion in 90 degree bend pipeline. Int. J. Hydrogen Energy 2013, 38, 14115–14120. [Google Scholar] [CrossRef]
- Li, Y.; Bi, M.; Li, B.; Zhou, Y.; Gao, W. Effects of hydrogen and initial pressure on flame characteristics and explosion pressure of methane/hydrogen fuels. Fuel 2018, 233, 269–282. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Q.; Chen, J.; Huang, Y.; Shi, Y. Effects of hydrogen on combustion characteristics of methane in air. Int. J. Hydrogen Energy 2014, 39, 11291–11298. [Google Scholar] [CrossRef]
- Coppens, F.H.V.; De Ruyck, J.; Konnov, A.A. Effects of hydrogen enrichment on adiabatic burning velocity and NO formation in methane+air flames. Exp. Therm. Fluid Sci. 2007, 31, 437–444. [Google Scholar] [CrossRef]
- Di Sarli, V.; Benedetto, A.D. Laminar burning velocity of hydrogen–methane/air premixed flames. Int. J. Hydrogen Energy 2007, 32, 637–646. [Google Scholar] [CrossRef]
- Hu, E.; Huang, Z.; He, J.; Zheng, J.; Miao, H. Measurements of laminar burning velocities and onset of cellular instabilities of methane–hydrogen–air flames at elevated pressures and temperatures. Int. J. Hydrogen Energy 2009, 34, 5574–5584. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Y.; Zeng, K.; Liu, B.; Wang, Q.; Jiang, D. Measurements of laminar burning velocities for natural gas–hydrogen–air mixtures. Combust. Flame 2006, 146, 302–311. [Google Scholar] [CrossRef]
- Ueda, A.; Nisida, K.; Matsumura, Y.; Ichikawa, T.; Nakashimada, Y.; Endo, T.; Kim, W. Effects of hydrogen and carbon dioxide on the laminar burning velocities of methane–air mixtures. J. Energy Inst. 2021, 99, 178–185. [Google Scholar] [CrossRef]
- Ren, F.; Chu, H.; Xiang, L.; Han, W.; Gu, M. Effect of hydrogen addition on the laminar premixed combustion characteristics the main components of natural gas. J. Energy Inst. 2019, 92, 1178–1190. [Google Scholar] [CrossRef]
- Hu, E.; Huang, Z.; He, J.; Miao, H. Experimental and numerical study on lean premixed methane–hydrogen–air flames at elevated pressures and temperatures. Int. J. Hydrogen Energy 2009, 34, 6951–6960. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.; Tang, C.; Miao, H.; Wang, X. Numerical study of the effect of hydrogen addition on methane–air mixtures combustion. Int. J. Hydrogen Energy 2009, 34, 1084–1096. [Google Scholar] [CrossRef]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W.C. GRI-Mech 3.0. Available online: http://combustion.berkeley.edu/gri-mech/version30/text30.html (accessed on 25 December 2022).
- Sieradzka, M.; Rajca, P.; Zajemska, M.; Mlonka-Mędrala, A.; Magdziarz, A. Prediction of gaseous products from refuse derived fuel pyrolysis using chemical modelling software—Ansys Chemkin-Pro. J. Clean. Prod. 2020, 248, 119277. [Google Scholar] [CrossRef]
- Bougrine, S.; Richard, S.; Nicolle, A.; Veynante, D. Numerical study of laminar flame properties of diluted methane-hydrogen-air flames at high pressure and temperature using detailed chemistry. Int. J. Hydrogen Energy 2011, 36, 12035–12047. [Google Scholar] [CrossRef]
- Khan, A.R.; Ravi, M.R.; Ray, A. Experimental and chemical kinetic studies of the effect of H2 enrichment on the laminar burning velocity and flame stability of various multicomponent natural gas blends. Int. J. Hydrogen Energy 2019, 44, 1192–1212. [Google Scholar] [CrossRef]
- Wang, J.; Liang, Y.; Tian, F.; Chen, C. A numerical study on the effect of CO2 addition for methane explosion reaction kinetics in confined space. Sci. Rep. 2021, 11, 20733. [Google Scholar] [CrossRef]
- Chemkin Overview. Volume 4. 2015. Available online: https://www.reactiondesign.com/products/chemkin (accessed on 23 December 2022).
- Takizawa, K.; Takahashi, A.; Tokuhashi, K.; Kondo, S.; Sekiya, A. Burning velocity measurement of fluorinated compounds by the spherical-vessel method. Combust. Flame 2005, 141, 298–307. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Gao, W. Experimental research on unconfined hydrogen explosion of different gas scale and the overpressure prediction method. Int. J. Hydrogen Energy 2023, 48, 30985–30996. [Google Scholar] [CrossRef]
- Shen, X.; Xiu, G.; Wu, S. Experimental study on the explosion characteristics of methane/air mixtures with hydrogen addition. Appl. Therm. Eng. 2017, 120, 741–747. [Google Scholar] [CrossRef]
- Eckart, S.; Pizzuti, L.; Fritsche, C.; Krause, H. Experimental study and proposed power correlation for laminar burning velocity of hydrogen-diluted methane with respect to pressure and temperature variation. Int. J. Hydrogen Energy 2022, 47, 6334–6348. [Google Scholar] [CrossRef]
- Bosschaart, K.J.; de Goey, L.P.H. The laminar burning velocity of flames propagating in mixtures of hydrocarbons and air measured with the heat flux method. Combust. Flame 2004, 136, 261–269. [Google Scholar] [CrossRef]
- Hermanns, R.T.E.; Konnov, A.A.; Bastiaans, R.J.M.; de Goey, L.P.H.; Lucka, K.; Köhne, H. Effects of temperature and composition on the laminar burning velocity of CH4+ H2+ O2+ N2 flames. Fuel 2010, 89, 114–121. [Google Scholar] [CrossRef]
- Hu, E.; Huang, Z.; He, J.; Jin, C.; Zheng, J. Experimental and numerical study on laminar burning characteristics of premixed methane–hydrogen–air flames. Int. J. Hydrogen Energy 2009, 34, 4876–4888. [Google Scholar] [CrossRef]
- Cai, X.; Wang, J.; Bian, Z.; Zhao, H.; Zhang, M.; Huang, Z. Self-similar propagation and turbulent burning velocity of CH4/H2/air expanding flames: Effect of Lewis number. Combust. Flame 2020, 212, 1–12. [Google Scholar] [CrossRef]
- Nilsson, E.J.K.; van Sprang, A.; Larfeldt, J.; Konnov, A.A. The comparative and combined effects of hydrogen addition on the laminar burning velocities of methane and its blends with ethane and propane. Fuel 2017, 189, 369–376. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yu, D.; Li, P.; Sun, X.; Chen, X. Characteristics of Explosion Hazards in Methane–Air Mixtures Diluted by Hydrogen. Energies 2023, 16, 6416. https://doi.org/10.3390/en16186416
Liu J, Yu D, Li P, Sun X, Chen X. Characteristics of Explosion Hazards in Methane–Air Mixtures Diluted by Hydrogen. Energies. 2023; 16(18):6416. https://doi.org/10.3390/en16186416
Chicago/Turabian StyleLiu, Jiajia, Danyang Yu, Ping Li, Xuxu Sun, and Xianfeng Chen. 2023. "Characteristics of Explosion Hazards in Methane–Air Mixtures Diluted by Hydrogen" Energies 16, no. 18: 6416. https://doi.org/10.3390/en16186416
APA StyleLiu, J., Yu, D., Li, P., Sun, X., & Chen, X. (2023). Characteristics of Explosion Hazards in Methane–Air Mixtures Diluted by Hydrogen. Energies, 16(18), 6416. https://doi.org/10.3390/en16186416






