Effect of Combustion Conditions and Blending Ratio on Aero-Engine Emissions
Abstract
:1. Introduction
2. Model Development
2.1. Fuel Substitution Model
2.2. Combustion Chamber Model
2.3. Mathematical Models
- : Generation of turbulent kinetic energy due to mean velocity gradient;
- : Turbulent kinetic energy generated by buoyancy;
- : Contribution of fluctuating expansion to the total dissipation rate in compressible turbulence;
- : constants, which are ;
- : Turbulent Prandtl number, 1.0, 1.3, respectively;
- which:
- : Constant, taken as 0.09
3. Results and Discussion
3.1. Feasibility Verification
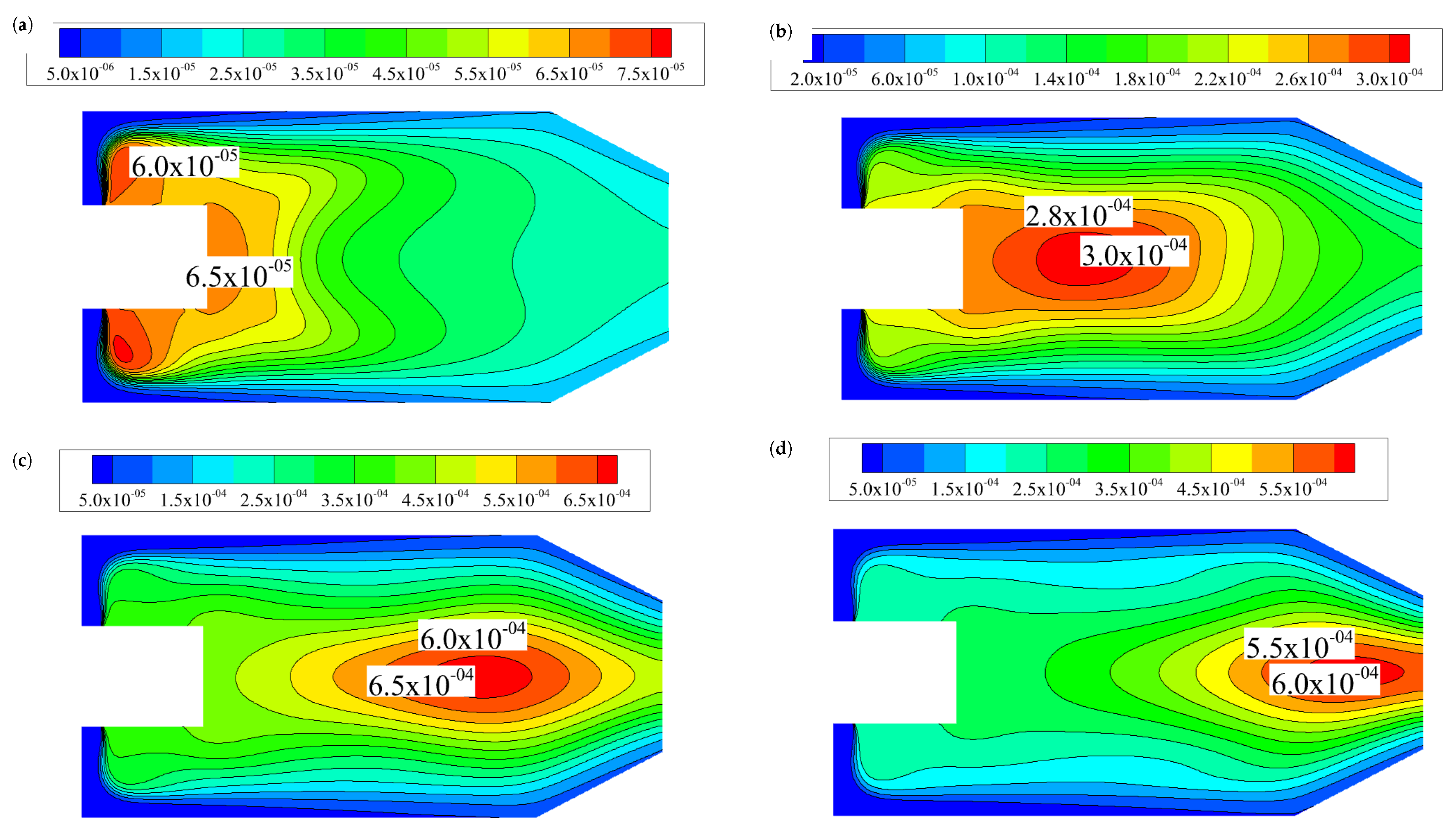
3.1.1. Temperature and CO, CO2 Distributions in the Combustion Chamber
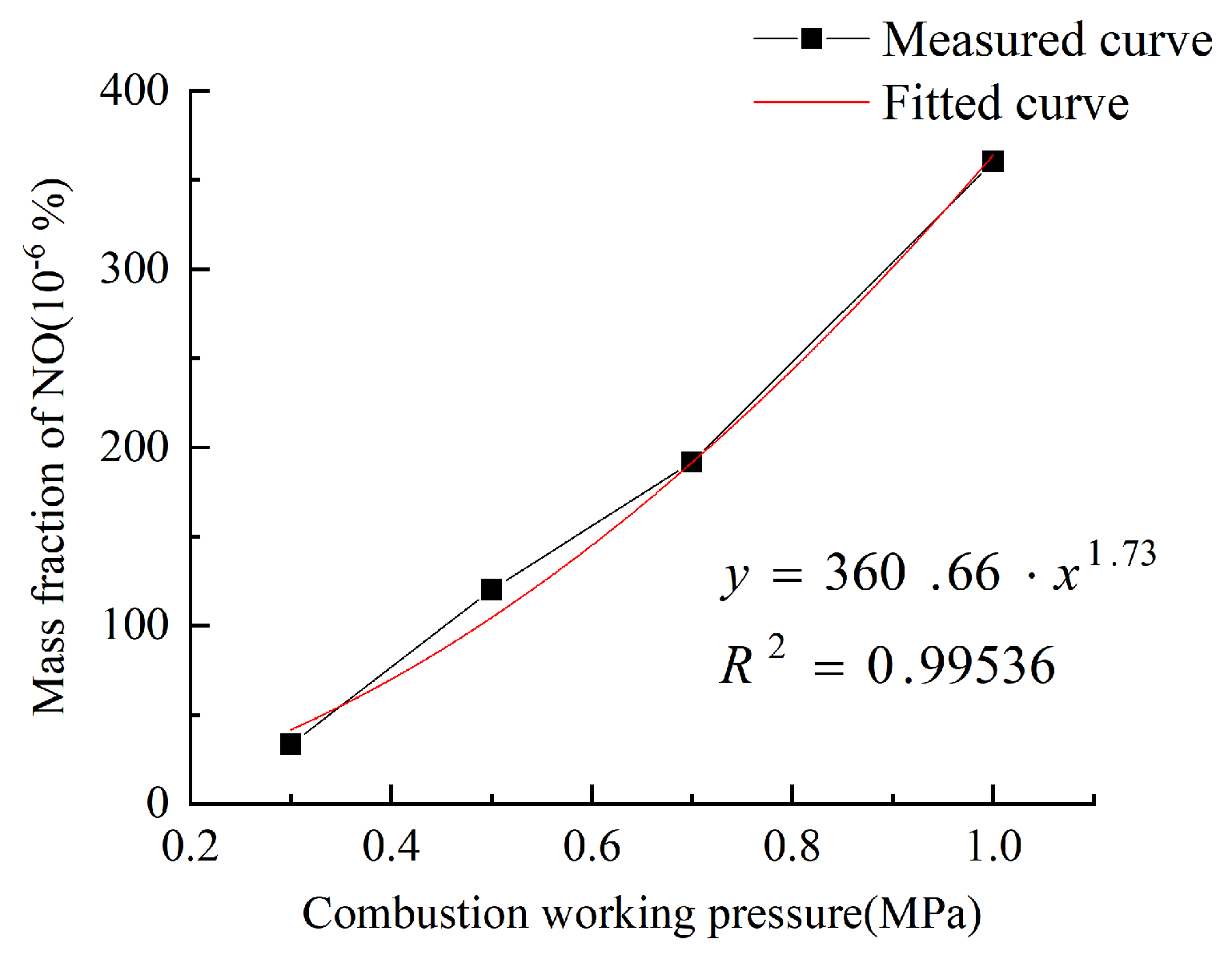
3.1.2. Influence of Combustion Chamber Pressure on NO Emissions
3.2. Influence of Combustion Conditions and Blending Ratio on COx Emissions
3.2.1. Influence of Combustion Chamber Pressure
3.2.2. Influence of Air Inlet Temperature
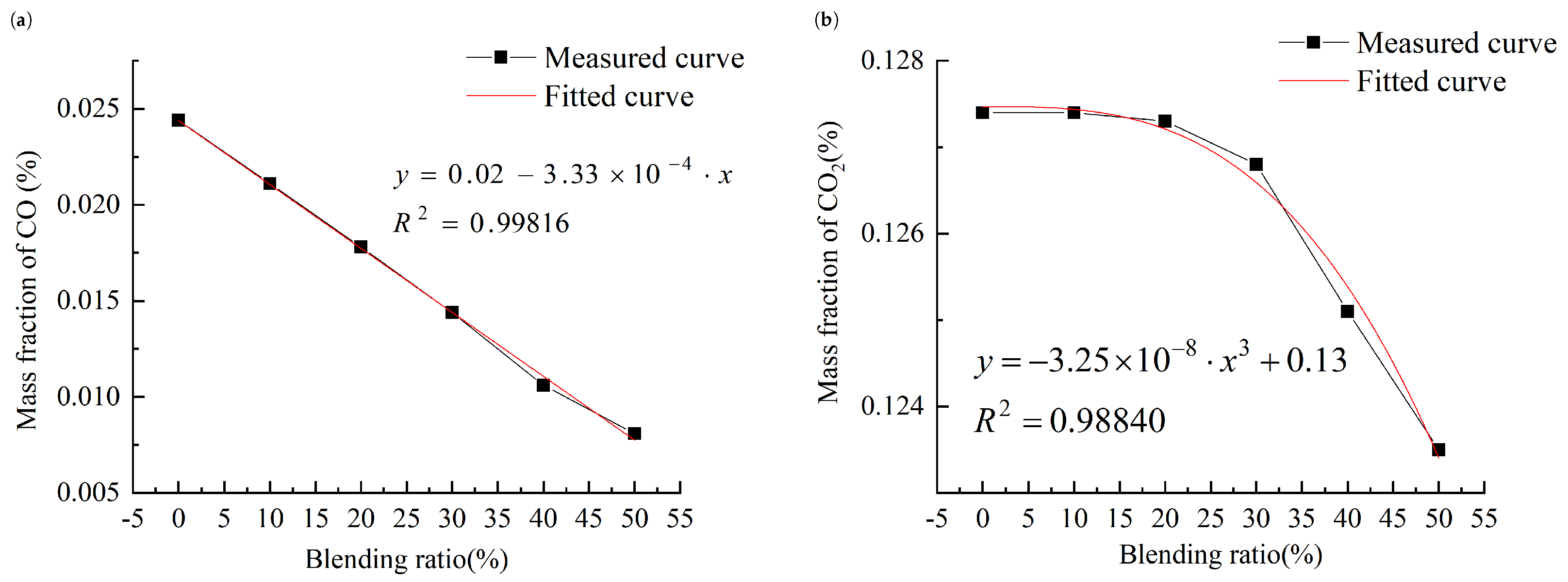
3.2.3. Analysis of the Influence of Blending Ratio
3.3. Analysis of Effects of Combustion Conditions and Biofuel Blending Ratio on NO Emissions
3.3.1. Analysis of the Influence of Combustion Chamber Pressure
3.3.2. Analysis of the Influence of Air Inlet Temperature
3.3.3. Analysis of the Impact of Bio-Fuel Blending Ratio
3.4. Analysis of the Effects of Air Inlet Temperature and Biofuel Blending Ratio on Soot
3.4.1. Analysis of the Influence of Air Inlet Temperature
3.4.2. Analysis of the Effect of Bio-Fuel Blending Ratio
4. Conclusions
- As the working pressure of the combustion chamber increases, the distribution of NO in the combustion chamber is gradually shifted backwards, the emissions of NO are in an exponential relationship with the working pressure, with the growth rate gradually increasing. When the operating pressure of the combustion chamber is greater than 0.5 MPa, the emission of CO increases rapidly. The CO2 emissions increase exponentially with the combustion chamber operating pressure, and the growth rate decreases when the combustion chamber operating pressure is greater than 0.7 MPa.
- As the inlet air temperature increases, the emission of CO gradually increases when the inlet air temperature is above 550 K. When the inlet air temperature is above 450 K, the growth rate of CO2 emission decreases. The generation of NO increases rapidly with the increase of inlet air temperature. However, when the temperature reaches 550 K, the emission rate of NO starts to decrease. At this point, the emission of carbon soot at the outlet approaches zero. When the inlet air temperature exceeds 550 K, the emission of carbon soot increases rapidly.
- When the blending ratio of bio-fuel exceeds 30%, the emissions of CO2 gradually decrease with the increase in the blending ratio. And when the mixing ratio is below 30%, it is largely unaffected by the mixing ratio. With the increase in the blending ratio of bio-fuel, the emissions of CO and carbon particles show a decreasing trend. At the same time, the emissions of NO follow an exponential function with the blending ratio.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Turgut, E.T.; Cavcar, M.; Usanmaz, O.; Yay, O.D.; Dogeroglu, T.; Armutlu, K. Investigating actual landing and takeoff operations for time-in-mode, fuel and emissions parameters on domestic routes in Turkey. Transp. Res. Part D Transp. Environ. 2017, 53, 249–262. [Google Scholar] [CrossRef]
- Parthasarathy, R.N.; Gollahalli, S.; Attaphong, C. Combustion Properties of Jet A/Ethanol Blends in a Porous Media Burner. In Proceedings of the 11th International Energy Conversion Engineering Conference, San Jose, CA, USA, 14–17 July 2013; p. 3677. [Google Scholar]
- Wang, X.; He, Y.; Zhou, T.; Chen, Q.; Ding, C.; Wang, J. Experimental study on fire behaviors of kerosene/additive blends. Fire Technol. 2018, 54, 1841–1869. [Google Scholar]
- Muelas, Á.; Remacha, P.; Ballester, J. Combustion characteristics of isolated free-falling droplets of Jet A blended with ethanol and butanol. In Proceedings of the Turbo Expo: Power for Land, Sea, and Air, Oslo, Norway, 11–15 June 2018; American Society of Mechanical Engineers: New York, NY, USA, 2018; Volume 51067, p. V04BT04A038. [Google Scholar]
- Zheng, L.; Ling, C.; Ubogu, E.A.; Cronly, J.; Ahmed, I.; Zhang, Y.; Khandelwal, B. Effects of alternative fuel properties on particulate matter produced in a gas turbine combustor. Energy Fuels 2018, 32, 9883–9897. [Google Scholar] [CrossRef]
- Lefebvre, A.H. Fuel effects on gas turbine combustion-liner temperature, pattern factor, and pollutant emissions. J. Aircr. 1984, 21, 887–898. [Google Scholar] [CrossRef]
- Jiang, C. Combustion Process Simulation and Analysis of BED Multi-Component Fuel. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2014. [Google Scholar]
- Fantozzi, F.; Laranci, P.; Bianchi, M.; De Pascale, A.; Pinelli, M.; Cadorin, M. CFD simulation of a microturbine annular combustion chamber fuelled with methane and biomass pyrolysis syngas: Preliminary results. In Proceedings of the Turbo Expo: Power for Land, Sea, and Air, Orlando, FL, USA, 8–12 June 2009; Volume 48838, pp. 811–822. [Google Scholar]
- Franco, A.; Giannini, N. Perspectives for the use of biomass as fuel in combined cycle power plants. Int. J. Therm. Sci. 2005, 44, 163–177. [Google Scholar]
- Edwards, T. “Kerosene” Fuels for Aerospace Propulsion-Composition and Properties. In Proceedings of the 38th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Indianapolis, IN, USA, 7–10 July 2002; p. 3874. [Google Scholar]
- Wang, T.S. Thermophysics characterization of kerosene combustion. J. Thermophys. Heat Transf. 2001, 15, 140–147. [Google Scholar] [CrossRef]
- Xiao, B.G. Detailed and simplified chemical kinetic models for the combustion of RP-3 aviation kerosene. J. Aerosp. Power 2010, 34, 1948–1955. (In Chinese) [Google Scholar]
- Li, D.P. Research on the Working Process of the Combustion Chamber of Kerosene Dual-Mode Ramjet Engine. Ph.D. Thesis, National University of Defense Technology, Changsha, China, 2006. (In Chinese). [Google Scholar]
- Huang, S.H.; Xu, S.L.; Liu, X.Y. Numerical study of two-phase flow field in kerosene ramjet engine with afterburning (III) Multi-step chemical reaction characteristics of kerosene in afterburning flow field. J. Propuls. Technol. 2005, 26, 101–105. (In Chinese) [Google Scholar]
- Zhang, Y.H. Analysis of the Combustion Characteristics of Biofuels in the DGEN380 Engine. Ph.D. Thesis, Civil Aviation University of China, Tianjin, China, 2019. (In Chinese). [Google Scholar]
- Li, M.; Wang, Q.; Zhao, Y.; Dai, X.; Shang, W. Combustion and emission characteristics of a novel staged combustor for aero gas turbine engine. Aerosp. Sci. Technol. 2023, 134, 108169. [Google Scholar] [CrossRef]
- Shen, Y.G.; Nie, K.; Xu, J.S. Combustion and emission characteristics of aviation piston engines under different altitudes. J. Propuls. Technol. 2022, 43, 248–256. (In Chinese) [Google Scholar]
- Chen, X.X. Reaction Kinetics Research on the Combustion and Emissions Formation of Aviation Engines. Ph.D Thesis, Shenyang Aerospace University, Shenyang, China, 2013. (In Chinese). [Google Scholar]
- Wang, S.Y. Study on the Combustion Characteristics of Biofuels in a Certain Type of Engine. Ph.D. Thesis, Civil Aviation University of China, Tianjin, China, 2018. (In Chinese). [Google Scholar]
- He, P.; Deng, X.Y.; E, Y.J. Research on NOx emission test and prediction method of low emission combustion chamber. J. Propuls. Technol. 2019, 40, 2762–2770. (In Chinese) [Google Scholar]
- Zhao, J.X. Pollution emissions of civil engines and development trends of low pollution combustion technology. J. Aerosp. Power 2008, 23, 986–996. (In Chinese) [Google Scholar]
- Zheng, L.K.; Zhu, Y.C.; Zhou, Y.B. The influence of the type and content of aromatics on the emissions of aviation engine combustion. J. Aerosp. Power 2021, 36, 1244–1252. (In Chinese) [Google Scholar]
- Li, J.; Zhu, H.; Meng, N. Research on the emission characteristics of biodiesel-ethanol and biodiesel-methanol mixed fuels. Small Intern. Combust. Engine Veh. Technol. 2022, 51, 51–54. (In Chinese) [Google Scholar]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, W.; Zhou, H.; Mao, J.; Ding, Q.; Cui, Y.; Zhao, F.; Xiong, C.; Li, H. Effect of Combustion Conditions and Blending Ratio on Aero-Engine Emissions. Energies 2023, 16, 7060. https://doi.org/10.3390/en16207060
Shan W, Zhou H, Mao J, Ding Q, Cui Y, Zhao F, Xiong C, Li H. Effect of Combustion Conditions and Blending Ratio on Aero-Engine Emissions. Energies. 2023; 16(20):7060. https://doi.org/10.3390/en16207060
Chicago/Turabian StyleShan, Wenjuan, Hanwei Zhou, Jiabing Mao, Qingmiao Ding, Yanyu Cui, Fang Zhao, Changhong Xiong, and Hailong Li. 2023. "Effect of Combustion Conditions and Blending Ratio on Aero-Engine Emissions" Energies 16, no. 20: 7060. https://doi.org/10.3390/en16207060
APA StyleShan, W., Zhou, H., Mao, J., Ding, Q., Cui, Y., Zhao, F., Xiong, C., & Li, H. (2023). Effect of Combustion Conditions and Blending Ratio on Aero-Engine Emissions. Energies, 16(20), 7060. https://doi.org/10.3390/en16207060





