CO2 Mineralized Sequestration and Assistance by Microorganisms in Reservoirs: Development and Outlook
Abstract
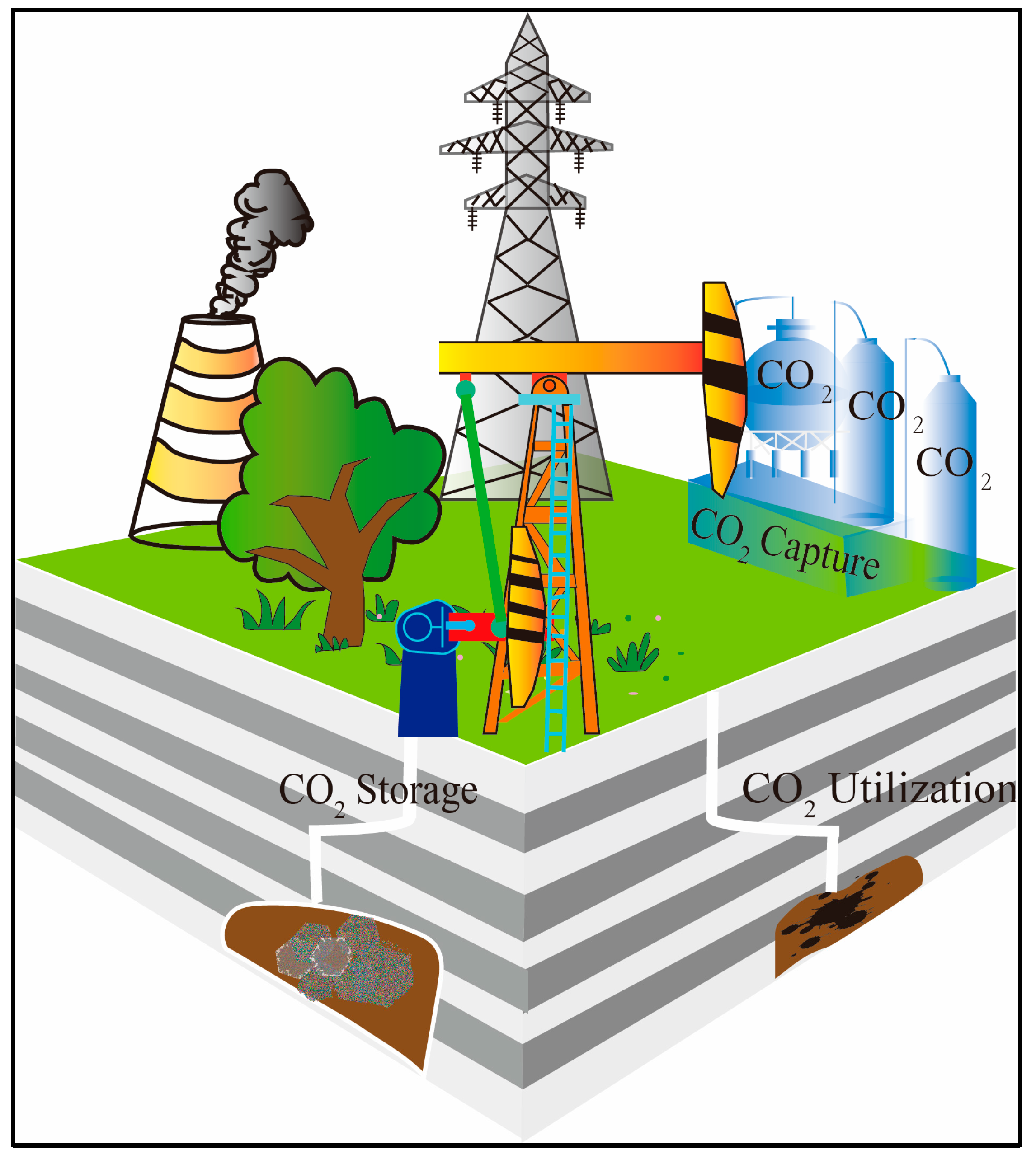
:1. Introduction
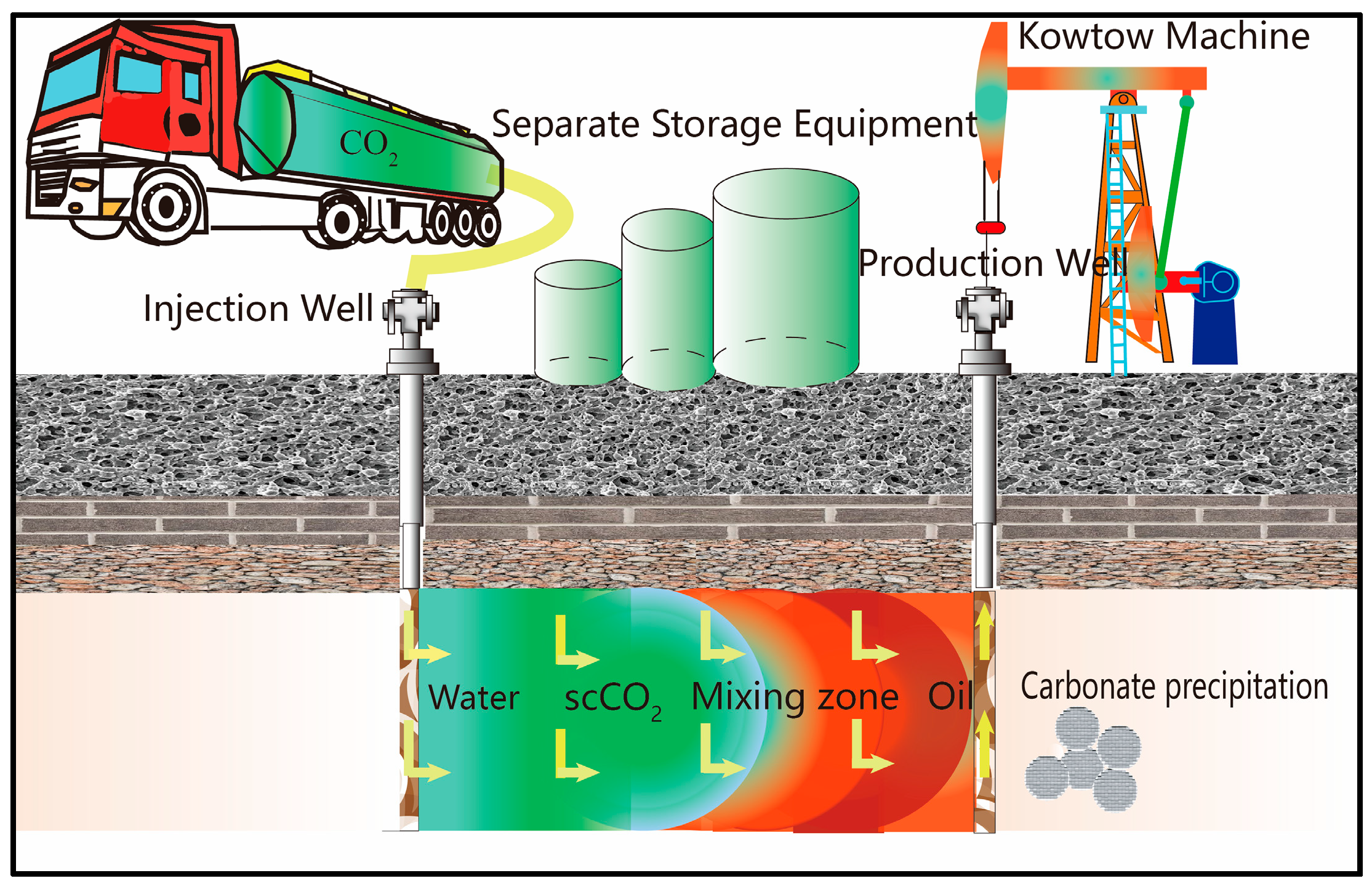
2. Sequestration Mechanism of Carbon Dioxide Mineralization
- (1)
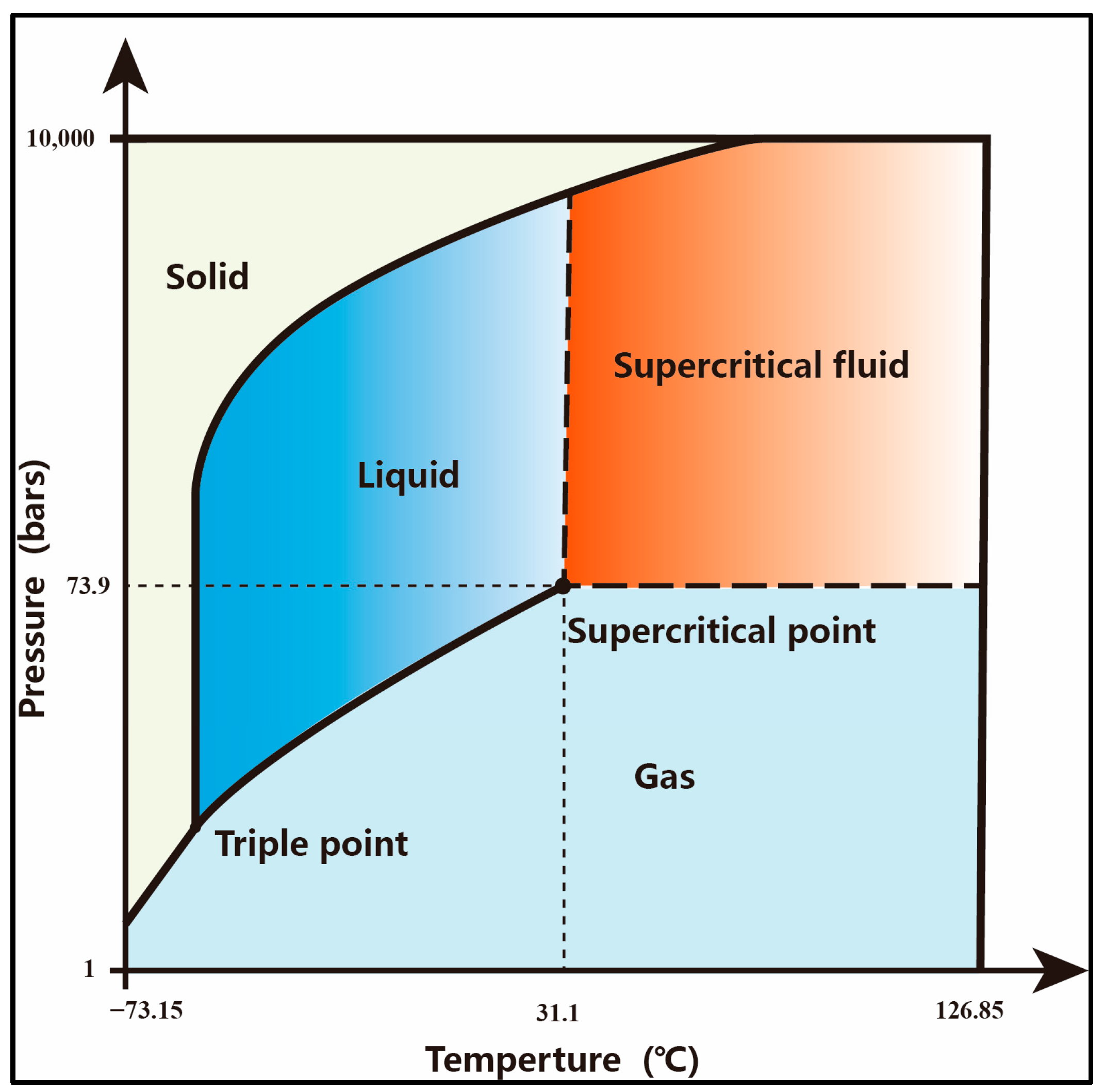
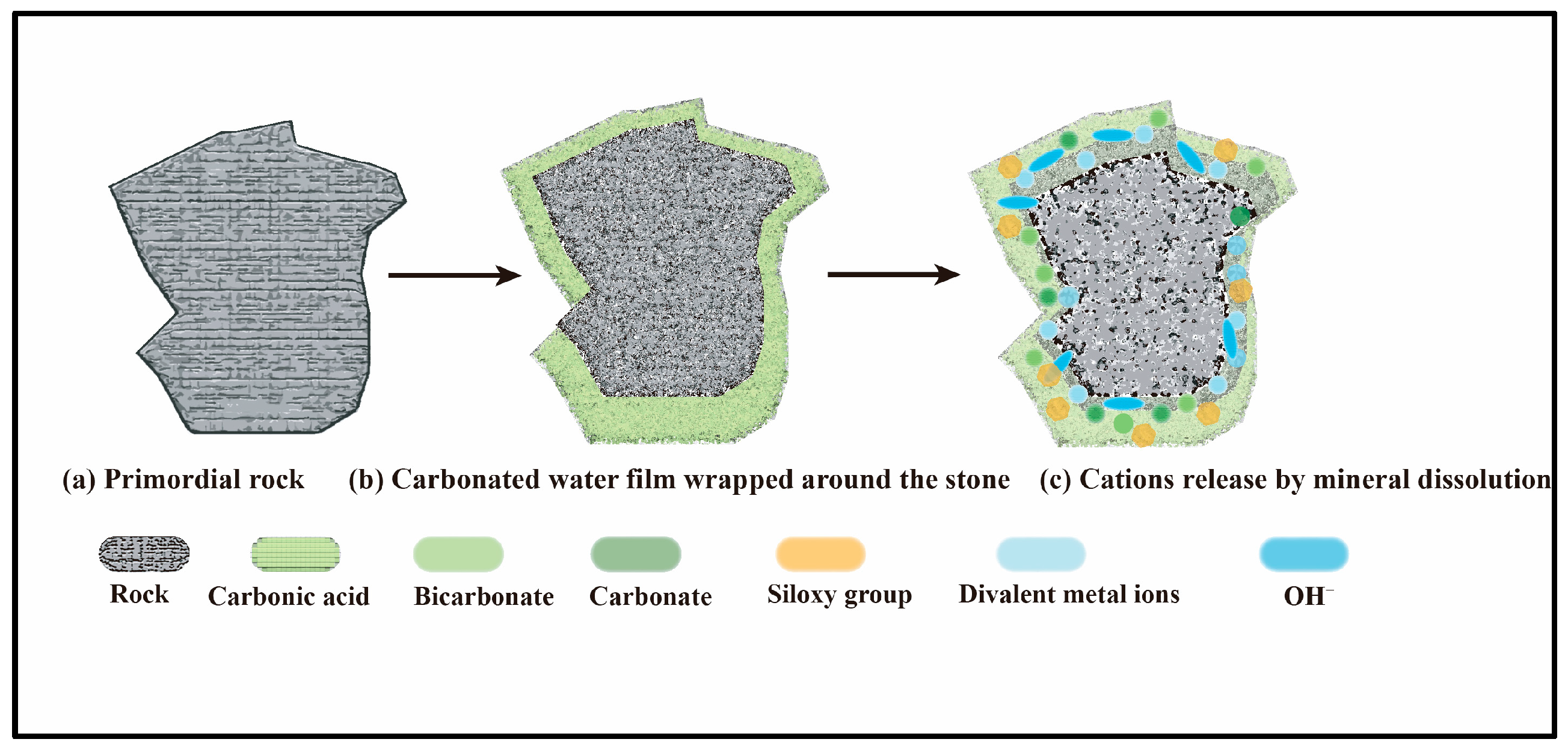
- Dissolution and ionization of carbon dioxide
- (2)
- Mineral corrosion
- (3)
- Carbonate mineral deposition
| Region | Felsic Mineral Content/% | Carbonate Mineral Content/% | Clay Mineral Content/% | Reference |
|---|---|---|---|---|
| Cambrian Yurtus Formation, Tarim Basin, China | 21.2–94.8 57.8 (Mean) | 0.1–69.6 16.9 (Mean) | 0.8–48.5 15.7 (Mean) | [52] |
| Cretaceous Qingshankou Formation, Songliao Basin, China | 3.7–73.4 49.4 (Mean) | 0.1–93.5 13.1 (Mean) | 2.5–49.6 33.6 (Mean) | [53] |
| Permian Luchaogou Formation, Junggar Basin, China | 0.1–73.5 49.4 (Mean) | 0.1–91.5 32.8 (Mean) | 0.8–48.5 11.9 (Mean) | [54] |
| Paleogene Kongdian Formation, Cangdong Sag, Bohai Bay Basin, China | 0.1–56.0 36.7 (Mean) | 0.1–95.0 32.8 (Mean) | 0.8–48.5 15.7 (Mean) | [55] |
| Permian Longtan Formation, Southeast Xiang-tan Depression, China | 9.0–43.0 27.0 (Mean) | 23.0–50.0 38.0 (Mean) | 0.8–48.5 15.7 (Mean) | [56] |
| Devonian–Mississippian of the Western Canadian Basin | 21.2–94.8 57.8 (Mean) | 0.1–85.4 4.7 (Mean) | 0.8–48.5 15.7 (Mean) | [57] |
| Barnett Shale, Fort Worth Basin, USA | 51.9 | 8.1 | 35.0 | [58] |
| Upper Jurassic Haynesville Shale, Gulf of Mexico Basin, USA | 31.8 | 22.7 | 45.5 | [59] |
| West Philippine Sea | 22.7–75.4 66.3 (Mean) | 14.6–41.5 28.0 (Mean) | 5.3–54.3 21.5 (Mean) | [60] |
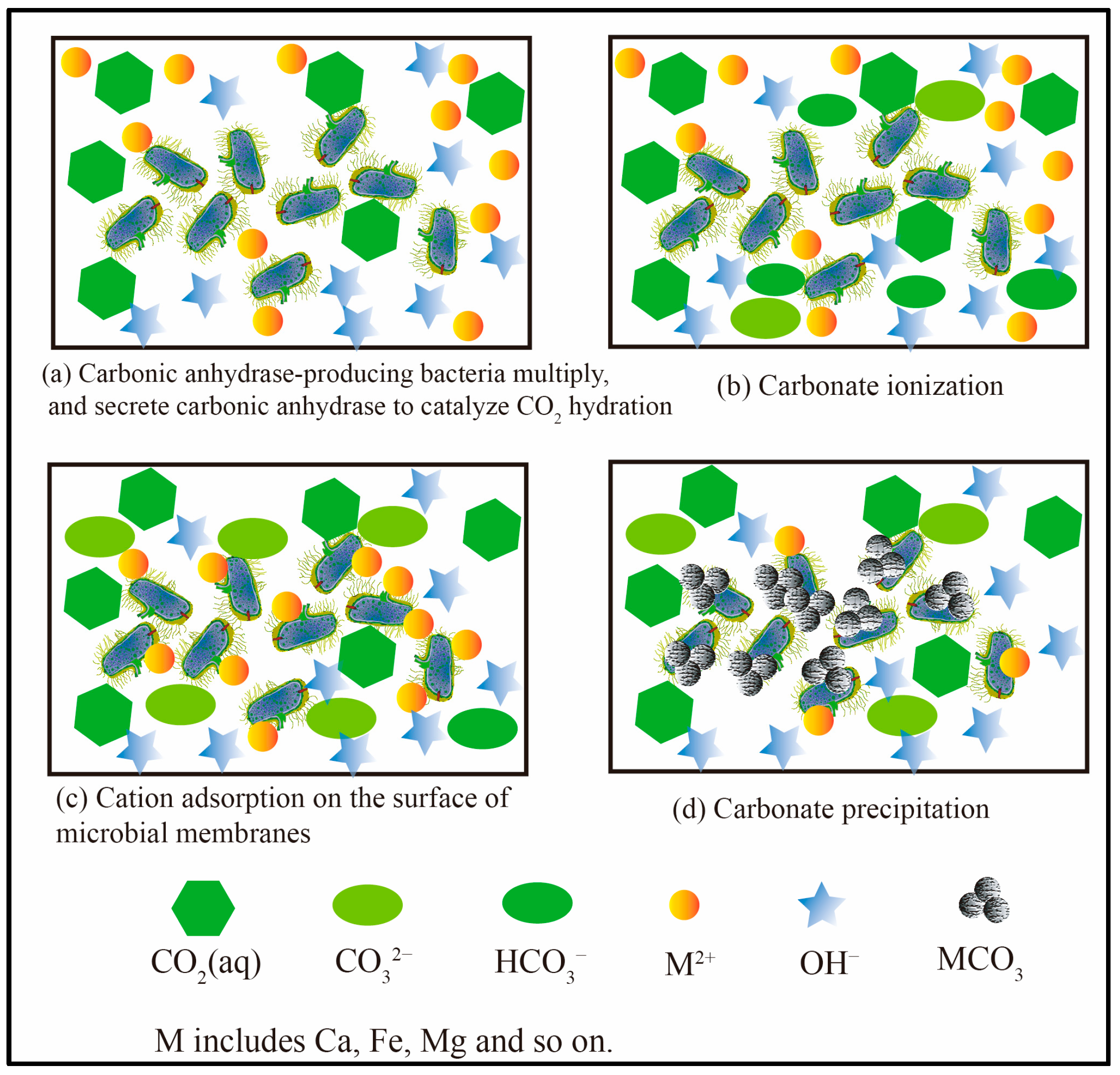
3. Mechanism of Microbial-Assisted Mineralization
- (1)
- Reservoir microbial function in carbon dioxide mineralization
- (2)
- Key microbial enzymes in CO2 mineralization
3.1. Catalytic Mechanism of Carbonic Anhydrase
3.2. Carbonic Anhydrase Catalytic Activity and Stability Factors
3.3. Application of Carbonic Anhydrase Immobilization in CO2 Mineralization
4. Comparisons of the Natural and Microbial-Catalyzed Sequestration Processes
5. Outlook
6. Conclusions
- (1)
- The geophysical–chemical process of carbon dioxide mineralization in reservoirs primarily encompasses three stages: carbon dioxide dissolution ionization, mineral dissolution, and carbonate mineral precipitation.
- (2)
- The rate of CO2 mineralization sequestration in alkaline environments is influenced by factors such as carbon anion concentrations; concentrations of divalent metal cations like calcium, magnesium, and iron; and the availability of mineral nucleation sites.
- (3)
- Microbial induction can expedite the mineralization process by enhancing the precipitation environment and providing nucleation sites. Additionally, the carbonic anhydrase produced during microbial metabolism is a pivotal enzyme in the process of CO2 mineralization induced by bacteria.
- (4)
- Carbonic anhydrase primarily catalyzes the carbon dioxide hydration reaction, influencing the CO2 mineralization process. Its enzyme activity and stability are impacted by factors like the structure of the active region, the environmental temperature, and the pressure. Utilizing covalent bonding and other methods to prepare immobilized carbonic anhydrase by chemo-mimicry can augment enzyme catalytic activity, thereby enhancing the rate of bio-induced mineralization.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soeder, D.J. Greenhouse gas sources and mitigation strategies from a geosciences perspective. Adv. Geo Energy Res. 2021, 5, 274–285. [Google Scholar] [CrossRef]
- IPCC, E. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: New York, NY, USA, 2005; p. 442. [Google Scholar]
- Tapia, J.F.D.; Lee, J.-Y.; Ooi, R.E.; Foo, D.C.; Tan, R.R. A review of optimization and decision-making models for the planning of CO2 capture, utilization and storage (CCUS) systems. Sustain. Prod. Consum. 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, S.; Yang, Z.; Pan, S.; Wang, G.; Jiang, X.; Guan, M.; Yu, C.; Yu, Z.; Shen, Y. Progress, challenge and significance of building carbon industrial system in the context of carbon neutral strategy. Pet. Explor. Dev. 2023, 50, 190–205. [Google Scholar]
- Jin, L.; Zhou, D.; Liu, R. Research on ecological protection compensation mechanism for adapting to carbon peak and carbon neutrality—Based on the perspective of carbon sink value. Proc. Chin. Acad. Sci. USA 2022, 37, 1623–1634. [Google Scholar]
- Liu, H.; Were, P.; Li, Q.; Gou, Y.; Hou, Z. Worldwide status of CCUS technologies and their development and challenges in China. Geofluids 2017, 2017, 6126505. [Google Scholar] [CrossRef]
- Núñez-López, V.; Gil-Egui, R.; Hosseini, S.A. Environmental and operational performance of CO2-EOR as a CCUS technology: A Cranfield example with dynamic LCA considerations. Energies 2019, 12, 448. [Google Scholar] [CrossRef]
- Xu, T.; Tian, H.; Zhu, H.; Cai, J. China actively promotes CO2 capture, utilization and storage research to achieve carbon peak and carbon neutrality. Adv. Geo Energy Res. 2022, 6, 1–3. [Google Scholar] [CrossRef]
- Cui, G.; Hu, Z.; Ning, F.; Jiang, S.; Wang, R. A review of salt precipitation during CO2 injection into saline aquifers and its potential impact on carbon sequestration projects in China. Fuel 2023, 334, 126615. [Google Scholar] [CrossRef]
- Gunter, W.; Bachu, S.; Law, D.-S.; Marwaha, V.; Drysdale, D.; MacDonald, D.; McCann, T. Technical and economic feasibility of CO2 disposal in aquifers within the Alberta Sedimentary Basin, Canada. Energy Convers. Manag. 1996, 37, 1135–1142. [Google Scholar] [CrossRef]
- Shukla, R.; Ranjith, P.; Haque, A.; Choi, X. A review of studies on CO2 sequestration and caprock integrity. Fuel 2010, 89, 2651–2664. [Google Scholar] [CrossRef]
- Emami-Meybodi, H.; Hassanzadeh, H.; Green, C.P.; Ennis-King, J. Convective dissolution of CO2 in saline aquifers: Progress in modeling and experiments. Int. J. Greenh. Gas Control 2015, 40, 238–266. [Google Scholar]
- Iglauer, S. Dissolution Trapping of Carbon Dioxide in Reservoir Formation Brine—A Carbon Storage Mechanism; INTECH Open Access Publisher: London, UK, 2011; pp. 233–262. [Google Scholar]
- Li, L.; Zhao, N.; Wei, W.; Sun, Y. A review of research progress on CO2 capture, storage, and utilization in Chinese Academy of Sciences. Fuel 2013, 108, 112–130. [Google Scholar] [CrossRef]
- Zhang, L.; Soong, Y.; Dilmore, R.; Lopano, C. Numerical simulation of porosity and permeability evolution of Mount Simon sandstone under geological carbon sequestration conditions. Chem. Geol. 2015, 403, 1–12. [Google Scholar] [CrossRef]
- Song, Y.; Sung, W.; Jang, Y.; Jung, W. Application of an artificial neural network in predicting the effectiveness of trapping mechanisms on CO2 sequestration in saline aquifers. Int. J. Greenh. Gas Control 2020, 98, 103042. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, W.; Jiang, J.; Shang, F.; He, H.; Wang, S. Remaining oil distribution and development strategy for offshore unconsolidated sandstone reservoir at ultrahigh water-cut stage. Geofluids 2022, 2022, 6856298. [Google Scholar] [CrossRef]
- Huang, S.; Wu, Y.; Meng, X.; Liu, L.; Ji, W. Recent advances on microscopic pore characteristics of low permeability sandstone reservoirs. Adv. Geo Energy Res. 2018, 2, 122–134. [Google Scholar] [CrossRef]
- Shiyi, Y.; Qiang, W. New progress and prospect of oilfields development technologies in China. Pet. Explor. Dev. 2018, 45, 698–711. [Google Scholar]
- Guo, H.; Wang, Z.; Wang, B.; Zhang, Y.; Meng, H.; Sui, H. Molecular dynamics simulations of oil recovery from dolomite slit nanopores enhanced by CO2 and N2 injection. Adv. Geo Energy Res. 2022, 6, 306–313. [Google Scholar] [CrossRef]
- Xinmin, S.; Feng, W.; Desheng, M.; Ming, G.; Zhang, Y. Progress and prospect of carbon dioxide capture, utilization and storage in CNPC oilfields. Pet. Explor. Dev. 2023, 50, 229–244. [Google Scholar]
- Yang, B.; Shao, C.; Hu, X.; Ngata, M.R.; Aminu, M.D. Advances in Carbon Dioxide Storage Projects: Assessment and Perspectives. Energy Fuels 2023, 37, 1757–1776. [Google Scholar] [CrossRef]
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joyce, E.L., Jr.; Sharp, D.H. Carbon dioxide disposal in carbonate minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Seifritz, W. CO2 disposal by means of silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Saito, T.; Sakai, E.; Morioka, M.; Otsuki, N. Carbonation of γ-Ca2SiO4 and the Mechanism of Vaterite Formation. J. Adv. Concr. Technol. 2010, 8, 273–280. [Google Scholar] [CrossRef]
- Bachu, S. Sequestration of CO2 in geological media: Criteria and approach for site selection in response to climate change. Energy Convers. Manag. 2000, 41, 953–970. [Google Scholar] [CrossRef]
- Xu, R.; Li, R.; Ma, J.; He, D.; Jiang, P. Effect of mineral dissolution/precipitation and CO2 exsolution on CO2 transport in geological carbon storage. Acc. Chem. Res. 2017, 50, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Glatz, G.; Gholami, R.; Mahmoud, M.; Alafnan, S. Carbon mineralization and geological storage of CO2 in basalt: Mechanisms and technical challenges. Earth Sci. Rev. 2022, 229, 104036. [Google Scholar] [CrossRef]
- Kou, Z.; Wang, H.; Alvarado, V.; Nye, C.; Bagdonas, D.A.; McLaughlin, J.F.; Quillinan, S.A. Effects of carbonic acid-rock interactions on CO2/Brine multiphase flow properties in the upper minnelusa sandstones. SPE J. 2023, 28, 754–767. [Google Scholar] [CrossRef]
- Campbell, J.S.; Foteinis, S.; Furey, V.; Hawrot, O.; Pike, D.; Aeschlimann, S.; Maesano, C.N.; Reginato, P.L.; Goodwin, D.R.; Looger, L.L. Geochemical negative emissions technologies: Part I. Review. Front. Clim. 2022, 4, 879133. [Google Scholar] [CrossRef]
- Khosrokhavar, R.; Elsinga, G.; Farajzadeh, R.; Bruining, H. Visualization and investigation of natural convection flow of CO2 in aqueous and oleic systems. J. Pet. Sci. Eng. 2014, 122, 230–239. [Google Scholar] [CrossRef]
- Gibbons, B.H.; Edsall, J.T. Rate of hydration of carbon dioxide and dehydration of carbonic acid at 25. J. Biol. Chem. 1963, 238, 3502–3507. [Google Scholar] [CrossRef]
- Ho, C.; Sturtevant, J.M. The kinetics of the hydration of carbon dioxide at 25. J. Biol. Chem 1963, 238, 3499–3501. [Google Scholar] [CrossRef] [PubMed]
- Dennard, A.; Williams, R. The catalysis of the reaction between carbon dioxide and water. J. Chem. Soc. A Inorg. Phys. Theor. 1966, 812–816. [Google Scholar] [CrossRef]
- Bhaduri, G.A. Catalytic Enhancement of Hydration of CO₂ Using Nickel Nanoparticles for Carbon Capture and Storage. Ph.D. Thesis, Newcastle University, Newcastle upon Tyne, UK, 2018. [Google Scholar]
- Verma, M.; Bhaduri, G.A.; Phani Kumar, V.S.; Deshpande, P.A. Biomimetic catalysis of CO2 hydration: A materials perspective. Ind. Eng. Chem. Res. 2021, 60, 4777–4793. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Miao, X.; Gan, M.; Li, X. Geochemistry in geologic CO2 utilization and storage: A brief review. Adv. Geo Energy Res. 2019, 3, 304–313. [Google Scholar] [CrossRef]
- André, L.; Audigane, P.; Azaroual, M.; Menjoz, A. Numerical modeling of fluid–rock chemical interactions at the supercritical CO2–liquid interface during CO2 injection into a carbonate reservoir, the Dogger aquifer (Paris Basin, France). Energy Convers. Manag. 2007, 48, 1782–1797. [Google Scholar] [CrossRef]
- Ketzer, J.; Iglesias, R.; Einloft, S.; Dullius, J.; Ligabue, R.; De Lima, V. Water–rock–CO2 interactions in saline aquifers aimed for carbon dioxide storage: Experimental and numerical modeling studies of the Rio Bonito Formation (Permian), Southern Brazil. Appl. Geochem. 2009, 24, 760–767. [Google Scholar] [CrossRef]
- Zhang, R.; Winterfeld, P.H.; Yin, X.; Xiong, Y.; Wu, Y.-S. Sequentially coupled THMC model for CO2 geological sequestration into a 2D heterogeneous saline aquifer. J. Nat. Gas Sci. Eng. 2015, 27, 579–615. [Google Scholar] [CrossRef]
- Khudhur, F.W.; MacDonald, J.M.; Macente, A.; Daly, L. The utilization of alkaline wastes in passive carbon capture and sequestration: Promises, challenges and environmental aspects. Sci. Total Environ. 2022, 823, 153553. [Google Scholar] [CrossRef]
- Jorat, M.E.; Goddard, M.A.; Manning, P.; Lau, H.K.; Ngeow, S.; Sohi, S.P.; Manning, D.A. Passive CO2 removal in urban soils: Evidence from brownfield sites. Sci. Total Environ. 2020, 703, 135573. [Google Scholar] [CrossRef]
- Ali, M.; Jha, N.K.; Pal, N.; Keshavarz, A.; Hoteit, H.; Sarmadivaleh, M. Recent advances in carbon dioxide geological storage, experimental procedures, influencing parameters, and future outlook. Earth Sci. Rev. 2022, 225, 103895. [Google Scholar] [CrossRef]
- Gerdemann, S.J.; O’Connor, W.K.; Dahlin, D.C.; Penner, L.R.; Rush, H. Ex situ aqueous mineral carbonation. Environ. Sci. Technol. 2007, 41, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Prigiobbe, V.; Costa, G.; Baciocchi, R.; Hänchen, M.; Mazzotti, M. The effect of CO2 and salinity on olivine dissolution kinetics at 120 °C. Chem. Eng. Sci. 2009, 64, 3510–3515. [Google Scholar] [CrossRef]
- Wigand, M.; Carey, J.; Schütt, H.; Spangenberg, E.; Erzinger, J. Geochemical effects of CO2 sequestration in sandstones under simulated in situ conditions of deep saline aquifers. Appl. Geochem. 2008, 23, 2735–2745. [Google Scholar] [CrossRef]
- Bénézeth, P.; Palmer, D.A.; Anovitz, L.M.; Horita, J. Dawsonite synthesis and reevaluation of its thermodynamic properties from solubility measurements: Implications for mineral trapping of CO2. Geochim. Cosmochim. Acta 2007, 71, 4438–4455. [Google Scholar] [CrossRef]
- Goldberg, P.; Chen, Z.-Y.; O’Connor, W.; Walters, R.; Ziock, H. CO2 Mineral Sequestration Studies in the US. In Proceedings of the National Conference on Carbon Sequestration, Washington, DC, USA, 15–17 May 2001; National Energy Technology Laboratory: Pittsburgh, PA, USA; Washington, DC, USA, 2001. [Google Scholar]
- Manning, D.A.; Renforth, P. Passive sequestration of atmospheric CO2 through coupled plant-mineral reactions in urban soils. Environ. Sci. Technol. 2013, 47, 135–141. [Google Scholar] [CrossRef]
- Xi, K.; Cao, Y.; Haile, B.G.; Zhu, N.; Liu, K.; Wu, S.; Hellevang, H. Diagenetic variations with respect to sediment composition and paleo-fluids evolution in conglomerate reservoirs: A case study of the Triassic Baikouquan Formation in Mahu Sag, Junggar Basin, Northwestern China. J. Pet. Sci. Eng. 2021, 197, 107943. [Google Scholar] [CrossRef]
- Liu, B.; Fu, X.; Li, Z. Impacts of CO2-brine-rock interaction on sealing efficiency of sand caprock: A case study of Shihezi formation in Ordos basin. Adv. Geo Energy Res. 2018, 2, 380–392. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Z.; Xue, L.; Bao, Y.; Fang, Y. Differential diagenetic evolution of the Lower Cambrian black rock system at the northwestern margin of the Tarim Basin and its influencing factors. J. Petrol. 2020, 36, 3463–3476. [Google Scholar]
- Li, S.Z.; Yang, J.G.; Liu, B.; Yao, Y.L.; Xiao, F.; Bai, L.H.; Huang, Y.M.; Li, A.; Zhang, L.Y. Petrologic characterization and petrographic delineation of mud shale in a section of Qingshankou Formation, Sanzhao Depression, Songliao Basin—An example from Songpai Oil Well No. 3. Geol. Resour. 2021, 30, 317–324. [Google Scholar]
- Alonso-Zarza, A.M.; Tanner, L.H. Carbonates in Continental Settings: Facies, Environments, and Processes; Elsevier: Amsterdam, The Netherlands, 2009; p. 371. [Google Scholar]
- Schieber, J.; Southard, J.; Thaisen, K. Accretion of mudstone beds from migrating floccule ripples. Science 2007, 318, 1760–1763. [Google Scholar] [CrossRef]
- Yan, J.H.; Deng, Y.; Pu, X.G.; Zhou, L.H.; Chen, S.Y.; Jiao, Y.X. Characteristics and controlling factors of fine-grained mixed sedimentary rocks in the Kong II section of the Cangdong Depression, Bohai Bay Basin. Oil Gas Geol. 2017, 38, 98–109. [Google Scholar]
- Liu, Y.Q.; Jiao, X.; Li, H.; Yuan, M.S.; Zhou, X.H.; Liang, H.; Zhou, D.W.; Zheng, C.Y.; Sun, Q. Permian mantle hydrothermal jet-type primary dolomite from Yuejin Gully, Santang Lake, Xinjiang. Chin. Sci. Earth Sci. 2011, 41, 1862–1871. [Google Scholar]
- Manger, K.C.; Curtis, J. Geologic influences on location and production of Antrim Shale gas. Devonian Gas Shales Technol. Rev. GRI 1991, 7, 5–16. [Google Scholar]
- Curtis, J.B. Fractured shale-gas systems. AAPG Bull. 2002, 86, 1921–1938. [Google Scholar]
- Shi, X.F.; Chen, L.R.; Li, K.Y.; Yang, H.L. Mineral assemblages of West Philippine Sea sediments and their geologic significance. Ocean. Lakes 1994, 25, 328–335. [Google Scholar]
- Snæbjörnsdóttir, S.Ó.; Sigfússon, B.; Marieni, C.; Goldberg, D.; Gislason, S.R.; Oelkers, E.H. Carbon dioxide storage through mineral carbonation. Nat. Rev. Earth Environ. 2020, 1, 90–102. [Google Scholar] [CrossRef]
- Al Kalbani, M.; Serati, M.; Hofmann, H.; Bore, T. A comprehensive review of enhanced in-situ CO2 mineralisation in Australia and New Zealand. Int. J. Coal Geol. 2023, 276, 104316. [Google Scholar] [CrossRef]
- Stokreef, S.; Sadri, F.; Stokreef, A.; Ghahreman, A. Mineral carbonation of ultramafic tailings: A review of reaction mechanisms and kinetics, industry case studies, and modelling. Clean. Eng. Technol. 2022, 8, 100491. [Google Scholar] [CrossRef]
- Matter, J.M.; Stute, M.; Snæbjörnsdottir, S.Ó.; Oelkers, E.H.; Gislason, S.R.; Aradottir, E.S.; Sigfusson, B.; Gunnarsson, I.; Sigurdardottir, H.; Gunnlaugsson, E. Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science 2016, 352, 1312–1314. [Google Scholar] [CrossRef]
- Ebigbo, A.; Gregory, S.P. The relevance of microbial processes in geo-energy applications. Adv. Geo Energy Res. 2021, 5, 5–7. [Google Scholar] [CrossRef]
- Herrmann, G.; Jayamani, E.; Mai, G.; Buckel, W. Energy conservation via electron-transferring flavoprotein in anaerobic bacteria. J. Bacteriol. 2008, 190, 784–791. [Google Scholar] [CrossRef]
- Jones, D.; Head, I.; Gray, N.; Adams, J.; Rowan, A.; Aitken, C.; Bennett, B.; Huang, H.; Brown, A.; Bowler, B. Crude-oil biodegradation via methanogenesis in subsurface petroleum reservoirs. Nature 2008, 451, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Boquet, E.; Boronat, A.; Ramos-Cormenzana, A. Production of calcite (calcium carbonate) crystals by soil bacteria is a general phenomenon. Nature 1973, 246, 527–529. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Zhong, M.; Jing, C.; Guo, B. Research status and development of microbial induced calcium carbonate mineralization technology. PLoS ONE 2022, 17, e0271761. [Google Scholar] [CrossRef] [PubMed]
- Mirjafari, P. Sequestration of Carbon Dioxide: A Biological Approach to Mineralization of Carbon Dioxide in Depleted Oil and Gas Reservoirs; University of Regina: Regina, SK, Canada, 2006. [Google Scholar]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, N.; Cai, G.; Jin, Y.; Ding, N.; Shen, D. Review of ground improvement using microbial induced carbonate precipitation (MICP). Mar. Georesources Geotechnol. 2017, 35, 1135–1146. [Google Scholar] [CrossRef]
- Jain, S.; Fang, C.; Achal, V. A critical review on microbial carbonate precipitation via denitrification process in building materials. Bioengineered 2021, 12, 7529–7551. [Google Scholar] [CrossRef]
- Semple, K.; Westlake, D. Characterization of iron-reducing Alteromonas putrefaciens strains from oil field fluids. Can. J. Microbiol. 1987, 33, 366–371. [Google Scholar] [CrossRef]
- Personna, Y.R.; Ntarlagiannis, D.; Slater, L.; Yee, N.; O’Brien, M.; Hubbard, S. Spectral induced polarization and electrodic potential monitoring of microbially mediated iron sulfide transformations. J. Geophys. Res. Biogeosciences 2008, 113. [Google Scholar] [CrossRef]
- Han, Z.; Zhao, Y.; Yan, H.; Zhao, H.; Han, M.; Sun, B.; Sun, X.; Hou, F.; Sun, H.; Han, L. Struvite precipitation induced by a novel sulfate-reducing bacterium Acinetobacter calcoaceticus SRB4 isolated from river sediment. Geomicrobiol. J. 2015, 32, 868–877. [Google Scholar] [CrossRef]
- Wang, F.; Zheng, S.; Qiu, H.; Cao, C.; Tang, X.; Hao, L.; Liu, F.; Li, J. The process by which the iron-reducing bacterium Shewanella oneidensis MR-4 induces hydrated iron oxide to form cyanite. J. Microbiol. Biotechnol. 2018, 58, 573–583. [Google Scholar]
- Mobley, H.L. Urease. In Helicobacter Pylori: Physiology and Genetics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; pp. 177–191. [Google Scholar]
- Lalucat, J.; Bennasar, A.; Bosch, R.; García-Valdés, E.; Palleroni, N.J. Biology of Pseudomonas stutzeri. Microbiol. Mol. Biol. Rev. 2006, 70, 510–547. [Google Scholar] [CrossRef]
- Zeikus, J. The biology of methanogenic bacteria. Bacteriol. Rev. 1977, 41, 514–541. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.D.; Mota, A.; Teixeira, J.A.; Vicente, A.A. Continuous cultivation of photosynthetic microorganisms: Approaches, applications and future trends. Biotechnol. Adv. 2015, 33, 1228–1245. [Google Scholar] [CrossRef]
- Kwon, S.-W.; Kim, B.-Y.; Song, J.; Weon, H.-Y.; Schumann, P.; Tindall, B.J.; Stackebrandt, E.; Fritze, D. Sporosarcina koreensis sp. nov. and Sporosarcina soli sp. nov., isolated from soil in Korea. Int. J. Syst. Evol. Microbiol. 2007, 57, 1694–1698. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef]
- Rajkhowa, S.; Sarma, J. Biosurfactant: An Alternative Towards Sustainability. In Innovative Bio-Based Technologies for Environmental Remediation; CRC Press: Boca Raton, FL, USA, 2022; pp. 377–402. [Google Scholar]
- Thanigaivelan, R.; Prakash, S.; Maniraj, S. Surface Modification Techniques for Bio-Materials: An Overview. Adv. Manuf. Tech. Eng. Eng. Mater. 2022, 42–60. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Fernández-Remolar, D.; Amils, R.; Sánchez-Navas, A.; Schmid, T.; Martin-Uriz, P.S.; Rodríguez, N.; McKenzie, J.A.; Vasconcelos, C. Microbial mediated formation of Fe-carbonate minerals under extreme acidic conditions. Sci. Rep. 2014, 4, 4767. [Google Scholar] [CrossRef]
- Jun, Y.-S.; Zhu, Y.; Wang, Y.; Ghim, D.; Wu, X.; Kim, D.; Jung, H. Classical and nonclassical nucleation and growth mechanisms for nanoparticle formation. Annu. Rev. Phys. Chem. 2022, 73, 453–477. [Google Scholar] [CrossRef]
- Benzerara, K.; Skouri-Panet, F.; Li, J.; Férard, C.; Gugger, M.; Laurent, T.; Couradeau, E.; Ragon, M.; Cosmidis, J.; Menguy, N. Intracellular Ca-carbonate biomineralization is widespread in cyanobacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 10933–10938. [Google Scholar] [CrossRef]
- Yan, H.; Owusu, D.C.; Han, Z.; Zhao, H.; Ji, B.; Zhao, Y.; Tucker, M.E.; Zhao, Y. Extracellular, surface, and intracellular biomineralization of Bacillus subtilis Daniel-1 bacteria. Geomicrobiol. J. 2021, 38, 698–708. [Google Scholar] [CrossRef]
- Hammes, F.; Boon, N.; de Villiers, J.; Verstraete, W.; Siciliano, S.D. Strain-specific ureolytic microbial calcium carbonate precipitation. Appl. Environ. Microbiol. 2003, 69, 4901–4909. [Google Scholar] [CrossRef] [PubMed]
- Larachi, F. Kinetic model for the reversible hydration of carbon dioxide catalyzed by human carbonic anhydrase II. Ind. Eng. Chem. Res. 2010, 49, 9095–9104. [Google Scholar] [CrossRef]
- Khajepour, H.; Mahmoodi, M.; Biria, D.; Ayatollahi, S. Investigation of wettability alteration through relative permeability measurement during MEOR process: A micromodel study. J. Pet. Sci. Eng. 2014, 120, 10–17. [Google Scholar] [CrossRef]
- Khalifah, R.G. The carbon dioxide hydration activity of carbonic anhydrase: I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Seijo, J.A.; Borchert, M.S.; Navarro-Poulsen, J.-C.; Schnorr, K.M.; Mortensen, S.B.; Leggio, L.L. Structure of a dimeric fungal α-type carbonic anhydrase. FEBS Lett. 2011, 585, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Lindskog, S. Structure and mechanism of carbonic anhydrase. Pharmacol. Ther. 1997, 74, 1–20. [Google Scholar] [CrossRef]
- Meldrum, N.U.; Roughton, F.J. Carbonic anhydrase. Its preparation and properties. J. Physiol. 1933, 80, 113. [Google Scholar] [CrossRef]
- Hassan, M.I.; Shajee, B.; Waheed, A.; Ahmad, F.; Sly, W.S. Structure, function and applications of carbonic anhydrase isozymes. Bioorganic Med. Chem. 2013, 21, 1570–1582. [Google Scholar] [CrossRef]
- Rowlett, R.S. Structure and catalytic mechanism of the β-carbonic anhydrases. Biochim. Biophys. Acta Proteins Proteom. 2010, 1804, 362–373. [Google Scholar] [CrossRef]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Bose, H.; Satyanarayana, T. Microbial carbonic anhydrases in biomimetic carbon sequestration for mitigating global warming: Prospects and perspectives. Front. Microbiol. 2017, 8, 1615. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; Fisher, G.M.; Andrews, K.T.; Poulsen, S.-A.; Capasso, C.; Supuran, C.T. Discovery of a new family of carbonic anhydrases in the malaria pathogen Plasmodium falciparum—The η-carbonic anhydrases. Bioorganic Med. Chem. Lett. 2014, 24, 4389–4396. [Google Scholar] [CrossRef] [PubMed]
- Lane, T.W.; Morel, F.M. A biological function for cadmium in marine diatoms. Proc. Natl. Acad. Sci. USA 2000, 97, 4627–4631. [Google Scholar] [CrossRef]
- Lapointe, M.; MacKenzie, T.D.; Morse, D. An external δ-carbonic anhydrase in a free-living marine dinoflagellate may circumvent diffusion-limited carbon acquisition. Plant Physiol. 2008, 147, 1427–1436. [Google Scholar] [CrossRef]
- Jensen, E.L.; Maberly, S.C.; Gontero, B. Insights on the functions and ecophysiological relevance of the diverse carbonic anhydrases in microalgae. Int. J. Mol. Sci. 2020, 21, 2922. [Google Scholar] [CrossRef]
- Boone, C.D.; Habibzadegan, A.; Tu, C.; Silverman, D.N.; McKenna, R. Structural and catalytic characterization of a thermally stable and acid-stable variant of human carbonic anhydrase II containing an engineered disulfide bond. Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 1414–1422. [Google Scholar] [CrossRef]
- An, H.; Tu, C.; Duda, D.; Montanez-Clemente, I.; Math, K.; Laipis, P.J.; McKenna, R.; Silverman, D.N. Chemical rescue in catalysis by human carbonic anhydrases II and III. Biochemistry 2002, 41, 3235–3242. [Google Scholar] [CrossRef]
- Tu, C.; Silverman, D.N.; Forsman, C.; Jonsson, B.H.; Lindskog, S. Role of histidine 64 in the catalytic mechanism of human carbonic anhydrase II studied with a site-specific mutant. Biochemistry 1989, 28, 7913–7918. [Google Scholar] [CrossRef]
- Jönsson, B.; Håkansson, K.; Liljas, A. The structure of human carbonic anhydrase II in complex with bromide and azide. FEBS Lett. 1993, 322, 186–190. [Google Scholar] [CrossRef]
- Thoms, S. Hydrogen bonds and the catalytic mechanism of human carbonic anhydrase II. J. Theor. Biol. 2002, 215, 399–404. [Google Scholar] [CrossRef]
- Silverman, D.N.; Lindskog, S. The catalytic mechanism of carbonic anhydrase: Implications of a rate-limiting protolysis of water. Acc. Chem. Res. 1988, 21, 30–36. [Google Scholar] [CrossRef]
- Park, D.; Lee, M.S. Kinetic study of catalytic CO2 hydration by metal-substituted biomimetic carbonic anhydrase model complexes. R. Soc. Open Sci. 2019, 6, 190407. [Google Scholar] [CrossRef] [PubMed]
- Boone, C.D.; Rasi, V.; Tu, C.; McKenna, R. Structural and catalytic effects of proline substitution and surface loop deletion in the extended active site of human carbonic anhydrase II. FEBS J. 2015, 282, 1445–1457. [Google Scholar] [CrossRef]
- Lindskog, S.; Coleman, J.E. The catalytic mechanism of carbonic anhydrase. Proc. Natl. Acad. Sci. USA 1973, 70, 2505–2508. [Google Scholar] [CrossRef] [PubMed]
- Liszka, M.J.; Clark, M.E.; Schneider, E.; Clark, D.S. Nature versus nurture: Developing enzymes that function under extreme conditions. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 77–102. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Steiner, W. The biocatalytic potential of extremophiles and extremozymes. Food Technol. Biotechnol. 2004, 42, 223–225. [Google Scholar]
- Sharma, T.; Sharma, S.; Kamyab, H.; Kumar, A. Energizing the CO2 utilization by chemo-enzymatic approaches and potentiality of carbonic anhydrases: A review. J. Clean. Prod. 2020, 247, 119138. [Google Scholar] [CrossRef]
- Rasouli, H.; Nguyen, K.; Iliuta, M.C. Recent advancements in carbonic anhydrase immobilization and its implementation in CO2 capture technologies: A review. Sep. Purif. Technol. 2022, 296, 121299. [Google Scholar] [CrossRef]
- de Oliveira Maciel, A.; Christakopoulos, P.; Rova, U.; Antonopoulou, I. Carbonic anhydrase to boost CO2 sequestration: Improving carbon capture utilization and storage (CCUS). Chemosphere 2022, 299, 134419. [Google Scholar] [CrossRef]
- Ozdemir, E. Biomimetic CO2 sequestration: 1. Immobilization of carbonic anhydrase within polyurethane foam. Energy Fuels 2009, 23, 5725–5730. [Google Scholar] [CrossRef]
- Molina-Fernández, C.; Luis, P. Immobilization of carbonic anhydrase for CO2 capture and its industrial implementation: A review. J. CO2 Util. 2021, 47, 101475. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, F.; Li, H.; Su, S.; Gao, H.; Han, X.; Ren, S. Potential application of the immobilization of carbonic anhydrase based on metal organic framework supports. Process Biochem. 2022, 122, 214–223. [Google Scholar] [CrossRef]
- Rodriguez, L.C.; Restrepo-Sánchez, N.; Pelaez, C.; Bernal, C. Enhancement of the catalytic activity of Carbonic Anhydrase by covalent immobilization on Magnetic Cellulose Crystals. Bioresour. Technol. Rep. 2023, 21, 101380. [Google Scholar] [CrossRef]
- Ray, B. Purification and immobilization of human carbonic anhydrase B by using polyacrylamide gel. Experientia 1977, 33, 1439–1440. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Yang, Y. Characterization of the diatomite binding domain in the ribosomal protein L2 from E. coli and functions as an affinity tag. Appl. Microbiol. Biotechnol. 2013, 97, 2541–2549. [Google Scholar] [CrossRef]
- Chen, Q.; Kenausis, G.L.; Heller, A. Stability of oxidases immobilized in silica gels. J. Am. Chem. Soc. 1998, 120, 4582–4585. [Google Scholar] [CrossRef]
- Zhang, S.; Du, M.; Shao, P.; Wang, L.; Ye, J.; Chen, J.; Chen, J. Carbonic anhydrase enzyme-MOFs composite with a superior catalytic performance to promote CO2 absorption into tertiary amine solution. Environ. Sci. Technol. 2018, 52, 12708–12716. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Wanjari, S.; Prabhu, C.; Kumar, V.; Labhsetwar, N.; Satyanarayanan, T.; Kotwal, S.; Rayalu, S. Immobilized carbonic anhydrase for the biomimetic carbonation reaction. Energy Fuels 2010, 24, 6198–6207. [Google Scholar] [CrossRef]
- Vinoba, M.; Kim, D.H.; Lim, K.S.; Jeong, S.K.; Lee, S.W.; Alagar, M. Biomimetic sequestration of CO2 and reformation to CaCO3 using bovine carbonic anhydrase immobilized on SBA-15. Energy Fuels 2011, 25, 438–445. [Google Scholar] [CrossRef]
- Krevor, S.; De Coninck, H.; Gasda, S.E.; Ghaleigh, N.S.; de Gooyert, V.; Hajibeygi, H.; Juanes, R.; Neufeld, J.; Roberts, J.J.; Swennenhuis, F. Subsurface carbon dioxide and hydrogen storage for a sustainable energy future. Nat. Rev. Earth Environ. 2023, 4, 102–118. [Google Scholar] [CrossRef]
- Joseph, E.T.; Ashok, A.; Singh, D.; Ranganathan, A.; Pandey, G.; Bhan, U.; Singh, Y. Carbon sequestration: Capture, storage & utilization of CO2 emissions from anthropogenic sources. AIP Conf. Proc. 2023, 2521, 030022. [Google Scholar]
- Mwakipunda, G.C.; Ngata, M.R.; Mgimba, M.M.; Yu, L. Carbon Dioxide Sequestration in Low Porosity and Permeability Deep Saline Aquifer: Numerical Simulation Method. J. Energy Resour. Technol. 2023, 145, 073401. [Google Scholar] [CrossRef]
- White, S.K.; Spane, F.A.; Schaef, H.T.; Miller, Q.R.; White, M.D.; Horner, J.A.; McGrail, B.P. Quantification of CO2 mineralization at the Wallula basalt pilot project. Environ. Sci. Technol. 2020, 54, 14609–14616. [Google Scholar] [CrossRef]
- Polites, E.G.; Schaef, H.T.; Horner, J.A.; Owen, A.T.; Holliman, J.E., Jr.; McGrail, B.P.; Miller, Q.R. Exotic carbonate mineralization recovered from a deep basalt carbon storage demonstration. Environ. Sci. Technol. 2022, 56, 14713–14722. [Google Scholar] [CrossRef]
- Pogge von Strandmann, P.A.; Burton, K.W.; Snæbjörnsdóttir, S.O.; Sigfússon, B.; Aradóttir, E.S.; Gunnarsson, I.; Alfredsson, H.A.; Mesfin, K.G.; Oelkers, E.H.; Gislason, S.R. Rapid CO2 mineralisation into calcite at the CarbFix storage site quantified using calcium isotopes. Nat. Commun. 2019, 10, 1983. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Y.; Hou, M.Z.; Xiong, Y.; Wu, X.; Lüddeke, C.T.; Huang, L. Advances in subsea carbon dioxide utilization and storage. Energy Rev. 2023, 2, 100016. [Google Scholar] [CrossRef]
- Xu, F.; Wang, D. Review on Soil Solidification and Heavy Metal Stabilization by Microbial-Induced Carbonate Precipitation (MICP) Technology. Geomicrobiol. J. 2023, 40, 503–518. [Google Scholar] [CrossRef]
- Khodabandeh, M.A.; Nagy, G.; Török, Á. Stabilization of collapsible soils with nanomaterials, fibers, polymers, industrial waste, and microbes: Current trends. Constr. Build. Mater. 2023, 368, 130463. [Google Scholar] [CrossRef]
- Lu, C.; Ge, H.; Li, Z.; Zheng, Y. Effect evaluation of microbial mineralization for repairing load-induced crack in concrete with a cyclic injection-immersion process. Case Stud. Constr. Mater. 2022, 17, e01702. [Google Scholar] [CrossRef]
- Carter, M.S.; Tuttle, M.J.; Mancini, J.A.; Martineau, R.; Hung, C.-S.; Gupta, M.K. Microbially induced calcium carbonate precipitation by sporosarcina pasteurii: A case study in optimizing biological CaCO3 precipitation. Appl. Environ. Microbiol. 2023, 89, e01794-22. [Google Scholar] [CrossRef] [PubMed]
- Seifan, M.; Berenjian, A. Microbially induced calcium carbonate precipitation: A widespread phenomenon in the biological world. Appl. Microbiol. Biotechnol. 2019, 103, 4693–4708. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.F.; Li, Q.; Zhang, X. China Carbon Dioxide Capture, Utilization and Storage (CCUS) Annual Report (2021)—A Study of CCUS Pathways in China; Environmental Planning Institute of the Ministry of Ecology and Environment, Wuhan Institute of Geotechnics, Chinese Academy of Sciences: Wuhan, China, 2021. [Google Scholar]







| Types of Microorganisms that Assist Mineralization | Typical Microbial Representative | Main Mechanism | Environment | Reference |
|---|---|---|---|---|
| Sulfate-reducing bacteria | Acinetobacter calcoaceticus SRB4 | Consumption of specific electron donors, forming a metal sulfide precipitate | Anaerobic environment rich in organic matter, calcium, and sulfate; can survive in oil reservoirs | [76] |
| Iron-reducing bacteria | Shewanella oneidensis MR-4 | Consumption of specific electron donors, adjusting Eh value, promoting siderite precipitation | Anaerobic environment; most of them are thermophilic bacteria, and a few can survive in oil reservoirs | [77] |
| Urea-decomposing bacteria | Thermoanaerobacterium | Decomposition of urea | Aerobic environment | [78] |
| Denitrifying bacteria | Pseudomonas stutzeri | Consumption of specific electron donors | Anaerobic environment; can survive in oil reservoirs | [79] |
| Methanogenic bacteria | Methanococcales | Oxidization of methane, producing carbon anions | Anaerobic environment; can survive in oil reservoirs | [55,80] |
| Photosynthetic microorganisms | Cyanobacteria | Consumption of CO2, promoting carbon anion generation | Aerobic environment, light conditions | [81] |
| Microorganisms producing carbonic anhydrase | Sporosarcina Kluyver | Accelerating CO2 hydration, increasing carbon anion concentration | Aerobic environment | [82] |
| Key Enzyme for Carbon Dioxide Mineralization | Main Role | Catalytic Rate | Maintain Active Environment | Application | Reference |
|---|---|---|---|---|---|
| Urease | Decomposes urea and increases the pH | - | pH 7.0, 40 °C catalyzed the conversion of urea to ammonium carbonate; the optimal pH is 7.4. | Ecological restoration, soil reinforcement | [78] |
| Carbonic anhydrase | Accelerates CO2 hydration and increases CO32− ion concentration | Kcat 104–106/s | The pH value between 4.0 and 9.0 and the temperature below 65 °C can maintain high activity and stability. | Fixed CO2, biological monitoring | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, S.; Lv, W.; Ji, Z.; Wang, K. CO2 Mineralized Sequestration and Assistance by Microorganisms in Reservoirs: Development and Outlook. Energies 2023, 16, 7571. https://doi.org/10.3390/en16227571
Ni S, Lv W, Ji Z, Wang K. CO2 Mineralized Sequestration and Assistance by Microorganisms in Reservoirs: Development and Outlook. Energies. 2023; 16(22):7571. https://doi.org/10.3390/en16227571
Chicago/Turabian StyleNi, Shumin, Weifeng Lv, Zemin Ji, and Kai Wang. 2023. "CO2 Mineralized Sequestration and Assistance by Microorganisms in Reservoirs: Development and Outlook" Energies 16, no. 22: 7571. https://doi.org/10.3390/en16227571
APA StyleNi, S., Lv, W., Ji, Z., & Wang, K. (2023). CO2 Mineralized Sequestration and Assistance by Microorganisms in Reservoirs: Development and Outlook. Energies, 16(22), 7571. https://doi.org/10.3390/en16227571






