Hydrogen and Corrosion Resistance of Nickel Superalloys for Gas Turbines, Engines Cooled Blades
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. High-Temperature Salt Corrosion of Alloys
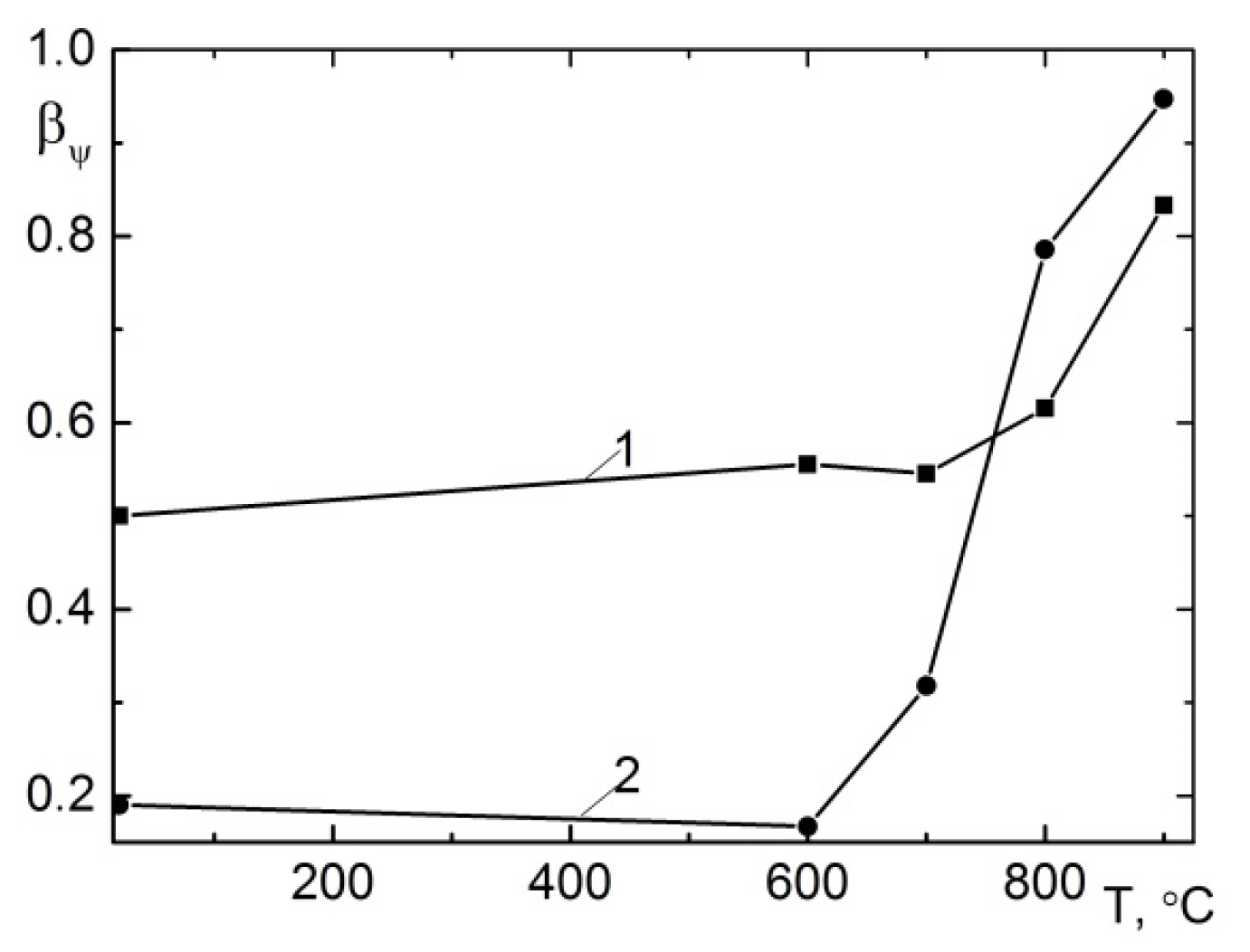
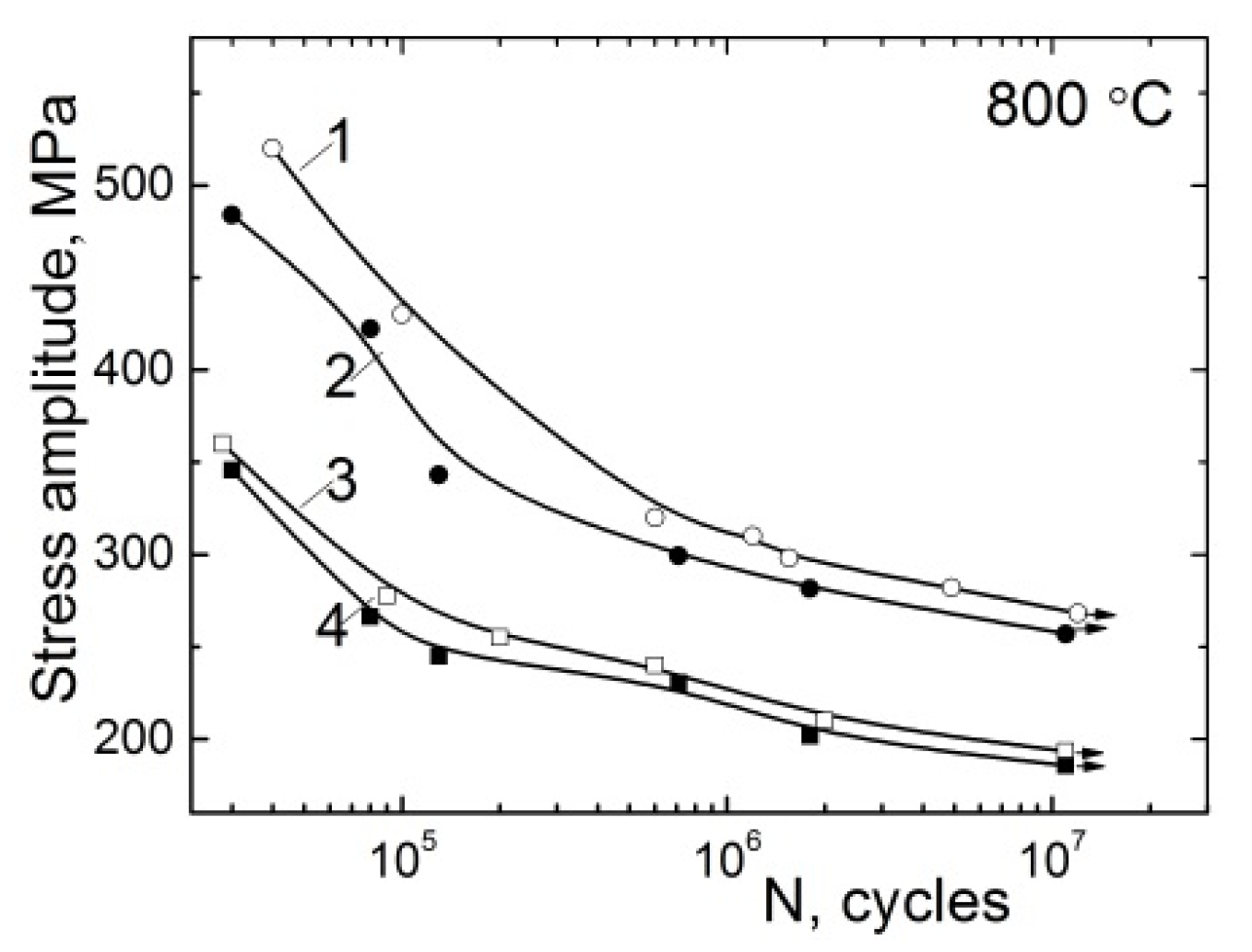
3.2. The Influence of Hydrogen on the Properties of Alloys for Gas Turbine Blades
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature and Abbreviations
| σu | ultimate tensile strength (UTS) |
| σ0,2 | yield strength (YS) |
| σ-1 | fatigue limit (FL) |
| N | number of cycles |
| δ | elongation |
| ψ | reduction of area |
| CH | hydrogen concentration |
| wppm | weight parts per millions |
| GTE | gas turbine engine |
| GET | environmentally ‘greener’ hydrogen energetic turbine |
| HCF | high-cycle fatigue |
| LCF | low-cycle fatigue |
| RPM | rotation per minute |
| HD | hydrogen durability |
| HCE | hydrogen-containing environment |
| HE | hydrogen embrittlement |
| MO | metal oxide |
| MS | metal sulphides and selenides |
| TA | turboaggregate (turbine + turbogenerator) |
References
- Nagano, S.; Kitajima, T.; Yoshida, K.; Kazao, Y.; Kabata, Y.; Murata, D.; Nagakura, K. Development of world’s largest hydrogen-cooled turbine generator. IEEE Power Eng. Soc. Summer Meet. 2002, 2, 657–663. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A. Assessment of impact of hydrogen cooled generator on power system loadability enhancement. In Proceedings of the 2015 International Conference on Energy, Power and Environment: Towards Sustainable Growth (ICEPE), Shillong, India, 12–13 June 2015; pp. 1–6. [Google Scholar] [CrossRef]
- Dhivya, S. 210 MW turbo generator’s hydrogen gas cooling system online monitoring and controlling using node red flow based IOT. Malaya J. Mat. 2020, S, 3225–3231. [Google Scholar] [CrossRef]
- Griebel, P. Gas Turbines and Hydrogen. In Hydrogen Science and Engineering: Materials, Processes, Systems and Technology; Wiley: Hoboken, NJ, USA, 2016; Volume 2, pp. 1011–1032. [Google Scholar] [CrossRef]
- Stefan, E.; Talic, B.; Larring, Y.; Gruber, A.; Peters, T.A. Materials challenges in hydrogen-fuelled gas turbines. Int. Mater. Rev. 2022, 67, 461–486. [Google Scholar] [CrossRef]
- Bothien, M.R.; Ciani, A.; Wood, J.P.; Fruechtel, T.G. Decarbonized power generation with gas turbines by using sequential combustion for burning hydrogen. J. Eng. Gas Turbines Power 2019, 141, 121013. [Google Scholar] [CrossRef]
- Chiesa, P.; Lozza, G.; Mazzocchi, L. Using hydrogen as gas turbine fuel. J. Eng. Gas Turbines Power 2005, 127, 73–80. [Google Scholar] [CrossRef]
- Lambert, H.; Roche, R.; Jemeï, S.; Ortega, P.; Hissel, D. Combined cooling and power management strategy for a standalone house using hydrogen and solar energy. Hydrogen 2021, 2, 207–224. [Google Scholar] [CrossRef]
- Balitskii, A.; Krohmalny, O.; Ripey, I. Hydrogen cooling of turbogenerators and the problem of rotor retaining ring materials degradation. Int. J. Hydrog. Energy 2000, 25, 167–171. [Google Scholar] [CrossRef]
- Unnikrishnan, U.; Yang, V. A review of cooling technologies for high temperature rotating components in gas turbine. Propuls. Power Res. 2022, 11, 293–310. [Google Scholar] [CrossRef]
- Technical Database for Hydrogen Compatibility of Materials; Sandia National Laboratories: California, CA, USA, 2022; Available online: https://h2tools.org/technical-database-hydrogencompatibility-materials (accessed on 27 November 2022).
- Wee, S.; Do, J.; Kim, K.; Lee, C.; Seok, C.; Choi, B.-G.; Kim, W. Review on mechanical thermal properties of superalloys and thermal barrier coating used in gas turbines. Appl. Sci. 2020, 10, 5476. [Google Scholar] [CrossRef]
- Glotka, A. Prediction thermo-physical characteristics heat-resistant nickel alloys directional crystallization. Acta Metall. Slovaca 2021, 27, 68–71. [Google Scholar] [CrossRef]
- Sheykhlari, F.A.; Moghanaki, K.S.; Moattari, M.; Shafiei, A.; Amirjan, M. On the failure behavior of fifth stage gas turbine blade. Eng. Fail. Anal. 2020, 116, 104766. [Google Scholar] [CrossRef]
- Klochikhin, V.; Naumyk, V. Improvement of technological processes obtaining a heat-resistant nickel alloys for turbine blades using foundry return. Mater. Sci. Technol. 2019, 1, 1454. [Google Scholar] [CrossRef]
- Rayapati, S. Gas turbine blade failure scenario due to thermal loads in case of Nickel based super alloys. Mater. Today Proc. 2021, 46, 8119–8126. [Google Scholar] [CrossRef]
- Kuznetsov, P.; Lesnikov, V.P.; Konakova, I.P.; Popov, N.A.; Kvasnitskaya, Y.G. Structural and phase transformations in single-crystal rhenium- and ruthenium-alloyed nickel alloy under testing for long-term strength. Met. Sci. Heat Treat. 2015, 57, 503–506. [Google Scholar] [CrossRef]
- Wu, X.; Makineni, S.K.; Liebscher, C.H.; Dehm, G.; Mianroodi, J.R. Unveiling the Re effect in Ni-based single crystal superalloys. Nat. Commun. 2020, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Hlotka, А.А.; Haiduk, S.V. Prediction of the thermodynamic processes of phase separation in single-crystal refractory alloys based on nickel. Mater. Sci. 2020, 54, 878–883. [Google Scholar] [CrossRef]
- Rajabinezhad, M.; Bahrami, A.; Mousavinia, M.; Seyedi, S.J.; Taheri, P. Corrosion-fatigue failure of gas-turbine blades in an oil and gas production plant. Materials 2020, 13, 900. [Google Scholar] [CrossRef]
- Kvasnytska, Y.H.; Ivaskevych, L.М.; Balytskyi, О.І.; Maksyuta, І.І.; Myalnitsa, H.P. High-temperature salt corrosion of a heat-resistant nickel alloy. Mater. Sci. 2020, 56, 432. [Google Scholar] [CrossRef]
- Reising, R.F. High temperature corrosion of nickel by sodium sulffat. Corrosion 1977, 33, 84–91. [Google Scholar] [CrossRef]
- Sims, C.T.; Stoloff, N.S.; Hagel, W.C. Superalloys II: High-Temperature Materials for Aerospace and Industrial Power; John Wiley & Sons: New York, NY, USA, 1987; 640p, Available online: https://www.wiley.com/en-us/Superalloys+II%3A+High+Temperature+Materials+for+Aerospace+and+Industrial+Power-p-9780471011477 (accessed on 27 November 2022).
- Makineni, S.K.; Lenz, M.; Neumeier, S.; Spiecker, E.; Raabe, D.; Gault, B. Elemental segregation to antiphase boundaries in a crept CoNi-based single crystal superalloy. Scr. Mater. 2018, 157, 62–66. [Google Scholar] [CrossRef]
- Balitskii, O.I.; Kvasnytska, Y.H.; Ivaskevych, L.M.; Mialnitsa, H.P.; Kvasnytska, K.H. Fatigue fracture of the blades of gas turbine engine made of a new refractory nickel alloy. Mater. Sci. 2022, 57, 475–483. [Google Scholar] [CrossRef]
- Kvasnytska, Y.H.; Ivaskevich, L.M.; Balitskii, A.I.; Kvasnytska, K.H.; Mialnitsa, H.P. Structural and mechanical properties of the nickel alloy of gas-turbine engine blades. Mater. Sci. 2022, 57, 688–694. [Google Scholar] [CrossRef]
- Balyts’kyi, O.I.; Chmiel, J.; Krause, P.; Niekrasz, J.; Maciag, M. Role of hydrogen in the cavitation fracture of 45 steel in lubricating media. Mater. Sci. 2009, 45, 651–654. [Google Scholar] [CrossRef]
- Balyts’kyi, O.I.; Ivas’kevych, L.M.; Mochul’s’kyi, V.M.; Holiyan, O.M. Influence of hydrogen on the crack resistance of 10Kh15N27T3V2MR steel. Mater. Sci. 2009, 45, 258–267. [Google Scholar] [CrossRef]
- Balyts’kyi, A.I.; Kvasnyts’ka, Y.H.; Ivas’kevich, L.M.; Myal’nitsa, H.P. Corrosion and Hydrogen-Resistance of Heat-Resistant Blade Nickel-Cobalt Alloys. Mater. Sci. 2018, 54, 230–239. [Google Scholar] [CrossRef]
- Zhang, Z.; Obasi, G.; Morana, R.; Preuss, M. In-Situ observation of hydrogen induced crack initiation in a nickel-based superalloy. Scr. Mater. 2017, 140, 40–44. [Google Scholar] [CrossRef]
- Šmíd, M.; Horník, V.; Kunz, L.; Hrbáček, K.; Hutař, P. High cycle fatigue data transferability of MAR-M 247 superalloy from separately cast specimens to real gas turbine blade. Metals 2020, 10, 1460. [Google Scholar] [CrossRef]
- Mytsyk, B.G.; Ivanytskyi, Y.L.; Balitskii, A.I.; Kost’, Y.P.; Sakharuk, O.M. Study of hydrogen influence on 1020 steel by low deformation method. Mater. Lett. 2016, 184, 328–331. [Google Scholar] [CrossRef]
- Zhoua, P.J.; Yub, J.J.; Sunb, X.F.; Guanb, H.R.; Hec, X.M.; Hub, Z.Q. Influence of Y on stress rupture property of a Ni-based superalloy. Mat. Sci. Eng. A 2012, 551, 236–240. [Google Scholar] [CrossRef]
- Amouyal, Y.; Seidman, D.N. The role of hafnium in the formation of misoriented defects in Ni-based superalloys: An atomprobe tomographic study. Acta Mater. 2011, 59, 3321–3333. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, M.; Rong, L. Effect of grain size on the hydrogen embrittlement sensitivity of a precipitation strengthened Fe-Ni based alloy. Mat. Sci. Eng. A 2014, 594, 98–102. [Google Scholar] [CrossRef]
- Tkachov, V.I.; Levina, I.M.; Ivas’kevych, L.M. Distinctive features of hydrogen hydrogen degradation of heat-resistance alloys based on nickel. Mater. Sci. 1997, 33, 524–531. [Google Scholar] [CrossRef]
- Balitskii, A. Hydrogen assisted crack initiation and propagation in nickel-cobalt heat resistant superalloys. Procedia Struct. Integr. 2019, 16, 134–140. [Google Scholar] [CrossRef]
- Lee, A. Hydrogen embrittlement of nickel, cobalt and iron-based superalloys. In The Problem, Its Characterization and Effects on Particular Alloy Classes. Gaseous Hydrogen Embrittlement of Materials in Energy Technologies, Volume 1; Gangloff, R.P., Somerday, B.P., Eds.; Woodhead Publishing Limited.: Oxford, UK; Cambridge, UK; Philadelphia, PA, USA; New Delhi, India, 2012; pp. 624–667. Available online: https://www.sciencedirect.com/science/article/pii/B978184569677150017X?via%3Dihub (accessed on 27 November 2022). [CrossRef]
- Balitskii, A.; Ivaskevich, L.; Mochulskyi, V.; Eliasz, J.; Skolozdra, O. Influence of high pressure and high temperature hydrogen on fracture toughness of Ni-containing steels and alloys. Arch. Mech. Eng. 2014, 61, 129–138. [Google Scholar] [CrossRef]
- Taji, I.; Hajilou, T.; Karimi, S.; Schott, F.; Plesiutschnig, E.; Barnoush, A.; Johnsen, R. Role of grain boundaries in hydrogen embrittlement of alloy 725: Single and bi-crystal microcantilever bending study. Int. J. Hydrog. Energy 2022, 47, 12771–12781. [Google Scholar] [CrossRef]
- Taji, I.; Hajilou, T.; Ebner, A.S.; Scheiber, D.; Karimi, S.; Plesiutschnig, E.; Ecker, W.; Barnoush, A.; Maier-Kiener, V.; Johnsen, R.; et al. Hydrogen assisted intergranular cracking of alloy 725: The effect of boron and copper alloying. Corros. Sci. 2022, 203, 110331. [Google Scholar] [CrossRef]
- Balitskii, A.I.; Ivaskevich, L.M. Hydrogen effect on cumulation of failure, mechanical properties, and fracture toughness of Ni-Cr alloys. Adv. Mat. Sci. Eng. 2019, 2019, 3680253. [Google Scholar] [CrossRef]
- ASTM G129-2000(R2013); Standard Practice for Slow Strain Rate Testing to Evaluate the Susceptibility of Metallic Materials to Environmentally Assisted Cracking. American Society for Testing Material: West Conshohocken, PA, USA, 2013; p. 129. Available online: https://webstore.ansi.org/standards/astm/astmg129002013 (accessed on 27 November 2022).
- Djukic, M.; Curtin, W.A.; Zhang, Z.; Sedmak, A. Recent advances on hydrogen embrittlement understanding and future research framework. Eng. Fract. Mech. 2021, 241, 107439. [Google Scholar] [CrossRef]
- Ogawa, Y.; Noguchi, K.; Takakuwa, O. Criteria for hydrogen-assisted crack initiation in Ni-based superalloy 718. Acta Mater. 2022, 229, 117789. [Google Scholar] [CrossRef]
- Harris, Z.D.; Bhattacharyya, J.J.; Ronevich, J.A.; Agnew, S.R.; Burns, J.T. The combined effects of hydrogen and aging condition on the deformation and fracture behavior of a precipitation-hardened nickel-base superalloy. Acta Mater. 2020, 186, 616–630. [Google Scholar] [CrossRef]
- Balyts’kyi, O.I.; Ivas’kevych, L.M.; Eliasz, J.J. Static Crack Resistance of Heat-Resistant KhN43MBTYu Nickel-Chromium Alloy in Gaseous Hydrogen. Strength Mater. 2020, 52, 386–397. [Google Scholar] [CrossRef]
- Balitskii, A.I.; Ivaskevich, L.M.; Balitskii, O.A. Rotor steels crack resistance and fracture behavior for hydrogen targeted materials ever-widening database. Eng. Fract. Mech. 2022, 260, 108168. [Google Scholar] [CrossRef]
- Panasyuk, V.V.; Dmytrakh, I.M.; Toth, L.; Bilyi, O.L.; Syrotyuk, A.M. A method for the assessment of the serviceability and fracture hazard for structural elements with cracklike defects. Mater. Sci. 2014, 49, 565–576. [Google Scholar] [CrossRef]
- Balitskii, A.I.; Dmytryk, V.V.; Ivaskevich, L.M.; Balitskii, O.A.; Glushko, A.V.; Medovar, L.B.; Abramek, K.F.; Stovpchenko, G.P.; Eliasz, J.J.; Krolikowski, M.A. Improvement of the Mechanical Characteristics, Hydrogen Crack Resistance and Durability of Turbine Rotor Steels Welded Joints. Energies 2022, 15, 6006. [Google Scholar] [CrossRef]
- Aksimentyeva, O.I.; Demchenko, P.Y.; Savchyn, V.P.; Balitskii, O.A. The chemical exfoliation phenomena in layered GaSe-polyaniline composite. Nanoscale Res. Lett. 2013, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Rozumek, D.; Macha, E. Elastic-plastic fatigue crack growth in 18G2A steel under proportional bending with torsion loading. Fatigue Fract. Eng. Mater. Struct. 2006, 29, 135–145. [Google Scholar] [CrossRef]
- Moustabchir, H.; Azari, Z.; Hairi, S.; Dmytrakh, I. Experimental and computed stress distribution ahead of notch in pressure vessel: Application of T-stress conception. Comput. Mater. Sci. 2012, 58, 59–66. [Google Scholar] [CrossRef]
- Karpenko, G.V.; Litvin, A.K.; Tkachev, V.I.; Soshko, A.I. Mechanism of hydrogen embrittlement. Sov. Mater. Sci. 1975, 9, 367–371. [Google Scholar] [CrossRef]
- Rozumek, D.; Marciniak, Z. Fatigue properties of notched specimens made of FeP04 Steel. Mater. Sci. 2012, 47, 462–469. [Google Scholar] [CrossRef]
- Kozak, L.Y. Discrete models of plastic deformation of solids under the action of high hydrostatic pressure. Mater. Sci. 2016, 52, 108–112. [Google Scholar] [CrossRef]
- Balitskii, O.A.; Savchyn, V.P.; Savchyn, P.V. Thermal oxidation of indium and gallium sulphides. Phys. B Condens. Matter 2005, 355, 365–369. [Google Scholar] [CrossRef]
- Romaniv, O.N.; Nikiforchin, G.N.; Kozak, L.Y. Structural sensitivity of the cyclic crack resistance of rotor steel in gaseous hydrogen. Sov. Mater. Sci. 1984, 20, 424–429. [Google Scholar] [CrossRef]
- Romaniv, O.N.; Nikiforchin, G.N.; Kozak, L.Y. Cyclic rack resistance of constructional steel in gaseous hydrogen. Sov. Mater. Sci. 1987, 23, 439–450. [Google Scholar] [CrossRef]
- Syrotyuk, A.M.; Dmytrakh, I.M. Methods for the evaluation of fracture and strength of pipeline steels and structures under the action of working media. Part II: Influence of hydrogen-containing media. Mater. Sci. 2015, 50, 475–487. [Google Scholar] [CrossRef]
- Dmytrakh, I.M.; Leshchak, R.L.; Syrotyuk, A.M.; Barna, R.A. Effect of hydrogen concentration on fatigue crack growth behaviour in pipeline steel. Int. J. Hydrog. Energy 2017, 42, 6401–6408. [Google Scholar] [CrossRef]
- Zima, Y.V.; Kozak, L.Y. Fractographic aspects of the cyclic crack resistance of 35KhN3MFA steel in vacuum, air, and hydrogen. Mater. Sci. 1986, 22, 268–275. [Google Scholar] [CrossRef]
- Bihun, R.I.; Stasyuk, Z.V.; Balitskii, O.A. Crossover from quantum to classical electron transport in ultrathin metal films. Phys. B Condens. Matter 2016, 487, 73–77. [Google Scholar] [CrossRef]
- Capelle, J.; Dmytrakh, I.; Gilgert, J.; Jodin, P.; Pluvinage, G. A comparison of experimental results and computations for ctacked tubes subjected to internal pressure. Mat. Technol. 2006, 40, 233–237. [Google Scholar]
- Benghalia, M.A.; Faces, C.; Khadrauui, A.; Meliani, M.H.; Obot, I.B.; Sorrour, A.; Dmytrakh, M.; Azari, Z. Performance evaluation of a natural and synthetic compound as corrosion inhibitors of API 5l X52 steel in hydrochloric acid media. Moroc. J. Chem. 2018, 6, 51–61. [Google Scholar] [CrossRef]
- Akid, R.; Dmytrakh, I.M.; Gonzalez-Sanchez, J. Fatigue damage accumulation: The role of corrosion on the early stages of crack development. Corros. Eng. Sci. Technol. 2006, 41, 328–335. [Google Scholar] [CrossRef]
- Olmi, G.; Freddi, A. Low cycle fatigue behavior and anisotropy of two steels for turbogenerator coil retaining ring and rotors. In Proceedings of the 9th Youth Symposium on Experimental Solid Mechanics, Trieste, Italy, 7–10 July 2010; pp. 188–192, ISBN 978-88-95940-30-4. Available online: https://www.semanticscholar.org/paper/Low-cycle-fatigue-behaviour-and-anisotropy-of-two-Olmi-Freddi/6a7b4c86f579967fa26aa7062db32c01119a2743 (accessed on 27 November 2022).
- Olmi, G. An efficient method for the determination of the probability of failure on the basis of LCF data: Application to turbogenerator design. Struct. Durab. Health Monit. 2012, 8, 61–89. [Google Scholar]
- Krasovskii, A.Y.; Vainshtok, V.A. Use of fracture mechanics to evaluate the bearing capacity and remaining life of rotors in turbomachinery. Strength Mater. 1982, 14, 997–1005. [Google Scholar] [CrossRef]
- Eckardt, D.; Rufli, P. Advanced gas turbine technology: ABB/BCC historical first. Trans. ASME 2002, 124, 542–549. [Google Scholar] [CrossRef]
- Balitskii, O.; Borowiak-Palen, E.; Konicki, W. Synthesis and characterization of colloidal gallium selenide nanowires. Cryst. Res. Technol. 2011, 46, 417–420. [Google Scholar] [CrossRef]
- Dmytrakh, I.M.; Akid, R.; Miller, K.J. Electrochemistry of deformed smooth surfaces and short corrosion fatigue crack growth behaviour. Br. Corros. J. 1997, 32, 138–144. [Google Scholar] [CrossRef]
- Syrotyuk, А.M.; Dmytrakh, I.M. Methods for the evaluation of fracture and strength of pipeline steels and structures under the action of working media. Part І. Influence of the corrosion factor. Mater. Sci 2014, 50, 324–339. [Google Scholar] [CrossRef]
- Charpagne, M.A.; Polonsky, A.T.; Echlin, M.P.; Jacomet, S.; De Jaeger, J.; De Graef, N.; Bozzolo, T.; Pollock, M. Growth accidents induced by primary γ′ precipitates in a polycrystalline nickel-based superalloy. Scr. Mater. 2020, 186, 109–113. [Google Scholar] [CrossRef]
- Balitska, V.; Shpotyuk, Y.; Filipecki, J.; Shpotyuk, O.; Iovu, M. Post-irradiation relaxation in vitreous arsenic/antimony trisulphides. J. Non-Cryst. Solids 2011, 357, 487–489. [Google Scholar] [CrossRef]
- Ogawa, Y.; Hosoi, H.; Tsuzaki, K.; Redarce, T.; Takakuwa, O.; Matsunaga, H. Hydrogen, as an alloying element, enables a greater strength-ductility balance in an Fe-Cr-Ni-based, stable austenitic stainless steel. Acta Mater. 2020, 199, 181–192. [Google Scholar] [CrossRef]
- Michler, T.; Naumann, J.; Balogh, M.P. Hydrogen environment embrittlement of solution treated Fe–Cr–Ni super alloys. Mat. Sci. Eng. A. 2014, 607, 71–80. [Google Scholar] [CrossRef]
- Zhang, Z.; Obasis, G.; Morana, R.; Preuss, M. Hydrogen assisted crack initiation and propagation in a nickel-based superalloy. Acta Mater. 2016, 113, 272–283. [Google Scholar] [CrossRef]
- Guédou, J.-Y.; Augustins-Lecallier, I.; Nazé, L.; Caron, P.; Locq, D. Development of a new fatigue and creep resistant PM nickel-base superalloy for disk applications. Superalloys 2008, 1, 21–30. [Google Scholar] [CrossRef]
- Furrer, D.; Fecht, H. Ni-based superalloys for turbine discs. JOM 1999, 51, 14–17. [Google Scholar] [CrossRef]
- Drexler, A.; He, S.; Pippan, R.L.; Romaner, V.; Razumovskiy, I.; Ecker, W. Hydrogen segregation near a crack tip in nickel. Scr. Mater. 2021, 194, 113697. [Google Scholar] [CrossRef]
- Tarzimoghadam, Z.; Rohwerder, M.; Merzlikin, S.V.; Bashir, A.; Yedra, L.; Eswara, S.; Ponge, D.; Raabe, D. Multi-scale and spatially resolved hydrogen mapping in a Ni–Nb model alloy reveals the role of the δ phase in hydrogen embrittlement of alloy 718. Acta Mater. 2016, 109, 69–81. [Google Scholar] [CrossRef]
- Ma, D.; Blazej Grabowski, B.; Körmann, F.; Neugebauer, J.; Raabe, D. Ab initio thermodynamics of the CoCrFeMnNi high entropy alloy: Importance of entropy contributions beyond the configurational one. Acta Mater. 2015, 100, 90–97. [Google Scholar] [CrossRef]
- Shpotyuk, O.I.; Balitska, V.O.; Vakiv, M.M.; Shpotyuk, L.I. Sensors of high-energy radiation based on amorphous chalcogenides. Sens. Actuators A Phys. 1998, 68, 356–358. [Google Scholar] [CrossRef]
- Tarzimoghadam, Z.; Ponge, D.; Klöwer, J.; Raabe, D. Hydrogen-assisted failure in Ni-based superalloy 718 studied under in situ hydrogen charging: The role of localized deformation in crack propagation. Acta Mater. 2017, 128, 365–374. [Google Scholar] [CrossRef]
- Povstugar, I.; Choi, P.-P.; Neumeier, S.; Bauer, A.; Christopher, H.; Göken, Z.M.; Raabe, D. Elemental partitioning and mechanical properties of Ti- and Ta-containing Co–Al–W-base superalloys studied by atom probe tomography and nanoindentation. Acta Mater. 2014, 78, 78–85. [Google Scholar] [CrossRef]
- Michler, T. Influence of gaseous hydrogen on the tensile properties of Fe–36Ni INVAR alloy. Int. J. Hydrogen Energy 2014, 39, 11807–11809. [Google Scholar] [CrossRef]
- Ma, D.; Yao, M.; Pradeep, V.; Tasan, C.C.; Springer, H.; Raabe, D. Phase stability of non-equiatomic CoCrFeMnNi high entropy alloys. Acta Mater. 2015, 98, 288–296. [Google Scholar] [CrossRef]
- Chauvet, E.; Kontis, P.; Jägle, E.A.; Gault, B.; Raabe, D.; Tassin, C.; Blandin, J.-J.; Dendievel, R.; Vayre, B.; Abed, S.; et al. Hot cracking mechanism affecting a non-weldable Ni-based superalloy produced by selective electron beam melting. Acta Mater. 2018, 142, 82–94. [Google Scholar] [CrossRef]
- Tytko, D.; Choi, P.-P.; Klöwer, J.; Kostka, A.; Inden, G.; Raabe, D. Microstructural evolution of a Ni-based superalloy (617B) at 700°C studied by electron microscopy and atom probe tomography. Acta Mater. 2012, 60, 1731–1740. [Google Scholar] [CrossRef]
- Matuszewski, K.; Rettig, R.; Matysiak, H.; Peng, Z.; Povstugar, I.; Choi, P.; Müller, J.; Raabe, D.; Spiecker, E.; Kurzydłowski, K.J.; et al. Effect of ruthenium on the precipitation of topologically close packed phases in Ni-based superalloys of 3rd and 4th generation. Acta Mater. 2015, 95, 274–283. [Google Scholar] [CrossRef]
- Kontis, P.; Chauvet, E.; Peng, Z.; He, J.; Kwiatkowski, A.; Silva, D.; Raabe, D.; Tassin, C.; Blandin, J.J.; Abed, S.; et al. Atomic-scale grain boundary engineering to overcome hot-cracking in additively-manufactured superalloys. Acta Mater. 2019, 177, 209–221. [Google Scholar] [CrossRef]
- Haghighat, S.M.H.; Eggeler, G.; Raabe, D. Effect of climb on dislocation mechanisms and creep rates in γ′-strengthened Ni base superalloy single crystals: A discrete dislocation dynamics study. Acta Mater. 2013, 61, 3709–3723. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Zeravcic, V.S.; Sedmak, A.; Rajicic, B. The synergistic action and interplay of hydrogen embrittlement mechanisms in steels and iron: Localized plasticity and decohesion. Eng. Fract. Mech. 2019, 216, 106528. [Google Scholar] [CrossRef]
- Wasim, M.; Djukic, M.; Ngo, T.D. Influence of hydrogen-enhanced plasticity and decohesion mechanisms of hydrogen embrittlement on the fracture resistance of steel. Eng. Fail. Anal. 2021, 123, 105312. [Google Scholar] [CrossRef]
- Glotka, О.А. Distribution of alloying elements in carbides of refractory nickel alloys under the conditions of equiaxial crystallization. Mater. Sci. 2021, 56, 714–721. [Google Scholar] [CrossRef]
- Wasim, M.; Djukic, M. Hydrogen embrittlement of low carbon structural steel at macro-, micro- and nano-levels. Int. J. Hydrogen Energy 2020, 45, 2145–2156. [Google Scholar] [CrossRef]
- Prakash, A.; Guénolé, J.; Wang, J.; Müller, J.; Spiecker, E.; Mills, M.J.; Povstugar, I.; Choi, P.; Raabe, D.; Bitzek, E. Atom probe informed simulations of dislocation–precipitate interactions reveal the importance of local interface curvature. Acta Mater. 2015, 92, 33–45. [Google Scholar] [CrossRef]
- Ram, F.; Li, Z.; Zaefferer, S.; Masood, S.; Haghighat, H.; Zhu, Z.; Raabe, D.; Roger, C.; Reed, R.C. On the origin of creep dislocations in a Ni-base, single-crystal superalloy: An ECCI, EBSD, and dislocation dynamics-based study. Acta Mater. 2016, 109, 151–161. [Google Scholar] [CrossRef]
- Neeraj, T.; Srinivasan, R.; Li, J. Hydrogen embrittlement of ferritic steels: Observations on deformation microstructure, nanoscale dimples and failure by nanovoiding. Acta Mater. 2012, 60, 5160–5171. [Google Scholar] [CrossRef]
- Teus, S.M.; Mazanko, V.F.; Plive, J.-M.; Gavrilijuk, V.G. Grain boundary migration of substitutional and interstitial atoms in α-iron. Acta Mater. 2012, 69, 105–113. [Google Scholar] [CrossRef]
- Van Sluytman, J.S.; Pollock, T.M. Optimal precipitate shapes in nickel-base γ–γ′ alloys. Acta Mater. 2012, 60, 1771–1783. [Google Scholar] [CrossRef]
- Bezold, A.; Volz, N.; Lenz, M.; Karpstein, N.; Zenk, C.H.; Spiecker, E.; Göken, M.; Neumeier, S. Quantification of the temperature-dependent evolution of defect structures in a CoNi-base superalloy. Acta Mater. 2022, 227, 117702. [Google Scholar] [CrossRef]
- Zhang, X.; Sergiy, V.; Divinski, S.V.; Blazej Grabowski, B. Ab initio prediction of vacancy energetics in HCP Al-Hf-Sc-Ti-Zr high entropy alloys and the subsystems. Acta Mater. 2022, 227, 117677. [Google Scholar] [CrossRef]
- Balitska, V.; Filipecki, J.; Shpotyuk, O.; Swiatek, J.; Vakiv, M. Dynamic radiation-induced effects in chalcogenide vitreous compounds. J. Non-Cryst. Solids 2001, 287, 216–221. [Google Scholar] [CrossRef]
- Glotka, O.A.; Gayduk, S.V.; Olshanetskiy, V.Y. The distribution of alloying elements in secondary carbides of heat-resistant nickel alloys. Metalozn. Obrobka Met. 2020, 26, 25–36. [Google Scholar] [CrossRef]
- Glotka, A.A.; Haiduk, S.V.; Ol’shanetskii, V.Y. Modeling thermophysical characteristics of nickel-based superalloys. J. Eng. Phys. Thermophys. 2021, 94, 1363–1368. [Google Scholar] [CrossRef]
- Rezende, C.; Araujo, L.S.; Gabriel, S.B.; dos Santos, D.S.; de Almeida, L.H. Hydrogen embrittlement in nickel-based superalloy 718: Relationship between γ′ + γ″ precipitation and the fracture mode. Int. J. Hydrogen Energy 2015, 40, 17075–17083. [Google Scholar] [CrossRef]
- New Energy and Industrial Technology Development Organization. International Clean Energy Network Using Hydrogen Conversion (WE-NET). 1993 Annual Summary Report on Results (NEDO-WE-NET-93); New Energy and Industrial Technology Development Organization: Kawasaki, Japan, 1994; 84p. Available online: https://www.osti.gov/etdeweb/servlets/purl/20316293 (accessed on 27 November 2022).
- Kozakiewicz, A.; Jóźwiak, S.; Jóźwiak, P.; Kachel, S. Material origins of the accelerated operational wear of RD-33 engine blades. Materials 2021, 14, 336. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Raabe, D.; Roters, F.; Arsenlis, A. Interfacial dislocation motion and interactions in single-crystal superalloys. Acta Mater. 2014, 79, 216–233. [Google Scholar] [CrossRef]
- Ryś, J. Quantitative Metallography; AGH: Krakow, Poland, 1983; 280p. [Google Scholar]
- Gao, Z.; Tan, J.; Guo, W.; Zhang, C.; Zhang, W. Characterization of the microstructure evolution of Ni-based superalloy at liquidus temperature by electromagnetic field. Metals 2018, 8, 748. [Google Scholar] [CrossRef]
- Pearson, K. The Grammar of Science; Dover Phoenix Editions; Cosimo Classics: New York, NY, USA, 2004; 416p. [Google Scholar]
- Wahl, J.B.; Harris, K. CMSX-4 plus single alloy development, characterization and application development. In Superalloys 2016: Proceedings of the 13th International Symposium on Superalloys, TMS (the Minerals, Metals & Materials Society), Seven Springs, PA, USA, 11–15 September 2016; Wiley: Hoboken, NJ, USA, 2016; pp. 25–33. [Google Scholar]


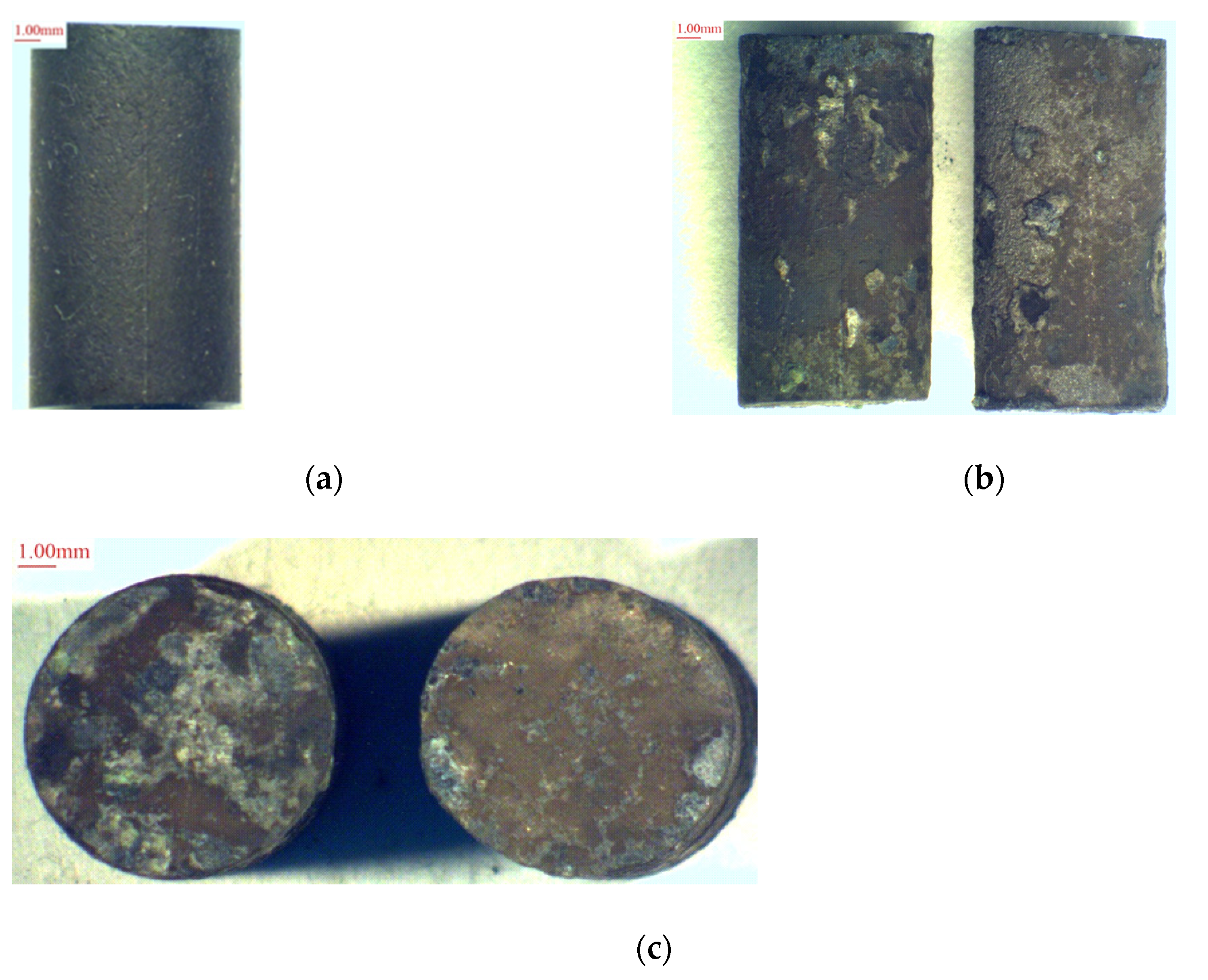
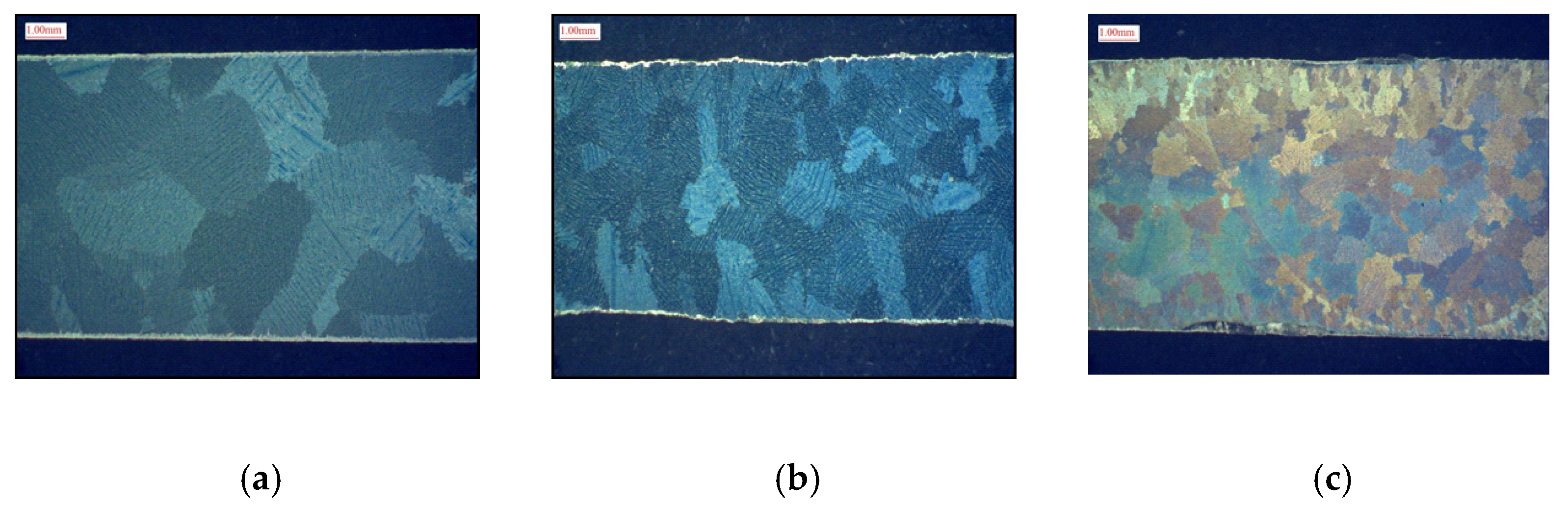

| Alloy | Experimental | CM88Y | SDP 3-A | |||
|---|---|---|---|---|---|---|
| Sample Number | 1 | 2 | 3 | 4 | 5 | 6 |
| Grain size in the alloy, ×10−3 m | 1.0–2.0 | 1.5–3.0 | 1.0–3.0 | 3.0–4.0 | 0.5–1.0 | 0.5–1.0 |
| Specific mass loss, after 30 h, kg/m2 | 0.287 | 0.278 | 0.353 | 0.289 | 0.053 | 0.121 |
| Corrosion rate, Vq, kg/m2 h | 0.0089 | 0.0096 | 0.0118 | 0.0096 | 0.0018 | 0.004 |
| Depth of external corrosion, d × 10−3 m | 0.28 | 0.05 | 0.05 | 0.24 | 0.30 | 0.27 |
| Depth of internal corrosion, h ×10−3 m | 0.05–0.10 | 0.05–0.10 | 0.12–0.15 | 0.10–0.12 | 0.20–0.40 | 0.05–0.30 |
| Alloy | Content of Elements, wt.% (Ni-Balance) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Cr | Co | Mo | Ti | Al | W | Nb | Hf | Y | B | Zr | Ce | |
| CM88Y | 0.07 | 15.6 | 11.0 | 2.0 | 4.20 | 3.8 | 5.90 | 0.2 | 0.3 | 0.3 | 0.07 | 0.05 | – |
| ZhS3DK | 0.07 | 12.0 | 10.3 | 4.2 | 2.99 | 4.4 | 2.96 | – | – | – | 0.01 | – | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balitskii, A.I.; Kvasnytska, Y.H.; Ivaskevych, L.M.; Kvasnytska, K.H.; Balitskii, O.A.; Shalevska, I.A.; Shynskii, O.Y.; Jaworski, J.M.; Dowejko, J.M. Hydrogen and Corrosion Resistance of Nickel Superalloys for Gas Turbines, Engines Cooled Blades. Energies 2023, 16, 1154. https://doi.org/10.3390/en16031154
Balitskii AI, Kvasnytska YH, Ivaskevych LM, Kvasnytska KH, Balitskii OA, Shalevska IA, Shynskii OY, Jaworski JM, Dowejko JM. Hydrogen and Corrosion Resistance of Nickel Superalloys for Gas Turbines, Engines Cooled Blades. Energies. 2023; 16(3):1154. https://doi.org/10.3390/en16031154
Chicago/Turabian StyleBalitskii, Alexander I., Yuliia H. Kvasnytska, Lyubomir M. Ivaskevych, Kateryna H. Kvasnytska, Olexiy A. Balitskii, Inna A. Shalevska, Oleg Y. Shynskii, Jaroslaw M. Jaworski, and Jakub M. Dowejko. 2023. "Hydrogen and Corrosion Resistance of Nickel Superalloys for Gas Turbines, Engines Cooled Blades" Energies 16, no. 3: 1154. https://doi.org/10.3390/en16031154
APA StyleBalitskii, A. I., Kvasnytska, Y. H., Ivaskevych, L. M., Kvasnytska, K. H., Balitskii, O. A., Shalevska, I. A., Shynskii, O. Y., Jaworski, J. M., & Dowejko, J. M. (2023). Hydrogen and Corrosion Resistance of Nickel Superalloys for Gas Turbines, Engines Cooled Blades. Energies, 16(3), 1154. https://doi.org/10.3390/en16031154









