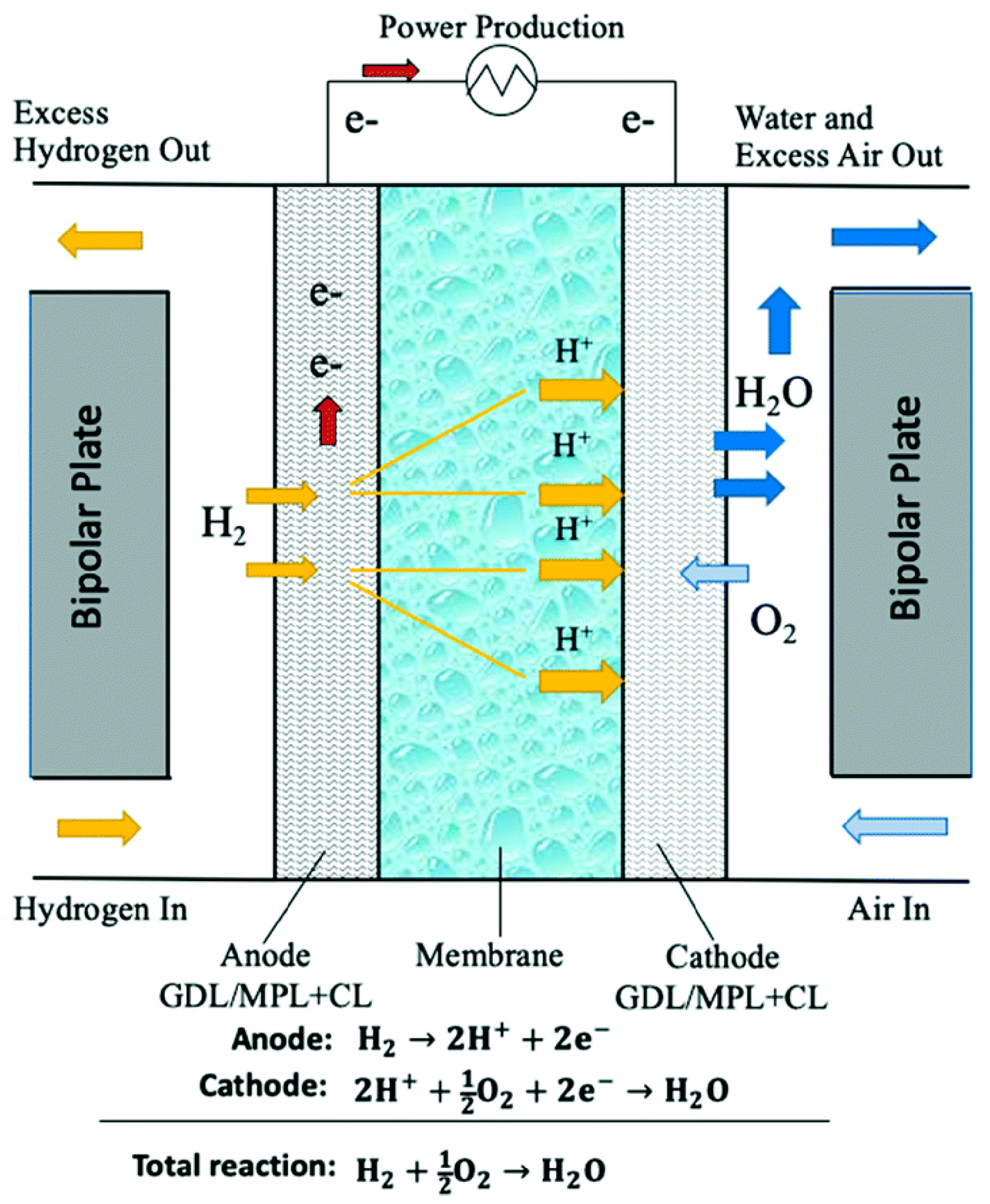
3.3.1. Fuel Cell Stack
The inventory of the FC stack was mainly conducted by adapting the work of Evangelisti et al. [
12] to the considered parameters. It was assumed that the FC stack is the same for architectures A and B (
Figure 2), while a different stack was considered for architecture C since it had to be adjusted to the requests of power and voltage due to the lack of a converter to regulate its output.
Table 2 reports the parameters assumed for the two stacks, while
Table 3 and
Table 4 report the inventory for the production of the fuel cell stacks for the different configurations.
Concerning the proton exchange membrane of the fuel cell, it must present a high proton conductivity, be chemically and mechanically stable in the fuel cell, and offer a barrier to the mixing of reactants. Perfluoro sulfonic acid (PFSA), in particular Nafion
®, produced by Chemours, is the most used material [
20]. Nafion
® can be classified as a copolymer of tetrafluoroethylene (TFE) and unsaturated perfluoroalkyl sulfonyl fluoride, where the former supplies the required mechanical strength while the latter provides the hydrophilic proton-carrying sulfonic acid groups at the end of the side chains [
19]. The membrane considered in this work is a dispersion-cast membrane, NRE-212, with a thickness of 50 µm [
12]. Since there is no available LCA dataset for Nafion
®, a proxy process was assumed, with 57.4 wt% of TFE and 42.6 wt% sulfuric acid for the PSFA. To account for the dispersion cast process, two proxy processes were used: a coil laminating process and a laminated foil process [
12]. The inventory for the manufacture of the membrane is shown in
Supplementary Materials, Table S1.
Moving to the outer part of the electrode, i.e., the Gas Diffusion Layer (GDL), the one considered in this study is composed of a carbon cloth material coated with 10 wt% polytetrafluoroethylene (PTFE) and 5 wt% carbon black for the microporous layer (MPL), assuming a carbon cloth made from carbon fibers [
30,
38]. Since there are no direct datasets in Ecoinvent 3.7 for carbon fibers and PTFE, their production was modeled following the processes considered by Evangelisti [
12] and reported in the inventory. Then, the preparation of an MPL, consisting of a hydrophobic solution of PTFE and carbon powder (hydrophobic ink) with a solid content of 27 wt%, was considered, followed by its deposition on the GDL and the subsequent heat treatment [
12]. The inventory for the thermoforming of the gas diffusion layer is reported in
Table S2, while the inventory of PTFE and carbon fiber is described in
Table S3.
The catalyst layer is the part of the cell where the reactions of hydrogen oxidation (HOR) and oxygen reduction (ORR) take place. The most commonly used catalyst for PEMFC is platinum (Pt), and even if other materials are proven to offer similar results, platinum is still preferred because of its stability, activity, and selectivity [
12]. Platinum mining and refining generate large overall impacts when considering the manufacture of a PEMFC, and reducing the amount needed in the catalyst layer is one of the main focuses of recent studies aiming at lowering the cost and environmental impacts of fuel cell technology [
39]. The Pt-loading considered in this work was the same as the one in the Toyota Mirai, i.e., 0.4 mg/cm
2 [
40], with carbon black being the support material, owing to its porous and conductive nature that allows surface area and contact with the membrane to be maximized. Before the deposition of the catalyst, carbon must undergo a pre-treatment. The entire process to manufacture 1 g of Pt/C was modeled based on the work by Evangelisti et al. [
12].
The catalyst is usually prepared in the form of ink. The process model in this study is based on ball mill production with a wet catalyst solution composed of 6 wt% Pt/C, 9 wt% carbon black, 72 wt% Nafion
® DE-521 solution (Nafion
®, Wilmington, DE, USA), 6.5 wt% deionized water, and 6.5 wt% methanol, which ultimately corresponds to a dry catalyst composition of 48 wt% carbon black, 32 wt% Pt/C, and 20 wt% Nafion
® [
12]. The catalyst is assumed to be applied directly to the membrane using a spraying catalyst decaling method. This procedure is composed of two steps: the thinner anode layer is spray-coated directly on the hydrated membrane and dried, while the thicker cathode layer is spray-coated onto a transfer substrate, followed by drying. Then, the two catalyst layers are heated and roll pressed with the transfer substrate, usually made from polyester, which is subsequently peeled away from the deposited cathode layer [
12].
Table S4 reports the inventory of the production of the catalyst layer. Additional information for the inventory of Nafion
® DE-521 solution and the catalyst in carbon (Pt/C) can be found in
Table S5.
The combination of two GDLs, the membrane, and two catalyst layers produces the membrane electrode assembly (MEA). The catalyzed membrane is hot-pressed between the two gas diffusion layers and die-cut to the final cell dimensions. Pressing of the membrane and GDL is performed at around 7 bar and 100 °C for 124 s to achieve the best contact possible [
12]. The inventory of this process is reported in
Table S6.
In addition, bipolar plates (BPP) are needed when assembling a multi-cell stack since their primary role is to electrically connect the anode of one cell to the cathode of the adjacent cell. They are typically made of graphite composite materials, flexible graphite foil, and stainless steel alloys. The material considered in this study is a compression-molded graphite composite, assumed to be composed of 70% graphite and 30% vinyl ester. To model the vinyl ester, a proxy based on vinyl acetate monomer was considered, while the ‘graphite, battery grade’ process was used for graphite [
12]. The inventory of the production of the bipolar plates is reported in
Table S7.
All the previous components are held together and sealed by gaskets, end plates, and tie-rods, preventing the leakage of the reactants and minimizing the contact resistance among the layers [
20]. In this study, it was assumed that the gaskets are steel rule dies cut from a silicon roll. The outline of the gasket is laid out and cut into a board, and strip steel is embedded into the board at a uniform height and mounted on a small-stroke, fast-acting press. The bulk gasket material is fed into the press, and the material is cut. The process considered to manufacture the end plates is cellular manufacturing, involving an A465-cast aluminum block as the material. To model the material production, the ‘aluminum, production mix, cast alloy, at plant’ process in Ecoinvent 3.7 was used [
12].
Table S8 reports the inventory model for the production of these components.
3.3.2. Balance of Plant
The fuel cell system is formed by other components besides the fuel cell stack, which are auxiliaries that have four main tasks: heat management, fuel management, air management, and water management [
38].
The heat management system comprises high-temperature radiators and a high-temperature coolant pump. The air management system is composed of an air filtration system and a compressor expander module (CEM) for air supply. Since the air reaching the cathode comes from outside, it needs to be filtered; otherwise, there could be the possibility of poisoning the catalyst, which is sensitive to carbon monoxide and other particulates [
30]. The water management system includes an enthalpy wheel humidifier for air and a membrane humidifier for hydrogen to keep the level of humidity homogeneous in the cell [
12,
30]. Finally, the fuel management system includes a hydrogen recirculation blower and ejectors [
12]. Other auxiliaries of the FC system include the AC/DC inverter and the DC/DC converters. The inventory of the inverter was based on the work by Habermacher [
41], while to model the materials and components of the balance of the plant, the work by Candelaresi et al. [
13] was taken into account, adjusting the data for a 111 kW FC system.
Table S9 reports the inventory for the production of the balance of the plant.
3.3.4. Hydrogen Tank
The hydrogen tank is a major contributor to the environmental impacts of the fuel cell system and also one of the key challenges for the whole FCEV industry [
12]. According to various studies, the environmental impacts of the tank are comparable to the impacts of major components such as the glider or the fuel cell stack [
33], with evidence that the tank alone could contribute even up to 50% to the overall impacts of the production phase when not considering the impacts of the glider itself [
17]. Because of the low density presented by hydrogen, storage is one of the main issues in adopting and developing this technology, especially in the automotive field [
33]. Hydrogen tanks must satisfy several parameters to provide optimal storage and safety, such as high volumetric and gravimetric energy densities, a good rate of fuel release, and being lightweight yet robust enough to ensure safety from flammability, explosions, or projections. Finally, the materials must not be toxic to human health. These characteristics are met by compressed gas tanks now available on the market, although their geometry requirements and positioning in the vehicle present some technological issues that are still being faced [
44].
The main issue in modeling the hydrogen tank for LCA is the scarce availability of first-hand and freely available data about the production of carbon fiber, which is the main component of the tank [
33]. The one considered in this study was modeled by taking into account the characteristics of the type IV tank of the Toyota Mirai. Type IV tanks present a multi-layered structure, with the first layer made of a polymeric material coated in aluminum, the second layer made mainly of carbon fiber, and finally a glass fiber layer combined with resin. The first layer is called the liner and blocks the passage of hydrogen completely; the second one provides mechanical resistance; and the final one is a protective layer [
30]. The Mirai is equipped with two Type IV tanks with a total capacity of 122.4 L and approximately 5 kg of stored hydrogen, with a storage density of 5.7 wt% and an operating pressure of 700 bar [
45]. For the sake of simplicity, a single tank with these characteristics was modeled for a total weight of 87.7 kg.
Table S11 reports the considered inventory.
3.3.5. Battery
In FCEVs, the battery plays an important role in improving the efficiency of the vehicle and covers various tasks. It allows for the recovery of energy from regenerative braking and helps the fuel cell system deliver the required power [
11,
26]. The battery can increase the efficiency of the system but also reduce the size of the fuel cell stack and extend the maximum range [
46].
As previously explained, the battery model in this work is a lithium manganese oxide lithium-ion battery (LMO Li-ion), referring to the study by Colnago et al. [
11,
35].
Because of the presence or absence of the converter linking the battery to the motor, the nominal voltage of the battery varies with the architecture considered. The ones having the battery converter (architectures A and C) use a battery pack with a nominal voltage of 325 V, while architecture B is equipped with a 650 V battery. This consideration does not influence the inventory of this study since the final weight and number of cells are the same for both types of batteries.
The battery pack was modeled following the study of Zhao et al. [
47] for what concerns the composition of anode, cathode, and electrolyte, while it is based on the work by Ellingsen et al. [
48] to model the cell, the auxiliaries, and the pack itself, adapting the data to the parameters considered by Colnago et al. [
11], with a final weight of 42.24 kg. The whole inventory for the manufacturing of the battery pack can be seen in
Tables S12–S17.
3.3.6. Glider and Electric Motor
The glider considered in this work includes the body of the vehicle, steering, braking, and suspension systems; tires; gearbox; cockpit equipment; fluids necessary for vehicle operation (brake fluid, transmission fluid, powertrain coolant, windscreen fluid, and adhesives); electronics nonrelated to the powertrain; and the hydrogen distribution system. The reference to model the gearbox, tires, fluids, and hydrogen distribution system was the work by Candelaresi et al. [
13], while the rest of the components were described according to the model developed by Habermacher [
41], adapted for a vehicle of 2130 kg (without battery), which is the weight assumed by Colnago et al. [
11].
The electric motor was modeled with the powertrain, which also included the AC/DC inverter. The former was described according to Candelaresi et al. [
13] and adapted for the considered power of 111 kW, while the inverter inventory was designed according to Habermacher [
41] for a total weight of about 118 kg. The considered motor is common to all three configurations. The inventories for the glider, the motor, the inverter, and other auxiliaries are reported in the
Supplementary Materials (Tables S18–S24).
3.3.7. Hydrogen Production
Finally, the production of hydrogen was modeled to account for its consumption during the use phase. For the purpose of this study, only the direct processes for the production of the fuel were considered, leaving upstream processes outside of the system boundaries.
The production of hydrogen as a fuel and the development of FCEV technology in the market are strictly connected, since, without the first, the second would not be competitive with the traditional means of transport. On the other hand, technologies for hydrogen production still have to overcome technical and economic issues, mainly concerning the infrastructure dedicated to hydrogen and the impacts of hydrogen policies on consumers [
9].
Although the possibility of a renewable future for hydrogen production is present, its current state is still far from being environmentally neutral [
8]. Currently, approximately 97% of the total hydrogen production is based on fossil primary energy, and only 3% is based on renewable energies [
9].
For the purpose of this study, considering all these factors, the produced hydrogen was modeled as a mixture of hydrogen produced with the three most utilized production technologies, namely, steam methane reforming, coal gasification, and electrolysis [
7], in proportions considered taking into account the study of Garbe et al. [
49]: for each kilogram of hydrogen, hydrogen produced from SMR contributes 58%, while it accounts for 20% and 22%, respectively, from coal gasification and electrolysis.
The main feedstock of SMR is natural gas, consisting mostly of methane (CH
4), with other heavier hydrocarbons and carbon dioxide [
50,
51]. Conversely, the gasification of coal exploits the natural abundance of coal as feedstock, as well as the high efficiency and reliability of the process [
50,
51]. Regarding water electrolysis, this study considered hydrogen as produced by an electrolytic cell based on a proton exchange membrane. The process of electrolysis can be considered environmentally friendly because no carbon feedstock is directly employed to produce hydrogen; however, there can still be impacts on climate change when considering upstream processes, such as the manufacturing of infrastructure and, more importantly, the source of electricity [
8,
51]. To account for the present situation of the production of electricity, the process utilized in the inventory was the production of electricity mix for Italy, as presented in Ecoinvent 3.7.
Tables S25–S27 report the inventory of hydrogen production from steam methane reforming, coal gasification, and electrolysis, respectively. The distribution phase was excluded from the system boundaries since it was not useful for the comparison of the different power systems considered.