1. Introduction
Despite the gradual transition of the world from fossil fuels to renewables, it is expected that oil and gas will still be used for many decades, particularly in sectors that are difficult to decarbonize, such as the aviation sector [
1]. A significant portion of oil and gas is produced on offshore platforms, particularly in countries like Norway. But most offshore oil and gas platforms (OOGPs), especially those more than 100 km from shore, are usually powered by single-cycle gas turbines (GT), which have low efficiency and contribute about 80–85% of the platforms’ carbon emissions [
2,
3]. These OOGPs are not electrified with power from shore either because it is technically or economically not feasible, which leads to high carbon emissions for OOGPs. Consequently, many economies in Europe have taken an approach to decarbonizing the operation of OOGPs by using the carbon emission tax or emission trading system to encourage fewer carbon emissions [
4,
5]. Thus, operators of OOGPs are considering various means to reduce their carbon emissions.
Battery energy storage systems (BESS) have inevitably a significant role to play in carbon emissions reduction. In particular, the decrease in the cost of batteries makes them an attractive choice for various grid services in an OOGP [
6]. BESS can be sized to provide various grid services aimed at carbon emissions reduction, such as spinning reserve, which allows one GT to shut down permanently, leading to high carbon emissions reduction. BESS can also be sized to provide peak shaving and wind power capacity firming.
An OOGP usually has 3 to 4 GTs, and in most cases, 2 to 3 GTs are running while the last GT is for redundancy. The load on an OOGP can be in the range of 20–100 MW. Thus, the energy requirement for these services can easily enter the MWh range. However, before the BESS is sized, there is a need for a technology suitability assessment (TSA) of the available battery chemistries to be able to make the best choice of battery technology.
The OOGP presents unique challenges for the integration of BESS, and these need to be carefully considered in the TSA of the battery chemistries. The most critical factors that need to be considered include the volumetric energy density, the gravimetric energy density, and the safety of the battery chemistry. The OOGP has limited allowance for space and weight; thus, a battery chemistry with high energy densities is preferred. Also, the battery chemistry must be very safe, as the OOGP is a highly flammable environment. Other important factors include cost per kWh, lifespan, cycle life, and maintenance requirements. The OOGP is a difficult place to access as access depends on the availability of a favorable weather window, which does not occur often in offshore locations. Thus, a battery chemistry that requires little or no maintenance is ideal for this application. Also, a long cycle life and lifespan are desired in order to reduce recurrent replacements (again due to limited access) on the platform. All these factors need to be considered in a proper TSA procedure to determine the most suitable battery chemistry.
Chapaloglou et al. sized BESS for increasing wind power penetration levels and carbon emissions reduction in an OOGP but did not consider the choice of battery chemistry [
7]. Ardal et al. sized BESS for spinning reserve but only considered three battery chemistries [
8]. Also, only weight and maintenance requirements were considered. Farhadi et al. performed TSA, but this was done for high-power ES only, and energy-intensive energy storage (ES) was not considered [
9]. Adeyemo et al. proposed a 7-step procedure for performing the TSA of ES for OOGP [
10], but the TSA procedure was only applied to high-power ES. Similar to step 7 of the TSA procedure in [
10], Oliveira et al. assessed the suitability of various ES technologies for application in a micro-grid by creating a table with scores for energy density, power density, life span, safety, and financial feasibility [
11], but they did not specifically address the suitability of ES for offshore micro-grids, which have more stringent constraints on power and energy density and safety. Georgious et al. performed a review of ES systems and classified them based on physical form and also based on discharge duration [
12]. They noted that the classification is an initial guide to selecting the appropriate ES system for a given application. They also provided updated data on various characteristics of an ES, which is needed for selecting the most suitable ES. However, they did not consider the critical constraints of offshore deployment, which require a holistic estimation of the weight and space that an ES will require, i.e., estimating the weight and space of the ES and all associated ES components. They also did not review the operational experience, safety, or cost of the ES. Mutarraf et al. reviewed ES systems for shipboard microgrids, which have similar issues with offshore platforms but failed to capture a broader list of ES technologies [
13]. To the best of the authors’ knowledge, the review of the current state-of-the-art of high-energy BESS as it relates to the selection of the most suitable ES technology for OOGP applications has not yet been addressed in the literature. Thus, this work seeks to fill that gap and provide a comprehensive review of the current state-of-the-art of battery chemistry suitable for high-energy applications on OOGPs. The review in this paper provides relevant information that can be used to determine the most suitable battery chemistry for peak shaving and spinning reserve in OOGPs. This paper goes one step further by improving the 7-step procedure proposed by Adeyemo et al. in [
10] and applying it to two case studies.
The battery chemistries that are considered in detail in this work include Li-ion (lithium iron phosphate (LFP), lithium titanate (LTO), lithium nickel manganese cobalt oxide (NMC)), nickel cadmium (NiCd), nickel metal-hydride (NiMH), sodium sulfur (NaS), sodium nickel chloride (ZEBRA), flow batteries (vanadium redox (VRB) and zinc bromine (ZnBr)), metal-air (zinc air), and lithium-ion capacitor.
The main contributions of this paper are:
- -
Review of the current state-of-the-art of consolidated and emerging battery chemistries
- -
Overview of the application of nine battery chemistries for grid-scale services in offshore and onshore locations
- -
Improvement of the 7-step procedure proposed in [
10] by including a coefficient of variation to make the computation of the TSA weighted score as objective as possible. More score points are also used to make the TSA-weighted score more accurate.
- -
Application of the modified 7-step procedure to two case studies (peak shaving and spinning reserve) based on a real OOGP in the North Sea
The structure of this paper is as follows: before the review of the battery chemistries, the technology suitability assessment procedure proposed in [
10] is briefly introduced in
Section 2.
Section 3 presents the typical energy-intensive grid services that BESS can provide.
Section 4 reviews the state-of-the-art in battery chemistry.
Section 5 reviews the operational experience in battery chemistry.
Section 6 presents a case study where ES sizing, weight and space estimation, life cycle cost analysis, and the calculation of a weighted TSA score are done.
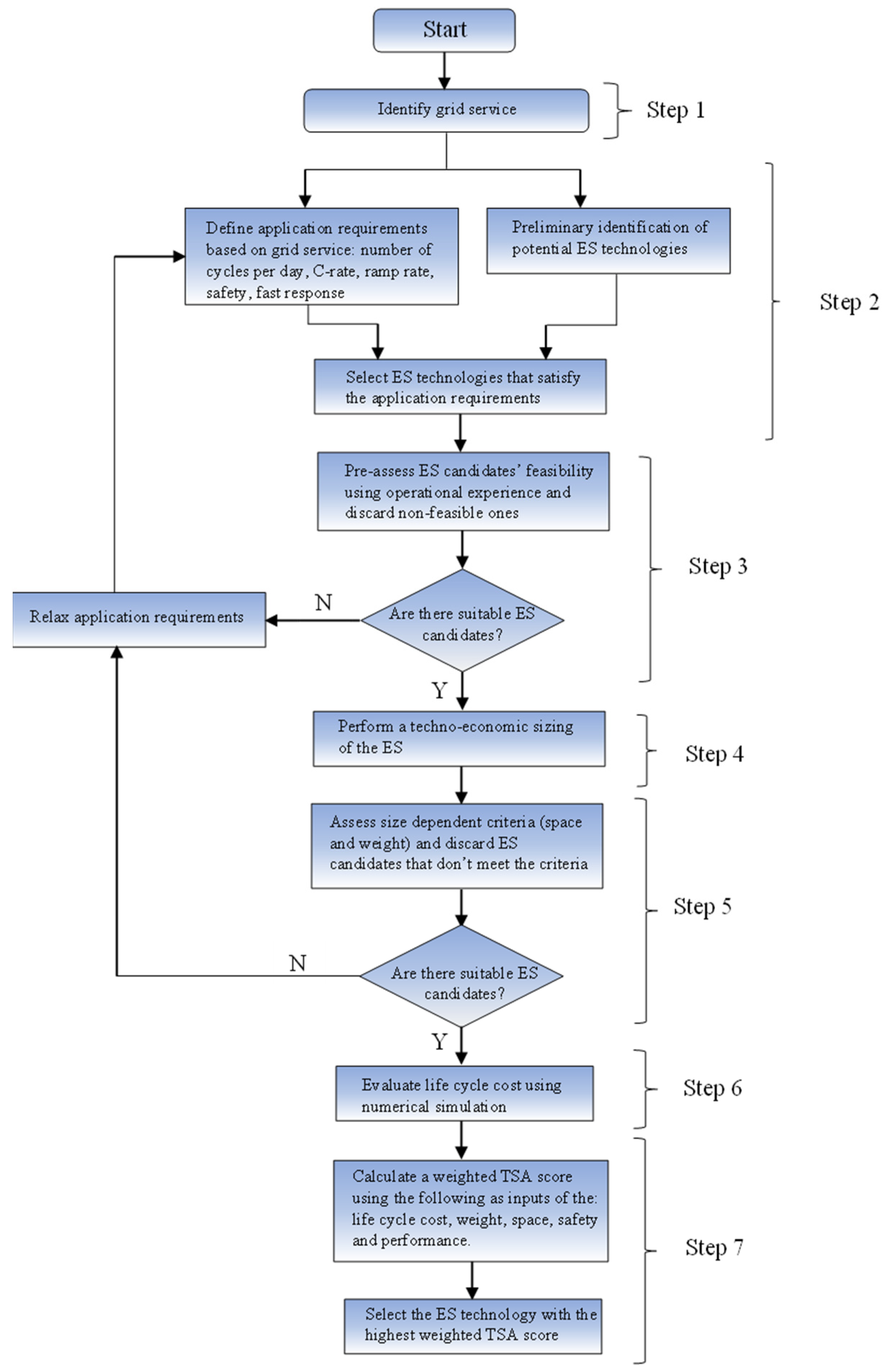
2. Technology Suitability Assessment Procedure
The technology suitability assessment procedure is a systematic way of selecting the most suitable ES technology (battery chemistry) based on some criteria. The considered TSA procedure was presented in [
10] and is here recalled for the sake of completeness. It consists of seven steps.
2.1. Step 1: Identification of the Grid Service
The grid service to be offered by the ES is identified. This is used to determine the application requirements in step 2.
2.2. Step 2: Choose the Potential ES Technologies That Satisfy the Application Requirements Imposed by the Grid Service from a Preliminary List of ES Technologies
This step entails the identification of application requirements based on the grid service to be offered by the ES. A list of ES technologies that satisfy these application requirements is then defined.
2.2.1. Application Requirements
These are characteristics that the ES must possess to be able to deliver the grid service being considered. They could include:
- -
Number of cycles per day: This is the average number of full cycles that the ES must deliver per day. This has huge significance for the choice of ES technology. If the application requires a high number of cycles per day, then an ES technology with a low life cycle is not suitable.
- -
Safety: This is the safety requirement for the ES technology. Given that an OOGP is a highly flammable environment, safety is very important, as the risk of fire or thermal runaway in an ES cannot be tolerated. Thus, an ES technology of high safety must be chosen if the application environment is an OOGP.
- -
C-rate: C-rate is a term used to define the maximum current a battery can deliver. For example, a 1 Ah battery rated at 30 C can deliver 30 A. The C-rate of the application determines the ES technologies that are suitable. For example, spinning reserve requires a high C-rate (greater than 1 C) battery, while peak shaving requires a low C-rate (less than or equal to 1 C) battery. Some battery chemistries can only deliver a low C-rate, while others can deliver a high C-rate.
- -
Fast response: This refers to how quickly an ES can start delivering power when called upon. If the application demands fast response, an ES technology with fast response must be selected. For example, black start is a service that demands fast response [
14], so an ES such as flow batteries with slow response will not be suitable for this kind of grid service.
- -
Ramp rate: Ramp rate is how quickly an ES can increase or decrease power once it is operating. The ramp rate required by the grid service also determines which ES technologies are suitable.
- -
Life span: The project life determines the life span that is required from the battery chemistry, as replacement operations are discouraged.
2.2.2. Identification of Potential ES Technologies
A broad preliminary list of ES technologies is defined. From this list, the ES technologies that satisfy the application requirements are selected as potential candidates.
2.3. Step 3: Selection of Potential ES Technologies Using Operational Experience
From the list of ES technologies that satisfy the application requirements in Step 2, potential ES technologies are further selected using proven operational experience. Operational experience with ES technologies in marine environments, proximal fields, and grid-scale applications demonstrates the techno-economic feasibility of their application. The ES technologies that satisfy the application requirements and have good operational experience are selected as potential candidate solutions.
After the ES technologies have been selected based on application requirements and operational experience, the required ES size is estimated.
2.4. Step 4: Techno-Economic Sizing of the ES
The nominal power and nominal energy of the ES are estimated in this step. This is needed to determine the ES’s weight, space, and life cycle cost.
2.5. Step 5: Estimation of ES Size Dependent Constraints
After the size of the ES has been determined, the two main criteria that must be satisfied (i.e. weight and space) are assessed.
- -
Space: In an OOGP, there is limited space. Thus, the overall footprint of the ES and all associated components (balance of plant) must be less than a certain value. Thus, it is important to use an ES with a high volumetric energy density in energy-intensive applications such as peak shaving. For other grid services requiring high power and high energy, such as spinning reserve, an ES with a high volumetric power density (high C-rate) and a high volumetric energy density should be used to limit the space required.
- -
Weight: Just as with space, there is limited weight that can be supported in an OOGP. The overall weight of the ES must not exceed a certain value. An ES with a high gravimetric energy density must be used for energy-intensive applications such as peak shaving. For other grid services requiring high power and high energy, such as spinning reserve, an ES with a high gravimetric power density (high C-rate) and a high gravimetric energy density needs to be used.
2.6. Step 6: ES Cost Assessment
After the potential ES technologies have been selected based on application requirements, operational experience, and weight and space constraints, the ES life cycle cost is estimated. The size of the ES determined in step 4 is used to estimate the life cycle cost.
2.7. Step 7: Decision Making on the Most Suitable ES Technology Using a Weighted TSA Score
After the ES life cycle cost has been estimated, a weighted TSA score is computed, and the ES with the highest weighted TSA score is selected. The criteria considered in the weighted TSA score for our use case are: weight, space, safety, performance, and life cycle cost. This weighted TSA score is calculated in
Section 6.4.4 and
Section 6.5.4. A coefficient of variation is introduced in this step different from the method in [
10]. The coefficient of variation is used to assign weight to the ES attributes. The use of the coefficient of variation allows the assignment of scores to the ES to be as objective as possible. The coefficient of variation is explained in
Section 6.4.4.
5. Operational Experiences and Practical Deployments in Offshore Environment and Proximal Fields
In this section, the operational experience and practical deployment of the various battery chemistries in offshore environments are specifically reviewed. However, due to the limited application of batteries in offshore isolated power systems, practical deployments in onshore grids are also considered. The technical feasibility of the battery chemistry can be assessed using operational experience.
BESS constitute less than 10% of global installed grid ES capacity in 2020, with pumped hydro storage taking up over 90% of global installed grid ES capacity [
82]. However, in recent years, BESS installation for grid-scale applications has grown at a fast pace. As of 2020, the global installed grid-scale BESS was 17 GW [
83]. 5 GW of BESS was added in 2020 alone, with lithium-ion taking a share of 93%. This shows that lithium-ion will continue to dominate the energy storage market for grid-connected BESS. Hence, Li-ion has plenty of operational experience.
Table 3 shows the practical deployments of various battery chemistries for grid applications for both offshore and onshore.
The largest lithium-ion grid-connected ES solution in the world is at Moss Landing, California, USA, with a 400 MW/1600 MWh rating [
84]. The BESS’s primary functions are to perform peak shaving and renewable energy time shifts. This enormous deployment of lithium-ion batteries proves the technical and economic feasibility of their application for grid services. Another large BESS has also been brought online in Moss Landing, California, USA, with a capacity of 182.5 MW/730 MWh [
85]. The unit is composed of 256 Tesla Megapacks on 33 concrete slabs. The grid services performed by this BESS are peak shaving and renewable energy time shift. More importantly, Li-ion batteries have been deployed offshore. 6 MW/166 MWh Li-ion BESS was deployed on the West Mira drilling rig in the North Sea in 2018 [
19]. The Li-ion BESS helps to perform peak shaving, which leads to a 42% reduction in the diesel engine runtime. This corresponds to a 15% CO
2 emissions reduction and a 12% NOx emissions reduction. A Li-ion battery was also deployed in Goodwyn A OOGP on the Australian North West Shelf in 2019 [
86]. The Li-ion BESS is a 1 MW/1 MWh Powerstore battery that was deployed for spinning reserve. The platform has four gas turbines that are always running. The deployment of the battery allows the platform to run on three gas turbines, with the fourth gas turbine shut down. The integration of the Li-ion BESS yields a fuel gas consumption reduction of 3000 tonnes per year and a CO
2 emission reduction of 4%.
NaS batteries have also been applied to large-scale grid services. NGK insulators is the sole manufacturer of NaS batteries and has successfully installed NaS batteries in several locations around the world, many of them in Japan. The largest NaS installation is a 108 MW/648 MWh installation in Abu Dhabi, UAE [
94]. It is a virtual battery plant composed of 15 batteries located in 10 locations in the city. All the batteries are operated as a single ES unit using a communication link. The major grid service performed by this BESS unit is peak shaving. Similarly, NaS has been deployed in other large installations, which are given in
Table 3. The major reason NaS is being used for large installations is the relative abundance of sodium and sulfur, which make them cheap raw materials unlike the rare and expensive lithium and cobalt used in Li-ion batteries. However, pure sodium burns spontaneously in the presence of air and moisture, and a robust protection layer must be used to house the molten sodium and sulfur. This leads to increased manufacturing costs.
ZEBRA batteries have also been used for several grid services, although at lower power ratings compared to those of NaS. The largest ZEBRA battery installation in the world is on Prince Edward Island, Canada, with a rating of 10 MW/20 MWh [
87]. The BESS is based on the Durathon battery supplied by General Electric. The BESS is used for renewable energy time shifts, which help to use more renewable energy. The BESS has been operating since 2013. ZEBRA batteries have also been applied in OOGP as a backup power supply for critical equipment, but with low power ratings. Other ZEBRA applications are given in
Table 3.
VRB is the most deployed flow battery for grid applications. VRB has been used in grid applications as low as 100 kW [
105] and in multi-MW applications. The largest VRB installation is a 200 MW/800 MWh BESS in Dalian, China [
98]. The major reason VRB is used is that it does not lose capacity after cycling thousands of times and is non-flammable. There are several other deployments of VRB, some of which are shown in
Table 3. These practical deployments demonstrate the techno-economic suitability of VRB for grid-scale applications. The main challenge for the application of VRB in an OOGP is its low gravimetric and volumetric energy density, which requires large space and weight.
ZnBr is less used compared to VRB, and as such, there are few grid-scale applications of ZnBr. The most notable deployment of ZnBr is a 500 kW/2 MWh BESS at a microgrid at the Rialto bioenergy facility owned and operated by waste recovery company Anaergia in San Bernadino County, California [
101]. The BESS is supplied by Australian ZnBr manufacturer Redflow. The BESS consists of 192 Redflow ZnBr batteries (each rated at 10 kWh). The primary function of the BESS is to reduce the peak load drawn by the microgrid. In general, ZnBr has much less operational experience and practical deployments compared to VRB, and as such, its techno-economic feasibility has not been robustly demonstrated.
Nickel-based batteries have the fewest grid-scale practical deployments among commercial battery chemistries. The only known grid-scale applications of NiCd are: a 27 MW/14.6 MWh BESS at Fairbanks, Alaska, USA [
103] and a 3 MW/6 MWh BESS at Bonaire Island, Netherlands [
104]. Both deployments are used for spinning reserves. The 3 MW/6 MWh BESS is also used for frequency regulation. The only known grid-scale application of NiMH is a 0.3 MW/0.08 MWh BESS at Okinawa, Japan [
89]. The BESS is used for frequency regulation. NiMH is typically not used for grid applications, but it is more readily used in plug-in hybrid electric vehicles due to its high energy density and lower life cycle cost compared to Li-ion batteries. Thus, both NiCd and NiMH have limited operational experience, and their techno-economic feasibility has not been robustly demonstrated.
Zinc-air batteries are mostly in the research and development phase and have not been commercialized. Thus, there is no operational experience. Likewise, even though there are few LiC products on the market, LiC has not attained full commercial status, and thus there is no operational experience for it. Due to this, both zinc–air batteries and LiC are discarded and are not investigated further.
7. Conclusions
This paper performed a technology suitability assessment study of multiple battery chemistries for application in OOGPs. An improved 7-step procedure for determining the most suitable battery chemistry is proposed. In order to implement the 7-step procedure, the following battery chemistries were reviewed in detail: LFP, LTO, NMC, NiCd, NiMH, NaS, ZEBRA, VRB, ZnBr, Zn–air, and LiC. As part of the 7-step procedure, the operational experiences of these battery chemistries were also reviewed and used to evaluate the suitability of the battery chemistries for peak shaving and spinning reserve. Based on the lack of sufficient operational experience, Zn–air and LiC were discarded while the remaining battery chemistries were investigated further.
In general, this work highlights the need for proper TSA for selecting a battery chemistry due to the harsh and unique conditions the offshore environment presents. This is exemplified using the 7-step procedure on two case study applications (peak shaving and spinning reserve), which are based on a real OOGP in the North Sea. Although several grid services were reviewed, the case study only considers two grid services, which are peak shaving and spinning reserve.
A TSA-weighted score is calculated. For peak shaving, ZEBRA has the lowest TSA-weighted score. ZEBRA’s low TSA-weighted score is due to its poor performance in weight and space. LFP has the highest TSA-weighted score and is deemed the most suitable battery chemistry for peak shaving. For spinning reserve, LTO has the lowest TSA-weighted score due to its high life cycle cost and requirement for high weight and space. LFP and NMC have the highest TSA-weighted scores and are deemed the most suitable battery chemistries for spinning reserve.
Note that even though the two grid services have been treated separately, one BESS can be deployed to deliver both grid services. If one BESS serves both grid services, it is possible to use a BESS size that is less than the sum of the two capacities for the grid services.