Comparative Study of Hydrogen Production from Organic Fraction of Municipal Solid Waste and Its Challenges: A Review
Abstract
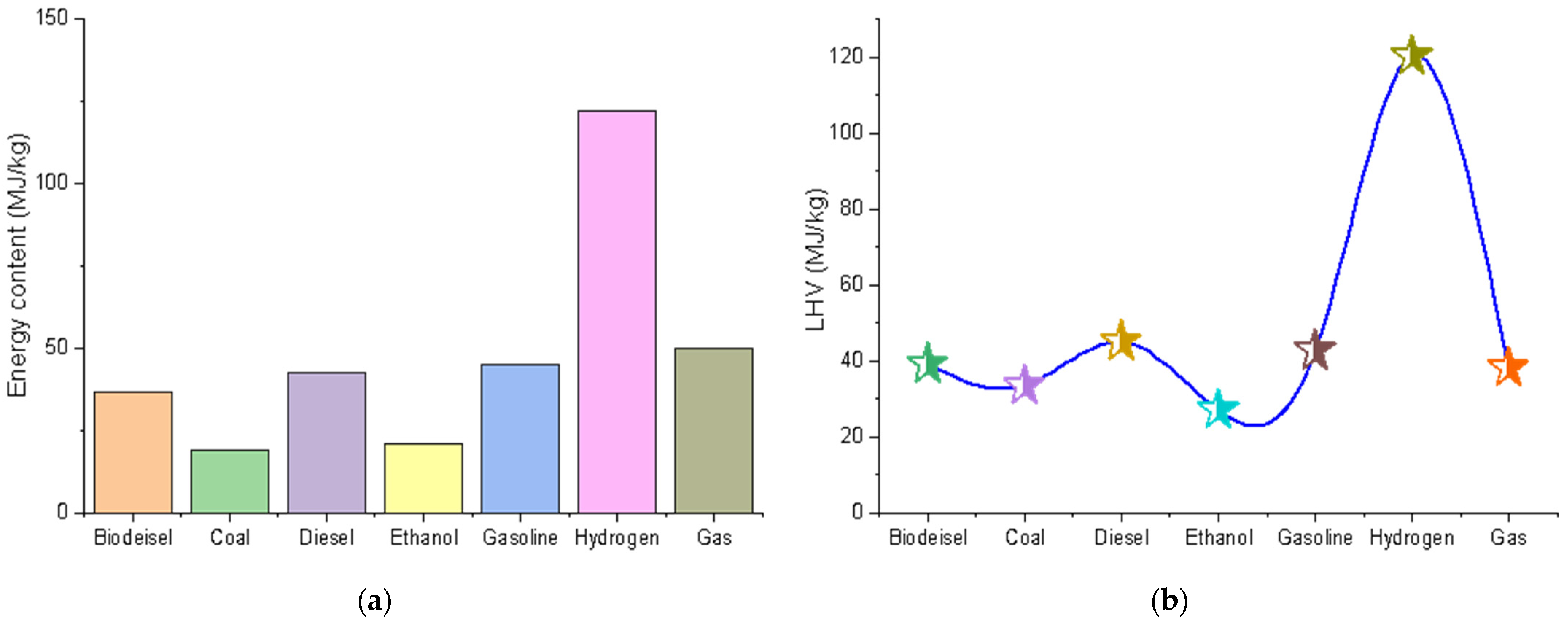
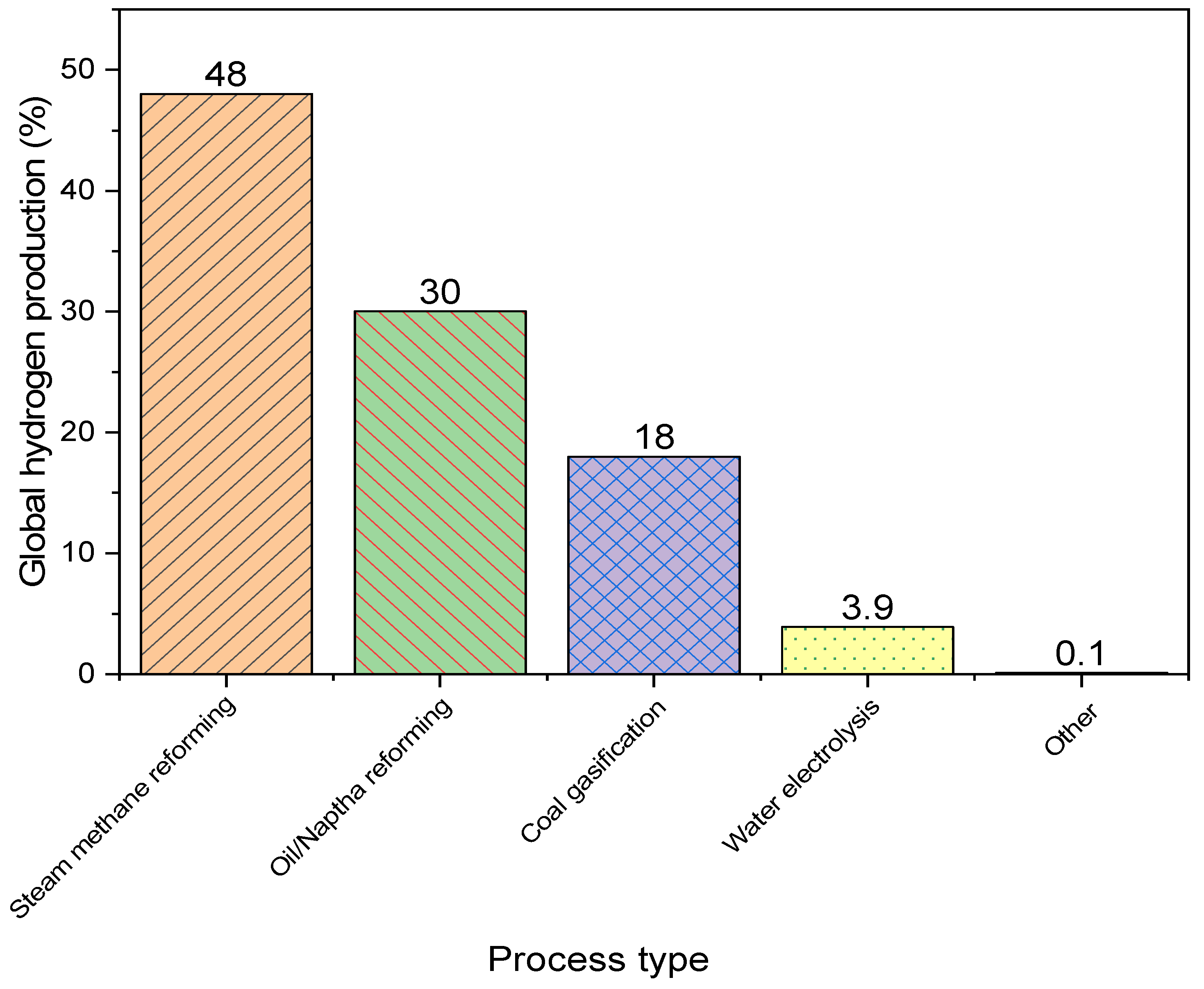
:1. Introduction
2. Feedstocks: Collection and Pretreatment Process
2.1. Collection Process
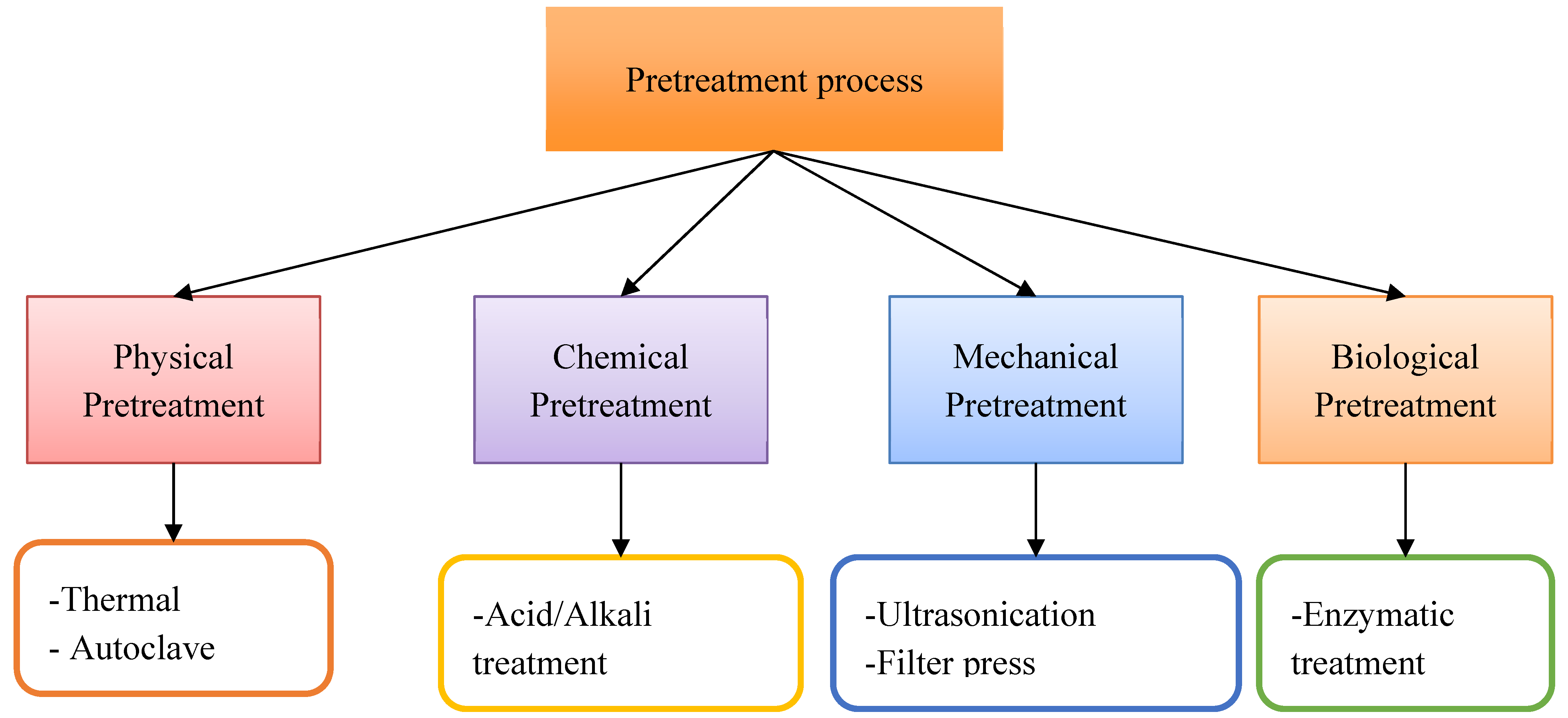
2.2. Pretreatment Process for Hydrogen Production
3. Hydrogen Fuel Production Process
3.1. Electrocatalytic Hydrogen Evolution
3.2. Photobiological Hydrogen Production
3.2.1. Photo Fermentation
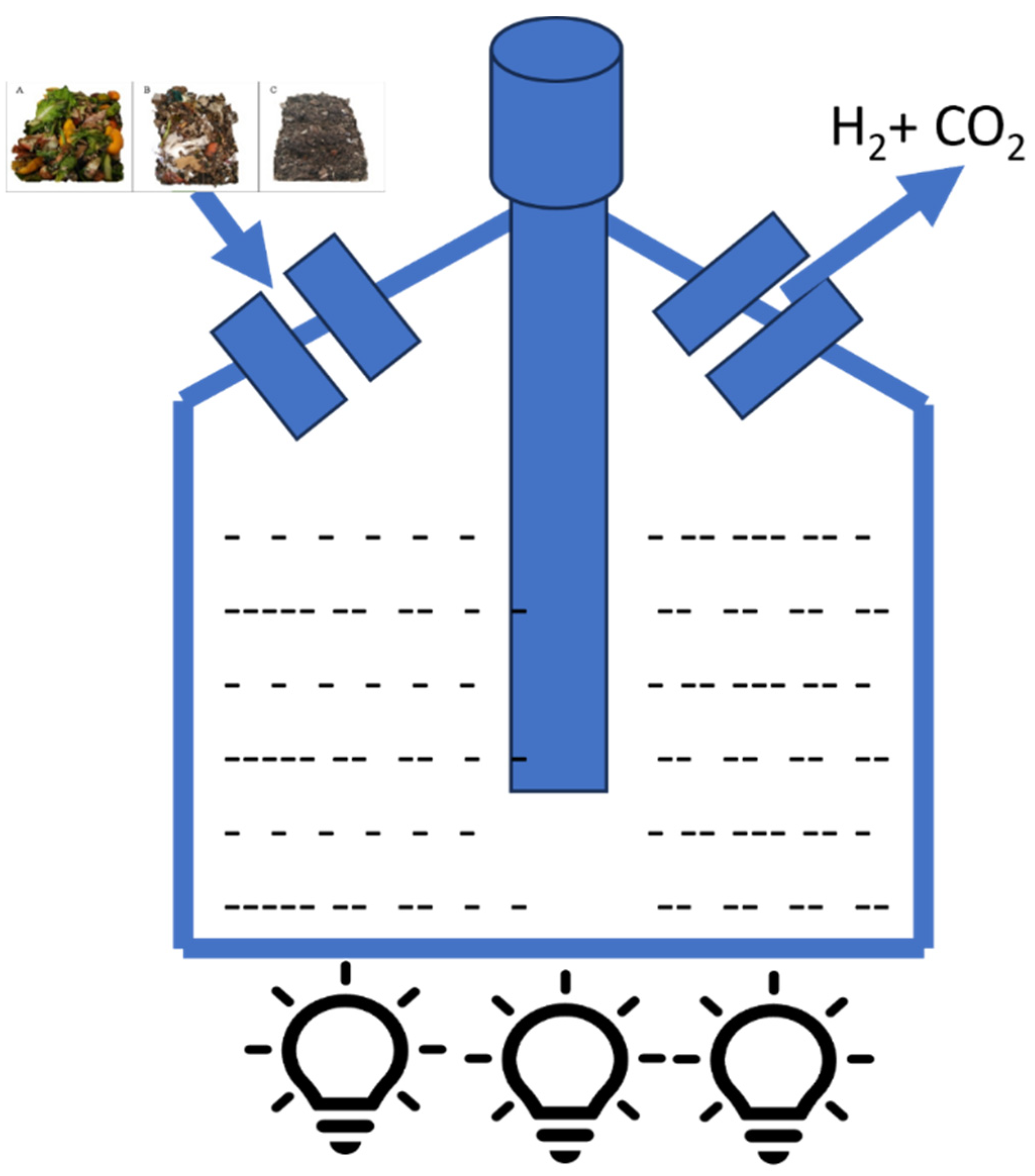
3.2.2. Dark Fermentation
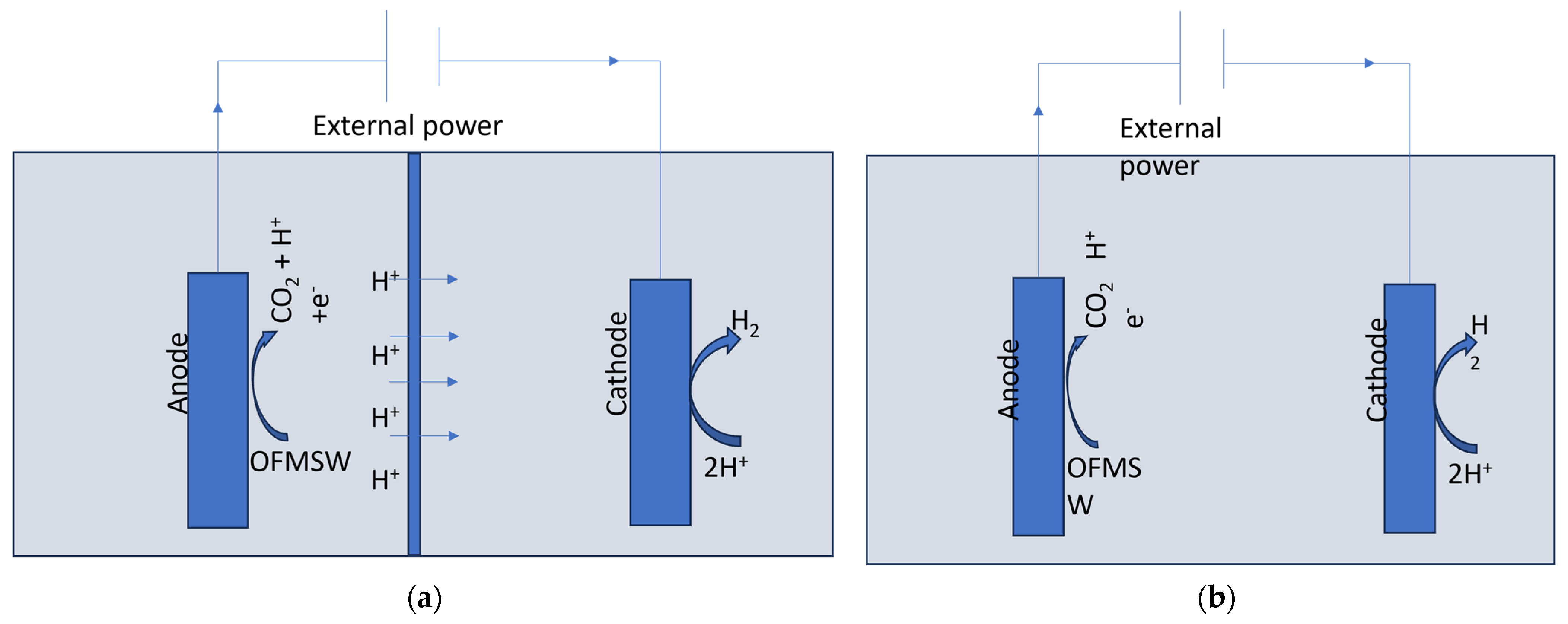
3.3. Microbial Electrolysis Cell (MEC)
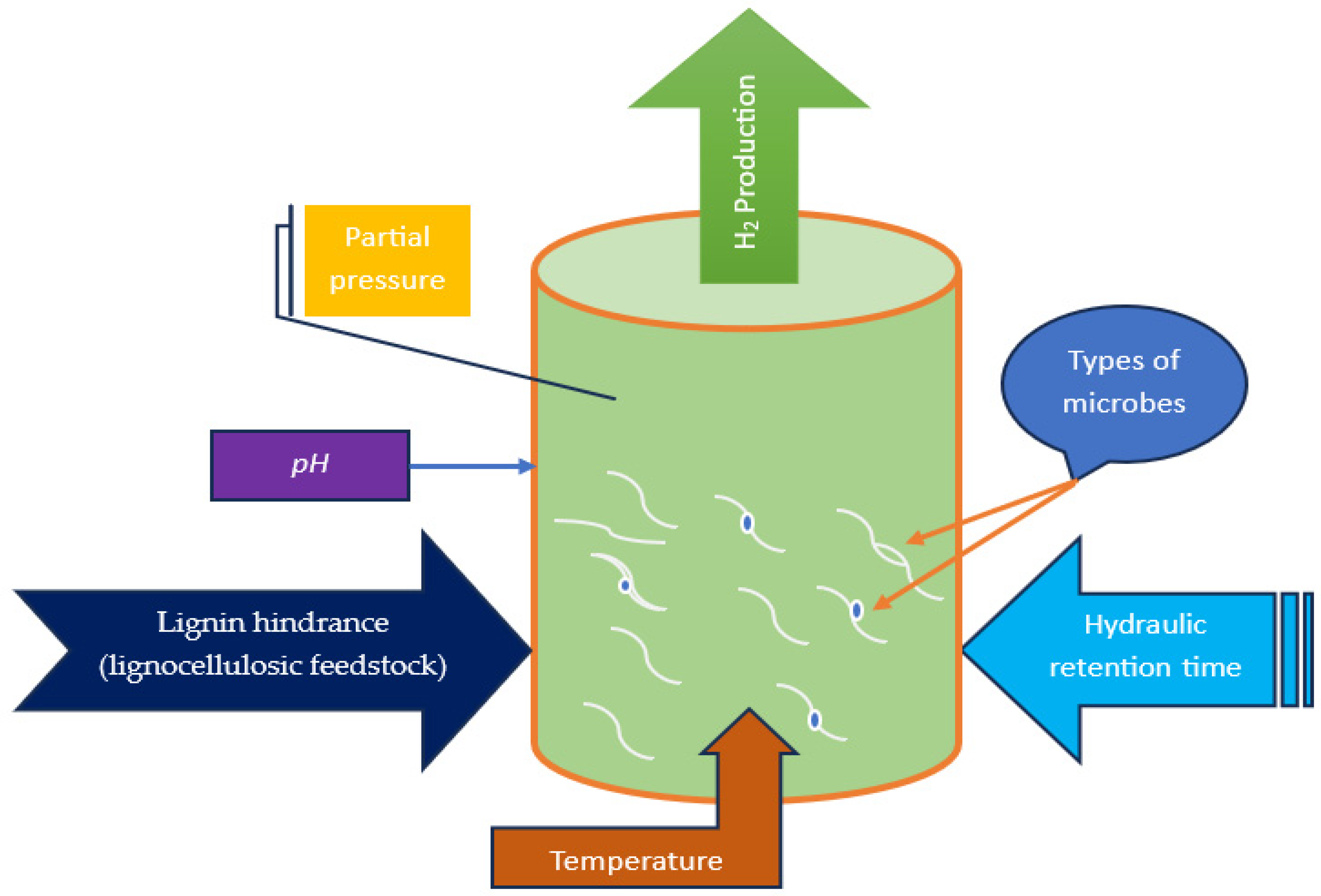
4. Critical Process Parameters and Challenges of Hydrogen Production
5. Conclusions and Recommendation
Author Contributions
Funding
Conflicts of Interest
References
- Kaza, S.; Yao, L. At a Glance: A Global Picture of Solid Waste Management. In What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; The World Bank: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- USEPA. National Overview: Facts and Figures on Materials, Wastes and Recycling; USEPA: Washington, DC, USA, 2015. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures-materials (accessed on 1 August 2023).
- Roberts, M.; Allen, S.; Clarke, J.; Searle, J.; Coley, D. Understanding the global warming potential of circular design strategies: Life cycle assessment of a design-for-disassembly building. Sustain. Prod. Consum. 2023, 37, 331–343. [Google Scholar] [CrossRef]
- Iravani, A.; Akbari, M.H.; Zohoori, M. Advantages and disadvantages of green technology; goals, challenges and strengths. Int. J. Sci. Eng. Appl. 2017, 6, 272–284. [Google Scholar] [CrossRef]
- Task, I.B. Methane eMissions from Biogas Plants; IEA Bioenergy: Paris, France, 2017. [Google Scholar]
- Akratos, C.S.; Tekerlekopoulou, A.G.; Vasiliadou, I.A.; Vayenas, D.V. Cocomposting of olive mill waste for the production of soil amendments. In Olive Mill Waste; Elsevier: Amsterdam, The Netherlands, 2017; pp. 161–182. [Google Scholar]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Balat, M. Potential importance of hydrogen as a future solution to environmental and transportation problems. Int. J. Hydrogen Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Vendruscolo, F. Biohydrogen production from starch residues. World Acad. Sci. Eng. Technol. Int. J. Bioeng. Life Sci. 2014, 8, 1287–1293. [Google Scholar]
- Ahmed, K.; Farrok, O.; Rahman, M.M.; Ali, M.S.; Haque, M.M.; Azad, A.K. Proton exchange membrane hydrogen fuel cell as the grid connected power generator. Energies 2020, 13, 6679. [Google Scholar] [CrossRef]
- Sarker, A.K.; Azad, A.K.; Rasul, M.G.; Doppalapudi, A.T. Prospect of Green Hydrogen Generation from Hybrid Renewable Energy Sources: A Review. Energies 2023, 16, 1556. [Google Scholar] [CrossRef]
- Abánades, A.; Rubbia, C.; Salmieri, D. Technological challenges for industrial development of hydrogen production based on methane cracking. Energy 2012, 46, 359–363. [Google Scholar] [CrossRef]
- Balachandar, G.; Khanna, N.; Das, D. Biohydrogen production from organic wastes by dark fermentation. In Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2013; pp. 103–144. [Google Scholar]
- Sebos, I. Fossil fraction of CO2 emissions of biofuels. Carbon Manag. 2022, 13, 154–163. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.; Xie, X.; Yu, Z.; Von Gadow, K.; Xu, J.; Zhao, S.; Yang, Y. Analysis of the global warming potential of biogenic CO2 emission in life cycle assessments. Sci. Rep. 2017, 7, 39857. [Google Scholar] [CrossRef]
- AlHumaidan, F.S.; Halabi, M.A.; Rana, M.S.; Vinoba, M. Blue hydrogen: Current status and future technologies. Energy Convers. Manag. 2023, 283, 116840. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Djellabi, R.; Vaccari, M.; Prasad, S.; Aminabhavi, T.M.; Rtimi, S. Emerging technologies and sustainable strategies for municipal solid waste valorization: Challenges of circular economy implementation. J. Clean. Prod. 2023, 423, 138708. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Hu, Z.; Liu, S.; Guan, Y.; Cai, L. Influence of particle size on pyrolysis and gasification performance of municipal solid waste in a fixed bed reactor. Bioresour. Technol. 2010, 101, 6517–6520. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Cai, C.; Liu, P.; Lin, W.; Liang, B.; Zhang, H.; Ma, Z.; Ma, H.; Xing, Y.; Liu, W. Supercritical water gasification of food waste: Effect of parameters on hydrogen production. Int. J. Hydrogen Energy 2020, 45, 14744–14755. [Google Scholar]
- Mazhar, A.; Khoja, A.H.; Azad, A.K.; Mushtaq, F.; Naqvi, S.R.; Shakir, S.; Hassan, M.; Liaquat, R.; Anwar, M. Performance Analysis of TiO2-Modified Co/MgAl2O4 Catalyst for Dry Reforming of Methane in a Fixed Bed Reactor for Syngas (H2, CO) Production. Energies 2021, 14, 3347. [Google Scholar] [CrossRef]
- Policastro, G.; Cesaro, A. Composting of Organic Solid Waste of Municipal Origin: The Role of Research in Enhancing Its Sustainability. Int. J. Environ. Res. Public Health 2023, 20, 312. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Li, J.; Yan, M.; Tong, Y.W.; Wang, C.; Wang, X. Organic waste to biohydrogen: A critical review from technological development and environmental impact analysis perspective. Appl. Energy 2019, 256, 113961. [Google Scholar] [CrossRef]
- Laurinavichene, T.; Tekucheva, D.; Laurinavichius, K.; Tsygankov, A. Utilization of distillery wastewater for hydrogen production in one-stage and two-stage processes involving photofermentation. Enzym. Microb. Technol. 2018, 110, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.; Cheng, J.; Ding, L.; Lin, R.; Song, W.; Zhou, J.; Cen, K. Enhancement of energy production efficiency from mixed biomass of Chlorella pyrenoidosa and cassava starch through combined hydrogen fermentation and methanogenesis. Appl. Energy 2014, 120, 23–30. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Rajamehala, M.; Karthigadevi, G.; Praveenkumar, R.; Bharathiraja, B. A review on feedstock, pretreatment methods, influencing factors, production and purification processes of bio-hydrogen production. Case Stud. Chem. Environ. Eng. 2020, 2, 100038. [Google Scholar] [CrossRef]
- Parmar, K.R.; Tuli, V.; Caiola, A.; Hu, J.; Wang, Y. Sustainable production of hydrogen and carbon nanotubes/nanofibers from plastic waste through microwave degradation. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Banu, J.R.; Chang, J.-S. Chapter 10—Biohydrogen Production from Renewable Biomass Resources. In Biohydrogen, 2nd ed.; Ashok Pandey, S., Mohan, V., Chang, J.-S., Hallenbeck, P.C., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Kumar, J.A.; Sathish, S.; Prabu, D.; Renita, A.A.; Saravanan, A.; Deivayanai, V.C.; Anish, M.; Jayaprabakar, J.; Baigenzhenov, O.; Hosseini-Bandegharaei, A. Agricultural waste biomass for sustainable bioenergy production: Feedstock, characterization and pre-treatment methodologies. Chemosphere 2023, 331, 138680. [Google Scholar] [CrossRef]
- Chang, K.-L.; Lin, Y.-C.; Shangdiar, S.; Chen, S.-C.; Hsiao, Y.-H. Hydrogen production from dry spirulina algae with downstream feeding in microwave plasma reactor assisted under atmospheric pressure. J. Energy Inst. 2020, 93, 1597–1601. [Google Scholar] [CrossRef]
- Acosta, N.; De Vrieze, J.; Sandoval, V.; Sinche, D.; Wierinck, I.; Rabaey, K. Cocoa residues as viable biomass for renewable energy production through anaerobic digestion. Bioresour. Technol. 2018, 265, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.S.; Khoo, K.S.; Show, P.-L.; Carolin, C.F.; Jackulin, C.F.; Jeevanantham, S.; Karishma, S.; Show, K.-Y.; Lee, D.-J.; et al. Biohydrogen from organic wastes as a clean and environment-friendly energy source: Production pathways, feedstock types, and future prospects. Bioresour. Technol. 2021, 342, 126021. [Google Scholar] [CrossRef] [PubMed]
- Rambabu, K.; Show, P.-L.; Bharath, G.; Banat, F.; Naushad, M.; Chang, J.-S. Enhanced biohydrogen production from date seeds by Clostridium thermocellum ATCC 27405. Int. J. Hydrogen Energy 2020, 45, 22271–22280. [Google Scholar] [CrossRef]
- Xie, L.; Dong, N.; Wang, L.; Zhou, Q. Thermophilic hydrogen production from starch wastewater using two-phase sequencing batch fermentation coupled with UASB methanogenic effluent recycling. Int. J. Hydrogen Energy 2014, 39, 20942–20949. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Leu, H.-J.; Lee, K.-H. Hydrogen production from beverage wastewater via dark fermentation and room-temperature methane reforming. Int. J. Hydrogen Energy 2016, 41, 21736–21746. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, A.; Long, Y.; Li, Q.; Zhang, Y. An overview of characteristics of municipal solid waste fuel in China: Physical, chemical composition and heating value. Renew. Sustain. Energy Rev. 2014, 36, 107–122. [Google Scholar] [CrossRef]
- Khandelwal, H.; Dhar, H.; Thalla, A.K.; Kumar, S. Application of life cycle assessment in municipal solid waste management: A worldwide critical review. J. Clean. Prod. 2019, 209, 630–654. [Google Scholar] [CrossRef]
- Salem, K.S.; Clayson, K.; Salas, M.; Haque, N.; Rao, R.; Agate, S.; Singh, A.; Levis, J.W.; Mittal, A.; Yarbrough, J.M. A critical review of existing and emerging technologies and systems to optimize solid waste management for feedstocks and energy conversion. Matter 2023, 6, 3348–3377. [Google Scholar] [CrossRef]
- Hannan, M.A.; Begum, R.A.; Al-Shetwi, A.Q.; Ker, P.J.; Al Mamun, M.A.; Hussain, A.; Hassan, B.; Mahlia, T.M.I. Waste collection route optimisation model for linking cost saving and emission reduction to achieve sustainable development goals. Sustain. Cities Soc. 2020, 62, 102393. [Google Scholar] [CrossRef]
- Gomes, A.P.; Matos, M.A.; Carvalho, I.C. Separate collection of the biodegradable fraction of MSW: An economic assessment. Waste Manag. 2008, 28, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.; Mishra, B.P.; Parida, K. A review on dimensionally controlled synthesis of g-C3N4 and formation of an isotype heterojunction for photocatalytic hydrogen evolution. Catal. Sci. Technol. 2021, 11, 7505–7524. [Google Scholar] [CrossRef]
- Wang, W.; Niu, Q.; Zeng, G.; Zhang, C.; Huang, D.; Shao, B.; Zhou, C.; Yang, Y.; Liu, Y.; Guo, H.; et al. 1D porous tubular g-C3N4 capture black phosphorus quantum dots as 1D/0D metal-free photocatalysts for oxytetracycline hydrochloride degradation and hexavalent chromium reduction. Appl. Catal. B Environ. 2020, 273, 119051. [Google Scholar] [CrossRef]
- Tahir, W.; Cheang, T.-Y.; Li, J.-H.; Ling, C.; Lu, X.-J.; Ullah, I.; Wang, G.; Xu, A.-W. Interfacial Ti≣N bonding of a g-C3N4/TiH1.92 type-II heterojunction photocatalyst significantly enhanced photocatalytic hydrogen evolution from water splitting. Catal. Sci. Technol. 2022, 12, 2023–2029. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Lv, X.; Zheng, S.; Wang, J.; Li, Z. Controllable synthesis of MOFs-derived porous and tubular bimetallic Fe–Ni phosphides for efficient electrocatalytic water splitting. Catal. Sci. Technol. 2023, 13, 1512–1517. [Google Scholar] [CrossRef]
- Zhou, Z.; Jia, Y.; Wang, Q.; Jiang, Z.; Xiao, J.; Guo, L. Recent Progress on Molybdenum Carbide-Based Catalysts for Hydrogen Evolution: A Review. Sustainability 2023, 15, 14556. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.X.; Li, B.; Zhu, H.M.; Shi, J.L. 3D interconnected nanoporous Ta3N5 films for photoelectrochemical water splitting: Thickness-controlled synthesis and insights into stability. Sci. China Mater. 2021, 64, 1876–1888. [Google Scholar] [CrossRef]
- Vadalà, M.; Kröll, E.; Küppers, M.; Lupascu, D.C.; Brunstermann, R. Hydrogen production via dark fermentation by bacteria colonies on porous PDMS-scaffolds. Int. J. Hydrogen Energy 2023, 48, 25274–25284. [Google Scholar] [CrossRef]
- Rao, R.; Basak, N. Optimization and modelling of dark fermentative hydrogen production from cheese whey by Enterobacter aerogenes 2822. Int. J. Hydrogen Energy 2021, 46, 1777–1800. [Google Scholar] [CrossRef]
- Gupta, H.; Barua, M.K. Identifying enablers of technological innovation for Indian MSMEs using best–worst multi criteria decision making method. Technol. Forecast. Soc. Change 2016, 107, 69–79. [Google Scholar] [CrossRef]
- Chen, H.; Wu, J.; Huang, R.; Zhang, W.; He, W.; Deng, Z.; Han, Y.; Xiao, B.; Luo, H.; Qu, W. Effects of temperature and total solid content on biohydrogen production from dark fermentation of rice straw: Performance and microbial community characteristics. Chemosphere 2022, 286, 131655. [Google Scholar] [CrossRef] [PubMed]
- Karadag, D.; Jaakko, A. Puhakka. Effect of changing temperature on anaerobic hydrogen production and microbial community composition in an open-mixed culture bioreactor. Int. J. Hydrogen Energy 2010, 35, 10954–10959. [Google Scholar] [CrossRef]
- Mu, Y.; Zheng, X.-J.; Yu, H.-Q.; Zhu, R.-F. Biological hydrogen production by anaerobic sludge at various temperatures. Int. J. Hydrogen Energy 2006, 31, 780–785. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Liu, H. Effect of pH on hydrogen production from glucose by a mixed culture. Bioresour. Technol. 2002, 82, 87–93. [Google Scholar] [CrossRef]
- Khanal, S.K.; Chen, W.-H.; Li, L.; Sung, S. Biological hydrogen production: Effects of pH and intermediate products. Int. J. Hydrogen Energy 2004, 29, 1123–1131. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Choi, J.-A.; Abou-Shanab, R.A.I.; Bhatnagar, A.; Min, B.; Song, H.; Kumar, E.; Choi, J.; Lee, E.S.; Kim, Y.J.; et al. Effect of pH and sulfate concentration on hydrogen production using anaerobic mixed microflora. Int. J. Hydrogen Energy 2009, 34, 9702–9710. [Google Scholar] [CrossRef]
- Molina-Peñate, E.; Artola, A.; Sánchez, A. Organic municipal waste as feedstock for biorefineries: Bioconversion technologies integration and challenges. Rev. Environ. Sci. Bio/Technol. 2022, 21, 247–267. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Show, K.Y.; Tay, J.H.; Liang, D.T.; Lee, D.J.; Jiang, W.J. Effect of hydraulic retention time on biohydrogen production and anaerobic microbial community. Process Biochem. 2006, 41, 2118–2123. [Google Scholar] [CrossRef]


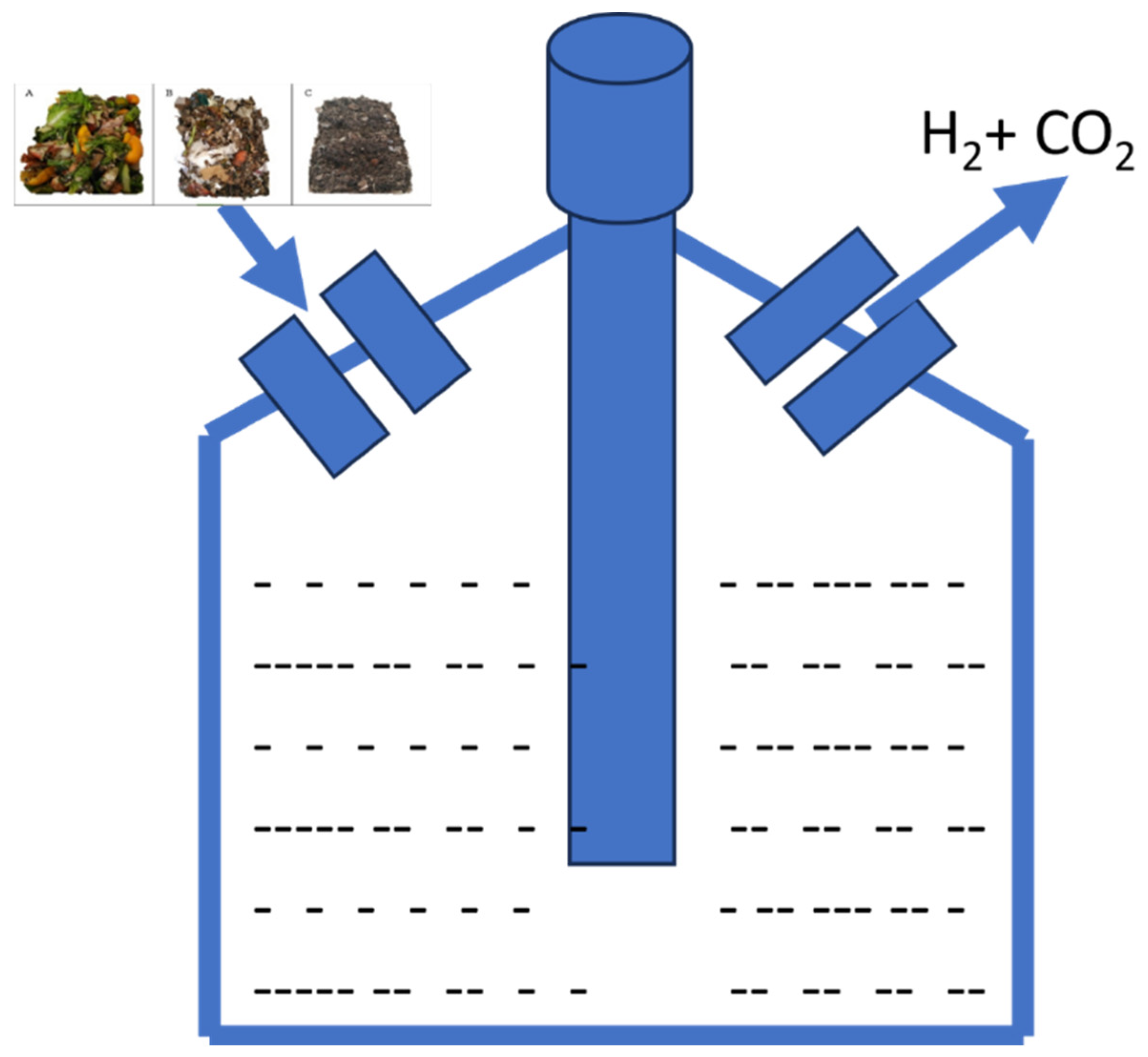
| Waste Type | Microorganism | Hydrogen Yield | Reference |
|---|---|---|---|
| Wastewater | Mixed culture | 1.6 ± 0.3 L/L | [23] |
| Agricultural waste | Mixed culture | 71.8 ± 5.19 mL/g | [24] |
| Food waste | Mixed culture | Controlled at 4.0–4.6 219.9 mL/g | [25] |
| Plastic waste | - | >92 vol% | [26] |
| Fruit and vegetable waste | Mixed culture | 3.46 mol/mol total sugar | [27] |
| Cone | Mixed culture | 107.7 kg/t-bio | [28] |
| Micro algae | Mixed culture | 31–36% | [29] |
| Cocoa waste | - | 107 L kg−1 | [30] |
| Pine tree (saw dust) | - | 0.1–0.4% | [28] |
| Palm oil mill effluent | Mixed culture | 108.35 mL/g –Reducing sugars | [31] |
| Date seed waste | Mixed culture | 103.97 mmol/L glucose | [32] |
| Starch wastewater | Mixed culture | 5.79 mmol/g—COD | [31,33] |
| Beverage wastewater | - | 1.53 mol/mol- hexose | [31,34] |
| OFMSW, including paper, cardboard | No external inoculum | 57.3 mL/g | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, N.; Azad, A.K. Comparative Study of Hydrogen Production from Organic Fraction of Municipal Solid Waste and Its Challenges: A Review. Energies 2023, 16, 7853. https://doi.org/10.3390/en16237853
Haque N, Azad AK. Comparative Study of Hydrogen Production from Organic Fraction of Municipal Solid Waste and Its Challenges: A Review. Energies. 2023; 16(23):7853. https://doi.org/10.3390/en16237853
Chicago/Turabian StyleHaque, Naimul, and Abul Kalam Azad. 2023. "Comparative Study of Hydrogen Production from Organic Fraction of Municipal Solid Waste and Its Challenges: A Review" Energies 16, no. 23: 7853. https://doi.org/10.3390/en16237853
APA StyleHaque, N., & Azad, A. K. (2023). Comparative Study of Hydrogen Production from Organic Fraction of Municipal Solid Waste and Its Challenges: A Review. Energies, 16(23), 7853. https://doi.org/10.3390/en16237853







