Chemical Looping Gasification of Wood Waste Using NiO-Modified Hematite as an Oxygen Carrier
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization
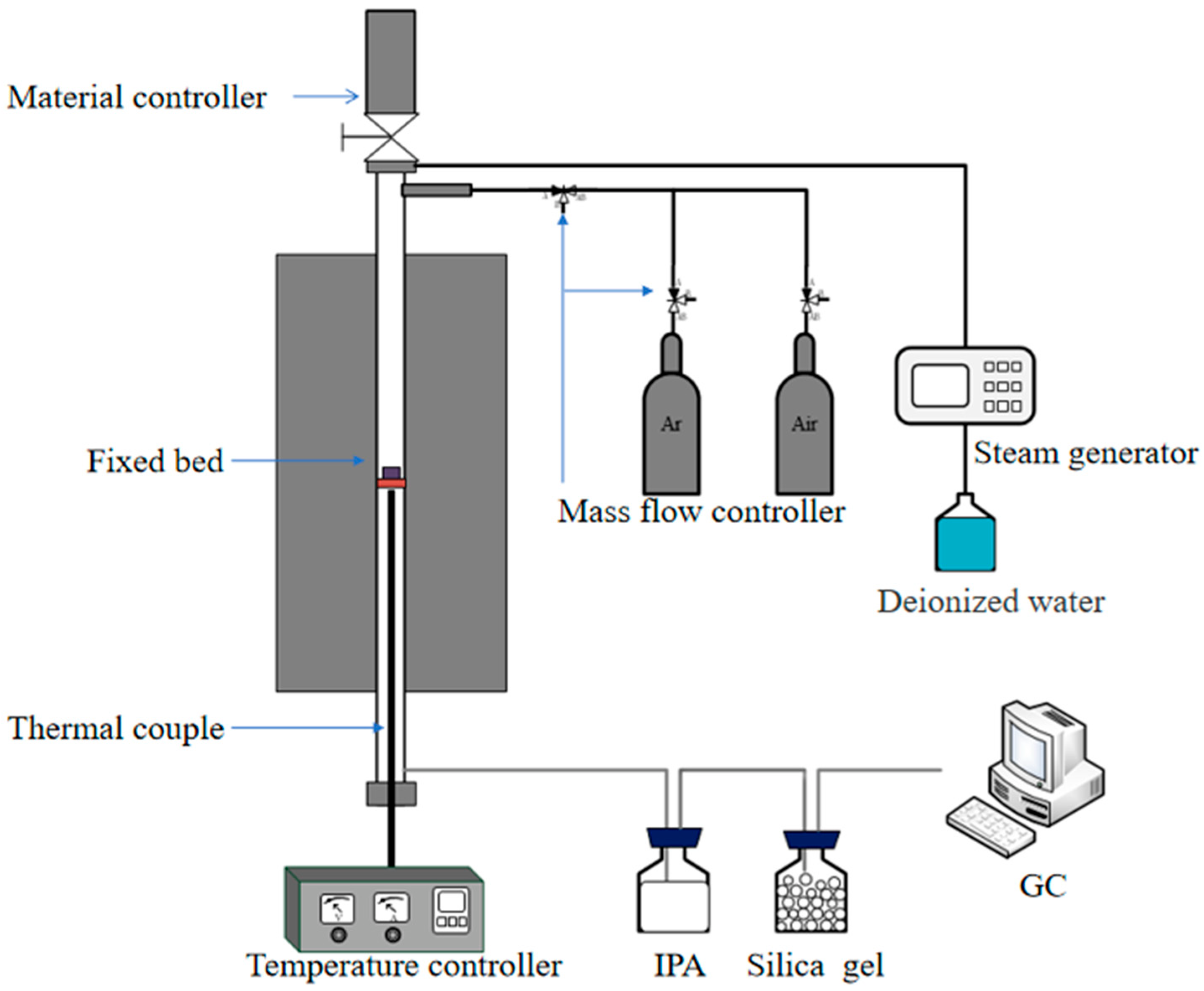
2.3. Experimental Set-Up and Procedure
2.4. Data Processing
3. Results and Discussion
3.1. The Effect of Various OCs
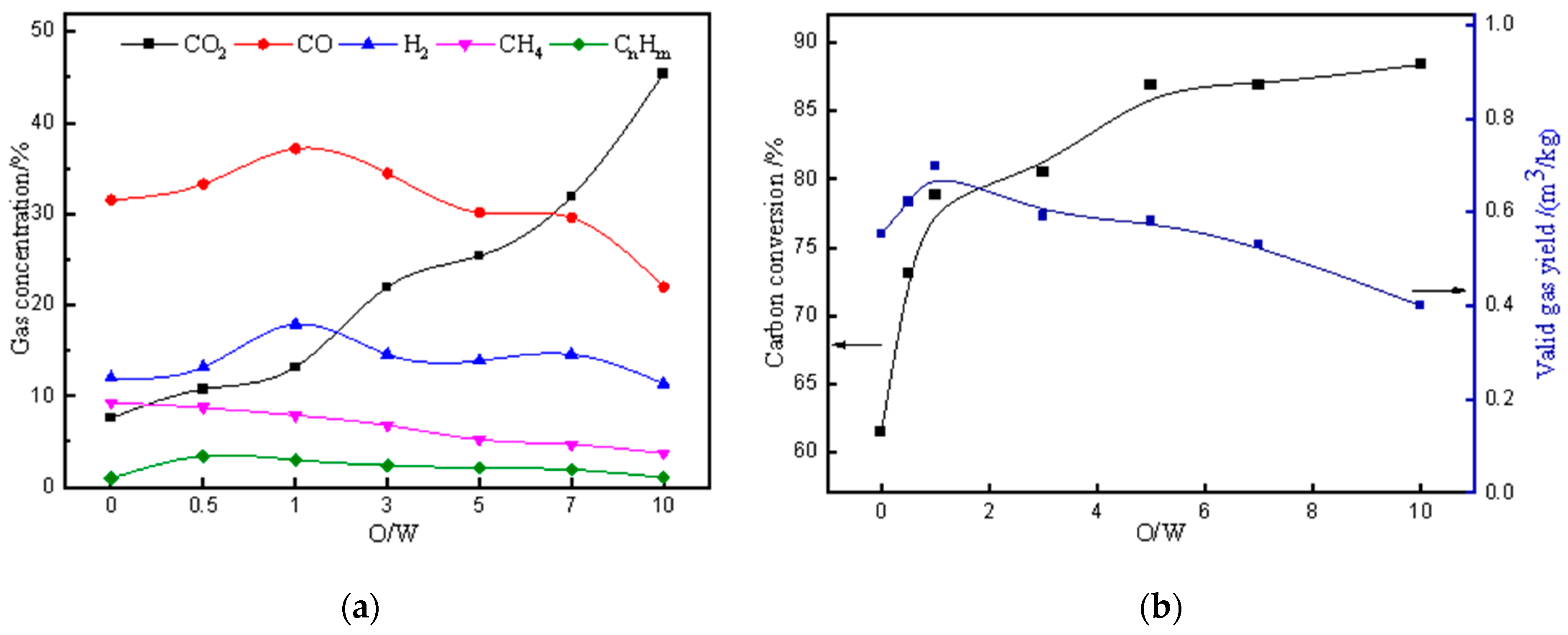
3.2. The Effect of Varying O/W
3.3. The Effects of Varying the Reaction Temperature
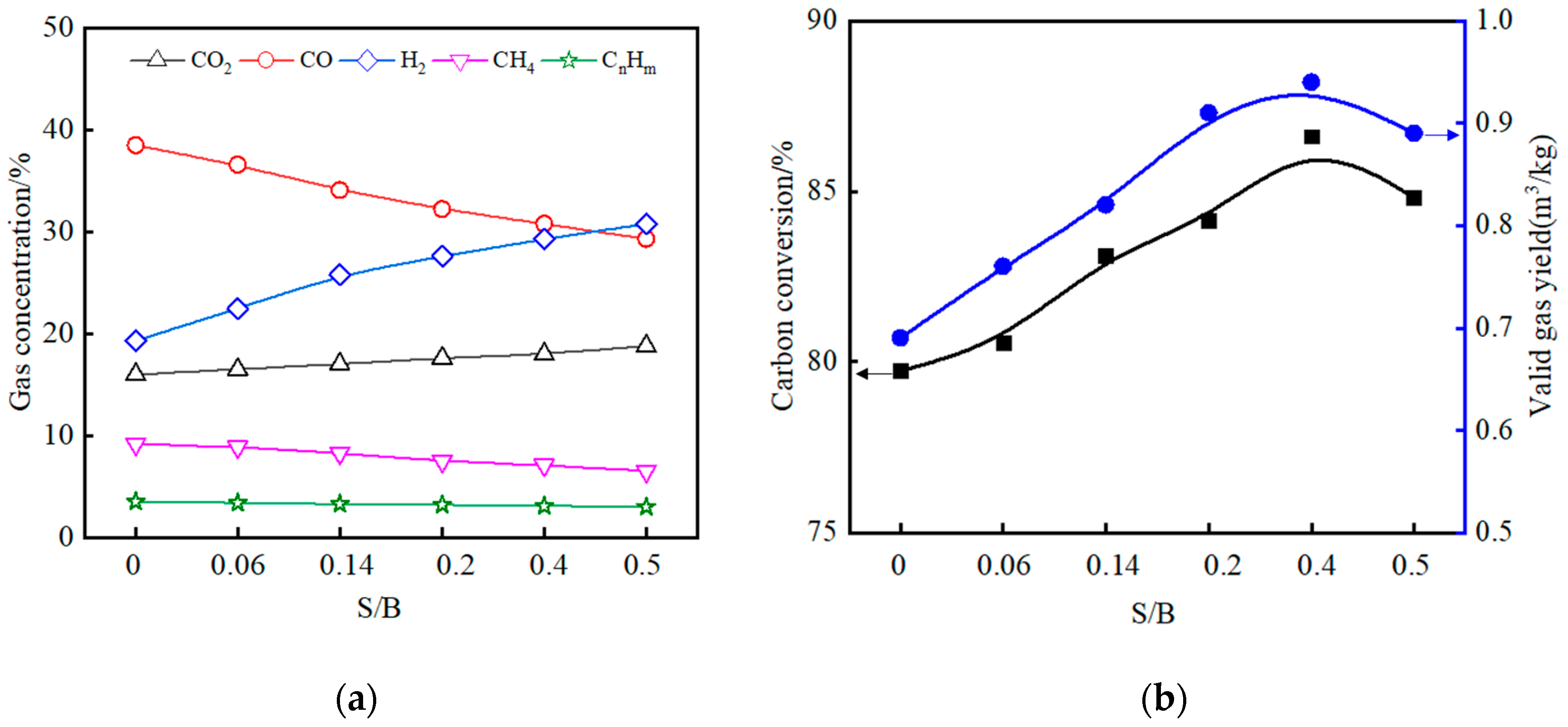
3.4. The Effects of Varying the S/B
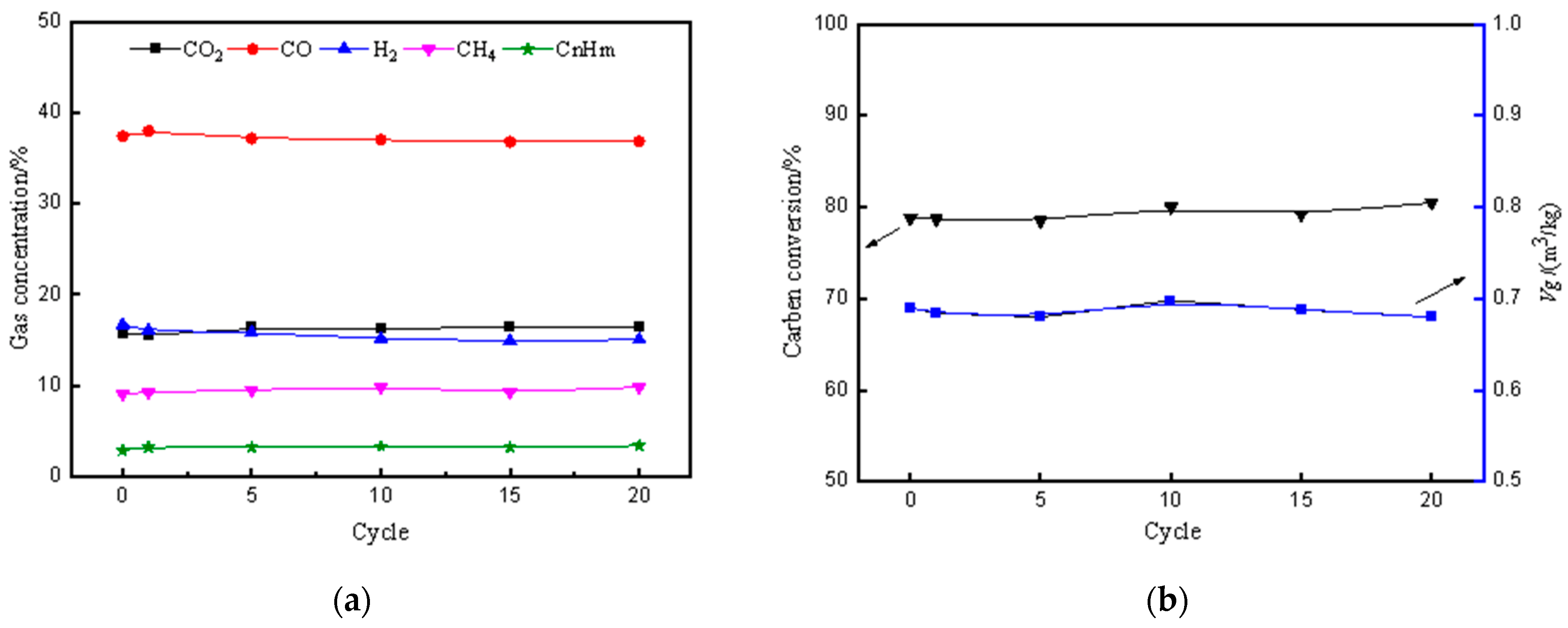
3.5. The Effects of the Number of Cycles
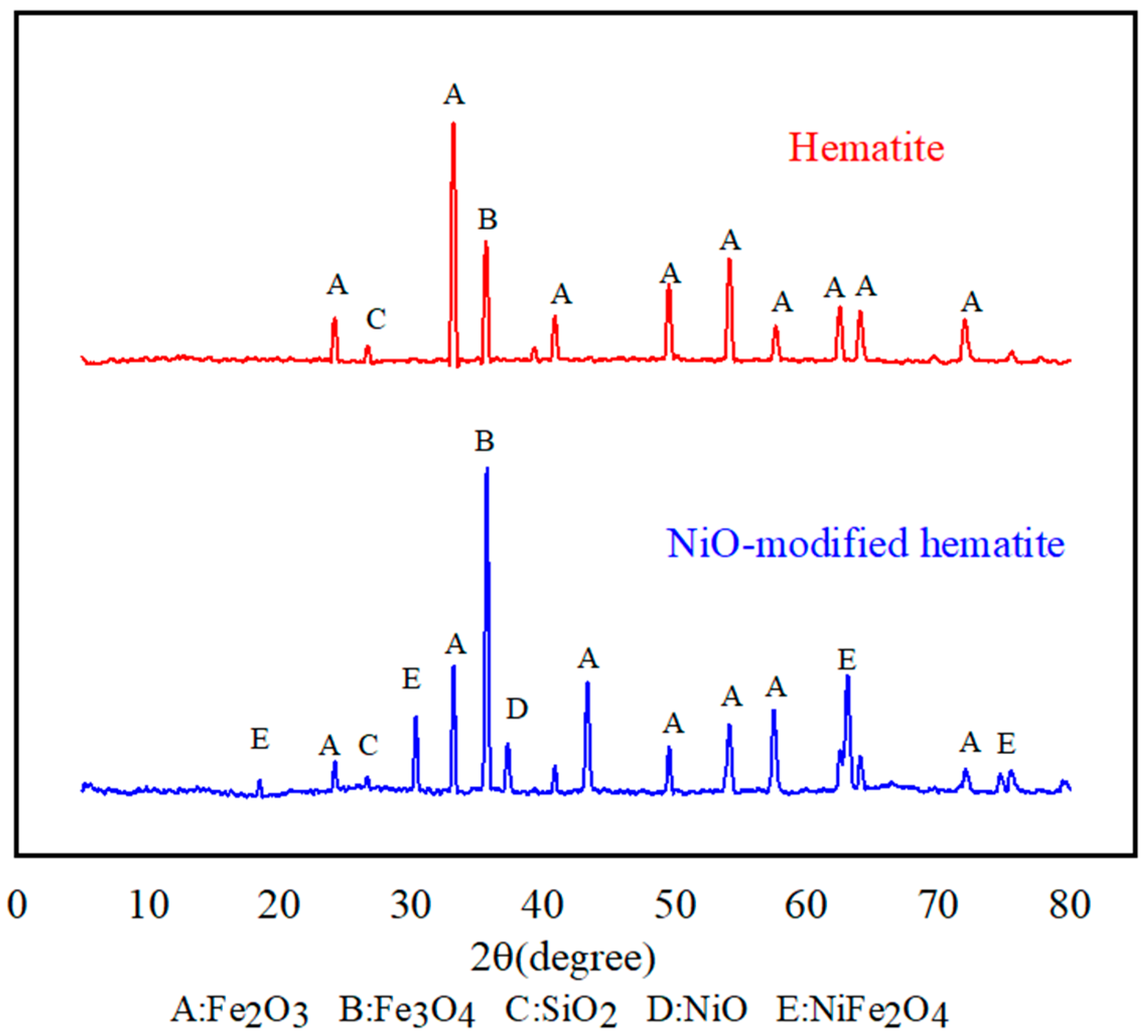
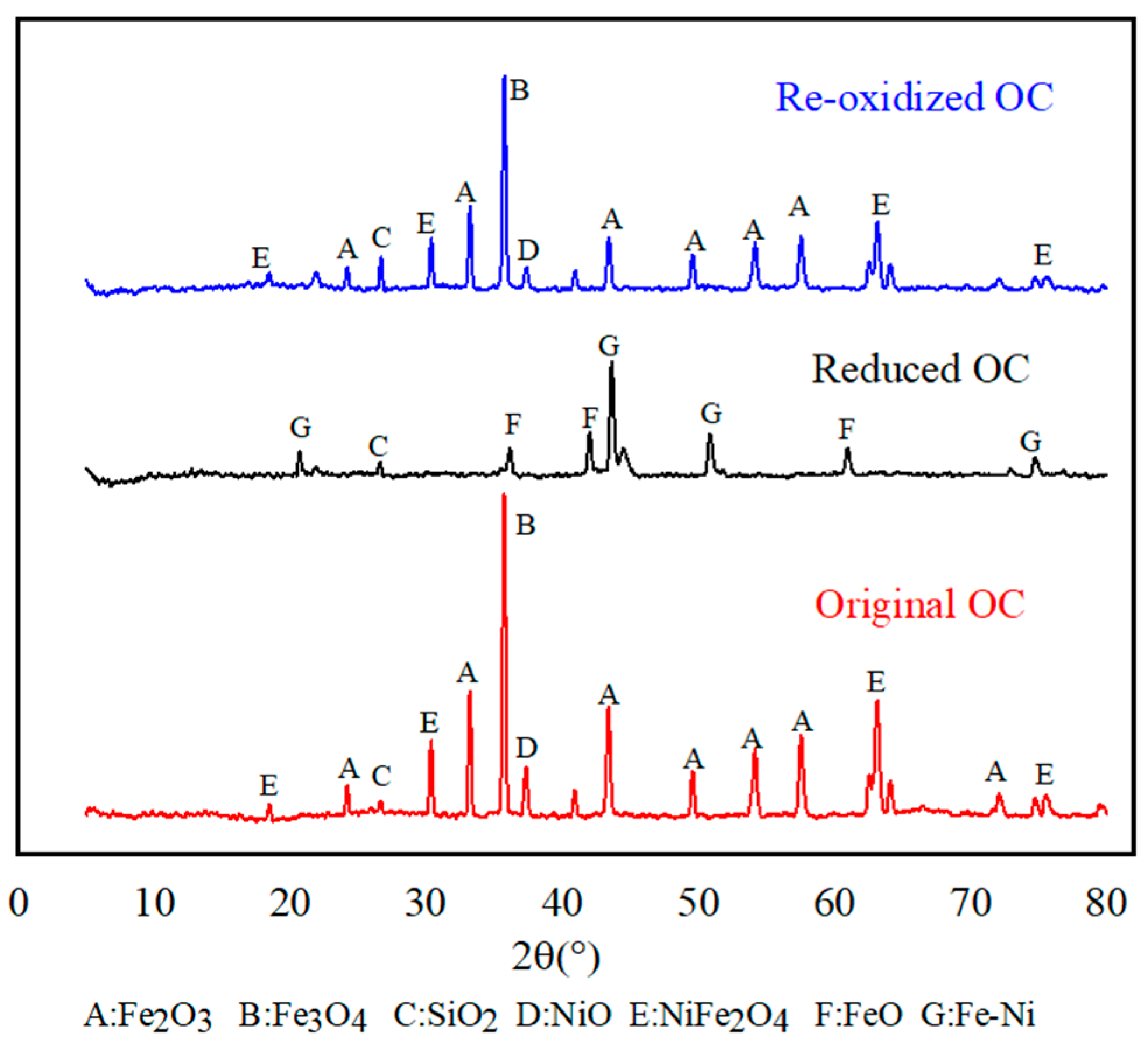
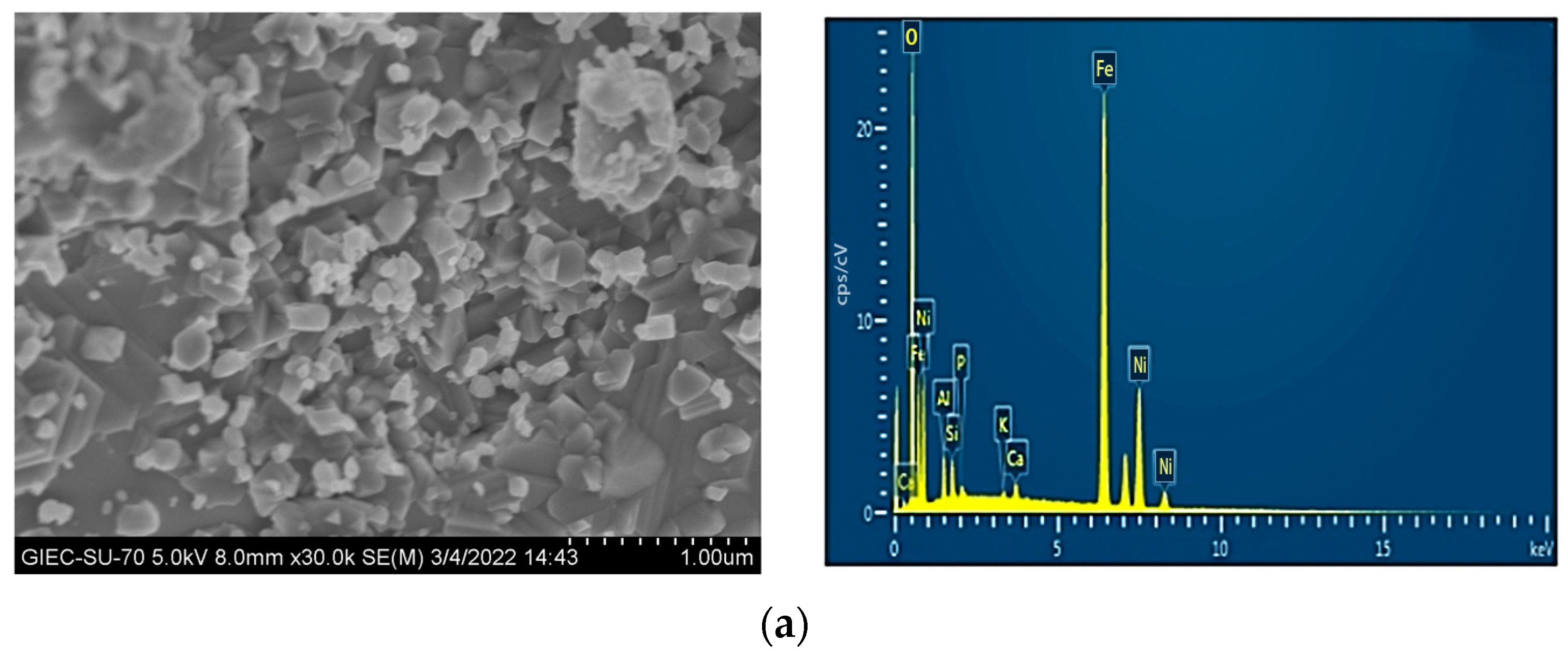
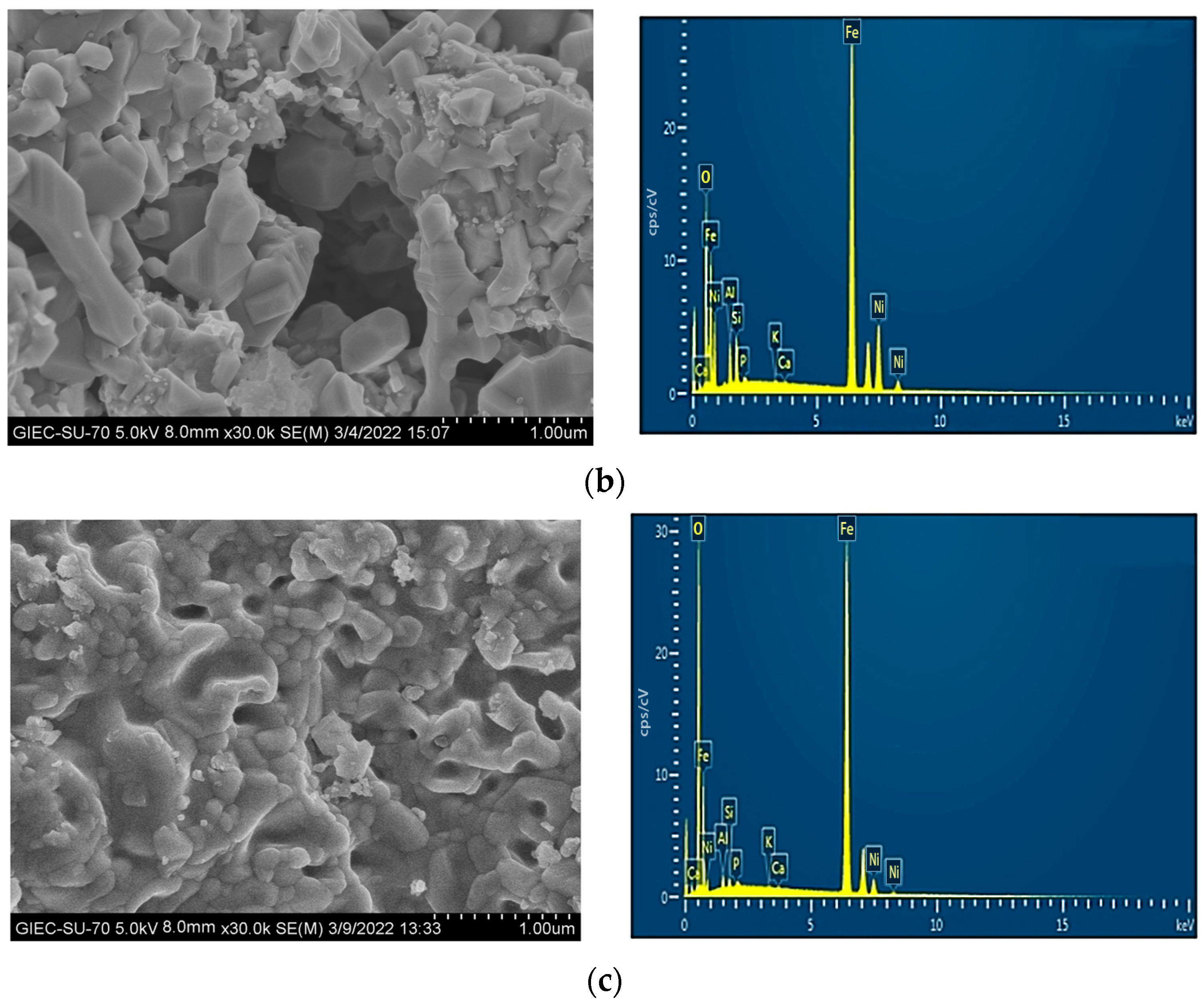
3.6. Characterization of the OC at Various Reaction Stages
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zeng, Q.Z.; Lu, Q.F.; Zhou, Y.H.; Chen, N.R.; Rao, J.P.; Fan, M.Z. Circular development of recycled natural fibers from medium density fiberboard wastes. J. Clean. Prod. 2018, 202, 456–464. [Google Scholar] [CrossRef]
- Khodaei, H.; Olson, C.; Patino, D.; Rico, J.; Jin, Q.; Boateng, A. Multi-objective utilization of wood waste recycled from construction and demolition (C&D): Products and characterization. Waste Manag. 2022, 149, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.S.; Chang, J.M.; Ren, X.Y.; Han, Y.X.; Si, H.; Huang, Y. Study on Clean Treatment of Urea-Formaldehyde Waste Using Fast Pyrolysis. Adv. Mater. Res. 2011, 194–196, 2097. [Google Scholar] [CrossRef]
- Paulino, R.F.S.; Essiptchouk, A.M.; Silveira, J.L. The use of syngas from biomedical waste plasma gasification systems for electricity production in internal combustion: Thermodynamic and economic issues. Energy 2020, 199, 117419. [Google Scholar] [CrossRef]
- Correa, P.S.P.; Lora, E.E.S.; Andrade, R.V.; Pinto, L.R.D.E.; Ratner, A. The Use of Natural Gas Blends with Syngas from Biomass in Gas Micro Turbine Thermal Performance and Emissions Tests. In Proceedings of the 25th European Biomass Conference, Stockholm, Schweden, 12–15 June 2017; OpenAgrar: Greifswald, Germany, 2017; pp. 714–724. [Google Scholar]
- Liu, M.; Pourquie, M.J.B.M.; Fan, L.; Halliop, W.; Cobas, V.R.M.; Verkooijen, A.H.M.; Aravind, P.V. The Use of Methane-Containing Syngas in a Solid Oxide Fuel Cell: A Comparison of Kinetic Models and a Performance Evaluation. Fuel Cells 2013, 13, 428–440. [Google Scholar] [CrossRef]
- Lv, P.M.; Xiong, Z.H.; Chang, J.; Wu, C.Z.; Chen, Y.; Zhu, J.X. An experimental study on biomass air-steam gasification in a fluidized bed. Bioresour. Technol. 2004, 95, 95–101. [Google Scholar] [CrossRef]
- Hejazi, B. Heat integration and waste minimization of biomass steam gasification in a bubbling fluidized bed reactor. Biomass Bioenergy 2022, 159, 106409. [Google Scholar] [CrossRef]
- Hoang, A.T.; Huang, Z.H.; Nizetic, S.; Pandey, A.; Nguyen, X.P.; Luque, R.; Ong, H.C.; Said, Z.; Le, T.H.; Pham, V.V. Characteristics of hydrogen production from steam gasification of plant-originated lignocellulosic biomass and its prospects in Vietnam. Int. J. Hydrogen Energy 2022, 47, 4394–4425. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Q.; Du, J.; Chen, J. Oxygen-enriched air gasification of biomass materials for high-quality syngas production. Energy Convers. Manag. 2019, 199, 111628. [Google Scholar] [CrossRef]
- Nunes, L.J.R. Biomass gasification as an industrial process with effective proof-of-concept: A comprehensive review on technologies, processes and future developments. Results Eng. 2022, 14, 100408. [Google Scholar] [CrossRef]
- Wang, M.; Wan, Y.L.; Guo, Q.H.; Bai, Y.H.; Yu, G.S.; Liu, Y.R.; Zhang, H.; Zhang, S.; Wei, J.T. Brief review on petroleum coke and biomass/coal co-gasification: Syngas production, reactivity characteristics, and synergy behavior. Fuel 2021, 304, 121517. [Google Scholar] [CrossRef]
- Mishra, S.U.R.K. Review on biomass gasification: Gasifiers, gasifying mediums, and operational parameters. Mater. Sci. Energy Technol. 2021, 4, 329–340. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Tippayawong, N.; Wei, G.Q.; Huang, Z.; Zhao, K.; Jiang, L.Q.; Zheng, A.Q.; Zhao, Z.L.; Li, H.B. Minimizing tar formation whilst enhancing syngas production by integrating biomass torrefaction pretreatment with chemical looping gasification. Appl. Energy 2020, 260, 114315. [Google Scholar] [CrossRef]
- Zeng, J.M.; Hu, J.W.; Qiu, Y.; Zhang, S.; Zeng, D.W.; Xiao, R. Multi-function of oxygen carrier for in-situ tar removal in chemical looping gasification: Naphthalene as a model compound. Appl. Energy 2019, 253, 113502. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, K.; Zhao, Z.L.; He, F.; Huang, Z.; Wei, G.Q. Identifying the roles of MFe2O4 (M = Cu, Ba, Ni, and Co) in the chemical looping reforming of char, pyrolysis gas and tar resulting from biomass pyrolysis. Int. J. Hydrogen Energy 2019, 44, 4674–4687. [Google Scholar] [CrossRef]
- Yan, X.Y.; Hu, J.J.; Zhang, Q.G.; Zhao, S.H.; Dang, J.T.; Wang, W. Chemical-looping gasification of corn straw with Fe-based oxygen carrier: Thermogravimetric analysis. Bioresour. Technol. 2020, 303, 122904. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, H.T.; Huang, Z.; Liu, M.; Wei, G.Q.; Zhao, Z.L.; Li, H.B.; Fang, Y.T. Chemical looping gasification coupled with steam reforming of biomass using NiFe2O4: Kinetic analysis of DAEM-TI, thermodynamic simulation of OC redox, and a loop test. Chem. Eng. J. 2020, 395, 125046. [Google Scholar] [CrossRef]
- Song, T.; Shen, L.H.; Xiao, J.; Chen, D.Q.; Gu, H.M.; Zhang, S.W. Nitrogen transfer of fuel-N in chemical looping combustion. Combust. Flame 2012, 159, 1286–1295. [Google Scholar] [CrossRef]
- Mendiara, T.; Izquierdo, M.T.; Abad, A.; de Diego, L.F.; Garcia-Labiano, F.; Gayan, P.; Adanez, J. Release of pollutant components in CLC of lignite. Int. J. Greenh. Gas Control 2014, 22, 15–24. [Google Scholar] [CrossRef]
- Song, T.; Guo, W.J.; Shen, L.H. Fuel Nitrogen Conversion in Chemical Looping with Oxygen Uncoupling of Coal with a CuO-Based Oxygen Carrier. Energy Fuels 2015, 29, 3820–3832. [Google Scholar] [CrossRef]
- Alam, S.; Kumar, J.P.; Rani, K.Y.; Sumana, C. Self-sustained process scheme for high purity hydrogen production using sorption enhanced steam methane reforming coupled with chemical looping combustion. J. Clean. Prod. 2017, 162, 687–701. [Google Scholar] [CrossRef]
- Torres, W.; Pansare, S.S.; Goodwin, J.G. Hot gas removal of tars, ammonia, and hydrogen sulfide from Biomass gasification gas. Catal. Rev. 2007, 49, 407–456. [Google Scholar] [CrossRef]
- Abu El-Rub, Z.; Bramer, E.A.; Brem, G. Review of catalysts for tar elimination in Biomass gasification processes. Ind. Eng. Chem. Res. 2004, 43, 6911–6919. [Google Scholar] [CrossRef]
- Bhavsar, S.; Najera, M.; Solunke, R.; Veser, G. Chemical looping: To combustion and beyond. Catal. Today 2014, 228, 96–105. [Google Scholar] [CrossRef]
- Rubel, A.; Liu, K.L.; Neathery, J.; Taulbee, D. Oxygen carriers for chemical looping combustion of solid fuels. Fuel 2009, 88, 876–884. [Google Scholar] [CrossRef]
- Linderholm, C.; Schmitz, M.; Biermann, M.; Hanning, M.; Lyngfelt, A. Chemical-looping combustion of solid fuel in a 100 kW unit using sintered manganese ore as oxygen carrier. Int. J. Greenh. Gas Control 2017, 65, 170–181. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in Chemical-Looping Combustion and Reforming technologies. Prog. Energy Combust. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Feng, Y.P.; Zhao, K.; Zheng, A.Q.; Chang, S.; Li, H.B. Synthesis gas production through biomass direct chemical looping conversion with natural hematite as an oxygen carrier. Bioresour. Technol. 2013, 140, 138–145. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Zhao, K.; Feng, Y.P.; Zheng, A.Q.; Chang, S.; Zhao, Z.L.; Li, H.B. Natural iron ore as an oxygen carrier for biomass chemical looping gasification in a fluidized bed reactor. J. Therm. Anal. Calorim. 2014, 116, 1315–1324. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Zheng, A.Q.; Zhao, K.; Chang, S.; Zhao, Z.L.; Li, H.B. Synthesis gas production from biomass gasification using steam coupling with natural hematite as oxygen carrier. Energy 2013, 53, 244–251. [Google Scholar] [CrossRef]
- Jia, L.; Cheng, P.; Yu, Y.; Chen, S.H.; Wang, C.X.; He, L.; Nie, H.T.; Wang, J.C.; Zhang, J.C.; Fan, B.G.; et al. Regeneration mechanism of a novel high-performance biochar mercury adsorbent directionally modified by multimetal multilayer loading. J. Environ. Manag. 2023, 326, 116790. [Google Scholar] [CrossRef]
- Nam, H.; Wang, Z.H.; Shanmugam, S.R.; Adhikari, S.; Abdoulmoumine, N. Chemical looping dry reforming of benzene as a gasification tar model compound with Ni- and Fe-based oxygen carriers in a fluidized bed reactor. Int. J. Hydrogen Energy 2018, 43, 18790–18800. [Google Scholar] [CrossRef]
- Ge, H.J.; Shen, L.H.; Feng, F.; Jiang, S.X. Experiments on biomass gasification using chemical looping with nickel-based oxygen carrier in a 25 kWth reactor. Appl. Therm. Eng. 2015, 85, 52–60. [Google Scholar] [CrossRef]
- Abad, A.; Garcia-Labiano, F.; de Diego, L.F.; Gayan, P.; Adanez, J. Reduction kinetics of Cu-, Ni-, and Fe-based oxygen carriers using syngas (CO + H-2) for chemical-looping combustion. Energy Fuel 2007, 21, 1843–1853. [Google Scholar] [CrossRef]
- Elgarni, M.M.; Tijani, M.M.; Mahinpey, N. Characterization, kinetics and stability studies of NiO and CuO supported by Al2O3, ZrO2, CeO2 and their combinations in chemical combustion. Catal. Today 2022, 397, 205–219. [Google Scholar] [CrossRef]
- Yang, J.; Liu, S.Y.; Ma, L.P.; Liu, H.P.; Yang, J.; Guo, Z.Y.; Ao, R.; Dai, Q.X. Syngas preparation by NiO-CaSO4-based oxygen carrier from chemical looping gasification technology. J. Energy Inst. 2021, 94, 191–198. [Google Scholar] [CrossRef]
- Feng, X.B.; Li, Z.Q.; Lin, S.; Tian, S.P.; Li, K.Z. Enhanced performance of red mud for chemical-looping combustion of coal by the modification of transition metal oxides. J Energy Inst. 2022, 102, 22–31. [Google Scholar] [CrossRef]
- Sun, Z.K.; Lu, D.Y.; Symonds, R.T.; Hughes, R.W. Chemical looping reforming of CH4 in the presence of CO2 using ilmenite ore and NiO-modified ilmenite ore oxygen carriers. Chem. Eng. J. 2020, 401, 123481. [Google Scholar] [CrossRef]
- Dong, N.H.; Huo, R.Q.; Liu, M.; Deng, L.S.; Deng, Z.B.; Chang, G.Z.; Huang, Z.; Huang, H.Y. Chemical looping gasification of sewage sludge using copper slag modified by NiO as an oxygen carrier. Chin. J. Chem. Eng. 2021, 29, 335–343. [Google Scholar] [CrossRef]
- Saha, C.; Roy, B.; Bhattacharya, S. Chemical looping combustion of Victorian brown coal using NiO oxygen carrier. Int. J. Hydrogen Energy 2011, 36, 3253–3259. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Feng, Y.P.; Zhao, K.; Zheng, A.Q.; Chang, S.; Wei, G.Q.; Zhao, Z.L.; Li, H.B. Biomass Char Direct Chemical Looping Gasification Using NiO-Modified Iron Ore as an Oxygen Carrier. Energy Fuel 2014, 28, 183–191. [Google Scholar] [CrossRef]
- Hu, Z.F.; Jiang, E.C.; Ma, X.Q. Microwave pretreatment on microalgae: Evolution of gas and char in chemical looping gasification. Int. J. Energy Res. 2019, 43, 956–969. [Google Scholar] [CrossRef]
- Zou, J.; Oladipo, J.; Fu, S.L.; Al-Rahbi, A.; Yang, H.P.; Wu, C.F.; Cai, N.; Williams, P.; Chen, H.P. Hydrogen production from cellulose catalytic gasification catalyst on CeO2/Fe2O3. Energy Convers. Manag. 2018, 171, 241–248. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, S.Y.; Russell, C.K.; Hu, J.; Rony, A.H.; Tan, G.; Chen, A.M.; Duan, L.B.; Boman, J.; Tang, J.K.; et al. Improvement of H-2-rich gas production with tar abatement from pine wood conversion over bi-functional Ca2Fe2O5 catalyst: Investigation of inner-looping redox reaction and promoting mechanisms. Appl. Energy 2018, 212, 931–943. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Chen, D.Z.; Zhao, K.; Wei, G.Q.; Zheng, A.Q.; Zhao, Z.L.; Li, H.B. Investigation on reactivity of iron nickel oxides in chemical looping dry reforming. Energy 2016, 116, 53–63. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, Z.B.; Fen, Y.H.; Chen, T.J.; Chen, D.Z.; Zheng, A.Q.; Wei, G.Q.; He, F.; Zhao, Z.L.; Wu, J.H.; et al. Exploring the Conversion Mechanisms of Toluene as a Biomass Tar Model Compound on NiFe2O4 Oxygen Carrier. ACS Sustain. Chem. Eng. 2019, 7, 16539–16548. [Google Scholar] [CrossRef]
- Wei, G.Q.; Yang, M.; Huang, Z.; Bai, H.C.; Chang, G.Z.; He, F.; Yi, Q.; Huang, Y.; Zheng, A.Q.; Zhao, K.; et al. Syngas production from lignite via chemical looping gasification with hematite oxygen carrier enhanced by exogenous metals. Fuel 2022, 321, 124119. [Google Scholar] [CrossRef]
- Nordgreen, T.; Liliedahl, T.; Sjostrom, K. Metallic iron as a tar breakdown catalyst related to atmospheric, fluidised bed gasification of biomass. Fuel 2006, 85, 689–694. [Google Scholar] [CrossRef]
- Gao, N.B.; Li, A.M.; Quan, C.; Qu, Y.; Mao, L.Y. Characteristics of hydrogen-rich gas production of biomass gasification with porous ceramic reforming. Int. J. Hydrogen Energy 2012, 37, 9610–9618. [Google Scholar] [CrossRef]
- Niu, X.; Shen, L.H. Ca- and Mg-rich waste as high active carrier for chemical looping gasification of biomass. Chin. J. Chem. Eng. 2021, 38, 145–154. [Google Scholar] [CrossRef]
- Yan, B.B.; Li, Z.Y.; Jiao, L.G.; Li, J.; Chen, G.Y.; Yang, G.X. Chemical looping gasification of Chlorella: Parametric optimization, reaction mechanisms, and nitrogen-containing pollutants emission. Fuel 2021, 289, 119987. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, G.L.; Deng, Z.B.; Zhao, K.; He, F.; Chen, D.Z.; Wei, G.Q.; Zheng, A.Q.; Zhao, Z.L.; Li, H.B. Investigation on gasification performance of sewage sludge using chemical looping gasification with iron ore oxygen carrier. Int. J. Hydrogen Energy 2017, 42, 25474–25491. [Google Scholar] [CrossRef]
- Niu, X.; Shen, L.H.; Gu, H.M.; Song, T.; Xiao, J. Sewage sludge combustion in a CLC process using nickel-based oxygen carrier. Chem. Eng. J. 2015, 260, 631–641. [Google Scholar] [CrossRef]
- De Andres, J.M.; Roche, E.; Narros, A.; Rodriguez, M.E. Characterisation of tar from sewage sludge gasification. Influence of gasifying conditions: Temperature, throughput, steam and use of primary catalysts. Fuel 2016, 180, 116–126. [Google Scholar] [CrossRef]
- Mu, L.; Zhao, L.; Hu, T.C.; Zhang, B.; Zhai, Z.D.; Shang, Y.; Yin, H.C. Modeling and Evaluation of Biomass-Based Chemical Looping Gasification-Integrated Power Generation Cycles with Focus on Energy and Exergy Analyses and Solar Energy Application. Ind. Eng. Chem. Res. 2021, 60, 15618–15634. [Google Scholar] [CrossRef]
- Condori, O.; Garcia-Labiano, F.; de Diego, L.F.; Izquierdo, M.T.; Abad, A.; Adanez, J. Biomass chemical looping gasification for syngas production using ilmenite as oxygen carrier in a 1.5 kW(th) unit. Chem. Eng. J. 2021, 405, 126679. [Google Scholar] [CrossRef]



| Ultimate Analysis | Proximate Analysis | Q/(kJ/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O * | Moisture | Volatiles | Fixed Carbon | Ash | |
| 45.89 | 5.07 | 3.72 | 0.18 | 45.14 | 8.02 | 73.67 | 16.61 | 1.70 | 17,472 |
| Content (%) | Fe | O * | Ni | Si | Al | K | Ca | Others |
|---|---|---|---|---|---|---|---|---|
| Pristine hematite | 62.44 | 32.38 | 0 | 3.04 | 0.13 | 0.02 | 0.04 | 1.90 |
| NiO-modified hematite | 50.85 | 29.06 | 15.56 | 2.54 | 0.20 | 0.24 | 0.14 | 0.31 |
| Item | Operating parameter |
|---|---|
| Oxygen carrier | NiO-modified hematite or pristine hematite |
| Reaction temperature | 750 °C, 800 °C, 850 °C, or 900 °C |
| Oxygen carrier mass | 0.25 g, 0.5 g, 1.5 g, 2.5 g, 3.5 g, or 5.0 g |
| Fuel | Wood waste |
| Fuel mass | 0.5 g |
| Inert gas | Ar |
| Inert gas flow rate | 100 mL/min |
| Gasification agent | H2O |
| Gasification agent mass (steam) | 0.03 g, 0.07 g, 0.1 g, 0.2 g, 0.25 g |
| Oxidation agent | O2 |
| Oxidation agent flow rate | 100 mL/min |
| Reduction time | 45 min |
| Purge time | 30 min |
| Oxidation time | 30 min |
| Number of cycles | 20 cycles (48 h) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Zhu, K.; Zhang, Z.; Chen, X.; Lin, Y.; Hu, J.; Xiong, Y.; Zhang, Y.; Huang, Z.; Huang, H. Chemical Looping Gasification of Wood Waste Using NiO-Modified Hematite as an Oxygen Carrier. Energies 2023, 16, 1847. https://doi.org/10.3390/en16041847
Xie J, Zhu K, Zhang Z, Chen X, Lin Y, Hu J, Xiong Y, Zhang Y, Huang Z, Huang H. Chemical Looping Gasification of Wood Waste Using NiO-Modified Hematite as an Oxygen Carrier. Energies. 2023; 16(4):1847. https://doi.org/10.3390/en16041847
Chicago/Turabian StyleXie, Jinlong, Kang Zhu, Zhen Zhang, Xinfei Chen, Yan Lin, Jianjun Hu, Ya Xiong, Yongqi Zhang, Zhen Huang, and Hongyu Huang. 2023. "Chemical Looping Gasification of Wood Waste Using NiO-Modified Hematite as an Oxygen Carrier" Energies 16, no. 4: 1847. https://doi.org/10.3390/en16041847
APA StyleXie, J., Zhu, K., Zhang, Z., Chen, X., Lin, Y., Hu, J., Xiong, Y., Zhang, Y., Huang, Z., & Huang, H. (2023). Chemical Looping Gasification of Wood Waste Using NiO-Modified Hematite as an Oxygen Carrier. Energies, 16(4), 1847. https://doi.org/10.3390/en16041847









