Hydrate Formation from Joule Thomson Expansion Using a Single Pass Flowloop
Abstract
:1. Introduction
2. Materials and Methods
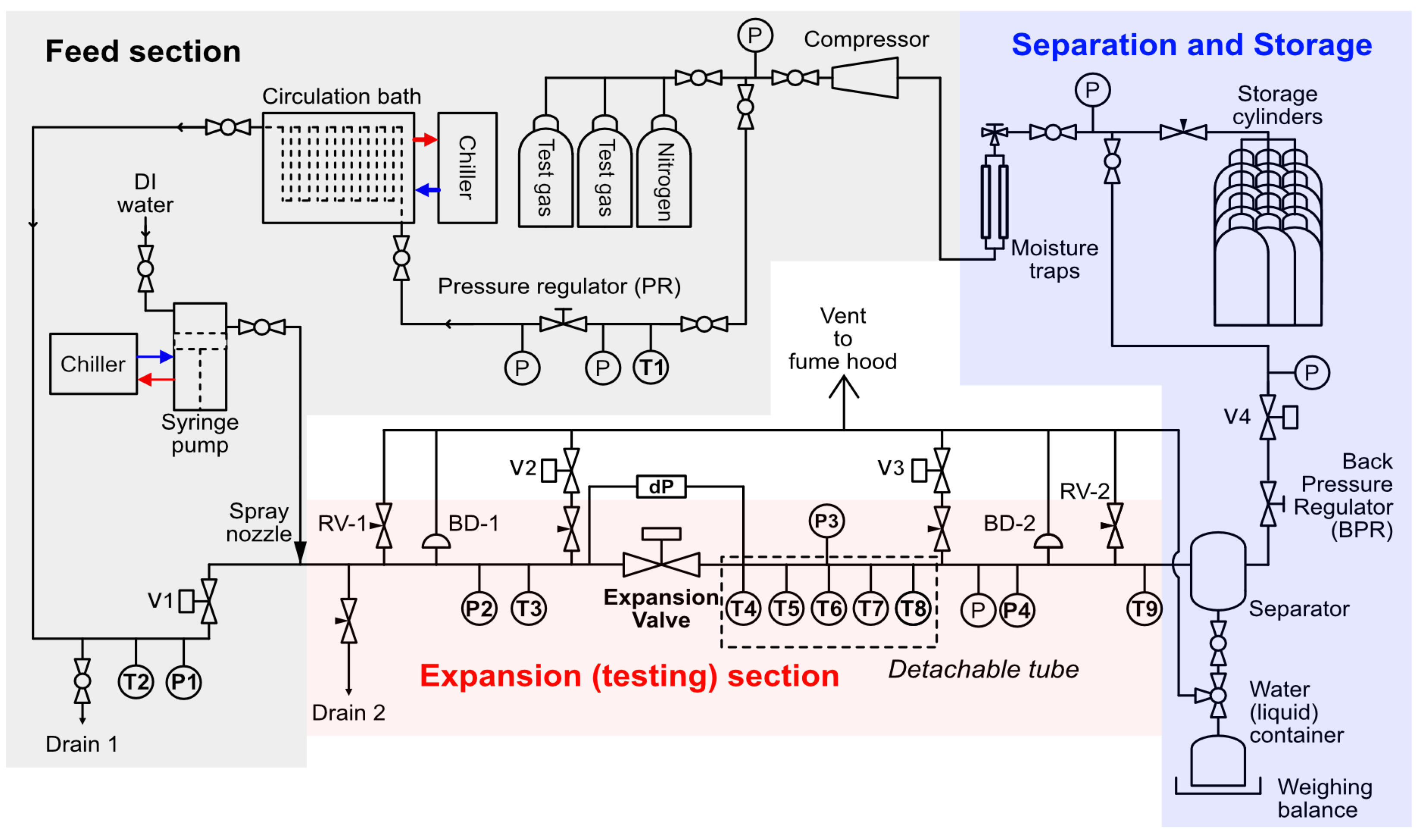
2.1. Joule Thomson Expansion Loop
2.2. Experimental Methods
3. Results
3.1. Dry Gas Experiments
3.2. Wet Gas Experiments
3.2.1. Immediate Hydrate Formation/Blockage Observed
3.2.2. Growth Rates in Excess of Current Kinetic Models
3.2.3. Hydrate Plug Mobility Observed
3.2.4. Plugging Potential and Water Rate Inversely Correlated
4. Discussion and Conclusions
- Hydrate formation occurs near-instantaneously (within ten seconds, as evidenced by the initial pressure slope change in plugging cases) during wet gas expansion over a valve. Contrary to pipeline flow, in which some induction time is expected, valves seem to act as effective spray deposition nozzles for hydrate formation resulting in surface areas at least an order of magnitude larger. Crystal formation is effectively immediate and severe.
- The growth rates of hydrate when expanded over a valve are not well represented by current models. Existing kinetic models fall short by an order of magnitude or more when attempting to estimate the rate at which hydrate deposits grow downstream of a valve due to poor surface area estimates. To close this gap, the interfacial area sub-models within hydrate growth algorithms require refinement. Further, the underlying fluid dynamic models must be updated to enable estimates of the interfacial area to be captured for both normal pipe flow and expansion cases simultaneously– as they presently require one or the other to be implemented.
- Hydrate plugs downstream of valves may be mobile, corresponding to a pressure-driven (approximately 1 MPa) plugging shift as shown in Experiment 26, though additional data are required to determine the factors controlling this behavior. The use of advanced instrumentation to spatially resolve the test section may enable a more granular description of plug movement.
- The rate of plug formation was shown to be inversely proportional to the water injection rate, where the plugging pressure was approximately halved as the water injection rate increased by an order of magnitude. This was likely due to the increased subcooling and/or finer atomization experienced in systems with lower water rate flows, which leads to shorter, faster-growing plugs immediately following the expansion valve.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sloan, E.D., Jr.; Koh, C.A. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Koh, C.A.; Sloan, E.D.; Sum, A.K.; Wu, D.T. Fundamentals and Applications of Gas Hydrates. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.D.; Koh, C.; Sum, A.K.; Ballard, A.L.; Creek, J.; Eaton, M.; Lachance, J.; McMullen, N.; Palermo, T.; Shoup, G.; et al. Natural Gas Hydrates in Flow Assurance; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Englezos, P. Clathrate hydrates. Ind. Eng. Chem. Res. 1993, 32, 1251–1274. [Google Scholar] [CrossRef]
- Cha, M.; Shin, K.; Kim, J.; Chang, D.; Seo, Y.; Lee, H.; Kang, S.-P. Thermodynamic and kinetic hydrate inhibition performance of aqueous ethylene glycol solutions for natural gas. Chem. Eng. Sci. 2013, 99, 184–190. [Google Scholar] [CrossRef]
- Lim, V.W.; Metaxas, P.J.; Johns, M.L.; Aman, Z.M.; May, E.F. The impact of mono-ethylene glycol and kinetic inhibitors on methane hydrate formation. Chem. Eng. J. 2022, 427, 131531. [Google Scholar] [CrossRef]
- Wang, Y.; Koh, C.A.; White, J.; Patel, Z.; Zerpa, L.E. Hydrate formation management simulations with anti-agglomerants and thermodynamic inhibitors in a subsea tieback. Fuel 2019, 252, 458–468. [Google Scholar] [CrossRef]
- Sloan, E.D. A changing hydrate paradigm—From apprehension to avoidance to risk management. Fluid Phase Equilibria 2005, 228–229, 67–74. [Google Scholar] [CrossRef]
- Norris, B.W.E.; Charlton, T.B.; Johns, M.L.; May, E.F.; Aman, Z.M. Risk-Based Flow Assurance Design for Natural Gas Hydrate Production Systems. In Proceedings of the Offshore Technology Conference Asia, Kuala Lumpur, Malaysia, 20–23 March 2018. [Google Scholar]
- Gao, S. Hydrate Risk Management at High Watercuts with Anti-agglomerant Hydrate Inhibitors. Energy Fuels 2009, 23, 2118–2121. [Google Scholar] [CrossRef]
- Kelland, M.A. Production Chemicals for the Oil and Gas Industry; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Jeong, K.; Metaxas, P.J.; Chan, J.; Kuteyi, T.O.; Aman, Z.M.; Stanwix, P.L.; Johns, M.L.; May, E.F. Hydrate nucleation and growth on water droplets acoustically-levitated in high-pressure natural gas. Phys. Chem. Chem. Phys. 2019, 21, 21685–21688. [Google Scholar] [CrossRef]
- Jeong, K.; Metaxas, P.J.; Helberg, A.; Johns, M.L.; Aman, Z.M.; May, E.F. Gas hydrate nucleation in acoustically levitated water droplets. Chem. Eng. J. 2022, 433, 133494. [Google Scholar] [CrossRef]
- Barwood, M.T.J.; Metaxas, P.J.; Lim, V.W.S.; Sampson, C.C.; Johns, M.L.; Aman, Z.M.; May, E.F. Extracting nucleation rates from ramped temperature measurements of gas hydrate formation. Chem. Eng. J. 2022, 450, 137895. [Google Scholar] [CrossRef]
- Herath, D.; Khan, F.; Rathnayaka, S.; Rahman, M.A. Probabilistic estimation of hydrate formation. J. Pet. Sci. Eng. 2015, 135, 32–38. [Google Scholar] [CrossRef]
- Xu, H.; Khan, F.; Jung, S.; Wang, Q. Probabilistic model for hydrate and wax risk assessment in oil and gas pipelines. Process Saf. Environ. Prot. 2023, 170, 11–18. [Google Scholar] [CrossRef]
- Sohn, Y.h.; Kim, J.; Shin, K.; Chang, D.; Seo, Y.; Aman, Z.M.; May, E.F. Hydrate plug formation risk with varying watercut and inhibitor concentrations. Chem. Eng. Sci. 2015, 126, 711–718. [Google Scholar] [CrossRef]
- Jamaluddin, A.; Kalogerakis, N.; Bishnoi, P. Hydrate plugging problems in undersea natural gas pipelines under shutdown conditions. J. Pet. Sci. Eng. 1991, 5, 323–335. [Google Scholar] [CrossRef]
- Marić, I. The Joule–Thomson effect in natural gas flow-rate measurements. Flow Meas. Instrum. 2005, 16, 387–395. [Google Scholar] [CrossRef]
- She, H.; Cui, X.; Weng, J.; Chang, Z. Study on the influence of distributed Joule-Thomson effect on the performance of microchannel cryocooler. Appl. Therm. Eng. 2022, 213, 118795. [Google Scholar] [CrossRef]
- Cao, H.S.; Vanapalli, S.; Holland, H.J.; Vermeer, C.H.; ter Brake, H.J.M. A micromachined Joule–Thomson cryogenic cooler with parallel two-stage expansion. Int. J. Refrig. 2016, 69, 223–231. [Google Scholar] [CrossRef]
- Lemmon, E.; Bell, I.H.; Huber, M.; McLinden, M. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 10.0; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2018. [Google Scholar]
- Schoen, M. The Joule–Thomson effect in confined fluids. Phys. A Stat. Mech. Its Appl. 1999, 270, 353–379. [Google Scholar] [CrossRef]
- Tarom, N.; Hossain, M.M.; Rohi, A. A new practical method to evaluate the Joule–Thomson coefficient for natural gases. J. Pet. Explor. Prod. Technol. 2018, 8, 1169–1181. [Google Scholar] [CrossRef]
- Budenholzer, R.; Sage, R.; Lacey, W. Phase equilibria in hydrocarbon systems Joule-Thomson coefficients for gaseous mixtures of methane and n-butane. Ind. Eng. Chem. 1940, 32, 384–387. [Google Scholar] [CrossRef]
- Roebuck, J.; Murrell, T.; Miller, E. The Joule-Thomson effect in carbon dioxide. J. Am. Chem. Soc. 1942, 64, 400–411. [Google Scholar] [CrossRef]
- Roebuck, J.; Osterberg, H. The Joule-Thomson effect in nitrogen. Phys. Rev. 1935, 48, 450. [Google Scholar] [CrossRef]
- Hendricks, R.C.; Peller, I.C.; Baron, A.K. Joule-Thomson Inversion Curves and Related Coefficients for Several Simple Fluids; NASA: Washington, DC, USA, 1972. [Google Scholar]
- Wood, S.A.; Spera, F.J. Adiabatic decompression of aqueous solutions: Applications to hydrothermal fluid migration in the crust. Geology 1984, 12, 707–710. [Google Scholar] [CrossRef]
- Stauffer, P.H.; Lewis, K.; Stein, J.S.; Travis, B.J.; Lichtner, P.; Zyvoloski, G. Joule–Thomson effects on the flow of liquid water. Transp. Porous Media 2014, 105, 471–485. [Google Scholar] [CrossRef]
- Johal, K.; Teh, C.; Cousins, A. An alternative economic method to riserbase gas lift for deep water subsea oil/gas field developments. In Proceedings of the SPE Offshore Europe, Aberdeen, UK, 9–12 September 1997. [Google Scholar]
- Lovell, D.; Pakulski, M. Hydrate inhibition in gas wells treated with two low dosage hydrate inhibitors. In Proceedings of the SPE Gas Technology Symposium, Calgary, AB, Canada, 30 April–2 May 2002. [Google Scholar]
- Szymczak, S.; Sanders, K.; Pakulski, M.; Higgins, T. Chemical compromise: A thermodynamic and low-dose hydrate-inhibitor solution for hydrate control in the Gulf of Mexico. SPE Proj. Facil. Constr. 2006, 1, 1–5. [Google Scholar] [CrossRef]
- Pakulski, M. Accelerating effect of surfactants on gas hydrates formation. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 28 February–2 March 2007. [Google Scholar]
- Liao, Y.; Zheng, J.; Wang, Z.; Sun, B.; Sun, X.; Linga, P. Modeling and characterizing the thermal and kinetic behavior of methane hydrate dissociation in sandy porous media. Appl. Energy 2022, 312, 118804. [Google Scholar] [CrossRef]
- Yang, L.; Shi, K.; Qu, A.; Liang, H.; Li, Q.; Lv, X.; Leng, S.; Liu, Y.; Zhang, L.; Liu, Y.; et al. The locally varying thermodynamic driving force dominates the gas production efficiency from natural gas hydrate-bearing marine sediments. Energy 2023, 276, 127545. [Google Scholar] [CrossRef]
- Guan, D.; Qu, A.; Gao, P.; Fan, Q.; Li, Q.; Zhang, L.; Zhao, J.; Song, Y.; Yang, L. Improved temperature distribution upon varying gas producing channel in gas hydrate reservoir: Insights from the Joule-Thomson effect. Appl. Energy 2023, 348, 121542. [Google Scholar] [CrossRef]
- Mathias, S.A.; Gluyas, J.G.; Oldenburg, C.M.; Tsang, C.-F. Analytical solution for Joule–Thomson cooling during CO2 geo-sequestration in depleted oil and gas reservoirs. Int. J. Greenh. Gas Control 2010, 4, 806–810. [Google Scholar] [CrossRef]
- Oldenburg, C.M. Joule-Thomson cooling due to CO2 injection into natural gas reservoirs. Energy Convers. Manag. 2007, 48, 1808–1815. [Google Scholar] [CrossRef]
- Ziabakhsh-Ganji, Z.; Kooi, H. Sensitivity of Joule–Thomson cooling to impure CO2 injection in depleted gas reservoirs. Appl. Energy 2014, 113, 434–451. [Google Scholar] [CrossRef]
- LabVIEW, National Instruments: Austin, TX, USA, 2020.
- Multiflash 7.0, KBC Advanced Technologies Ltd.: Walton-on-Thames, UK, 2018.
- Smith, R.L. Joule-Thomson Coefficients of Propane and N-Butane. Ph.D. Thesis, California Institute of Technology, Pasadena, CA, USA, 1970. [Google Scholar]
- Charlton, T.B. Predicting Hydrate Blockages in Gas-Dominant Flowlines. Ph.D. Thesis, The University of Western Australia, Perth, Australia, 2021. [Google Scholar]
- Charlton, T.B.; Zerpa, L.E.; Koh, C.A.; May, E.F.; Aman, Z.M. Predicting hydrate blockage formation in gas-dominant systems. In Proceedings of the Offshore Technology Conference Asia, Kuala Lumpur, Malaysia, 20–23 March 2018. [Google Scholar]
- Yin, Z.; Khurana, M.; Tan, H.K.; Linga, P. A review of gas hydrate growth kinetic models. Chem. Eng. J. 2018, 342, 9–29. [Google Scholar] [CrossRef]
- Wu, P.K.; Tseng, L.K. Primary breakup in gas/liquid mixing layers for turbulent liquids. At. Sprays 1992, 2, 295–317. [Google Scholar] [CrossRef]












| Exp. | Gas Type | Upstream Pressure (MPa) | Upstream Temperature (K) | BPR (Downstream) Setpoint (MPa) | Water Injection Rate (mL/min) | Min. Downstream Temperature (K) |
|---|---|---|---|---|---|---|
| 1 | Dry Gas Mixture C1:C2, 68:32 mol% | 8.8 | 295 | 2.9 | N/A | 259.3 |
| 2 | 3.1 | 259.8 | ||||
| 3 | 3.9 | 266.2 | ||||
| 4 | 3.9 | 266.5 | ||||
| 5 | 3.9 | 266.1 | ||||
| 6 | 4.0 | 268.0 | ||||
| 7 | 4.0 | 267.6 | ||||
| 8 | 4.0 | 267.8 | ||||
| 9 | 4.0 | 268.1 | ||||
| 10 | 4.1 | 268.0 | ||||
| 11 | 5.0 | 273.6 | ||||
| 12 | 5.0 | 273.0 | ||||
| 13 | 5.0 | 272.8 | ||||
| 14 | 5.1 | 273.8 | ||||
| 15 | 5.3 | 274.8 | ||||
| 16 | 5.5 | 275.0 | ||||
| 17 | 5.6 | 276.5 | ||||
| 18 | Wet Gas Mixture C1:C2, 68:32 mol% | 4.0 | 10 | 267.9 | ||
| 19 | 269.6 | |||||
| 20 | 269.0 | |||||
| 21 | 25 | 278.2 | ||||
| 22 | 271.8 | |||||
| 23 | 277.1 | |||||
| 24 | 50 | 270.2 | ||||
| 25 | 274.2 | |||||
| 26 | 271.4 | |||||
| 27 | 100 | 275.4 | ||||
| 28 | 275.1 | |||||
| 29 | 275.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, K.; Norris, B.W.E.; May, E.F.; Aman, Z.M. Hydrate Formation from Joule Thomson Expansion Using a Single Pass Flowloop. Energies 2023, 16, 7594. https://doi.org/10.3390/en16227594
Jeong K, Norris BWE, May EF, Aman ZM. Hydrate Formation from Joule Thomson Expansion Using a Single Pass Flowloop. Energies. 2023; 16(22):7594. https://doi.org/10.3390/en16227594
Chicago/Turabian StyleJeong, Kwanghee, Bruce W. E. Norris, Eric F. May, and Zachary M. Aman. 2023. "Hydrate Formation from Joule Thomson Expansion Using a Single Pass Flowloop" Energies 16, no. 22: 7594. https://doi.org/10.3390/en16227594





