Exploration of Oil/Water/Gas Occurrence State in Shale Reservoir by Molecular Dynamics Simulation
Abstract
:1. Introduction
2. Models and Methodology
2.1. Modeling
2.2. Simulation Details
3. Results and Discussion
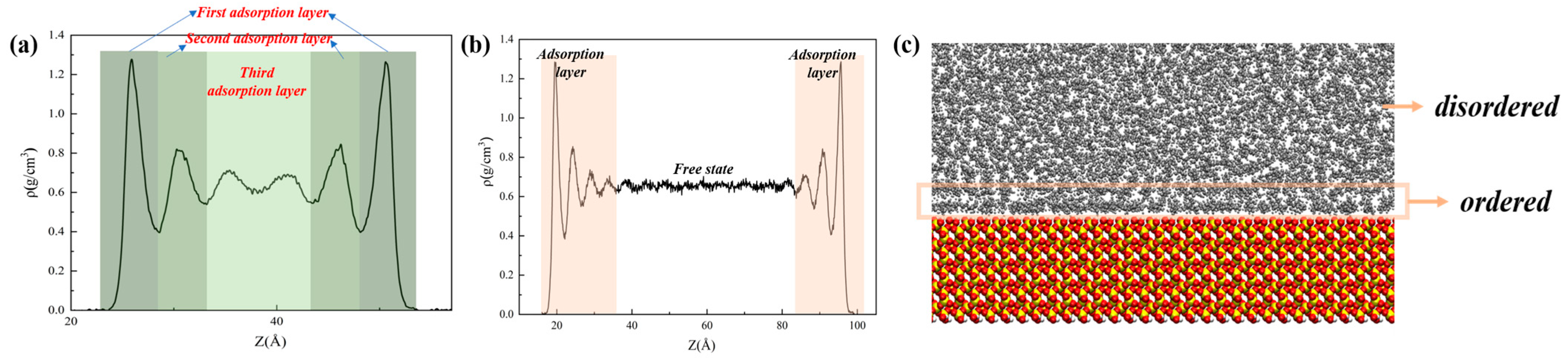
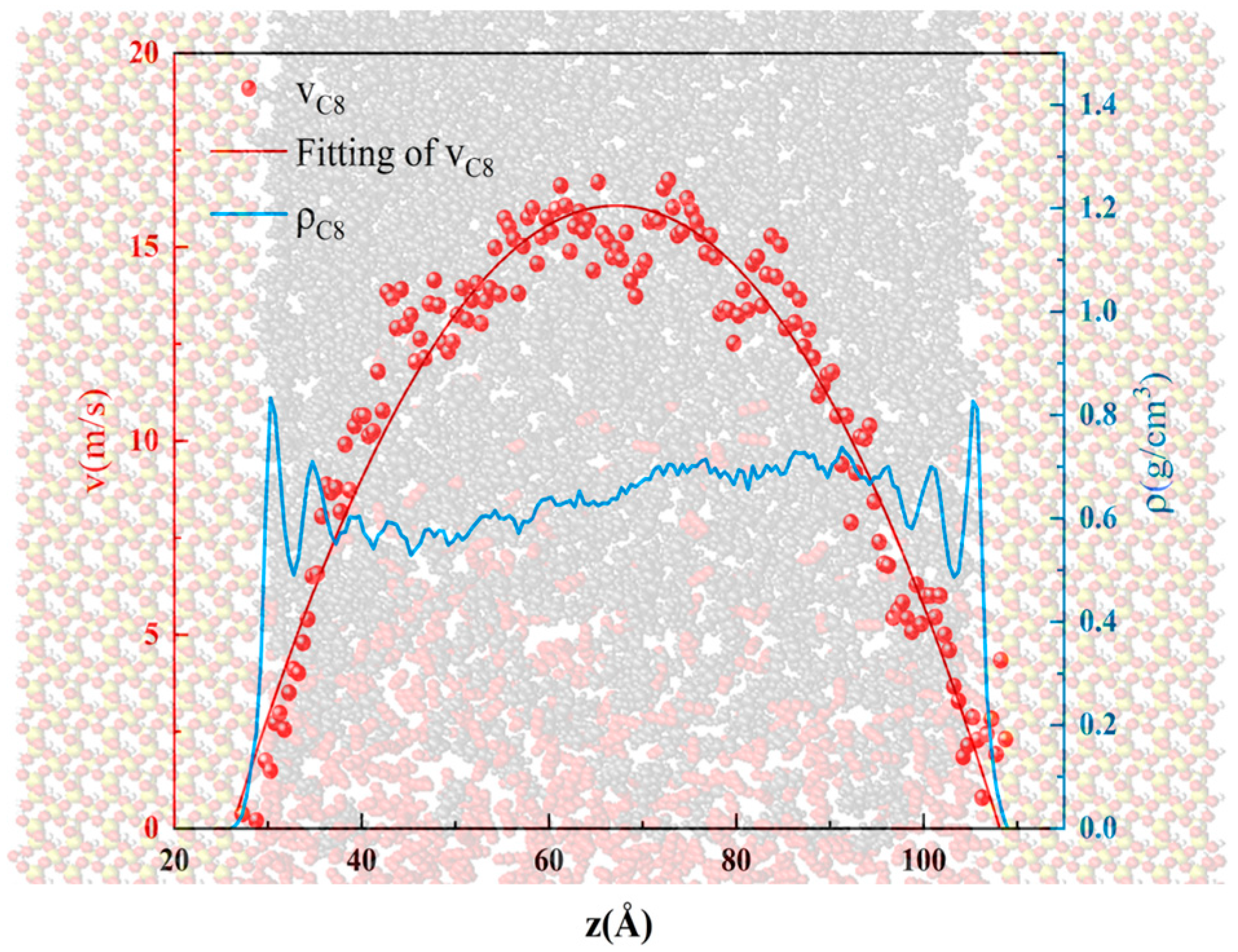
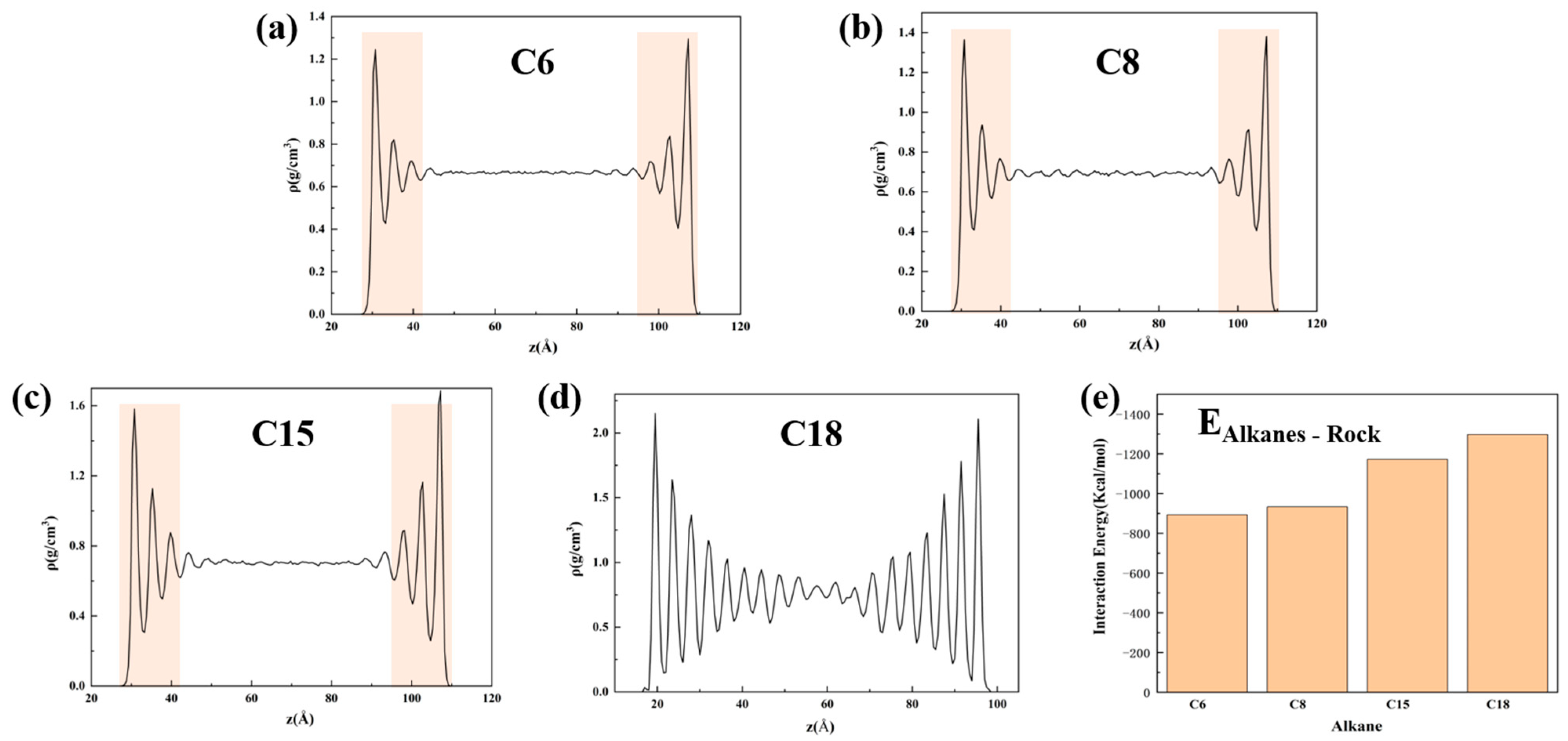
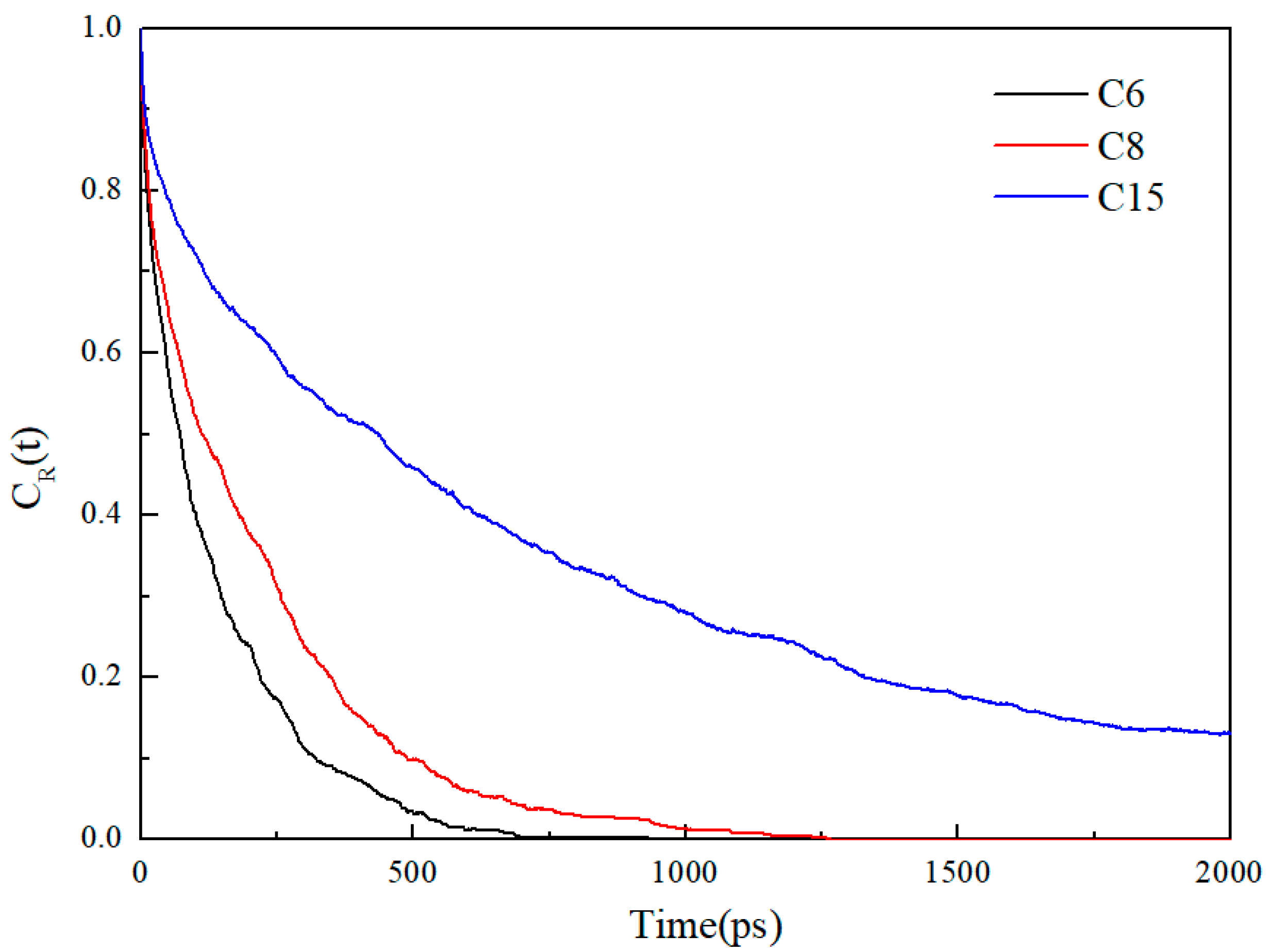
3.1. Oil Occurrence Characteristics in Shale Pores
3.2. Water Occurrence Characteristics in Shale Pores
3.3. Gas Occurrence Characteristics in Shale Pores
3.4. Effect of Different Shale Reservoir Conditions
4. Conclusions
- (1)
- When the alkane is light oil, in narrow pores with width of less than 3 nm, the space is occupied by the oil adsorption layer, with almost none in a free state. In pores larger than 3 nm, the oil is in both adsorbed and free states. The oil molecules in the free state have significantly higher mobility than those in the adsorbed layer due to the strong interaction with the pore wall. In addition, oil molecules with longer carbon chains tend to stay in the adsorption layer, and those with shorter chains are more likely to be peeled off from the surface to form free oil.
- (2)
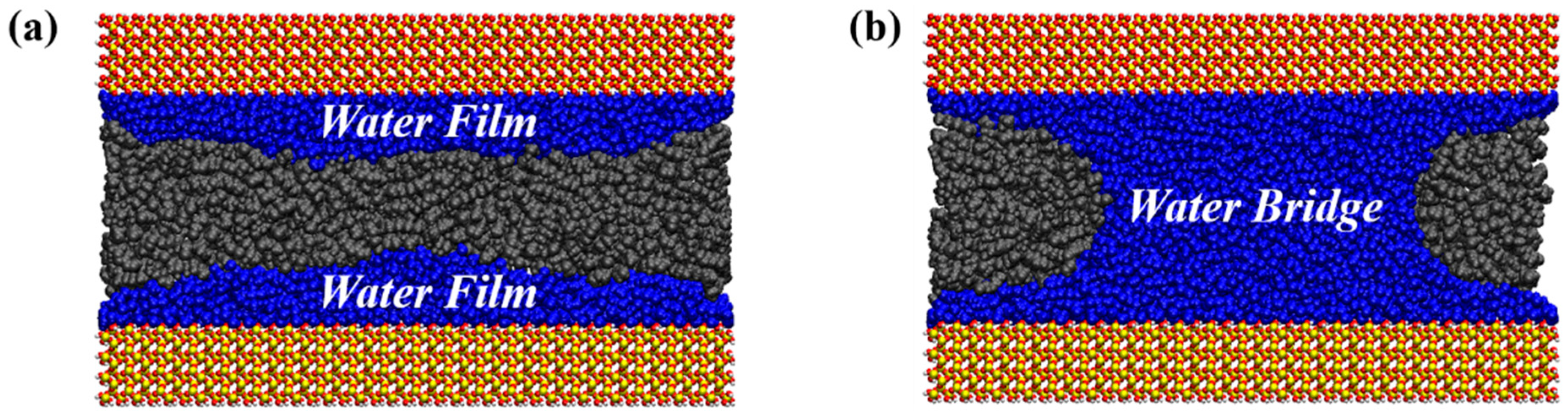
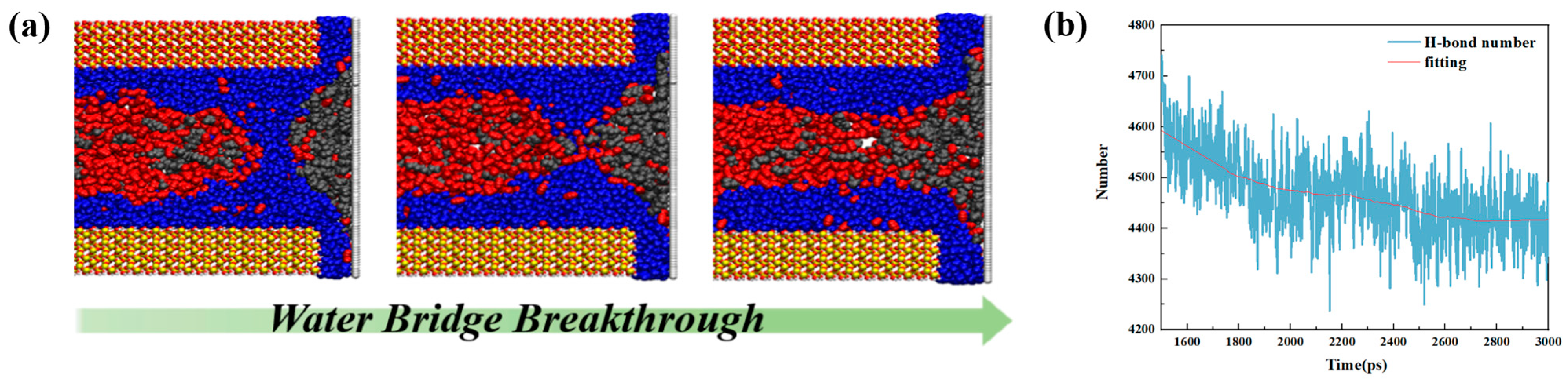
- The presence of water in shale nanopores affects the adsorption behavior of oil and water on the surface. Due to the stronger competitive adsorption with the rock surface than oil, water tends to occupy the adsorption sites near the rock, reducing the adsorbed oil molecules. The content of water in the pore determines whether it exists in the form of water film or water bridge. The injection of CO2 gas can displace free oil molecules out of the pore and affect the occurrence state of the oil phase, as well as breaking water bridges and changing the occurrence state of water.
- (3)
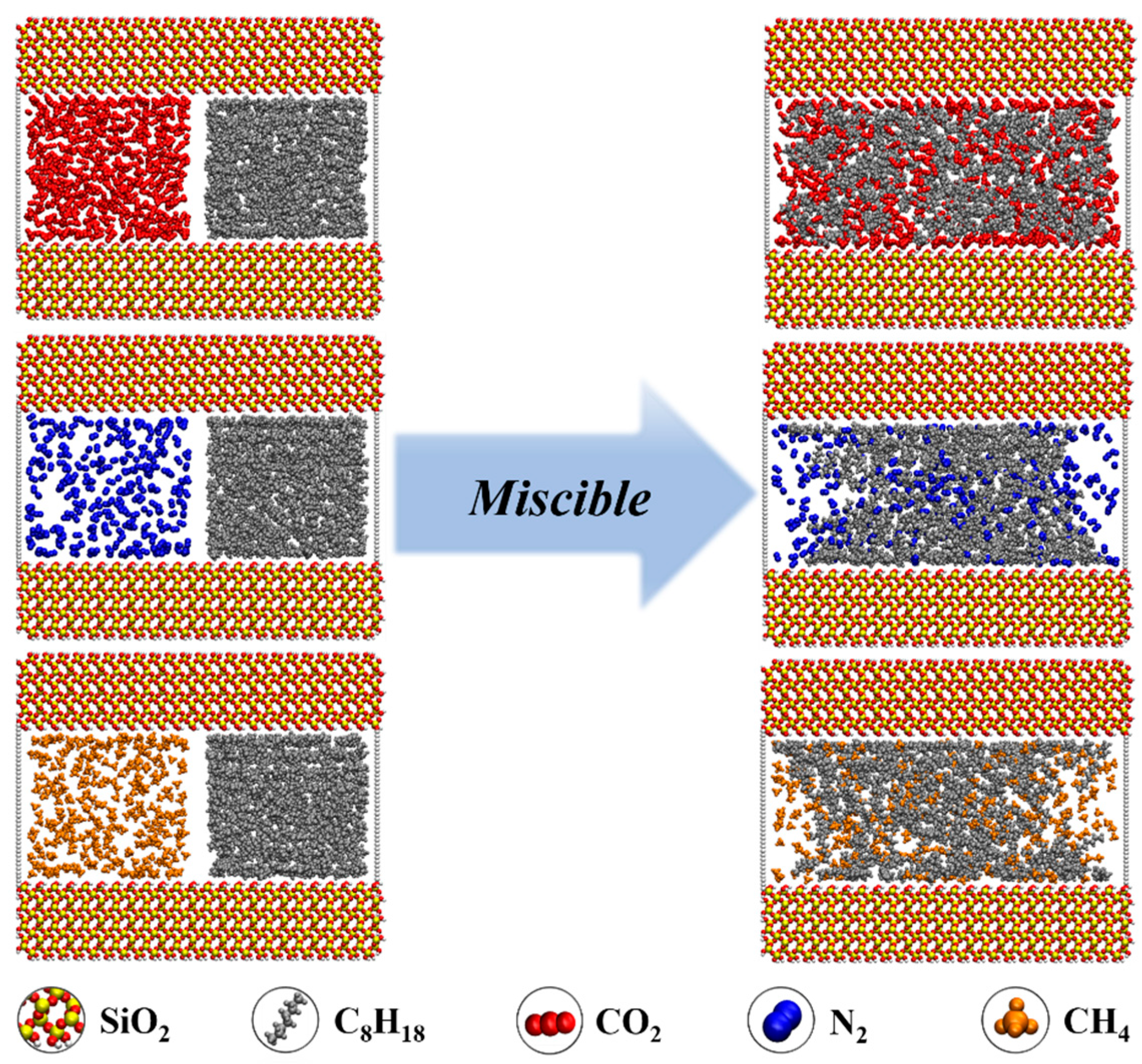
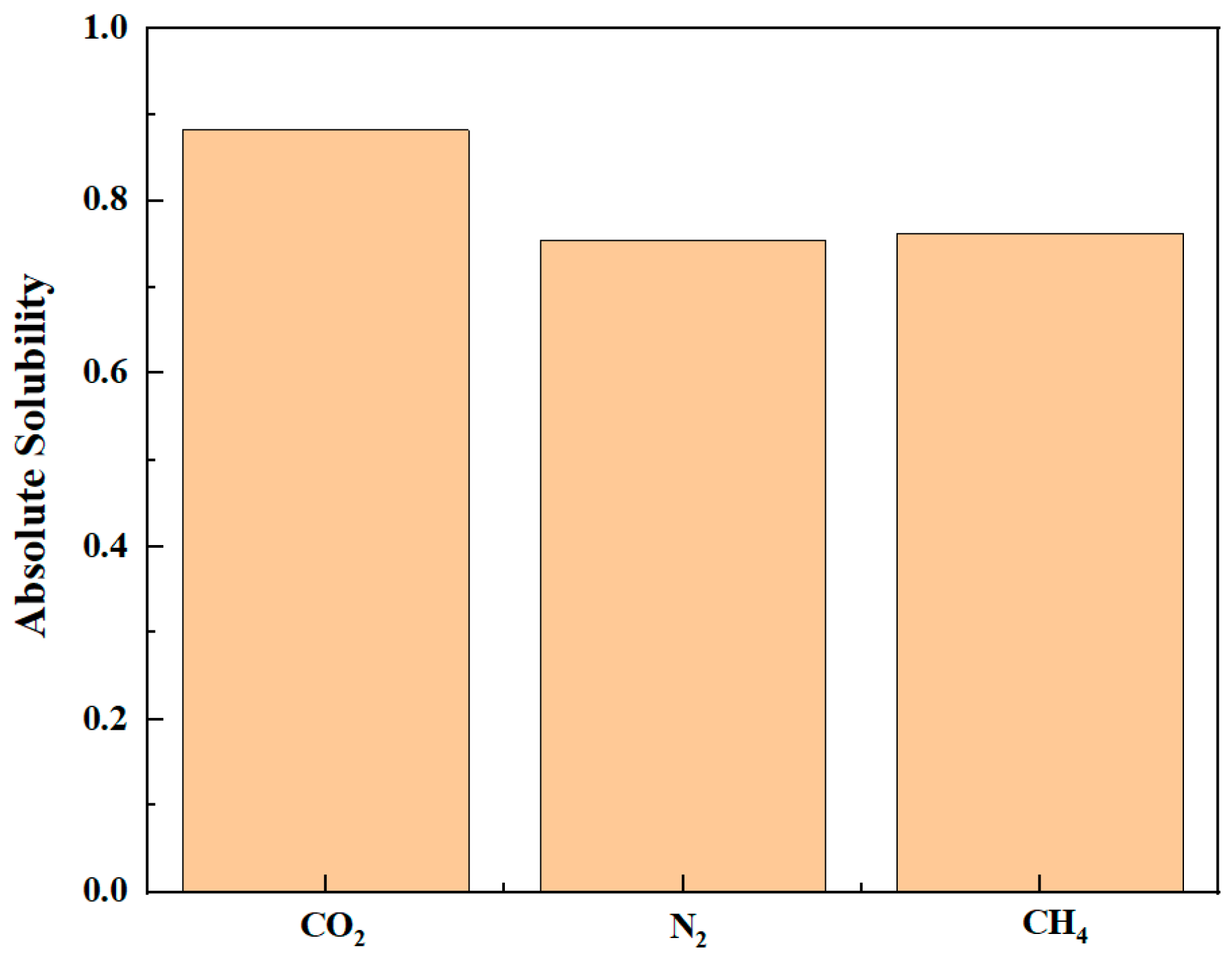
- During the displacement process, the oil/gas occurrence state is influenced by the type of gas. Due to the strong adsorption capacity, CO2 can separate the intertwined oil molecules to peels them from the rock and form a CO2 adsorption layer on the rock surface; the maximum adsorption peak of CO2 can reach 1.45 g/cm3, which is higher than the density peak of oil (1.29 g/cm3). The simulation results show that the solubility of octane molecules in different injected gases is in the following order: CO2 (88.1%) > CH4 (76.8%) > N2 (75.4%). Comparatively, N2 and CH4 have weak competitive adsorption on the rock. N2 forms a gas channel between oil molecules, and CH4 forms an oil adsorption layer again after peeling off the oil.
- (4)
- At high temperature or low pressure, oil/gas molecules can enter the free state or be displaced more easily, whereas at low temperature or high pressure, oil/gas molecules are more tightly adsorbed on the reservoir rock surface.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, C.; Qiu, Z.; Zhang, J.; Li, Z.; Wei, H.; Liu, B.; Zhao, J.; Yang, T.; Zhu, S.; Tao, H.; et al. Unconventional petroleum sedimentology: A key to understanding unconventional hydrocarbon accumulation. Engineering 2022, 18, 62–78. [Google Scholar] [CrossRef]
- Demirbas, A.; Bafail, A.; Zytoon, A.M.; Al Sayed, N. Unconventional energy sources: Safety impacts, opportunities, and economic challenges. Energy Sources Part B Econ. Plan. Policy 2017, 12, 387–393. [Google Scholar] [CrossRef]
- Rana, A.; Arfaj, M.K.; Saleh, T.A. Advanced developments in shale inhibitors for oil production with low environmental footprints—A review. Fuel 2019, 247, 237–249. [Google Scholar] [CrossRef]
- Kang, W.L.; Zhou, B.B.; Issakhov, M.; Gabdullin, M. Advances in enhanced oil recovery technologies for low permeability reservoirs. Pet. Sci. 2022, 19, 1622–1640. [Google Scholar] [CrossRef]
- Caineng, Z.; Guangya, Z.; Shizhen, T.; Suyun, H.; Xiaodi, L.; Jianzhong, L.; Dazhong, D.; Rukai, Z.; Xuanjun, Y.; Lianhua, H.; et al. Geological features, major discoveries and unconventional petroleum geology in the global petroleum exploration. Pet. Explor. Dev. 2010, 37, 129–145. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, J.; Zhang, G.; Zhou, D.; Chen, L.; Viswanathan, H.; Kang, Q. Minireview on Lattice Boltzmann Modeling of Gas Flow and Adsorption in Shale Porous Media: Progress and Future Direction. Energy Fuels 2023, 37, 1511–1524. [Google Scholar] [CrossRef]
- Lu, L.; Li, Y.; Zhang, X. Effects of cracks and geometric parameters on the flow in shale. ACS Omega 2021, 6, 4619–4629. [Google Scholar] [CrossRef]
- Zha, X.; Lai, F.; Gao, X.; Gao, Y.; Jiang, N.; Luo, L.; Li, Y.; Wang, J.; Peng, S.; Luo, X.; et al. Characteristics and Genetic Mechanism of Pore Throat Structure of Shale Oil Reservoir in Saline Lake—A Case Study of Shale Oil of the Lucaogou Formation in Jimsar Sag, Junggar Basin. Energies 2021, 14, 8450. [Google Scholar] [CrossRef]
- Zang, Q.; Liu, C.; Awan, R.S.; Yang, X.; Lu, Z.; Li, G.; Wu, Y.; Feng, D.; Ran, Y. Comparison of Pore Size Distribution, Heterogeneity and Occurrence Characteristics of Movable Fluids of Tight Oil Reservoirs Formed in Different Sedimentary Environments: A Case Study of the Chang 7 Member of Ordos Basin, China. Nat. Resour. Res. 2022, 31, 415–442. [Google Scholar] [CrossRef]
- Yu, H.; Xu, H.Y.; Fan, J.C.; Zhu, Y.-B.; Wang, F.; Wu, H. Transport of shale gas in microporous/nanoporous media: Molecular to pore-scale simulations. Energy Fuels 2020, 35, 911–943. [Google Scholar] [CrossRef]
- Alfi, M.; Barrufet, M.; Killough, J. Effect of pore sizes on composition distribution and enhance recovery from liquid shale—Molecular sieving in low permeability reservoirs. Fuel 2019, 235, 1555–1564. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Z.; Zou, C. Shale Oil Geological Characteristics and Resource Potential of Mesozoic Yanchang Formation in Ordos Basin, North-Central China. In Proceedings of the International Conference and Exhibition, Melbourne, Australia, 13–16 September 2015; Society of Exploration Geophysicists and American Association of Petroleum Geologists: Tulsa, OK, USA, 2015; Volume 89, p. 333. [Google Scholar]
- Dong, H.; Zhu, Q.; Wang, L.; Yue, X.; Fang, H.; Wang, Z.; Liu, S.; Wei, S.; Lu, X. Effects of Shale Pore Size and Connectivity on scCO2 Enhanced Oil Recovery: A Molecular Dynamics Simulation Investigation. Langmuir 2023, 39, 6287–6299. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zeng, J.; Qiao, J.; Liu, Y.; Liu, S.; Jia, W.; Cao, W.; Wang, C.; Geng, F.; Wei, W. Role of Organic Matter, Mineral, and Rock Fabric in the Full-Scale Pore and Fracture Network Development in the Mixed Lacustrine Source Rock System. Energy Fuels 2022, 36, 8161–8179. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, K.; Zhang, L.; Sun, H.; Zhang, K.; Ma, J. Pore-scale simulation of shale oil flow based on pore network model. Fuel 2019, 251, 683–692. [Google Scholar] [CrossRef]
- Xu, Y.; Lun, Z.; Pan, Z.; Wang, H.; Zhou, X.; Zhao, C.; Zhang, D. Occurrence space and state of shale oil: A review. J. Pet. Sci. Eng. 2022, 211, 110183. [Google Scholar] [CrossRef]
- Gu, Z.; Lu, T.; Li, Z.; Li, B.; Du, L.; Zhang, C. Analysis on the mechanism and characteristics of nanofluid imbibition in low permeability sandstone core pore surface: Application in reservoir development engineering. Colloids Surf. A Physicochem. Eng. Asp. 2023, 659, 130774. [Google Scholar] [CrossRef]
- Huang, K.; Yu, F.; Zhang, W.; Tong, K.; Guo, J.; Li, S.; Chen, S.; Dai, Z. Relationship between capillary water absorption mechanism and pore structure and microfracture of red-layer mudstone in central Sichuan. Bull. Eng. Geol. Environ. 2023, 82, 100. [Google Scholar] [CrossRef]
- Liu, B.; Liu, W.; Pan, Z.; Yu, L.; Xie, Z.; Lv, G.; Zhao, P.; Chen, D.; Fang, W. Supercritical CO2 breaking through a water bridge and enhancing shale oil recovery: A molecular dynamics simulation study. Energy Fuels 2022, 36, 7558–7568. [Google Scholar] [CrossRef]
- Dai, C.; Sun, X.; Sun, Y.; Zhao, M.; Du, M.; Zou, C.; Guan, B. The effect of supercritical CO2 fracturing fluid retention-induced permeability alteration of tight oil reservoir. J. Pet. Sci. Eng. 2018, 171, 1123–1132. [Google Scholar] [CrossRef]
- Ma, C.; Lin, C.; Dong, C.; Elsworth, D.; Wu, S.; Wang, X.; Sun, X. Determination of the critical flow pore diameter of shale caprock. Mar. Pet. Geol. 2020, 112, 104042. [Google Scholar] [CrossRef]
- Zhang, D.; Han, M.; Zhou, Q.; Ye, T.; Zhou, Y.; Chang, J.; Lin, X. The Micro-Occurrence Mechanisms of Tight Oil: Fluid–Rock Interactions at Microscale Pores, Nanoscale Pores, and Mineral Surfaces. Energies 2023, 16, 3917. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, F.; Hu, T.; Chen, D.; Huang, L.; Jiang, Z.; Wang, X.; Liu, Z.; Wu, Y.; Lv, J.; et al. Oil occurrence state and quantity in alkaline lacustrine shale using a high-frequency NMR technique. Mar. Pet. Geol. 2023, 154, 106302. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, C.; Jiang, F.; Hu, T.; Awan, R.S.; Chen, Z.; Lv, J.; Zhang, C.; Hu, M.; Huang, R.; et al. Occurrence Space and State of Petroleum in Lacustrine Shale: Insights from Two-Step Pyrolysis and the N2 Adsorption Experiment. Energy Fuels 2022, 36, 10920–10933. [Google Scholar] [CrossRef]
- Nikolaev, M.Y.; Kazak, A.V. Liquid saturation evaluation in organic-rich unconventional reservoirs: A comprehensive review. Earth-Sci. Rev. 2019, 194, 327–349. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Z.; Tian, Y. An improved method to characterize the pore-throat structures in tight sandstone reservoirs: Combined high-pressure and rate-controlled mercury injection techniques. Energy Explor. Exploit. 2020, 38, 2389–2412. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, Z.; Sun, W.; Li, Y.; Zhang, X.; Zhu, L.; Wen, M. A multiscale comprehensive study on pore structure of tight sandstone reservoir realized by nuclear magnetic resonance, high pressure mercury injection and constant-rate mercury injection penetration test. Mar. Pet. Geol. 2019, 109, 208–222. [Google Scholar] [CrossRef]
- Xu, J.; Wang, R.; Zan, L. Shale oil occurrence and slit medium coupling based on a molecular dynamics simulation. J. Pet. Sci. Eng. 2023, 220, 111151. [Google Scholar] [CrossRef]
- Luo, Y.; Xiao, H.; Liu, X.; Zheng, T. Occurrence characteristics and influential factors of movable oil in nano-pores by molecular dynamics simulation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130320. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Liu, H.; Liu, F. Impacts of polar molecules of crude oil on spontaneous imbibition in calcite nanoslit: A molecular dynamics simulation study. Energy Fuels 2021, 35, 13671–13686. [Google Scholar] [CrossRef]
- Emmings, J.F.; Dowey, P.J.; Taylor, K.G.; Davies, S.J.; Vane, C.H.; Moss-Hayes, V.; Rushton, J.C. Origin and implications of early diagenetic quartz in the Mississippian Bowland Shale Formation, Craven Basin, UK. Mar. Pet. Geol. 2020, 120, 104567. [Google Scholar] [CrossRef]
- Xu, K.; Chen, S.; Lu, J.; Li, Y.; Yin, X.; Wu, X.; Li, C. Molecular Simulation Analysis of Methane Adsorption Micromechanisms and the Impact of Water Saturation on Methane Adsorption in Transitional Shale. Lithosphere 2022, 2022, 8195502. [Google Scholar] [CrossRef]
- Wang, S.; Javadpour, F.; Feng, Q. Molecular dynamics simulations of oil transport through inorganic nanopores in shale. Fuel 2016, 171, 74–86. [Google Scholar] [CrossRef]
- Skelton, A.A.; Fenter, P.; Kubicki, J.D.; Wesolowski, D.J.; Cummings, P.T. Simulations of the quartz (1011)/water interface: A comparison of classical force fields, ab initio molecular dynamics, and X-ray reflectivity experiments. J. Phys. Chem. C 2011, 115, 2076–2088. [Google Scholar] [CrossRef]
- Skelton, A.A.; Wesolowski, D.J.; Cummings, P.T. Investigating the quartz (1010)/water interface using classical and ab initio molecular dynamics. Langmuir 2011, 27, 8700–8709. [Google Scholar] [CrossRef]
- Wang, L.; Lyu, W.; Ji, Z.; Wang, L.; Liu, S.; Fang, H.; Yue, X.; Wei, S.; Liu, S.; Wang, Z.; et al. Molecular Dynamics Insight into the CO2 Flooding Mechanism in Wedge-Shaped Pores. Molecules 2022, 28, 188. [Google Scholar] [CrossRef]
- Koretsky, C.M.; Sverjensky, D.A.; Sahai, N. A model of surface site types on oxide and silicate minerals based on crystal chemistry; implications for site types and densities, multi-site adsorption, surface infrared spectroscopy, and dissolution kinetics. Am. J. Sci. 1998, 298, 349–438. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, G.; Liu, G.; Gao, B.; Liu, Q.; Wang, H.; Liang, C.; Wang, R. Research progress and key scientific issues of continental shale oil in China. Acta Pet. Sin. 2021, 42, 821–835. [Google Scholar]
- Liu, Z.; Zhu, W.; Sun, Q.; Jian, B.; Xu, X.; Zhang, H. Characteristics of geotemperature-geopressure systems in petroliferous basins of China. Acta Pet. Sin. 2012, 33, 1. [Google Scholar]
- Zhang, Y.; Guo, W. Molecular insight into the tight oil movability in nano-pore throat systems. Fuel 2021, 293, 120428. [Google Scholar] [CrossRef]
- Sun, P.Z.; Yang, Q.; Kuang, W.J.; Stebunov, Y.V.; Xiong, W.Q.; Yu, J.; Nair, R.R.; Katsnelson, M.I.; Yuan, S.J.; Grigorieva, I.V.; et al. Limits on gas impermeability of graphene. Nature 2020, 579, 229–232. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Beckedahl, D.; Obaga, E.O.; Uken, D.A.; Sergi, A.; Ferrario, M. On the configurational temperature Nosè–Hoover thermostat. Phys. A 2016, 461, 19–35. [Google Scholar] [CrossRef]
- Harris, J.G.; Yung, K.H. Carbon dioxide’s liquid-vapor coexistence curve and critical properties as predicted by a simple molecular model. J. Phys. Chem. 1995, 99, 12021–12024. [Google Scholar] [CrossRef]
- Headen, T.F.; Boek, E.S. Molecular dynamics simulations of asphaltene aggregation in supercritical carbon dioxide with and without limonene. Energy Fuels 2011, 25, 503–508. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Cygan, R.T.; Liang, J.J.; Kalinichev, A.G. Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Argüello-Luengo, J.; González-Tudela, A.; Shi, T.; Zoller, P.; Cirac, J.I. Analogue quantum chemistry simulation. Nature 2019, 574, 215–218. [Google Scholar] [CrossRef]
- Granados-Bazan, E.L.; Quinones-Cisneros, S.E.; Deiters, U.K. Structure and contact angle in sessile droplets of binary mixtures of Lennard-Jones chains: A molecular dynamics study. Langmuir 2021, 37, 10945–10957. [Google Scholar] [CrossRef]
- Forsman, J.; Woodward, C.E. Limitations of the Derjaguin approximation and the Lorentz–Berthelot mixing rule. Langmuir 2010, 26, 4555–4558. [Google Scholar] [CrossRef]
- Xiong, Y.; Winterfeld, P.; Wang, C.; Huang, Z.; Wu, Y.-S. Effect of Large Capillary Pressure on Fluid Flow and Transport in Stress-sensitive Tight Oil Reservoirs. In SPE Annual Technical Conference and Exhibition; Society of Petroleum Engineers: Houston, TX, USA, 2015; p. 26. [Google Scholar]
- Kuila, U.; Prasad, M. Surface area and pore-size distribution in clays and shales. Geophys. Prospect. 2013, 61, 341–362. [Google Scholar] [CrossRef]
- Ross DJ, K.; Bustin, R.M. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Wang, S.; Feng, Q.; Javadpour, F.; Hu, Q.; Wu, K. Competitive adsorption of methane and ethane in montmorillonite nanopores of shale at supercritical conditions: A grand canonical Monte Carlo simulation study. Chem. Eng. J. 2019, 355, 76–90. [Google Scholar] [CrossRef]
- Chen, G.; Lu, S.; Liu, K.; Xue, Q.; Xu, C.; Tian, S.; Li, J.; Zhang, Y.; Tong, M.; Pang, X.; et al. Investigation of pore size effects on adsorption behavior of shale gas. Mar. Pet. Geol. 2019, 109, 1–8. [Google Scholar] [CrossRef]
- Afsharpoor, A.; Javadpour, F. Liquid slip flow in a network of shale noncircular nanopores. Fuel 2016, 180, 580–590. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, J.; Hu, Q.; Wang, J.; Tian, T.; Chao, J.; Wang, M. Integrated NMR and FE-SEM methods for pore structure characterization of Shahejie shale from the Dongying Depression, Bohai Bay Basin. Mar. Pet. Geol. 2019, 100, 85–94. [Google Scholar] [CrossRef]
- Wu, K.; Chen, Z.; Li, X. Real gas transport through nanopores of varying cross-section type and shape in shale gas reservoirs. Chem. Eng. J. 2015, 281, 813–825. [Google Scholar] [CrossRef]
- Liu, B.; Qi, C.; Mai, T.; Zhang, J.; Zhan, K.; Zhang, Z.; He, J. Competitive adsorption and diffusion of CH4/CO2 binary mixture within shale organic nanochannels. J. Nat. Gas Sci. Eng. 2018, 53, 329–336. [Google Scholar] [CrossRef]
- Rocchi, C.; Bizzarri, A.R.; Cannistraro, S. Water dynamical anomalies evidenced by molecular-dynamics simulations at the solvent-protein interface. Phys. Rev. E 1998, 57, 3315–3325. [Google Scholar] [CrossRef]
- Li, X.; Xue, Q.; Zhu, L.; Jin, Y.; Wu, T.; Guo, Q.; Zheng, H.; Lu, S. How to select an optimal surfactant molecule to speed up the oil-detachment from solid surface: A computational simulation. Chem. Eng. Sci. 2016, 147, 47–53. [Google Scholar] [CrossRef]
- Fang, T.; Wang, M.; Wang, C.; Liu, B.; Shen, Y.; Dai, C.; Zhang, J. Oil detachment mechanism in CO2 flooding from silica surface: Molecular dynamics simulation. Chem. Eng. Sci. 2017, 16, 17–22. [Google Scholar] [CrossRef]
- Li, Q.; Han, Y.; Liu, X.; Ansari, U.; Cheng, Y.; Yan, C. Hydrate as a by-product in CO2 leakage during the long-term sub-seabed sequestration and its role in preventing further leakage. Environ. Sci. Pollut. Res. 2022, 29, 77737–77754. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, F.; Wang, Y.; Forson, K.; Cao, L.; Zhang, C.; Zhou, C.; Zhao, B.; Chen, J. Experimental investigation on the high-pressure sand suspension and adsorption capacity of guar gum fracturing fluid in low-permeability shale reservoirs: Factor analysis and mechanism disclosure. Environ. Sci. Pollut. Res. 2022, 29, 53050–53062. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xiao, Z.; Liu, X.; Ren, J.; Xing, T.; Li, Z.; Li, X.; Chen, Y. Strategic design of cellulose nanofibers@ zeolitic imidazolate frameworks derived mesoporous carbon-supported nanoscale CoFe2O4/CoFe hybrid composition as trifunctional electrocatalyst for Zn-air battery and self-powered overall water-splitting. J. Power Source 2022, 521, 230925. [Google Scholar] [CrossRef]
- Fei, J.; Wang, M.; Li, J.; Zhao, C.; Wang, X.; Zhang, L.; Yu, C.; Li, Y.; Dong, S. Molecular Dynamics Simulation of Adsorption and Absorption Behavior of Shale Oil in Realistic Kerogen Slits. Energy Fuels 2023, 37, 3654–3671. [Google Scholar] [CrossRef]
- Wu, C.; Xue, H.; Lu, S.; Tian, S. Measurement and Analysis of Oil-water Contact Angle on Several Common Minerals. Geoscience 2018, 32, 842–849. [Google Scholar]




| Interaction Energy | (kcal/mol) | (kcal/mol) | |
|---|---|---|---|
| Water Content | |||
| 55 wt% | −0.652585 | −5349.2 | |
| 80 wt% | −3.86542 | −5234.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Jia, N.; Feng, C.; Wang, L.; Liu, S.; Lyu, W. Exploration of Oil/Water/Gas Occurrence State in Shale Reservoir by Molecular Dynamics Simulation. Energies 2023, 16, 7253. https://doi.org/10.3390/en16217253
Sun L, Jia N, Feng C, Wang L, Liu S, Lyu W. Exploration of Oil/Water/Gas Occurrence State in Shale Reservoir by Molecular Dynamics Simulation. Energies. 2023; 16(21):7253. https://doi.org/10.3390/en16217253
Chicago/Turabian StyleSun, Linghui, Ninghong Jia, Chun Feng, Lu Wang, Siyuan Liu, and Weifeng Lyu. 2023. "Exploration of Oil/Water/Gas Occurrence State in Shale Reservoir by Molecular Dynamics Simulation" Energies 16, no. 21: 7253. https://doi.org/10.3390/en16217253




