Chemically and Physically Pretreated Straw in Moderate Conditions: Poor Correlation between Biogas Production and Commonly Used Biomass Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
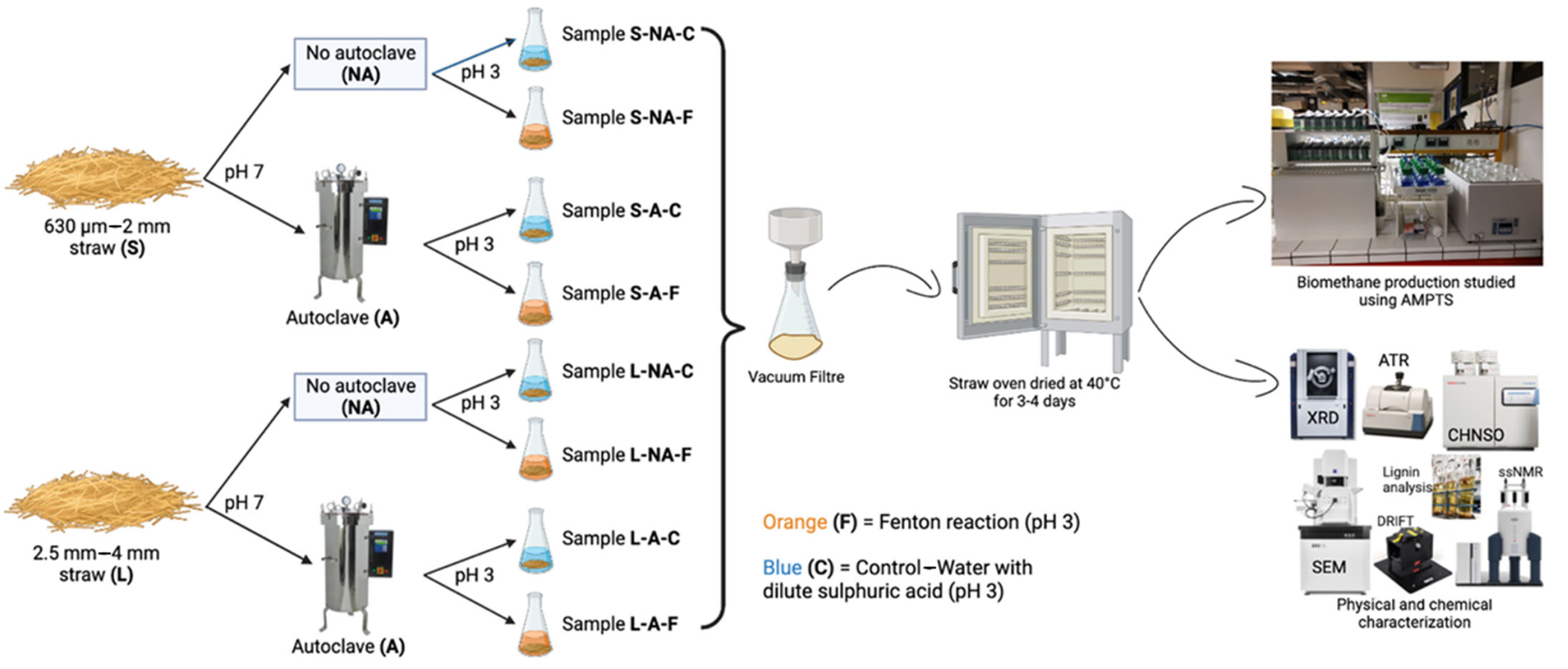
2.2. Pretreatment
2.2.1. Physical Pretreatment
2.2.2. Autoclaving Process
2.2.3. Fenton Pretreatment
2.3. Chemical and Physio-Chemical Characterization
2.3.1. Determination of the Total Solid and Volatile Contents of Samples
2.3.2. CHNSO Elemental Composition
2.3.3. Lignin Composition Analysis
2.4. Biochemical Methane Potential (BMP) Test
2.4.1. Realization of BMP Tests
2.4.2. Statistical Analysis and Kinetics of Biomethane Production
2.5. Advanced Characterization Techniques
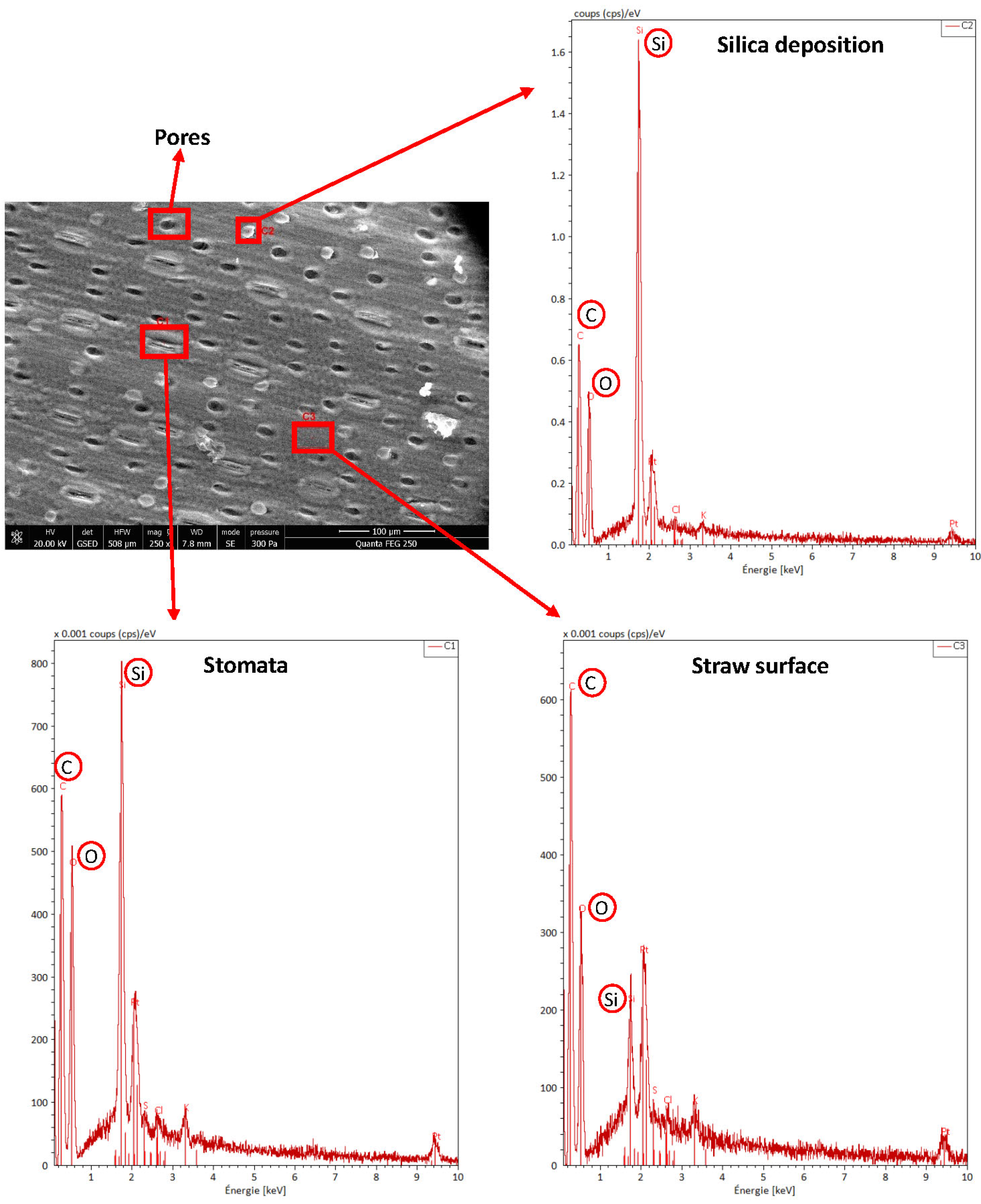
2.5.1. Scanning Electron Microscopy (SEM)—Energy Dispersive Spectroscopy (EDS)
2.5.2. Simons’ Staining
2.5.3. Infrared Spectroscopy
- Diffuse Reflectance (DRIFT)
- Attenuated Total Reflectance (ATR)
2.5.4. Wide-Angle X-ray Diffraction Analysis (WAXD)
2.5.5. Nuclear Magnetic Resonance Spectroscopy (NMR)
3. Results
3.1. Physio-Chemical and Chemical Characterization
3.1.1. TS, VS, and Elemental Composition
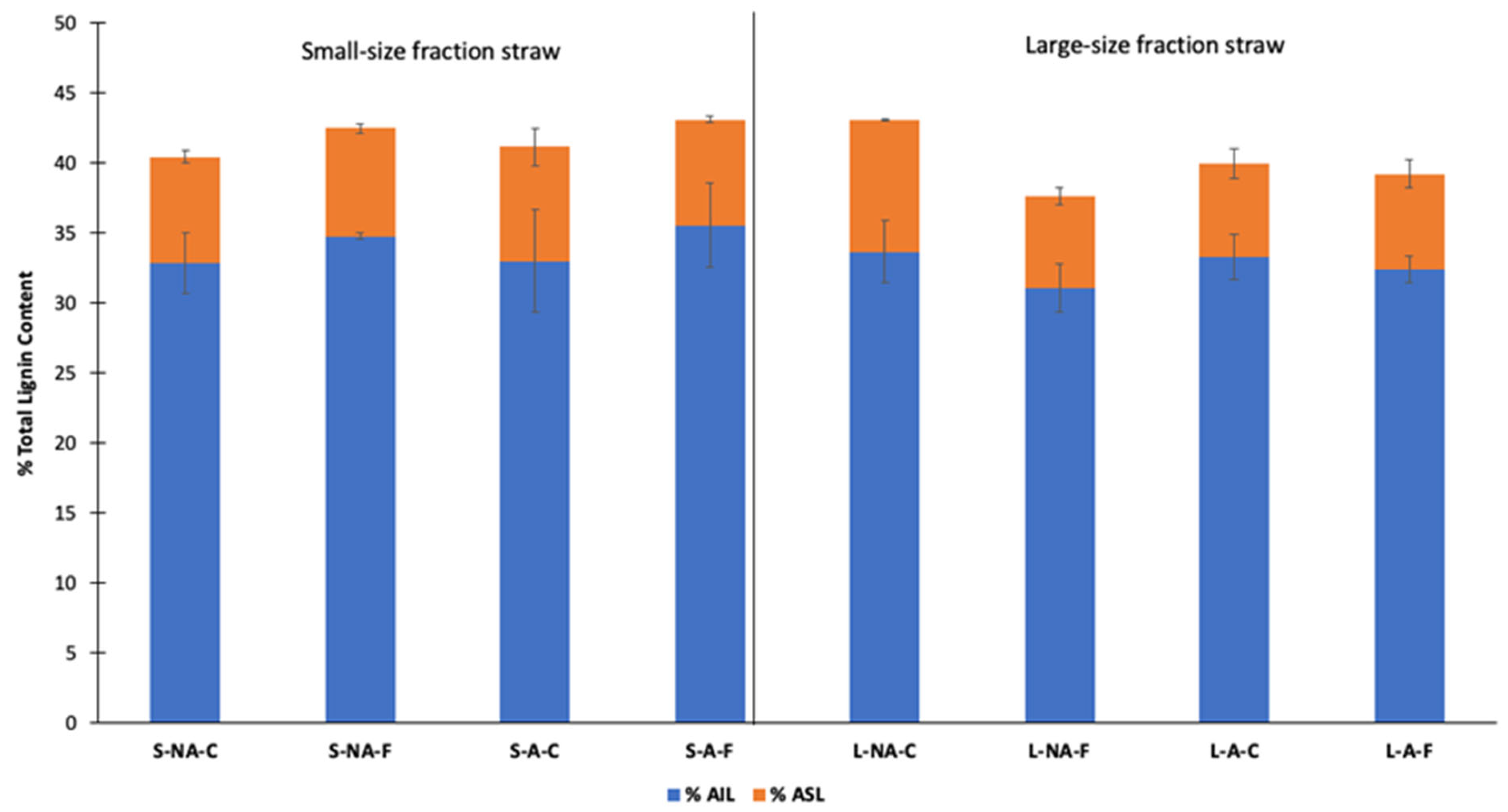
3.1.2. Lignin Content Changes in Straw
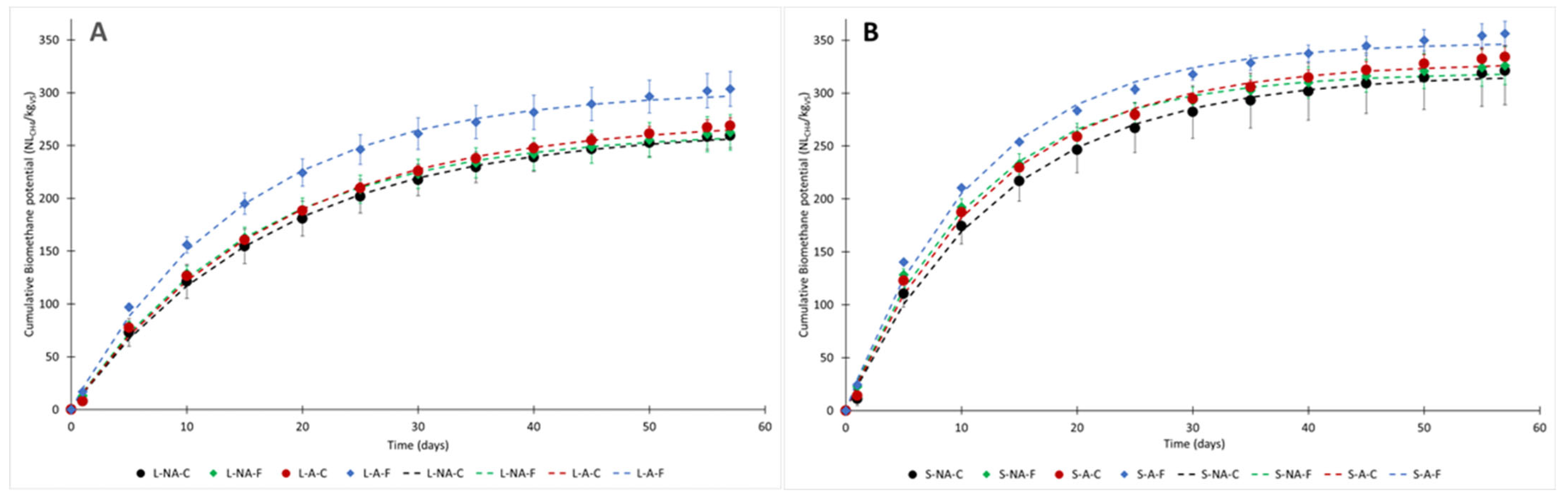
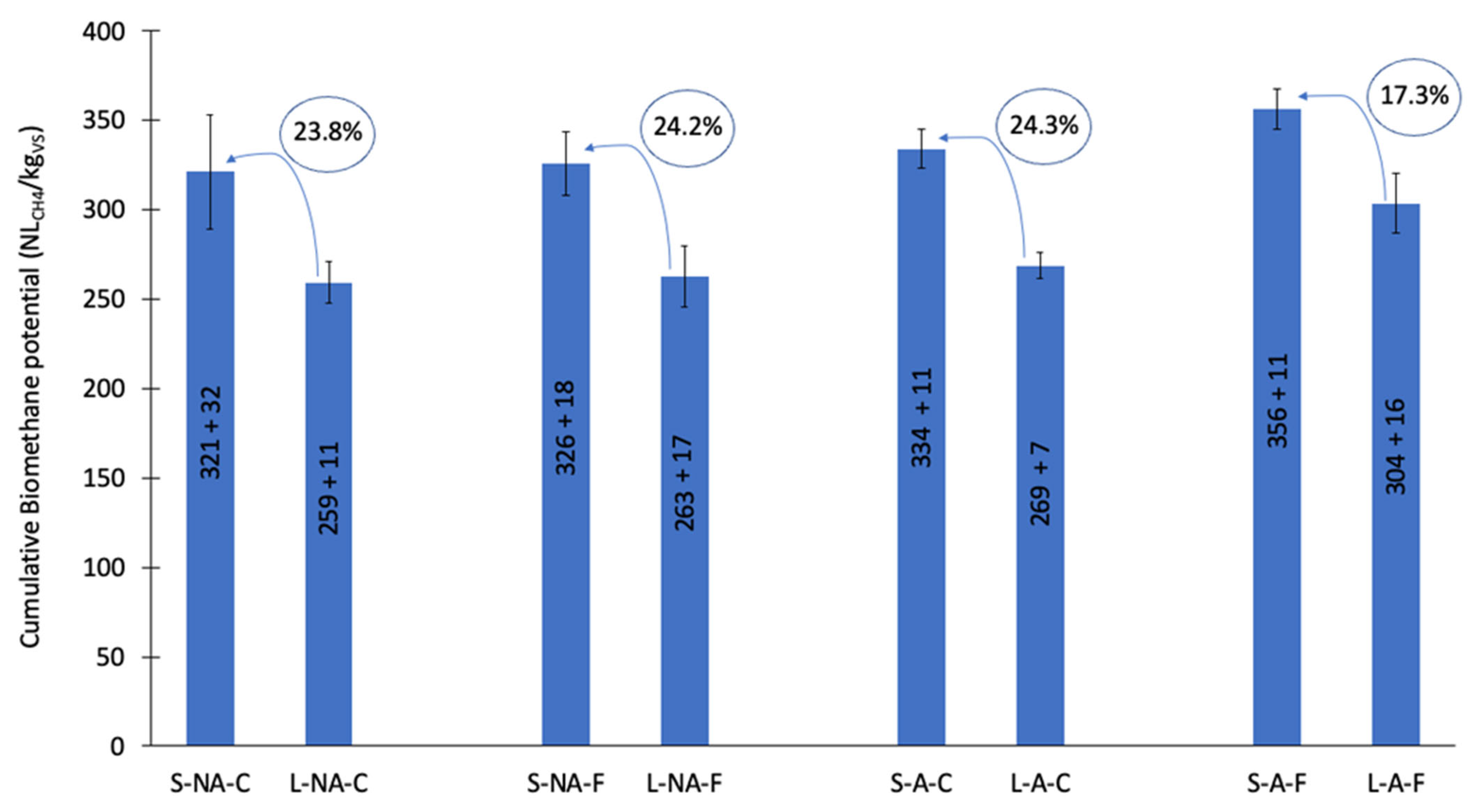
3.2. Biochemical Methane Potential (BMP)
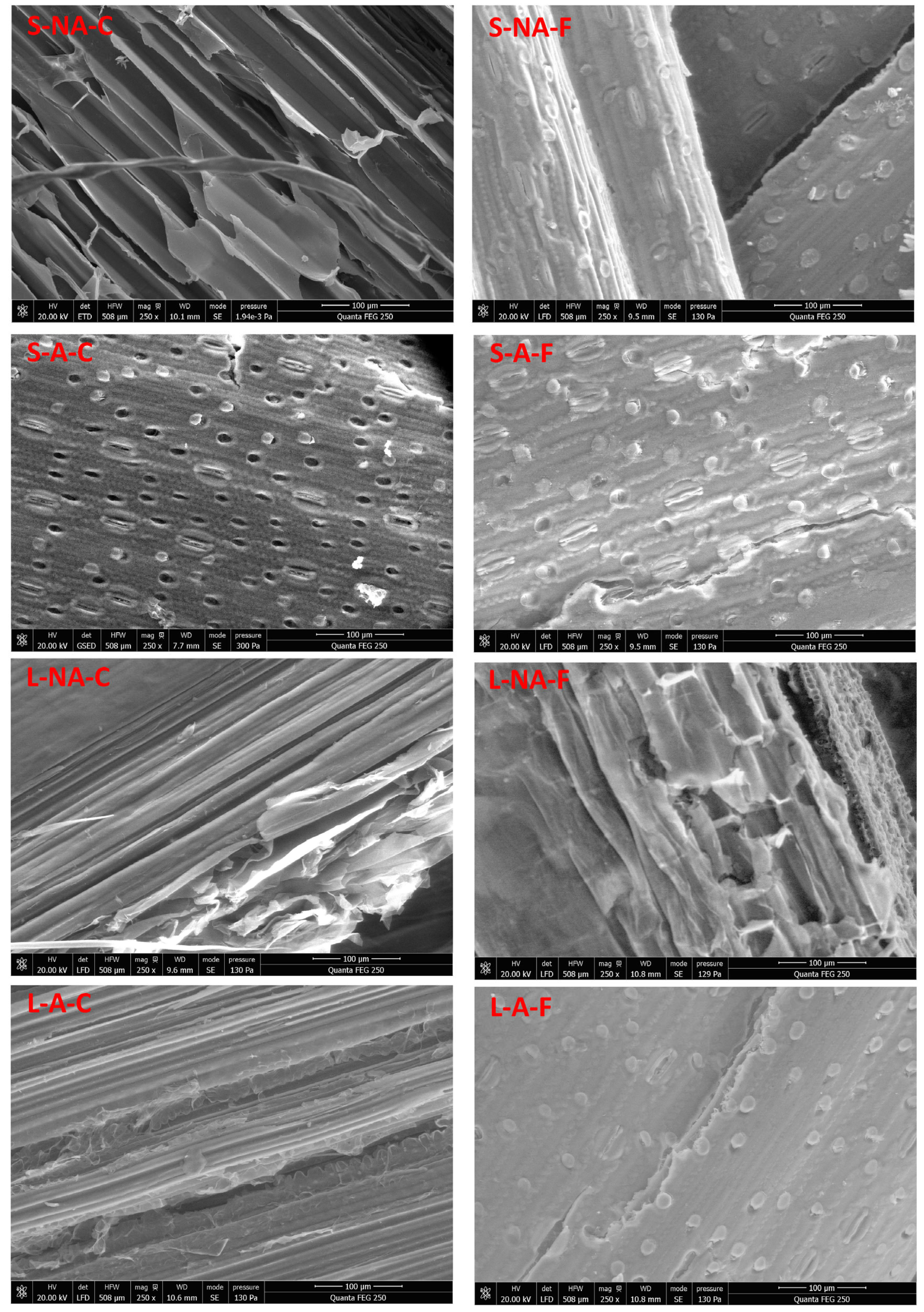
3.3. Physical Characterization of Straw
3.3.1. Surface Morphology Analysis by SEM-EDS
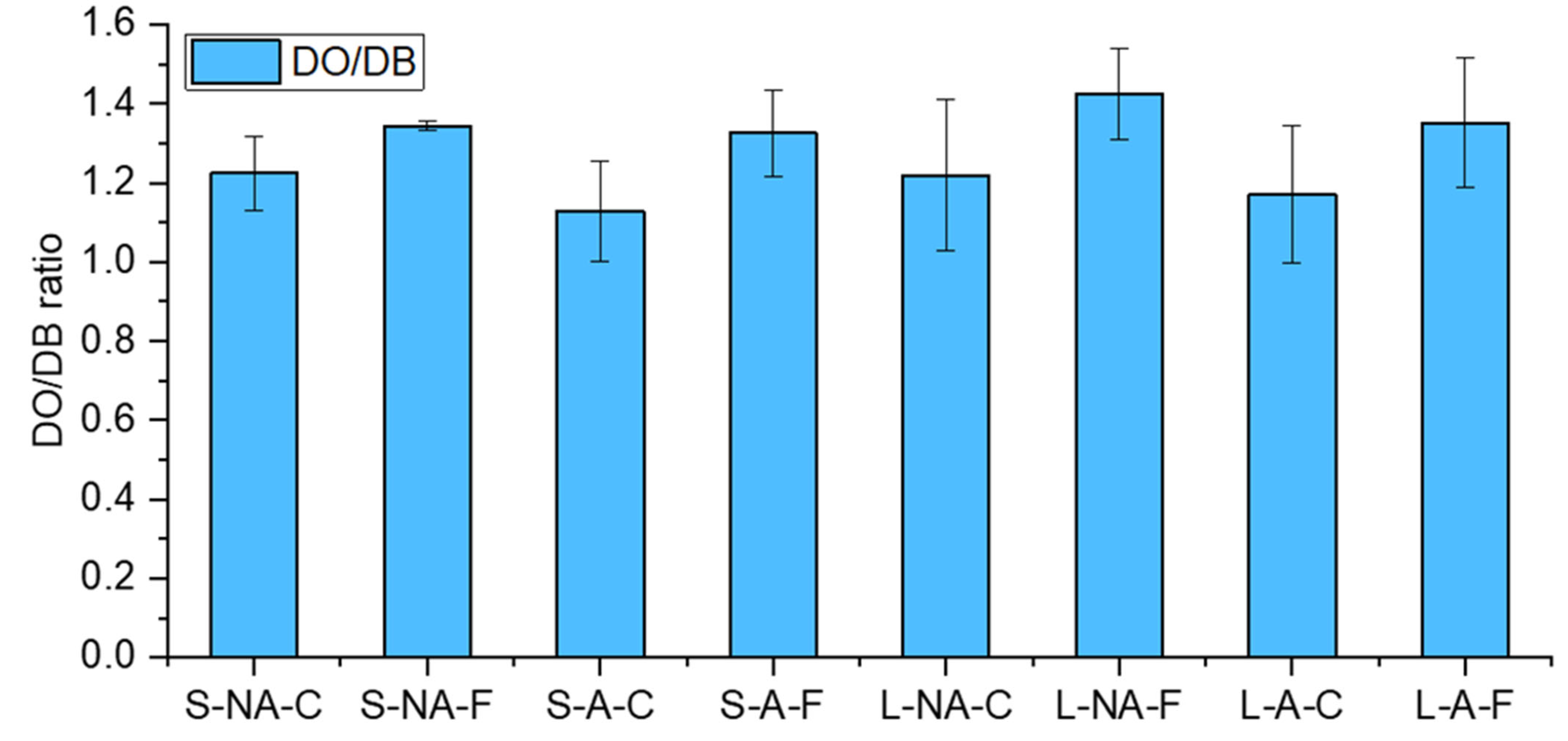
3.3.2. Porosity Indicator by Simon’s Staining
3.4. Spectroscopic Analysis of Straw
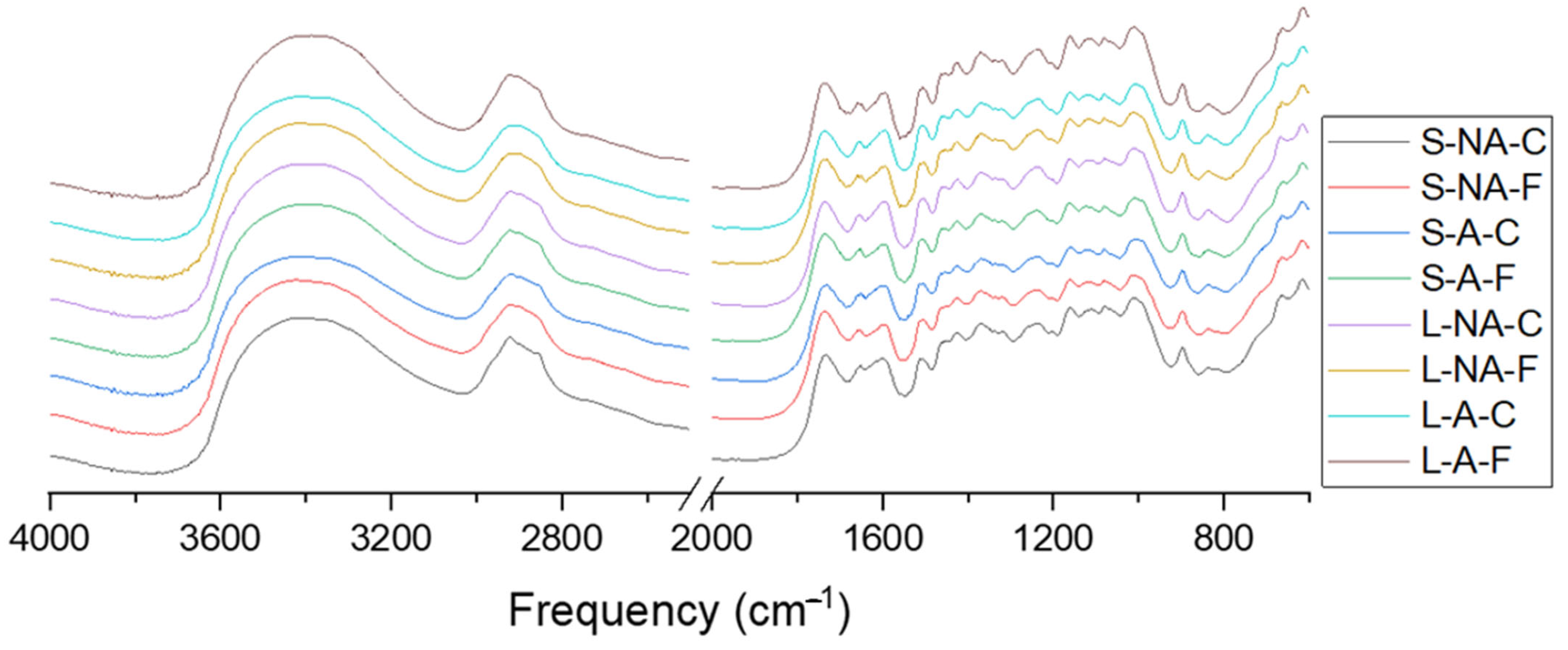
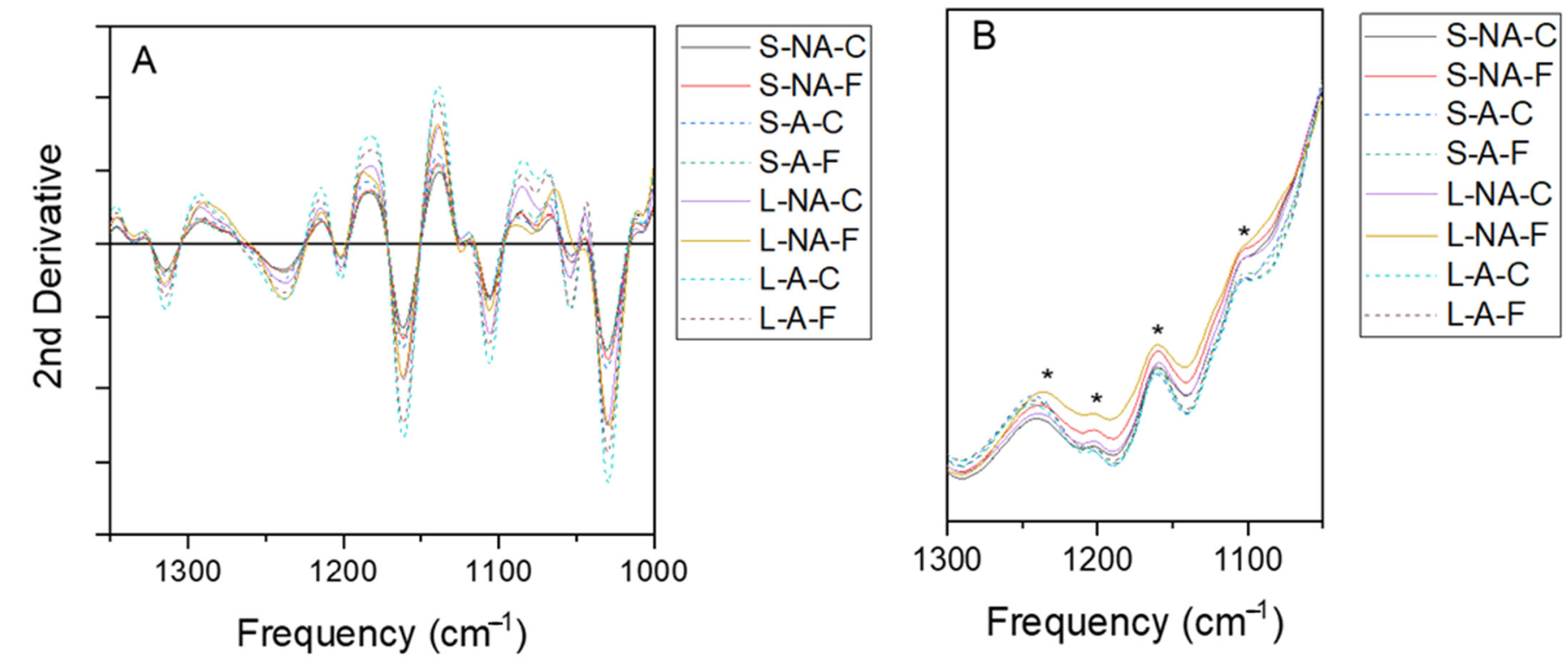
3.4.1. DRIFT Spectroscopy
3.4.2. ATR Spectroscopy
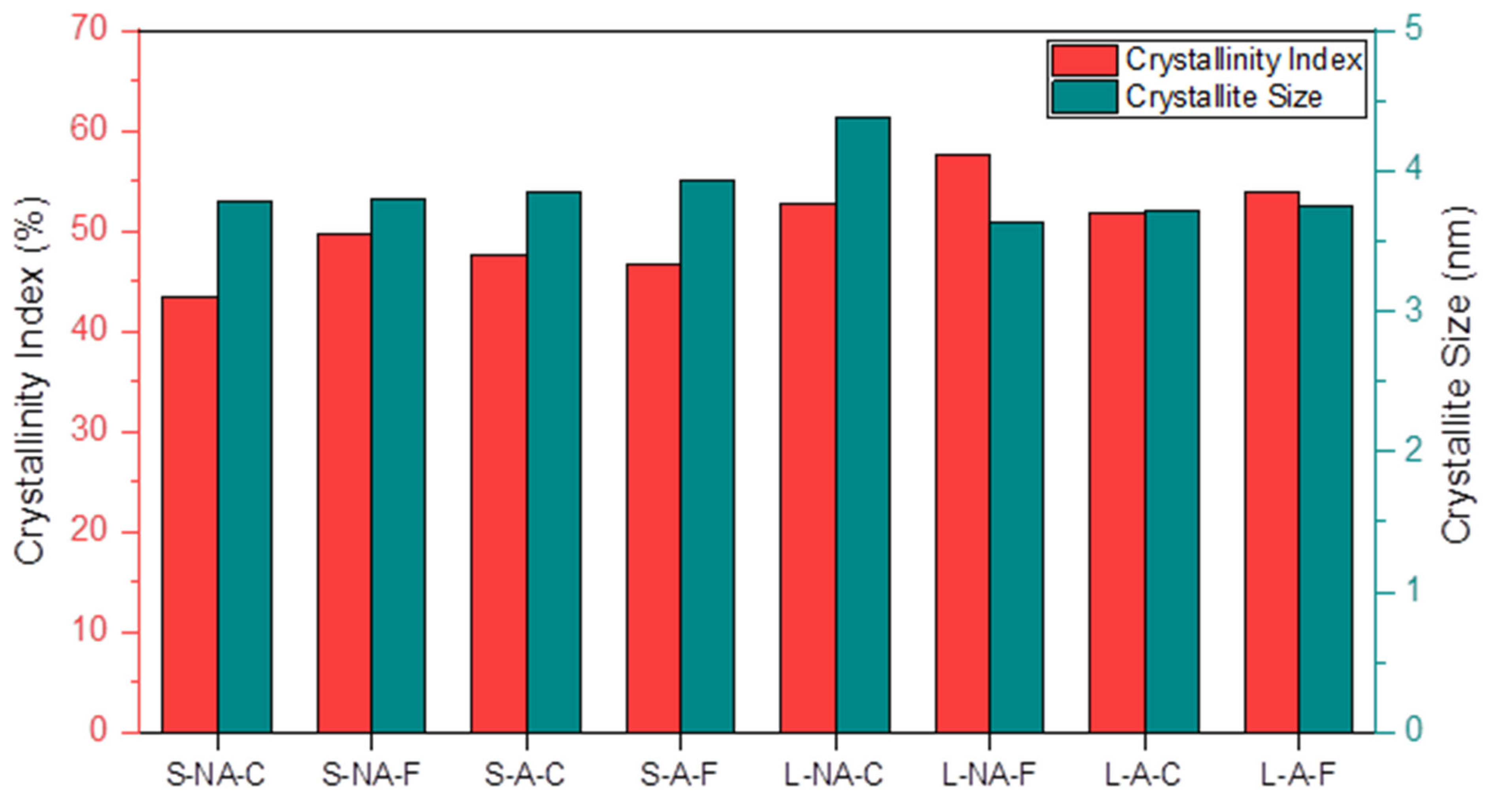
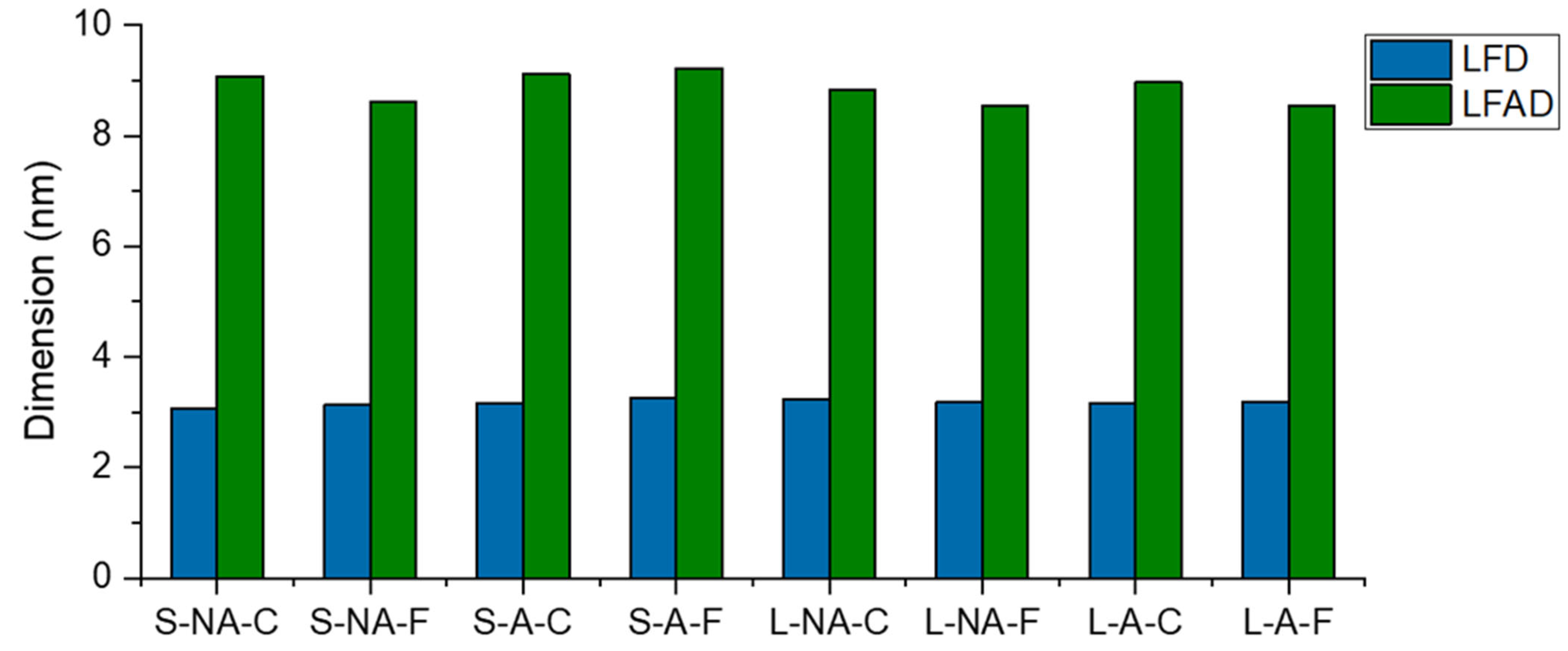
3.5. Cellulose Crystallinity and Crystallite Size by X-ray Diffraction
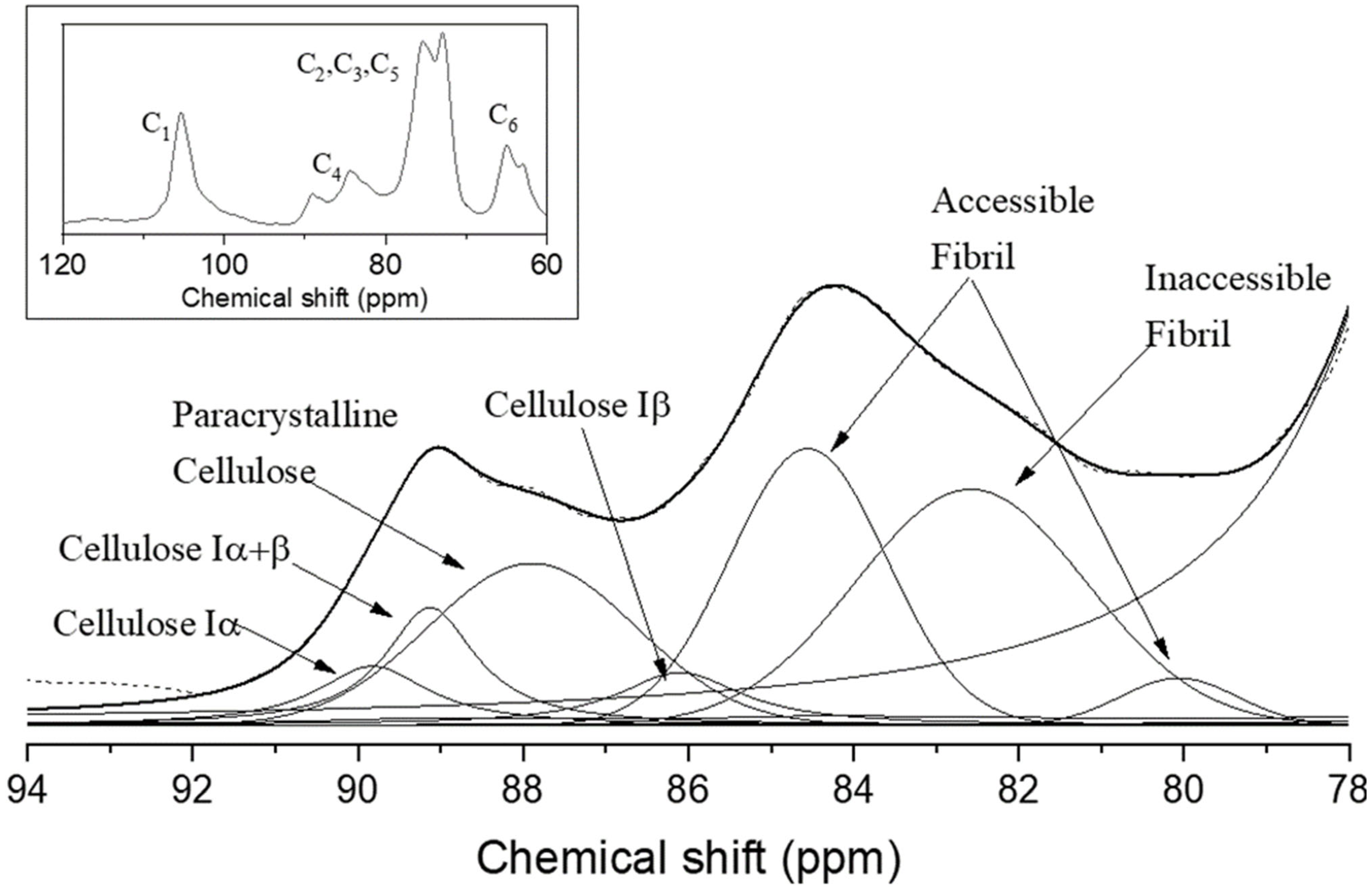
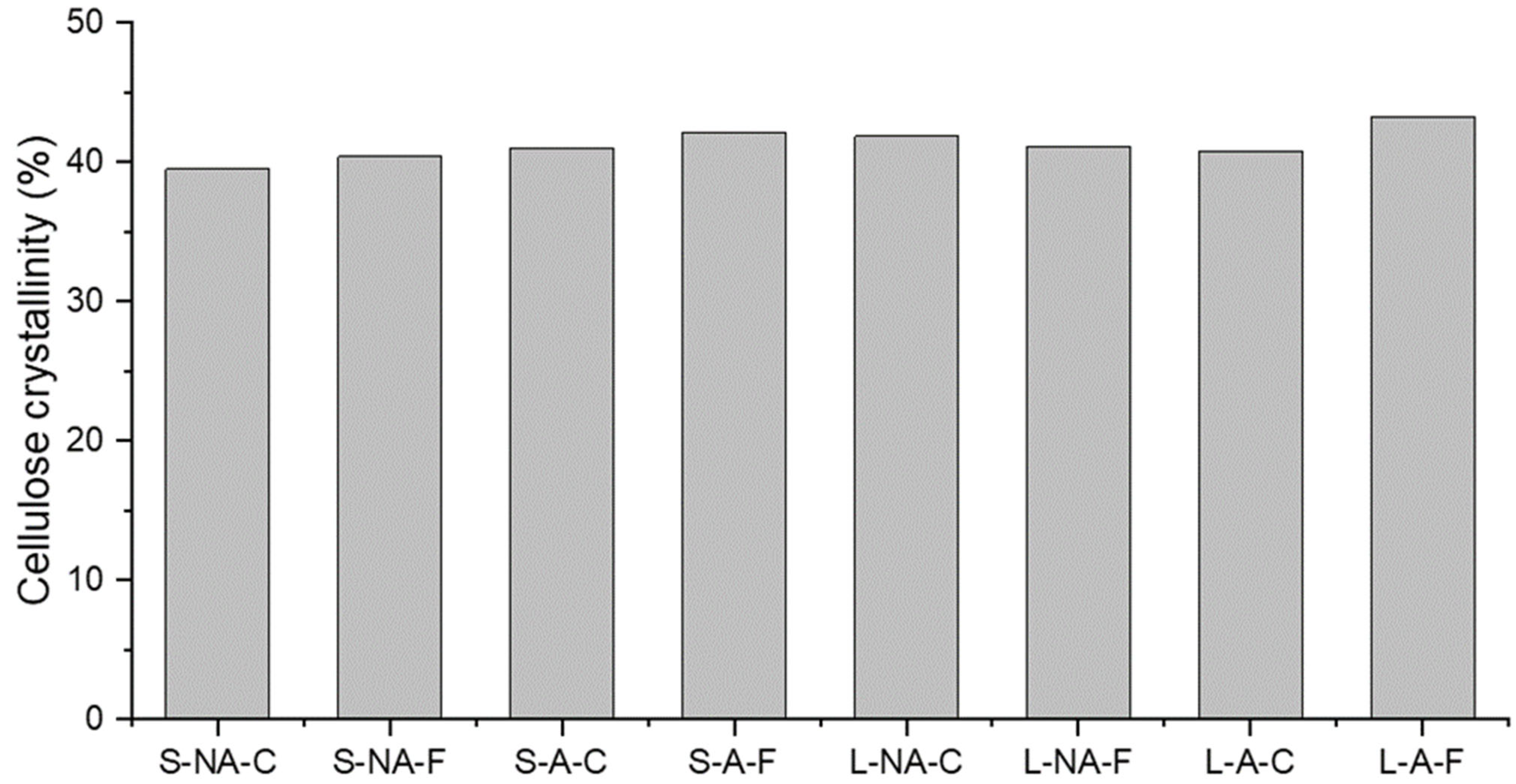
3.6. Multi-CP MAS 13C NMR Spectroscopy
3.7. Discussion and Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, L.; Müller, B.; Schnürer, A. Biogas Production from Wheat Straw: Community Structure of Cellulose-Degrading Bacteria. Energy Sustain. Soc. 2013, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Adney, W.S.; Rivard, C.J.; Shiang, M.; Himmel, M.E. Anaerobic Digestion of Lignocellulosic Biomass and Wastes: Cellulases and Related Enzymes. Appl. Biochem. Biotechnol. 1991, 30, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.; Hu, Y.; Du, B.; Meng, J.; Wu, G.; Liu, H.; Zhan, X. Distinguishing Responses of Acetoclastic and Hydrogenotrophic Methanogens to Ammonia Stress in Mesophilic Mixed Cultures. Water Res. 2022, 224, 119029. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Wu, J. Enhancement of Methane Production in Anaerobic Digestion Process: A Review. Appl. Energy 2019, 240, 120–137. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Coarita Fernandez, H.; Amaya Ramirez, D.; Teixeira Franco, R.; Buffière, P.; Bayard, R. Methods for the Evaluation of Industrial Mechanical Pretreatments before Anaerobic Digesters. Molecules 2020, 25, 860. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M. Effect of Particle Size on Enhancement of Biogas Production from Crop Residue. Mater. Today Proc. 2022, 57, 1950–1954. [Google Scholar] [CrossRef]
- Feng, R.; Li, Q.; Zaidi, A.A.; Peng, H.; Shi, Y. Effect of Autoclave Pretreatment on Biogas Production through Anaerobic Digestion of Green Algae. Period. Polytech. Chem. Eng. 2021, 65, 483–492. [Google Scholar] [CrossRef]
- Antczak, A.; Szadkowski, J.; Szadkowska, D.; Zawadzki, J. Assessment of the Effectiveness of Liquid Hot Water and Steam Explosion Pretreatments of Fast-Growing Poplar (Populus trichocarpa) Wood. Wood Sci. Technol. 2022, 56, 87–109. [Google Scholar] [CrossRef]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Effect of Substrate Pretreatment on Biogas Production through Anaerobic Digestion of Food Waste. Int. J. Hydrogen Energy 2017, 42, 26522–26528. [Google Scholar] [CrossRef]
- Abraham, A.; Mathew, A.K.; Park, H.; Choi, O.; Sindhu, R.; Parameswaran, B.; Pandey, A.; Park, J.H.; Sang, B.-I. Pretreatment Strategies for Enhanced Biogas Production from Lignocellulosic Biomass. Bioresour. Technol. 2020, 301, 122725. [Google Scholar] [CrossRef]
- Anukam, A.; Berghel, J. Biomass Pretreatment and Characterization: A Review. In Biotechnological Applications of Biomass; Peixoto Basso, T., Olitta Basso, T., Carlos Basso, L., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-83881-180-8. [Google Scholar]
- Kumar, A.K.; Sharma, S. Recent Updates on Different Methods of Pretreatment of Lignocellulosic Feedstocks: A Review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Meenakshisundaram, S.; Fayeulle, A.; Leonard, E.; Ceballos, C.; Pauss, A. Fiber Degradation and Carbohydrate Production by Combined Biological and Chemical/Physicochemical Pretreatment Methods of Lignocellulosic Biomass—A Review. Bioresour. Technol. 2021, 331, 125053. [Google Scholar] [CrossRef]
- Uellendahl, H.; Wang, G.; Møller, H.B.; Jørgensen, U.; Skiadas, I.V.; Gavala, H.N.; Ahring, B.K. Energy Balance and Cost-Benefit Analysis of Biogas Production from Perennial Energy Crops Pretreated by Wet Oxidation. Water Sci. Technol. 2008, 58, 1841–1847. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, P. Structure of Lignocellulosic Biomass. In Pretreatment of Lignocellulosic Biomass for Biofuel Production; Springer Briefs in Molecular Science; Springer: Singapore, 2016; ISBN 978-981-10-0687-6. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Van Soest, P.J.; Wine, R.H. Determination of Lignin and Cellulose in Acid-Detergent Fiber with Permanganate. J. Assoc. Off. Anal. Chem. 1968, 51, 780–785. [Google Scholar] [CrossRef]
- Technical Committee ISO/TC 6. Paper, board and pulps. In Pulps—Determination of Lignin Content—Acid Hydrolysis Method; ISO: Geneva, Switzerland, 2020. [Google Scholar]
- Meenakshisundaram, S.; Léonard, E.; Ceballos, C.; Fayeulle, A. Lignin Fungal Depolymerization: From Substrate Characterization to Oligomers Valorization. In Fungal Biopolymers and Biocomposites; Deshmukh, S.K., Deshpande, M.V., Sridhar, K.R., Eds.; Springer Nature: Singapore, 2022; pp. 329–391. ISBN 978-981-19099-9-3. [Google Scholar]
- Dayton, D.C.; Foust, T.D. Biomass Characterization. In Analytical Methods for Biomass Characterization and Conversion; Elsevier: Amsterdam, The Netherlands, 2020; pp. 19–35. ISBN 978-0-12-815605-6. [Google Scholar]
- Oyedeji, O.; Gitman, P.; Qu, J.; Webb, E. Understanding the Impact of Lignocellulosic Biomass Variability on the Size Reduction Process: A Review. ACS Sustain. Chem. Eng. 2020, 8, 2327–2343. [Google Scholar] [CrossRef]
- Liu, X.; Hiligsmann, S.; Gourdon, R.; Bayard, R. Anaerobic Digestion of Lignocellulosic Biomasses Pretreated with Ceriporiopsis Subvermispora. J. Environ. Manag. 2017, 193, 154–162. [Google Scholar] [CrossRef]
- Jung, Y.H.; Kim, H.K.; Park, H.M.; Park, Y.-C.; Park, K.; Seo, J.-H.; Kim, K.H. Mimicking the Fenton Reaction-Induced Wood Decay by Fungi for Pretreatment of Lignocellulose. Bioresour. Technol. 2015, 179, 467–472. [Google Scholar] [CrossRef]
- Bhange, V.P.; William, S.P.; Sharma, A.; Gabhane, J.; Vaidya, A.N.; Wate, S.R. Pretreatment of Garden Biomass Using Fenton’s Reagent: Influence of Fe2+ and H2O2 Concentrations on Lignocellulose Degradation. J. Environ. Health Sci. Eng. 2015, 13, 12. [Google Scholar] [CrossRef] [Green Version]
- Kato, D.M.; Elía, N.; Flythe, M.; Lynn, B.C. Pretreatment of Lignocellulosic Biomass Using Fenton Chemistry. Bioresour. Technol. 2014, 162, 273–278. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; de Wilde, V.; et al. Towards a Standardization of Biomethane Potential Tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Nkuna, R.; Roopnarain, A.; Rashama, C.; Adeleke, R. Insights into Organic Loading Rates of Anaerobic Digestion for Biogas Production: A Review. Crit. Rev. Biotechnol. 2021, 42, 487–507. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, W.; Sun, M.; Xu, X.; Zhang, B.; Sun, Y. Evaluation of Biochemical Methane Potential and Kinetics on the Anaerobic Digestion of Vegetable Crop Residues. Energies 2018, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Chandra, R.; Ewanick, S.; Hsieh, C.; Saddler, J.N. The Characterization of Pretreated Lignocellulosic Substrates Prior to Enzymatic Hydrolysis, Part 1: A Modified Simons’ Staining Technique. Biotechnol. Prog. 2008, 24, 1178–1185. [Google Scholar] [CrossRef]
- Chandra, R.P.; Esteghlalian, A.R.; Saddler, J.N. Assessing Substrate Accessibility to Enzymatic Hydrolysis by Cellulases. In Characterization of Lignocellulosic Materials; Hu, T.Q., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2008; pp. 60–80. ISBN 978-1-4443-0542-5. [Google Scholar]
- Yu, X.; Atalla, R.H. A Staining Technique for Evaluating the Pore Structure Variations of Microcrystalline Cellulose Powders. Powder Technol. 1998, 98, 135–138. [Google Scholar] [CrossRef]
- Kubelka, P. New Contributions to the Optics of Intensely Light-Scattering Materials. Part I. J. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar] [CrossRef]
- Sirita, J.; Phanichphant, S.; Meunier, F.C. Quantitative Analysis of Adsorbate Concentrations by Diffuse Reflectance FT-IR. Anal. Chem. 2007, 79, 3912–3918. [Google Scholar] [CrossRef] [Green Version]
- Ahvenainen, P.; Kontro, I.; Svedström, K. Comparison of Sample Crystallinity Determination Methods by X-ray Diffraction for Challenging Cellulose I Materials. Cellulose 2016, 23, 1073–1086. [Google Scholar] [CrossRef] [Green Version]
- Murthy, N.S.; Minor, H. General Procedure for Evaluating Amorphous Scattering and Crystallinity from X-ray Diffraction Scans of Semicrystalline Polymers. Polymer 1990, 31, 996–1002. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A General-purpose Peak Fitting Program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Pearson India Education Services: Bengaluru, India, 2015; ISBN 978-93-325-3516-9. [Google Scholar]
- Johnson, R.L.; Schmidt-Rohr, K. Quantitative Solid-State 13C NMR with Signal Enhancement by Multiple Cross Polarization. J. Magn. Reson. 2014, 239, 44–49. [Google Scholar] [CrossRef]
- Croce, S.; Wei, Q.; D’Imporzano, G.; Dong, R.; Adani, F. Anaerobic Digestion of Straw and Corn Stover: The Effect of Biological Process Optimization and Pre-Treatment on Total Bio-Methane Yield and Energy Performance. Biotechnol. Adv. 2016, 34, 1289–1304. [Google Scholar] [CrossRef] [PubMed]
- Achinas, S.; Euverink, G.J.W. Theoretical Analysis of Biogas Potential Prediction from Agricultural Waste. Resour.-Effic. Technol. 2016, 2, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Guerriero, G.; Hausman, J.-F.; Legay, S. Silicon and the Plant Extracellular Matrix. Front. Plant Sci. 2016, 7, 463. [Google Scholar] [CrossRef] [Green Version]
- Shtein, I.; Shelef, Y.; Marom, Z.; Zelinger, E.; Schwartz, A.; Popper, Z.A.; Bar-On, B.; Harpaz-Saad, S. Stomatal Cell Wall Composition: Distinctive Structural Patterns Associated with Different Phylogenetic Groups. Ann. Bot. 2017, 119, 1021–1033. [Google Scholar] [CrossRef] [Green Version]
- Mulat, D.G.; Horn, S.J. Biogas Production from Lignin via Anaerobic Digestion. In Energy and Environment Series; Beckham, G.T., Ed.; Royal Society of Chemistry: Cambridge, UK, 2018; ISBN 978-1-78262-554-4. [Google Scholar]
- Menardo, S.; Airoldi, G.; Balsari, P. The Effect of Particle Size and Thermal Pre-Treatment on the Methane Yield of Four Agricultural by-Products. Bioresour. Technol. 2012, 104, 708–714. [Google Scholar] [CrossRef]
- Dumas, C.; Silva Ghizzi Damasceno, G.; Barakat, A.; Carrère, H.; Steyer, J.-P.; Rouau, X. Effects of Grinding Processes on Anaerobic Digestion of Wheat Straw. Ind. Crop. Prod. 2015, 74, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Scherzinger, M.; Kulbeik, T.; Kaltschmitt, M. Autoclave Pre-Treatment of Green Wastes—Effects of Temperature, Residence Time and Rotation Speed on Fuel Properties. Fuel 2020, 273, 117796. [Google Scholar] [CrossRef]
- Ma, H.; Fu, P.; Zhao, J.; Lin, X.; Wu, W.; Yu, Z.; Xia, C.; Wang, Q.; Gao, M.; Zhou, J. Pretreatment of Wheat Straw Lignocelluloses by Deep Eutectic Solvent for Lignin Extraction. Molecules 2022, 27, 7955. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, P.; Xiu, H.; Li, J.; Yang, X.; Ma, F.; Li, X.; Zhang, X.; Kozliak, E.; Ji, Y. Morphological Changes of Lignin during Separation of Wheat Straw Components by the Hydrothermal-Ethanol Method. Bioresour. Technol. 2019, 294, 122157. [Google Scholar] [CrossRef] [PubMed]
- Ueno, O.; Agarie, S. Silica Deposition in Cell Walls of the Stomatal Apparatus of Rice Leaves. Plant Prod. Sci. 2005, 8, 71–73. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A Critical Review on Analysis in Pretreatment of Lignocelluloses: Degree of Polymerization, Adsorption/Desorption, and Accessibility. Bioresour. Technol. 2016, 203, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Simons, F.L. A Stain for Use in the Microscopy of Beaten Fibers. Tappi J. 1950, 33, 312–314. [Google Scholar]
- Esteghlalian, A.R.; Bilodeau, M.; Mansfield, S.D.; Saddler, J.N. Do Enzymatic Hydrolyzability and Simons’ Stain Reflect the Changes in the Accessibility of Lignocellulosic Substrates to Cellulase Enzymes? Biotechnol. Prog. 2001, 17, 1049–1054. [Google Scholar] [CrossRef]
- Yu, X.; Minor, J.L.; Atalla, R.H. Mechanism of Action of Simons’ Stain. Tappi J. 1995, 78, 175–180. [Google Scholar]
- Rodrigues, R.C.L.B.; Green Rodrigues, B.; Vieira Canettieri, E.; Acosta Martinez, E.; Palladino, F.; Wisniewski, A., Jr.; Rodrigues, D., Jr. Comprehensive Approach of Methods for Microstructural Analysis and Analytical Tools in Lignocellulosic Biomass Assessment—A Review. Bioresour. Technol. 2022, 348, 126627. [Google Scholar] [CrossRef]
- Mamilla, J.L.K.; Novak, U.; Grilc, M.; Likozar, B. Natural Deep Eutectic Solvents (DES) for Fractionation of Waste Lignocellulosic Biomass and Its Cascade Conversion to Value-Added Bio-Based Chemicals. Biomass Bioenergy 2019, 120, 417–425. [Google Scholar] [CrossRef]
- Auxenfans, T.; Crônier, D.; Chabbert, B.; Paës, G. Understanding the Structural and Chemical Changes of Plant Biomass Following Steam Explosion Pretreatment. Biotechnol. Biofuels 2017, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Foston, M.; Meng, X.; Sawada, D.; Pingali, S.V.; O’Neill, H.M.; Li, H.; Wyman, C.E.; Langan, P.; Ragauskas, A.J.; et al. Effect of Lignin Content on Changes Occurring in Poplar Cellulose Ultrastructure during Dilute Acid Pretreatment. Biotechnol. Biofuels 2014, 7, 150. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Pang, Y.; Liu, Y.; Li, X.; Wang, K. Physicochemical Characterization of Rice Straw Pretreated with Sodium Hydroxide in the Solid State for Enhancing Biogas Production. Energy Fuels 2008, 22, 2775–2781. [Google Scholar] [CrossRef]
- Stewart, D.; Wilson, H.M.; Hendra, P.J.; Morrison, I.M. Fourier-Transform Infrared and Raman Spectroscopic Study of Biochemical and Chemical Treatments of Oak Wood (Quercus Rubra) and Barley (Hordeum Vulgare) Straw. J. Agric. Food Chem. 1995, 43, 2219–2225. [Google Scholar] [CrossRef]
- Gogna, M.; Goacher, R.E. Comparison of Three Fourier Transform Infrared Spectroscopy Sampling Techniques for Distinction between Lignocellulose Samples. BioResources 2017, 13, 846–860. [Google Scholar] [CrossRef]
- Larkin, P. Infrared and Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 978-0-12-386984-5. [Google Scholar]
- Faix, O.; Böttcher, J.H. The Influence of Particle Size and Concentration in Transmission and Diffuse Reflectance Spectroscopy of Wood. Holz Als Roh-Werkst. 1992, 50, 221–226. [Google Scholar] [CrossRef]
- Krongtaew, C.; Messner, K.; Ters, T.; Fackler, K. Characterization of Key Parameters for Biotechnological Lignocellulose Conversion Assessed by FT-NIR Spectroscopy. Part I: Qualitative Analysis of Pretreated Straw. BioResources 2010, 5, 2063–2080. [Google Scholar]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of Short-Time Vibratory Ball Milling on the Shape of FT-IR Spectra of Wood and Cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of Certain Infrared Bands to Cellulose Crystallinity and Crystal Latticed Type. Part I. Spectra of Lattice Types I, II, III and of Amorphous Cellulose. J. Appl. Polym. Sci. 1964, 8, 1311–1324. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose Crystallinity Index: Measurement Techniques and Their Impact on Interpreting Cellulase Performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Bernardinelli, O.D.; Lima, M.A.; Rezende, C.A.; Polikarpov, I.; de Azevedo, E.R. Quantitative 13C MultiCP Solid-State NMR as a Tool for Evaluation of Cellulose Crystallinity Index Measured Directly inside Sugarcane Biomass. Biotechnol. Biofuels 2015, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- Larsson, P.T.; Wickholm, K.; Iversen, T. A CP/MAS13C NMR Investigation of Molecular Ordering in Celluloses. Carbohydr. Res. 1997, 302, 19–25. [Google Scholar] [CrossRef]
- Wickholm, K.; Larsson, P.T.; Iversen, T. Assignment of Non-Crystalline Forms in Cellulose I by CP/MAS 13C NMR Spectroscopy. Carbohydr. Res. 1998, 312, 123–129. [Google Scholar] [CrossRef]
- Hult, E.-L.; Larsson, P.T.; Iversen, T. A Comparative CP/MAS 13C-NMR Study of Cellulose Structure in Spruce Wood and Kraft Pulp. Cellulose 2000, 7, 35–55. [Google Scholar] [CrossRef]
- Foston, M.B.; Hubbell, C.A.; Ragauskas, A.J. Cellulose Isolation Methodology for NMR Analysis of Cellulose Ultrastructure. Materials 2011, 4, 1985–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atalla, R.H.; VanderHart, D.L. The Role of Solid State 13C NMR Spectroscopy in Studies of the Nature of Native Celluloses. Solid State Nucl. Magn. Reson. 1999, 15, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rezende, C.A.; de Lima, M.A.; Maziero, P.; de Azevedo, E.R.; Garcia, W.; Polikarpov, I. Chemical and Morphological Characterization of Sugarcane Bagasse Submitted to a Delignification Process for Enhanced Enzymatic Digestibility. Biotechnol. Biofuels 2011, 4, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, M.; Prajapati, B.P.; Suryawanshi, R.K.; Kishor Dey, K.; Kango, N. Study of the Effect of Enzymatic Deconstruction on Natural Cellulose by NMR Measurements. Chem. Phys. Lett. 2019, 727, 105–115. [Google Scholar] [CrossRef]
- Liu, R.; Yu, H.; Huang, Y. Structure and Morphology of Cellulose in Wheat Straw. Cellulose 2005, 12, 25–34. [Google Scholar] [CrossRef]
- Hult, E.-L.; Larsson, P.; Iversen, T. Cellulose Fibril Aggregation—An Inherent Property of Kraft Pulps. Polymer 2001, 42, 3309–3314. [Google Scholar] [CrossRef]
- Zuckerstätter, G.; Terinte, N.; Sixta, H.; Schuster, K.C. Novel Insight into Cellulose Supramolecular Structure through 13C CP-MAS NMR Spectroscopy and Paramagnetic Relaxation Enhancement. Carbohydr. Polym. 2013, 93, 122–128. [Google Scholar] [CrossRef]
- Chunilall, V.; Bush, T.; Larsson, P.T.; Iversen, T.; Kindness, A. A CP/MAS 13C-NMR Study of Cellulose Fibril Aggregation in Eucalyptus Dissolving Pulps during Drying and the Correlation between Aggregate Dimensions and Chemical Reactivity. Holzforschung 2010, 64, 693–698. [Google Scholar] [CrossRef]
- Sun, Q.; Foston, M.; Sawada, D.; Pingali, S.V.; O’Neill, H.M.; Li, H.; Wyman, C.E.; Langan, P.; Pu, Y.; Ragauskas, A.J. Comparison of Changes in Cellulose Ultrastructure during Different Pretreatments of Poplar. Cellulose 2014, 21, 2419–2431. [Google Scholar] [CrossRef]
- Moset, V.; de Almeida Neves Xavier, C.; Feng, L.; Wahid, R.; Møller, H.B. Combined Low Thermal Alkali Addition and Mechanical Pre-Treatment to Improve Biogas Yield from Wheat Straw. J. Clean. Prod. 2018, 172, 1391–1398. [Google Scholar] [CrossRef]
- Beig, B.; Riaz, M.; Raza Naqvi, S.; Hassan, M.; Zheng, Z.; Karimi, K.; Pugazhendhi, A.; Atabani, A.E.; Thuy Lan Chi, N. Current Challenges and Innovative Developments in Pretreatment of Lignocellulosic Residues for Biofuel Production: A Review. Fuel 2021, 287, 119670. [Google Scholar] [CrossRef]
- Dai, X.; Hua, Y.; Dai, L.; Cai, C. Particle Size Reduction of Rice Straw Enhances Methane Production under Anaerobic Digestion. Bioresour. Technol. 2019, 293, 122043. [Google Scholar] [CrossRef]
- Sharma, S.K.; Mishra, I.M.; Sharma, M.P.; Saini, J.S. Effect of Particle Size on Biogas Generation from Biomass Residues. Biomass 1988, 17, 251–263. [Google Scholar] [CrossRef]
- Chandler, J.; Jewell, W. Predicting Methane Fermentation Biodegradability; Final Report; Department of Agricultural Engineering Cornell University Ithaca: New York, NY, USA, 1980. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Xin, F.; Zhou, J.; Jia, H.; Xu, J.; Jiang, M.; Dong, W. Biomethane Production from Lignocellulose: Biomass Recalcitrance and Its Impacts on Anaerobic Digestion. Front. Bioeng. Biotechnol. 2019, 7, 191. [Google Scholar] [CrossRef]
- Hall, M.; Bansal, P.; Lee, J.H.; Realff, M.J.; Bommarius, A.S. Cellulose Crystallinity—A Key Predictor of the Enzymatic Hydrolysis Rate: Cellulose Crystallinity. FEBS J. 2010, 277, 1571–1582. [Google Scholar] [CrossRef]
- Ma, S.; Wang, H.; Li, J.; Fu, Y.; Zhu, W. Methane Production Performances of Different Compositions in Lignocellulosic Biomass through Anaerobic Digestion. Energy 2019, 189, 116190. [Google Scholar] [CrossRef]
- Taherdanak, M.; Zilouei, H. Improving Biogas Production from Wheat Plant Using Alkaline Pretreatment. Fuel 2014, 115, 714–719. [Google Scholar] [CrossRef]
- Meng, X.; Foston, M.; Leisen, J.; DeMartini, J.; Wyman, C.E.; Ragauskas, A.J. Determination of Porosity of Lignocellulosic Biomass before and after Pretreatment by Using Simons’ Stain and NMR Techniques. Bioresour. Technol. 2013, 144, 467–476. [Google Scholar] [CrossRef]
- Peciulyte, A.; Karlström, K.; Larsson, P.T.; Olsson, L. Impact of the Supramolecular Structure of Cellulose on the Efficiency of Enzymatic Hydrolysis. Biotechnol. Biofuels 2015, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.N.; Chen, H.-C.; Grethlein, H.E. Comparison of Pretreatment Methods on the Basis of Available Surface Area. Bioresour. Technol. 1992, 39, 155–163. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Nilsen, P.J.; Fdz-Polanco, F.; Pérez-Elvira, S.I. Biomethane Potential of Wheat Straw: Influence of Particle Size, Water Impregnation and Thermal Hydrolysis. Chem. Eng. J. 2014, 242, 254–259. [Google Scholar] [CrossRef]
- Hsu, T.-C.; Guo, G.-L.; Chen, W.-H.; Hwang, W.-S. Effect of Dilute Acid Pretreatment of Rice Straw on Structural Properties and Enzymatic Hydrolysis. Bioresour. Technol. 2010, 101, 4907–4913. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, Z.; Zhang, K.; Si, M.; Liu, M.; Chai, L.; Liu, X.; Shi, Y. Bacteria-Enhanced Dilute Acid Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2017, 245, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Grethlein, H.E. The Effect of Pore Size Distribution on the Rate of Enzymatic Hydrolysis of Cellulosic Substrates. Nat. Biotechnol. 1985, 3, 155–160. [Google Scholar] [CrossRef]
- Kim, S.; Holtzapple, M.T. Effect of Structural Features on Enzyme Digestibility of Corn Stover. Bioresour. Technol. 2006, 97, 583–591. [Google Scholar] [CrossRef]
- Pu, Y.; Hu, F.; Huang, F.; Davison, B.H.; Ragauskas, A.J. Assessing the Molecular Structure Basis for Biomass Recalcitrance during Dilute Acid and Hydrothermal Pretreatments. Biotechnol. Biofuels 2013, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-H.P.; Lynd, L.R. Toward an Aggregated Understanding of Enzymatic Hydrolysis of Cellulose: Noncomplexed Cellulase Systems. Biotechnol. Bioeng. 2004, 88, 797–824. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to Enhance the Digestibility of Lignocellulosic Biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Zollitsch, W.; Mayer, K.; Gruber, L. Biogas Production from Maize and Dairy Cattle Manure—Influence of Biomass Composition on the Methane Yield. Agric. Ecosyst. Environ. 2007, 118, 173–182. [Google Scholar] [CrossRef]
- Dandikas, V.; Heuwinkel, H.; Lichti, F.; Drewes, J.E.; Koch, K. Correlation between Biogas Yield and Chemical Composition of Energy Crops. Bioresour. Technol. 2014, 174, 316–320. [Google Scholar] [CrossRef]
- Thomsen, S.T.; Spliid, H.; Østergård, H. Statistical Prediction of Biomethane Potentials Based on the Composition of Lignocellulosic Biomass. Bioresour. Technol. 2014, 154, 80–86. [Google Scholar] [CrossRef]
- Triolo, J.M.; Sommer, S.G.; Møller, H.B.; Weisbjerg, M.R.; Jiang, X.Y. A New Algorithm to Characterize Biodegradability of Biomass during Anaerobic Digestion: Influence of Lignin Concentration on Methane Production Potential. Bioresour. Technol. 2011, 102, 9395–9402. [Google Scholar] [CrossRef]

| Sample | %TS | VS (%TS) | % C (%TS) | % H (%TS) | % N (%TS) | % S (%TS) | % O (%TS) |
|---|---|---|---|---|---|---|---|
| S-NA-C | 97.8 ± 0.4 | 98.0 ± 0.0 | 44.7 ± 0.5 | 5.8 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.5 | 40.4 ± 0.1 |
| S-NA-F | 97.0 ± 0.5 | 98.1 ± 0.3 | 44.7 ± 0.6 | 5.7 ± 0.0 | 0.3 ± 0.0 | 0.1 ± 0.1 | 42.7 ± 0.2 |
| S-A-C | 96.0 ± 0.5 | 98.6 ± 0.3 | 45.1 ± 1.1 | 5.7 ± 0.1 | 0.3 ± 0.0 | 0.4 ± 0.3 | 43.6 ± 0.5 |
| S-A-F | 96.0 ± 0.9 | 98.6 ± 0.3 | 43.6 ± 0.6 | 5.5 ± 0.1 | 0.3 ± 0.0 | 0.0 ± 0.0 | 44.2 ± 0.2 |
| L-NA-C | 95.8 ± 0.3 | 98.7 ± 0.4 | 44.8 ± 0.1 | 5.7 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.2 | 40.1 ± 0.1 |
| L-NA-F | 94.4 ± 0.3 | 98.6 ± 0.3 | 44.3 ± 0.5 | 5.6 ± 0.0 | 0.2 ± 0.1 | 0.0 ± 0.0 | 43.0 ± 0.9 |
| L-A-C | 96.2 ± 0.3 | 99.5 ± 0.0 | 45.1 ± 1.2 | 5.7 ± 0.1 | 0.2 ± 0.1 | 0.0 ± 0.0 | 39.6 ± 0.8 |
| L-A-F | 94.7 ± 0.3 | 98.8 ± 0.3 | 44.2 ± 0.4 | 5.6 ± 0.1 | 0.4 ± 0.3 | 0.3 ± 0.3 | 43.6 ± 0.6 |
| Sample | Vmax (NLCH4/kg VS) | k (d−1) | R2 (–) |
|---|---|---|---|
| S-NA-C | 318 ± 29 | 0.076 ± 0 | 0.997 |
| S-NA-F | 320 ± 16 | 0.089 ± 0.002 | 0.995 |
| S-A-C | 329 ± 10 | 0.081 ± 0.005 | 0.996 |
| S-A-F | 348 ± 10 | 0.089 ± 0.004 | 0.994 |
| L-NA-C | 266 ± 6 | 0.058 ± 0.008 | 0.998 |
| L-NA-F | 264 ± 17 | 0.064 ± 0.002 | 0.998 |
| L-A-C | 274 ± 8 | 0.059 ± 0.003 | 0.998 |
| L-A-F | 303 ± 17 | 0.069 ± 0.002 | 0.997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meenakshisundaram, S.; Calcagno, V.; Ceballos, C.; Fayeulle, A.; Léonard, E.; Herledan, V.; Krafft, J.-M.; Millot, Y.; Liu, X.; Jolivalt, C.; et al. Chemically and Physically Pretreated Straw in Moderate Conditions: Poor Correlation between Biogas Production and Commonly Used Biomass Characterization. Energies 2023, 16, 1146. https://doi.org/10.3390/en16031146
Meenakshisundaram S, Calcagno V, Ceballos C, Fayeulle A, Léonard E, Herledan V, Krafft J-M, Millot Y, Liu X, Jolivalt C, et al. Chemically and Physically Pretreated Straw in Moderate Conditions: Poor Correlation between Biogas Production and Commonly Used Biomass Characterization. Energies. 2023; 16(3):1146. https://doi.org/10.3390/en16031146
Chicago/Turabian StyleMeenakshisundaram, Shruthi, Vincenzo Calcagno, Claire Ceballos, Antoine Fayeulle, Estelle Léonard, Virginie Herledan, Jean-Marc Krafft, Yannick Millot, Xiaojun Liu, Claude Jolivalt, and et al. 2023. "Chemically and Physically Pretreated Straw in Moderate Conditions: Poor Correlation between Biogas Production and Commonly Used Biomass Characterization" Energies 16, no. 3: 1146. https://doi.org/10.3390/en16031146






