Preparation of Biomass Hydrochar and Application Analysis of Blast Furnace Injection
Abstract
:1. Introduction
2. Experiment
2.1. Experimental Materials
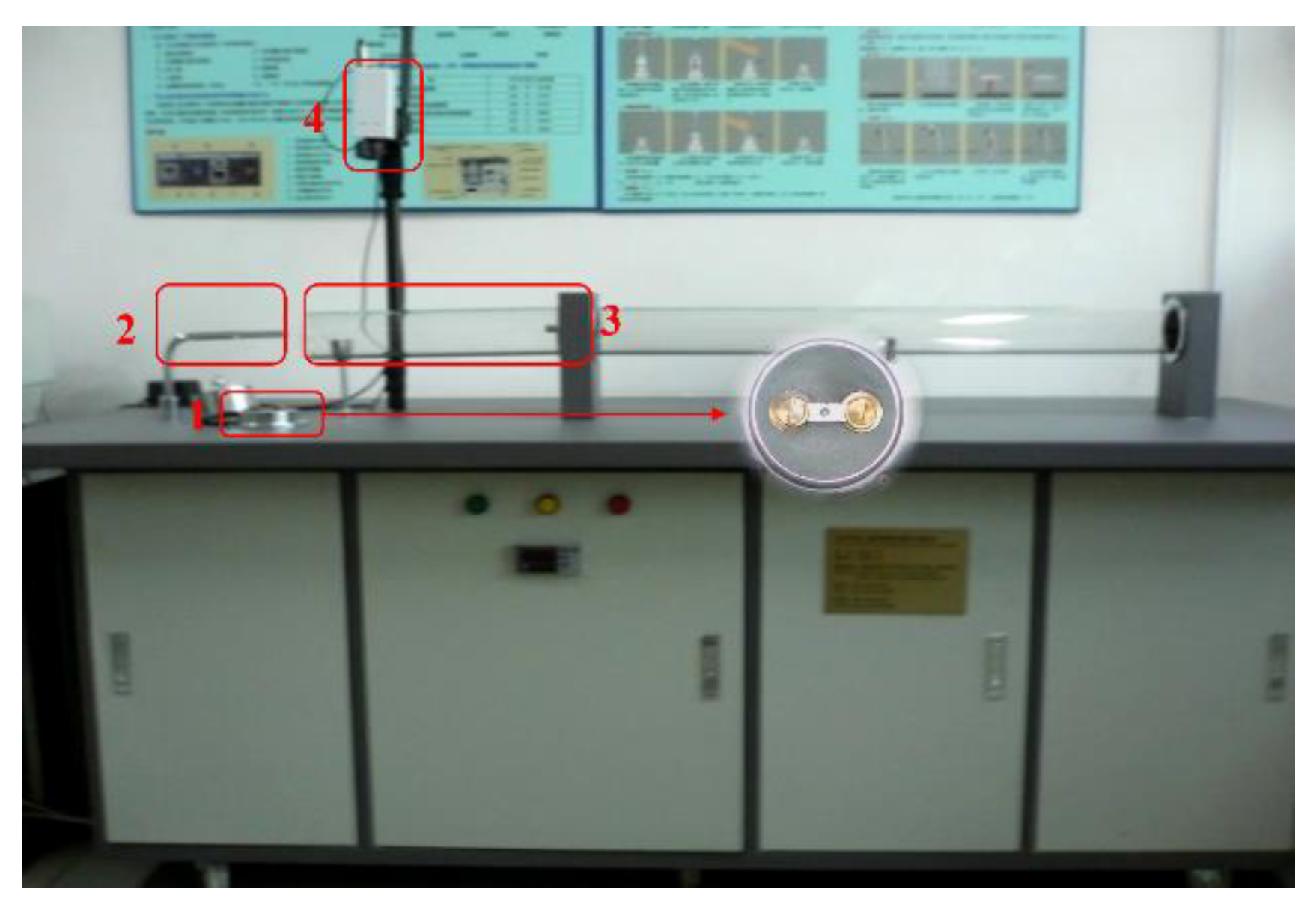
2.2. Experimental Method
2.3. Analytical Methods
3. Experimental Results and Discussion
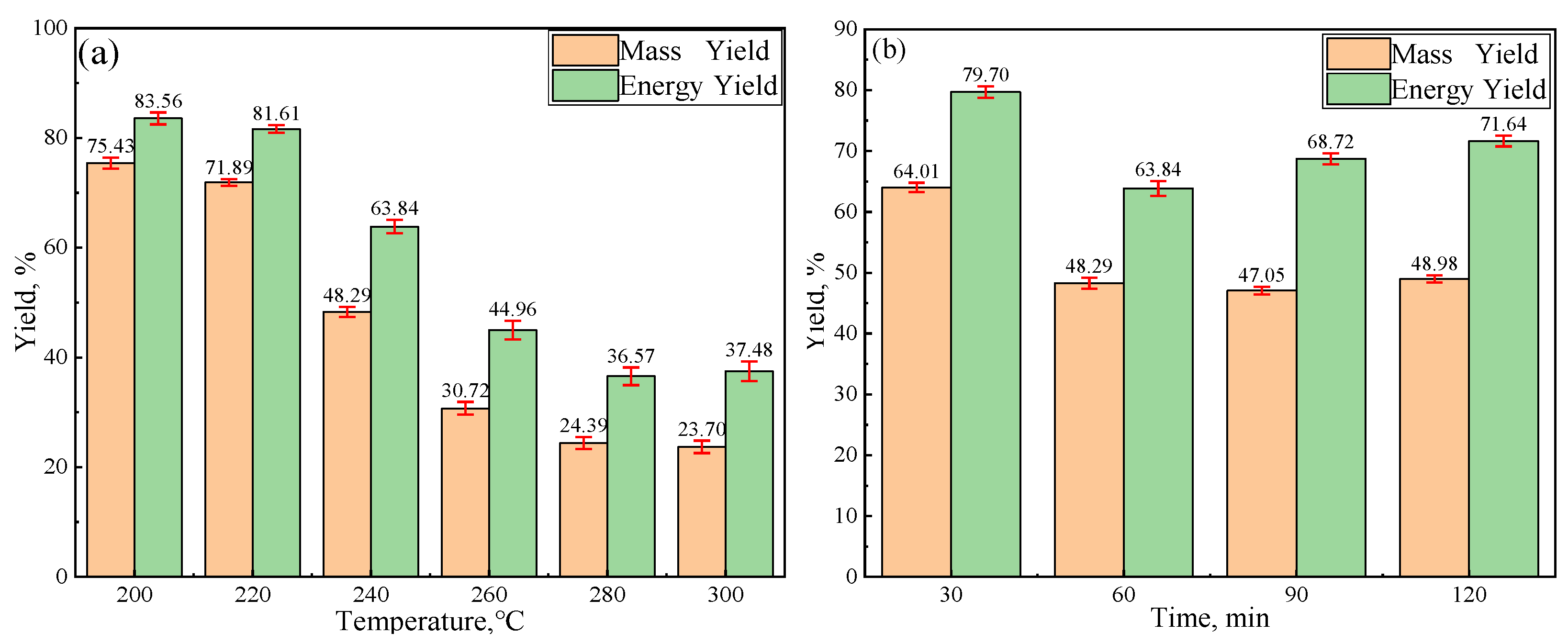
3.1. Yield and Morphology Analysis
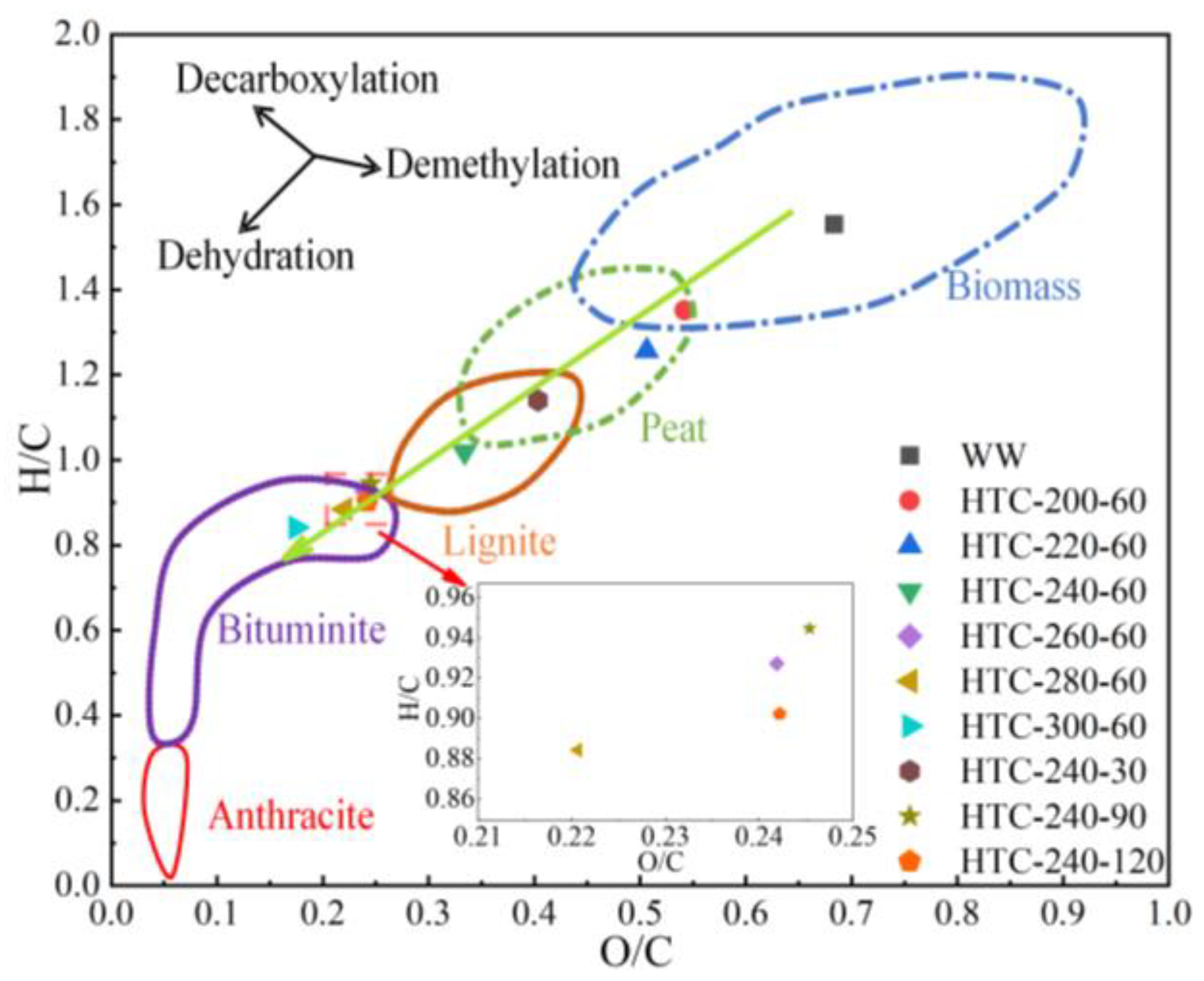
3.2. Proximate Analysis and Ultimate Analysis
3.3. Structural Analysis of Hydrochar
3.4. Combustion Behavior Analysis
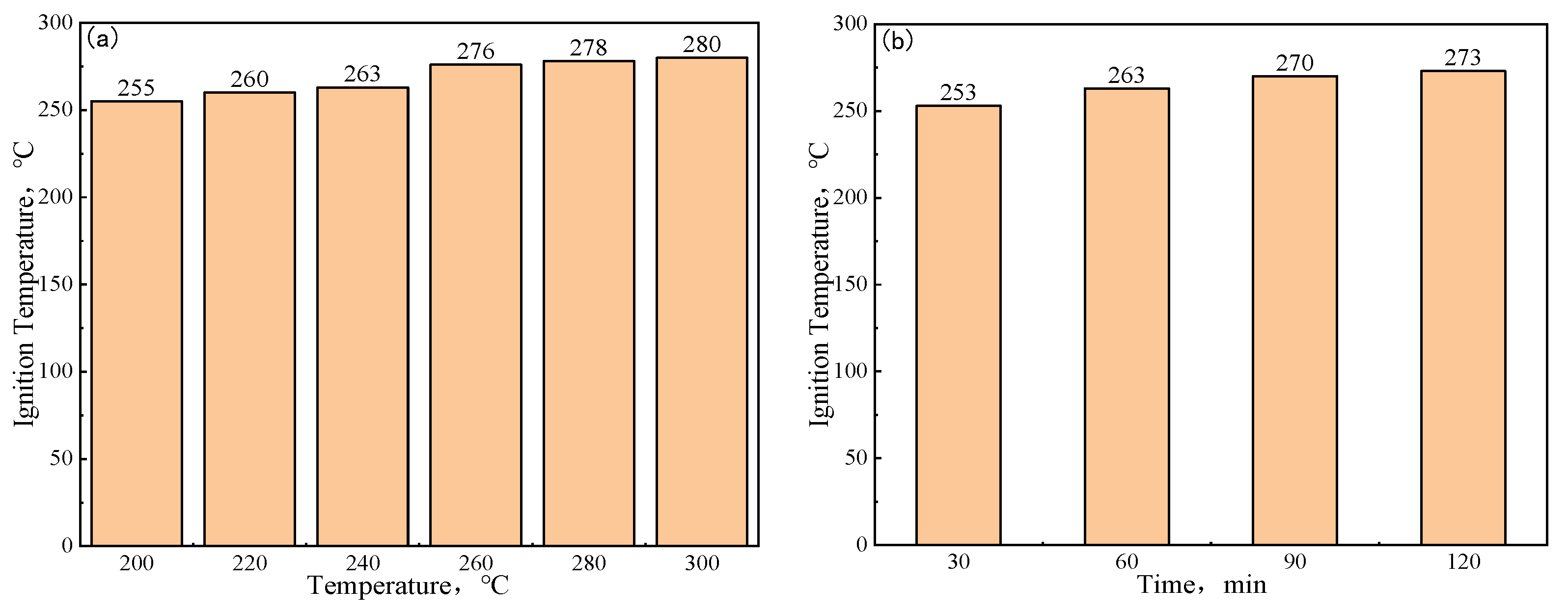
3.5. Ignition Temperature and Explosive Analysis
3.6. Alkali Metal Content Analysis
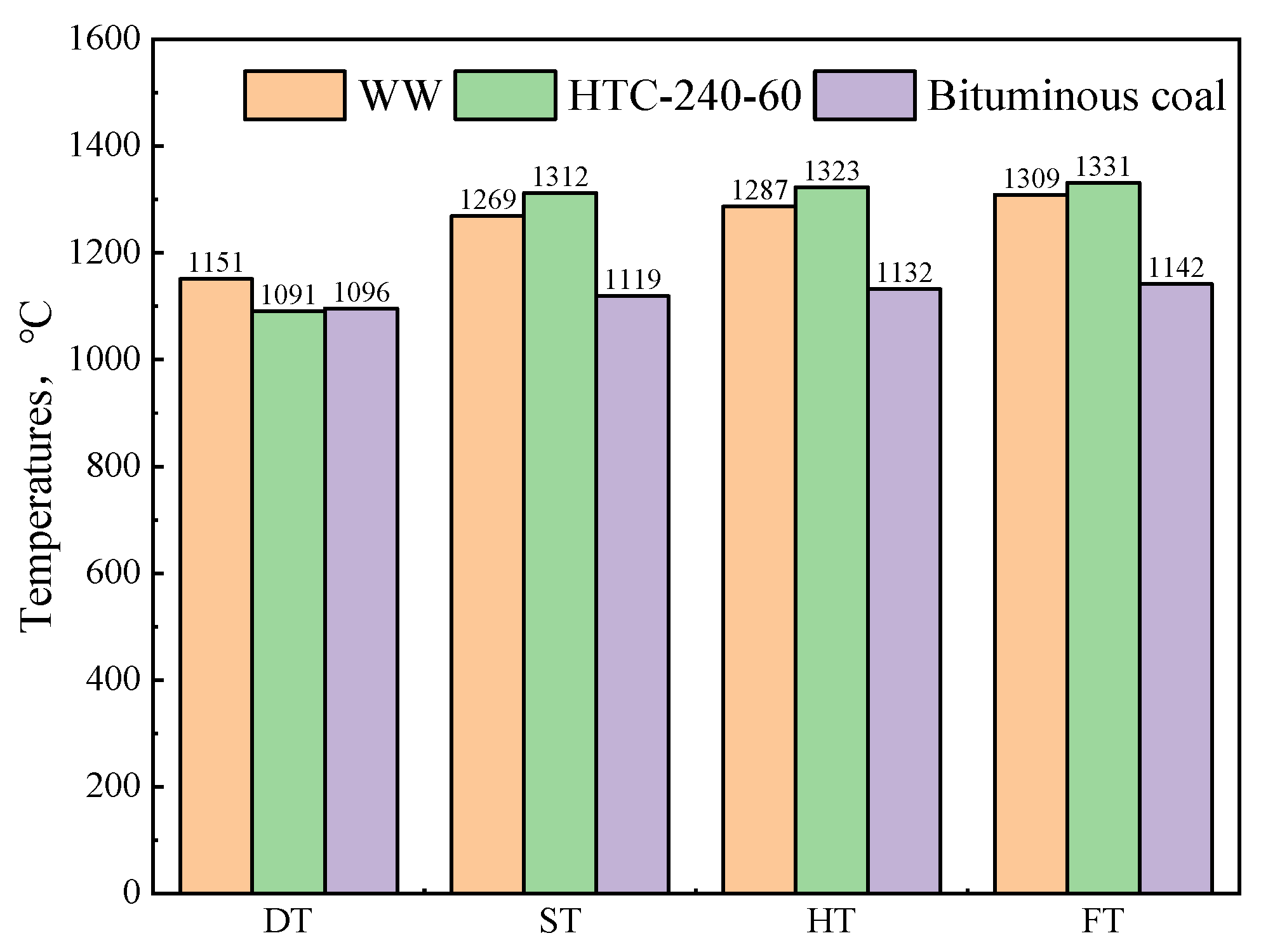
3.7. Analysis of Ash Composition and Ash Melt Characteristics
3.8. CO2 Emissions Reduction Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- National Bureau of Statistics of China. Statistical Communiqué of the People’s Republic of China on National Economic and Social Development in 2020. China Stat. 2021, 3, 8–22. [Google Scholar]
- Quader, M.A.; Ahmed, S.; Dawal, S.Z.; Nukman, Y. Present needs, recent progress and future trends of energy-efficient Ultra-Low Carbon Dioxide (CO2) Steelmaking (ULCOS) program. Renew. Sustain. Energ. Rev. 2016, 55, 537–549. [Google Scholar] [CrossRef]
- Zhao, J.; Zuo, H.B.; Wang, Y.J.; Wang, J.S.; Xue, Q.G. Review of green and low-carbon ironmaking technology. Ironmak. Steelmak. 2020, 47, 296–306. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Kemppainen, A.; Haapakangas, J.; Fabritius, T. Extensive review of the opportunities to use biomass-based fuels in iron and steelmaking processes. J. Clean. Prod. 2017, 148, 709–734. [Google Scholar] [CrossRef]
- Cao, L.C.; Zhang, C.; Chen, H.H.; Tsang, D.C.W.; Luo, G.; Zhang, S.C.; Chen, J. Hydrothermal liquefaction of agricultural and forestry wastes: State-of-the-art review and future prospects. Bioresour. Technol. 2017, 245, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Abbasi T, Abbasi S A. Biomass energy and the environmental impacts associated with its production and utilization. Renew. Sustain. Energy Rev. 2010, 14, 919–937. [Google Scholar] [CrossRef]
- Mousa, E.; Wang, C.; Riesbeck, J.; Larsson, M. Biomass applications in iron and steel industry: An overview of challenges and opportunities. Renew. Sustain. Energy Rev. 2016, 65, 1247–1266. [Google Scholar] [CrossRef]
- Orre, J.; Ökvist, L.S.; Bodén, A.; Björkman, B. Understanding of Blast Furnace Performance with Biomass Introduction. Minerals 2021, 11, 157. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.S.; Han, Y.J.; Li, Y.J.; Gao, P. Review on coal-based reduction and magnetic separation for refractory iron-bearing resources. Int. J. Miner. Metall. Mater. 2022, 29, 2087–2105. [Google Scholar] [CrossRef]
- Cholico, G.D.; Lara, N.O.; Miranda, M.A.S.; Estrella, R.M.; García, R.E.; Patiño, C.A.L. Efficient metallization of magnetite concentrate by reduction with agave bagasse as a source of reducing agents. Int. J. Miner. Metall. Mater. 2021, 28, 603–611. [Google Scholar] [CrossRef]
- Zandi, M.; Martinez-Pacheco, M.; Fray, T.A.T. Biomass for iron ore sintering. Miner. Eng. 2010, 23, 1139–1145. [Google Scholar] [CrossRef]
- Ooi, T.C.; Aries, E.; Bruce, C.R.E.; Dennis, T.; David, R.A.; Ray, F.; Fray, T.; Tognarelli, D. The study of sunflower seed husks as a fuel in the iron ore sintering process. Miner. Eng. 2008, 21, 167–177. [Google Scholar] [CrossRef]
- Zhou, D.; Wei, R.; Long, H.; Li, J.X.; Qi, L.Y.; Xu, C.C. Combustion characteristics and kinetics of different food solid wastes treatment by blast furnace. Renew. Energy 2020, 145, 530–541. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, J.; Wang, G.; Wang, C.; Mao, X.; Ning, X.; Zhang, N.; Teng, H.; Li, J.; Wang, C. Feasibility analysis of plastic and biomass hydrochar for blast furnace injection. Energy 2023, 263, 125903. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Xiao, L.; Zhang, X.Y.; Tong, S.; Wu, C.; Ashida, R.; Liu, W.; Miura, K.; Yao, H. Novel carbon-rich additives preparation by degradative solvent extraction of biomass wastes for coke-making. Bioresour. Technol. 2016, 207, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, J.L.; Wu, S.L.; Wu, J.L.; Xu, J.Y.; Ning, X.J.; Wang, G. Feasibility Analysis of Biomass Hydrochar Blended Coal Injection for Blast Furnace. Sustainability 2022, 14, 10885. [Google Scholar] [CrossRef]
- Zhang, J.L.; Fu, H.Y.; Liu, Y.X.; Dang, H.; Ye, L.; Alberto, N.C.; Xu, R.S. Review on biomass metallurgy: Pretreatment technology, metallurgical mechanism and process design. Int. J. Miner. Metall. Mater. 2022, 29, 1133–1149. [Google Scholar] [CrossRef]
- Khan, T.A.; Saud, A.S.; Jamari, S.S.; Rahim, M.H.A.; Park, J.W.; Kim, H.J. Hydrothermal carbonization of lignocellulosic biomass for carbon rich material preparation: A review. Biomass. Bioenergy 2019, 130, 105384. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Khiari, B.; Jellali, S.; Ghimbeu, C.M.; Jeguirim, M. Hydrochars production, characterization and application for wastewater treatment: A review. Renew. Sustain. Energy Rev. 2020, 127, 109882. [Google Scholar] [CrossRef]
- Sharma, H.B.; Sarmah, A.K.; Dubey, B. Hydrothermal carbonization of renewable waste biomass for solid biofuel production: A discussion on process mechanism, the influence of process parameters, environmental performance and fuel properties of hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Hydrothermal carbonization of anaerobically digested sludge for solid fuel production and energy recovery. Fuel 2014, 130, 120–125. [Google Scholar] [CrossRef]
- Yang, G.Y.; Liu, H.; Li, Y.; Zhou, Q.Y.; Jin, M.H.; Xiao, H.; Yao, H. Kinetics of hydrothermal carbonization of kitchen waste based on multi-component reaction mechanism. Fuel 2022, 324, 124693. [Google Scholar] [CrossRef]
- He, Q.; Cheng, C.; Abdul, R.; Ding, L.; Lam, S.A.; Yu, G.S. Effect of hydrothermal carbonization on woody biomass: From structure to reactivity. Fuel 2022, 330, 125586. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, X.; Peng, X.; Yu, Z.S. Hydrothermal carbonization of typical components of municipal solid waste for deriving hydrochars and their combustion behavior. Bioresour. Technol. 2017, 243, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Lee, K.; Kim, D. Characterized hydrochar of algal biomass for producing solid fuel through hydrothermal carbonization. Bioresour. Technol. 2018, 258, 119–124. [Google Scholar] [CrossRef]
- Ning, X.J.; Liang, W.; Zhang, J.L.; Wang, G.W.; Li, Y.J.; Jiang, C.H. Effect of ash on coal structure and combustibility. Int. J. Miner. Metall. Mater. 2019, 26, 973–982. [Google Scholar] [CrossRef]
- Zhu, G.; Yang, L.; Gao, Y.; Xu, J.Y.; Chen, H.J.; Zhu, Y.Z.; Wang, Y.; Liao, C.; Lu, C.; Zhu, C. Characterization and pelletization of cotton stalk hydrochar from HTC and combustion kinetics of hydrochar pellets by TGA. Fuel 2019, 244, 479–491. [Google Scholar] [CrossRef]
- Yan, K.; Liu, C.; Liu, L.; Xiong, M.; Chen, J.T.; Zhang, Z.T.; Zhong, S.; Xu, Z.; Huang, J. Pyrolysis behaviour and combustion kinetics of waste printed circuit boards. Int. J. Miner. Metall. Mater. 2022, 29, 1722–1732. [Google Scholar] [CrossRef]
- Yu, C.M.; Ren, S.; Wang, G.W.; Xu, J.J.; Teng, H.P.; Li, T.; Huang, C.; Wang, C. Kinetic analysis and modeling of maize straw hydrochar combustion using a multi-Gaussian-distributed activation energy model. Int. J. Miner. Metall. Mater. 2022, 29, 464–472. [Google Scholar] [CrossRef]
- Jin, L.Z.; Niu, X.M. Micromorphology and safety properties of meager and meager-lean coal for blast furnace injection. Int. J. Miner. Metall. Mater. 2021, 28, 774–781. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Upgrading the characteristics of biochar from cellulose, lignin, and xylan for solid biofuel production from biomass by hydrothermal carbonization. J. Ind. Eng. Chem. 2016, 42, 95–100. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuel Bioprod. Bioresour. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Sermyagina, E.; Saari, J.; Kaikko, J.; Vakkilainen, E. Hydrothermal carbonization of coniferous biomass: Effect of process parameters on mass and energy yields. J. Anal. Appl. Pyrol. 2015, 113, 551–556. [Google Scholar] [CrossRef]
- Zhang, D.L.; Wang, F.; Shen, X.L.; Yi, W.M.; Li, Z.H.; Li, Y.J.; Tian, C. Comparison study on fuel properties of hydrochars produced from corn stalk and corn stalk digestate. Energy 2018, 165, 527–536. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Q.; Xie, W.L.; Wang, Y.F.; Kang, J.M. Effects of temperature, time and acidity of hydrothermal carbonization on the hydrochar properties and nitrogen recovery from corn stover. Biomass Bioenergy 2019, 122, 175–182. [Google Scholar] [CrossRef]
- Liang, W.; Wang, G.W.; Jiao, K.X.; Ning, X.J.; Zhang, J.L.; Guo, X.M.; Li, J.; Wang, C. Conversion mechanism and gasification kinetics of biomass char during hydrothermal carbonization. Renew. Energy 2021, 173, 318–328. [Google Scholar] [CrossRef]
- Ning, X.J.; Liang, W.; Wang, G.W.; Xu, R.S.; Wang, P.; Zhang, J.L.; Guo, X.; Jiang, C.; Li, J.; Wang, C. Effect of pyrolysis temperature on blast furnace injection performance of biochar. Fuel 2022, 313, 122648. [Google Scholar] [CrossRef]
- Wang, C.; Mellin, P.; Lövgren, J.; Nilsson, L.; Yang, W.H.; Salman, H.; Hultgren, A.; Larsson, M. Biomass as blast furnace injectant–Considering availability, pretreatment and deployment in the Swedish steel industry. Energy Convers. Manag. 2015, 102, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [Green Version]
- Román, S.; Nabais JM, V.; Laginhas, C.; Ledesma, B.; González, J.F. Hydrothermal carbonization as an effective way of densifying the energy content of biomass. Fuel Process. Technol. 2012, 103, 78–83. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, C.; Xia, J.; Yang, S. Experimental study on the comprehensive behavior of combustion for blended coals. Asia-Pac. J. Chem. Eng. 2010, 5, 435–440. [Google Scholar] [CrossRef]
- Cai, J.J.; Wang, S.Z.; Kuang, C.; Tang, X.Y. Insight into the kinetic analysis of catalytic combustion for biomass after alkaline metals loaded pretreatment. Fuel 2017, 203, 501–513. [Google Scholar] [CrossRef]
- GB/T 32151.5-2015; Greenhouse Gas Emissions Accounting and Reporting Requirements Part 5: Steel Producers. National Standards of People’s Republic of China: Beijing, China, 2015.
- GB/T 32150-2015; General Guideline of the Green House Gas Emissions Accounting and Reporting for Industrial Enterprises. National Standards of People’s Republic of China: Beijing, China, 2015.





| Sample | Proximate Analysis (wt.%) | Ultimate Analysis (wt.%) | H/C | O/C | HHV (MJ/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ad | Vd | FCd * | Cd | Hd | Od * | Nd | Sd | ||||
| WW | 1.98 | 81.85 | 16.17 | 47.89 | 6.20 | 43.64 | 0.25 | 0.04 | 1.55 | 0.68 | 19.47 |
| HTC-200-60 | 2.23 | 73.01 | 24.76 | 53.10 | 5.98 | 38.38 | 0.27 | 0.04 | 1.35 | 0.54 | 21.57 |
| HTC-220-60 | 1.92 | 70.75 | 27.33 | 54.98 | 5.76 | 37.11 | 0.20 | 0.03 | 1.26 | 0.51 | 22.10 |
| HTC-240-30 | 2.02 | 65.01 | 32.97 | 59.89 | 5.69 | 32.22 | 0.14 | 0.04 | 1.14 | 0.40 | 24.24 |
| HTC-240-60 | 1.84 | 58.21 | 39.95 | 63.95 | 5.43 | 28.48 | 0.28 | 0.02 | 1.02 | 0.33 | 25.74 |
| HTC-240-90 | 1.72 | 56.44 | 41.84 | 69.78 | 5.49 | 22.83 | 0.16 | 0.02 | 0.94 | 0.25 | 28.44 |
| HTC-240-120 | 1.21 | 55.32 | 43.47 | 70.50 | 5.30 | 22.77 | 0.21 | 0.01 | 0.90 | 0.24 | 28.48 |
| HTC-260-60 | 1.59 | 52.70 | 45.71 | 70.13 | 5.42 | 22.62 | 0.21 | 0.03 | 0.93 | 0.24 | 28.50 |
| HTC-280-60 | 1.21 | 52.30 | 46.49 | 72.05 | 5.31 | 21.20 | 0.20 | 0.03 | 0.88 | 0.22 | 29.19 |
| HTC-300-60 | 1.20 | 50.01 | 48.79 | 75.59 | 5.30 | 17.60 | 0.28 | 0.03 | 0.84 | 0.17 | 30.79 |
| Sample | St (m2/g) | Vt (cm3/g) | Da (nm) |
|---|---|---|---|
| HTC-200-60 | 3.09 | 0.0122 | 15.81 |
| HTC-220-60 | 2.51 | 0.0103 | 16.48 |
| HTC-240-30 | 1.03 | 0.0038 | 14.64 |
| HTC-240-60 | 1.36 | 0.0051 | 15.05 |
| HTC-240-90 | 0.98 | 0.0031 | 12.55 |
| HTC-240-120 | 0.75 | 0.0021 | 11.05 |
| HTC-260-60 | 1.18 | 0.0041 | 13.84 |
| HTC-280-60 | 1.15 | 0.0040 | 13.78 |
| HTC-300-60 | 0.79 | 0.0024 | 12.01 |
| Sample | Ti/°C | Tf/°C | TM1/°C | TM2/°C | RM1/s−1 | RM2/s−1 |
|---|---|---|---|---|---|---|
| WW | 235 | 378 | 272 | 362 | 0.0121 | 0.0061 |
| HTC-200-60 | 267 | 495 | 322 | 363 | 0.0093 | 0.0061 |
| HTC-220-60 | 268 | 484 | 329 | 393 | 0.0052 | 0.0061 |
| HTC-240-30 | 267 | 498 | 341 | 415 | 0.0047 | 0.0056 |
| HTC-240-60 | 278 | 508 | 348 | 449 | 0.0035 | 0.0056 |
| HTC-240-90 | 287 | 528 | – | 498 | – | 0.0061 |
| HTC-240-120 | 321 | 540 | – | 504 | – | 0.0062 |
| HTC-260-60 | 312 | 520 | – | 481 | – | 0.0057 |
| HTC-280-60 | 323 | 542 | – | 487 | – | 0.0059 |
| HTC-300-60 | 355 | 542 | – | 499 | – | 0.0068 |
| Sample | K, wt.% | Ca, wt.% | Mg, wt.% |
|---|---|---|---|
| WW | 0.171 | 1.099 | 0.058 |
| HTC-240-60 | 0.042 | 0.968 | 0.036 |
| DE | 88.09 | 57.45 | 70.18 |
| Sample | CaO | K2O | MgO | SiO2 | P2O5 | SO3 | Al2O3 | Fe2O3 | Na2O |
|---|---|---|---|---|---|---|---|---|---|
| WW | 77.60 | 10.40 | 4.83 | 1.93 | 1.44 | 1.41 | 0.47 | 0.41 | 0.29 |
| HTC-240-60 | 73.57 | 2.76 | 3.21 | 5.70 | 5.52 | 2.53 | 1.59 | 3.78 | 0.56 |
| Bituminous coal | 24.48 | 0.37 | 1.53 | 32.08 | – | 13.04 | 14.81 | 8.67 | 3.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Li, R.; Dan, J.; Yuan, X.; Shao, J.; Liu, J.; Xu, K.; Li, T.; Ning, X.; Wang, C. Preparation of Biomass Hydrochar and Application Analysis of Blast Furnace Injection. Energies 2023, 16, 1216. https://doi.org/10.3390/en16031216
Wang G, Li R, Dan J, Yuan X, Shao J, Liu J, Xu K, Li T, Ning X, Wang C. Preparation of Biomass Hydrochar and Application Analysis of Blast Furnace Injection. Energies. 2023; 16(3):1216. https://doi.org/10.3390/en16031216
Chicago/Turabian StyleWang, Guangwei, Renguo Li, Jiayun Dan, Xiang Yuan, Jiugang Shao, Jiawen Liu, Kun Xu, Tao Li, Xiaojun Ning, and Chuan Wang. 2023. "Preparation of Biomass Hydrochar and Application Analysis of Blast Furnace Injection" Energies 16, no. 3: 1216. https://doi.org/10.3390/en16031216




