Selective Catalytic Hydrogenation of Vegetable Oils over Copper-Based Catalysts Supported on Amorphous Silica
Abstract
:1. Introduction
- Synthesize new Cu-based catalysts supported on amorphous silica following green chemistry principles. For this reason, two different synthesis methods are used and compared: hydrolysis precipitation (HP) and ammonia-evaporation (AE);
- Perform an experimental test campaign in a semi-batch reactor to evaluate the conversion and selectivity as a function of temperature, pressure, and catalyst/oil mass ratio, with particular attention to low pressure and a low loading of the catalyst given with the scope of reducing the impact of the process;
- Fully characterize the developed catalysts correlating their properties to the activities and selectivities;
- Select the best-performing catalyst for designing an industrial-scale plant and optimizing the operating parameters, with particular attention to the reaction time, avoiding complete fatty acid saturation;
- Design a semi-continuous process, including the introduction of a loop reactor to improve mass transfer, optimize heat recovery, and reduce operative costs.
2. Materials and Methods
2.1. Hydrolysis Precipitation Method (HP)
- A required amount of Cu(NO3)2·3H2O was dissolved in deionized water (20 mL), and the solution was then added to a solution of TEOS. The TEOS:EtOH:H2O ratio was 1:1:1 by weight. After stirring for 1.5 h at 400 rpm, the mixture and an (NH4)2CO3 solution (0.25 M) were added dropwise to deionized water at 80 °C. The pH was maintained between 7 and 7.5.
- The resulting suspension was stirred at 80 °C for 18 h, separated with filtration, and washed with deionized water, checking the conductivity of the permeate.
- The recovered solid was dried for 24 h at 105 °C and then calcined at 550 °C under static air, with a heating rate of 5 °C/min and 6 h of dwell.
2.2. Ammonia-Evaporation Method (AE)
- Cu(NO3)2·3H2O was dissolved in distilled water (50 mL), and ammonia solution was added till the resulting solution presented a pH between 11 and 12. The solution was stirred at room temperature for 30 min, forming the tri-ammonium copper nitrate.
- The amount of silica precursor was added to the copper ammonia complex solution and stirred for 4 h at room temperature, then heated at 90 °C to evaporate the ammonia up to a pH value between 6 and 7.
- The precipitate was separated with filtration and washed with deionized water, and the recovered solid was dried for 24 h at 105 °C, then calcined at 550 °C under static air.
2.3. Characterization Methods
- V.A.R.I.A.N. 720-ES ICP-AES ICP-AES (Inductively Coupled Plasma—Atomic Emission Spectroscopy), equipped with a custom-designed charge coupled device (CCD) highly sensitive photon detector. The estimated detection limit for the quantified Cu was 5 μg/L. The samples were mineralized in a mixture of concentrated strong acids, then diluted in deionized water to be ready for nebulization operated by the device.
- A MICROMERITICS ASAP 2420 to determine surface area and porosity, recording N2 adsorption and desorption isotherms at −196 °C. ASAP 2420 software v2.09 performed calculations of surface area (SBET), pore volume (VBJH, des), average pore diameters (Dav, BJH) and pore size distribution. Degassing of the powder was performed before the analysis under a weak vacuum with a 10 °C min−1 heating ramp until 250 °C, and it dwelled overnight (at least 8 h).
- X-ray diffractometer BRUKER AXS D8 ADVANCED, using CuKα1 radiation to detect crystalline phases with a Bragg–Brentano geometry. XRD spectra were recorded in a Bragg angle range from 20° and 90° at 0.0158° scanning step and a sampling time of 1s per step (3 s for amorphous phases). Phase identification was performed with the DIFRAC.EVA V5.2.0.3 software, including the JCDPS (Joint Committee on Powder Diffraction Standards) database. The spectra acquired on powder samples allowed for the estimation of average crystallite sizes (L) of the main detected phases with Scherrer equation [55].
- Raman spectrometer HORIBA JOBIN YVON LabRam ARAMIS, equipped with two lasers at 532 nm (green) and 785 nm (red) and coupled with an optical confocal microscope. The range of acquired signals was from 200 cm−1 to 1850 cm−1,, and the spectra were compared to results from the literature for copper [56] and silica catalysts [57,58,59,60,61] and online databases collected by RRUFF Project [62], the WURM Project [63], and the database of the University of Parma [64].
- FT-IR NEXUS 870 THERMONICOLET: Fast Fourier Transform Infrared Spectroscopy Attenuated Total Reflectance (FTIR-ATR). Measures were obtained in the medium infrared field, ranging from 400 cm−1 to 4000 cm−1.
- MICROMERITICS AUTOCHEM II 2920 analyzer for temperature programmed reduction (TPR) and temperature programmed desorption (TPD-N2O) of 200mg of catalyst (50–150 µm). TPR started with a 10 °C min−1 ramp heating up to 450 °C, letting it dwell for 2 h at 450 °C under 50 NmL min−1 flow of reducing gas (10%vol H2 in Ar). A TCD (Thermal Conductivity Detector) measured H2 consumption and then recorded temperature versus time profile, elaborated with AUTOCHEM II software v4.02. The metallic copper surface was characterized by N2O temperature programmed desorption of 400 mg of catalyst (50–150 µm) reduced under 10%vol H2 in Ar (50 NmL min−1 flow rate) at 300 °C overnight after a 3 °C min−1 ramp [65,66]. Before the N2O TPD, a flow rate of 2% N2O in Ar (50 NmL min−1) was sent to the TCD detector; the TCD signal was acquired until a stable signal was reached. The loop was therefore purged with Ar, and then, the sample was oxidized with the same flowrate of 2% N2O in Ar (50 NmL min−1) for 1 h at 50 °C.
- THERMO VG MULTILAB 2000, which performed XPS (X-ray Photoelectron Spectroscopy), determining silica-supported catalysts’ superficial elemental composition and electronic state. The instrument could operate under an ultra-high vacuum.
- ZEISS GEMINI SEM 500 device equipped with OXFORD ENERGY 250 INCAx-act detector and combined with scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS), operating at 15 kV, BSE (Back Scattering Electron) Z-contrast mode.
- JEOL 2100 LaB6 (lanthanum hexaboride filament), operating a 200 kV, with punctual resolution equal to 0.2 nm in parallel mode and 2–3 nm works in STEM (scanning transmission electron microscopy) mode, equipped with an SDD detector (30 mm2) for elemental analyses with in situ EDS.
2.4. Reaction Tests
3. Results and Discussion
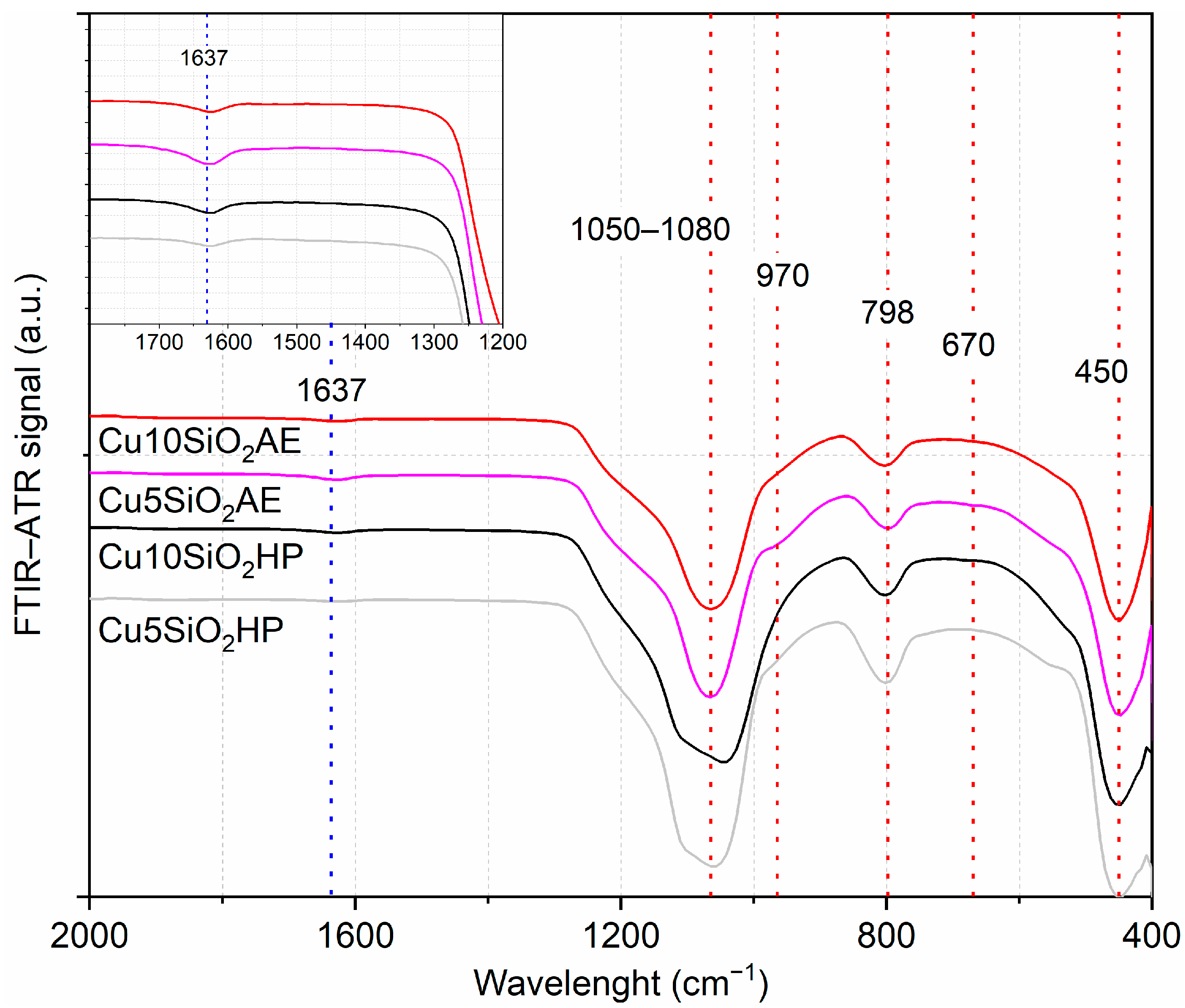
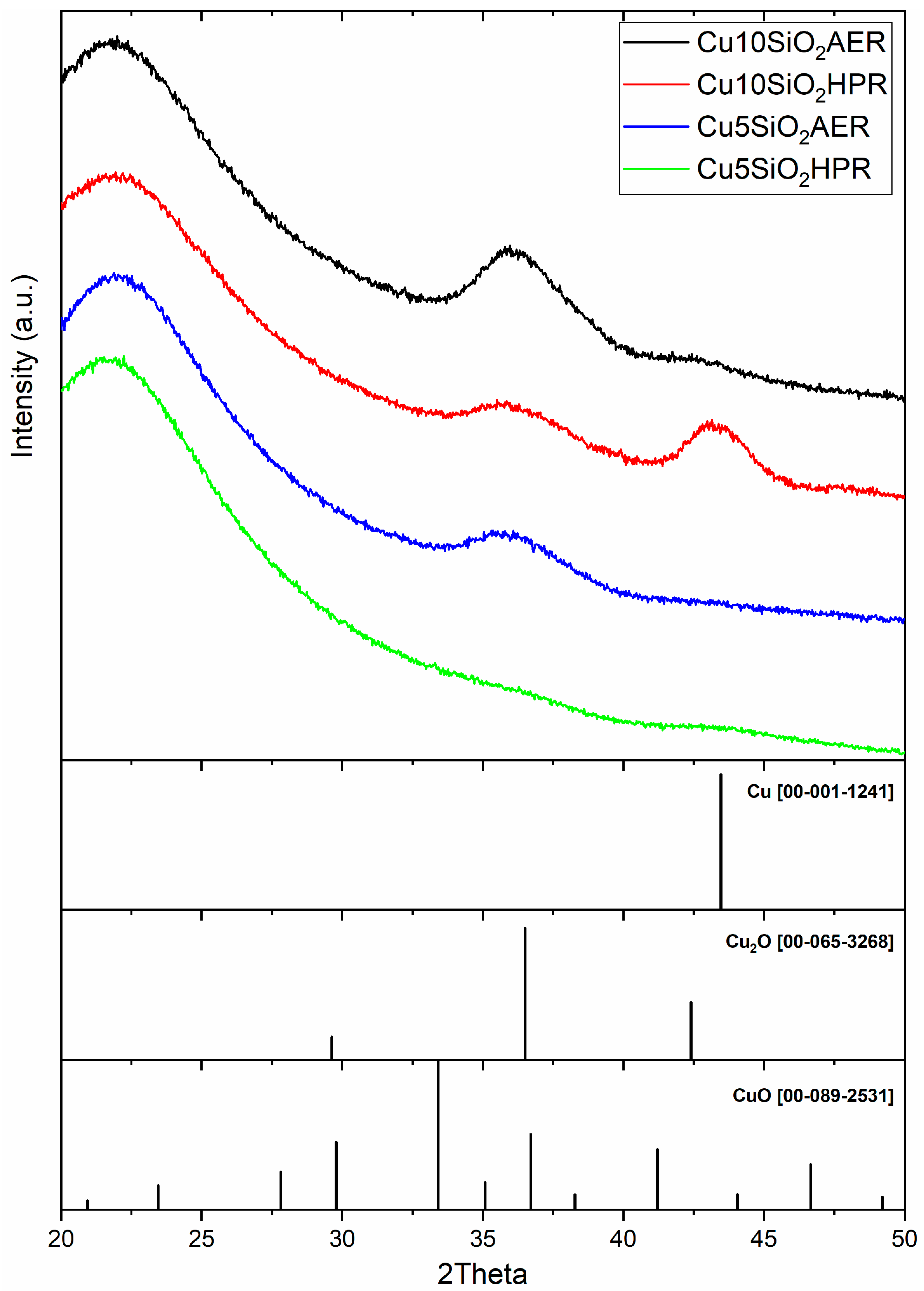
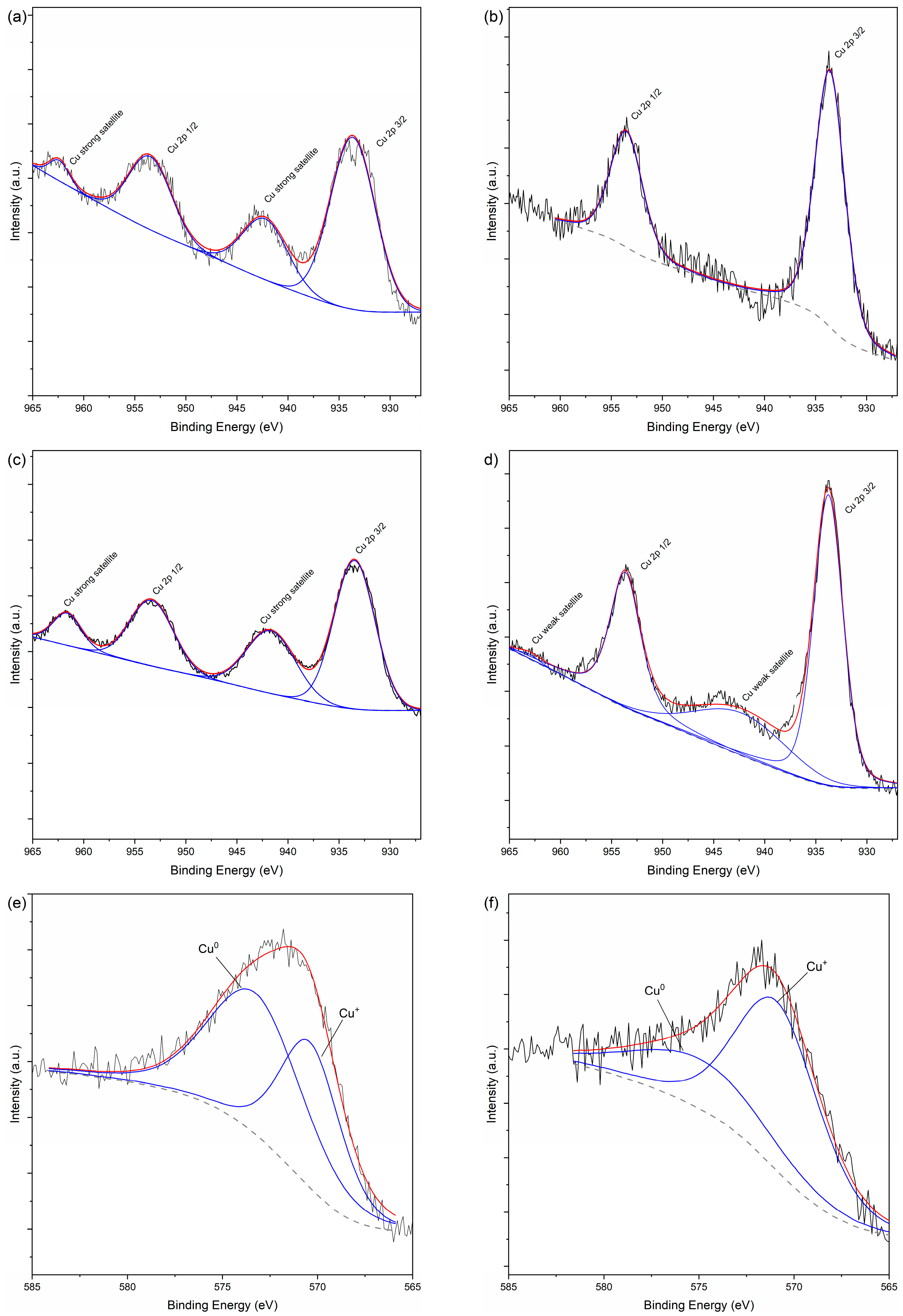
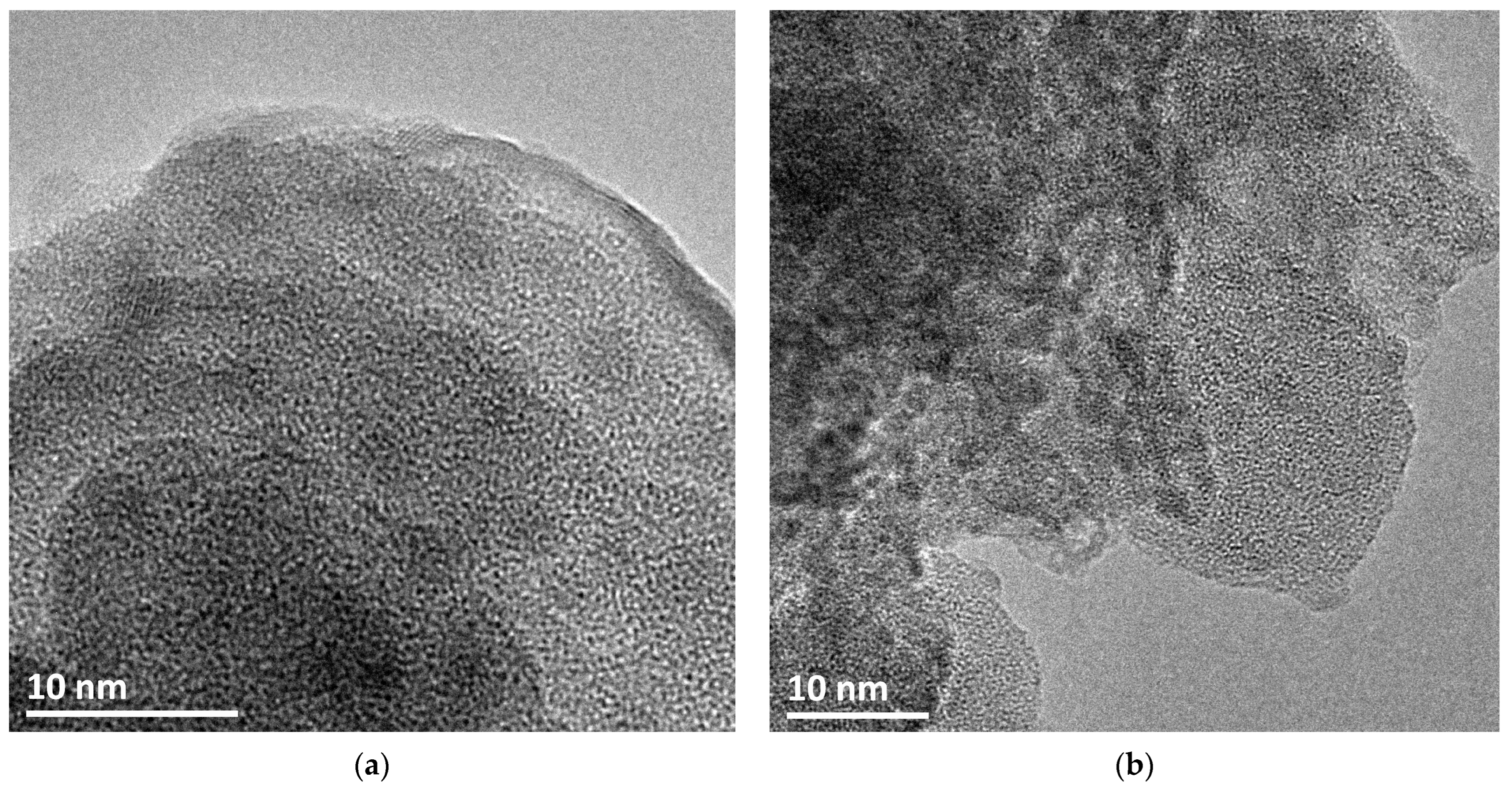
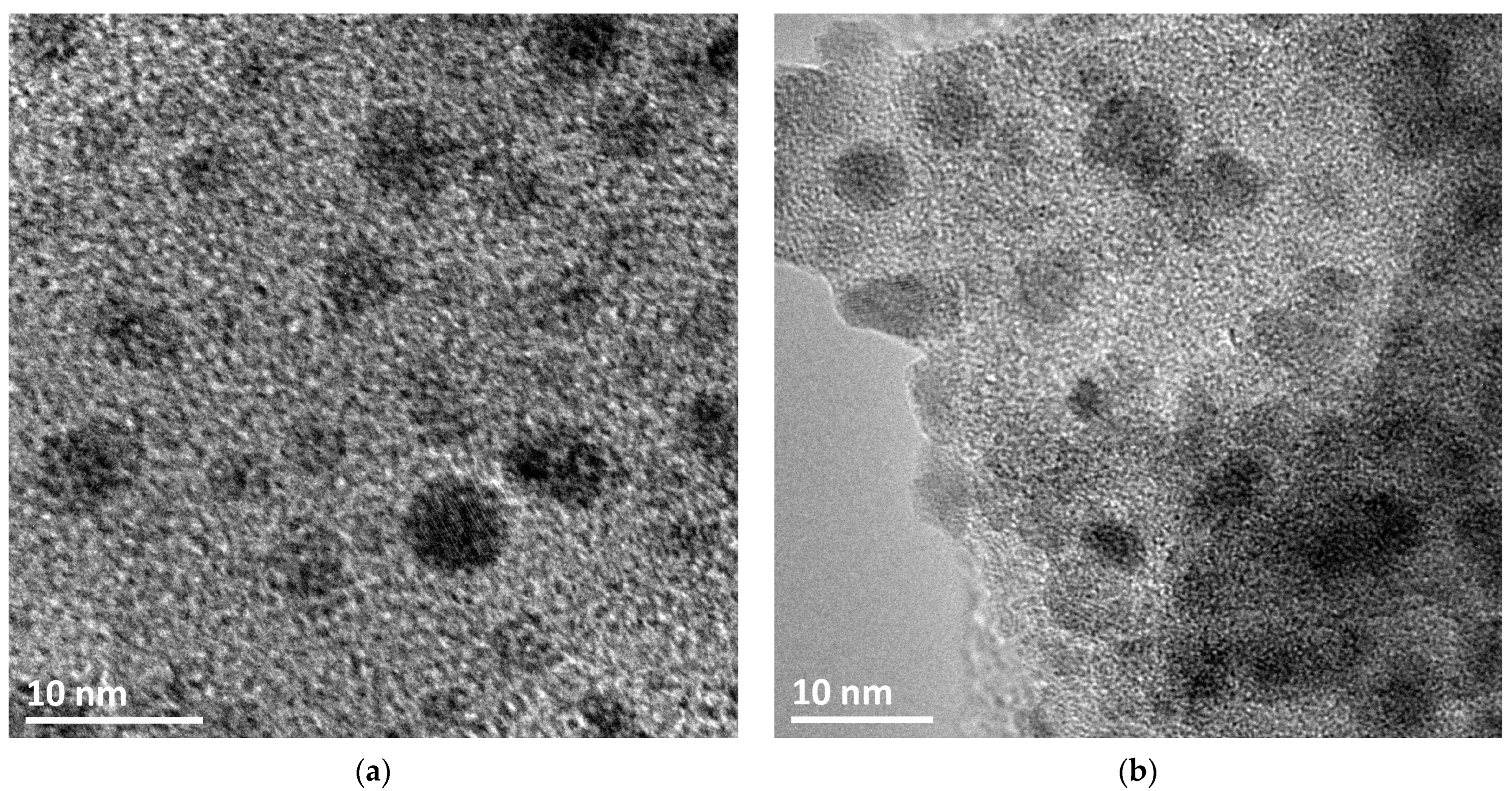
3.1. Characterization of Catalysts
- At 450 cm−1 and 800 cm−1, the symmetric stretching of siloxane groups (Si-O-Si);
- At 1060 cm−1, the asymmetric stretching of the same siloxane groups;
- The shoulder at 960 cm−1, the angular deformation of the Si-OH silanol group.
- Raman spectroscopy is combined with FTIR analysis to integrate the information between the two techniques for copper-based catalysts. As-synthetized samples present the Raman bands of copper phyllosilicate called chrysocolla [78,79] (Figure 3). Chrysocolla and copper hydroxide structures are usually found together; sometimes, the copper hydroxide could be reformed by decomposing the copper phyllosilicate [60]. The reduced samples (HPR and AER, respectively) show less intense bands, and the Cu+ oxide bands appear formed from the reduction of chrysocolla or phyllosilicates-like phases, as illustrated in the literature [50,54,80].
3.2. Catalyst Reactivity Tests
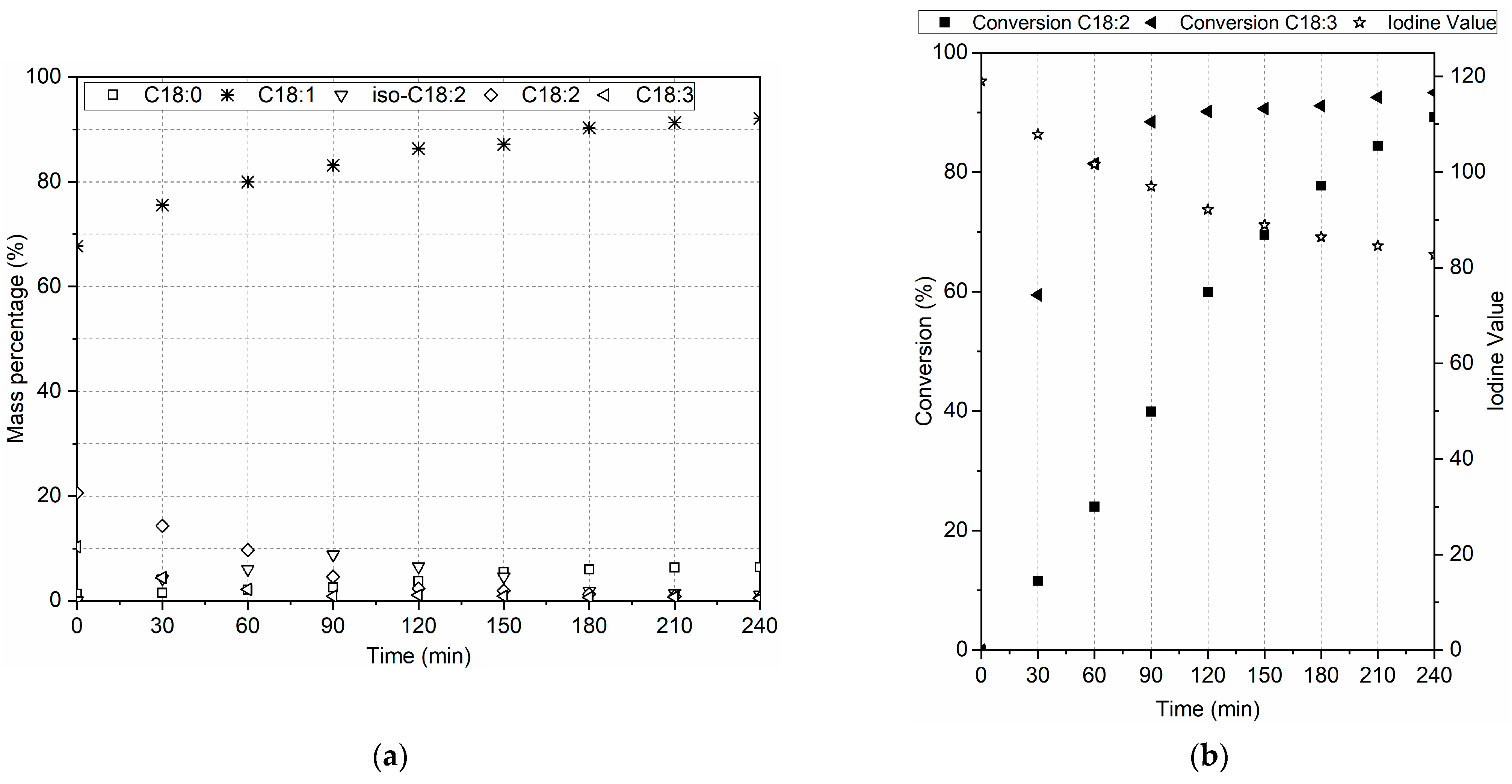
3.2.1. Effect of Copper Loading, Temperature, and Pressure
3.2.2. Effect of Catalyst Concentration
3.2.3. Effect of Different Oil
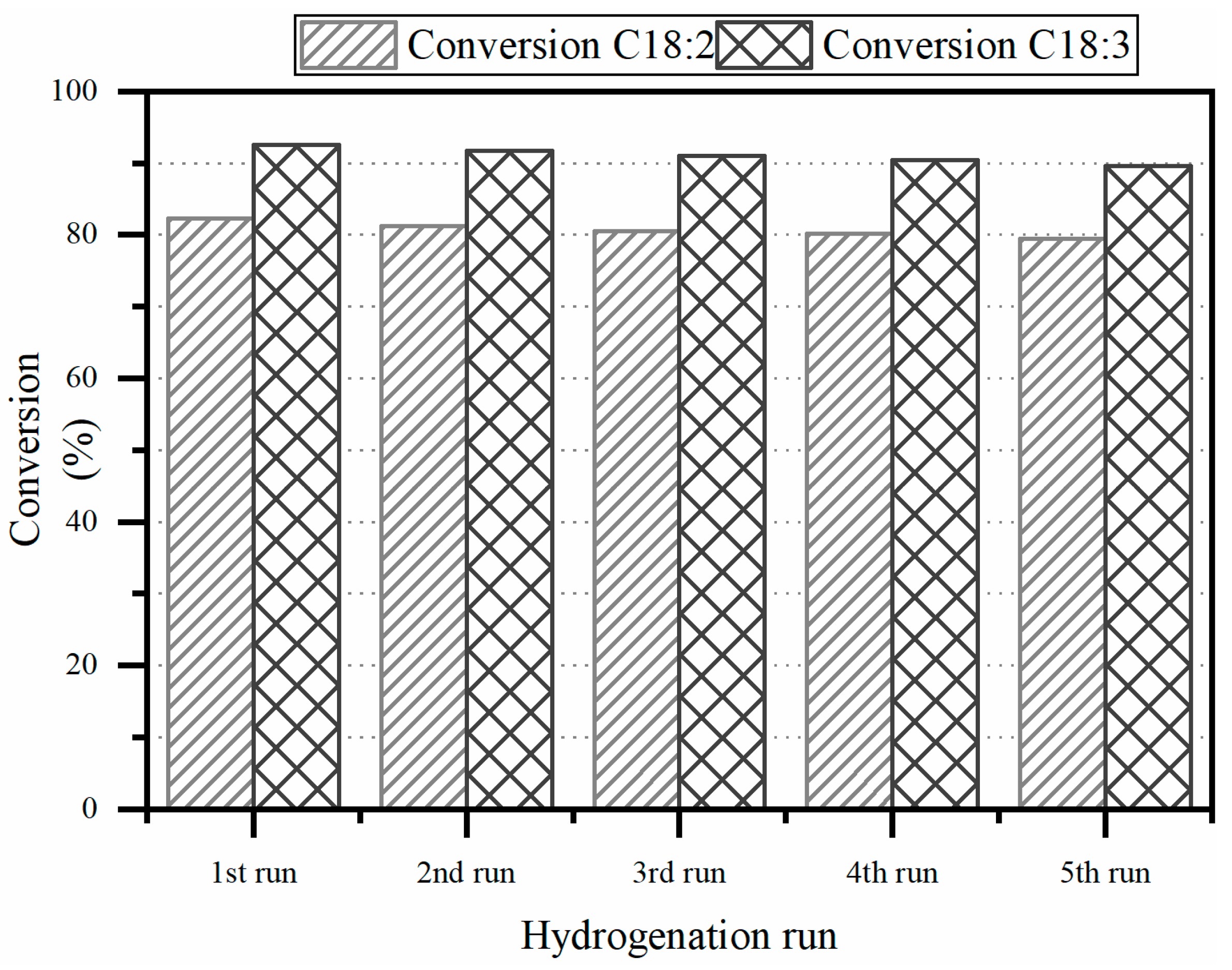
3.2.4. Reusability and Stability of the Catalyst: Cyclic Tests
3.3. Industrial Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leading Health Organizations. Rally around Call to Action to Protect People’s Health from Climate Change—Public Health Institute. Available online: https://www.phi.org/news-events/1494/leading-health-organizations-rally-around-call-to-action-to-protect-peoples-health-from-climate-change (accessed on 6 July 2019).
- Murtaugh, P.A.; Schlax, M.G. Reproduction and the Carbon Legacies of Individuals. Glob. Environ. Chang. 2009, 19, 14–20. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; The World Bank: Washington, DC, USA, 2018; ISBN 978-1-4648-1329-0. [Google Scholar]
- Mulvaney, D.; Gibbs, B.J. Green Metrics; Constable, D.J.C., Ed.; WILEY-VCH: Hoboken, NJ, USA, 2012; Volume 11, ISBN 9780470413265. [Google Scholar]
- UNFCC. Katowice Climate Change Conference, Katowice, Poland, 2–14 December 2018. Available online: https://unfccc.int/katowice (accessed on 24 January 2019).
- OECD. Improving Plastics Management: Trends, Policy Responses and the Role of International Co-Operation and Trade; Policy Paper no. 12; OECD: Paris, France, 2018. [Google Scholar]
- Lieberman, D.; Doherty from the Center for Resource Solutions, S.; Commission for Environmental Cooperation. Renewable Energy as a Hedge against Fuel Price Fluctuation: How to Capture the Benefits; Commission for Environmental Cooperation: Montreal, QC, Canada, 2018. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Berenblyum, A.S.; Danyushevsky, V.Y.; Kuznetsov, P.S.; Katsman, E.A.; Shamsiev, R.S. Catalytic Methods for the Manufacturing of High-Production Volume Chemicals from Vegetable Oils and Fats (Review). Pet. Chem. 2016, 56, 663–671. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). Biorefineries: Adding Value to the Sustanable Utilisation of Biomass. IEA Bioenergy 2009, T42, 1–16. [Google Scholar]
- Astruc, D. The Metathesis Reactions: From a Historical Perspective to Recent Developments. New J. Chem. 2005, 29, 42–56. [Google Scholar] [CrossRef]
- Erhan, S.Z.; Sharma, B.K.; Perez, J.M. Oxidation and Low Temperature Stability of Vegetable Oil-Based Lubricants. Ind. Crops Prod. 2006, 24, 292–299. [Google Scholar] [CrossRef]
- Behr, A.; Westfechtel, A.; Pérez Gomes, J. Catalytic Processes for the Technical Use of Natural Fats and Oils. Chem. Eng. Technol. 2008, 31, 700–714. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid Oxidation, 2nd ed.; Oily Press: Philadelphia, PA, USA, 2005; ISBN 9780857097927. [Google Scholar]
- Trasarti, A.F.; Segobia, D.J.; Apesteguía, C.R.; Santoro, F.; Zaccheria, F.; Ravasio, N. Selective Hydrogenation of Soybean Oil on Copper Catalysts as a Tool towards Improved Bioproducts. J. Am. Oil Chem. Soc. 2012, 89, 2245–2252. [Google Scholar] [CrossRef]
- Knothe, G. “Designer” Biodiesel: Optimizing Fatty Ester Composition to Improve Fuel Properties. Energy Fuels 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- Moser, B.R.; Haas, M.J.; Winkler, J.K.; Jackson, M.A.; Erhan, S.Z.; List, G.R. Evaluation of Partially Hydrogenated Methyl Esters of Soybean Oil as Biodiesel. Eur. J. Lipid Sci. Technol. 2007, 109, 17–24. [Google Scholar] [CrossRef]
- Bouriazos, A.; Mouratidis, K.; Psaroudakis, N.; Papadogianakis, G. Catalytic Conversions in Aqueous Media. Part 2. A Novel and Highly Efficient Biphasic Hydrogenation of Renewable Methyl Esters of Linseed and Sunflower Oils to High Quality Biodiesel Employing Rh/TPPTS Complexes. Catal. Lett. 2008, 121, 158–164. [Google Scholar] [CrossRef]
- Liu, W.; Tian, F.; Yu, J.; Bi, Y. Magnetic Mesopourous Palladium Catalyzed Selective Hydrogenation of Sunflower Oil. J. Oleo Sci. 2016, 15, 451–458. [Google Scholar] [CrossRef]
- Veldsink, J.W.; Bouma, M.J.; Schöön, N.H.; Beenackers, A.A.C.M. Heterogeneous Hydrogenation of Vegetable Oils: A Literature Review. Catal. Rev. 1997, 39, 253–318. [Google Scholar] [CrossRef]
- Numwong, N.; Luengnaruemitchai, A.; Chollacoop, N.; Yoshimura, Y. Effect of Metal Type on Partial Hydrogenation of Rapeseed Oil-Derived FAME. J. Am. Oil Chem. Soc. 2013, 90, 1431–1438. [Google Scholar] [CrossRef]
- Belkacemi, K.; Kemache, N.; Hamoudi, S.; Arul, J. Hydrogenation of Sunflower Oil over Bimetallic Supported Catalysts on Mesostructured Silica Material. Int. J. Chem. React. Eng. 2007, 5, 1–25. [Google Scholar] [CrossRef]
- Simakova, I.L.; Simakova, O.A.; Romanenko, A.V.; Murzin, D.Y. Hydrogenation of Vegetable Oils over Pd on Nanocomposite Carbon Catalysts. Ind. Eng. Chem. 2008, 47, 7219–7225. [Google Scholar] [CrossRef]
- Cheng, H.N.; Dowd, M.K.; Easson, M.W.; Condon, B.D. Hydrogenation of Cottonseed Oil with Nickel, Palladium and Platinum Catalysts. JAOCS J. Am. Oil Chem. Soc. 2012, 89, 1557–1566. [Google Scholar] [CrossRef]
- McArdle, S.; Leahy, J.J.; Curtin, T.; Tanner, D. Hydrogenation of Sunflower Oil over Pt-Ni Bimetallic Supported Catalysts: Preparation, Characterization and Catalytic Activity. Appl. Catal. A Gen. 2014, 474, 78–86. [Google Scholar] [CrossRef]
- List, G.R.; King, J.W. Hydrogenation of Fats and Oils: Theory and Practice, 2nd ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands; AOCS Press: Urbana, IL, USA, 2015; ISBN 9780128043493. [Google Scholar]
- Dijkstra, A.J. Revisiting the Formation Oftrans Isomers during Partial Hydrogenation of Triacylglycerol Oils. Eur. J. Lipid Sci. Technol. 2006, 108, 249–264. [Google Scholar] [CrossRef]
- Numwong, N.; Prabnasak, P.; Prayoonpunratn, P.; Triphatthanaphong, P.; Thunyaratchatanon, C.; Mochizuki, T.; Chen, S.Y.; Luengnaruemitchai, A.; Sooknoi, T. Effect of Pd Particle Size on Activity and Cis-Trans Selectivity in Partial Hydrogenation of Soybean Oil-Derived FAMEs over Pd/SiO2 Catalysts. Fuel Process. Technol. 2020, 203, 106393. [Google Scholar] [CrossRef]
- Na Rungsi, A.; Luengnaruemitchai, A.; Chollacoop, N.; Chen, S.Y.; Mochizuki, T.; Takagi, H.; Yoshimura, Y. Performance and Sulfur Poisoning of SiO2, γ-Al2O3, and SiO2-Al2O3-Supported Bimetallic Pd-Pt Catalysts in Selective Hydrogenation of Soybean Oil-Derived Fatty Acid Methyl Esters. Fuel 2023, 331, 125919. [Google Scholar] [CrossRef]
- Quaranta, E.; Dibenedetto, A.; Colucci, A.; Cornacchia, D. Partial Hydrogenation of FAMEs with High Content of C18:2 Dienes. Selective Hydrogenation of Tobacco Seed Oil-Derived Biodiesel. Fuel 2022, 326, 125030. [Google Scholar] [CrossRef]
- Numwong, N.; Luengnaruemitchai, A.; Chollacoop, N.; Yoshimura, Y. Effect of SiO2 Pore Size on Partial Hydrogenation of Rapeseed Oil-Derived FAMEs. Appl. Catal. A Gen. 2012, 441, 72–78. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered Porous Materials for Emerging Applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Zhao, D. On the Controllable Soft-Templating Approach to Mesoporous Silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef]
- Dijkstra, A.J. On the Mechanism of the Copper-Catalysed Hydro-Genation; a Reinterpretation of Published Data. Eur. J. Lipid Sci. Technol. 2002, 104, 29–35. [Google Scholar] [CrossRef]
- Koritala, S.; Dutton, H.J. Selective Hydrogenation of Soybean Oil. II. Copper-Chromium Catalysts. J. Am. Oil Chem. Soc. 1966, 43, 556–558. [Google Scholar] [CrossRef]
- Popescu, O.; Koritala, S.; Dutton, H.J. High Oleic Oils by Selective Hydrogenation of Soybean Oil. J. Am. Oil Chem. Soc. 1969, 46, 97–99. [Google Scholar] [CrossRef]
- Koritala, S. Selective Hydrogenation of Soybean Oil: V. A Novel Copper Catalyst with Excellent Re-Use Properties. J. Am. Oil Chem. Soc. 1970, 47, 106–107. [Google Scholar] [CrossRef]
- Koritala, S. Selective Hydrogenation of Soybean Oil. VI. Copper-on-Silica Gel Catalysts. J. Am. Oil Chem. Soc. 1972, 49, 83–84. [Google Scholar] [CrossRef]
- Koritala, S.; Selke, E.; Dutton, H.J. Deuteration of Methyl Linoleate with Nickel, Palladium, Platinum and Copper-Chromite Catalysts. JAOCS J. Am. Oil Chem. Soc. 1973, 50, 310–316. [Google Scholar] [CrossRef]
- Koritala, S. Selective Hydrogenation of Soybean Oil: VII. Poisons and Inhibitors for Copper Catalysts. J. Am. Oil Chem. Soc. 1975, 52, 240–243. [Google Scholar] [CrossRef]
- Mounts, T.L.; Koritala, S.; Friedrich, J.P.; Dutton, H.J. Selective Hydrogenation of Soybean Oil: IX. Effect of Pressure in Copper Catalysis. J. Am. Oil Chem. Soc. 1978, 55, 402–406. [Google Scholar] [CrossRef]
- Koritala, S.; Friedrich, J.P.; Mounts, T.L. Selective Hydrogenation of Soybean Oil: X. Ultra High Pressure and Low Pressure. J. Am. Oil Chem. Soc. 1980, 57, 1–5. [Google Scholar] [CrossRef]
- Koritala, S.; Frankel, E.N. Selective Conjugation of Soybean Esters to Increase Hydrogenation Selectivity. J. Am. Oil Chem. Soc. 1981, 58, 553–556. [Google Scholar] [CrossRef]
- Pecchia, P.; Galasso, I.; Mapelli, S.; Bondioli, P.; Zaccheria, F.; Ravasio, N. Stabilisation of Camelina Oil Methyl Esters through Selective Hydrogenation. Ind. Crops Prod. 2013, 51, 306–309. [Google Scholar] [CrossRef]
- Sitthisa, S.; Sooknoi, T.; Ma, Y.; Balbuena, P.B.; Resasco, D.E. Kinetics and Mechanism of Hydrogenation of Furfural on Cu/SiO2 Catalysts. J. Catal. 2011, 277, 306–309. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Modica, V.H.; Ravasio, N. Catalytic Selective Reduction of NO with Ethylene over a Series of Copper Catalysts on Amorphous Silicas. Appl. Catal. B Environ. 2000, 28, 175–185. [Google Scholar] [CrossRef]
- Li, K.; Wang, H.; Wei, Y.; Liu, M. Catalytic Performance of Cerium Iron Complex Oxides for Partial Oxidation of Methane to Synthesis Gas. J. Rare Earths 2008, 26, 705–710. [Google Scholar] [CrossRef]
- Ding, T.; Tian, H.; Liu, J.; Wu, W.; Zhao, B. Effect of Promoters on Hydrogenation of Diethyl Malonate to 1,3-Propanediol over Nano Copper-Based Catalysts. Catal. Commun. 2016, 74, 10–15. [Google Scholar] [CrossRef]
- Huang, X.; Ma, M.; Miao, S.; Zheng, Y.; Chen, M.; Shen, W. Hydrogenation of Methyl Acetate to Ethanol over a Highly Stable Cu/SiO2 Catalyst: Reaction Mechanism and Structural Evolution. Appl. Catal. A Gen. 2017, 531, 79–88. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.; Wang, Y.; Shan, B.; Zhang, J.; Wang, S.; Ma, X. Efficient Tuning of Surface Copper Species of Cu/SiO2 Catalyst for Hydrogenation of Dimethyl Oxalate to Ethylene Glycol. Chem. Eng. J. 2017, 313, 759–768. [Google Scholar] [CrossRef]
- Zhang, B.; Hui, S.; Zhang, S.; Ji, Y.; Li, W.; Fang, D. Effect of Copper Loading on Texture, Structure and Catalytic Performance of Cu/SiO2 Catalyst for Hydrogenation of Dimethyl Oxalate to Ethylene Glycol. J. Nat. Gas Chem. 2012, 21, 563–570. [Google Scholar] [CrossRef]
- Dijkstra, A.J. Kinetics and Mechanism of the Hydrogenation Process—The State of the Art. Eur. J. Lipid Sci. Technol. 2012, 114, 985–998. [Google Scholar] [CrossRef]
- Zaccheria, F.; Psaro, R.; Ravasio, N. Selective Hydrogenation of Alternative Oils: A Useful Tool for the Production of Biofuels. Green Chem. 2009, 11, 462–465. [Google Scholar] [CrossRef]
- Li, F.; Wang, L.; Han, X.; He, P.; Cao, Y.; Li, H.; Takakusagi, S.; Asakura, K.; Sheppard, E.W.; Wang, D.; et al. Influence of Support on the Performance of Copper Catalysts for the Effective Hydrogenation of Ethylene Carbonate to Synthesize Ethylene Glycol and Methanol. RSC Adv. 2016, 6, 45894–45906. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R.; Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 02, 154–160. [Google Scholar] [CrossRef]
- Deng, Y.; Handoko, A.D.; Du, Y.; Xi, S.; Yeo, B.S. In Situ Raman Spectroscopy of Copper and Copper Oxide Surfaces during Electrochemical Oxygen Evolution Reaction: Identification of CuIII Oxides as Catalytically Active Species. ACS Catal. 2016, 6, 2473–2481. [Google Scholar] [CrossRef]
- Dong, X.; Ma, X.; Xu, H.; Ge, Q.; Sio, C. From Chip-in-a-Lab to Lab-on-a-Chip: Towards a Single Handheld Electronic System for Multiple Application-Specific Lab-on-a-Chip (ASLOC). Catal. Sci. Technol. 2014, 6, 4151. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Fan, W.; Wang, J.; Li, Y. A Highly Efficient and Robust Cu/SiO2 Catalyst Prepared by the Ammonia Evaporation Hydrothermal Method for Glycerol Hydrogenolysis to 1,2-Propanediol. Catal. Sci. Technol. 2015, 5, 1169–1180. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, Y.; Ding, G.; Zhu, S.; Zheng, H.; Li, Y. Highly Selective Synthesis of Ethylene Glycol and Ethanol via Hydrogenation of Dimethyl Oxalate on Cu Catalysts: Influence of Support. Appl. Catal. A Gen. 2013, 468, 296–304. [Google Scholar] [CrossRef]
- Hope, G.A.; Buckley, A.N.; Parker, G.K.; Numprasanthai, A.; Woods, R.; McLean, J. The Interaction of N-Octanohydroxamate with Chrysocolla and Oxide Copper Surfaces. Miner. Eng. 2012, 36–38, 2–11. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Zhu, Y.; Zheng, H.; Li, Y. Promoting Effect of Boron Oxide on Cu/SiO2 Catalyst for Glycerol Hydrogenolysis to 1,2-Propanediol. J. Catal. 2013, 303, 70–79. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The Power of Databases: The RRUFF Project. In Highlights in Mineralogical Crystallography; De Gruyter: Berlin, Germany, 2016. [Google Scholar]
- Caracas, R.; Bobocioiu, E. The WURM Project-a Freely Available Web-Based Repository of Computed Physical Data for Minerals. Am. Mineral. 2011, 96, 437–443. [Google Scholar] [CrossRef]
- Raman Database. University of Parma. Available online: http://www.fis.unipr.it/phevix/ramandb.php (accessed on 7 June 2019).
- Hinrichsen, O.; Genger, T.; Muhler, M. Chemisorption of N2O and H2 for the Surface Determination of Copper Catalysts. Chem. Eng. Technol. 2000, 23, 956–959. [Google Scholar] [CrossRef]
- Sagar, G.V.; Rao, P.V.R.; Chakravartula, S.; Srikanth, A.; Chary, K.V.R. Dispersion and Reactivity of Copper Catalysts Supported on Al2O3−ZrO2. J. Phys. Chem. B 2006, 110, 13881–13888. [Google Scholar] [CrossRef]
- Laverdura, U.P.; Rossi, L.; Ferella, F.; Courson, C.; Zarli, A.; Alhajyoussef, R.; Gallucci, K. Selective Catalytic Hydrogenation of Vegetable Oils on Lindlar Catalyst. ACS Omega 2020, 5, 22901–22913. [Google Scholar] [CrossRef]
- IUPAC AOAC. AOAC Official Method 969.33 Fatty Acids in Oils and Fats: Preparation of Methyl Esters Boron Trifluoride Method; AOAC International: Washington, DC, USA, 2000. [Google Scholar]
- ISO/TC 34/SC 11. ISO 3961:2018; Animal and Vegetable Fats and Oils—Determination of Iodine Value. ISO: Geneva, Switzerland, 2018.
- Albright, L.F.; Wisniak, J. Selectivity and Isomerization during Partial Hydrogenation of Cottonseed Oil and Methyl Oleate: Effect of Operating Variables. J. Am. Oil Chem. Soc. 1962, 39, 14–19. [Google Scholar] [CrossRef]
- Albright, L.F. Quantitative Measure of Selectivity of Hydrogenation of Triglycerides. J. Am. Oil Chem. Soc. 1965, 42, 250–253. [Google Scholar] [CrossRef]
- Bailey, A.E. Some Additional Notes on the Kinetics and Theory of Fatty Oil Hydrogenation. J. Am. Oil Chem. Soc. 1949, 26, 644–648. [Google Scholar] [CrossRef]
- Philippaerts, A.; Jacobs, P.; Sels, B. Catalytic Hydrogenation of Vegetable Oils. In Catalytic Hydrogenation for Biomass Valorization; Energy and Environment Series; Rinaldi, R., Ed.; Royal Society of Chemistry RSC: Cambridge, UK, 2015; pp. 223–241. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Boza, A.F.; Kupfer, V.L.; Oliveira, A.R.; Radovanovic, E.; Rinaldi, A.W.; Meneguin, J.G.; Domingues, N.L.C.; Moisés, M.P.; Favaro, S.L. Synthesis of α-Aminophosphonates Using a Mesoporous Silica Catalyst Produced from Sugarcane Bagasse Ash. RSC Adv. 2016, 6, 23981–23986. [Google Scholar] [CrossRef]
- Rovani, S.; Santos, J.J.; Corio, P.; Fungaro, D.A. An Alternative and Simple Method for the Preparation of Bare Silica Nanoparticles Using Sugarcane Waste Ash, an Abundant and Despised Residue in the Brazilian Industry. Artic. J. Braz. Chem. Soc. 2019, 30, 1524–1533. [Google Scholar] [CrossRef]
- Hu, S.; Hsieh, Y.-L. Preparation of Activated Carbon and Silica Particles from Rice Straw. ACS Sustain. Chem. Eng. 2014, 2, 726–734. [Google Scholar] [CrossRef]
- Toupance, T.; Kermarec, M.; Louis, C. Metal Particle Size in Silica-Supported Copper Catalysts. Influence of the Conditions of Preparation and of Thermal Pretreatments. J. Phys. Chem. B 2000, 104, 965–972. [Google Scholar] [CrossRef]
- Dong, F.; Ding, G.; Zheng, H.; Xiang, X.; Chen, L.; Zhu, Y.; Li, Y. Highly Dispersed Cu Nanoparticles as an Efficient Catalyst for the Synthesis of the Biofuel 2-Methylfuran. Catal. Sci. Technol. 2016, 6, 767–779. [Google Scholar] [CrossRef]
- Ravasio, N.; Zaccheria, F.; Gargano, M.; Recchia, S.; Fusi, A.; Poli, N.; Psaro, R. Environmental Friendly Lubricants through Selective Hydrogenation of Rapeseed Oil over Supported Copper Catalysts. Appl. Catal. A Gen. 2002, 233, 1–6. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Inaba, M.; Takahara, I. Synthesis of Ethanol from Methanol and Syngas through an Indirect Route Containing Methanol Dehydrogenation, DME Carbonylation, and Methyl Acetate Hydrogenolysis. Fuel Process. Technol. 2013, 110, 206–213. [Google Scholar] [CrossRef]
- Chary, K.V.R.; Sagar, G.V.; Chakravarthula, S.; Srikanth, A.; Rao, V.V. Characterization and Catalytic Functionalities of Copper Oxide Catalysts Supported on Zirconia. J. Phys. Chem. B 2006, 111, 543–550. [Google Scholar] [CrossRef]
- Schneider, M.P. Plant-Oil-Based Lubricants and Hydraulic Fluids. J. Sci. Food Agric. 2006, 86, 1769–1780. [Google Scholar] [CrossRef]
- Pasqual Laverdura, U. Study of Selective Catalytic Hydrogenation of Triglycerides and Their Derivatives. Ph.D. Thesis, Université de Strasbourg, L’Aquila, Italie, 2019. [Google Scholar]
- Peters, M.S.; Timmerhaus, K.D. Plant Design and Economics for Chemicals Engineers, 4th ed.; McGraw Hill International Editions: New York, NY, USA, 1997; Volume 2, ISBN 0070496137. [Google Scholar]





| Characteristic | |
|---|---|
| Activity | Pd > Rh > Pt >> Ir > Ru = Ni >> Cu |
| Selectivity | Cu > Pd > Rh > Pt >> Ir > Ru = Ni |
| Cis/trans selectivity | Cu ≈ Pd > Rh > Ru = Ni >> Ir > Pt |
| Name | Nominal Cu Loading [%w/w] |
|---|---|
| Cu5SiO2HP | 5 |
| Cu10SiO2HP | 10 |
| Cu5SiO2AE | 5 |
| Cu10SiO2AE | 10 |
| Test Number | Catalysts | Oil | Catalyst Concentration (mg/mLoil) | Temperature (°C) | Pressure (bar) | Test Duration (min) |
|---|---|---|---|---|---|---|
| 1 | Cu5SiO2AE | Canola | 4 | 180 | 4 | 240 |
| 2 | 12 | |||||
| 3 | 200 | 4 | ||||
| 4 | 12 | |||||
| 5 | Cu5SiO2HP | Canola | 4 | 180 | 4 | 240 |
| 6 | 12 | |||||
| 7 | 200 | 4 | ||||
| 8 | 12 | |||||
| 9 | Cu10SiO2AE | Canola | 4 | 180 | 4 | 240 |
| 10 | 12 | |||||
| 11 | 200 | 4 | ||||
| 12 | 12 | |||||
| 13 | Cu10SiO2AE | Canola | 8 | 180 | 4 | 240 |
| 14 | 12 | |||||
| 15 | 200 | 4 | ||||
| 16 | 12 | |||||
| 17 | Cu10SiO2AE | Canola | 2 | 180 | 4 | 240 |
| 18 | 12 | |||||
| 19 | 200 | 4 | ||||
| 20 | Cu10SiO2AE | Sunflower | 4 | 180 | 4 | 360 |
| 21 | 12 | |||||
| 22 | 200 | 4 | ||||
| 23 | Cu10SiO2HP | Canola | 4 | 180 | 4 | 240 |
| 24 | 12 | |||||
| 25 | 200 | 4 | ||||
| 26 | 12 | |||||
| 27 | Cu10SiO2HP | Canola | 8 | 180 | 4 | 240 |
| 28 | 12 | |||||
| 29 | 200 | 4 | ||||
| 30 | 12 | |||||
| 31 | Cu10SiO2HP | Canola | 2 | 180 | 4 | 240 |
| 32 | 12 | |||||
| 33 | 200 | 4 | ||||
| 34 | Cu10SiO2HP | Sunflower | 4 | 180 | 4 | 360 |
| 35 | 12 | |||||
| 36 | 200 | 4 |
| Component | Retention Time [min] |
|---|---|
| Myristic FAME | 4.12 |
| Palmitic FAME | 5.23 |
| Stearic FAME | 6.81 |
| Elaidic FAME | 7.33 |
| Oleic FAME | 7.62 |
| t,t Linoleic FAME | 8.23 |
| c,t Linoleic FAME | 8.58 |
| t,c Linoleic FAME | 8.70 |
| c,c Linoleic FAME | 9.12 |
| Behenic FAME | 9.65 |
| Linolenic FAME | 10.81 |
| Erucic FAME | 15.22 |
| Sample | Nominal Loading | ICP-AES Measure |
|---|---|---|
| Cu5SiO2HP | 5 | 5.8 ± 0.2 |
| Cu10SiO2HP | 10 | 10.8 ± 0.3 |
| Cu5SiO2AE | 5 | 6.5 ± 0.1 |
| Cu10SiO2AE | 10 | 11.7 ± 0.2 |
| Materials | SBET [m2g−1] | VBJH, des [cm3g−1] | Dav, BJH [nm] |
|---|---|---|---|
| Cu5SiO2HP | 342 | 1.58 | 18.7 |
| Cu10SiO2HP | 359 | 1.71 | 20.3 |
| Cu5SiO2AE | 208 | 0.55 | 10.6 |
| Cu10SiO2AE | 256 | 0.69 | 10.8 |
| Material | Cu Tred (°C) | Cu Surface (m2Cu/gCu) |
|---|---|---|
| Cu5SiO2HP | 242 | - |
| Cu10SiO2HP | 239 | 55 |
| Cu5SiO2AE | 232 | - |
| Material | (Cu+/Cu0 + Cu) ∗ 100 |
|---|---|
| Cu10SiO2HPR | 45.4 ± 0.2% |
| Cu10SiO2AER | 62.0 ± 0.2% |
| Test Number | Catalyst | Catalyst Concentration (mg/mLoil) | T (°C) | P (bar) | Oil 1 | (C18:1)max (%) | SLn | SII | χC18:3 (%) | χC18:2 (%) | TTmax (%) | TSmax (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cu5SiO2AE | 4 | 180 | 4 | C | 70.1 | 6.7 | 0.18 | 15.8 | 7.3 | 0.6 | 2.1 |
| 2 | 180 | 12 | C | 71.4 | 6.1 | 0.13 | 30.8 | 15.5 | 2.2 | 4.0 | ||
| 3 | 200 | 4 | C | 79.1 | 6.9 | 0.26 | 76.7 | 26.8 | 4.0 | 3.4 | ||
| 4 | 200 | 12 | C | 78.5 | 6.8 | 0.17 | 78.5 | 30.4 | 2.7 | 5.0 | ||
| 5 | Cu5SiO2HP | 4 | 180 | 4 | C | 68.3 | 6.1 | 0.32 | 65.3 | 20.5 | 9.0 | 1.6 |
| 6 | 180 | 12 | C | 69.7 | 6.5 | 0.35 | 66.3 | 23.5 | 7.8 | 1.5 | ||
| 7 | 200 | 4 | C | 79.1 | 6.1 | 0.31 | 82.3 | 36.7 | 9.5 | 1.7 | ||
| 8 | 200 | 12 | C | 80.2 | 6.5 | 0.35 | 83.1 | 38.9 | 8.5 | 1.9 | ||
| 9 | Cu10SiO2AE | 4 | 180 | 4 | C | 71.9 | 6.9 | 0.32 | 35.8 | 13.4 | 12.1 | 2.2 |
| 10 | 180 | 12 | C | 78.8 | 6.0 | 0.39 | 79.8 | 33.4 | 8.2 | 4.0 | ||
| 11 | 200 | 4 | C | 78.7 | 6.3 | 0.35 | 82.2 | 35.8 | 15.4 | 3.4 | ||
| 12 | 200 | 12 | C | 79.9 | 6.7 | 0.42 | 83.3 | 48.7 | 12.3 | 5.4 | ||
| 13 | 8 | 180 | 4 | C | 77.5 | 7.0 | 0.56 | 67.8 | 45.7 | 14.8 | 3.5 | |
| 14 | 180 | 12 | C | 87.8 | 6.8 | 0.48 | 80.3 | 65.2 | 20.2 | 3.1 | ||
| 15 | 200 | 4 | C | 78.6 | 6.8 | 0.58 | 69.8 | 57.6 | 17.3 | 4.7 | ||
| 16 | 200 | 12 | C | 88.6 | 6.7 | 0.54 | 84.5 | 79.5 | 24.0 | 5.3 | ||
| 17 | 2 | 180 | 4 | C | 71.4 | - | - | 21.1 | 5.8 | 4.8 | 2.1 | |
| 18 | 180 | 12 | C | 73.2 | - | - | 24.3 | 8.3 | 6.8 | 2.1 | ||
| 19 | 200 | 4 | C | 78.4 | 7.1 | 0.18 | 54.3 | 23.3 | 11.8 | 2.9 | ||
| 20 | 4 | 180 | 4 | S | 59.6 | - | 0.87 | - | 42.1 | 27.0 | 6.0 | |
| 21 | 180 | 12 | S | 67.2 | - | 0.89 | - | 60.2 | 28.5 | 4.7 | ||
| 22 | 200 | 4 | S | 69.1 | - | 0.88 | - | 62.2 | 33.4 | 8.0 | ||
| 23 | Cu10SiO2HP | 4 | 180 | 4 | C | 76.2 | 5.1 | 0.42 | 73.0 | 35.0 | 10.9 | 3.6 |
| 24 | 180 | 12 | C | 80.1 | 6.5 | 0.50 | 74.5 | 38.4 | 9.7 | 5.2 | ||
| 25 | 200 | 4 | C | 84.5 | 5.4 | 0.51 | 86.4 | 48.5 | 14.6 | 4.1 | ||
| 26 | 200 | 12 | C | 86.2 | 6.3 | 0.58 | 87.5 | 61.3 | 12.4 | 5.5 | ||
| 27 | 8 | 180 | 4 | C | 88.8 | 5.8 | 0.75 | 89.8 | 78.5 | 13.2 | 4.1 | |
| 28 | 180 | 12 | C | 89.2 | 5.7 | 0.62 | 91.8 | 82.5 | 14.8 | 5.4 | ||
| 29 | 200 | 4 | C | 90.7 | 5.5 | 0.82 | 92.6 | 82.3 | 18.8 | 5.5 | ||
| 30 | 200 | 12 | C | 91.3 | 5.7 | 0.69 | 93.3 | 90.4 | 23.0 | 5.3 | ||
| 31 | 2 | 180 | 4 | C | 69.2 | - | - | 17.5 | 4.2 | 3.8 | 1.9 | |
| 32 | 180 | 12 | C | 71.0 | - | - | 20.1 | 7.2 | 4.5 | 2.0 | ||
| 33 | 200 | 4 | C | 72.3 | 6.0 | 0.20 | 37.2 | 26.2 | 12.5 | 2.1 | ||
| 34 | 4 | 180 | 4 | S | 67.3 | - | 0.51 | - | 48.2 | 15.2 | 7.0 | |
| 35 | 180 | 12 | S | 69.1 | - | 0.72 | - | 55.1 | 23.4 | 4.1 | ||
| 36 | 200 | 4 | S | 70.6 | - | 0.83 | - | 60.4 | 28.1 | 6.3 |
| Test Number | Catalyst | Catalyst Concentration (mg/mLoil) | T (°C) | P (bar) | t (min) | IV | SII | C18:0 (%) | C18:1 (%) |
|---|---|---|---|---|---|---|---|---|---|
| 9 | Cu10SiO2AE | 4 | 180 | 4 | - | - | - | - | - |
| 10 | 12 | 209 | 110.2 | 0.25 | 1.8 | 67.5 | |||
| 11 | 200 | 4 | 168 | 109.4 | 0.26 | 2.0 | 68.3 | ||
| 12 | 12 | 135 | 111.3 | 0.17 | 1.8 | 66.0 | |||
| 13 | 8 | 180 | 4 | 109 | 110.5 | 0.35 | 2.5 | 67.4 | |
| 14 | 12 | 91 | 110.0 | 0.27 | 3.1 | 67.8 | |||
| 15 | 200 | 4 | 86 | 109.7 | 0.40 | 4.3 | 68.9 | ||
| 16 | 12 | 74 | 109.4 | 0.36 | 3.7 | 69.1 | |||
| 23 | Cu10SiO2HP | 4 | 180 | 4 | 159 | 110.1 | 0.23 | 1.5 | 67.9 |
| 24 | 12 | 137 | 110.6 | 0.17 | 1.5 | 68.7 | |||
| 25 | 200 | 4 | 124 | 109.2 | 0.26 | 1.5 | 69.0 | ||
| 26 | 12 | 111 | 108.9 | 0.18 | 1.8 | 69.3 | |||
| 27 | 8 | 180 | 4 | 82 | 109.6 | 0.31 | 1.6 | 68.1 | |
| 28 | 12 | 74 | 108.6 | 0.24 | 2.0 | 67.9 | |||
| 29 | 200 | 4 | 55 | 109.2 | 0.45 | 3.0 | 69.7 | ||
| 30 | 12 | 56 | 110.3 | 0.41 | 2.8 | 69.9 |
| Time (min) | C18:0 | t-C18:1 | C18:1 | iso-C18:2 | C18:2 | C18:3 |
|---|---|---|---|---|---|---|
| 0 | 1.3 | 0.00 | 67.8 | 0.1 | 20.6 | 10.3 |
| 180 | 3.7 | 13.5 | 90.2 | 2.9 | 2.2 | 0.9 |
| 240 | 3.9 | 17.1 | 92.1 | 2.0 | 1.2 | 0.8 |
| Investment Cost | € | |
|---|---|---|
| Equipment cost (EC) | 365,000 € | |
| Installation cost | 36,500 € | 10% EC |
| Piping, Instruments and controls | 73,000 € | 20% EC |
| Electric system | 18,250 € | 5% EC |
| Total direct costs TDC | 495,000 € | |
| Engineering, supervision, site | 49,000 | 10% TDC |
| Construction expenses | 24,750 | 5% TDC |
| Total costs direct + indirect | 570,000 € | |
| Contractor’s fee | 68,500 | 12% TDC + TIC |
| Contingencies | 28,500 | 5% TDC + TIC |
| Working Capital (Total Investment) | 667,000 € |
| Entry | m.u. | |
|---|---|---|
| Sell revenues | 4,392,000 | € |
| Costs | 3,060,000 | € |
| R-C | 1,332,000 | € |
| Depreciation (linear 5 years) | 133,400 | € |
| ROIm = (Net Revenues)/CAPEX | 1.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasqual Laverdura, U.; Rossi, L.; Courson, C.; Zarli, A.; Gallucci, K. Selective Catalytic Hydrogenation of Vegetable Oils over Copper-Based Catalysts Supported on Amorphous Silica. Energies 2023, 16, 7201. https://doi.org/10.3390/en16207201
Pasqual Laverdura U, Rossi L, Courson C, Zarli A, Gallucci K. Selective Catalytic Hydrogenation of Vegetable Oils over Copper-Based Catalysts Supported on Amorphous Silica. Energies. 2023; 16(20):7201. https://doi.org/10.3390/en16207201
Chicago/Turabian StylePasqual Laverdura, Umberto, Leucio Rossi, Claire Courson, Antonio Zarli, and Katia Gallucci. 2023. "Selective Catalytic Hydrogenation of Vegetable Oils over Copper-Based Catalysts Supported on Amorphous Silica" Energies 16, no. 20: 7201. https://doi.org/10.3390/en16207201
APA StylePasqual Laverdura, U., Rossi, L., Courson, C., Zarli, A., & Gallucci, K. (2023). Selective Catalytic Hydrogenation of Vegetable Oils over Copper-Based Catalysts Supported on Amorphous Silica. Energies, 16(20), 7201. https://doi.org/10.3390/en16207201








