Two-Phase Modeling and Simulations of a Polymer Electrolyte Membrane Water Electrolyzer Considering Key Morphological and Geometrical Features in Porous Transport Layers
Abstract
:1. Introduction
2. Multi-Phase PEM Electrolysis Model
2.1. Model Assumptions
- Ideal gas law was assumed for gas mixtures because of the low pressure in the cell;
- A laminar and incompressible flow was considered for the gaseous phase;
- Effects of gravity were neglected;
- Porous components, including PTLs and CLs, were assumed to be isotropic and were represented using effective porosity and permeability;
- The effect of bubble blockage on cell performance was neglected. Under this assumption, only tiny bubbles form and travel easily through the anode PTL (aPTL).
2.2. Governing Equations
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| a | Water activity; |
| C | Molar concentration of species, ; |
| D | Species diffusivity, ; |
| E | Thermodynamic equilibrium potential, ; |
| e | Euler’s number; |
| F | Faraday’s constant, 96,487 ; |
| H | Henry’s constant, ; |
| Exchange current density, ; | |
| I | Operating current density, ; |
| Diffusive mass flux of liquid phase; | |
| Diffusive mass flux of gas phase; | |
| j | Current density, ; |
| Relative permeability; | |
| n | Number of electrons transferred in the electrode reaction; |
| Electro-osmotic drag coefficient for water; | |
| M | Molecular weight, ; |
| mf | Mole fraction; |
| P | Pressure, ; |
| Bubble nucleation radius, ; | |
| Universal gas constant, 8.314 ; | |
| Liquid saturation; | |
| S | Source term in transport equation; |
| T | Temperature, ; |
| V | Cell voltage, ; |
| Greek | |
| α | Transfer coefficient; |
| δ | Thickness, ; |
| ε | Porosity; |
| η | Overpotential, ; |
| Density, ; | |
| κ | Proton conductivity, ; |
| τ | Tortuosity; |
| σ | Surface tension, ; |
| θ | Contact angle, °; |
| λ | Membrane water content, ; |
| Relative mobility of phase α; | |
| ν | Kinematic viscosity, ; |
| Superscripts | |
| eff | Effective; |
| g | Gas; |
| l | Liquid; |
| mem | Membrane; |
| ref | Reference value; |
| sat | Saturation; |
| Subscripts | |
| Anode; | |
| aCL | Anode catalyst layer; |
| act | Activation; |
| aPTL | Anode porous transport layer; |
| bn | Bubble nucleation; |
| c | Cathode; |
| cap | Capillary; |
| cCL | Cathode catalyst layer; |
| chan | Channel; |
| CL | Catalyst layer; |
| conc | Concentration; |
| cPTL | Cathode porous transport layer; |
| d | Diffusion; |
| e | Electrolyte; |
| g | Gas; |
| i | Species; |
| in | Inlet; |
| Hydrogen; | |
| l | Liquid; |
| mem | Membrane; |
| Oxygen; | |
| ohm | Ohmic; |
| op | Operating; |
| PTL | Porous transport layer; |
| r | Relative; |
| sat | Saturation; |
| w | Water; |
| 0 | Standard conditions—298.15 K and 101.3 kPa (1 atm); |
References
- Maier, M.; Smith, K.; Dodwell, J.; Hinds, G.; Shearing, P.R.; Brett, D.J.L. Mass transport in PEM water electrolysers: A review. Int. J. Hydrogen Energy 2022, 47, 30–56. [Google Scholar] [CrossRef]
- Falcão, D.S.; Pinto, A.M.F.R. A review on PEM electrolyzer modelling: Guidelines for beginners. J. Clean. Prod. 2020, 261, 121184. [Google Scholar] [CrossRef]
- Kim, H.; Park, M.; Lee, K.S. One-dimensional dynamic modeling of a high-pressure water electrolysis system for hydrogen production. Int. J. Hydrogen Energy 2013, 38, 2596–2609. [Google Scholar] [CrossRef]
- Abdol Rahim, A.H.; Tijani, A.S.; Kamarudin, S.K.; Hanapi, S. An overview of polymer electrolyte membrane electrolyzer for hydrogen production: Modeling and mass transport. J. Power Sources 2016, 309, 56–65. [Google Scholar] [CrossRef]
- Toghyani, S.; Afshari, E.; Baniasadi, E.; Atyabi, S.A.; Naterer, G.F. Thermal and electrochemical performance assessment of a high temperature PEM electrolyzer. Energy 2018, 152, 237–246. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Kang, Z.; Mo, J.; Yang, G.; Retterer, S.T.; Cullen, D.A.; Toops, T.J.; Green, J.B., Jr.; Mench, M.M.; Zhang, F.Y. Investigation of thin/well-tunable liquid/gas diffusion layers exhibiting superior multifunctional performance in low-temperature electrolytic water splitting. Energy Environ. Sci. 2017, 10, 166–175. [Google Scholar] [CrossRef]
- Han, B.; Mo, J.; Kang, Z.; Zhang, F.Y. Effects of membrane electrode assembly properties on two-phase transport and performance in proton exchange membrane electrolyzer cells. Electrochim. Acta 2016, 188, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.K.; Lee, C.H.; Bazylak, A. Pore network modelling to enhance liquid water transport through porous transport layers for polymer electrolyte membrane electrolyzers. J. Power Sources 2019, 437, 226910. [Google Scholar] [CrossRef]
- Zhan, Z.; Xiao, J.; Zhang, Y.; Pan, M.; Yuan, R. Gas diffusion through differently structured gas diffusion layers of PEM fuel cells. Int. J. Hydrogen Energy 2007, 32, 4443–4451. [Google Scholar] [CrossRef]
- Zhang, Y.; Verma, A.; Pitchumani, R. Optimum design of polymer electrolyte membrane fuel cell with graded porosity gas diffusion layer. Int. J. Hydrogen Energy 2016, 41, 8412–8426. [Google Scholar] [CrossRef] [Green Version]
- Kang, Z.; Yu, S.; Yang, G.; Li, Y.; Bender, G.; Pivovar, B.S.; Green Jr, J.B.; Zhang, F.Y. Performance improvement of proton exchange membrane electrolyzer cells by introducing in-plane transport enhancement layers. Electrochim. Acta 2019, 316, 43–51. [Google Scholar] [CrossRef]
- Forner-Cuenca, A.; Biesdorf, J.; Gubler, L.; Kristiansen, P.M.; Schmidt, T.J.; Boillat, P. Engineered Water Highways in Fuel Cells: Radiation Grafting of Gas Diffusion Layers. Adv. Mater. 2015, 27, 6317–6322. [Google Scholar] [CrossRef]
- Forner-Cuenca, A.; Biesdorf, J.; Manzi-Orezzoli, V.; Gubler, L.; Schmidt, T.J.; Boillat, P. Advanced Water Management in PEFCs: Diffusion Layers with Patterned Wettability III. Operando Characterization with Neutron Imaging. J. Electrochem. Soc. 2016, 163, F1389–F1398. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Mo, J.; Kang, Z.; Yang, G.; Barnhill, W.; Zhang, F.Y. Modeling of two-phase transport in proton exchange membrane electrolyzer cells for hydrogen energy. Int. J. Hydrogen Energy 2017, 42, 4478–4489. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Fujigaya, T.; Nakajima, H.; Inada, A.; Ito, K. Optimum structural properties for an anode current collector used in a polymer electrolyte membrane water electrolyzer operated at the boiling point of water. J. Power Sources 2016, 332, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Kang, Z.; Alia, S.M.; Young, J.L.; Bender, G. Effects of various parameters of different porous transport layers in proton exchange membrane water electrolysis. Electrochim. Acta 2020, 354, 136641. [Google Scholar] [CrossRef]
- Suermann, M.; Gimpel, T.; Bühre, L.V.; Schade, W.; Bensmann, B.; Hanke-Rauschenbach, R. Femtosecond laser-induced surface structuring of the porous transport layers in proton exchange membrane water electrolysis. J. Mater. Chem. A 2020, 8, 9–4898. [Google Scholar] [CrossRef]
- Marangio, F.; Santarelli, M.; Calì, M. Theoretical model and experimental analysis of a high pressure PEM water electrolyser for hydrogen production. Int. J. Hydrogen Energy 2009, 34, 1143–1158. [Google Scholar] [CrossRef]
- Han, B.; Steen, S.M.; Mo, J.; Zhang, F.Y. Electrochemical performance modeling of a proton exchange membrane electrolyzer cell for hydrogen energy. Int. J. Hydrogen Energy 2015, 40, 7006–7016. [Google Scholar] [CrossRef]
- Wang, C.Y.; Cheng, P. A multiphase mixture model for multiphase, multicomponent transport in capillary porous media—I. Model development. Int. J. Heat Mass Transf. 1996, 39, 3607–3618. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Witteman, L.; Wrubel, J.A.; Bender, G. A comprehensive modeling method for proton exchange membrane electrolyzer development. Int. J. Hydrogen Energy 2021, 46, 17627–17643. [Google Scholar] [CrossRef]
- Diaz, D.F.R.; Valenzuela, E.; Wang, Y. A component-level model of polymer electrolyte membrane electrolysis cells for hydrogen production. Appl. Energy 2022, 321, 119398. [Google Scholar] [CrossRef]
- Sawada, S.; Yamaki, T.; Maeno, T.; Asano, M.; Suzuki, A.; Terai, T.; Maekawa, Y. Solid polymer electrolyte water electrolysis systems for hydrogen production based on our newly developed membranes, Part I: Analysis of voltage–current characteristics. Prog. Nucl. Energy 2008, 50, 443–448. [Google Scholar] [CrossRef]
- Kopitzke, R.; Linkous, C.A.; Anderson, H.R.; Nelson, G.L. Conductivity and water uptake of aromatic-based proton exchange membrane electrolytes. J. Electrochem. Soc. 2000, 147, 1677–1681. [Google Scholar] [CrossRef]
- Zawodzinski, T.Z.; Derouin, C.; Radzinski, S.; Sherman, R.J.; Smith, V.T.; Springer, T. Water uptake by and transport through Nafion 117 membranes. J. Electrochem. Soc. 1993, 140, 1041–1047. [Google Scholar] [CrossRef]
- Biaku, C.Y.; Dale, N.V.; Mann, M.D.; Salehfar, H.; Peters, A.J.; Han, T. A semiempirical study of the temperature dependence of the anode charge transfer coefficient of a 6 kW PEM electrolyzer. Int. J. Hydrogen Energy 2008, 33, 4247–4254. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Srinivasan, S.; Appleby, A.J.; Martin, C.R. Temperature Dependence of the Electrode Kinetics of Oxygen Reduction at the Platinum/Nafion® Interface—A Microelectrode Investigation. J. Electrochem. Soc. 1992, 139, 2530–2537. [Google Scholar] [CrossRef]
- Weber, C.C.; Schuler, T.; Bruycker, R.D.; Gubler, L.; Büchi, F.N.; Angelis, S.D. On the role of porous transport layer thickness in polymer electrolyte water electrolysis. J. Power Sources Adv. 2022, 15, 100095. [Google Scholar] [CrossRef]
- Kulkarni, D.; Huynh, A.; Satjaritanun, P.; O’Brien, M.; Shimpalee, S.; Parkinson, D.; Shevchenko, P.; DeCarlo, F.; Danilovic, N.; Ayers, K.E.; et al. Elucidating effects of catalyst loadings and porous transport layer morphologies on operation of proton exchange membrane water electrolyzers. Appl. Catal. B 2022, 308, 121213. [Google Scholar] [CrossRef]
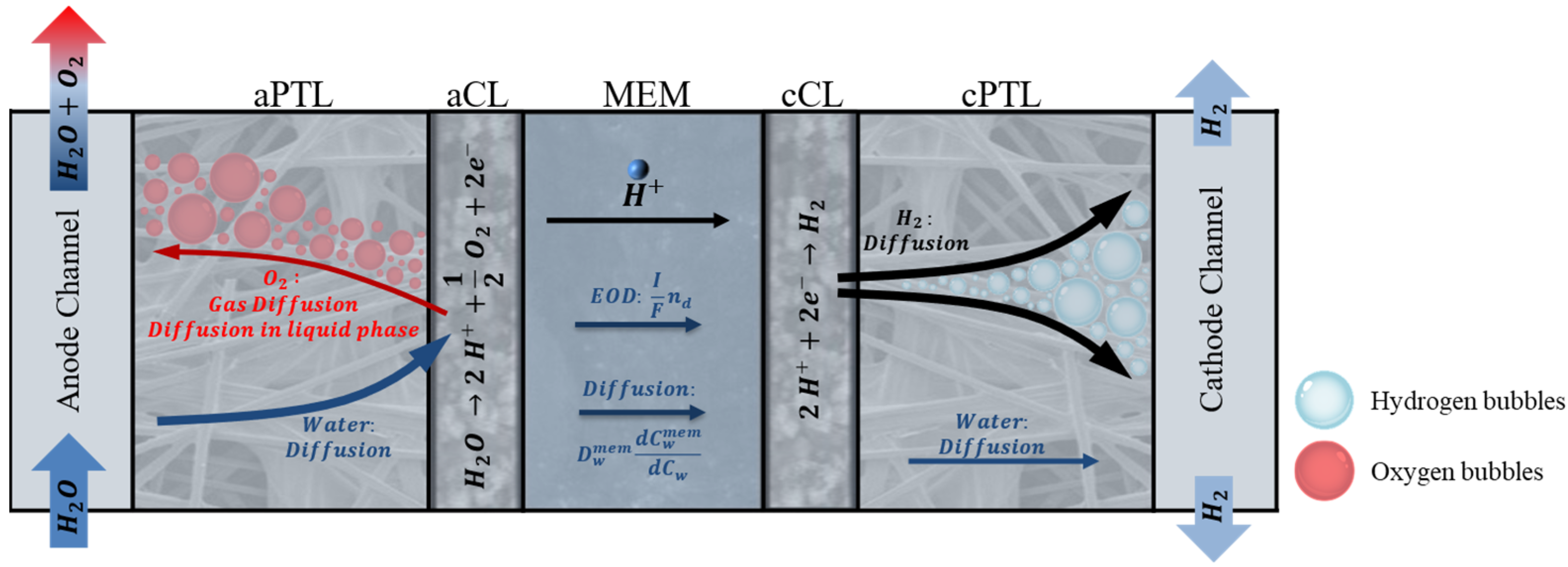
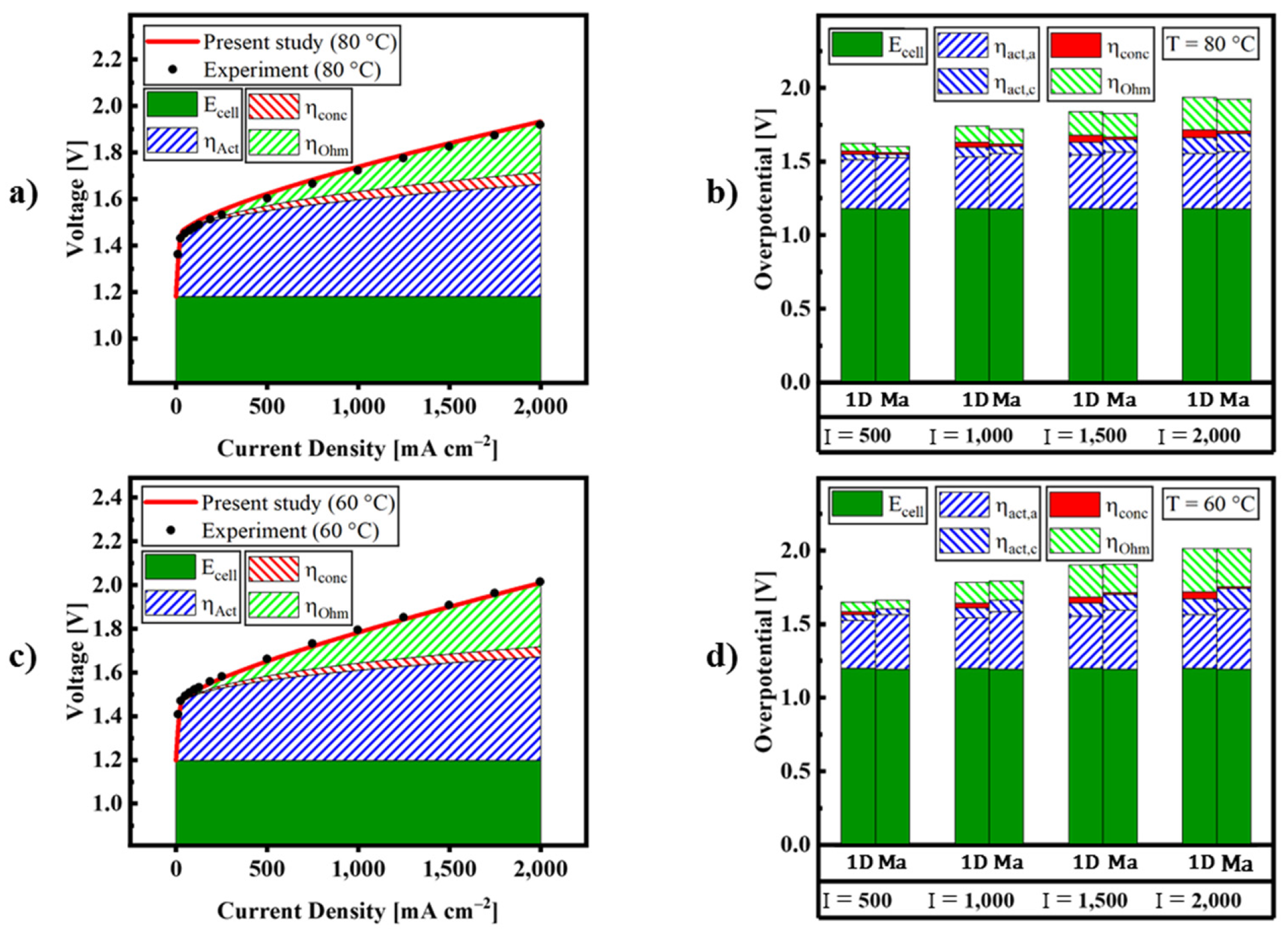
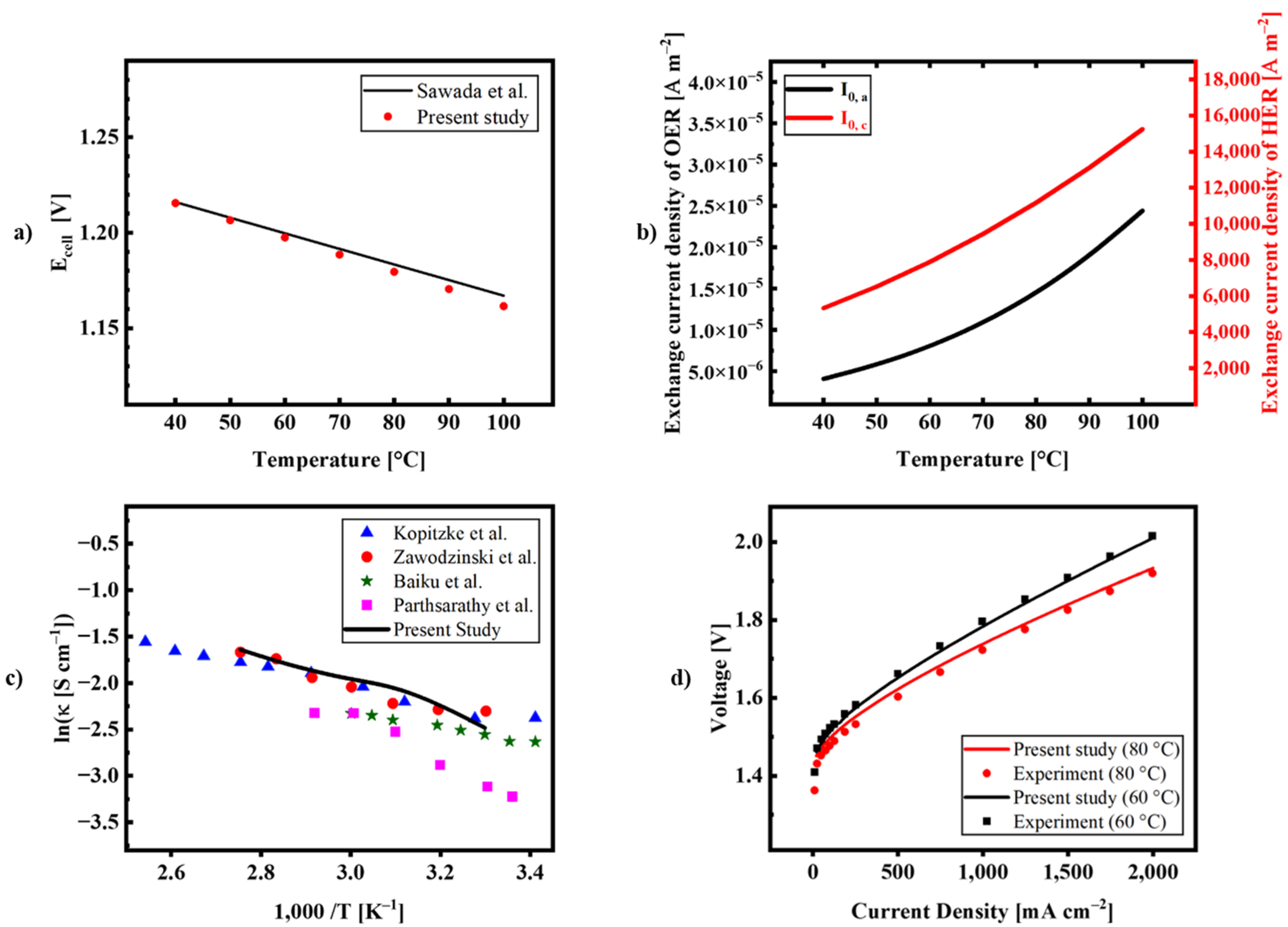

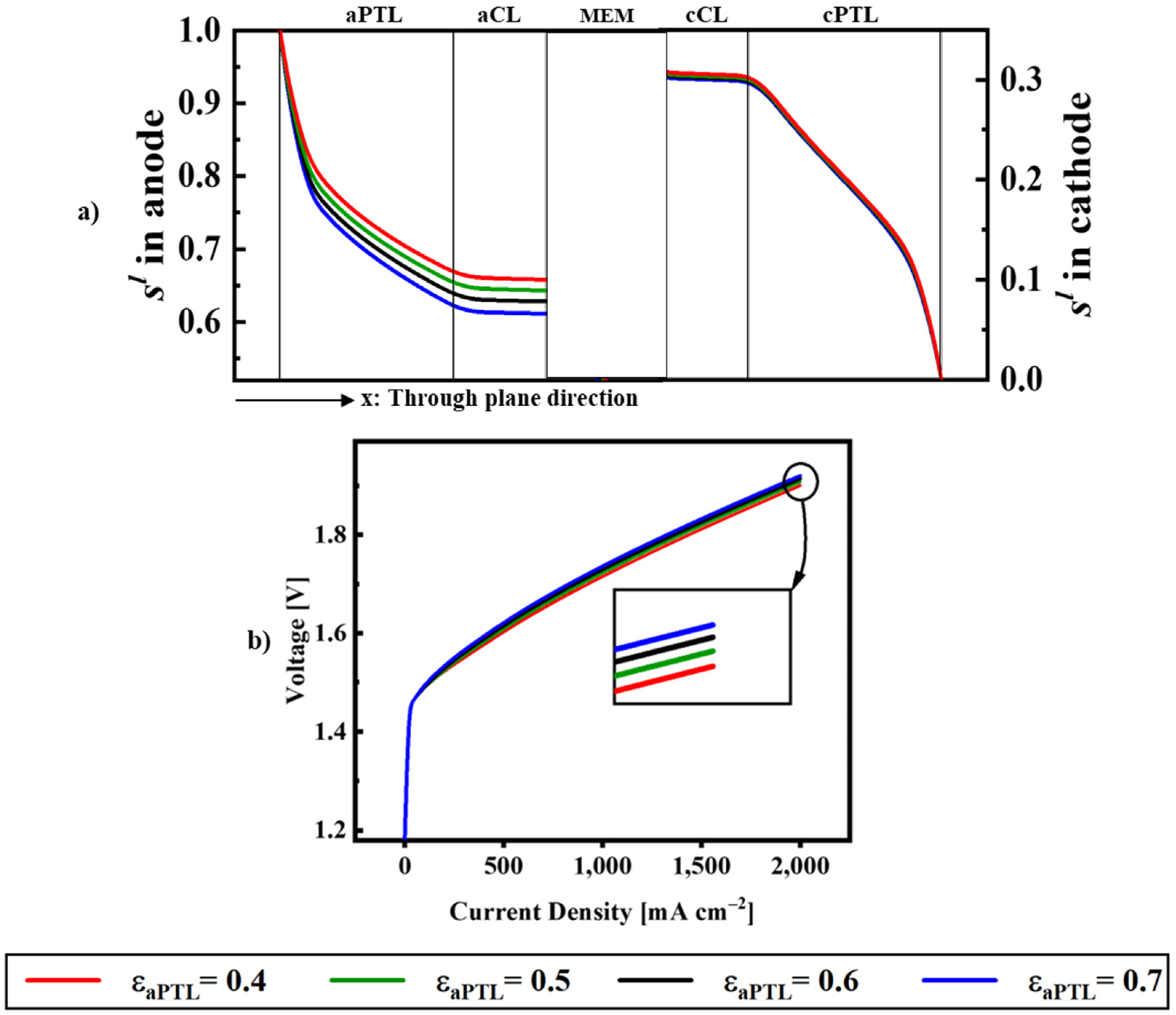
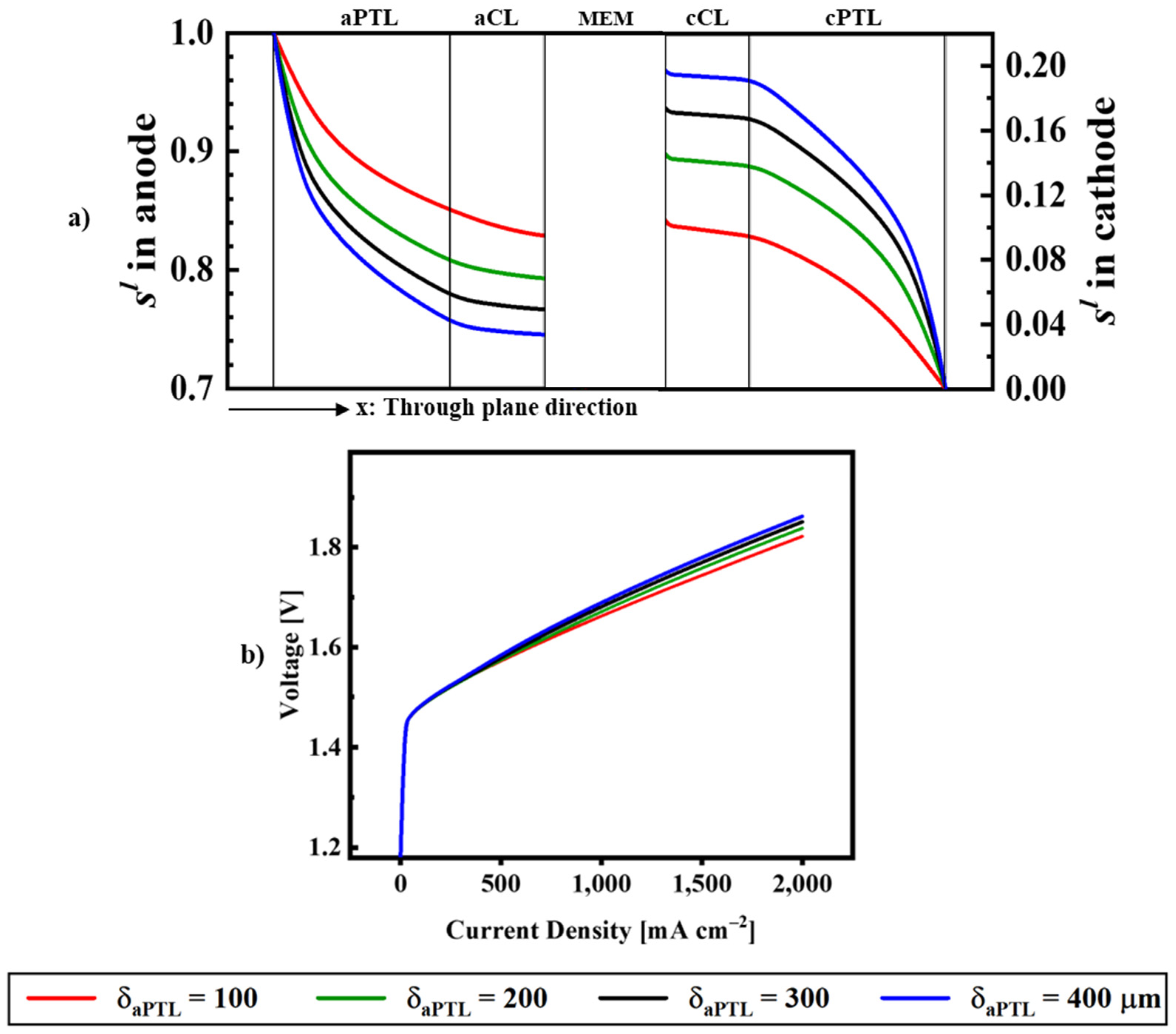

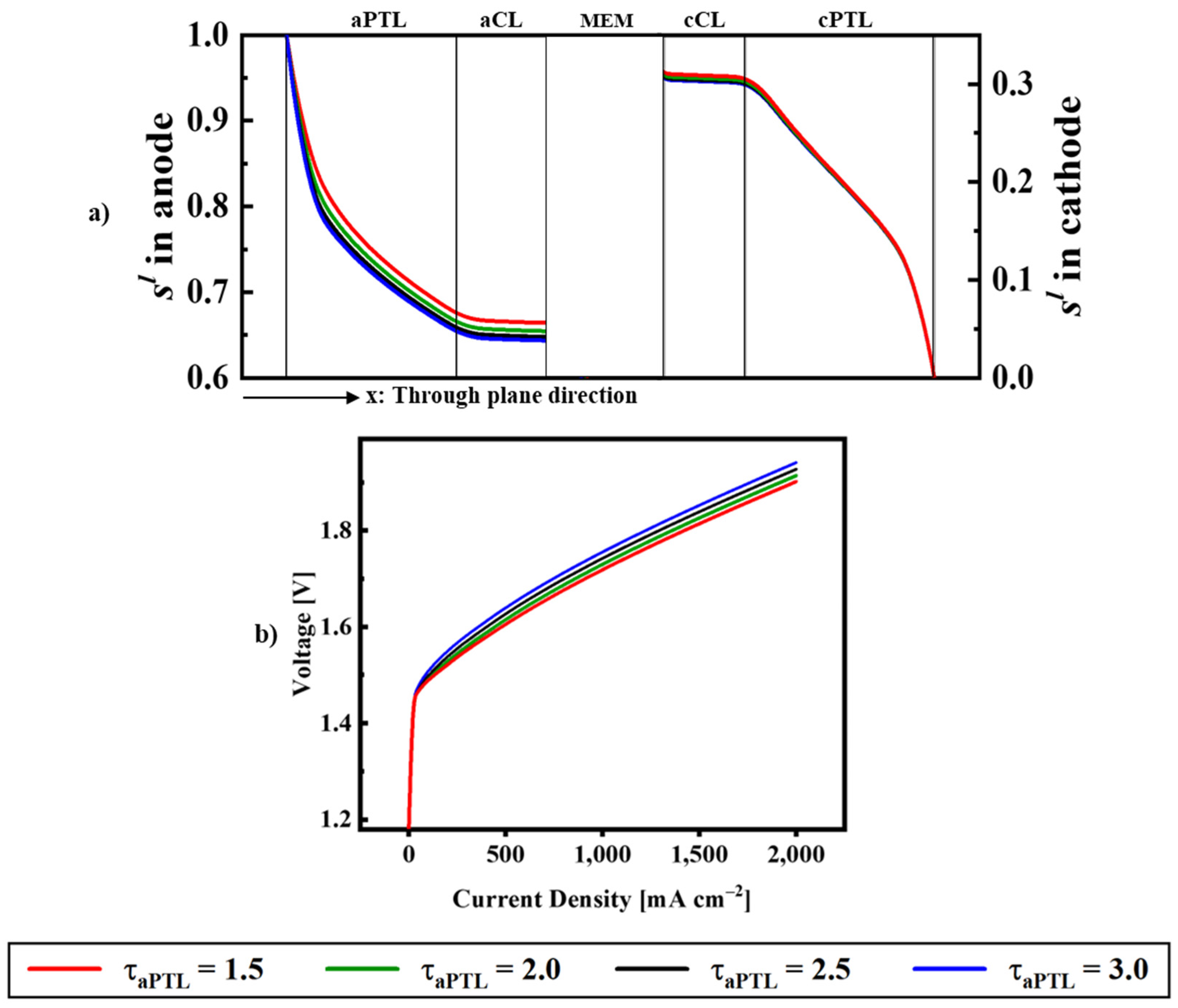
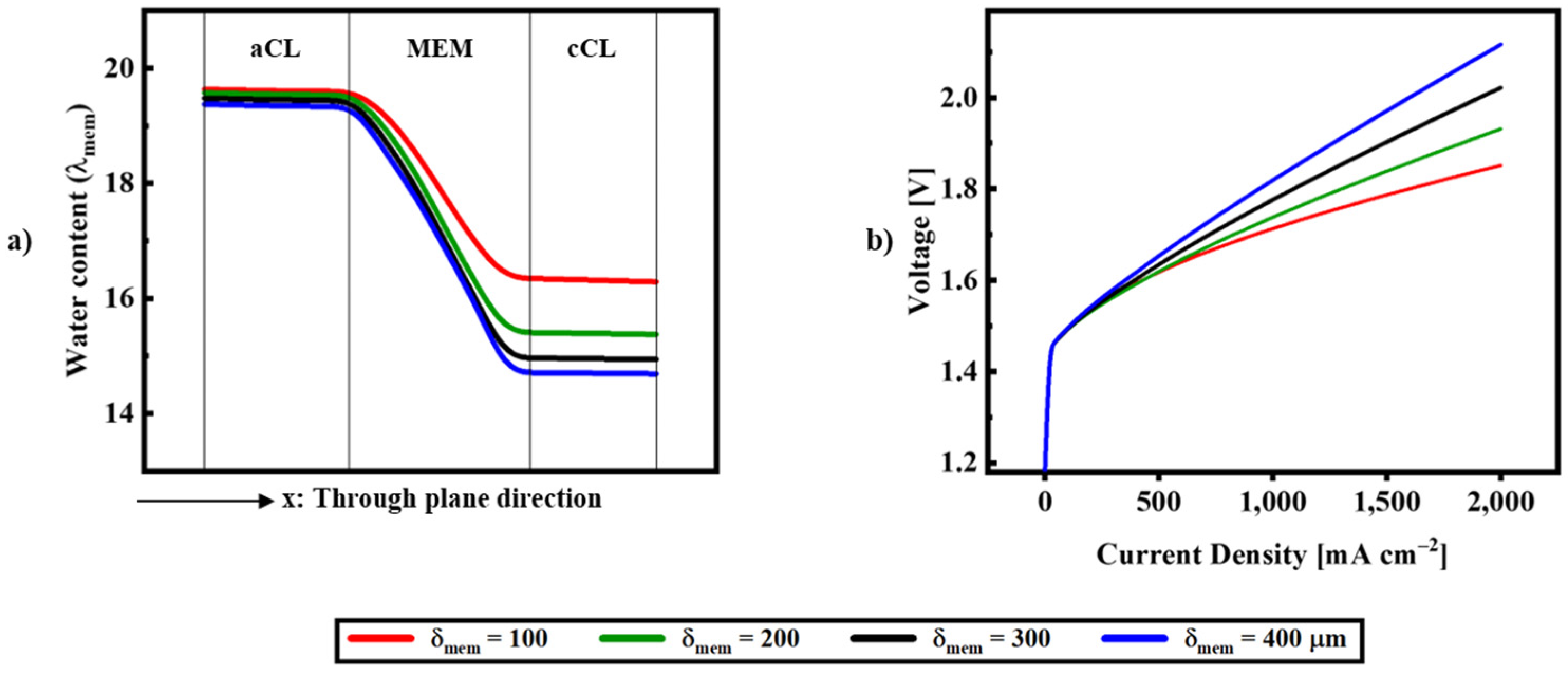
| Description | Equations | Equation # | |
|---|---|---|---|
| Source/sink terms | Anode CL | (6) | |
| (7) | |||
| Cathode CL | (8) | ||
| (9) | |||
| Boundary conditions | Interfacial surface between channel and PTL | (10) | |
| Interface of membrane and CLs | (11) | ||
| Description | Value |
|---|---|
| Thickness of the PTL, | 1.1 m |
| Thickness of the CL, | 12 m |
| Thickness of the membrane, | 183 m |
| Pressure (anode/cathode), | 1/1 bar |
| Operating temperature, | 80/60 ℃ |
| Inlet temperature, | 80/60 ℃ |
| Anode flow rate | 50 mL/min |
| Description | Value |
|---|---|
| Area of MEA | |
| 0.64/0.3 | |
| Contact angle of aPTL/aCL/cCL/cPTL, | 60°/60°/100°/100° |
| Permeability of the PTL/CL/membrane, | / |
| 2.0 | |
| 0.5 | |
| Equivalent weight of electrolyte in the membrane, EW | |
| Faraday constant, F | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salihi, H.; Ju, H. Two-Phase Modeling and Simulations of a Polymer Electrolyte Membrane Water Electrolyzer Considering Key Morphological and Geometrical Features in Porous Transport Layers. Energies 2023, 16, 766. https://doi.org/10.3390/en16020766
Salihi H, Ju H. Two-Phase Modeling and Simulations of a Polymer Electrolyte Membrane Water Electrolyzer Considering Key Morphological and Geometrical Features in Porous Transport Layers. Energies. 2023; 16(2):766. https://doi.org/10.3390/en16020766
Chicago/Turabian StyleSalihi, Hassan, and Hyunchul Ju. 2023. "Two-Phase Modeling and Simulations of a Polymer Electrolyte Membrane Water Electrolyzer Considering Key Morphological and Geometrical Features in Porous Transport Layers" Energies 16, no. 2: 766. https://doi.org/10.3390/en16020766





