Evaluation of a Simplified Model for Three-Phase Equilibrium Calculations of Mixed Gas Hydrates
Abstract
1. Introduction
- (i)
- When fluid mixtures are involved, performing experiments that consider all the possible mixture compositions can be significantly expensive and time-consuming. Therefore, it is of utmost importance to develop alternative thermodynamic tools that can be used to calculate the required properties of the pure components and their mixtures. Such are the cases of atomistic scale simulations, and the models based on continuum-scale theories.
- (ii)
- Molecular simulations (e.g., Molecular Dynamics [38,39,40,41,42,43,44,45,46] or Monte Carlo [47,48,49,50,51,52,53,54,55,56,57] simulations) can use very detailed physics at the microscopic level; however, they are very computationally demanding, and therefore, the size of the system under consideration is very small, and the considered times are usually short (e.g., up to several microseconds). Therefore, such types of simulations are hard to use during process design or process-optimization schemes.
- (iii)
- Models based on continuum-scale (macroscopic) theories, which are related to hydrate-forming systems, include those developed for the calculation of the three-phase (Hydrate–Liquid water–Vapor; H–Lw–V) equilibrium pressure and temperature conditions via a methodology that couples the van der Waals and Platteeuw statistical theory [58] with an Equation of State (EoS) [59,60,61,62,63,64]. The particular coupling is considered as one of the best examples of the application of Statistical Thermodynamic Theory toward the solution of a real industrial problem. Parrish and Prausnitz [59] pioneered the earlier development of the methodology, and an extensive review of the earlier studies was provided by Holder et al. [61] and Englezos [65]. While a significant number of studies considered the use of cubic EoS [59,60,61,62,66,67,68,69,70], during recent years, non-cubic EoS (e.g., SAFT-type [63,64,71,72]) has also been considered. Medeiros et al. [73] and Khan et al. [74] provided comprehensive reviews of hydrate equilibrium calculations using the van der Waals–Platteeuw theory, while the aspects of the particular theory under confinement have been discussed in [19,75].
2. Methodology
2.1. Calculation of Hydrate Dissociation Pressures
2.2. Parameter Estimation
3. Results and Discussion
4. Model Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| % absolute average deviation, defined by Equation (4) | |
| Component-specific parameter of Equation (3) | |
| Component-specific parameter of Equation (3) | |
| Component-specific parameter of Equation (3) | |
| Component-specific parameter of Equation (3) | |
| Symbol denoting a function | |
| n | Number of components in the gas mixture |
| ndp | Number of experimental data points |
| Hydrate equilibrium pressure (Pa) | |
| Temperature (K) | |
| Hydrate equilibrium temperature (K) | |
| y | Gas composition expressed as mole fraction (–) |
| z | Initial feed composition expressed as mole fraction (–) |
| Subscripts: | |
| i | Component i of a mixture with n gas components |
| gas-mixture | Denotes property of the gas mixture |
| H | Hydrate |
| Superscripts: | |
| calc | Calculated |
| eq | Equilibrium |
| exp | Experimental |
References
- Dendy Sloan, E.; Koh, C. Clathrate Hydrates of Natural Gases, 3rd ed.; Taylor & Francis, Ed.; Chemical Industries; CRC Press: Boca Raton, FL, USA, 2007; Volume 20074156, ISBN 978-0-8493-9078-4. [Google Scholar]
- Sloan, E.D. Fundamental Principles and Applications of Natural Gas Hydrates. Nature 2003, 426, 353. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.A. Towards a Fundamental Understanding of Natural Gas Hydrates. Chem. Soc. Rev. 2002, 31, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.R.; Hancock, S.H.; Wilson, S.J.; Patil, S.L.; Moridis, G.J.; Boswell, R.; Collett, T.S.; Koh, C.A.; Sloan, E.D. Preliminary Report on the Commercial Viability of Gas Production from Natural Gas Hydrates. Energy Econ. 2009, 31, 815–823. [Google Scholar] [CrossRef]
- Moridis, G.J.; Collett, T.S.; Boswell, R.; Kurihara, M.; Reagan, M.T.; Koh, C.; Sloan, E.D. Toward Production From Gas Hydrates: Current Status, Assessment of Resources, and Simulation-Based Evaluation of Technology and Potential. SPE Reserv. Eval. Eng. 2009, 12, 745–771. [Google Scholar] [CrossRef]
- Collett, T.; Bahk, J.J.; Baker, R.; Boswell, R.; Divins, D.; Frye, M.; Goldberg, D.; Husebø, J.; Koh, C.; Malone, M.; et al. Methane Hydrates in Nature-Current Knowledge and Challenges. J. Chem. Eng. Data 2015, 60, 319–329. [Google Scholar] [CrossRef]
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X.S. Review of Natural Gas Hydrates as an Energy Resource: Prospects and Challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Tsimpanogiannis, I.N.; Costandy, J.; Kastanidis, P.; El Meragawi, S.; Michalis, V.K.; Papadimitriou, N.I.; Karozis, S.N.; Diamantonis, N.I.; Moultos, O.A.; Romanos, G.E.; et al. Using Clathrate Hydrates for Gas Storage and Gas-Mixture Separations: Experimental and Computational Studies at Multiple Length Scales. Mol. Phys. 2018, 116, 2041–2060. [Google Scholar] [CrossRef]
- Kennett, J.P.; Cannariato, K.G.; Hendy, I.L.; Behl, R.J. Methane Hydrates in Quaternary Climate Change: The Clathrate Gun Hypothesis; American Geophysical Union: Washington, DC, USA, 2003; ISBN 0-87590-296-0. [Google Scholar]
- Ruppel, C.D.; Kessler, J.D. The Interaction of Climate Change and Methane Hydrates. Rev. Geophys. 2017, 55, 126–168. [Google Scholar] [CrossRef]
- Kelland, M.A. History of the Development of Low Dosage Hydrate Inhibitors. Energy Fuels 2006, 20, 825–847. [Google Scholar] [CrossRef]
- Zerpa, L.E.; Salager, J.-L.; Koh, C.A.; Sloan, E.D.; Sum, A.K. Surface Chemistry and Gas Hydrates in Flow Assurance. Ind. Eng. Chem. Res. 2011, 50, 188–197. [Google Scholar] [CrossRef]
- Kelland, M.A. A Review of Kinetic Hydrate Inhibitors from an Environmental Perspective. Energy Fuels 2018, 32, 12001–12012. [Google Scholar] [CrossRef]
- Vanneste, M.; Sultan, N.; Garziglia, S.; Forsberg, C.F.; L’Heureux, J.-S. Seafloor Instabilities and Sediment Deformation Processes: The Need for Integrated, Multi-Disciplinary Investigations. Mar. Geol. 2014, 352, 183–214. [Google Scholar] [CrossRef]
- McConnell, D.R.; Zhang, Z.; Boswell, R. Review of Progress in Evaluating Gas Hydrate Drilling Hazards. Mar. Pet. Geol. 2012, 34, 209–223. [Google Scholar] [CrossRef]
- Milkov, A.V. Global Estimates of Hydrate-Bound Gas in Marine Sediments: How Much Is Really Out There? Earth Sci. Rev. 2004, 66, 183–197. [Google Scholar] [CrossRef]
- Klauda, J.B.; Sandler, S.I. Global Distribution of Methane Hydrate in Ocean Sediment. Energy Fuels 2005, 19, 459–470. [Google Scholar] [CrossRef]
- Piñero, E.; Marquardt, M.; Hensen, C.; Haeckel, M.; Wallmann, K. Estimation of the Global Inventory of Methane Hydrates in Marine Sediments Using Transfer Functions. Biogeosciences 2013, 10, 959–975. [Google Scholar] [CrossRef]
- Li, X.S.; Zhang, Y.; Li, G.; Chen, Z.Y.; Yan, K.F.; Li, Q.P. Gas Hydrate Equilibrium Dissociation Conditions in Porous Media Using Two Thermodynamic Approaches. J. Chem. Thermodyn. 2008, 40, 1464–1474. [Google Scholar] [CrossRef]
- Yang, L.; Shi, K.; Qu, A.; Liang, H.; Li, Q.; Lv, X.; Leng, S.; Liu, Y.; Zhang, L.; Liu, Y.; et al. The Locally Varying Thermodynamic Driving Force Dominates the Gas Production Efficiency from Natural Gas Hydrate-Bearing Marine Sediments. Energy 2023, 276, 127545. [Google Scholar] [CrossRef]
- Waite, W.F.; Santamarina, J.C.; Cortes, D.D.; Dugan, B.; Espinoza, D.N.; Germaine, J.; Jang, J.; Jung, J.W.; Kneafsey, T.J.; Shin, H.; et al. Physical Properties of Hydrate-Bearing Sediments. Rev. Geophys. 2009, 47, RG4003. [Google Scholar] [CrossRef]
- Tsimpanogiannis, I.N. Study of the Critical Gas Saturation during Methane Hydrate Dissociation at the Single-Pore Scale: Analytical Solutions for Large Pores. J. Nat. Gas Sci. Eng. 2020, 83, 103577. [Google Scholar] [CrossRef]
- Tsimpanogiannis, I.N.; Stamatakis, E.; Stubos, A.K. Study of the Critical Pore Radius That Results in Critical Gas Saturation during Methane Hydrate Dissociation at the Single-Pore Scale: Analytical Solutions for Small Pores and Potential Implications to Methane Production from Geological Media. Energies 2022, 15, 210. [Google Scholar] [CrossRef]
- Li, X.S.; Xu, C.G.; Zhang, Y.; Ruan, X.K.; Li, G.; Wang, Y. Investigation into Gas Production from Natural Gas Hydrate: A Review. Appl. Energy 2016, 172, 286–322. [Google Scholar] [CrossRef]
- Khokhar, A.A.; Gudmundsson, J.S.; Sloan, E.D. Gas Storage in Structure H Hydrates. Fluid Phase Equilib. 1998, 150–151, 383–392. [Google Scholar] [CrossRef]
- Susilo, R.; Alavi, S.; Ripmeester, J.; Englezos, P. Tuning Methane Content in Gas Hydrates via Thermodynamic Modeling and Molecular Dynamics Simulation. Fluid Phase Equilib. 2008, 263, 6–17. [Google Scholar] [CrossRef]
- Mao, W.L.; Mao, H.-K.K.; Goncharov, A.F.; Struzhkin, V.V.; Guo, Q.; Hu, J.; Shu, J.; Hemley, R.J.; Somayazulu, M.; Zhao, Y. Hydrogen Clusters in Clathrate Hydrate. Science 2002, 297, 2247–2249. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Kumar, R.; Linga, P. Hydrogen Storage in Clathrate Hydrates: Current State of the Art and Future Directions. Appl. Energy 2014, 122, 112–132. [Google Scholar] [CrossRef]
- Tsimpanogiannis, I.N.; Economou, I.G.; Stubos, A.K. A Practical Methodology to Estimate the H2 Storage Capacity of Pure and Binary Hydrates Based on Monte Carlo Simulations. J. Chem. Eng. Data 2020, 65, 1289–1299. [Google Scholar] [CrossRef]
- Kang, S.-P.; Lee, H. Recovery of CO2 from Flue Gas Using Gas Hydrate: Thermodynamic Verification through Phase Equilibrium Measurements. Environ. Sci. Technol. 2000, 34, 4397–4400. [Google Scholar] [CrossRef]
- Seo, Y.-T.; Moudrakovski, I.L.; Ripmeester, J.A.; Lee, J.; Lee, H. Efficient Recovery of CO2 from Flue Gas by Clathrate Hydrate Formation in Porous Silica Gels. Environ. Sci. Technol. 2005, 39, 2315–2319. [Google Scholar] [CrossRef]
- Dabrowski, N.; Windmeier, C.; Oellrich, L.R. Purification of Natural Gases with High CO2 Content Using Gas Hydrates. Energy Fuels 2009, 23, 5603–5610. [Google Scholar] [CrossRef]
- Adeyemo, A.; Kumar, R.; Linga, P.; Ripmeester, J.; Englezos, P. Capture of Carbon Dioxide from Flue or Fuel Gas Mixtures by Clathrate Crystallization in a Silica Gel Column. Int. J. Greenh. Gas Control. 2010, 4, 478–485. [Google Scholar] [CrossRef]
- Babu, P.; Kumar, R.; Linga, P. Unusual Behavior of Propane as a Co-Guest during Hydrate Formation in Silica Sand: Potential Application to Seawater Desalination and Carbon Dioxide Capture. Chem. Eng. Sci. 2014, 117, 342–351. [Google Scholar] [CrossRef]
- Babu, P.; Nambiar, A.; He, T.; Karimi, I.A.; Lee, J.D.; Englezos, P.; Linga, P. A Review of Clathrate Hydrate Based Desalination to Strengthen Energy-Water Nexus. ACS Sustain. Chem. Eng. 2018, 6, 8093–8107. [Google Scholar] [CrossRef]
- Tsimpanogiannis, I.N.; Jamali, S.H.; Economou, I.G.; Vlugt, T.J.H.; Moultos, O.A. On the Validity of the Stokes–Einstein Relation for Various Water Force Fields. Mol. Phys. 2020, 118, e1702729. [Google Scholar] [CrossRef]
- Tsimpanogiannis, I.N.; Maity, S.; Celebi, A.T.; Moultos, O.A. Engineering Model for Predicting the Intradiffusion Coefficients of Hydrogen and Oxygen in Vapor, Liquid, and Supercritical Water Based on Molecular Dynamics Simulations. J. Chem. Eng. Data 2021, 66, 3226–3244. [Google Scholar] [CrossRef]
- Vatamanu, J.; Kusalik, P.G. Molecular Insights into the Heterogeneous Crystal Growth of SI Methane Hydrate. J. Phys. Chem. B 2006, 110, 15896–15904. [Google Scholar] [CrossRef] [PubMed]
- English, N.J.; Phelan, G.M. Molecular Dynamics Study of Thermal-Driven Methane Hydrate Dissociation. J. Chem. Phys. 2009, 131, 074704. [Google Scholar] [CrossRef]
- Alavi, S.; Ripmeester, J.A. Nonequilibrium Adiabatic Molecular Dynamics Simulations of Methane Clathrate Hydrate Decomposition. J. Chem. Phys. 2010, 132, 144703. [Google Scholar] [CrossRef]
- Conde, M.M.; Vega, C. Determining the Three-Phase Coexistence Line in Methane Hydrates Using Computer Simulations. J. Chem. Phys. 2010, 133, 064507. [Google Scholar] [CrossRef]
- Jacobson, L.C.; Molinero, V. Can Amorphous Nuclei Grow Crystalline Clathrates? The Size and Crystallinity of Critical Clathrate Nuclei. J. Am. Chem. Soc. 2011, 133, 6458–6463. [Google Scholar] [CrossRef]
- Michalis, V.K.; Costandy, J.; Tsimpanogiannis, I.N.; Stubos, A.K.; Economou, I.G. Prediction of the Phase Equilibria of Methane Hydrates Using the Direct Phase Coexistence Methodology. J. Chem. Phys. 2015, 142, 044501. [Google Scholar] [CrossRef] [PubMed]
- Costandy, J.; Michalis, V.K.; Tsimpanogiannis, I.N.; Stubos, A.K.; Economou, I.G. The Role of Intermolecular Interactions in the Prediction of the Phase Equilibria of Carbon Dioxide Hydrates. J. Chem. Phys. 2015, 143, 094506. [Google Scholar] [CrossRef] [PubMed]
- Michalis, V.K.; Tsimpanogiannis, I.N.; Stubos, A.K.; Economou, I.G. Direct Phase Coexistence Molecular Dynamics Study of the Phase Equilibria of the Ternary Methane–Carbon Dioxide–Water Hydrate System. Phys. Chem. Chem. Phys. 2016, 18, 23538–23548. [Google Scholar] [CrossRef] [PubMed]
- Tsimpanogiannis, I.N.; Michalis, V.K.; Economou, I.G. Enthalpy of Dissociation of Methane Hydrates at a Wide Pressure and Temperature Range. Fluid Phase Equilib. 2019, 489, 30–40. [Google Scholar] [CrossRef]
- Finney, A.R.; Rodger, P.M. Applying the Z Method to Estimate Temperatures of Melting in Structure II Clathrate Hydrates. Phys. Chem. Chem. Phys. 2011, 13, 19979. [Google Scholar] [CrossRef]
- Wierzchowski, S.J.; Monson, P.A. Calculation of Free Energies and Chemical Potentials for Gas Hydrates Using Monte Carlo Simulations. J. Phys. Chem. B 2007, 111, 7274–7282. [Google Scholar] [CrossRef]
- Sizov, V.V.; Piotrovskaya, E.M. Computer Simulation of Methane Hydrate Cage Occupancy. J. Phys. Chem. B 2007, 111, 2886–2890. [Google Scholar] [CrossRef]
- Papadimitriou, N.I.; Tsimpanogiannis, I.N.; Papaioannou, A.T.; Stubos, A.K. Evaluation of the Hydrogen-Storage Capacity of Pure H2 and Binary H2-THF Hydrates with Monte Carlo Simulations. J. Phys. Chem. C 2008, 112, 10294–10302. [Google Scholar] [CrossRef]
- Papadimitriou, N.I.; Tsimpanogiannis, I.N.; Stubos, A.K.; Martin, A.; Rovetto, L.J.; Peters, C.J. Unexpected Behavior of Helium as Guest Gas in SII Binary Hydrates. J. Phys. Chem. Lett. 2010, 1, 1014–1017. [Google Scholar] [CrossRef]
- Jensen, L.; Thomsen, K.; von Solms, N.; Wierzchowski, S.; Walsh, M.R.; Koh, C.A.; Sloan, E.D.; Wu, D.T.; Sum, A.K. Calculation of Liquid Water−Hydrate−Methane Vapor Phase Equilibria from Molecular Simulations. J. Phys. Chem. B 2010, 114, 5775–5782. [Google Scholar] [CrossRef]
- Papadimitriou, N.I.; Tsimpanogiannis, I.N.; Stubos, A.K.; Martín, A.; Rovetto, L.J.; Florusse, L.J.; Peters, C.J. Experimental and Computational Investigation of the SII Binary He−THF Hydrate. J. Phys. Chem. B 2011, 115, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, N.I.; Tsimpanogiannis, I.N.; Economou, I.G.; Stubos, A.K. Storage of Methane in Clathrate Hydrates: Monte Carlo Simulations of SI Hydrates and Comparison with Experimental Measurements. J. Chem. Eng. Data 2016, 61, 2886–2896. [Google Scholar] [CrossRef]
- Brumby, P.E.; Yuhara, D.; Wu, D.T.; Sum, A.K.; Yasuoka, K. Cage Occupancy of Methane Hydrates from Gibbs Ensemble Monte Carlo Simulations. Fluid Phase Equilib. 2016, 413, 242–248. [Google Scholar] [CrossRef]
- Papadimitriou, N.I.; Tsimpanogiannis, I.N.; Economou, I.G.; Stubos, A.K. Influence of Combining Rules on the Cavity Occupancy of Clathrate Hydrates by Monte Carlo Simulations. Mol. Phys. 2014, 112, 2258–2274. [Google Scholar] [CrossRef]
- Tsimpanogiannis, I.N.; Economou, I.G. Monte Carlo Simulation Studies of Clathrate Hydrates: A Review. J. Supercrit. Fluids 2018, 134, 51–60. [Google Scholar] [CrossRef]
- van der Waals, J.H.; Platteeuw, J.C. Clathrate Solutions. Adv. Chem. Phys. 1959, 2, 1–57. [Google Scholar]
- Parrish, W.R.; Prausnitz, J.M. Dissociation Pressures of Gas Hydrates Formed by Gas Mixtures. Ind. Eng. Chem. Process Des. Dev. 1972, 11, 26–35. [Google Scholar] [CrossRef]
- Holder, G.D.; Corbin, G.; Papadopoulos, K.D. Thermodynamic and Molecular Properties of Gas Hydrates from Mixtures Containing Methane, Argon, and Krypton. Ind. Eng. Chem. Fundam. 1980, 19, 282–286. [Google Scholar] [CrossRef]
- Holder, G.D.; Zetts, S.P.; Pradhan, N. Phase Behavior in Systems Containing Clathrate Hydrates: A Review. Rev. Chem. Eng. 1988, 5, 1–70. [Google Scholar] [CrossRef]
- Klauda, J.B.; Sandler, S.I. Modeling Gas Hydrate Phase Equilibria in Laboratory and Natural Porous Media. Ind. Eng. Chem. Res. 2001, 40, 4197–4208. [Google Scholar] [CrossRef]
- Dufal, S.; Galindo, A.; Jackson, G.; Haslam, A.J. Modelling the Effect of Methanol, Glycol Inhibitors and Electrolytes on the Equilibrium Stability of Hydrates with the SAFT-VR Approach. Mol. Phys. 2012, 110, 1223–1240. [Google Scholar] [CrossRef]
- El Meragawi, S.; Diamantonis, N.I.; Tsimpanogiannis, I.N.; Economou, I.G. Hydrate—Fluid Phase Equilibria Modeling Using PC-SAFT and Peng–Robinson Equations of State. Fluid Phase Equilib. 2016, 413, 209–219. [Google Scholar] [CrossRef]
- Englezos, P. Clathrate Hydrates. Ind. Eng. Chem. Res. 1993, 32, 1251–1274. [Google Scholar] [CrossRef]
- Ballard, A.L.; Sloan, E.D. The next Generation of Hydrate Prediction I. Hydrate Standard States and Incorporation of Spectroscopy. Fluid Phase Equilib. 2002, 194–197, 371–383. [Google Scholar] [CrossRef]
- Jager, M.D.; Ballard, A.L.; Sloan, E.D. The next Generation of Hydrate Prediction: II. Dedicated Aqueous Phase Fugacity Model for Hydrate Prediction. Fluid Phase Equilib. 2003, 211, 85–107. [Google Scholar] [CrossRef]
- Ballard, A.L.; Sloan, E.D. The next Generation of Hydrate Prediction Part III. Gibbs Energy Minimization Formalism. Fluid Phase Equilib. 2004, 218, 15–31. [Google Scholar] [CrossRef]
- Vinš, V.; Jäger, A.; Span, R.; Hrubý, J. Model for Gas Hydrates Applied to CCS Systems Part I. Parameter Study of the van der Waals and Platteeuw Model. Fluid Phase Equilib. 2016, 427, 268–281. [Google Scholar] [CrossRef]
- Vinš, V.; Jäger, A.; Hrubý, J.; Span, R. Model for Gas Hydrates Applied to CCS Systems Part II. Fitting of Parameters for Models of Hydrates of Pure Gases. Fluid Phase Equilib. 2017, 435, 104–117. [Google Scholar] [CrossRef]
- Li, X.S.; Wu, H.J.; Englezos, P. Prediction of Gas Hydrate Formation Conditions in the Presence of Methanol, Glycerol, Ethylene Glycol, and Triethylene Glycol with the Statistical Associating Fluid Theory Equation of State. Ind. Eng. Chem. Res. 2006, 45, 2131–2137. [Google Scholar] [CrossRef]
- Paricaud, P. Modeling the Dissociation Conditions of Salt Hydrates and Gas Semiclathrate Hydrates: Application to Lithium Bromide, Hydrogen Iodide, and Tetra-n-Butylammonium Bromide + Carbon Dioxide Systems. J. Phys. Chem. B 2011, 115, 288–299. [Google Scholar] [CrossRef]
- Medeiros, F.D.A.; Segtovich, I.S.V.; Tavares, F.W.; Sum, A.K. Sixty Years of the van Der Waals and Platteeuw Model for Clathrate Hydrates—A Critical Review from Its Statistical Thermodynamic Basis to Its Extensions and Applications. Chem. Rev. 2020, 120, 13349–13381. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Warrier, P.; Peters, C.J.; Koh, C.A. Advancements in Hydrate Phase Equilibria and Modeling of Gas Hydrates Systems. Fluid Phase Equilib. 2018, 463, 48–61. [Google Scholar] [CrossRef]
- Llamedo, M.; Anderson, R.; Tohidi, B. Thermodynamic Prediction of Clathrate Hydrate Dissociation Conditions in Mesoporous Media. Am. Mineral. 2004, 89, 1264–1270. [Google Scholar] [CrossRef]
- Lipenkov, V.Y.; Istomin, V.A. On the Stability of Air Clathrate-Hydrate Crystals in Subglacial Lake Vostok, Antarctica. Mater. Glyatsiol. Issled. 2001, 91, 138–149. [Google Scholar]
- McKay, C.P.; Hand, K.P.; Doran, P.T.; Andersen, D.T.; Priscu, J.C. Clathrate Formation and the Fate of Noble and Biologically Useful Gases in Lake Vostok, Antarctica. Geophys. Res. Lett. 2003, 30, 1702. [Google Scholar] [CrossRef]
- Hand, K.P.; Chyba, C.F.; Carlson, R.W.; Cooper, J.F. Clathrate Hydrates of Oxidants in the Ice Shell of Europa. Astrobiology 2006, 6, 463–482. [Google Scholar] [CrossRef]
- Thomas, C.; Mousis, O.; Picaud, S.; Ballenegger, V. Variability of the Methane Trapping in Martian Subsurface Clathrate Hydrates. Planet Space Sci. 2009, 57, 42–47. [Google Scholar] [CrossRef]
- Tsimpanogiannis, I.N.; Thomas, D.; Economou, I.G.; Stubos, A.K. Evaluation of a Simple Model for Hydrate Equilibrium Calculations of Binary Gas Hydrates with Application to Gas-Mixture Separation. In Proceedings of the 8th International Conference on Gas Hydrates (ICGH8), Beijing, China, 28 July 2014. [Google Scholar]
- Song, K.Y.; Kobayashi, R. The Water Content of Ethane, Propane and Their Mixtures in Equilibrium with Liquid Water or Hydrates. Fluid Phase Equilib. 1994, 95, 281–298. [Google Scholar] [CrossRef]
- Von Stackelberg, M. Solid Gas Hydrates. Naturwissenschaften 1949, 36, 327–359. [Google Scholar]
- Diepen, G.A.M.; Scheffer, F.E.C. The Ethene-Water System. Recl. Trav. Chim. Pays-Bas 2010, 69, 593–603. [Google Scholar] [CrossRef]
- Reamer, H.H.; Selleck, F.T.; Sage, B.H. Some Properties of Mixed Paraffinic and Olefinic Hydrates. J. Pet. Technol. 1952, 4, 197–202. [Google Scholar] [CrossRef]
- Sugahara, T.; Morita, K.; Ohgaki, K. Stability Boundaries and Small Hydrate-Cage Occupancy of Ethylene Hydrate System. Chem. Eng. Sci. 2000, 55, 6015–6020. [Google Scholar] [CrossRef]
- Kubota, H.; Shimizu, K.; Tanaka, Y.; Makita, T. Thermodynamic Properties of R13 (CClF3), R23 (CHF3), R152a (C2H4F2), and Propane Hydrates for Desalination of Sea Water. J. Chem. Eng. Jpn. 1984, 17, 423–429. [Google Scholar] [CrossRef]
- Mooijer-van den Heuvel, M.M. Phase Behaviour and Structural Aspects of Ternary Clathrate Hydrate Systems: The Role of Additives. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2004. [Google Scholar]
- Mohammadi, A.H.; Anderson, R.; Tohidi, B. Carbon Monoxide Clathrate Hydrates: Equilibrium Data and Thermodynamic Modeling. AIChE J. 2005, 51, 2825–2833. [Google Scholar] [CrossRef]
- Miller, S.L. Clathrate Hydrates of Air in Antarctic Ice. Science 1969, 165, 489–490. [Google Scholar] [CrossRef] [PubMed]
- van Cleeff, A.; Diepen, G.A.M. Gas Hydrates of Nitrogen and Oxygen. II. Recl. Trav. Chim. Pays-Bas 1965, 84, 1085–1093. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Richon, D. Ice−Clathrate Hydrate−Gas Phase Equilibria for Air, Oxygen, Nitrogen, Carbon Monoxide, Methane, or Ethane + Water System. Ind. Eng. Chem. Res. 2010, 49, 3976–3979. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Tohidi, B.; Burgass, R.W. Equilibrium Data and Thermodynamic Modeling of Nitrogen, Oxygen, and Air Clathrate Hydrates. J. Chem. Eng. Data 2003, 48, 612–616. [Google Scholar] [CrossRef]
- Byk, S.S.; Fomina, V.I. Gas Hydrates. Russ. Chem. Rev. 1968, 37, 469–491. [Google Scholar] [CrossRef]
- Tamman, G.; Krige, G.J. Equilibrium pressures of gas hydrates. Zeit. Anorg. Und Algem. Chem. 1925, 146, 179–195. [Google Scholar]
- Schroeder, W. Die Geschichte Der Gas Hydrate. Sammlung. Chem. Tech. Vortrage 1927, 29, 90–98. [Google Scholar]
- Dyadin, Y.A.; Larionov, E.G.; Manakov, A.Y.; Zhurko, F.V.; Aladko, E.Y.; Mikina, T.V.; Komarov, V.Y. Clathrate Hydrates of Hydrogen and Neon. Mendeleev Commun. 1999, 9, 209–210. [Google Scholar] [CrossRef]
- Kastanidis, P.; Tsimpanogiannis, I.N.; Romanos, G.E.; Stubos, A.K.; Economou, I.G. Recent Advances in Experimental Measurements of Mixed-Gas Three-Phase Hydrate Equilibria for Gas Mixture Separation and Energy-Related Applications. J. Chem. Eng. Data 2019, 64, 4991–5016. [Google Scholar] [CrossRef]
- Olsen, B.; Majumdar, A.; Bishnoi, R. Experimental Studies Dioxide on Hydrate and Its Systems. Int. J. Mat. Eng. Resour. 1999, 7, 17–23. [Google Scholar]
- Seo, Y.-T.; Kang, S.-P.; Lee, H.; Lee, C.-S.; Sung, W.-M. Hydrate Phase Equilibria for Gas Mixtures Containing Carbon Dioxide: A Proof-of-Concept to Carbon Dioxide Recovery from Multicomponent Gas Stream. Korean J. Chem. Eng. 2000, 17, 659–667. [Google Scholar] [CrossRef]
- Kang, S.P.; Lee, H.; Lee, C.S.; Sung, W.M. Hydrate Phase Equilibria of the Guest Mixtures Containing CO2, N2 and Tetrahydrofuran. Fluid Phase Equilib. 2001, 185, 101–109. [Google Scholar] [CrossRef]
- Linga, P.; Kumar, R.; Englezos, P. The Clathrate Hydrate Process for Post and Pre-Combustion Capture of Carbon Dioxide. J. Hazard. Mater. 2007, 149, 625–629. [Google Scholar] [CrossRef]
- Bruusgaard, H.; Beltra, J.G.; Servio, P. Vapor-Liquid Water-Hydrate Equilibrium Data for the System N2 + CO2 + H2O. J. Chem. Eng. Data 2008, 53, 2594–2597. [Google Scholar] [CrossRef]
- Herri, J.M.; Bouchemoua, A.; Kwaterski, M.; Fezoua, A.; Ouabbas, Y.; Cameirao, A. Gas Hydrate Equilibria for CO2-N2 and CO2-CH4 Gas Mixtures-Experimental Studies and Thermodynamic Modelling. Fluid Phase Equilib. 2011, 301, 171–190. [Google Scholar] [CrossRef]
- Belandria, V.; Eslamimanesh, A.; Mohammadi, A.; Richon, D. Gas Hydrate Formation in Carbon Dioxide + Nitrogen + Water System: Compositional Analysis of Equilibrium Phases. Ind. Eng. Chem. Res. 2011, 50, 4722–4730. [Google Scholar] [CrossRef]
- Lang, F.; Servio, P. Solubility Measurements for the N2 + CO2 + H2O System under Hydrate–Liquid–Vapor Equilibrium. J. Chem. Eng. Data 2014, 59, 2547–2550. [Google Scholar] [CrossRef]
- Le Quang, D.; Le Quang, D.; Bouillot, B.; Herri, J.M.; Glenat, P.; Duchet-Suchaux, P. Experimental Procedure and Results to Measure the Composition of Gas Hydrate, during Crystallization and at Equilibrium, from N2-CO2-CH4-C2H6-C3H8-C4H10 Gas Mixtures. Fluid Phase Equilib. 2016, 413, 10–21. [Google Scholar] [CrossRef]
- Jarrahian, A.; Nakhaee, A. Hydrate–Liquid–Vapor Equilibrium Condition of N2 + CO2 + H2O System: Measurement and Modeling. Fuel 2019, 237, 769–774. [Google Scholar] [CrossRef]
- Fan, S.-S.; Guo, T.-M. Hydrate Formation of CO2-Rich Binary and Quaternary Gas Mixtures in Aqueous Sodium Chloride Solutions. J. Chem. Eng. Data 1999, 44, 829–832. [Google Scholar] [CrossRef]
- Kim, S.H.; Seo, M.D.; Kang, J.W.; Lee, C.S. Hydrate-Containing Phase Equilibria for Mixed Guests of Carbon Dioxide and Nitrogen. Fluid Phase Equilib. 2011, 306, 229–233. [Google Scholar] [CrossRef]
- Sfaxi, I.B.A.; Belandria, V.; Mohammadi, A.H.; Lugo, R.; Richon, D. Phase Equilibria of CO2 + N2 and CO2 + CH4 Clathrate Hydrates: Experimental Measurements and Thermodynamic Modelling. Chem. Eng. Sci. 2012, 84, 602–611. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Lee, J.; Seo, Y. Structure Identification and Dissociation Enthalpy Measurements of the CO2 + N2 Hydrates for Their Application to CO2 Capture and Storage. Chem. Eng. J. 2014, 246, 20–26. [Google Scholar] [CrossRef]
- Chapoy, A.; Burgass, R.; Tohidi, B.; Alsiyabi, I. Hydrate and Phase Behavior Modeling in CO2-Rich Pipelines. J. Chem. Eng. Data 2015, 60, 447–453. [Google Scholar] [CrossRef]
- Du, J.; Wang, L. Phase Equilibrium Measurements for Clathrate Hydrates of Flue Gas (CO2 + N2 + O2) in the Presence of Tetra-n-Butyl Ammonium Bromide or Tri-n-Butylphosphine Oxide. J. Chem. Thermodyn. 2015, 88, 96–100. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, D.; Lee, J.W.; Seo, Y. Enclathration of CO2 as a Co-Guest of Structure H Hydrates and its Implications for CO2 Capture and Sequestration. Appl. Energy 2016, 163, 51–59. [Google Scholar] [CrossRef]
- Sadeq, D.; Iglauer, S.; Lebedev, M.; Smith, C.; Barifcani, A. Experimental Determination of Hydrate Phase Equilibrium for Different Gas Mixtures Containing Methane, Carbon Dioxide and Nitrogen with Motor Current Measurements. J. Nat. Gas. Sci. Eng. 2017, 38, 59–73. [Google Scholar] [CrossRef]
- Chazallon, B.; Pirim, C. Selectivity and CO2 Capture Efficiency in CO2-N2 Clathrate Hydrates Investigated by in-Situ Raman Spectroscopy. Chem. Eng. J. 2018, 342, 171–183. [Google Scholar] [CrossRef]
- Legoix, L.; Ruffine, L.; Deusner, C.; Haeckel, M. Experimental Study of Mixed Gas Hydrates from Gas Feed Containing CH4, CO2 and N2: Phase Equilibrium in the Presence of Excess Water and Gas Exchange. Energies 2018, 11, 1984. [Google Scholar] [CrossRef]
- Rho, W.G.; Kim, S.H.; Cho, D.-W.; Kang, J.W.; Lee, C.S. An Experimental and Modeling Study on Incipient Hydrate-Forming Conditions for Ternary Guests of Carbon Dioxide, Nitrogen and Sulfur Dioxide. Fluid Phase Equilib. 2018, 473, 127–131. [Google Scholar] [CrossRef]
- Sugahara, T.; Murayama, S.; Hashimoto, S.; Ohgaki, K. Phase Equilibria for H2 + CO2 + H2O System Containing Gas Hydrates. Fluid Phase Equilib. 2005, 233, 190–193. [Google Scholar] [CrossRef]
- Kumar, R.; Wu, H.; Englezos, P. Incipient Hydrate Phase Equilibrium for Gas Mixtures Containing Hydrogen, Carbon Dioxide and Propane. Fluid Phase Equilib. 2006, 244, 167–171. [Google Scholar] [CrossRef]
- Belandria, V.; Eslamimanesh, A.; Mohammadi, A.H.; Richon, D. Study of Gas Hydrate Formation in the Carbon Dioxide + Hydrogen + Water Systems: Compositional Analysis of the Gas Phase. Ind. Eng. Chem. Res. 2011, 50, 6455–6459. [Google Scholar] [CrossRef]
- Linga, P.; Kumar, R.; Englezos, P. Gas Hydrate Formation from Hydrogen/Carbon Dioxide and Nitrogen/Carbon Dioxide Gas Mixtures. Chem. Eng. Sci. 2007, 62, 4268–4276. [Google Scholar] [CrossRef]
- Chapoy, A.; Burgass, R.; Tohidi, B.; Austell, J.; Eickhoff, C. Effect of Common Impurities on the Phase Behavior of Carbon-Dioxide-Rich Systems: Minimizing the Risk of Hydrate Formation and Two-Phase Flow. SPE J. 2011, 16, 921–930. [Google Scholar] [CrossRef]
- Bouchafaa, W.; Dalmazzone, D.; Victor, B. Thermodynamic Equilibrium Data for Mixed Hydrates of CO2-N2, CO2-CH4 and CO2-H2 in Pure Water and TBAB Solutions. École Nationale Supérieure de Techniques Avancées Unité Chime et Procédés. In Proceedings of the 7th International Conference on Gas Hydrates (ICGH 2011), Edinburgh, UK, 17–21 July 2011. [Google Scholar]
- Pang, J.; Ng, H.J.; Zuo, J.; Zhang, D.; Ma, Q.; Chen, G. Hydrogen Gas Hydrate-Measurements and Predictions. Fluid Phase Equilib. 2012, 316, 6–10. [Google Scholar] [CrossRef]
- Kang, S.P.; Lee, J.; Seo, Y. Pre-Combustion Capture of CO2 by Gas Hydrate Formation in Silica Gel Pore Structure. Chem. Eng. J. 2013, 218, 126–132. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Eslamimanesh, A.; Richon, D. Semi-Clathrate Hydrate Phase Equilibrium Measurements for the CO2+H2/CH4+tetra-n-Butylammonium Bromide Aqueous Solution System. Chem. Eng. Sci. 2013, 94, 284–290. [Google Scholar] [CrossRef]
- Xia, Z.; Li, X.; Chen, Z.; Li, G.; Wang, Y.; Jing, C.; Li, Z.; Lv, Q. Hydrate-Based Synchronously Capture of CO2 and H2S for Clean H2 with New Synergic Additives. Energy Procedia 2017, 142, 3427–3432. [Google Scholar] [CrossRef]
- Lee, W.; Kim, Y.-S.; Kang, S.-P. Semiclathrate-Based CO2 Capture from Fuel Gas in the Presence of Tetra-n-Butyl Ammonium Bromide and Silica Gel Pore Structure. Chem. Eng. J. 2018, 331, 1–7. [Google Scholar] [CrossRef]
- Unruh, C.H.; Katz, D.L. Gas Hydrates of Carbon Dioxide-Methane Mixtures. J. Pet. Technol. 1949, 1, 83–86. [Google Scholar] [CrossRef]
- Adisasmito, S.; Frank, R.J.; Sloan, E.D. Hydrates of Carbon-Dioxide and Methane Mixtures. J. Chem. Eng. Data 1991, 36, 68–71. [Google Scholar] [CrossRef]
- Ohgaki, K.; Takano, K.; Sangawa, H.; Matsubara, T.; Nakano, S. Methane Exploitation by Carbon Dioxide from Gas Hydrates. Phase Equilibria for CO2-CH4 Mixed Hydrate System. J. Chem. Eng. Jpn. 1996, 29, 478–483. [Google Scholar] [CrossRef]
- Dholabhai, P.D.; Parent, J.S.; Bishnoi, P.R. Equilibrium Conditions for Hydrate Formation from Binary Mixtures of Methane and Carbon Dioxide in the Presence of Electrolytes, Methanol and Ethylene Glycol. Fluid Phase Equilib. 1997, 141, 235–246. [Google Scholar] [CrossRef]
- Servio, P.; Lagers, F.; Peters, C.; Englezos, P. Gas Hydrate Phase Equilibrium in the System Methane–Carbon Dioxide–Neohexane and Water. Fluid Phase Equilib. 1999, 158–160, 795–800. [Google Scholar] [CrossRef]
- Seo, Y.T.; Lee, H. Hydrate Phase Equilibria of the Carbon Dioxide, Methane, and Water System. J. Chem. Eng. Data 2001, 46, 381–384. [Google Scholar] [CrossRef]
- Beltrán, J.G.; Servio, P. Equilibrium Studies for the System Methane + Carbon Dioxide + Neohexane + Water. J. Chem. Eng. Data 2008, 53, 1745–1749. [Google Scholar] [CrossRef]
- Bruusgaard, H.; Beltrán, J.G.; Servio, P. Solubility Measurements for the CH4 + CO2 + H2O System under Hydrate–Liquid–Vapor Equilibrium. Fluid Phase Equilib. 2010, 296, 106–109. [Google Scholar] [CrossRef]
- Belandria, V.; Eslamimanesh, A.; Mohammadi, A.H.; Theveneau, P.; Legendre, H.; Richon, D. Compositional Analysis and Hydrate Dissociation Conditions Measurements for Carbon Dioxide plus Methane plus Water System. Ind. Eng. Chem. Res. 2011, 50, 5783–5794. [Google Scholar] [CrossRef]
- Xia, Z.; Chen, Z.; Li, X.; Zhang, Y.; Yan, K.; Lv, Q.; Xu, C.; Cai, J. Thermodynamic Equilibrium Conditions for Simulated Land Fi Ll Gas Hydrate Formation in Aqueous Solutions of Additives. J. Chem. Eng. Data. 2012, 57, 3290–3295. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.; Lee, Y.; Seo, Y. Thermodynamic and 13C NMR Spectroscopic Verification of Methane-Carbon Dioxide Replacement in Natural Gas Hydrates. Chem. Eng. J. 2013, 225, 636–640. [Google Scholar] [CrossRef]
- Bi, Y.; Yang, T.; Guo, K. Determination of the Upper-Quadruple-Phase Equilibrium Region for Carbon Dioxide and Methane Mixed Gas Hydrates. J. Pet. Sci. Eng. 2013, 101, 62–67. [Google Scholar] [CrossRef]
- Xia, Z.; Li, X.; Chen, Z.; Li, G.; Cai, J.; Wang, Y.; Yan, K.; Xu, C. Hydrate-Based Acidic Gases Capture for Clean Methane with New Synergic. Appl. Energy 2017, 207, 584–593. [Google Scholar] [CrossRef]
- Kastanidis, P.; Romanos, G.E.; Stubos, A.K.; Economou, I.G.; Tsimpanogiannis, I.N. Two- and Three-Phase Equilibrium Experimental Measurements for the Ternary CH4 + CO2 + H2O Mixture. Fluid Phase Equilib. 2017, 451, 96–105. [Google Scholar] [CrossRef]
- Seo, Y.T.; Lee, H. Multiple-Phase Hydrate Equilibria of the Ternary Carbon Dioxide, Methane, and Water Mixtures. J. Phys. Chem. B 2001, 105, 10084–10090. [Google Scholar] [CrossRef]
- Belandria, V.; Mohammadi, A.H.; Richon, D. Phase Equilibria of Clathrate Hydrates of Methane+Carbon Dioxide: New Experimental Data and Predictions. Fluid Phase Equilib. 2010, 296, 60–65. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.; Lee, J.; Lee, H.; Seo, Y. Experimental Verification of Methane-Carbon Dioxide Replacement in Natural Gas Hydrates Using a Differential Scanning Calorimeter. Environ. Sci. Technol. 2013, 47, 13184–13190. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.L.; Liang, D.Q.; Li, D.L. Phase Equilibrium Conditions for Simulated Landfill Gas Hydrate Formation in Aqueous Solutions of Tetrabutylammonium Nitrate. J. Chem. Thermodyn. 2014, 68, 322–326. [Google Scholar] [CrossRef]
- Obanijesu, E.O.; Barifcani, A.; Pareek, V.K.; Tade, M.O. Experimental Study on Feasibility of H2 and N2 as Hydrate Inhibitors in Natural Gas Pipelines. J. Chem. Eng. Data 2014, 59, 3756–3766. [Google Scholar] [CrossRef]
- Zhou, X.; Long, Z.; Liang, S.; He, Y.; Yi, L.; Li, D.; Liang, D. In Situ Raman Analysis on the Dissociation Behavior of Mixed CH4–CO2 Hydrates. Energy Fuels 2016, 30, 1279–1286. [Google Scholar] [CrossRef]
- Zang, X.; Liang, D. Phase Equilibrium Data for Semiclathrate Hydrate of Synthesized Binary CO2/CH4 Gas Mixture in Tetra-n-Butylammonium Bromide Aqueous Solution. J. Chem. Eng. Data 2017, 62, 851–856. [Google Scholar] [CrossRef]
- Legoix, L.; Ruffine, L.; Donval, J.-P.; Haeckel, M. Phase Equilibria of the CH4-CO2 Binary and the CH4-CO2-H2O Ternary Mixtures in the Presence of a CO2-Rich Liquid Phase. Energies 2017, 10, 2034. [Google Scholar] [CrossRef]
- Lim, J.; Choi, W.; Mok, J.; Seo, Y. Clathrate-Based CO2 Capture from CO2-Rich Natural Gas and Biogas. ACS Sustain. Chem. Eng. 2018, 6, 5627–5635. [Google Scholar] [CrossRef]
- Lang, F.; Servio, P. Bulk Liquid and Gas Mole Fraction Measurements during Hydrate Growth for the CH4 + CO2 + H2O System. J. Chem. Thermodyn. 2018, 117, 113–118. [Google Scholar] [CrossRef]
- Mu, L.; von Solms, N. Hydrate Thermal Dissociation Behavior and Dissociation Enthalpies in Methane-Carbon Dioxide Swapping Process. J. Chem. Thermodyn. 2018, 117, 33–42. [Google Scholar] [CrossRef]
- Matsui, Y.; Ogura, Y.; Miyauchi, H.; Makino, T.; Sugahara, T.; Ohgaki, K. Isothermal Phase Equilibria for Binary Hydrate Systems of Carbon Dioxide + Ethane and Carbon Dioxide + Tetrafluoromethane. J. Chem. Eng. Data 2010, 55, 3297–3301. [Google Scholar] [CrossRef]
- Adisasmito, S.; Sloan, E.D. Hydrates of Hydrocarbon Gases Containing Carbon Dioxide. J. Chem. Eng. Data 1992, 37, 343–349. [Google Scholar] [CrossRef]
- Maghsoodloo Babakhani, S.; Bouillot, B.; Douzet, J.; Ho-Van, S.; Herri, J.M. PVTx Measurements of Mixed Clathrate Hydrates in Batch Conditions under Different Crystallization Rates: Influence on Equilibrium. J. Chem. Thermodyn. 2018, 122, 73–84. [Google Scholar] [CrossRef]
- Kim, S.H.; Huh, C.; Kang, S.-G.; Kang, J.W.; Lee, C.S. Phase Equilibria Containing Gas Hydrate of Carbon Dioxide, Sulfur Dioxide, and Water Mixtures. J. Chem. Eng. Data 2013, 58, 1879–1882. [Google Scholar] [CrossRef]
- Sun, C.Y.; Ma, C.F.; Chen, G.J.; Zhang, S.X. Experimental and Simulation of Single Equilibrium Stage Separation of (Methane + Hydrogen) Mixtures via Forming Hydrate. Fluid Phase Equilib. 2007, 261, 85–91. [Google Scholar] [CrossRef]
- Ma, Q.-L.; Chen, G.-J.; Ma, C.-F.; Zhang, L.-W. Study of Vapor–Hydrate Two-Phase Equilibria. Fluid Phase Equilib. 2008, 265, 84–93. [Google Scholar] [CrossRef]
- Zhang, S.X.; Chen, G.J.; Ma, C.F.; Yang, L.Y.; Guo, T.M. Hydrate Formation of Hydrogen + Hydrocarbon Gas Mixtures. J. Chem. Eng. Data 2000, 45, 908–911. [Google Scholar] [CrossRef]
- Skiba, S.S.; Larionov, E.G.; Manakov, A.Y.; Kolesov, B.A.; Kosyakov, V.I. Investigation of Hydrate Formation in the System H2-CH4-H2O at a Pressure up to 250 MPa. J. Phys. Chem. B 2007, 111, 11214–11220. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Grim, R.G.; Khan, N.M.; Sugahara, T.; Ohgaki, K.; Sloan, E.D.; Koh, C.A.; Sum, A.K. Investigating the Thermodynamic Stabilities of Hydrogen and Methane Binary Gas Hydrates. J. Phys. Chem. C 2014, 118, 3783–3788. [Google Scholar] [CrossRef]
- Happel, J.; Hnatow, M.A.; Meyer, H. The Study of Separation of Nitrogen from Methane by Hydrate Formation Using a Novel Apparatus—Discussion. Ann. N. Y. Acad. Sci. 1994, 715, 425–429. [Google Scholar] [CrossRef]
- Mei, D.; Liao, J.; Yang, J.; Guo, T. Experimental and Modeling Studies on the Hydrate Formation of a Methane+ Nitrogen Gas Mixture in the Presence of Aqueous Electrolyte Solutions. Ind. Eng. Chem. Res. 1996, 35, 4342–4347. [Google Scholar] [CrossRef]
- Nixdorf, J.; Oellrich, L.R. Experimental Determination of Hydrate Equilibrium Conditions for Pure Gases, Binary and Ternary Mixtures and Natural Gases. Fluid Phase Equilib. 1997, 139, 325–333. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, D.Y.; Lee, H. Phase Behavior and Structure Transition of the Mixed Methane and Nitrogen Hydrates. Korean J. Chem. Eng. 2006, 23, 299–302. [Google Scholar] [CrossRef]
- Zhong, D.; Englezos, P. Methane Separation from Coal Mine Methane Gas by Tetra-n-Butyl Ammonium Bromide Semiclathrate Hydrate Formation. Energy Fuels 2012, 26, 2098–2106. [Google Scholar] [CrossRef]
- Zhong, D.L.; Daraboina, N.; Englezos, P. Coal Mine Methane Gas Recovery by Hydrate Formation in a Fixed Bed of Silica Sand Particles. Energy Fuels 2013, 27, 4581–4588. [Google Scholar] [CrossRef]
- Zhong, D.L.; Daraboina, N.; Englezos, P. Recovery of CH4 from Coal Mine Model Gas Mixture (CH4/N2) by Hydrate Crystallization in the Presence of Cyclopentane. Fuel 2013, 106, 425–430. [Google Scholar] [CrossRef]
- Zhong, D.; Sun, D.; Lu, Y.; Yan, J.; Wang, J. Adsorption−Hydrate Hybrid Process for Methane Separation from a CH4/N2/O2 Gas Mixture Using Pulverized Coal Particles. Ind. Eng. Chem. Res. 2014, 53, 15738–15746. [Google Scholar] [CrossRef]
- Lim, D.; Ro, H.; Seo, Y.; Seo, Y.; Lee, J.Y.; Kim, S.-J.; Lee, J.; Lee, H. Thermodynamic Stability and Guest Distribution of CH4/N2/CO2 Mixed Hydrates for Methane Hydrate Production Using N2/CO2 Injection. J. Chem. Thermodyn. 2017, 106, 16–21. [Google Scholar] [CrossRef]
- Lee, H.H.; Ahn, S.H.; Nam, B.U.; Kim, B.S.; Lee, G.W.; Moon, D.; Shin, H.J.; Han, K.W.; Yoon, J.H. Thermodynamic Stability, Spectroscopic Identification, and Gas Storage Capacity of CO2-CH4-N2 Mixture Gas Hydrates: Implications for Landfill Gas Hydrates. Environ. Sci. Technol. 2012, 46, 4184–4190. [Google Scholar] [CrossRef]
- Sabil, K.M.; Nasir, Q.; Partoon, B.; Seman, A.A. Measurement of H-LW-V and Dissociation Enthalpy of Carbon Dioxide Rich Synthetic Natural Gas Mixtures. J. Chem. Eng. Data 2014, 59, 3502–3509. [Google Scholar] [CrossRef]
- Seo, Y.; An, S.; Park, J.-W.; Kim, B.-S.; Komai, T.; Yoon, J.-H. Occupation and Release Behavior of Guest Molecules in CH4, CO2, N2, and Acetone Mixture Hydrates: An in-Situ Study by Raman Spectroscopy. Ind. Eng. Chem. Res. 2014, 53, 6179–6184. [Google Scholar] [CrossRef]
- Kakati, H.; Mandal, A.; Laik, S. Phase Stability and Kinetics of CH4 + CO2 + N2 Hydrates in Synthetic Seawater and Aqueous Electrolyte Solutions of NaCl and CaCl2. J. Chem. Eng. Data 2015, 60, 1835–1843. [Google Scholar] [CrossRef]
- Sun, D.; Ripmeester, J.; Englezos, P. Phase Equilibria for the CO2/CH4/N2/H2O System in the Hydrate Region under Conditions Relevant to Storage of CO2 in Depleted Natural Gas Reservoirs. J. Chem. Eng. Data 2016, 61, 4061–4067. [Google Scholar] [CrossRef]
- Sun, Y.-H.; Li, S.-L.; Zhang, G.-B.; Guo, W.; Zhu, Y.-H. Hydrate Phase Equilibrium of CH4 + N2 + CO2 Gas Mixtures and Cage Occupancy Behaviors. Ind. Eng. Chem. Res. 2017, 56, 8133–8142. [Google Scholar] [CrossRef]
- Zang, X.; Liang, D. Phase Equilibrium Data for the Hydrates of Synthesized Ternary CH4/CO2/N2 Biogas Mixtures. J. Chem. Eng. Data 2018, 63, 197–201. [Google Scholar] [CrossRef]
- Kwon, M.; Lee, J.; Lee, H. Temperature-Dependent Structural Transitions in Methane—Ethane Mixed Gas Hydrates. J. Phys. Chem. C 2014, 118, 28906–28913. [Google Scholar] [CrossRef]
- Soltanimehr, S.; Javanmardi, J.; Nasrifar, K. Liquid Water–Hydrate–Vapor Equilibrium for Methane + Ethane Gas Mixtures: Application of Gas Hydrates for Separation. J. Chem. Eng. Data 2017, 62, 2143–2148. [Google Scholar] [CrossRef]
- Song, K.Y.; Kobayashi, R. Final Hydrate Stability Conditions of a Methane and Propane Mixture in the Presence of Pure Water and Aqueous Solutions of Methanol and Ethylene Glycol. Fluid Phase Equilib. 1989, 47, 295–308. [Google Scholar] [CrossRef]
- Smith, C.; Pack, D.; Barifcani, A. Propane, n-Butane and i-Butane Stabilization Effects on Methane Gas Hydrates. J. Chem. Thermodyn. 2017, 115, 293–301. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.; Park, S.; Kim, Y.; Cha, I.; Seo, Y. Stability Conditions and Guest Distribution of the Methane + Ethane + Propane Hydrates or Semiclathrates in the Presence of Tetrahydrofuran or Quaternary Ammonium Salts. J. Chem. Thermodyn. 2013, 65, 113–119. [Google Scholar] [CrossRef]
- Holder, G.D.; Hand, J.H. Multiple-Phase Equilibria in Hydrates from Methane, Ethane, Propane and Water Mixtures. AIChE J. 1982, 28, 440–447. [Google Scholar] [CrossRef]
- Babakhani, S.M.; Bouillot, B.; Douzet, J.; Herri, J.M. A New Approach of Studying Mixed Gas Hydrates Involving Propane at Non-Equilibrium Conditions and Final State: An Experimental Study and Modeling. Chem. Eng. Sci. 2018, 179, 150–160. [Google Scholar] [CrossRef]
- Chen, L.; Lu, H.; Ripmeester, J.A. Dissociation Conditions and Raman Spectra of CO2 + SO2 and CO2 + H2S Hydrates. Ind. Eng. Chem. Res. 2015, 54, 5543–5549. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Richon, D. Hydrate Phase Equilibria of Gaseous Mixtures of Methane + Carbon Dioxide + Hydrogen Sulfide. Chem. Eng. Commun. 2015, 202, 629–633. [Google Scholar] [CrossRef]
- Ward, Z.T.; Marriott, R.A.; Sum, A.K.; Sloan, E.D.; Koh, C.A. Equilibrium Data of Gas Hydrates Containing Methane, Propane, and Hydrogen Sulfide. J. Chem. Eng. Data 2015, 60, 424–428. [Google Scholar] [CrossRef]
- Ma, Q.L.; Chen, G.J.; Sun, C.Y. Vapor-Liquid-Liquid-Hydrate Phase Equilibrium Calculation for Multicomponent Systems Containing Hydrogen. Fluid Phase Equilib. 2013, 338, 87–94. [Google Scholar] [CrossRef]
- Kuhs, W.F.; Klapproth, A.; Chazallon, B. Chemical Physics of Air Clathrate Hydrates. In Proceedings of the Physics of Ice Core Records, International Symposium on Physics of Ice Core Records, Shikotsukohan, Hokkaido, Japan, 14–17 September 1998; pp. 373–392. [Google Scholar]
- Yasuda, K.; Oto, Y.; Shen, R.; Uchida, T.; Ohmura, R. Phase Equilibrium Condition Measurements in Nitrogen and Air Clathrate Hydrate Forming Systems at Temperatures below Freezing Point of Water. J. Chem. Thermodyn. 2013, 67, 143–147. [Google Scholar] [CrossRef]
- Kastanidis, P.; Romanos, G.E.; Michalis, V.K.; Economou, I.G.; Stubos, A.K.; Tsimpanogiannis, I.N. Development of a Novel Experimental Apparatus for Hydrate Equilibrium Measurements. Fluid Phase Equilib. 2016, 424, 152–161. [Google Scholar] [CrossRef]
- Servio, P.; Englezos, P. Effect of Temperature and Pressure on the Solubility of Carbon Dioxide in Water in the Presence of Gas Hydrate. Fluid Phase Equilib. 2001, 190, 127–134. [Google Scholar] [CrossRef]

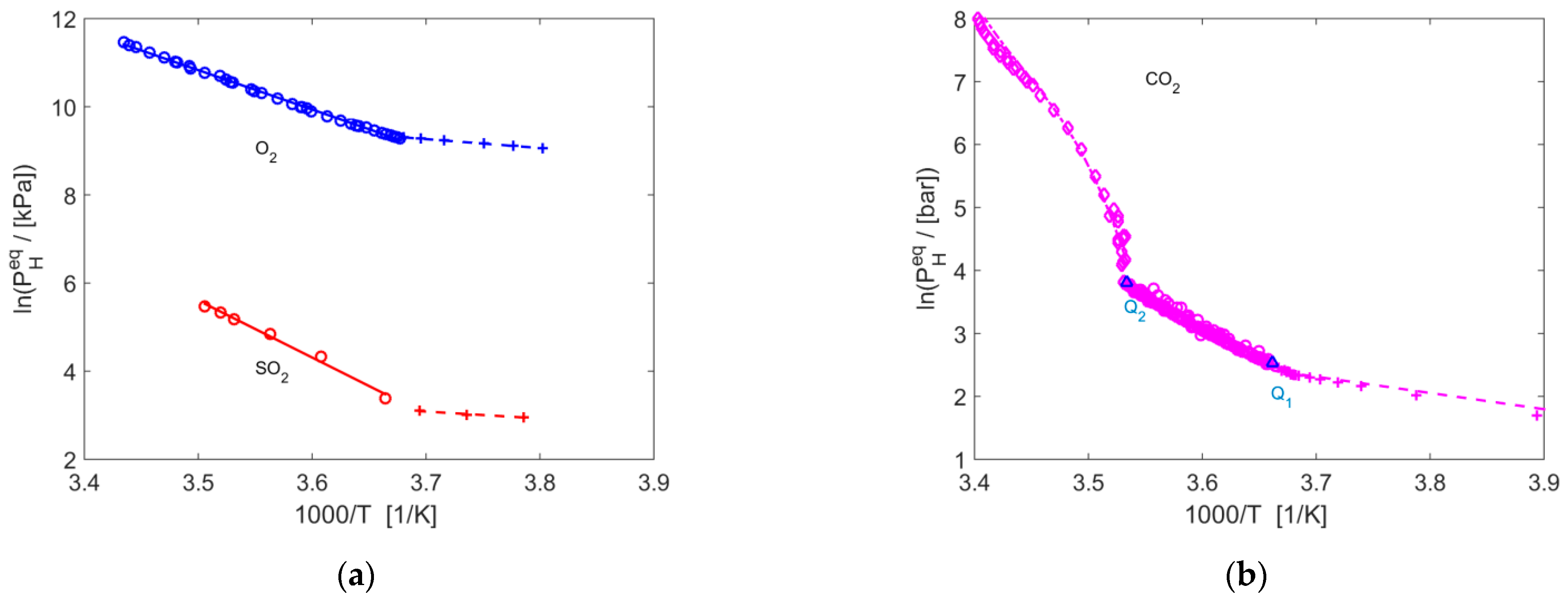
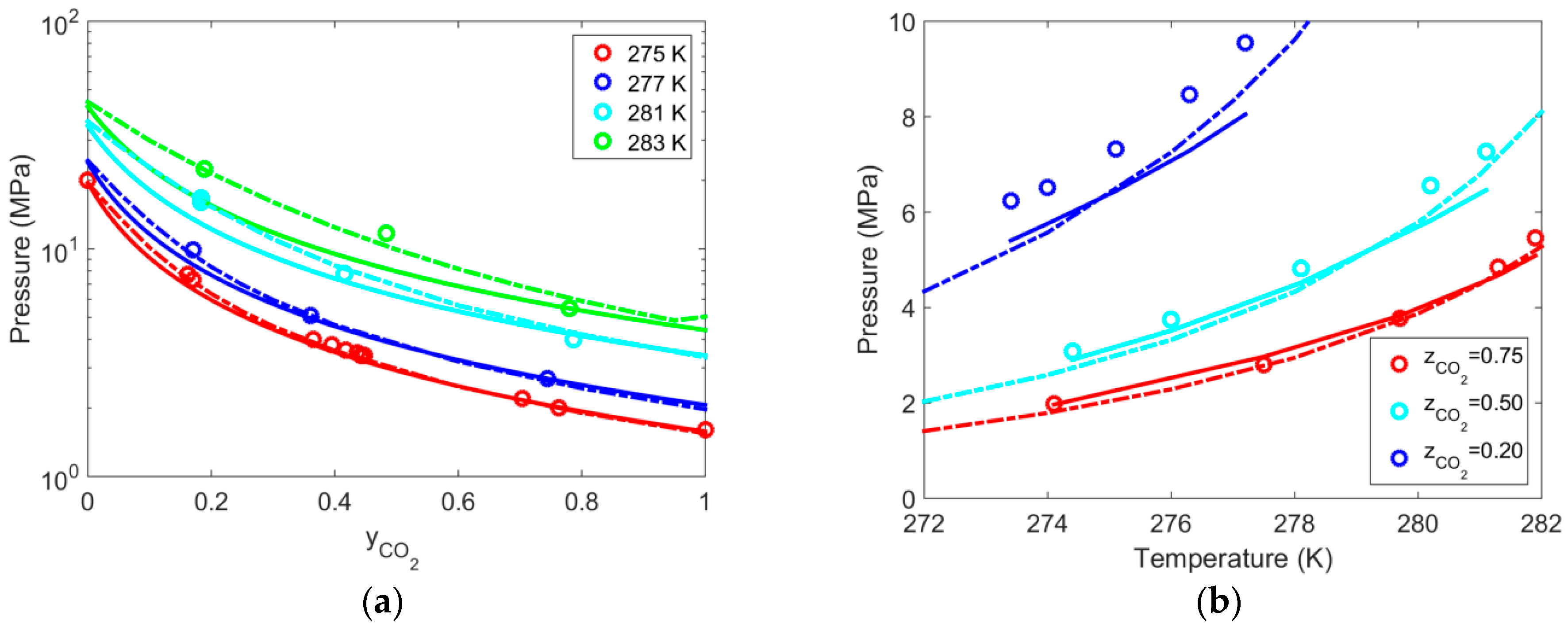
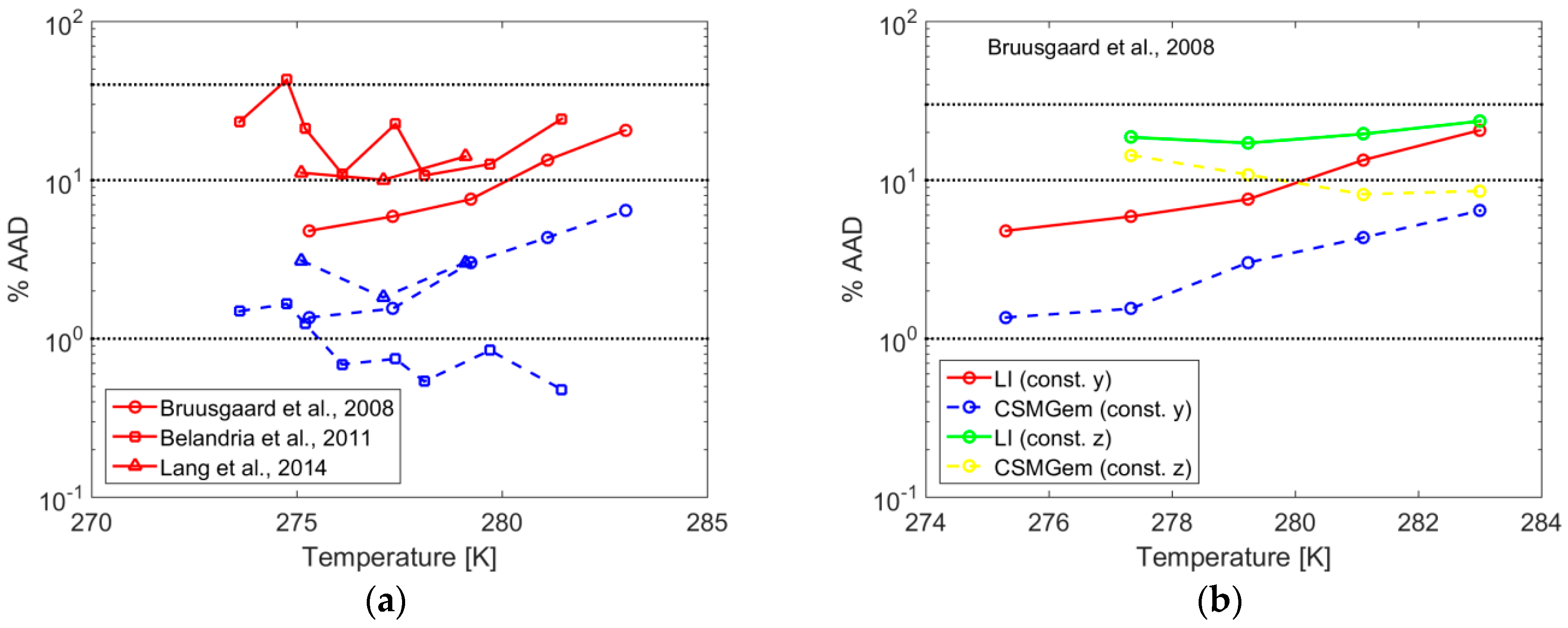
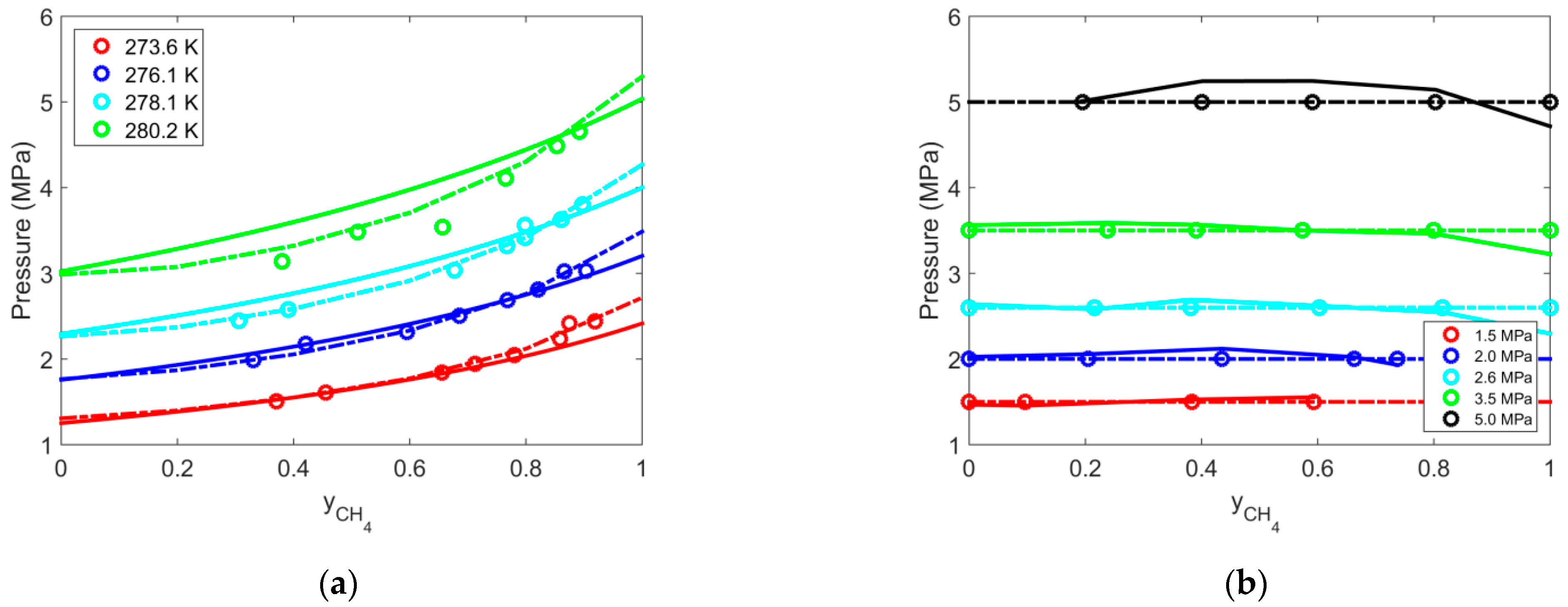
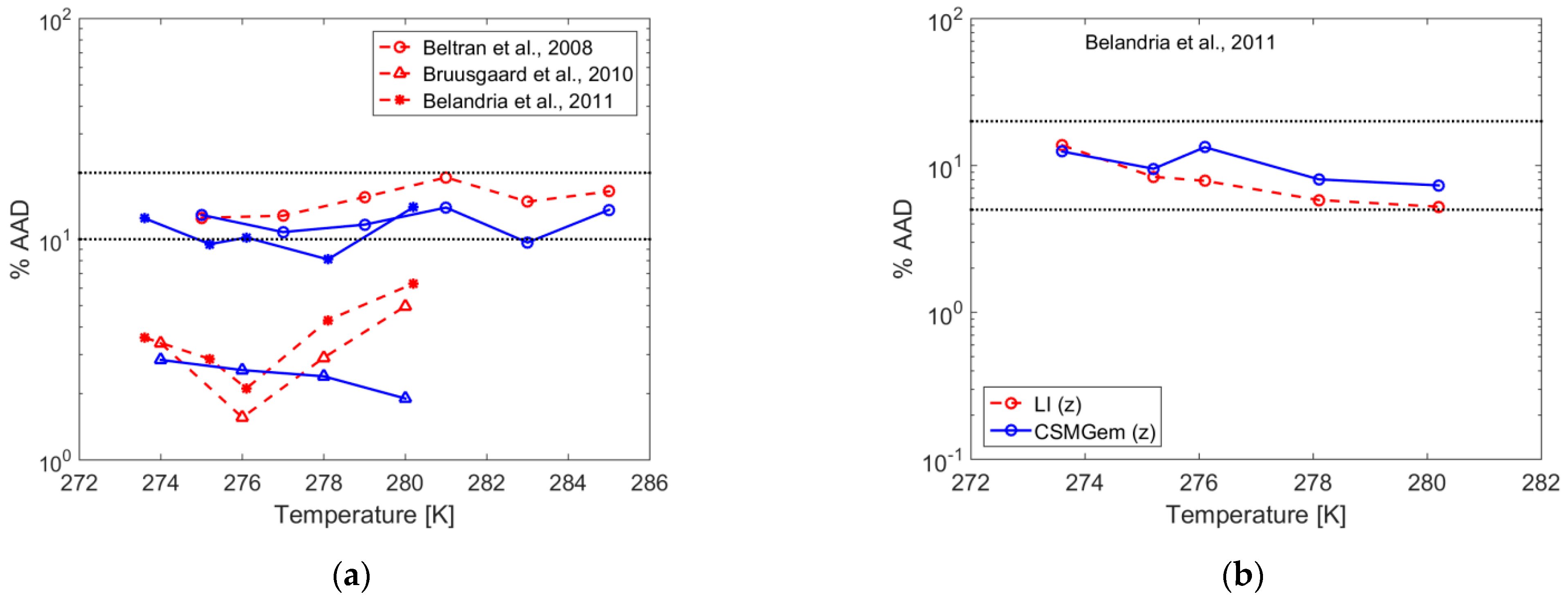
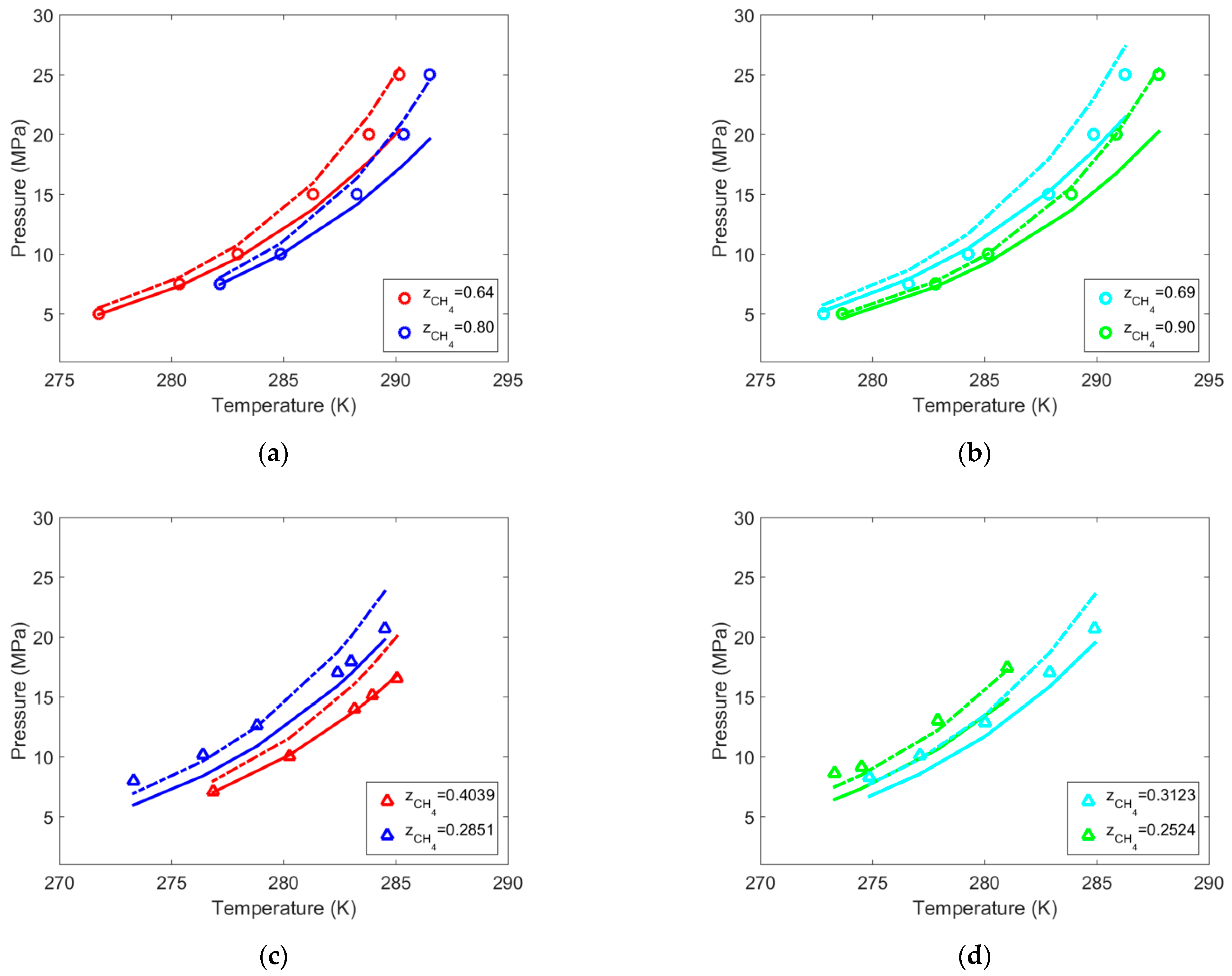
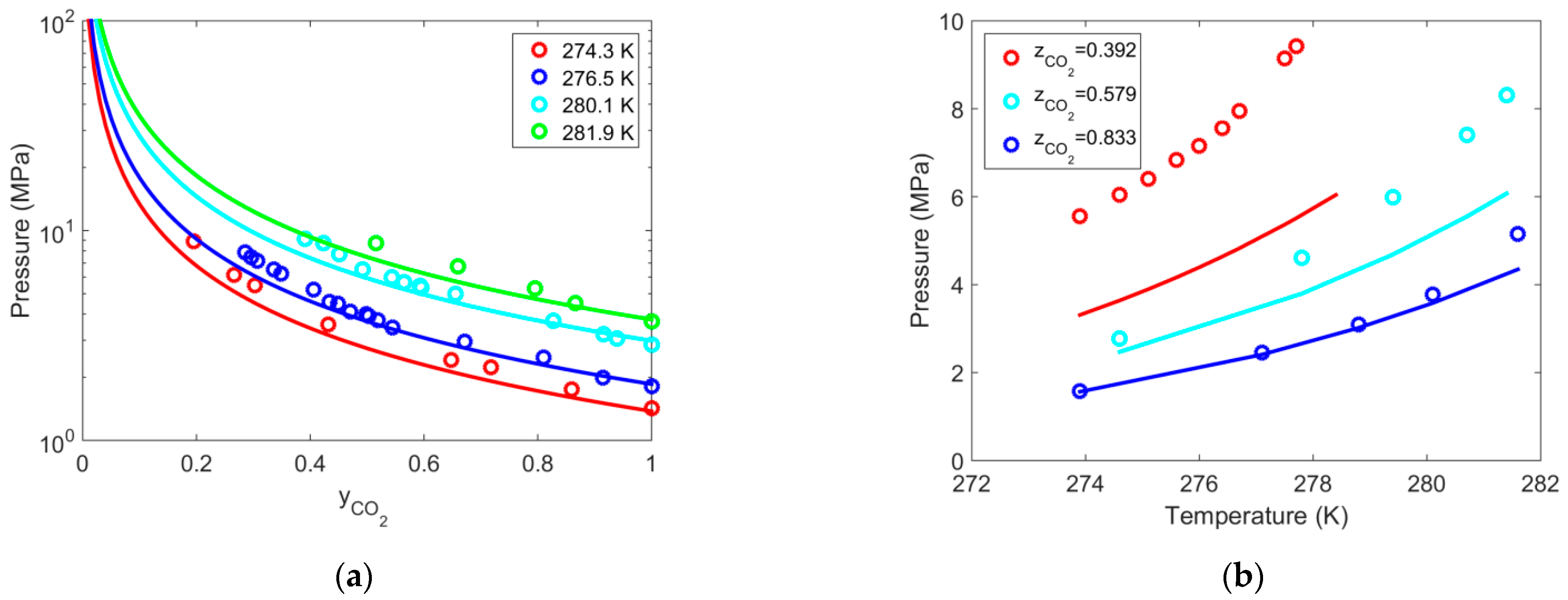

| Gas | T Range (K) | Type of Equilibria | Ai (−) | Bi (K) | Source | Refs. |
|---|---|---|---|---|---|---|
| CH4 | 248–273 | H–I–V | 14.7170 | −1886.79 | Holder et al. [61] | N/A |
| CH4 | 273–298 | H–Lw–V | 38.9803 | −8533.80 | Holder et al. [61] | N/A |
| C2H6 | 248–273 | H–I–V | 17.5110 | −3104.535 | Holder et al. [61] | N/A |
| C2H6 | 273–287 | H–Lw–V | 44.2728 | −10,424.248 | Holder et al. [61] | N/A |
| C2H6 | 287–304 | H–Lw–LH | 0.0367 | 10.867 | This work | [81] |
| C2H4 | 269–274 | H–Lw–V | 1.7055 | 466.056 | This work | [82,83,84] |
| C2H4 | 274–286 | H–Lw–V | 1.7384 | −11,138.677 490.000 | This work | [83,84,85] |
| C2H4 | 286–291 | H–Lw–V | 0.3859 | 109.283 | This work | [83,84,85] |
| C2H4 | 291–295 | H–Lw–LH | 2.8681 | −32,679.321 838.367 | This work | [83,85] |
| C3H8 | 248–273 | H–I–V | 17.1560 | −3269.646 | Holder et al. [61] | N/A |
| C3H8 | 273–278 | H–Lw–V | 67.1301 | −16,921.840 | Holder et al. [61] | N/A |
| C3H8 | 278–303 | H–Lw–LH | 0.0684 | 19.215 | This work | [86,87] |
| CO | 274–285 | H–Lw–V | 0.1108 | 31.071 | This work | [88] |
| CO2 | 248–273 | H–I–V | 18.5939 | −3161.410 | Holder et al. [61] | N/A |
| CO2 | 273–284 | H–Lw–V | 44.5776 | −10,245.010 | Holder et al. [61] | N/A |
| CO2 | 283–292 | H–Lw–LH | 4.0947 | −31,030.579 1171.400 | This work | [1] |
| N2 | 248–273 | H–I–V | 15.1289 | −1504.276 | Holder et al. [61] | N/A |
| N2 | 273–298 | H–Lw–V | 37.8079 | −7688.626 | Holder et al. [61] | N/A |
| H2S | 248–273 | H–I–V | 16.5597 | −3270.409 | Holder et al. [61] | N/A |
| H2S | 273–298 | H–Lw–V | 34.8278 | −8266.102 | Holder et al. [61] | N/A |
| O2 | 268–271.7 | H–I–V | 0.0871 | 23.298 | This work | [89,90,91] |
| O2 | 271.7–291 | H–Lw–V | 0.1963 | 54.799 | This work | [89,90,92] |
| SO2 | T < 270.65 | H–I–V | 1.3650 | 365.068 | This work | [93,94,95] |
| SO2 | 270.7–286 | H–Lw–V | 2.3810 | −12,940.097 667.712 | This work | [93,94,95] |
| H2 | 267–273.7 | H–I–V | 0.9598 | −11,120.167 259.541 | This work | [96] |
| H2 | 273.7–348 | H–Lw–V | 0.2126 | 64.707 | This work | [96] |
| Notation | System | Comment | References Examined |
|---|---|---|---|
| GM–1 | CO2 + N2 | “Flue Gas” A | [98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118] |
| GM–2 | CO2 + N2 + O2 | “Flue Gas” B | [113] |
| GM–3 | CO2 + H2 | Methane steam reforming | [101,119,120,121,122,123,124,125,126,127,128,129] |
| GM–4 | CO2 + CH4 | Biogas upgrade | [99,103,106,108,110,112,115,117,127,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154] |
| GM–5 | CO2 + gas (other) | CO2-containing mixtures | [108,112,155,156,157,158] |
| GM–6 | CH4 + H2 | – | [125,148,159,160,161,162,163] |
| GM–7 | CH4 + N2 | – | [115,164,165,166,167,168,169,170] |
| GM–8 | CH4 + N2 + O2 | “Coal-mine” gas mixture | [171] |
| GM–9 | CH4 + CO2 + N2 | – | [117,148,154,166,172,173,174,175,176,177,178,179] |
| GM–10 | CH4 + hydrocarbon gas mixtures | CH4-containing mixture A | [166,180,181,182,183,184] |
| GM–11 | CH4 + gas (other) | CH4-containing mixture B | (No system considered) |
| GM–12 | Light hydrocarbon binaries | Other cases | [166,185,186] |
| GM–13 | H2S + gas (other) | H2S-containing mixtures | [128,142,187,188,189] |
| GM–14 | H2 + natural gas mixtures | H2-containing mixtures | [128,148,160,161,190] |
| GM–15 | Air + gas (other) | Air/N2-containing mixtures | [91,92,191,192] |
| Gas Mixture System | # of Exper. Studies Examined | Total ndp | |||||
|---|---|---|---|---|---|---|---|
| CSMGem | Lipenkov–Istomin | ||||||
| y-Values | z-Values | y-Values | z-Values | ||||
| GM–1: CO2 + N2 | 20 | 432 | 15.73 | 25.74 | 7.40 | 17.54 | |
| GM–2: CO2 + N2 + O2 | 1 | 4 | N/A | N/A | - | 14.85 | |
| GM–3: CO2 + H2 | 12 | 194 | N/A | N/A | 9.24 | 34.45 | |
| GM–4: CO2 + CH4 | 36 | 719 | 10.79 | 14.12 | 7.44 | 13.23 | |
| GM–5: CO2 + gas (other) | CO2 + C2H6 | 3 | 127 | 1.40 | 3.42 | 5.66 | 6.11 |
| CO2 + C3H8 | 2 | 66 | - | 18.18 | - | 19.37 | |
| CO2 + C2H6 + C3H8 | 1 | 5 | - | 15.68 | - | 31.81 | |
| CO2 + CO | 1 | 6 | N/A | N/A | - | 91.45 | |
| CO2 + SO2 | 2 | 75 | N/A | N/A | - | 21.38 | |
| GM–6: CH4 + H2 | 7 | 338 | N/A | N/A | 9.38 | 27.61 | |
| GM–7: CH4 + N2 | 8 | 93 | 4.61 | 7.21 | 12.77 | 9.50 | |
| GM–8: CH4 + N2 + O2 | 1 | 6 | N/A | N/A | - | 25.31 | |
| GM–9: CH4 + CO2 + N2 | 12 | 146 | 33.77 | 14.11 | 15.79 | 9.66 | |
| GM–10: CH4 + hydrocarbon gas mixtures | CH4 + C2H6 | 3 | 35 | 3.15 | 18.72 | 14.38 | 17.30 |
| CH4 + C3H8 | 3 | 38 | - | 13.17 | - | 85.03 | |
| CH4 + C2H6 + C3H8 | 2 | 20 | - | 6.87 | - | 65.16 | |
| GM–11: CH4 + gas (other) | - | - | - | - | - | - | |
| GM–12: Light hydrocarbon binary | C2H6 + C3H8 | 3 | 78 | - | 13.16 | - | 25.28 |
| GM–13: H2S + gas (other) | H2S + CO2 | 2 | 19 | 90.97 | 25.92 | 3.11 | 15.02 |
| H2S + CH4 | 2 | 11 | - | 8.75 | - | 14.58 | |
| H2S + CH4 + CO2 | 2 | 13 | 8.60 | 9.93 | 15.52 | 3.30 | |
| H2S + CH4 + C3H8 | 1 | 10 | - | 128.72 | - | 78.83 | |
| H2S + H2 + CO2 | 1 | 4 | N/A | N/A | 24.65 | 48.74 | |
| GM–14: H2 + natural gas mixtures | H2 + CH4 + C3H8 | 1 | 22 | N/A | N/A | 49.51 | - |
| H2 + CH4 + C2H6 + C3H8 | 1 | 16 | N/A | N/A | 82.69 | - | |
| H2 + C2H4 | 1 | 6 | N/A | N/A | 21.49 | - | |
| H2 + CH4 + C2H4 | 1 | 4 | N/A | N/A | 37.33 | 3.73 | |
| H2 + CO2 + CH4 | 1 | 5 | N/A | N/A | 33.13 | - | |
| H2 + CO2 + H2S | 1 | 4 | N/A | N/A | 32.41 | 15.42 | |
| GM–15: Air + gas (other) | 4 | 20 | N/A | N/A | - | 13.90 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kastanidis, P.; Romanos, G.E.; Stubos, A.K.; Pappa, G.; Voutsas, E.; Tsimpanogiannis, I.N. Evaluation of a Simplified Model for Three-Phase Equilibrium Calculations of Mixed Gas Hydrates. Energies 2024, 17, 440. https://doi.org/10.3390/en17020440
Kastanidis P, Romanos GE, Stubos AK, Pappa G, Voutsas E, Tsimpanogiannis IN. Evaluation of a Simplified Model for Three-Phase Equilibrium Calculations of Mixed Gas Hydrates. Energies. 2024; 17(2):440. https://doi.org/10.3390/en17020440
Chicago/Turabian StyleKastanidis, Panagiotis, George E. Romanos, Athanasios K. Stubos, Georgia Pappa, Epaminondas Voutsas, and Ioannis N. Tsimpanogiannis. 2024. "Evaluation of a Simplified Model for Three-Phase Equilibrium Calculations of Mixed Gas Hydrates" Energies 17, no. 2: 440. https://doi.org/10.3390/en17020440
APA StyleKastanidis, P., Romanos, G. E., Stubos, A. K., Pappa, G., Voutsas, E., & Tsimpanogiannis, I. N. (2024). Evaluation of a Simplified Model for Three-Phase Equilibrium Calculations of Mixed Gas Hydrates. Energies, 17(2), 440. https://doi.org/10.3390/en17020440








