Preparation and Characterization of n-Octadecane@SiO2/GO and n-Octadecane@SiO2/Ag Nanoencapsulated Phase Change Material for Immersion Cooling of Li-Ion Battery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
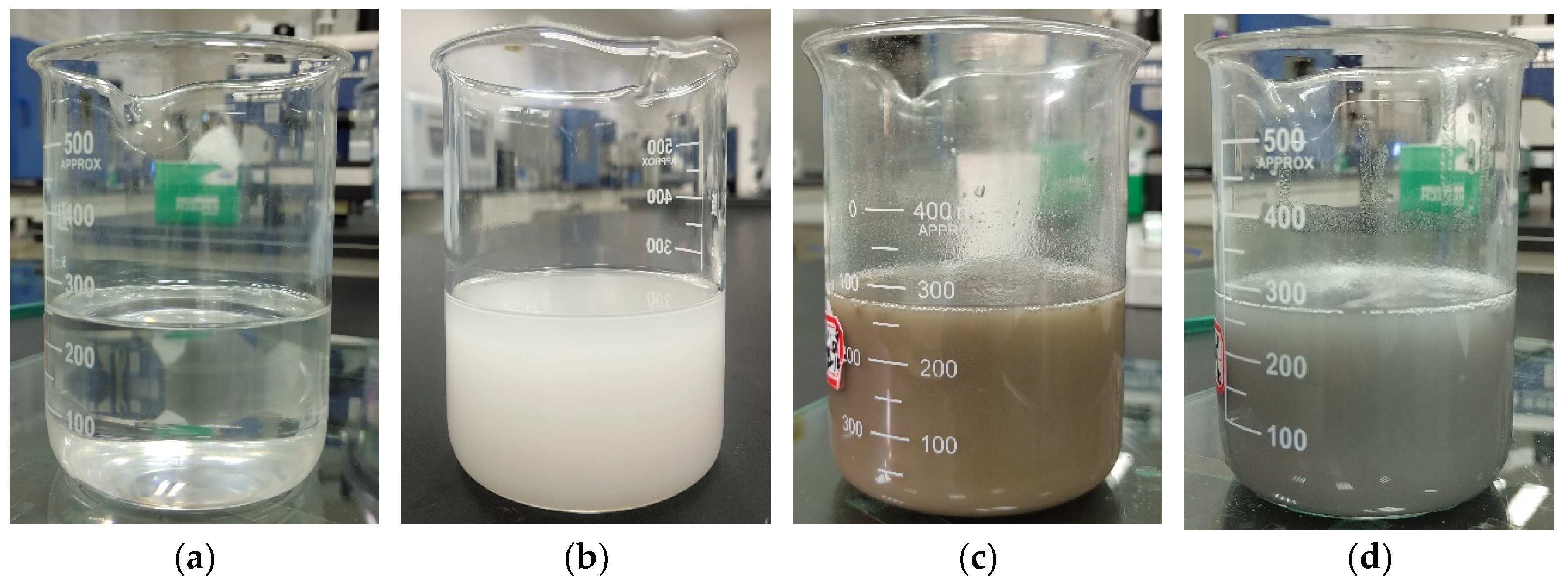
2.2. Preparation of Nanocapsules
2.3. Characterization
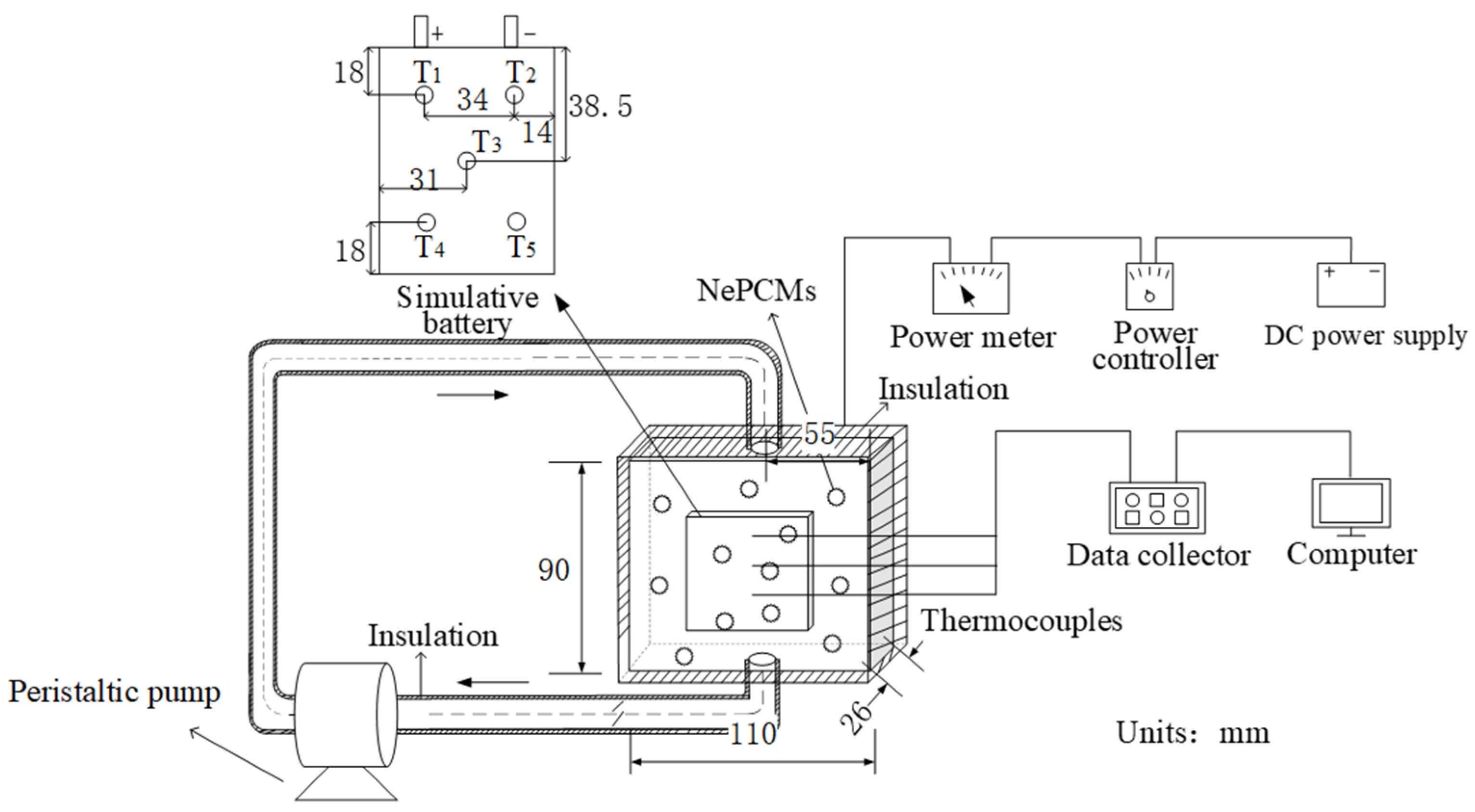
2.4. BTMS Experiment
3. Results and Discussion
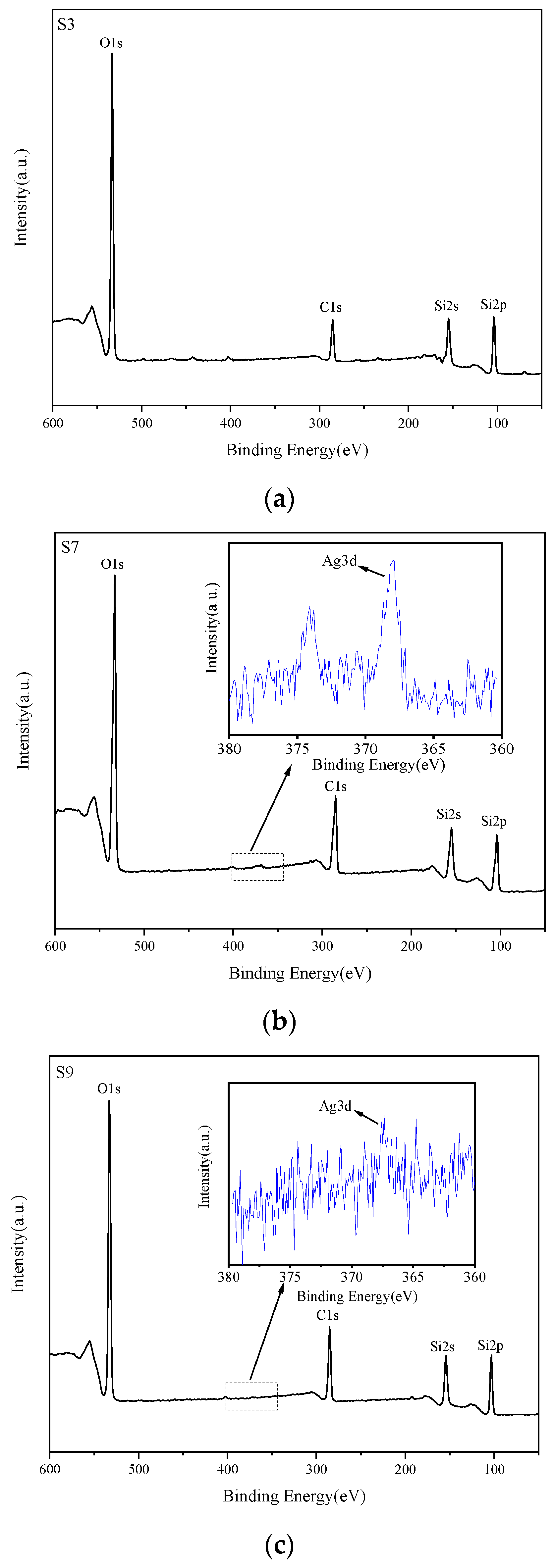
3.1. Chemical Composition and Crystal Structure
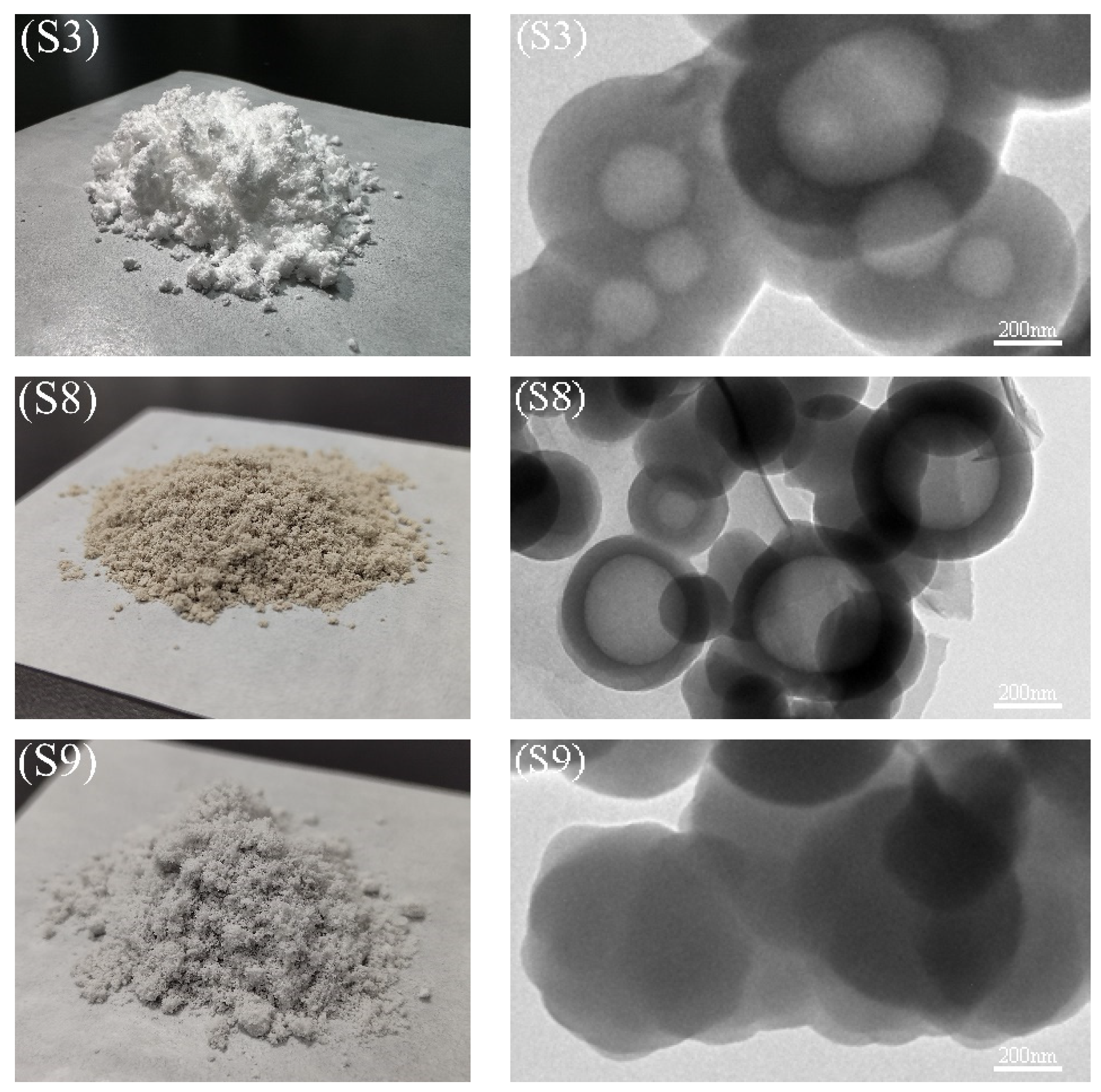
3.2. Morphology and Microstructure
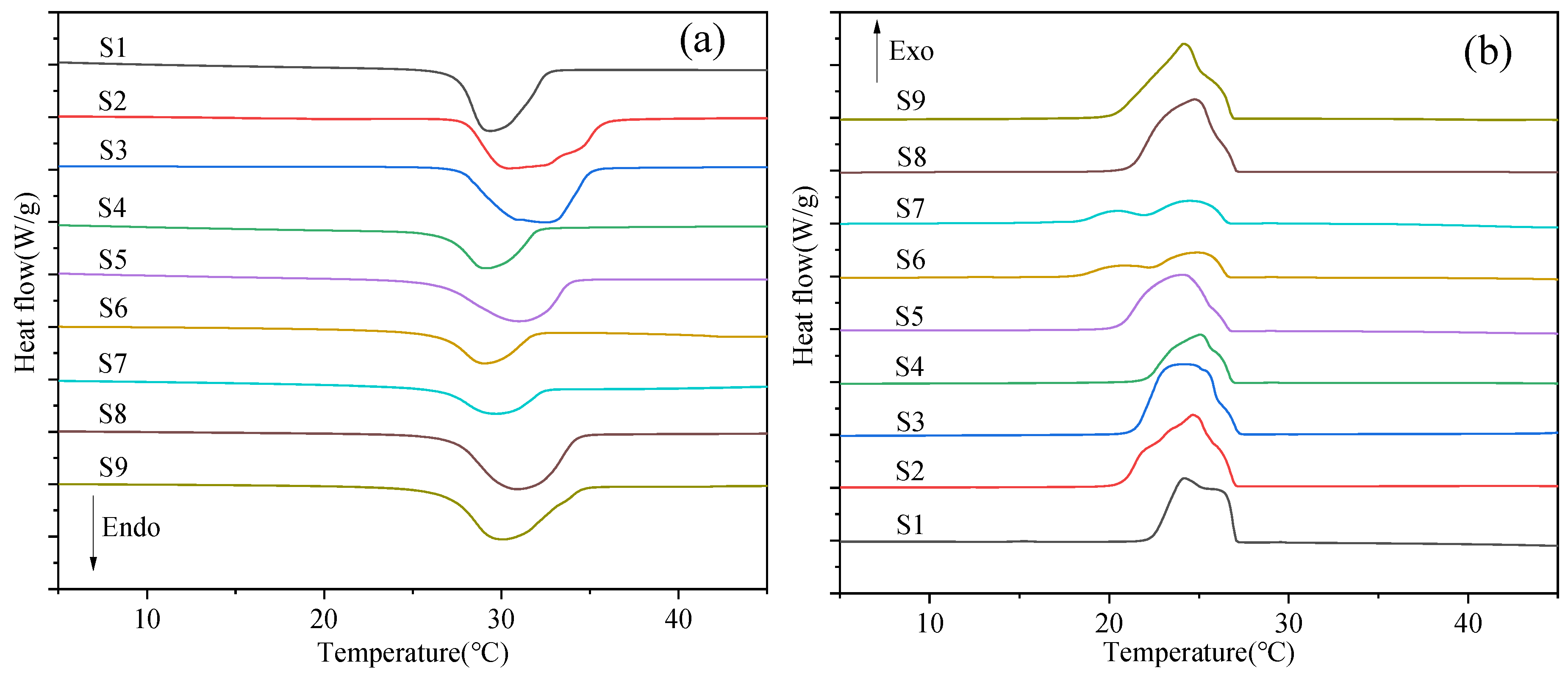
3.3. Phase Change Properties
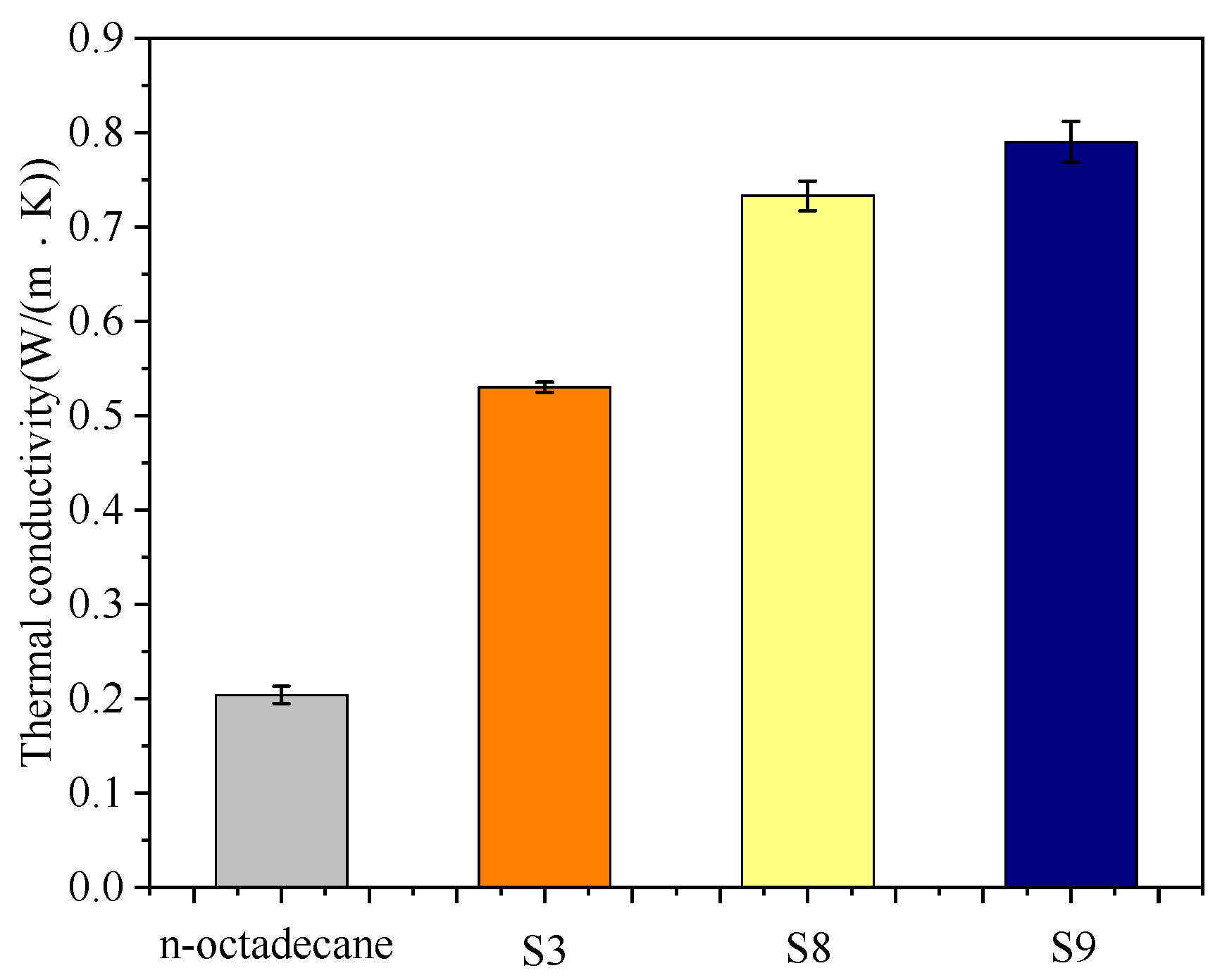
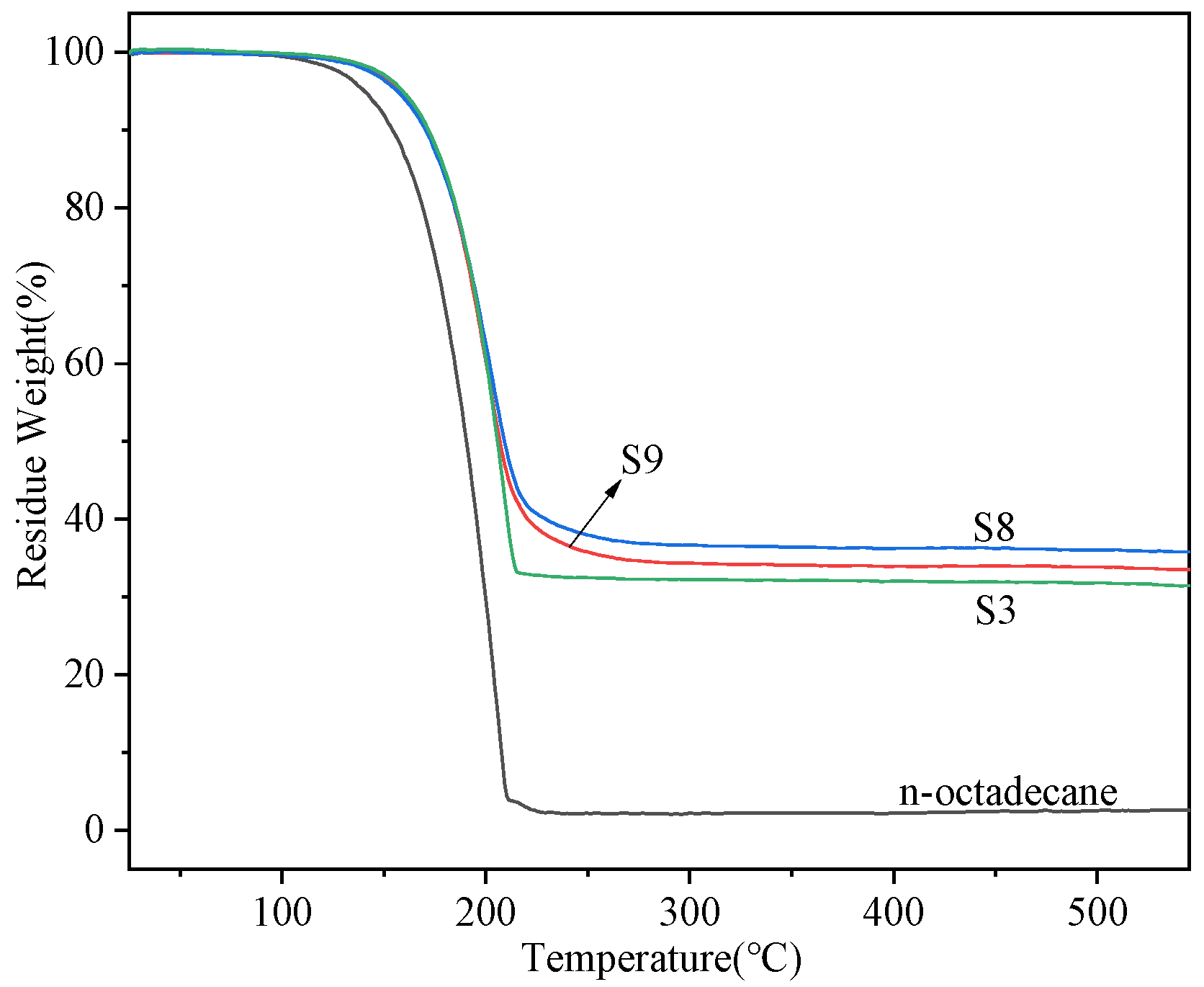
3.4. Thermal Conductivity and Thermal Stability of the Nanocapsules
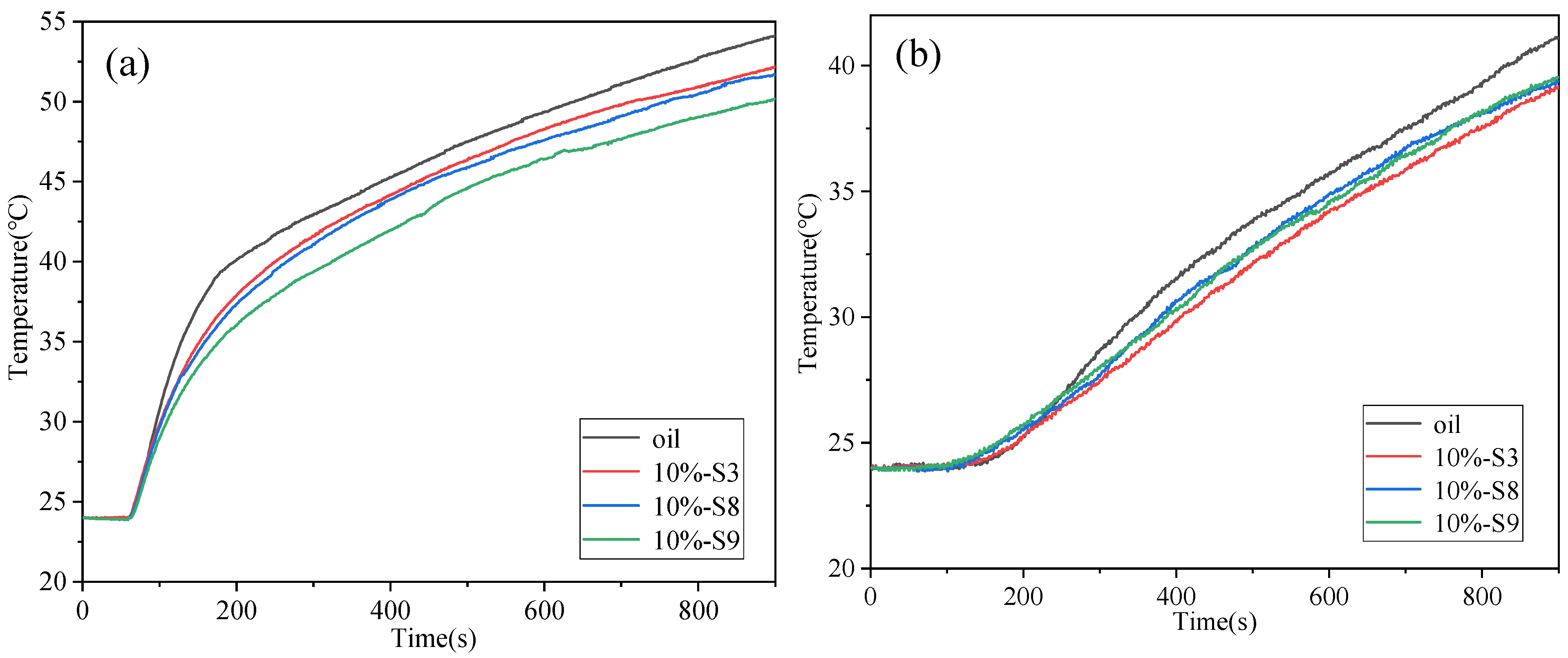
4. BTMS Test
5. Conclusions
- The core-to-shell ratio has a significant influence on the properties of the nanocapsules. When the ratio is 1:1, the samples possess a regular spherical shape, high enthalpy, and improved thermal stability.
- By doping the GO or Ag in the wall or shell materials, the performance of the nanoparticles can be further improved. When the Ag was doped in the shell materials, the thermal conductivity increased by 49%, while the supercooling degree decreased by 35.6% compared to the sample without the doping material.
- The addition of nanocapsules can significantly improve the specific heat capacity and thermal conductivity of the insulation oil. Compared to the simulative battery cooled with insulation oil, the temperature of the simulative battery cooled by nanocapsule slurries decreased by up to 3.95 °C.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, N.; Lior, N.; Jin, H. The energy situation and its sustainable development strategy in China. Energy 2011, 36, 3639–3649. [Google Scholar] [CrossRef]
- Lizana, J.; Chacartegui, R.; Barrios-Padura, A.; Valverde, J.M. Advances in thermal energy storage materials and their applications towards zero energy buildings: A critical review. Appl. Energy 2017, 203, 219–239. [Google Scholar] [CrossRef]
- Koohi-Fayegh, S.; Rosen, M.A. A review of energy storage types, applications and recent developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Guo, R.; Han, W. The effects of electrolytes, electrolyte/electrode interphase, and binders on lithium-ion batteries at low temperature. Mater. Today Sustain. 2022, 19, 100187. [Google Scholar] [CrossRef]
- Ling, Z.; Lin, W.; Zhang, Z.; Fang, X. Computationally efficient thermal network model and its application in optimization of battery thermal management system with phase change materials and long-term performance assessment. Appl. Energy 2020, 259, 114120. [Google Scholar] [CrossRef]
- Ling, Z.; Wen, X.; Zhang, Z.; Fang, X.; Gao, X. Thermal management performance of phase change materials with different thermal conductivities for Li-ion battery packs operated at low temperatures. Energy 2018, 144, 977–983. [Google Scholar] [CrossRef]
- Nazari, A.; Farhad, S. Heat generation in lithium-ion batteries with different nominal capacities and chemistries. Appl. Therm. Eng. 2017, 125, 1501–1517. [Google Scholar] [CrossRef]
- Heubner, C.; Schneider, M.; Michaelis, A. Detailed study of heat generation in porous LiCoO2 electrodes. J. Power Sources 2016, 307, 199–207. [Google Scholar] [CrossRef]
- Lin, J.; Liu, X.; Li, S.; Zhang, C.; Yang, S. A review on recent progress, challenges and perspective of battery thermal management system. Int. J. Heat Mass Transf. 2021, 167, 120834. [Google Scholar] [CrossRef]
- Lai, Y.; Wu, W.; Chen, K.; Wang, S.; Xin, C. A compact and lightweight liquid-cooled thermal management solution for cylindrical lithium-ion power battery pack. Int. J. Heat Mass Transf. 2019, 144, 118581. [Google Scholar] [CrossRef]
- Huang, Y.H.; Cheng, W.L.; Zhao, R. Thermal management of Li-ion battery pack with the application of flexible form-stable composite phase change materials. Energy Convers. Manag. 2019, 182, 9–20. [Google Scholar] [CrossRef]
- Rostami, S.; Afrand, M.; Shahsavar, A.; Sheikholeslami, M.; Kalbasi, R.; Aghakhani, S.; Shadloo, M.S.; Oztop, H.F. A review of melting and freezing processes of PCM/nano-PCM and their application in energy storage. Energy 2020, 211, 118698. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Fang, G. An overview of thermal energy storage systems. Energy 2018, 144, 341–378. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Lin, Y.; Alva, G.; Fang, G. Review on thermal performances and applications of thermal energy storage systems with inorganic phase change materials. Energy 2018, 165, 685–708. [Google Scholar] [CrossRef]
- Aftab, W.; Huang, X.; Wu, W.; Liang, Z.; Mahmood, A.; Zou, R. Nanoconfined phase change materials for thermal energy applications. Energy Environ. Sci. 2018, 11, 1392–1424. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, F.; Fang, X.; Gao, X.; Zhang, Z. A hybrid thermal management system for lithium ion batteries combining phase change materials with forced-air cooling. Appl. Energy 2015, 148, 403–409. [Google Scholar] [CrossRef]
- Park, S.; Jung, D. Battery cell arrangement and heat transfer fluid effects on the parasitic power consumption and the cell temperature distribution in a hybrid electric vehicle. J. Power Sources 2013, 227, 191–198. [Google Scholar] [CrossRef]
- Qaderi, A.; Veysi, F. Investigation of a water-NEPCM cooling thermal management system for cylindrical 18,650 Li-ion batteries. Energy 2022, 244, 122570. [Google Scholar] [CrossRef]
- Sundin, D.W.; Sponholtz, S. Thermal management of li-Ion batteries with single-phase liquid immersion cooling. IEEE Open J. Veh. Technol. 2020, 1, 82–92. [Google Scholar] [CrossRef]
- Patil, M.S.; Seo, J.H.; Lee, M.Y. A novel dielectric fluid immersion cooling technology for Li-ion battery thermal management. Energy Convers. Manag. 2021, 229, 113715. [Google Scholar] [CrossRef]
- Zhu, L.; Boehm, R.F.; Wang, Y.P.; Halford, C.; Sun, Y. Water immersion cooling of PV cells in a high concentration system. Sol. Energy Mater. Sol. Cells 2011, 95, 538–545. [Google Scholar] [CrossRef]
- Cheng, C.C.; Chang, P.C.; Li, H.C.; Hsu, F.I. Design of a single-phase immersion cooling system through experimental and numerical analysis. Int. J. Heat Mass Transf. 2020, 160, 120203. [Google Scholar] [CrossRef]
- Trimbake, A.; Singh, C.P.; Krishnan, S. Mineral Oil Immersion Cooling of Lithium-Ion Batteries: An Experimental Investigation. J. Electrochem. Energy Convers. Storage 2022, 19, 021007. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Ramachandran, T. Review of thermal energy storage of micro- and nanoencapsulated phase change materials. Mater. Res. Innov. 2013, 18, 541–554. [Google Scholar] [CrossRef]
- Wu, T.T.; Hu, Y.X.; Rong, H.Q.; Wang, C.H. SEBS-based composite phase change material with thermal shape memory for thermal management applications. Energy 2021, 221, 119900. [Google Scholar] [CrossRef]
- Liu, C.; Xu, D.; Weng, J.; Zhou, S.; Li, W.; Wan, Y.; Jiang, S.; Zhou, D.; Wang, J.; Huang, Q. Phase change materials application in battery thermal management system: A review. Materials 2020, 13, 4622. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, T.; Jiao, S.; Guo, C. Preparation of reduced graphene oxide modified magnetic phase change microcapsules and their application in direct absorption solar collector. Sol. Energy Mater. Sol. Cells 2020, 216, 110695. [Google Scholar] [CrossRef]
- Cao, J.; He, Y.; Feng, J.; Lin, S.; Ling, Z.; Zhang, Z.; Fang, X. Mini-channel cold plate with nano phase change material emulsion for Li-ion battery under high-rate discharge. Appl. Energy 2020, 279, 115808. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Liu, L.; Fang, G. Synthesis, characterization and applications of microencapsulated phase change materials in thermal energy storage: A review. Energy Build. 2017, 144, 276–294. [Google Scholar] [CrossRef]
- Liu, L.; Su, D.; Tang, Y.; Fang, G. Thermal conductivity enhancement of phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2016, 62, 305–317. [Google Scholar] [CrossRef]
- Khadiran, T.; Hussein, M.Z.; Zainal, Z.; Rusli, R. Encapsulation techniques for organic phase change materials as thermal energy storage medium: A review. Sol. Energy Mater. Sol. Cells 2015, 143, 78–98. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, X.; Wu, D. Design and synthesis of magnetic microcapsules based on n-eicosane core and Fe3O4/SiO2 hybrid shell for dual-functional phase change materials. Appl. Energy 2014, 134, 456–468. [Google Scholar] [CrossRef]
- Do, J.Y.; Son, N.; Shin, J.; Chava, R.K.; Joo, S.W.; Kang, M. n-Eicosane-Fe3O4@SiO2@Cu microcapsule phase change material and its improved thermal conductivity and heat transfer performance. Mater. Des. 2021, 198, 109357. [Google Scholar] [CrossRef]
- Zhu, Y.; Qin, Y.; Liang, S.; Chen, K.; Tian, C.; Wang, J.; Luo, X.; Zhang, L. Graphene/SiO2/n-octadecane nanoencapsulated phase change material with flower like morphology, high thermal conductivity, and suppressed supercooling. Appl. Energy 2019, 250, 98–108. [Google Scholar] [CrossRef]
- Singh , S.B.; De, M. Thermally exfoliated graphene oxide for hydrogen storage—ScienceDirect. Mater. Chem. Phys. 2020, 239, 122102. [Google Scholar] [CrossRef]
- Singh, S.B.; De, M. Effects of gaseous environments on physicochemical properties of thermally exfoliated graphene oxides for hydrogen storage: A comparative study. J. Porous Mater. 2021, 28, 875–888. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, W.; Jiang, Z.; He, F.; Zhang, K.; Fan, J.; Wu, J. Graphene oxide-modified microencapsulated phase change materials with high encapsulation capacity and enhanced leakage-prevention performance. Appl. Energy 2017, 197, 354–363. [Google Scholar] [CrossRef]
- He, L.; Mo, S.; Lin, P.; Jia, L.; Chen, Y.; Cheng, Z. D-mannitol@silica/graphene oxide nanoencapsulated phase change material with high phase change properties and thermal reliability. Appl. Energy 2020, 268, 115020. [Google Scholar] [CrossRef]
- Li, S.; Ji, W.; Zou, L.; Li, L.; Li, Y.; Cheng, X. Crystalline TiO2 shell microcapsules modified by Co3O4/GO nanocomposites for thermal energy storage and photocatalysis. Mater. Today Sustain. 2022, 19, 100197. [Google Scholar] [CrossRef]
- Shchukina, E.M.; Graham, M.; Zheng, Z.; Shchukin, D.G. Nanoencapsulation of phase change materials for advanced thermal energy storage systems. Chem. Soc. Rev. 2018, 47, 4156–4175. [Google Scholar] [CrossRef] [PubMed]
- Tahan Latibari, S.; Mehrali, M.; Mehrali, M.; Indra Mahlia, T.M.; Cornelis Metselaar, H.S. Synthesis, characterization and thermal properties of nanoencapsulated phase change materials via sol–gel method. Energy 2013, 61, 664–672. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Zhang, T.; Zhang, D.; Ji, H. Supercooling suppression and thermal behavior improvement of erythritol as phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2017, 171, 60–71. [Google Scholar] [CrossRef]
- Zhao, H. Research on Battery Thermal Management of Large-Capacity Lithium-Ion Battery Based on Phase Change Material and Heat Pipe. Master’s Thesis, North China Electric Power University, Beijing, China, 2022. [Google Scholar]
- Yang, D.; Shi, S.; Xiong, L.; Guo, H.; Zhang, H.; Chen, X.; Wang, C.; Chen, X. Paraffin/Palygorskite composite phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2016, 144, 228–234. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wu, D. Silica encapsulation of n-octadecane via sol-gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interface Sci. 2010, 343, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chi, Y.; Liang, S.; Luo, X.; Chen, K.; Tian, C.; Wang, J.; Zhang, L. Novel metal coated nanoencapsulated phase change materials with high thermal conductivity for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 176, 212–221. [Google Scholar] [CrossRef]
- Roy, S.; John, A.; Nagabooshanam, S.; Mishra, A.; Wadhwa, S.; Mathur, A.; Narang, J.; Singh, J.; Dilawar, N.; Davis, J. Self-aligned TiO2—Photo reduced graphene oxide hybrid surface for smart bandage application. Appl. Surf. Sci. 2019, 488, 261–268. [Google Scholar] [CrossRef]
- Tai, X.H.; Chook, S.W.; Lai, C.W.; Lee, K.M.; Yang, T.C.K.; Chong, S.; Juan, J.C. Effective photoreduction of graphene oxide for photodegradation of volatile organic compounds. RSC Adv. 2019, 9, 18076–18086. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, Y.; Liao, Y.; Tian, M.; Zou, H.; Zhang, L. Fabrication of silver-coated silica microspheres through mussel-inspired surface functionalization. J. Colloid Interface Sci. 2011, 358, 567–574. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.J. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, X.; Miao, X.; Zhang, Y.; Guo, M.; Cheng, F.; Zhang, M. Preparation and characterization of SiO2@n-octadecane capsules with controllable size and structure. Thermochim. Acta 2021, 705, 179037. [Google Scholar] [CrossRef]
- Tzou, D.Y. Thermal instability of nanofluids in natural convection. Int. J. Heat Mass Transf. 2008, 51, 2967–2979. [Google Scholar] [CrossRef]
- Tzou, D.Y. Instability of Nanofluids in Natural Convection. J. Heat Transf. 2008, 130, 072401. [Google Scholar] [CrossRef]
- Liang, S.; Li, Q.; Zhu, Y.; Chen, K.; Tian, C.; Wang, J.; Bai, R. Nanoencapsulation of n-octadecane phase change material with silica shell through interfacial hydrolysis and polycondensation in miniemulsion. Energy 2015, 93, 1684–1692. [Google Scholar] [CrossRef]
- Cao, F.; Yang, B. Supercooling suppression of microencapsulated phase change materials by optimizing shell composition and structure. Appl. Energy 2014, 113, 1512–1518. [Google Scholar] [CrossRef]
- Mo, S.; He, L.; Jia, L.; Chen, Y.; Cheng, Z. Thermophysical properties of a novel nanoencapsulated phase change material. Int. J. Thermophys. 2020, 41, 68. [Google Scholar] [CrossRef]






| Years | Author | Core | Shell | Size | ΔHm (kJ/kg) |
|---|---|---|---|---|---|
| 2014 | Jiang et al. [33] | n-eicosane | Fe3O4/SiO2 | 4–6 μm | 154.00 |
| 2017 | Zhang et al. [38] | paraffifin | GO/melamine-formaldehyde (MF) | 6.32–15.89 μm | 202.80 |
| 2019 | Zhu et al. [35] | n-octadecane | Graphene/SiO2 | 390 nm | 108.2 |
| 2020 | Gao et al. [28] | n-octadecane/Fe3O4 | rGO/MF | not available | 173.05 |
| 2020 | He et al. [39] | D-mannitol | GO/SiO2 | 100–400 nm | 216.7 |
| 2021 | Do et al. [34] | n-Eicosane/Fe3O4 | Cu/SiO2 | 631.0 nm | 153.94 |
| 2022 | Li et al. [40] | paraffin | Co3O4/GO/TiO2 | 2.71 μm | 140 |
| Samples | Core-Shell Mass Ratio | CTAB (g) | GO (mL) | Ag (g) | Doped Position |
|---|---|---|---|---|---|
| S1 | 1:3 | 0.9 | 0 | 0 | - |
| S2 | 2:3 | 0.9 | 0 | 0 | - |
| S3 | 1:1 | 0.9 | 0 | 0 | - |
| S4 | 4:3 | 0.9 | 0 | 0 | - |
| S5 | 1:1 | 0.45 | 0 | 0 | - |
| S6 | 1:1 | 0.9 | 5 | 0 | core |
| S7 | 1:1 | 0.9 | 0 | 0.1 | core |
| S8 | 1:1 | 0.9 | 5 | 0 | shell |
| S9 | 1:1 | 0.9 | 0 | 0.1 | shell |
| Samples | Core-Shell Mass Ratio | Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) |
|---|---|---|---|---|---|
| n-OD | - | 29.17 | 220.77 | 26.78 | 223.46 |
| S1 | 1:3 | 29.16 | 96.84 | 24.32 | 96.00 |
| S2 | 2:3 | 30.30 | 125.48 | 24.82 | 124.41 |
| S3 | 1:1 | 32.37 | 126.40 | 24.33 | 122.94 |
| S4 | 4:3 | 29.14 | 65.67 | 25.20 | 66.10 |
| S5 | 1:1 | 30.88 | 97.28 | 24.21 | 99.85 |
| S6 | 1:1 | 28.80 | 54.72 | 25.06 | 53.40 |
| S7 | 1:1 | 29.39 | 50.34 | 24.63 | 49.93 |
| S8 | 1:1 | 30.61 | 119.33 | 25.12 | 117.38 |
| S9 | 1:1 | 29.76 | 121.90 | 24.58 | 120.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Du, J.; Li, Y.; Li, J.; Chen, L.; Chai, Y.; Li, Y. Preparation and Characterization of n-Octadecane@SiO2/GO and n-Octadecane@SiO2/Ag Nanoencapsulated Phase Change Material for Immersion Cooling of Li-Ion Battery. Energies 2023, 16, 1498. https://doi.org/10.3390/en16031498
Gu J, Du J, Li Y, Li J, Chen L, Chai Y, Li Y. Preparation and Characterization of n-Octadecane@SiO2/GO and n-Octadecane@SiO2/Ag Nanoencapsulated Phase Change Material for Immersion Cooling of Li-Ion Battery. Energies. 2023; 16(3):1498. https://doi.org/10.3390/en16031498
Chicago/Turabian StyleGu, Jianhao, Jiajie Du, Yuxin Li, Jinpei Li, Longfei Chen, Yan Chai, and Yongli Li. 2023. "Preparation and Characterization of n-Octadecane@SiO2/GO and n-Octadecane@SiO2/Ag Nanoencapsulated Phase Change Material for Immersion Cooling of Li-Ion Battery" Energies 16, no. 3: 1498. https://doi.org/10.3390/en16031498






