La0.6Sr0.4MnO3-Based Fuel Electrode Materials for Solid Oxide Electrolysis Cells Operating under Steam, CO2, and Co-Electrolysis Conditions
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Powder Preparation
2.2. Powder Characterization
2.3. Cell Fabrication and Electrochemical Characterization
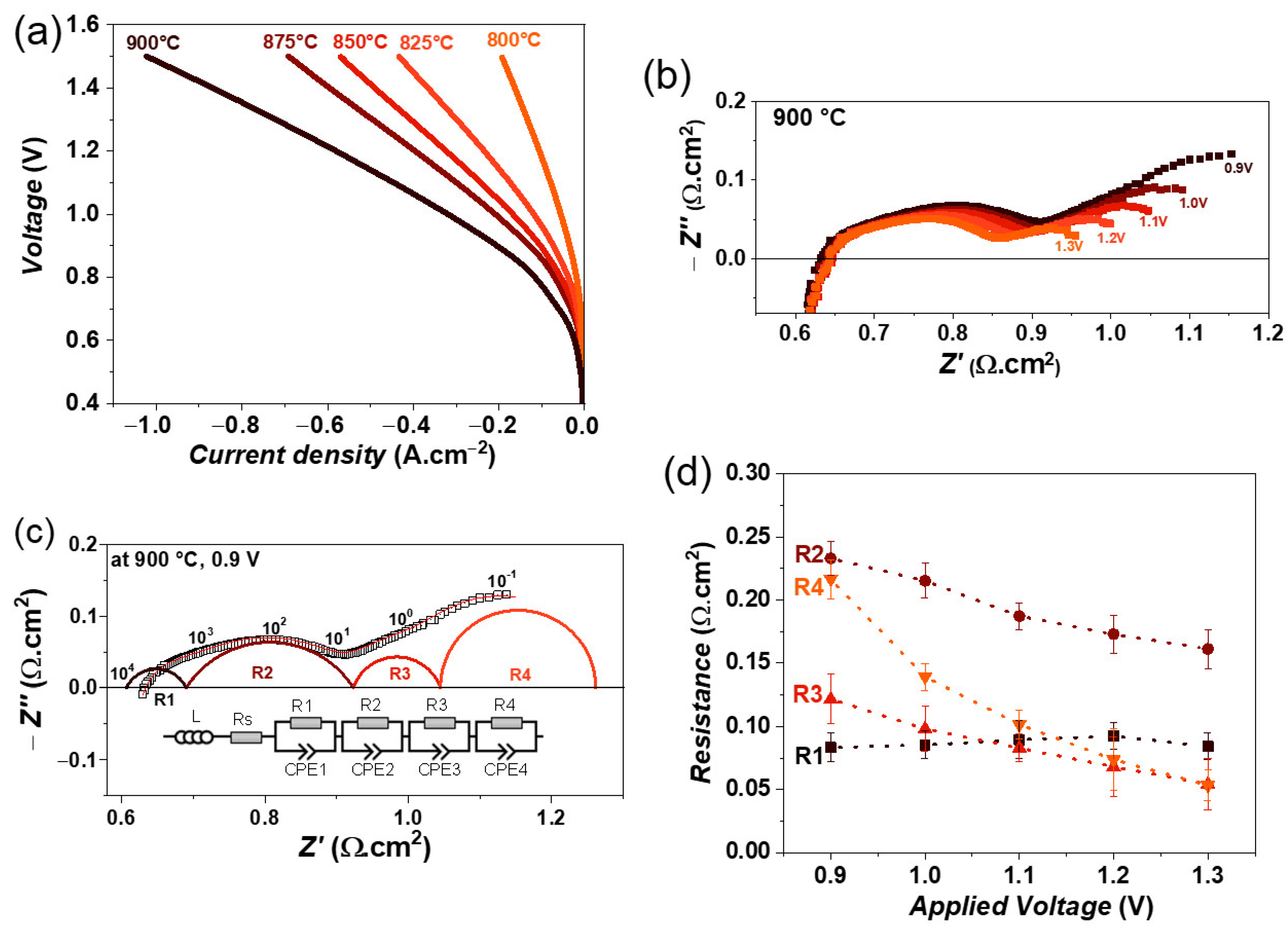
3. Results and Discussion
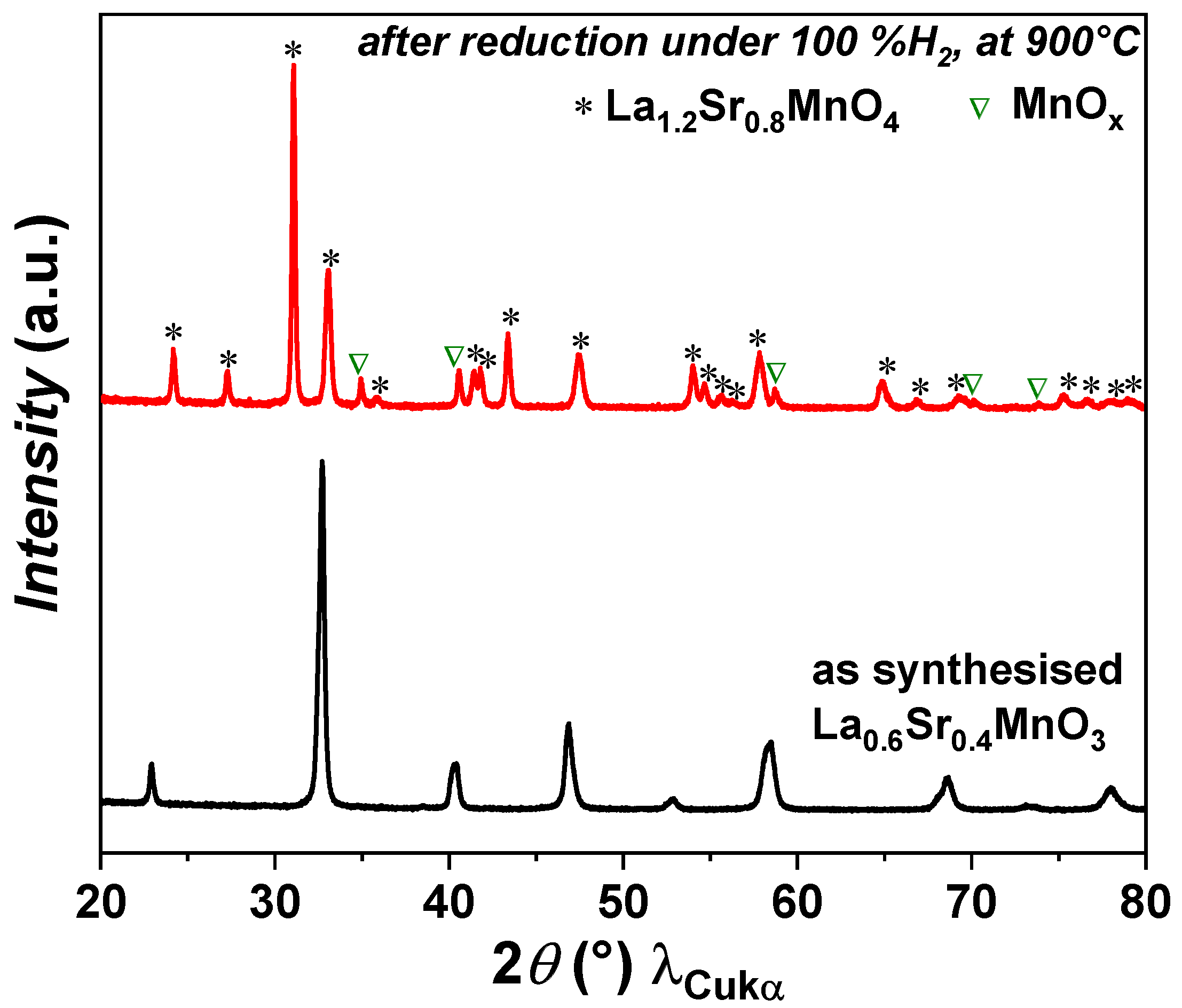
3.1. Phase Identification
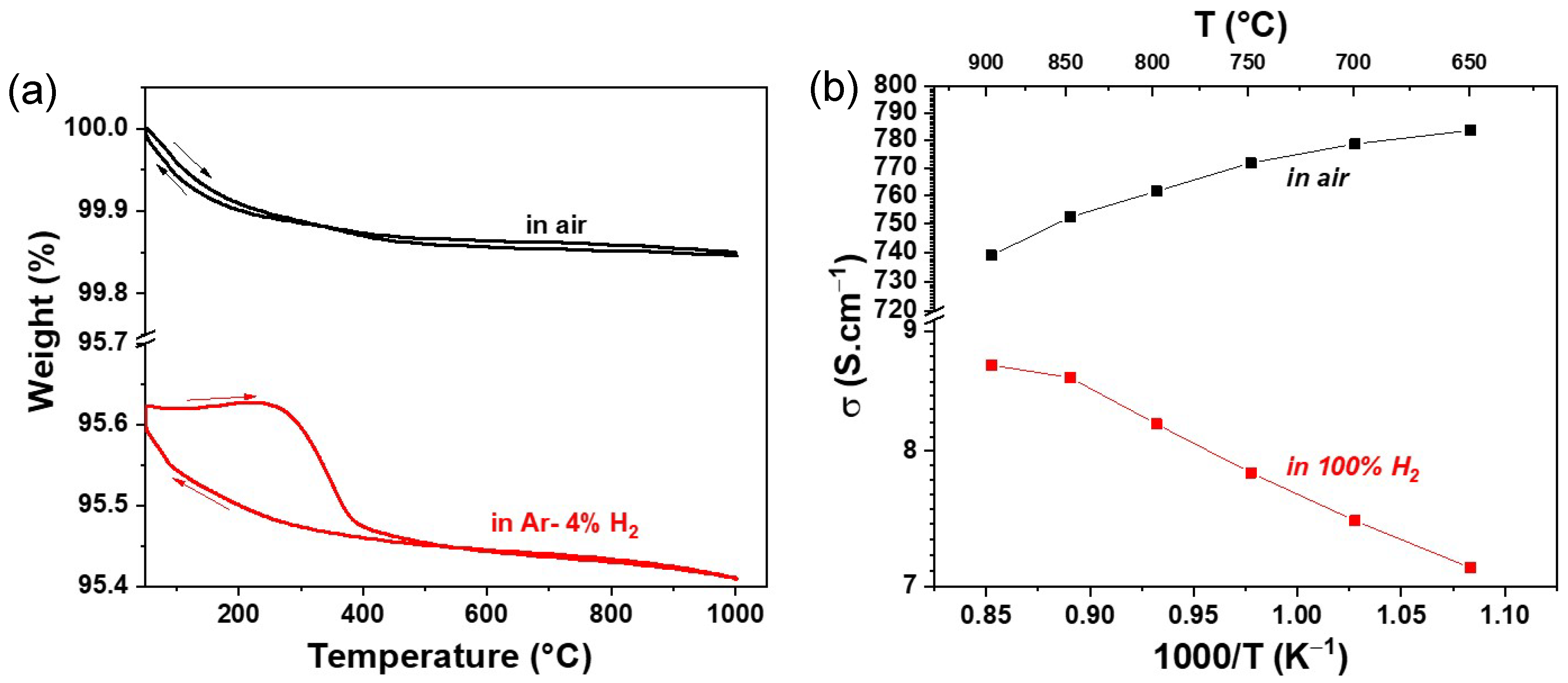
3.2. Thermal Variation of Weight% and DC Conductivity
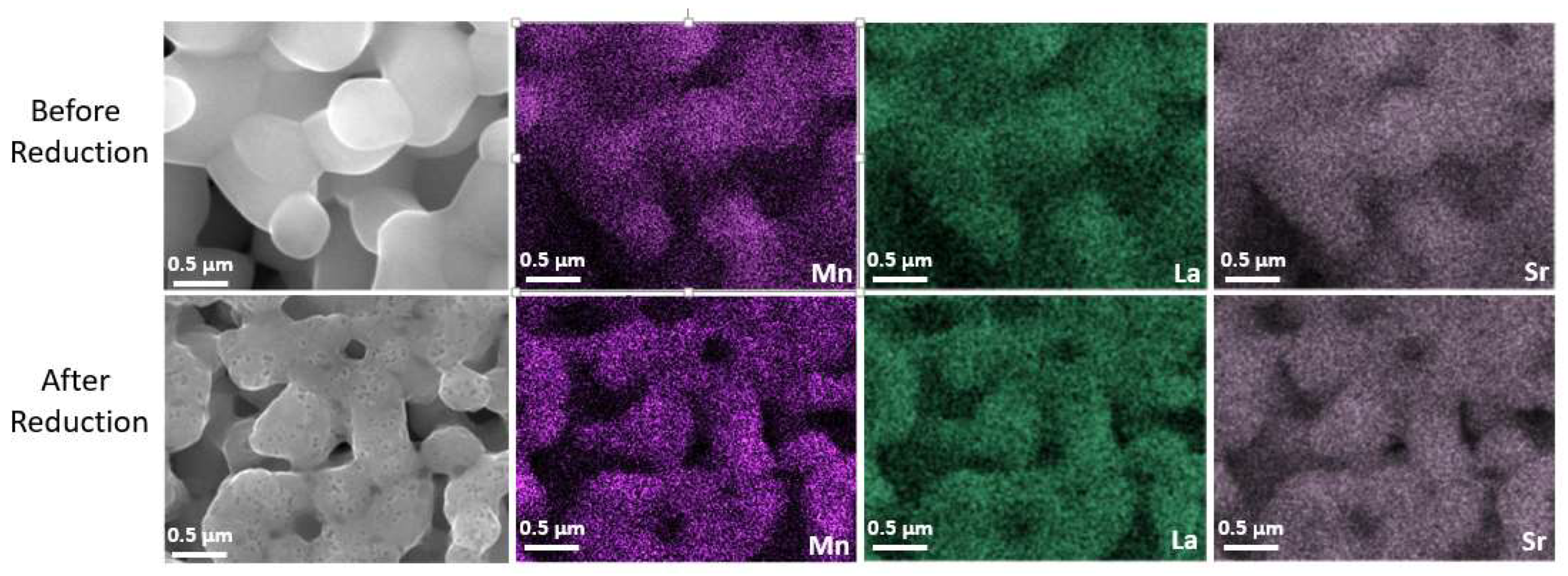
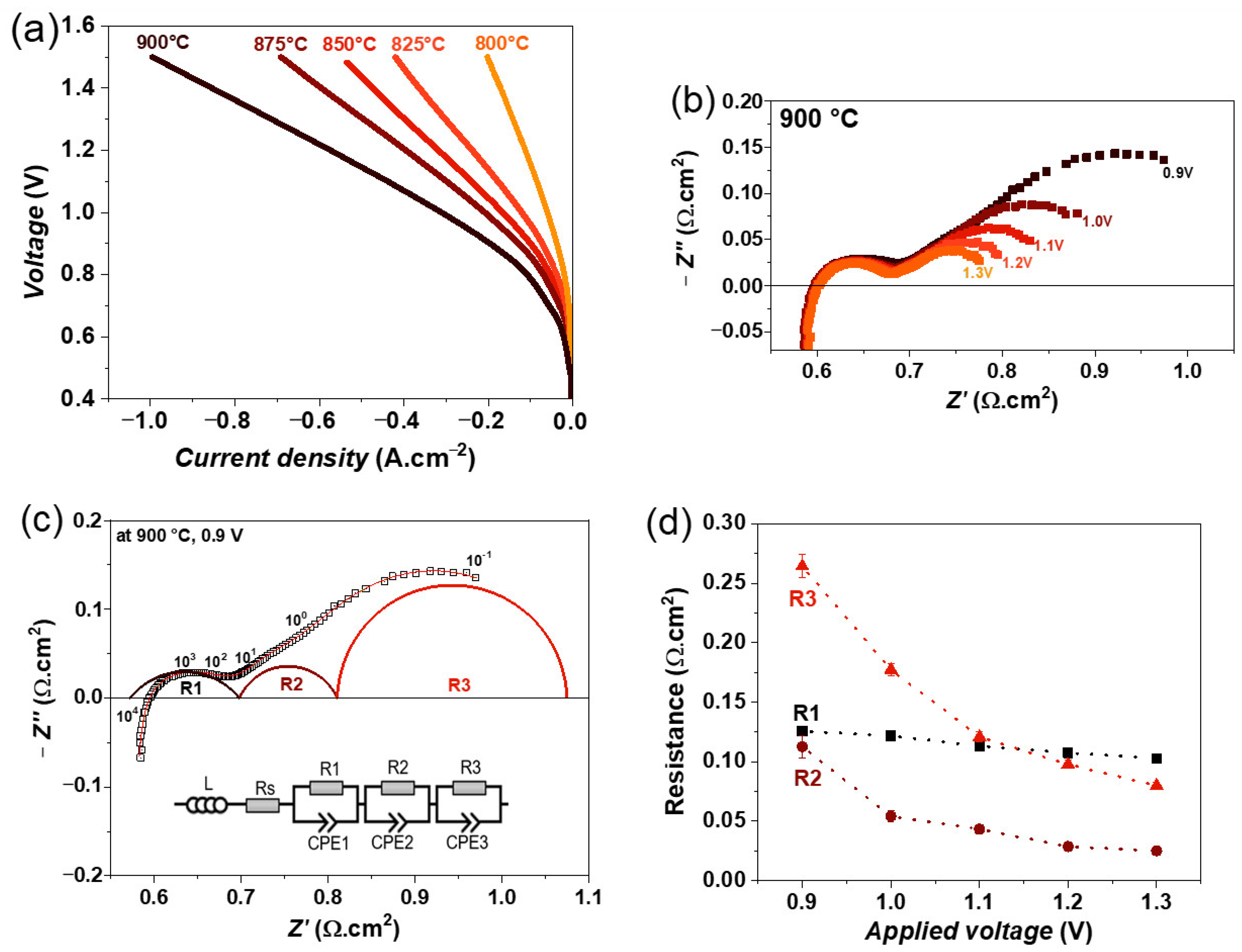
3.3. Single Cell Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Posdziech, O.; Schwarze, K.; Brabandt, J. Efficient hydrogen production for industry and electricity storage via high-temperature electrolysis. Int. J. Hydrogen Energy 2019, 44, 19089–19101. [Google Scholar] [CrossRef]
- Doenitz, W.; Schmidberger, R.; Steinheil, E.; Streicher, R. Hydrogen production by high temperature electrolysis of water vapour. Int. J. Hydrogen Energy 1980, 5, 55–63. [Google Scholar] [CrossRef]
- Wolf, S.E.; Winterhalder, F.E.; Vibhu, V.; de Haart, L.G.J.; Guillon, O.; Eichel, R.-A.; Menzler, N.H. Solid oxide electrolysis cells–current material development and industrial application. J. Mater. Chem. A 2023, 11, 17977–18028. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, S.P. Review—Materials Degradation of Solid Oxide Electrolysis Cells. J. Electrochem. Soc. 2016, 163, F3070–F3083. [Google Scholar] [CrossRef]
- Ma, Z.; Zhou, J.; Li, Y.; Liu, C.; Pu, J.; Chen, X. Developments in CO2 Electrolysis of Solid Oxide Electrolysis Cell with Different Cathodes. Fuel Cells 2020, 20, 650–660. [Google Scholar] [CrossRef]
- Jensen, S.H.; Larsen, P.H.; Mogensen, M. Hydrogen and synthetic fuel production from renewable energy sources. Int. J. Hydrogen Energy 2007, 32, 3253–3257. [Google Scholar] [CrossRef]
- Sala, E.M.; Mazzanti, N.; Mogensen, M.B.; Chatzichristodoulou, C. Current understanding of ceria surfaces for CO2 reduction in SOECs and future prospects–A review. Solid State Ion. 2022, 375, 115833. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Y.; Yu, B.; Wang, J.; Chen, J. Electrochemical characterization and mechanism analysis of high temperature Co-electrolysis of CO2 and H2O in a solid oxide electrolysis cell. Int. J. Hydrogen Energy 2017, 42, 29911–29920. [Google Scholar] [CrossRef]
- Ebbesen, S.D.; Sun, X.; Mogensen, M.B. Understanding the processes governing performance and durability of solid oxide electrolysis cells. Faraday Discuss. 2015, 182, 393–422. [Google Scholar] [CrossRef]
- Wolf, S.E.; Vibhu, V.; Tröster, E.; Vinke, I.C.; Eichel, R.-A.; de Haart, L.G.J. Steam Electrolysis vs. Co-Electrolysis: Mechanistic Studies of Long-Term Solid Oxide Electrolysis Cells. Energies 2022, 15, 5449. [Google Scholar] [CrossRef]
- Vafaeenezhad, S.; Hanifi, A.R.; Laguna-Bercero, M.A.; Etsell, T.H.; Sarkar, P. Microstructure and long-term stability of Ni–YSZ anode supported fuel cells: A review. Mater. Futures 2022, 1, 042101. [Google Scholar] [CrossRef]
- Graves, C.; Ebbesen, S.D.; Jensen, S.H.; Simonsen, S.B.; Mogensen, M.B. Eliminating degradation in solid oxide electrochemical cells by reversible operation. Nat. Mater. 2015, 14, 239–244. [Google Scholar] [CrossRef]
- Hauch, A.; Ebbesen, S.D.; Jensen, S.H.; Mogensen, M. Solid Oxide Electrolysis Cells: Microstructure and Degradation of the Ni/Yttria-Stabilized Zirconia Electrode. J. Electrochem. Soc. 2008, 155, B1184. [Google Scholar] [CrossRef]
- Jiang, S.P.; Chan, S.H. A review of anode materials development in solid oxide fuel cells. J. Mater. Sci. 2004, 39, 4405–4439. [Google Scholar] [CrossRef]
- Park, B.-K.; Scipioni, R.; Cox, D.; Barnett, S.A. Enhancement of Ni–(Y2O3)0.08(ZrO2)0.92 fuel electrode performance by infiltration of Ce0.8Gd0.2O2−δ nanoparticles. J. Mater. Chem. A 2020, 8, 4099–4106. [Google Scholar] [CrossRef]
- Trini, M.; Hauch, A.; De Angelis, S.; Tong, X.; Hendriksen, P.V.; Chen, M. Comparison of microstructural evolution of fuel electrodes in solid oxide fuel cells and electrolysis cells. J. Power Sources 2020, 450, 227599. [Google Scholar] [CrossRef]
- Hauch, A.; Blennow, P. Solid oxide electrolysis cells–Interplay between operating conditions, fuel electrode overpotential and degradation. Solid State Ion. 2023, 391, 116127. [Google Scholar] [CrossRef]
- Ebbesen, S.D.; Jensen, S.H.; Hauch, A.; Mogensen, M.B. High Temperature Electrolysis in Alkaline Cells, Solid Proton Conducting Cells, and Solid Oxide Cells. Chem. Rev. 2014, 114, 10697–10734. [Google Scholar] [CrossRef]
- Fu, Q.; Schefold, J.; Brisse, A.; Nielsen, J.U. Durability Testing of a High-Temperature Steam Electrolyzer Stack at 700 °C. Fuel Cells 2014, 14, 395–402. [Google Scholar] [CrossRef]
- Mougin, J.; Mansuy, A.; Chatroux, A.; Gousseau, G.; Petitjean, M.; Reytier, M.; Mauvy, F. Enhanced Performance and Durability of a High Temperature Steam Electrolysis Stack. Fuel Cells 2013, 13, 623–630. [Google Scholar] [CrossRef]
- Ebbesen, S.D.; Graves, C.; Mogensen, M. Production of Synthetic Fuels by Co-Electrolysis of Steam and Carbon Dioxide. Int. J. Green Energy 2009, 6, 646–660. [Google Scholar] [CrossRef]
- Budiman, R.A.; Konishi, R.; Bisaka, N.; Yashiro, K.; Kawada, T. Time-Dependence of Microstructural Evolution and Performance Degradation of Ni/YSZ Electrode in Co-Electrolysis SOEC. ECS Trans. 2023, 111, 1509. [Google Scholar] [CrossRef]
- Rorato, L.; Shang, Y.; Yang, S.; Hubert, M.; Couturier, K.; Zhang, L.; Vulliet, J.; Chen, M.; Laurencin, J. Understanding the Ni Migration in Solid Oxide Cell: A Coupled Experimental and Modeling Approach. J. Electrochem. Soc. 2023, 170, 034504. [Google Scholar] [CrossRef]
- Shang, Y.; Smitshuysen, A.L.; Yu, M.; Liu, Y.; Tong, X.; Jørgensen, P.S.; Rorato, L.; Laurencin, J.; Chen, M. 3D microstructural characterization of Ni/yttria-stabilized zirconia electrodes during long-term CO2 electrolysis. J. Mater. Chem. A 2023, 11, 12245–12257. [Google Scholar] [CrossRef]
- Mogensen, M.B.; Chen, M.; Frandsen, H.L.; Graves, C.; Hauch, A.; Hendriksen, P.V.; Jacobsen, T.; Jensen, S.H.; Skafte, T.L.; Sun, X. Ni migration in solid oxide cell electrodes: Review and revised hypothesis. Fuel Cells 2021, 21, 415–429. [Google Scholar] [CrossRef]
- Schefold, J.; Brisse, A.; Poepke, H. 23,000 h steam electrolysis with an electrolyte supported solid oxide cell. Int. J. Hydrogen Energy 2017, 42, 13415–13426. [Google Scholar] [CrossRef]
- Singh, V.; Muroyama, H.; Matsui, T.; Hashigami, S.; Inagaki, T.; Eguchi, K. Feasibility of alternative electrode materials for high temperature CO2 reduction on solid oxide electrolysis cell. J. Power Sources 2015, 293, 642–648. [Google Scholar] [CrossRef]
- Léon, A.; Micero, A.; Ludwig, B.; Brisse, A. Effect of scaling-up on the performance and degradation of long-term operated electrolyte supported solid oxide cell, stack and module in electrolysis mode. J. Power Sources 2021, 510, 230346. [Google Scholar] [CrossRef]
- Unachukwu, I.D.; Vibhu, V.; Uecker, J.; Vinke, I.C.; Eichel, R.-A.; de Haart, L.G.J. Electrochemical impedance analysis and degradation behavior of a Ni-GDC fuel electrode containing single cell in direct CO2 electrolysis. J. CO2 Util. 2023, 69, 102423. [Google Scholar] [CrossRef]
- Unachukwu, I.D.; Vibhu, V.; Vinke, I.C.; Eichel, R.-A.; de Haart, L.G.J. Electrochemical and degradation behaviour of single cells comprising Ni-GDC fuel electrode under high temperature steam- and co-electrolysis conditions. J. Power Sources 2023, 556, 232436. [Google Scholar] [CrossRef]
- Uecker, J.; Unachukwu, I.D.; Vibhu, V.; Vinke, I.C.; Eichel, R.-A.; de Haart, L.G.J. Performance, electrochemical process analysis and degradation of gadolinium doped ceria as fuel electrode material for solid oxide electrolysis cells. Electrochim. Acta 2023, 452, 142320. [Google Scholar] [CrossRef]
- Unachukwu, I.D.; Vibhu, V.; Uecker, J.; Vinke, I.C.; Eichel, R.-A.; de Haart, L.G.J. Comparison of the Electrochemical and Degradation Behaviour of Ni-YSZ and Ni-GDC Electrodes Under Steam, Co- and CO2 Electrolysis. ECS Trans. 2023, 111, 1445. [Google Scholar] [CrossRef]
- Vibhu, V.; Vinke, I.C.; Zaravelis, F.; Neophytides, S.G.; Niakolas, D.K.; Eichel, R.-A.; de Haart, L.G.J. Performance and Degradation of Electrolyte-Supported Single Cell Composed of Mo-Au-Ni/GDC Fuel Electrode and LSCF Oxygen Electrode during High Temperature Steam Electrolysis. Energies 2022, 15, 2726. [Google Scholar] [CrossRef]
- Bastidas, D.M.; Tao, S.; Irvine, J.T.S. A symmetrical solid oxide fuel cell demonstrating redox stable perovskite electrodes. J. Mater. Chem. 2006, 16, 1603–1605. [Google Scholar] [CrossRef]
- Slater, P.R.; Fagg, D.P.; Irvine, J.T.S. Synthesis and electrical characterisation of doped perovskite titanates as potential anode materials for solid oxide fuel cells. J. Mater. Chem. 1997, 7, 2495–2498. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, N.; Chuang, K.T.; Luo, J. Progress in La-doped SrTiO3 (LST)-based anode materials for solid oxide fuel cells. RSC Adv. 2014, 4, 118–131. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, C.; Dong, X.; Chen, F. Perovskite Sr2Fe1.5Mo0.5O6−δ as electrode materials for symmetrical solid oxide electrolysis cells. Int. J. Hydrogen Energy 2010, 35, 10039–10044. [Google Scholar] [CrossRef]
- Sengodan, S.; Choi, S.; Jun, A.; Shin, T.H.; Ju, Y.-W.; Jeong, H.Y.; Shin, J.; Irvine, J.T.S.; Kim, G. Layered oxygen-deficient double perovskite as an efficient and stable anode for direct hydrocarbon solid oxide fuel cells. Nat. Mater. 2015, 14, 205–209. [Google Scholar] [CrossRef]
- Tao, S.; Irvine, J.T.S. Study on the structural and electrical properties of the double perovskite oxide SrMn0.5Nb0.5O3−δ. J. Mater. Chem. 2002, 12, 2356–2360. [Google Scholar] [CrossRef]
- Ishihara, T.; Wang, S.; Wu, K.-T. Highly active oxide cathode of La(Sr)Fe(Mn)O3 for intermediate temperature CO2 and CO2-H2O co-electrolysis using LSGM electrolyte. Solid State Ion. 2017, 299, 60–63. [Google Scholar] [CrossRef]
- Jin, C.; Yang, C.; Zhao, F.; Cui, D.; Chen, F. La0.75Sr0.25Cr0.5Mn0.5O3 as hydrogen electrode for solid oxide electrolysis cells. Int. J. Hydrogen Energy 2011, 36, 3340–3346. [Google Scholar] [CrossRef]
- Mizusaki, J.; Yonemura, Y.; Kamata, H.; Ohyama, K.; Mori, N.; Takai, H.; Tagawa, H.; Dokiya, M.; Naraya, K.; Sasamoto, T.; et al. Electronic conductivity, Seebeck coefficient, defect and electronic structure of nonstoichiometric La1−xSrxMnO3. Solid State Ion. 2000, 132, 167–180. [Google Scholar] [CrossRef]
- Lee, H.-K. Electrochemical characteristics of La1−xSrxMnO3 for solid oxide fuel cell. Mater. Chem. Phys. 2003, 77, 639–646. [Google Scholar] [CrossRef]
- Filonova, E.; Pikalova, E. Overview of Approaches to Increase the Electrochemical Activity of Conventional Perovskite Air Electrodes. Materials 2023, 16, 4967. [Google Scholar] [CrossRef]
- Mizusaki, J.; Tagawa, H.; Naraya, K.; Sasamoto, T. Nonstoichiometry and thermochemical stability of the perovskite-type La1−xSrxMnO3−δ. Solid State Ion. 1991, 49, 111–118. [Google Scholar] [CrossRef]
- Chung, Y.S.; Kim, T.; Shin, T.H.; Yoon, H.; Park, S.; Sammes, N.M.; Kim, W.B.; Chung, J.S. In situ preparation of a La1.2Sr0.8Mn0.4Fe0.6O4 Ruddlesden–Popper phase with exsolved Fe nanoparticles as an anode for SOFCs. J. Mater. Chem. A 2017, 5, 6437–6446. [Google Scholar] [CrossRef]
- Tao, S.; Irvine, J.T.S. Synthesis and Characterization of (La0.75Sr0.25)Cr0.5Mn0.5O3−δ, a Redox-Stable, Efficient Perovskite Anode for SOFCs. J. Electrochem. Soc. 2004, 151, A252. [Google Scholar] [CrossRef]
- Qin, Q.; Xie, K.; Wei, H.; Qi, W.; Cui, J.; Wu, Y. Demonstration of efficient electrochemical biogas reforming in a solid oxide electrolyser with titanate cathode. RSC Adv. 2014, 4, 38474–38483. [Google Scholar] [CrossRef]
- Wolf, S.E.; Vibhu, V.; Coll, C.L.; Eyckeler, N.; Vinke, I.C.; Eichel, R.-A.; de Haart, L.G.J. Long-Term Stability of Perovskite-Based Fuel Electrode Material Sr2Fe2-XMoxO6−δ–GDC for Enhanced High-Temperature Steam and CO2 Electrolysis. ECS Trans. 2023, 111, 2119. [Google Scholar] [CrossRef]
- Marrero-López, D.; Peña-Martínez, J.; Ruiz-Morales, J.C.; Pérez-Coll, D.; Aranda, M.A.G.; Núñez, P. Synthesis, phase stability and electrical conductivity of Sr2MgMoO6−δ anode. Mater. Res. Bull. 2008, 43, 2441–2450. [Google Scholar] [CrossRef]
- Ge, B.; Ma, J.T.; Ai, D.; Deng, C.; Lin, X.; Xu, J. Sr2FeNbO6 Applied in Solid Oxide Electrolysis Cell as the Hydrogen Electrode: Kinetic Studies by Comparison with Ni-YSZ. Electrochim. Acta 2015, 151, 437–446. [Google Scholar] [CrossRef]
- Bernadet, L.; Moncasi, C.; Torrell, M.; Tarancón, A. High-performing electrolyte-supported symmetrical solid oxide electrolysis cells operating under steam electrolysis and co-electrolysis modes. Int. J. Hydrogen Energy 2020, 45, 14208–14217. [Google Scholar] [CrossRef]
- Sandoval, M.V.; Cardenas, C.; Capoen, E.; Roussel, P.; Pirovano, C.; Gauthier, G.H. Performance of La0.5Sr1.5MnO4±δ Ruddlesden-Popper manganite as electrode material for symmetrical Solid Oxide Fuel Cells. Part B. The Hydrogen Oxidation Reaction. Electrochim. Acta 2020, 353, 136494. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A.; Kilner, J.A.; Skinner, S.J. Performance and Characterization of (La, Sr)MnO3/YSZ and La0.6Sr0.4Co0.2Fe0.8O3 Electrodes for Solid Oxide Electrolysis Cells. Chem. Mater. 2010, 22, 1134–1141. [Google Scholar] [CrossRef]



| Materials | T (°C) | σ (Air), S·cm−1 | σ (Reducing Conditions), S·cm−1 | Ref. |
|---|---|---|---|---|
| LSCM (La0.75Sr0.25)Cr0.5Mn0.5O3-δ | 900 | 38.6 | 1.49 (in 5% H2/Ar) | [47] |
| LSMF La0.6Sr0.4Mn0.2Fe0.8O3 | 800 | 50.3 | 2.8 (in 20% H2/N2) | [46] |
| LST La0.2Sr0.8TiO3 | 800 | 0.7 | 1.1 (in 5% H2/Ar) | [48] |
| Fe doped LST | 800 | 0.58 | 1.54 (in 5% H2/Ar) | [48] |
| SFM Sr2Fe1.5Mo0.5O6-δ | 900 | 9.2 | 14.6 (in 100% H2) | [49] |
| Sr2Fe1.0Mo1.0O6-δ | 900 | 3.5 | 13.5 (in 100%H2) | [49] |
| Sr2MgMoO6−δ | 800 | 3 × 10−3 | 0.8 (in 5% H2/Ar) | [50] |
| Sr2FeNbO6−δ | 850 | 0.05 | 2.22 (wet 20% H2) | [51] |
| LSM | 900 | 740 | 8.7 (100% H2) | This work. |
| Electrolyte Supported Single Cells | Fuel Gas Composition | Current Density (A·cm−2) at 900 °C | Ref. |
|---|---|---|---|
| Sr2Fe1.5Mo0.5O6-δ (SFM)/GDC/YbScSZ/GDC/SFM | 90/10 of H2O/Ar | 1.4 A.cm−2 at 1.3 V | [52] |
| Sr2Fe1.5Mo0.5O6-δ (SFM)/GDC/YbScSZ/GDC/SFM | 75/25 of H2O/CO2 | 1.1 A.cm−2 at 1.3 V | [52] |
| Sr2Fe1.5Mo0.5O6-δ (SFM)/GDC/YSZ/GDC/LSCF | 50/50 of H2O/H2 | 1.42 A·cm−2 at 1.5 V | [49] |
| Sr2Fe1.0Mo1.0O6-δ (SFM)/GDC/YSZ/GDC/LSCF | 50/50 of H2O/H2 | 1.22 A·cm−2 at 1.5 V | [49] |
| Ni-GDC/8YSZ/GDC/LSCF | 50/50 of H2O/H2 | 1.31 A·cm−2 at 1.5 V | [30] |
| Ni-GDC/8YSZ/GDC/LSCF | 40/40/20 of H2O/CO2/CO | 1.37 A·cm−2 at 1.5 V | [30] |
| Ni-GDC/8YSZ/GDC/LSCF | 80/20 of CO2/CO | 1.16 A·cm−2 at 1.5 V | [29,32] |
| Ni-YSZ/8YSZ/GDC/LSCF | 50/50 of H2O/H2 | 0.91 A·cm−2 at 1.5 V | [32] |
| Ni-YSZ/8YSZ/GDC/LSCF | 40/40/20 of H2O/CO2/CO | 1.06 A·cm−2 at 1.5 V | [32] |
| Ni-YSZ/8YSZ/GDC/LSCF | 80/20 of CO2/CO | 0.63 A·cm−2 at 1.5 V | [29,32] |
| GDC/8YSZ/GDC/LSCF | 50/50 of H2O/H2 | 0.91 A·cm−2 at 1.5 V | [31] |
| GDC/8YSZ/GDC/LSCF | 40/40/20 of H2O/CO2/CO | 0.97 A·cm−2 at 1.5 V | [31] |
| La0.6Sr0.4Fe0.8Mn0.2O3-δ (LSFM)/LSGM/Ba0.6La0.4CoO3–δ(BLC) | 50/1/49 of CO2/CO/Ar | 0.52 A·cm−2 at 1.6 V | [40] |
| LSM/8YSZ/LSM + YSZ/LSM | 50/50 of H2O/N2 | 1 A·cm−2 at 1.5 V | This work |
| LSM/8YSZ/LSM + YSZ/LSM | 50/50 of H2O/CO2 | 1.02 A·cm−2 at 1.5 V | This work. |
| LSM/8YSZ/LSM + YSZ/LSM | 100% CO2 | 0.98 A·cm−2 at 1.5 V | This work. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vibhu, V.; Vinke, I.C.; Eichel, R.-A.; de Haart, L.G.J. La0.6Sr0.4MnO3-Based Fuel Electrode Materials for Solid Oxide Electrolysis Cells Operating under Steam, CO2, and Co-Electrolysis Conditions. Energies 2023, 16, 7115. https://doi.org/10.3390/en16207115
Vibhu V, Vinke IC, Eichel R-A, de Haart LGJ. La0.6Sr0.4MnO3-Based Fuel Electrode Materials for Solid Oxide Electrolysis Cells Operating under Steam, CO2, and Co-Electrolysis Conditions. Energies. 2023; 16(20):7115. https://doi.org/10.3390/en16207115
Chicago/Turabian StyleVibhu, Vaibhav, Izaak C. Vinke, Rüdiger-A. Eichel, and L. G. J. (Bert) de Haart. 2023. "La0.6Sr0.4MnO3-Based Fuel Electrode Materials for Solid Oxide Electrolysis Cells Operating under Steam, CO2, and Co-Electrolysis Conditions" Energies 16, no. 20: 7115. https://doi.org/10.3390/en16207115
APA StyleVibhu, V., Vinke, I. C., Eichel, R.-A., & de Haart, L. G. J. (2023). La0.6Sr0.4MnO3-Based Fuel Electrode Materials for Solid Oxide Electrolysis Cells Operating under Steam, CO2, and Co-Electrolysis Conditions. Energies, 16(20), 7115. https://doi.org/10.3390/en16207115








