Study of Chelating Agent—Surfactant Interactions on the Interphase as Possibly Useful for the Well Stimulation
Abstract
:1. Introduction
- Scale removal;
- Scale formation inhibition;
- Iron control during acid treatments;
- Hydraulic fracturing fluid additives;
- Well stimulation;
- Enhanced oil recovery (EOR).
2. Materials and Methods
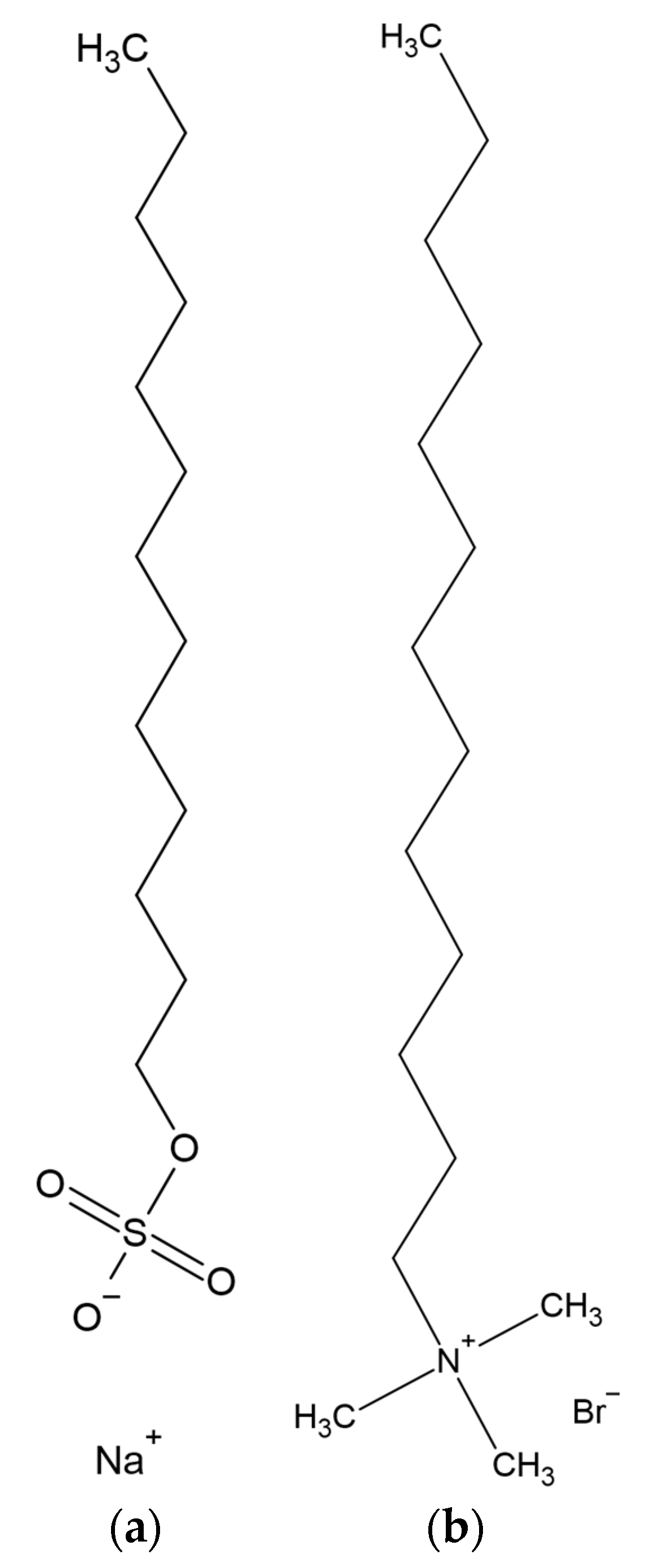
2.1. Materials
2.2. Methods
2.2.1. Solutions Preparation
2.2.2. IFT Measurement
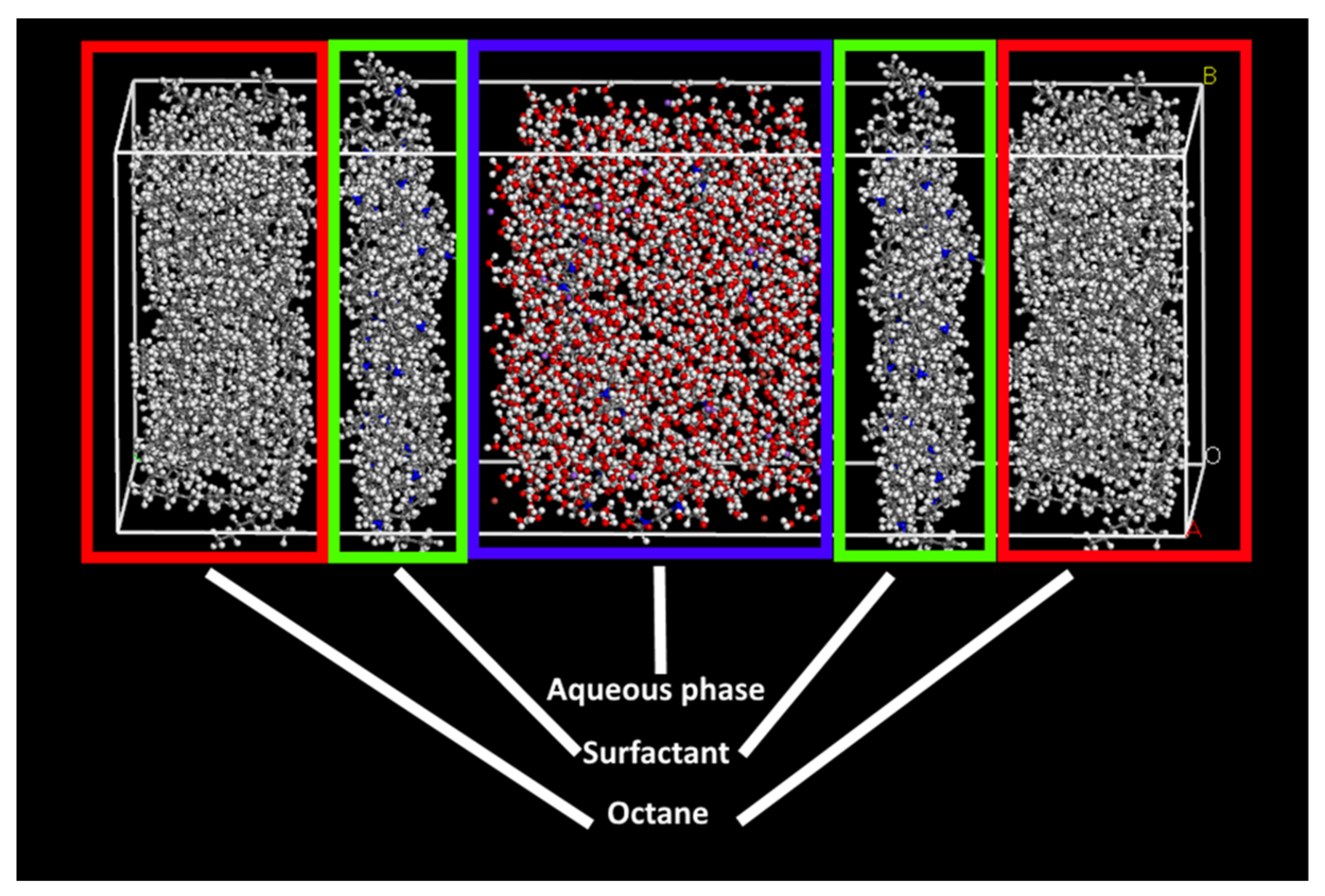
2.2.3. Simulation Details
- Water/octane/SDS;
- Water+chelate/octane/SDS;
- Water/octane/DTAB;
- Water+chelate/octane/DTAB.
3. Results
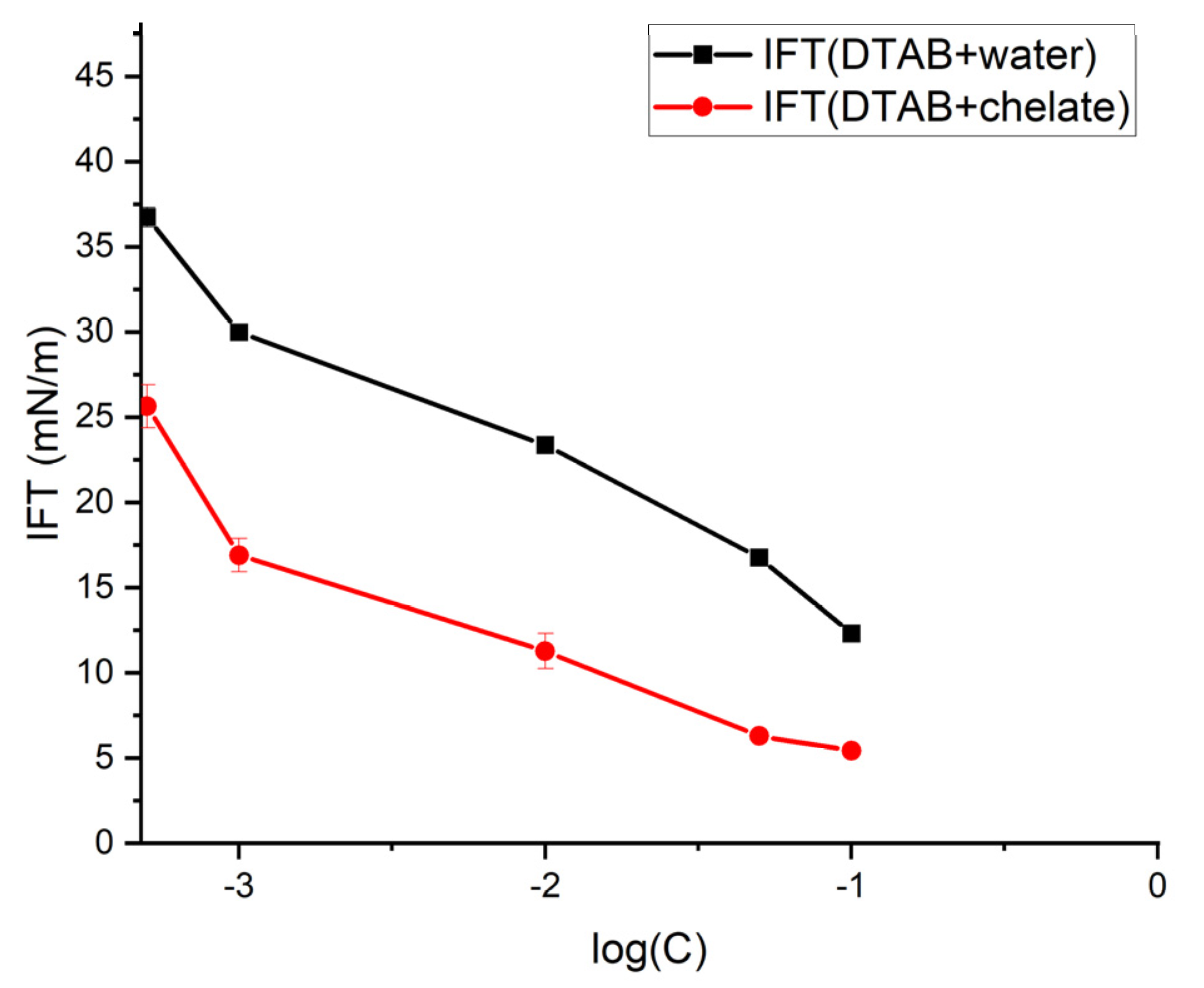
3.1. IFT Measurement
3.2. MD Results
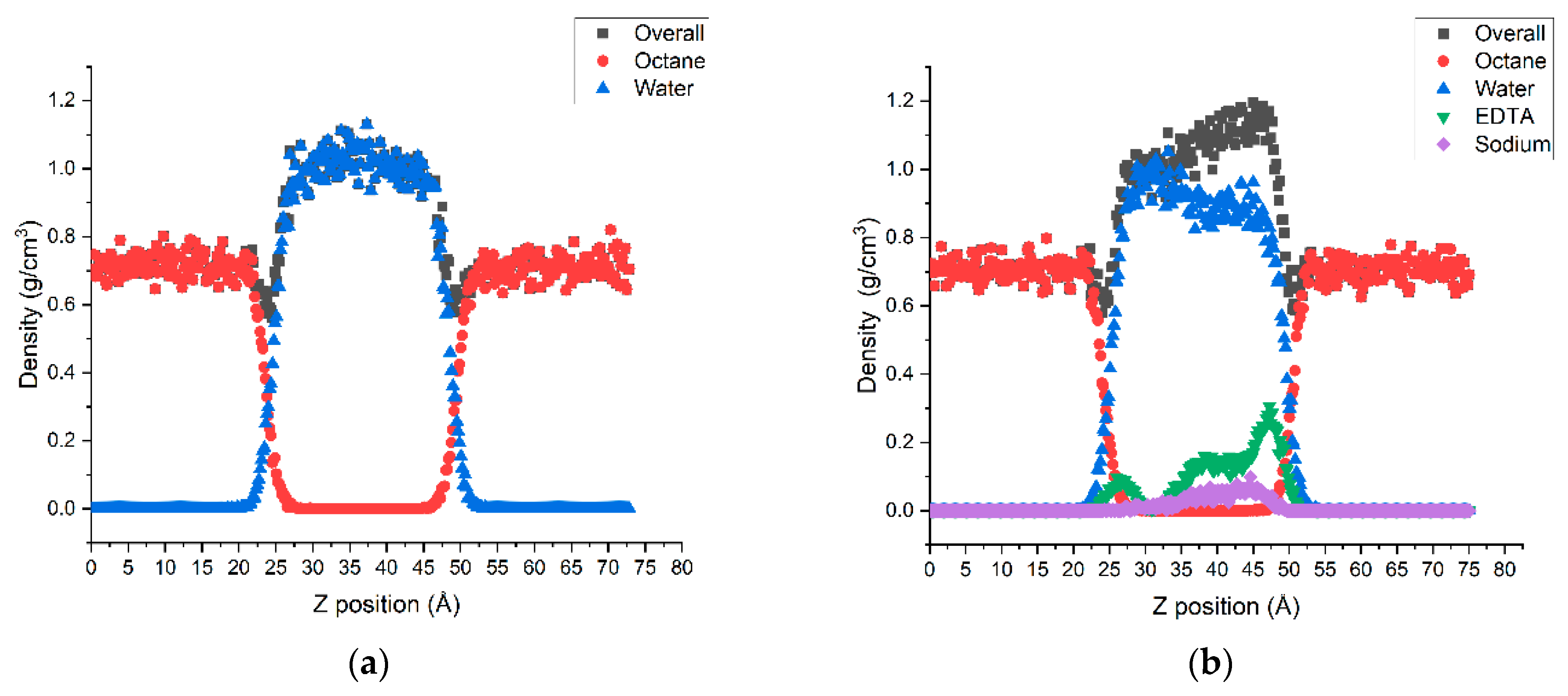
3.2.1. Density Profiles
3.2.2. Hydrogen Bonding
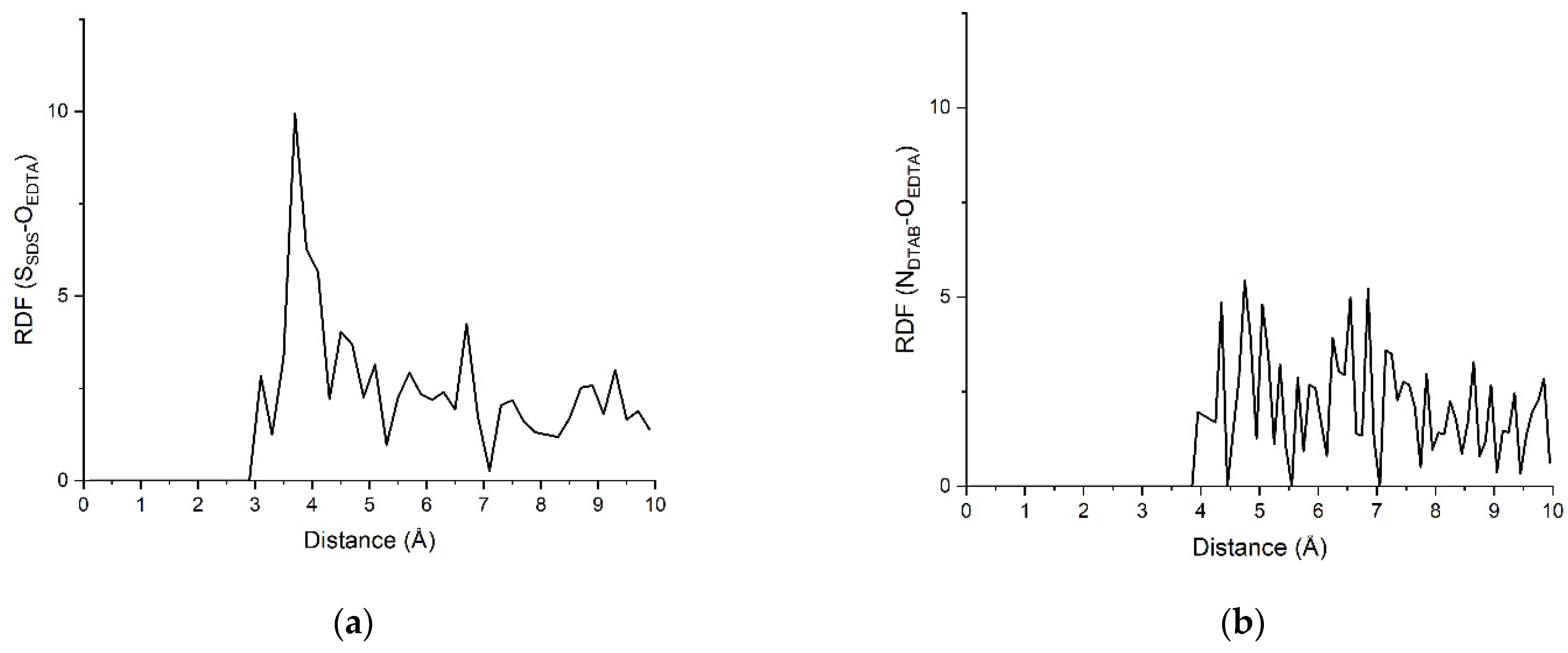
3.2.3. RDF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kołodyńska, D. Complexing Agents. In Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2019; pp. 1–26. [Google Scholar]
- Kołodyńska, D. The Effect of the Novel Complexing Agent in Removal of Heavy Metal Ions from Waters and Waste Waters. Chem. Eng. J. 2010, 165, 835–845. [Google Scholar] [CrossRef]
- Leštan, D.; Luo, C.-l.; Li, X.-d. The Use of Chelating Agents in the Remediation of Metal-Contaminated Soils: A Review. Environ. Pollut. 2008, 153, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Latif, N.A.; Al-Madfai, S.H.F.; Ghanim, A.N. Removal of Scale Deposited on Heat-Transfer Surfaces Using Chemical Methods. Ind. Eng. Chem. Res. 1988, 27, 1548–1551. [Google Scholar] [CrossRef]
- Teng, K.H.; Amiri, A.; Kazi, S.N.; Bakar, M.A.; Chew, B.T.; Al-Shamma’a, A.; Shaw, A. Retardation of Heat Exchanger Surfaces Mineral Fouling by Water-Based Diethylenetriamine Pentaacetate-Treated CNT Nanofluids. Appl. Therm. Eng. 2017, 110, 495–503. [Google Scholar] [CrossRef]
- Hassan, A.; Mahmoud, M.; Bageri, B.S.; Aljawad, M.S.; Kamal, M.S.; Barri, A.A.; Hussein, I.A. Applications of Chelating Agents in the Upstream Oil and Gas Industry: A Review. Energy Fuels 2020, 34, 15593–15613. [Google Scholar] [CrossRef]
- Fredd, C.N.; Scott Fogler, H. Influence of Transport and Reaction on Wormhole Formation in Porous Media. AIChE J. 1998, 44, 1933–1949. [Google Scholar] [CrossRef]
- Shafiq, M.U.; Ben Mahmud, H.K.; Ghasemi, M. Integrated Mineral Analysis of Sandstone and Dolomite Formations Using Different Chelating Agents during Matrix Acidizing. Petroleum 2019, 5, 67–76. [Google Scholar] [CrossRef]
- Mahmoud, M. Reaction of Chelating Agents and Catalyst with Sandstone Minerals During Matrix Acid Treatment. Arab. J. Sci. Eng. 2018, 43, 5745–5756. [Google Scholar] [CrossRef]
- Fredd, C.N.; Fogler, H.S. The Influence of Chelating Agents on the Kinetics of Calcite Dissolution. J. Colloid Interface Sci. 1998, 204, 187–197. [Google Scholar] [CrossRef]
- Fredd, C.N.; Fogler, H.S. Alternative Stimulation Fluids and Their Impact on Carbonate Acidizing. SPE J. 1998, 3, 34–40. [Google Scholar] [CrossRef]
- Frenier, W.; Brady, M.; Al-Harthy, S.; Arangath, R.; Chan, K.S.; Flamant, N.; Samuel, M. Hot Oil and Gas Wells Can Be Stimulated without Acids. In Proceedings of the SPE International Formation Damage Control Symposium, Lafayette, LA, USA, 18–20 February 2004. [Google Scholar]
- Huang, T.; McElfresh, P.M.; Gabrysch, A.D. Carbonate Matrix Acidizing Fluids at High Temperatures: Acetic Acid, Chelating Agents or Long-Chained Carboxylic Acids? In Proceedings of the SPE European Formation Damage Conference, Hague, The Netherlands, 13–14 May 2003. [Google Scholar]
- Panait, E.; Isac, C.; Marton, C.; Dos Santos, A.; Girardi, S. Effective Matrix Acidizing Based in Chelating Agents: A Case Study in Romanian Heavy Oil Reservoirs. In Proceedings of the SPE International Heavy Oil Conference and Exhibition, Kuwait City, Kuwait, 12 December 2018. [Google Scholar]
- Ng, J.H.; Almubarak, T.; Nasr-El-Din, H.A. Low-Carbon-Steel Corrosion at High Temperatures by Aminopolycarboxylic Acids. SPE Prod. Oper. 2018, 33, 131–144. [Google Scholar] [CrossRef]
- Almubarak, T.; Ng, J.H.; Nasr-El-Din, H. A Review of the Corrosivity & Degradability of Aminopolycarboxylic Acids. In Proceedings of the Annual Offshore Technology Conference, Houston, TX, USA, 1–4 May 2017. [Google Scholar]
- Kavipriya, K.; Rajendran, S.; Sathiyabama, J.; Suriya Prabha, A. A Critical Review of Corrosion Inhibition by Phosphonic Acids. Eur. Chem. Bull. 2012, 1, 366–374. [Google Scholar]
- Nasr-El-Din, H.A.; Al-Othman, A.M.; Taylor, K.C.; Al-Ghamdi, A.H. Surface Tension of HCl-Based Stimulation Fluids at High Temperatures. J. Pet. Sci. Eng. 2004, 43, 57–73. [Google Scholar] [CrossRef]
- Silin, M.A.; Magadova, L.A.; Tolstykh, L.I.; Davletshina, L.F.; Vlasova, V.D.; Yunusov, T.I.; Makarova, A.M. Aspects of Interaction of Surfactant—Acid Compositions at Phase Boundary with Hydrocarbons. Russ. J. Appl. Chem. 2019, 92, 1810–1819. [Google Scholar] [CrossRef]
- Mahmoud, M.; Attia, M.; Al-Hashim, H. EDTA Chelating Agent/Seawater Solution as Enhanced Oil Recovery Fluid for Sandstone Reservoirs. J. Pet. Sci. Eng. 2017, 152, 275–283. [Google Scholar] [CrossRef]
- Nasr El-Din Mahmoud, M.A.; Abdelgawad, K.Z. Chelating-Agent Enhanced Oil Recovery for Sandstone and Carbonate Reservoirs. SPE J. 2015, 20, 483–495. [Google Scholar] [CrossRef]
- Hassan, A.M.; Al-Hashim, H.S. Surface Charge Study of EDTA Interaction with Carbonate Rock during Chelating Agent Flooding. J. Pet. Sci. Eng. 2020, 191, 107163. [Google Scholar] [CrossRef]
- Kamal, M.S.; Hussein, I.A.; Sultan, A.S. Review on Surfactant Flooding: Phase Behavior, Retention, IFT, and Field Applications. Energy and Fuels 2017, 31, 7701–7720. [Google Scholar] [CrossRef]
- Zhang, Z.; Azad, M.S.; Trivedi, J.J. IFT or Wettability Alteration: What Is More Important for Oil Recovery in Oil-Wet Formation? Fuel 2021, 291, 119986. [Google Scholar] [CrossRef]
- Tatsumoto, N.; Fujii, S. The Effect of Ultrasonic Waves on the Chemical Dissolution of Calcium Salts: Urinary Stone and Gypsum. J. Acoust. Soc. Japan 1985, 5, 105–112. [Google Scholar] [CrossRef]
- Akcay, I.; Sen, B.H. The Effect of Surfactant Addition to EDTA on Microhardness of Root Dentin. J. Endod. 2012, 38, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Güzel, C.; Uzunoglu, E.; Dogan Buzoglu, H. Effect of Low–Surface Tension EDTA Solutions on the Bond Strength of Resin-Based Sealer to Young and Old Root Canal Dentin. J. Endod. 2018, 44, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Blas, N.; Jimenez-Relinque, E.; Castellote, M. Surfactants in Electrokinetic Remediation of Sediments to Enhance the Removal of Metals. J. Soils Sediments 2022, 22, 2853–2864. [Google Scholar] [CrossRef]
- Eastoe, J.; Dalton, J.S. Dynamic Surface Tension and Adsorption Mechanisms of Surfactants at the Air-Water Interface. Adv. Colloid Interface Sci. 2000, 85, 103–144. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Lu, J.R.; Thomas, R.K. Neutron Reflectivity Studies of the Adsorption of Aerosol-OT at the Air/Water Interface: The Surface Excess. Langmuir 1997, 13, 3681–3685. [Google Scholar] [CrossRef]
- Janjua, A.N.; Sultan, A.S.; Saikia, T.; Kamal, M.S.; Mahmoud, M. Experimental Investigation of Noble Viscoelastic Surfactant and Chelating Agent for Heavy Oil Enhanced Oil Recovery in High-Pressure–High-Temperature Carbonate Reservoirs. J. Surfactants Deterg. 2021, 24, 289–300. [Google Scholar] [CrossRef]
- Deng, X.; Patil, S.; Kamal, M.S.; Mahmoud, M.; Sultan, A.; Saikia, T. Wettability Alteration of Carbonate Rock by Chelating Agents and Viscoelastic Surfactants: Synergetic Impact. Energy Fuels 2022, 36, 7391–7401. [Google Scholar] [CrossRef]
- Chen, P.; Mohanty, K.K. Wettability Alteration in High Temperature Carbonate Reservoirs. In Proceedings of the 18th SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Chen, P.; Mohanty, K.K. Surfactant-Mediated Spontaneous Imbibition in Carbonate Rocks at Harsh Reservoir Conditions. SPE J. 2013, 18, 124–133. [Google Scholar] [CrossRef]
- Yeung, A.; Dabros, T.; Masliyah, J. Does Equilibrium Interfacial Tension Depend on Method of Measurement? J. Colloid Interface Sci. 1998, 208, 241–247. [Google Scholar] [CrossRef]
- Mirchi, V.; Saraji, S.; Akbarabadi, M.; Goual, L.; Piri, M. A Systematic Study on the Impact of Surfactant Chain Length on Dynamic Interfacial Properties in Porous Media: Implications for Enhanced Oil Recovery. Ind. Eng. Chem. Res. 2017, 56, 13677–13695. [Google Scholar] [CrossRef]
- Ghasemi, M.; Shafiei, A.; Foroozesh, J. A Systematic and Critical Review of Application of Molecular Dynamics Simulation in Low Salinity Water Injection. Adv. Colloid Interface Sci. 2022, 300, 102594. [Google Scholar] [CrossRef]
- Alonso, G.; Gamallo, P.; Mejía, A.; Sayós, R. Assessing Salt-Surfactant Synergistic Effects on Interfacial Tension from Molecular Dynamics Simulations. J. Mol. Liq. 2020, 299, 112223. [Google Scholar] [CrossRef]
- Kopanichuk, I.; Scerbacova, A.; Ivanova, A.; Cheremisin, A.; Vishnyakov, A. The Effect of the Molecular Structure of Alkyl Ether Carboxylate Surfactants on the Oil-Water Interfacial Tension. J. Mol. Liq. 2022, 360, 119525. [Google Scholar] [CrossRef]
- Silin, M.A.; Magadova, L.A.; Davletshina, L.F.; Yunusov, T.I.; Mikulov, V.A. Experimental and Theoretical Study of Carbonate Rocks Dissolution in Chelating Reagents. Proc. Gubkin Univ. 2022, 307, 20–36. [Google Scholar] [CrossRef]
- Kumar, B. Effect of Salinity on the Interfacial Tension of Model and Crude Oil Systems. Master’s Thesis, University of Calgary, Calgary, AB, Canada, 2012. [Google Scholar]
- Ahmadi, M.; Chen, Z. Comprehensive Molecular Scale Modeling of Anionic Surfactant-Asphaltene Interactions. Fuel 2021, 288, 119729. [Google Scholar] [CrossRef]
- Ahmadi, M.; Chen, Z. Insight into the Interfacial Behavior of Surfactants and Asphaltenes: Molecular Dynamics Simulation Study. Energy Fuels 2020, 34, 13536–13551. [Google Scholar] [CrossRef]
- Domínguez, H.; Rivera, M. Mixtures of Sodium Dodecyl Sulfate/Dodecanol at the Air/Water Interface by Computer Simulations. Langmuir 2005, 21, 7257–7262. [Google Scholar] [CrossRef]
- Lyttle, D.J.; Lu, J.R.; Su, T.J.; Thomas, R.K.; Penfold, J. Structure of a Dodecyltrimethylammonium Bromide Layer at the Air/Water Interface Determined by Neutron Reflection: Comparison of the Monolayer Structure of Cationic Surfactants with Different Chain Lengths. Langmuir 1995, 11, 1001–1008. [Google Scholar] [CrossRef]
- Karnanda, W.; Benzagouta, M.S.; AlQoraishi, A.; Amro, M.M. Effect of temperature, pressure, salinity, and surfactant concentration on IFT for surfactant flooding optimization. Arab. J. Geosci. 2013, 6, 3535–3544. [Google Scholar] [CrossRef]
- Hassan, A.; Mahmoud, M.; Patil, S. Impact of Chelating Agent Salt Type on the Enhanced Oil Recovery from Carbonate and Sandstone Reservoirs. Appl. Sci. 2021, 11, 7109. [Google Scholar] [CrossRef]
- Khan, A.M.; Shah, S.S. Determination of Critical Micelle Concentration of Sodium Dodecyl Sulfate (SDS) and the Effect of Low Concentration of Pyrene on Its CMC Using ORIGIN Software. J. Chem. Soc. Pak. 2008, 30, 186–191. [Google Scholar]
- Oremusová, J. Micellization of Alkyl Trimethyl Ammonium Bromides in Aqueous Solutions-Part 1: Critical Micelle Concentration (Cmc) and Ionization Degree. Tenside Surfactants Deterg. 2012, 49, 231–240. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Patel, H.A.; Bruce Nauman, E.; Garde, S. Molecular Structure and Hydrophobic Solvation Thermodynamics at an Octane-Water Interface. J. Chem. Phys. 2003, 119, 9199–9206. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, C.; Zhou, H.; Wang, Y.; Zhang, L.; Zhang, L.; Zhao, S. Characterizing the Impact of Surfactant Structure on Interfacial Tension: A Molecular Dynamics Study. J. Mol. Model. 2017, 23, 112. [Google Scholar] [CrossRef]
- Li, R.; Jiang, Z.; Chen, F.; Yang, H.; Guan, Y. Hydrogen Bonded Structure of Water and Aqueous Solutions of Sodium Halides: A Raman Spectroscopic Study. J. Mol. Struct. 2004, 707, 83–88. [Google Scholar] [CrossRef]
- Volkov, N.A.; Tuzov, N.V.; Shchekin, A.K. Molecular Dynamics Study of Salt Influence on Transport and Structural Properties of SDS Micellar Solutions. Fluid Phase Equilib. 2016, 424, 114–121. [Google Scholar] [CrossRef]
- Yuan, S.; Ma, L.; Zhang, X.; Zheng, L. Molecular Dynamics Studies on Monolayer of Cetyltrimethylammonium Bromide Surfactant Formed at the Air/Water Interface. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 289, 1–9. [Google Scholar] [CrossRef]





| System | Composition, Molecules Number | Dimensions | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Octane | Water | EDTA2− | EDTA3− | Na+ | Br− | CA | Surfactant | l × h × w, Å | |
| 1 | 118 × 2 | 1067 | 2 | 6 | 72 | 0 | 0 | 36 × 2 | 89 × 40 × 40 |
| 2 | 118 × 2 | 957 | 2 | 6 | 97 | 0 | 1 | 36 × 2 | 89 × 40 × 40 |
| 3 | 118 × 2 | 1593 | 3 | 9 | 0 | 72 | 0 | 36 × 2 | 111 × 40 × 40 |
| 4 | 118 × 2 | 1435 | 3 | 9 | 36 | 72 | 1 | 36 × 2 | 111 × 40 × 40 |
| Surfactant | Solution | Measured, % wt. | Reference Value, % wt. |
|---|---|---|---|
| SDS | Water | 0.145 | 0.230 [48] |
| Chelating agent | 0.0014 | - | |
| DTAB | Water | 0.533 | 0.547 [49] |
| Chelating agent | 0.061 | - |
| Surfactant | Solution | Interfacial Thickness, Å |
|---|---|---|
| SDS | Water | 18.18 |
| Chelating agent | 19.98 | |
| DTAB | Water | 31.43 |
| Chelating agent | 32.59 |
| Surfactant | Solution | Number of Hydrogen Bonds per Water Molecule | Number of Hydrogen Bonds per Surfactant Molecule |
|---|---|---|---|
| SDS | Water | 1.69 ± 0.02 | 4.35 ± 0.18 |
| Chelating agent | 1.59 ± 0.02 | 3.89 ± 0.13 | |
| DTAB | Water | 1.62 ± 0.01 | 0 |
| Chelating agent | 1.50 ± 0.014 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yunusov, T.I.; Davletshina, L.F.; Magadova, L.A.; Silin, M.A. Study of Chelating Agent—Surfactant Interactions on the Interphase as Possibly Useful for the Well Stimulation. Energies 2023, 16, 1679. https://doi.org/10.3390/en16041679
Yunusov TI, Davletshina LF, Magadova LA, Silin MA. Study of Chelating Agent—Surfactant Interactions on the Interphase as Possibly Useful for the Well Stimulation. Energies. 2023; 16(4):1679. https://doi.org/10.3390/en16041679
Chicago/Turabian StyleYunusov, Timur Ildarovich, Lyutsia Faritovna Davletshina, Lyubov Abdulaevna Magadova, and Mikhail Alexandrovich Silin. 2023. "Study of Chelating Agent—Surfactant Interactions on the Interphase as Possibly Useful for the Well Stimulation" Energies 16, no. 4: 1679. https://doi.org/10.3390/en16041679
APA StyleYunusov, T. I., Davletshina, L. F., Magadova, L. A., & Silin, M. A. (2023). Study of Chelating Agent—Surfactant Interactions on the Interphase as Possibly Useful for the Well Stimulation. Energies, 16(4), 1679. https://doi.org/10.3390/en16041679






