Ionic Storage Materials for Anodic Discoloration in Electrochromic Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Films
2.2. Fabrication of Electrochromic Devices (ECDs)
2.3. Experiment Details
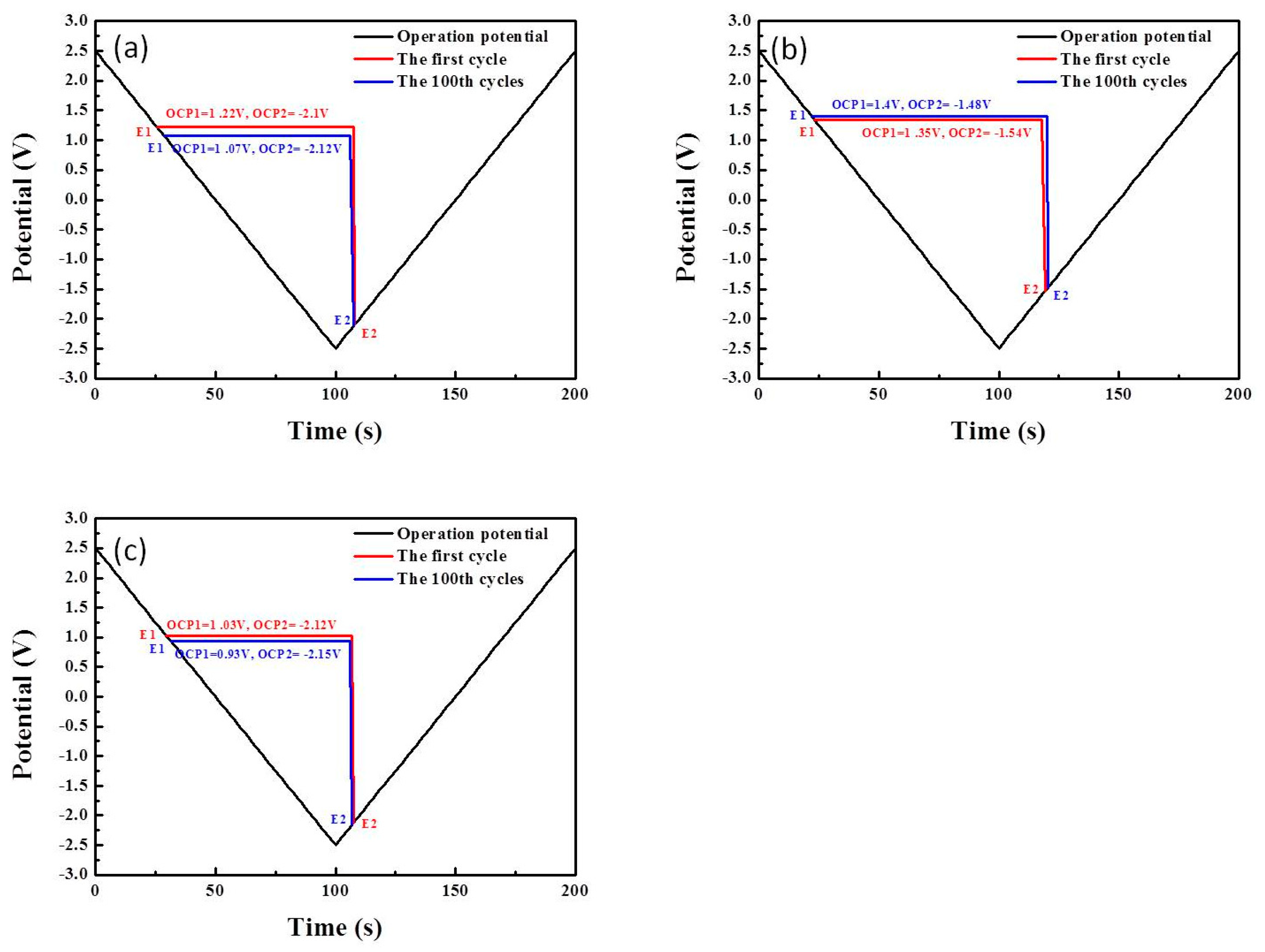
3. Results
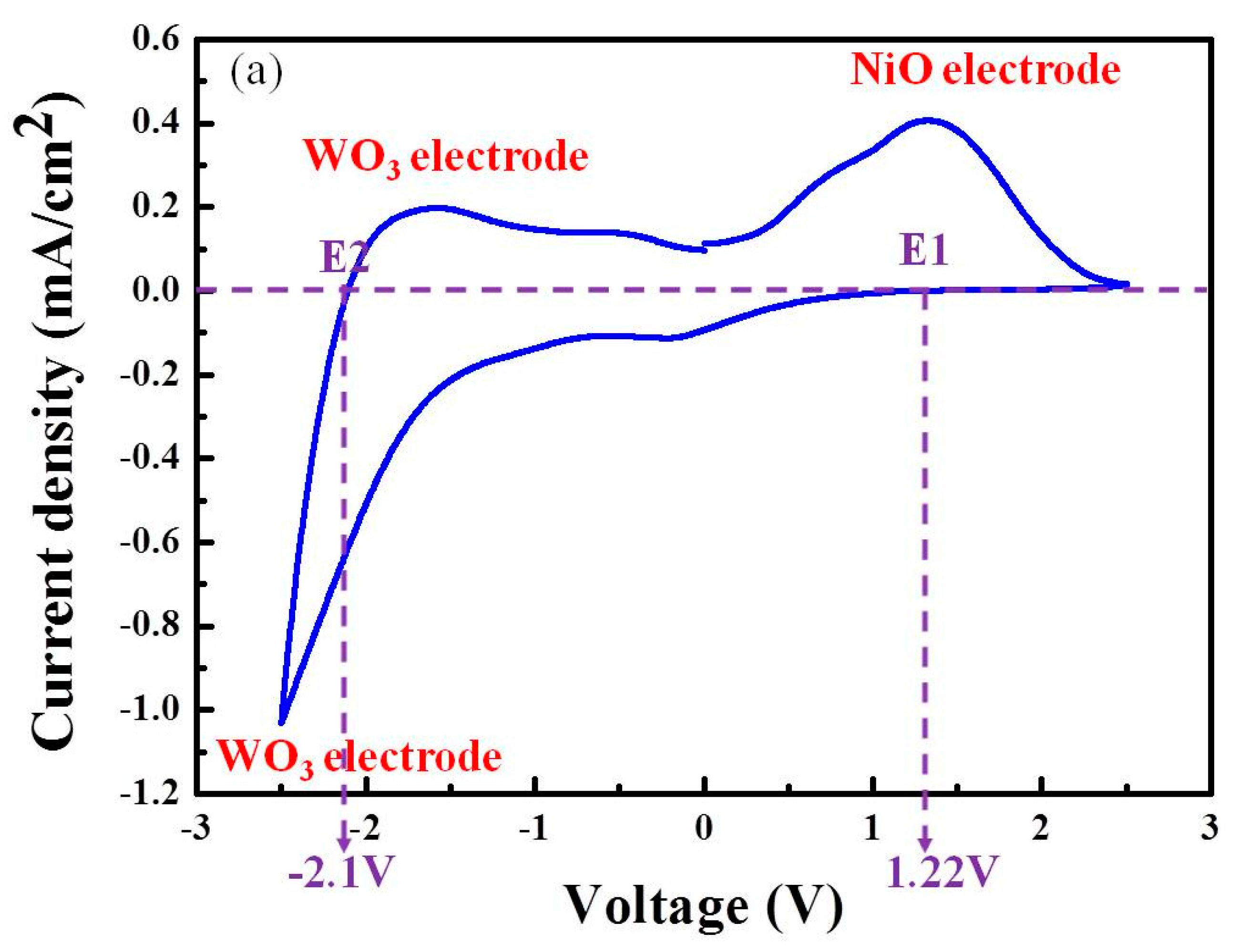
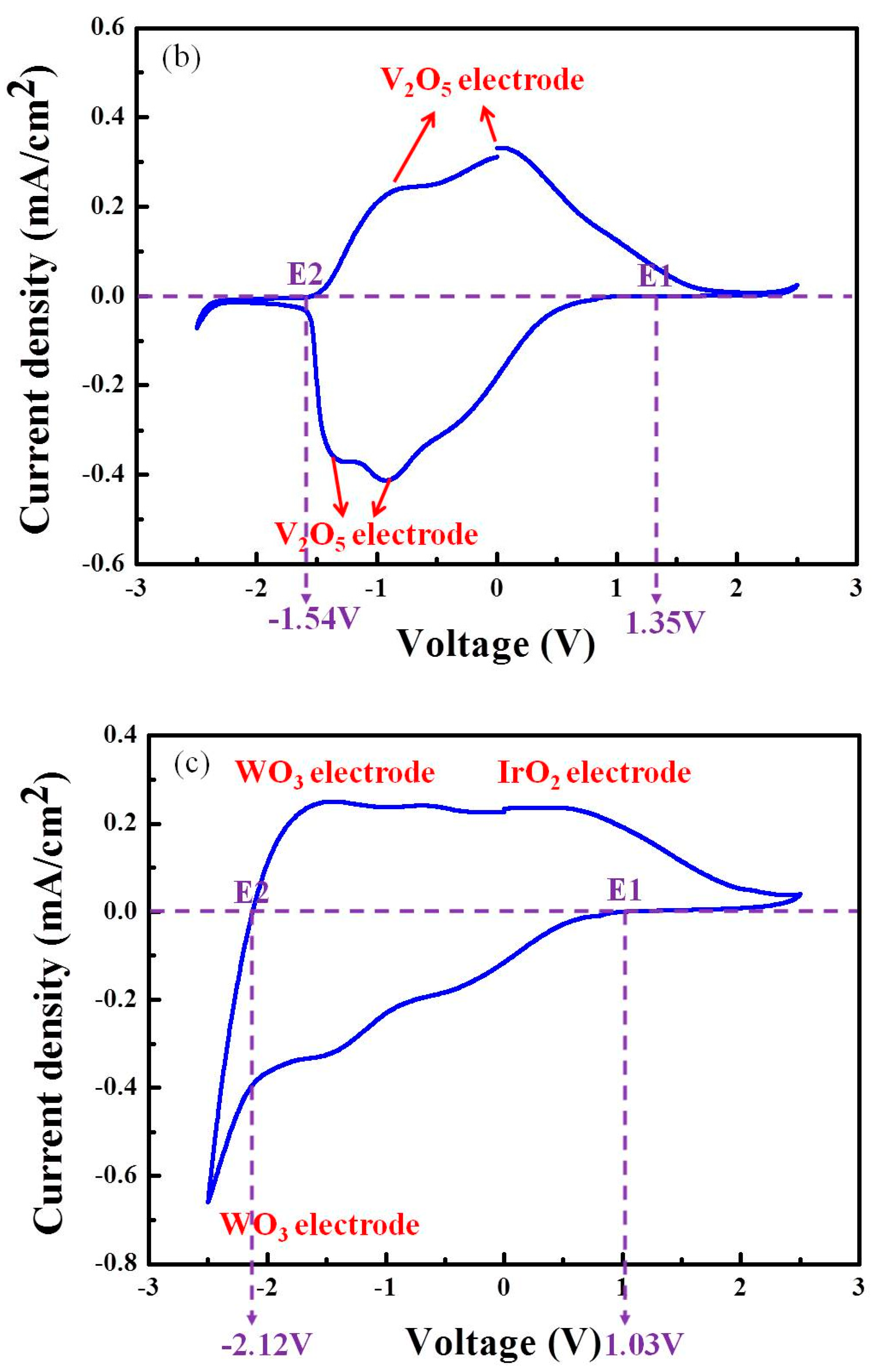
3.1. Mechanisms Underlying the Operations of Electrochromic Devices (ECD) Based on Counter Layers of NiO, V2O5, or IrO2
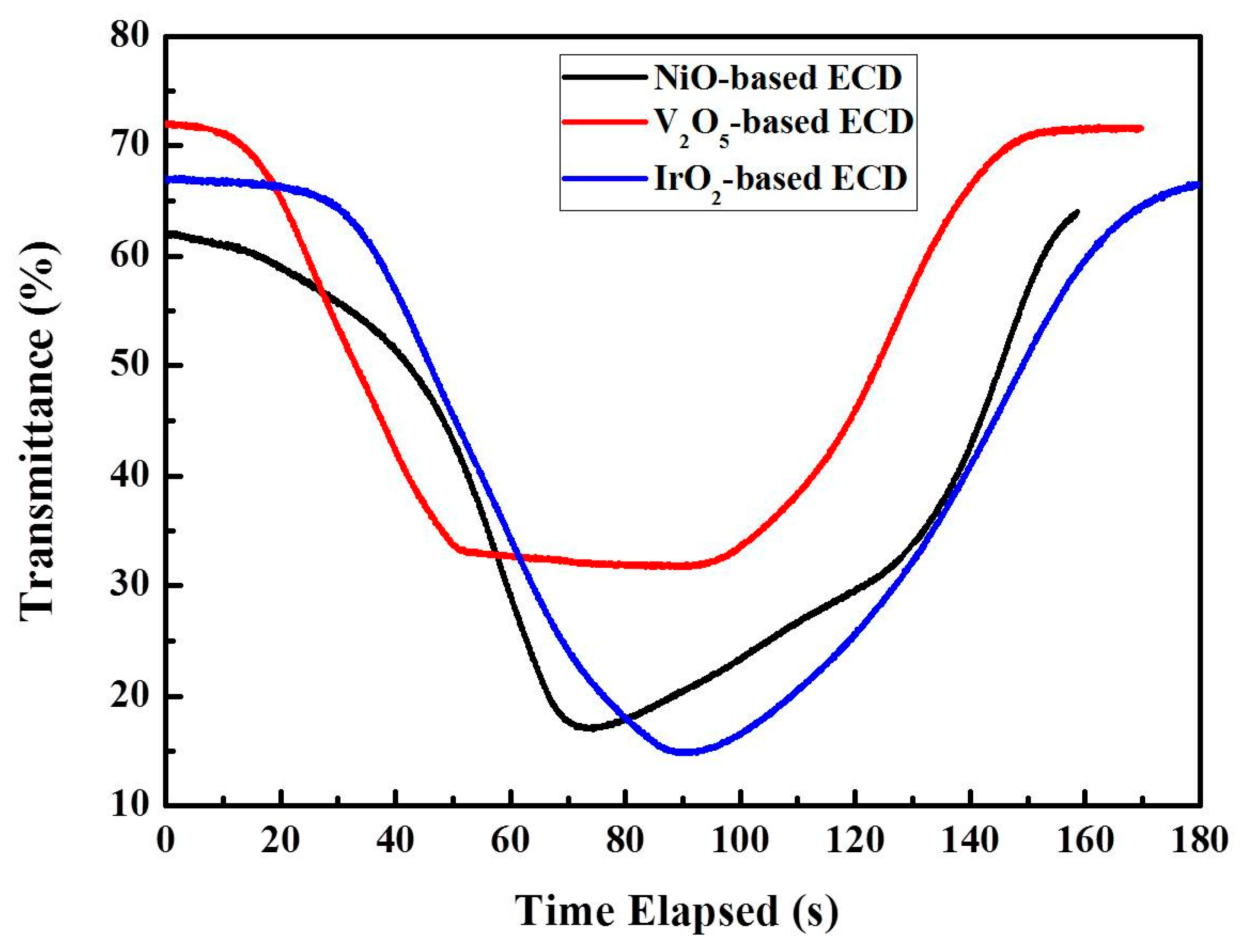
3.2. Electrochemical and Optical Properties as Functions of Counter Layer Types (NiO, V2O5, IrO2)
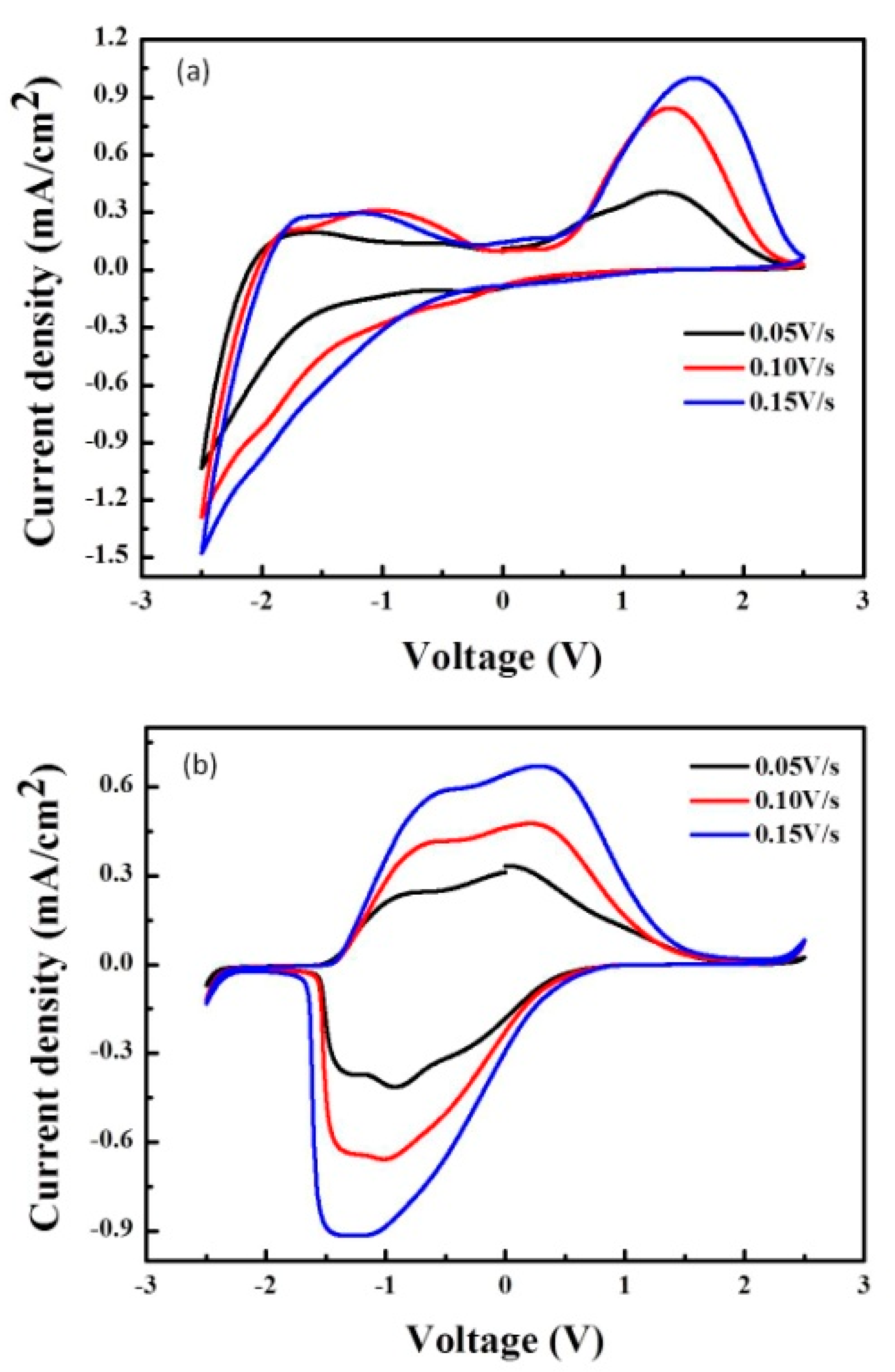
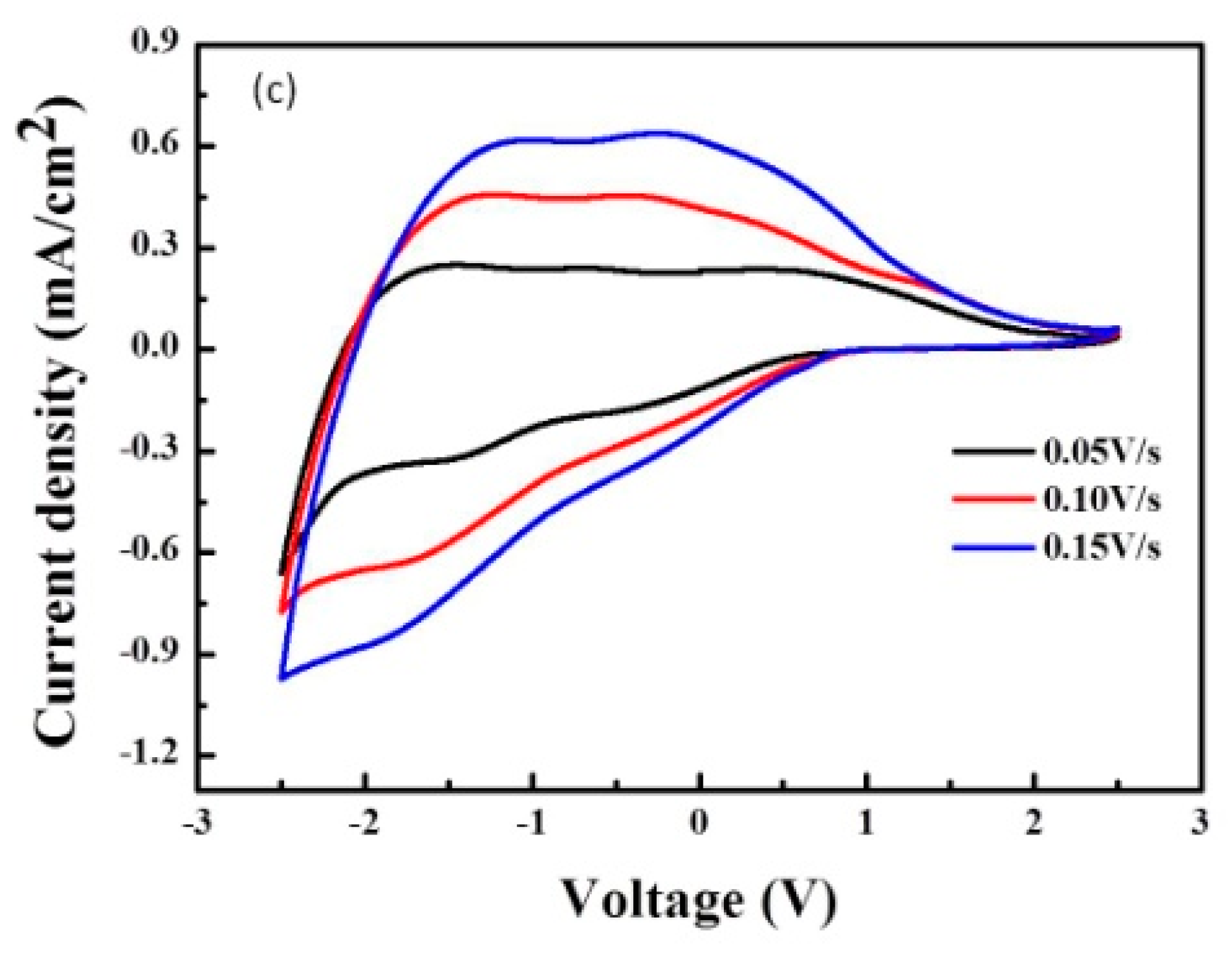
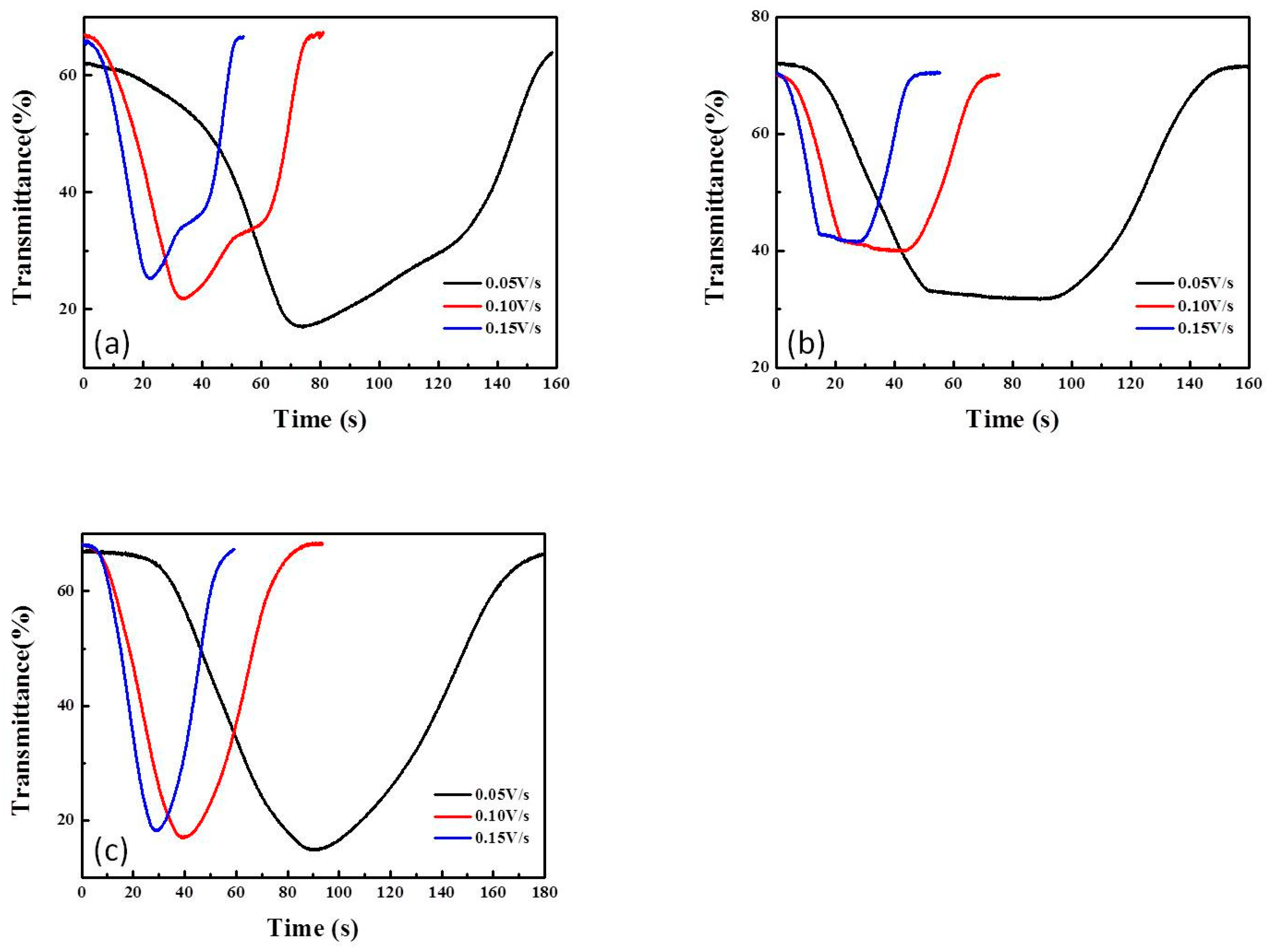
3.3. CV Curves of ECDs as a Function of Scanning Rate
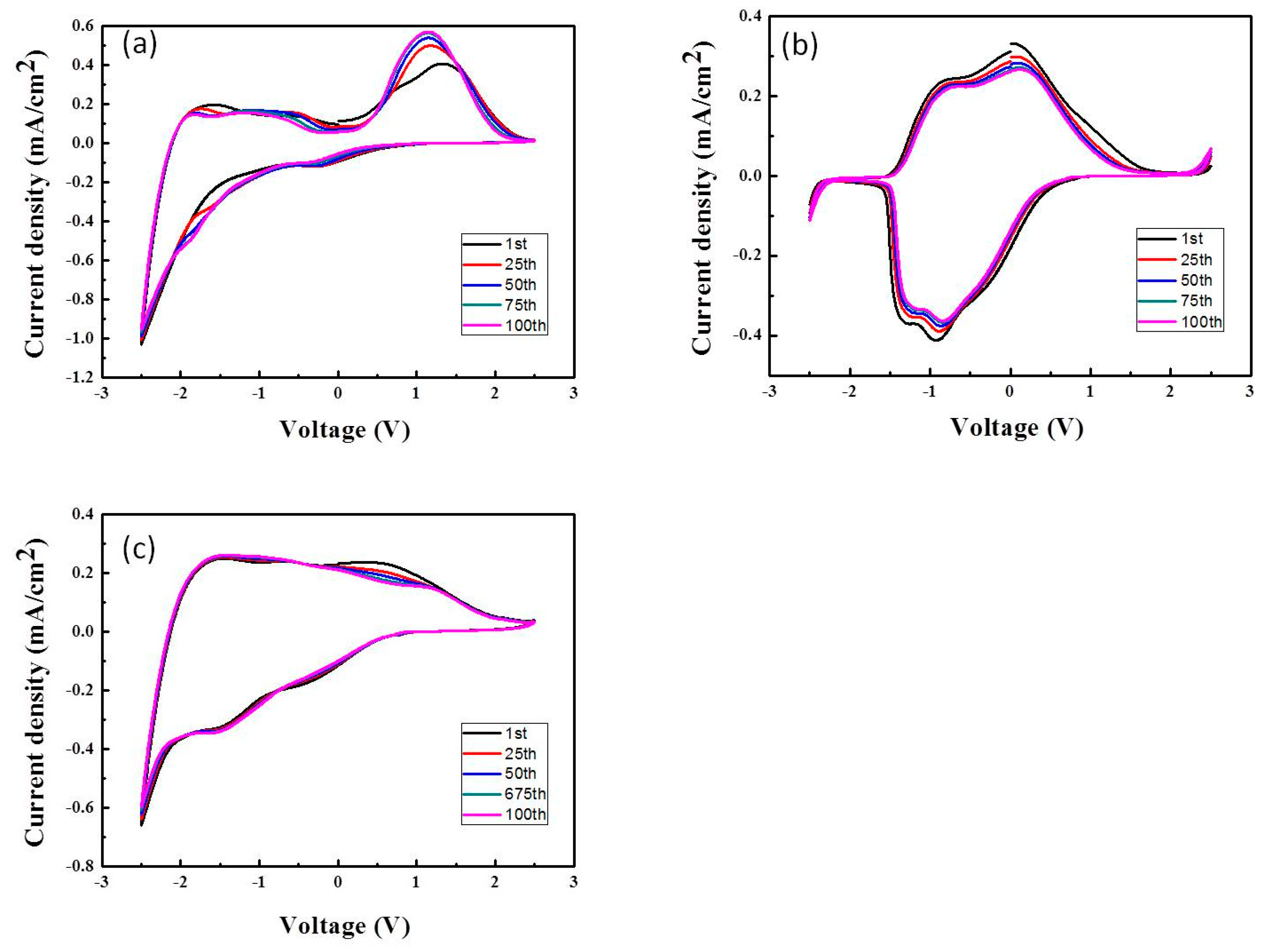
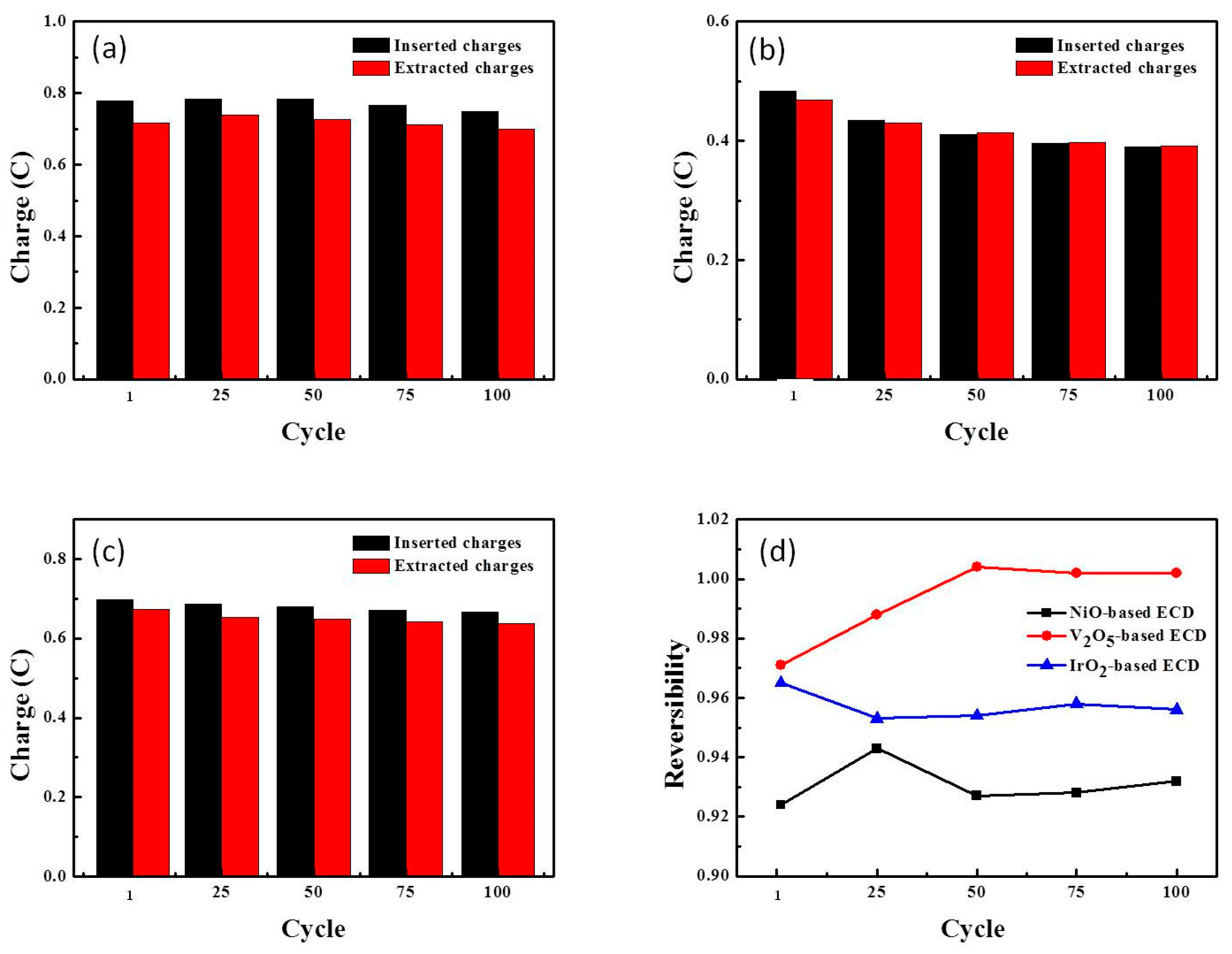
3.4. ECD Performance: Long-Term Stability and Durability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Granqvist, C.G. Electrochromics for smart windows: Oxide-based thin films and devices. Thin Solid Films 2014, 564, 1–38. [Google Scholar] [CrossRef]
- Chang, J.Y.; Chen, Y.C.; Wang, C.M.; Wang, W.N.; Wen, C.Y.; Lin, J.M. Electrochromic properties of Lithium-doped tungsten oxide prepared by electron beam evaporation. Coatings 2019, 9, 191. [Google Scholar] [CrossRef]
- Kim, K.H.; Koo, B.R.; Ahn, H.J. Sheet resistance dependence of fluorine-doped tin oxide films for high-performance electrochromic devices. Ceram. Int. 2018, 44, 9408–9413. [Google Scholar] [CrossRef]
- Jiang, B.; Lou, B.; Li, J.; Peng, P.; Chen, J.W.; Chu, L.H.; Li, Y.F.; Li, M.C. Electrochemical effect of graphite fluoride modification on Li-rich cathode material in lithium ion battery. Ceram. Int. 2019, 45, 160–167. [Google Scholar] [CrossRef]
- Yiying, H.; Zhuo, G.; Yufei, P.; Peng, G.; Yu, P.; Jiayu, T.; Xiao-Peng, Z.; Jian, L. Molecular engineering of π-extended viologens with four arms for efficient and tunable electrochromic devices. Dye. Pigment. 2023, 219, 111556. [Google Scholar]
- Yu, P.; Peng, G.; Hongjin, C.; Xiao-Peng, Z.; Yiying, H.; Zhuo, G.; Jinkun, X.; Rui, Z.; Jian, L. Electropolymerization of D-A type monomers consisting of mono-triphenylamine moiety for electrochromic devices and supercapacitors. J. Mol. Struct. 2023, 1292, 136182. [Google Scholar]
- Jiayu, T.; Hongjin, C.; Yiying, H.; Lang, P.; Xiao-Peng, Z.; Sheng, P.; Zhirong, W.; Haitao, L.; Jian, L. Electropolymerization of D-A-D type triphenlyamine-based monomers consisting of camphor substituted quinoxaline unit for efficient electrochromism and supercapacitors. Polymer 2023, 285, 126386. [Google Scholar]
- Phan, G.T.; Pham, D.V.; Patil, R.A.; Tsai, C.H.; Lai, C.C.; Yeh, W.C.; Liou, Y.; Ma, Y.R. Fast-switching electrochromic smart windows based on NiO-nanorods counter electrode. Sol. Energy Mater. Sol. Cells 2021, 231, 111306. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Yu, W.W.; Elezzabi, A.Y. Excitonic complexes and optical gain in two-dimensional molybdenum ditelluride well below the Mott transition. Light Sci. Appl. 2020, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Oh, Y.; Yoon, I.S.; Kim, S.H.; Ju, B.K.; Hong, J.M. Flash-induced nanowelding of silver nanowire networks for transparent stretchable electrochromic devices. Sci. Rep. 2018, 8, 2763. [Google Scholar] [CrossRef] [PubMed]
- Granqvist, C.G.; Arvizu, M.A.; Pehlivan, I.B.; Qu, H.Y.; Wen, R.T.; Niklasson, G.A. Electrochromic materials and devices for energy efficiency and human comfort in buildings: A critical review. Electrochim. Acta 2018, 259, 1170. [Google Scholar] [CrossRef]
- Wei, Y.X.; Ma, Y.B.; Chen, M.; Liu, W.M.; Li, L.; Yan, Y. Electrochemical investigation of electrochromic device based on WO3 and Ti doped V2O5 films by using electrolyte containing ferrocene. J. Electroanal. Chem. 2017, 807, 45–51. [Google Scholar] [CrossRef]
- Niklasson, G.A.; Granqvist, C.G. Sol-gel fabrication of NiO and NiO/WO3 based electrochromic device on ITO and flexible substrate. Ceram. Int. 2020, 46, 8631–8639. [Google Scholar]
- Chang, C.C.; Chi, P.W.; Chandan, P.; Lin, C.K. Electrochemistry and Rapid ElectrochromismControl of MoO3/V2O5 Hybrid Nano bilayers. Nat. Mater. 2019, 12, 2475. [Google Scholar] [CrossRef]
- Ding, J.; Abbas, S.A.; Hanmandlu, C.; Lin, L.; Lai, C.S.; Wang, P.C.; Li, L.J.; Chu, C.W.; Chang, C.C. Facile synthesis of carbon/MoO3 nanocomposites as stable battery anodes. J. Power Sources 2017, 348, 270. [Google Scholar] [CrossRef]
- Akkurt, N.; Pat, S.; Mohammadigharehbagh, R.; Özgür, M.; Demirkol, U.; Olkun, A.; Korkmaz, Ş. Investigation of TiO2 thin films as a cathodic material for electrochromic display devices. J. Mater. Sci. 2020, 31, 9568–9578. [Google Scholar] [CrossRef]
- Li, H.; McRae, L.; Elezzabi, A.Y. Solution-processed interfacial PEDOT: PSS assembly into porous tungsten molybdenum oxide nanocomposite films for electrochromic applications. ACS Appl. Mater. Interfaces 2018, 10, 10520–10527. [Google Scholar] [CrossRef]
- Eren, E.; Alver, C.; Karaca, G.Y.; Uygun, E.; Oksuz, L.; Oksuz, A.Y. High-performance Flexible Complementary Electrochromic Device Based on Plasma Modified WO3 Nano Hybrids and V2O5 Nanofilm with Low Operation Voltages. Electroanalysis 2018, 30, 2099–2109. [Google Scholar] [CrossRef]
- Kim, C.-Y.; Cho, S.-G.; Lim, T.-Y. Cycle test and degradation analysis of WO3/PC+LiClO4/CeO2·TiO2 electrochromic device. Sol. Energy Mater. Sol. Cells 2009, 93, 2056–2061. [Google Scholar] [CrossRef]
- Dong, D.; Wang, W.; Rougier, A.; Barnabé, A.; Dong, G.; Zhang, F.; Diao, X. Lithium trapping as a degradation mechanism of the electrochromic properties of all-solid-state WO3//NiO devices. J. Mater. Chem. C 2018, 6, 9875. [Google Scholar] [CrossRef]
- Wen, R.-T.; Granqvist, C.G.; Niklasson, G.A. Eliminating degradation and uncovering ion-trapping dynamics in electrochromic WO3 thin films. Nat. Mater. 2015, 14, 996. [Google Scholar] [CrossRef]
- Wen, R.-T.; Arvizu, M.A.; Morales-Luna, M.; Granqvist, C.G.; Niklasson, G.A. Ion Trapping and Detrapping in Amorphous Tungsten Oxide Thin Films Observed by Real-Time Electro-Optical Monitoring. Chem. Mater. 2016, 28, 4670. [Google Scholar] [CrossRef]
- Baloukas, B.; Arvizu, M.A.; Wen, R.-T.; Niklasson, G.A.; Granqvist, C.G.; Vernhes, R.; Klemberg-Sapieha, J.E.; Martinu, L. Galvanostatic Rejuvenation of Electrochromic WO3 Thin Films: Ion Trapping and Detrapping Observed by Optical Measurements and by Time-of-Flight Secondary Ion Mass Spectrometry. ACS Appl. Mater. Interfaces 2017, 9, 16995. [Google Scholar] [CrossRef]
- Runnerstrom, E.L.; Llordés, A.; Lounisac, S.D.; Milliron, D.J. Nanostructured electrochromic smart windows:traditional materials and NIR-selective plasmonic nanocrystals. Chem. Commun. 2014, 50, 10555–10572. [Google Scholar] [CrossRef]
- Li, H.; McRaea, L.; Firby, C.J.; Hussein, M.A.; Elezzabi, A.Y. Nanohybridization of molybdenum oxide with tungsten molybdenum oxide nanowires for solution-processed fully reversible switching of energy storing smart windows. Nano Energy 2020, 47, 130–139. [Google Scholar] [CrossRef]
- Chen, P.W.; Chang, C.T.; Ko, T.F.; Hsu, S.C.; Li, K.F.; Wu, J.Y. Fast response of complementary electrochromic device based on WO3/NiO electrodes. Sci. Rep. 2020, 10, 8430. [Google Scholar] [CrossRef]
- Li, Y.; McMaster, W.A.; Wei, H.; Chen, D.; Caruso, R.A. Enhanced electrochromic properties of WO3 Nanotree−like structures synthesized via a two-step sol-vothermal process showing promise for electrochromic window application. ACS Appl. 2018, 1, 2552–2558. [Google Scholar]
- Tritschler, U.; Beck, F.; Schlaad, H.; Cölfen, H. Electrochromic properties of self-organized multifunctional V2O5 polymer hybrid films. J. Mater. Chem. C 2015, 3, 950–954. [Google Scholar] [CrossRef]
- Chun, Y.T.; Neeves, M.; Smithwick, Q.; Placido, F.; Chu, D. High optical and switching performance electrochromic devices based on a zinc oxide nanowire with poly (methyl methacrylate) gel electrolytes. Appl. Phys. Lett. 2014, 105, 193301. [Google Scholar] [CrossRef]
- Xia, X.H.; Tu, J.P.; Zhang, J.; Wang, X.L.; Zhang, W.K.; Huang, H. Electrochromic properties of porous NiO thin flms prepared by a chemical bath deposition. Sol. Energy Mater Sol. Cells 2008, 92, 628–633. [Google Scholar] [CrossRef]
- Subrahmanyam, A.; Kumar, C.S.; Karuppasamy, K.M. A note on fast protonic solid state electrochromic device: NiOx/Ta2O5/WO3−x. Sol. Energy Mater Sol. Cells 2007, 91, 62–66. [Google Scholar] [CrossRef]
- Li, K.D.; Chen, P.W.; Chang, K.S.; Hsu, S.C.; Jan, D.J. Indium-Zinc-Tin-Oxide Film Prepared by Reactive Magnetron Sputtering for Electrochromic Applications. Materials 2018, 11, 2221. [Google Scholar] [CrossRef]
- Zhou, K.L.; Wang, H.; Liu, J.; Yan, H. The mechanism of trapped ions eroding the electrochromic performances of WO3 thin films. Int. J. Electrochem. Sci. 2018, 13, 7335. [Google Scholar] [CrossRef]





| Target | Ar/O2 (sccm) | W.P. (mTorr) | DC Power (W) | Deposition Time (min) | Deposition Rate (nm/min) | Deposition Temp (°C) | Thickness (nm) |
|---|---|---|---|---|---|---|---|
| ITO | 1/0 (Ar = 30) | 2.5 | 100 | 10 | 30 | RT | 300 |
| W Metal | 1/3 (Ar = 100) | 8 | 1500 | 15 | 13 | 50 | 200 |
| Ni Metal | 1/3 (Ar = 120) | 8 | 650 | 3 | 20 | 50 | 60 |
| V Metal | 1/3 (Ar = 20) | 2.5 | 1200 | 12 | 5 | 100 | 80 |
| Ir Metal | 1/3 (Ar = 120) | 8 | 650 | 3 | 20 | 50 | 60 |
| OCP1 | OCP2 | |
|---|---|---|
| NiO-based ECD | 1.22 V | −2.10 V |
| V2O5-based ECD | 1.35 V | −1.54 V |
| IrO2-based ECD | 1.03 V | −2.12 V |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-W.; Chang, C.-T.; Kuo, P.-H. Ionic Storage Materials for Anodic Discoloration in Electrochromic Devices. Energies 2023, 16, 8119. https://doi.org/10.3390/en16248119
Chen P-W, Chang C-T, Kuo P-H. Ionic Storage Materials for Anodic Discoloration in Electrochromic Devices. Energies. 2023; 16(24):8119. https://doi.org/10.3390/en16248119
Chicago/Turabian StyleChen, Po-Wen, Chen-Te Chang, and Po-Hsiu Kuo. 2023. "Ionic Storage Materials for Anodic Discoloration in Electrochromic Devices" Energies 16, no. 24: 8119. https://doi.org/10.3390/en16248119
APA StyleChen, P.-W., Chang, C.-T., & Kuo, P.-H. (2023). Ionic Storage Materials for Anodic Discoloration in Electrochromic Devices. Energies, 16(24), 8119. https://doi.org/10.3390/en16248119









