Investigation of the Properties of Anode Electrodes for Lithium–Ion Batteries Manufactured Using Cu, and Si-Coated Carbon Nanowall Materials
Abstract
:1. Introduction
2. Experimental Details
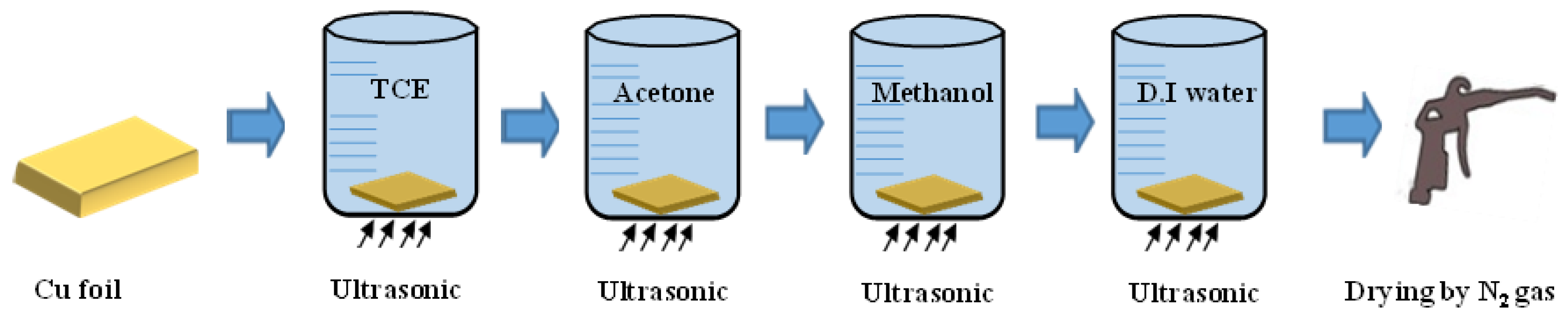
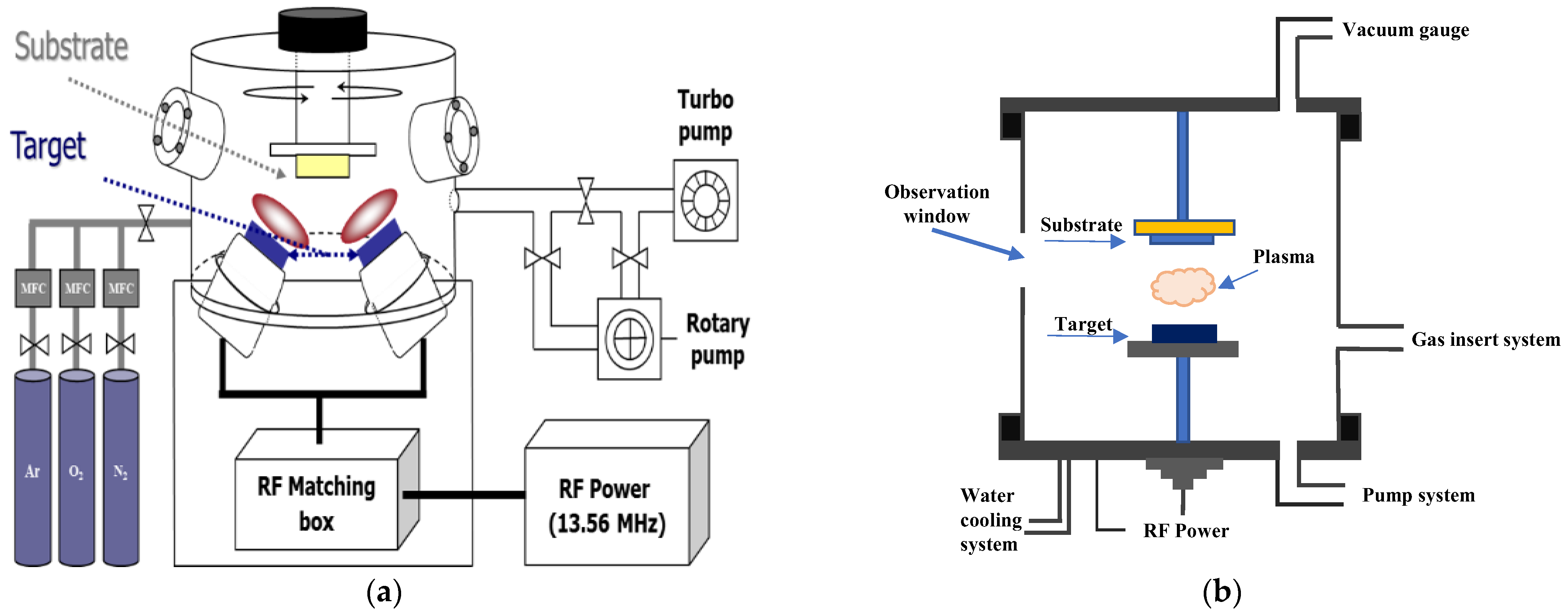
2.1. Preparing Substrate and Carbon Nanowall Growth
2.2. Preparation of Substrate and Carbon Nanowall Growth
2.3. Fabrication of Lithium–Ion Battery
2.4. Analysis and Measurement
3. Results and Discussion
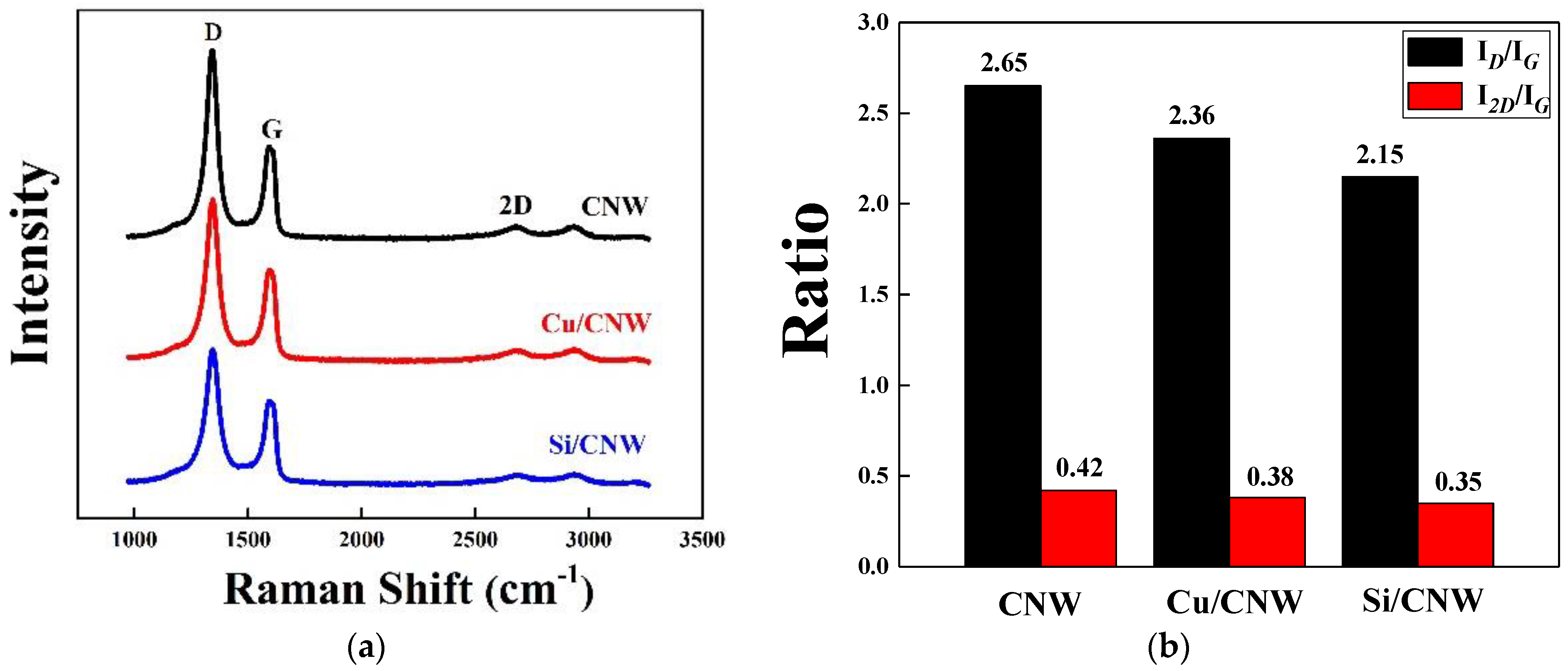
3.1. Morphology and Microstructure of the Synthesized Anode Materials
3.2. Electrochemical Performance of Cu, Si-Coated CNWs Based LIBs
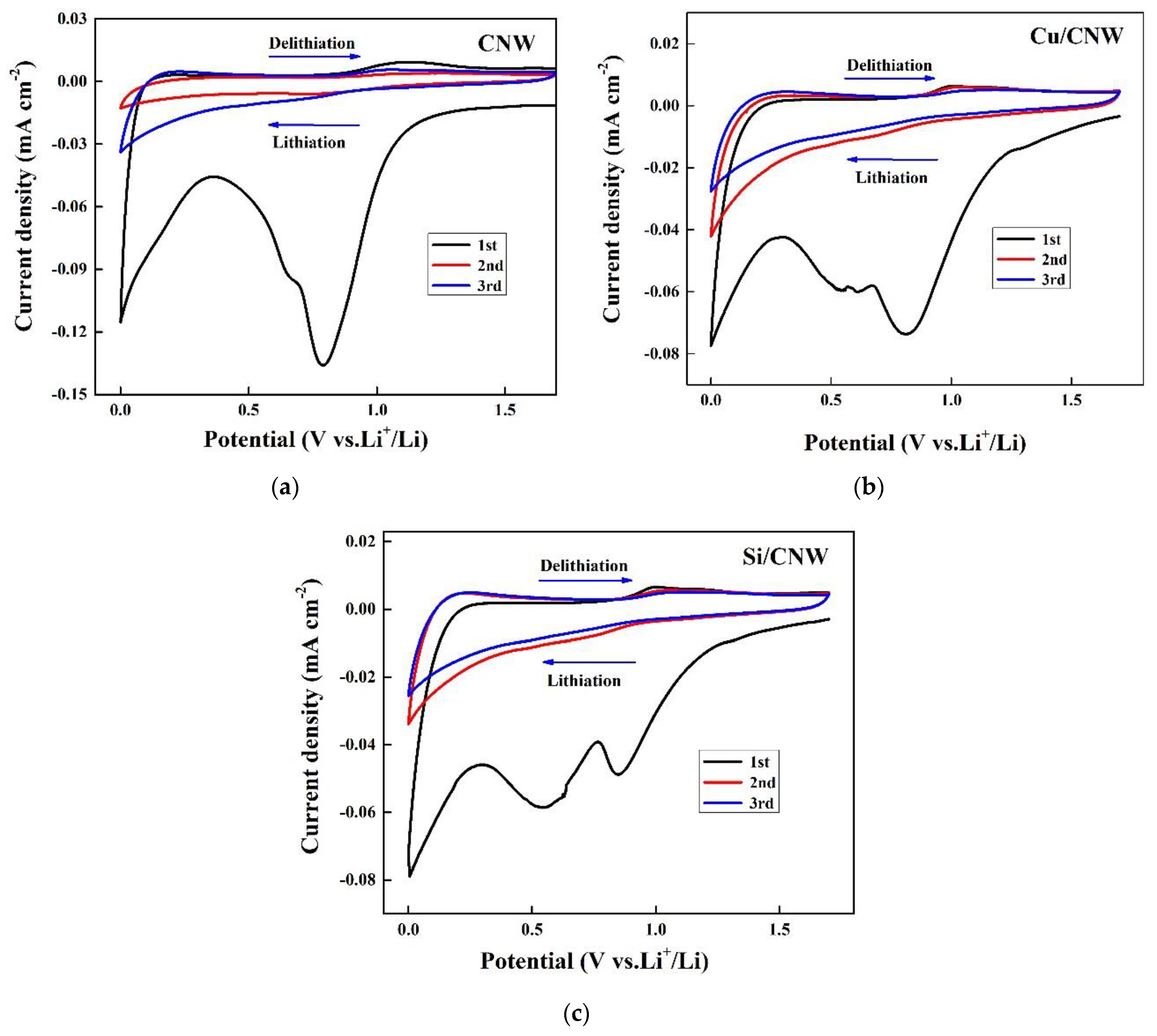
- Cyclic voltammetry of lithium–ion batteries
- Impedance analysis of lithium–ion batteries
- Charge–discharge behavior of lithium–ion batteries
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode materials for lithium-ion batteries: A review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Pargoletti, E.; Arnaboldi, S.; Cappelletti, G.; Longhi, M.; Meroni, D.; Minguzzi, A.; Mussini, P.R.; Rondinini, S.; Vertova, A. Smart interfaces in Li-ion batteries: Near-future key challenges. Electrochim. Acta 2022, 415, 140258. [Google Scholar] [CrossRef]
- Mageto, T.; Bhoyate, S.D.; de Souza, F.M.; Mensah-Darkwa, K.; Kumar, A.; Gupta, R.K. Developing practical solid-state rechargeable Li-ion batteries: Concepts, challenges, and improvement strategies. J. Energy Storage 2022, 55, 105688. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, C.; Yu, S.; Ge, D.; Zhou, H. Status and challenges facing representative anode materials for rechargeable lithium batteries. J. Energy Chem. 2022, 66, 260–294. [Google Scholar] [CrossRef]
- Zhao, F.; Zhao, M.; Dong, Y.; Ma, L.; Zhang, Y.; Niu, S.; Wei, L. Facile preparation of micron-sized silicon-graphite-carbon composite as anode material for high-performance lithium-ion batteries. Powder Technol. 2022, 404, 117455. [Google Scholar] [CrossRef]
- Lim, S.Y. Amorphous-silicon nanoshell on artificial graphite composite as the anode for lithium-ion battery. Solid State Sci. 2019, 93, 24–30. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, H.; Meng, T.; Yang, J.; Huang, B.; Gu, F.L.; Zhang, S.; Meng, C.; Tong, Y. Boosting electron transfer with heterointerface effect for high-performance lithium-ion storage. Energy Storage Mater. 2021, 36, 365–375. [Google Scholar] [CrossRef]
- Tran Thi, M.; Lee, S.; Choi, W. Adhesion and stability increased carbon nanowall for the application to lithium-ion batteries. J. Electr. Eng. Technol. 2022, 17, 3613–3618. [Google Scholar] [CrossRef]
- Kim, S.Y.; Choi, W.S.; Lee, J.H.; Hong, B. Substrate temperature effect on the growth of carbon nanowalls synthesized via microwave PECVD. Mater. Res. Bull. 2014, 58, 112–116. [Google Scholar] [CrossRef]
- Höltig, M.; Ruhmlieb, C.; Strelow, C.; Kipp, T.; Mews, A. Synthesis of carbon nanowalls and few-layer graphene sheets on transparent conductive substrates. Z. Phys. Chem. 2015, 229, 301–316. [Google Scholar] [CrossRef] [Green Version]
- Tran Thi, M.; Kwon, S.; Kang, H.; Kim, J.H.; Yoon, Y.K.; Choi, W. Growth properties of carbon nanowalls on nickel and titanium interlayers. Molecules 2022, 27, 406. [Google Scholar] [CrossRef]
- Yu, K.; Bo, Z.; Lu, G.; Mao, S.; Cui, S.; Zhu, Y.; Chen, X.; Ruoff, R.S.; Chen, J. Growth of carbon nanowalls at atmospheric pressure for one-step gas sensor fabrication. Nanoscale Res. Lett. 2011, 6, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mumlyakov, A.M.; Shibalov, M.V.; Timofeeva, E.R.; Trofimov, I.V.; Porokhov, N.V.; Evlashin, S.A.; Nekludova, P.A.; Pershina, E.A.; Anufriev, Y.V.; Tagachenkov, A.M.; et al. Fabrication and characterization of quasi-three-dimensional capacitor structure based on carbon nanowalls. Carbon 2021, 184, 698–705. [Google Scholar] [CrossRef]
- Choi, H.; Kwon, S.; Kang, H.; Kim, J.H.; Choi, W. Adhesion-increased carbon nanowalls for the electrodes of energy storage systems. Energies 2019, 12, 4759. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Sun, Q.; Zhang, H.; Cheng, J.; Li, Y.; Zeng, Z.; Zhang, S.; Xu, X.; Ji, F.; Li, D.; et al. The application road of silicon-based anode in lithium-ion batteries: From liquid electrolyte to solid-state electrolyte. Energy Storage Mater. 2023, 55, 244–263. [Google Scholar] [CrossRef]
- Silicon and Silicon–Copper Composite Nanorods for Anodes of Li-Ion Rechargeable Batteries—Tìm Trên Google. Available online: https://www.google.com/search?q=Silicon+and+silicon–copper+composite+nanorods+for+anodes+of+Li-ion+rechargeable+batteries.&oq=Silicon+and+silicon–copper+composite+nanorods+for+anodes+of+Li-ion+rechargeable+batteries.&aqs=chrome.0.69i59.610j0j4&sourceid=ch (accessed on 27 December 2022).
- Yang, Z.; Dong, Y.; Liu, C.; Feng, X.; Jin, H.; Ma, X.; Ding, F.; Li, B.; Bai, L.; Ouyang, Y.; et al. Design and synthesis of high-silicon silicon suboxide nanowires by radio-frequency thermal plasma for high-performance lithium-ion battery anodes. Appl. Surf. Sci. 2023, 614, 156235. [Google Scholar] [CrossRef]
- Wang, Q.; Meng, T.; Li, Y.; Yang, J.; Huang, B.; Ou, S.; Meng, C.; Zhang, S.; Tong, Y. Consecutive chemical bonds reconstructing surface structure of silicon anode for high-performance lithium-ion battery. Energy Storage Mater. 2021, 39, 354–364. [Google Scholar] [CrossRef]
- Ou, J.; Li, B.; Deng, H.; Li, K.; Wang, H. A carbon-covered silicon material modified by phytic acid with 3D conductive network as anode for lithium-ion batteries. Adv. Powder Technol. 2023, 34, 103891. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Hsieh, C.C.; Liu, W.R. Synthesis of double core-shell carbon/silicon/graphite composite anode materials for lithium-ion batteries. Surf. Coat. Technol. 2020, 387, 125528. [Google Scholar] [CrossRef]
- Uxa, D.; Jerliu, B.; Hüger, E.; Dörrer, L.; Horisberger, M.; Stahn, J.; Schmidt, H. On the lithiation mechanism of amorphous silicon electrodes in li-ion batteries. J. Phys. Chem. C 2019, 123, 22027–22039. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, K. Silicon-based anode materials applied in high specific energy lithium-ion batteries: A review. Wuji Cailiao Xuebao/J. Inorg. Mater. 2019, 34, 349–357. [Google Scholar] [CrossRef]
- Schmidt, H.; Jerliu, B.; Hüger, E.; Stahn, J. Volume expansion of amorphous silicon electrodes during potentiostatic lithiation of Li-ion batteries. Electrochem. Commun. 2020, 115, 106738. [Google Scholar] [CrossRef]
- Roiban, L.; Koneti, S.; Wada, T.; Kato, H.; Cadete Santos Aires, F.J.; Curelea, S.; Epicier, T.; Maire, E. Three dimensional analysis of nanoporous silicon particles for Li-ion batteries. Mater. Charact. 2017, 124, 165–170. [Google Scholar] [CrossRef]
- Au, M.; He, Y.; Zhao, Y.; Ghassemi, H.; Yassar, R.S.; Garcia-Diaz, B.; Adams, T. Silicon and silicon-copper composite nanorods for anodes of Li-ion rechargeable batteries. J. Power Sources 2011, 196, 9640–9647. [Google Scholar] [CrossRef]
- Gao, J.; Fu, L.J.; Zhang, H.P.; Zhang, T.; Wu, Y.P.; Wu, H.Q. Suppression of PC decomposition at the surface of graphitic carbon by Cu coating. Electrochem. Commun. 2006, 8, 1726–1730. [Google Scholar] [CrossRef]
- Nobili, F.; Dsoke, S.; Mancini, M.; Tossici, R.; Marassi, R. Electrochemical investigation of polarization phenomena and intercalation kinetics of oxidized graphite electrodes coated with evaporated metal layers. J. Power Sources 2008, 180, 845–851. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Jiang, H.; Li, X.; Cheng, Y.; Meng, C. Designed mesoporous hollow sphere architecture metal (Mn, Co, Ni) silicate: A potential electrode material for flexible all solid-state asymmetric supercapacitor. Chem. Eng. J. 2019, 362, 818–829. [Google Scholar] [CrossRef]
- Nguyen, C.C.; Song, S.W. Characterization of SEI layer formed on high performance Si-Cu anode in ionic liquid battery electrolyte. Electrochem. Commun. 2010, 12, 1593–1595. [Google Scholar] [CrossRef]
- Yen, J.P.; Chang, C.C.; Lin, Y.R.; Shen, S.T.; Hong, J.L. Sputtered copper coating on silicon/graphite composite anode for lithium ion batteries. J. Alloys Compd. 2014, 598, 184–190. [Google Scholar] [CrossRef]
- Koike, S.; Fujieda, T.; Sakai, T.; Higuchi, S. Characterization of sputtered vanadium oxide films for lithium batteries. J. Power Sources 1999, 81–82, 581–584. [Google Scholar] [CrossRef]
- Cui, L.; Chen, J.; Yang, B.; Sun, D.; Jiao, T. RF-PECVD synthesis of carbon nanowalls and their field emission properties. Appl. Surf. Sci. 2015, 357, 1–7. [Google Scholar] [CrossRef]
- Mori, S.; Ueno, T.; Suzuki, M. Synthesis of carbon nanowalls by plasma-enhanced chemical vapor deposition in a CO/H2 microwave discharge system. Diam. Relat. Mater. 2011, 20, 1129–1132. [Google Scholar] [CrossRef]
- Gao, J.M.; Song, X.F.; Hu, J.; Guo, S.C.; Fang, L.; Wu, F.; Wei, D.P. Superhydrophobic graphenic carbon nanowalls fabricated by one-step PECVD. Mater. Lett. 2016, 184, 273–277. [Google Scholar] [CrossRef]
- Lee, S.; Choi, W.S.; Yoo, J.; Lim, D.G.; Kim, H.J.; Lee, J.H.; Hong, B. Fabrication of metal-coated carbon nanowalls synthesized by microwave plasma enhanced chemical vapor deposition. J. Nanosci. Nanotechnol. 2014, 14, 9189–9193. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, S.; Kim, K.; Kang, H.; Ko, J.M.; Choi, W. Preparation of carbon nanowall and carbon nanotube for anode material of lithium-ion battery. Molecules 2021, 26, 6950. [Google Scholar] [CrossRef]
- Kwon, S.; Choi, H.; Lee, S.; Lee, G.; Kim, Y.; Choi, W.; Kang, H. Room temperature gas sensor application of carbon nanowalls using electrical resistance change by surface adsorption of toxic gases. Mater. Res. Bull. 2021, 141, 111377. [Google Scholar] [CrossRef]
- Kwon, S.H.; Kim, H.J.; Choi, W.S.; Kang, H. Structural and electrical characteristics of carbon nanowalls synthesized on the polyimide film. J. Nanosci. Nanotechnol. 2018, 18, 6309–6311. [Google Scholar] [CrossRef]
- Bhasin, V.; Nayak, C.; Biswas, A.; Halankar, K.K.; Tokas, R.B.; Ghosh, S.K.; Bahadur, J.; Bhattacharyya, D. Remarkably high capacity Li ion batteries with rf sputter deposited TiO2 thin film anodes on SS substrates. Appl. Surf. Sci. 2022, 592, 153273. [Google Scholar] [CrossRef]
- Liao, C.L.; Wu, M.T.; Yen, J.H.; Leu, I.C.; Fung, K.Z. Preparation of RF-sputtered lithium cobalt oxide nanorods by using porous anodic alumina (PAA) template. J. Alloys Compd. 2006, 414, 302–309. [Google Scholar] [CrossRef]
- Choi, H.; Choi, C.; Kwon, S.H.; Park, Y.; Choi, W. Characteristics of Carbon Nanowalls According to the Indium Tin Oxide Interlayer Thickness. Sci. Adv. Mater. 2020, 12, 1261–1264. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Kim, C.; Kim, K.; Kwon, S.; Kang, H.; Hong, B.; Choi, W. Innovative variation in the morphological characteristics of carbon nanowalls grown on a molybdenum disulfide interlayer. Nanomaterials 2022, 12, 4334. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Shin, S.K.; Kim, H.; Joung, Y.H.; Kang, H.; Kim, J.H.; Kim, H.J.; Choi, W.S. Properties of carbon nanowalls grown on glass substrates according to the etching microwave power. J. Nanosci. Nanotechnol. 2016, 16, 10740–10743. [Google Scholar] [CrossRef]
- Kwon, S.; Choi, H.; Choi, W.; Kang, H. Wettability of CNW/ITO micro structure for modification of surface hydrophilicity. Appl. Sci. 2020, 10, 142. [Google Scholar] [CrossRef] [Green Version]
- Tröltzsch, U.; Kanoun, O.; Tränkler, H.R. Characterizing aging effects of lithium ion batteries by impedance spectroscopy. Electrochim. Acta 2006, 51, 1664–1672. [Google Scholar] [CrossRef]
- Singh, P.; Vinjamuri, R.; Wang, X.; Reisner, D. Fuzzy logic modeling of EIS measurements on lithium-ion batteries. Electrochim. Acta 2006, 51, 1673–1679. [Google Scholar] [CrossRef]
- Xie, Y.; Li, J.; Yuan, C. Mathematical modeling of the electrochemical impedance spectroscopy in lithium ion battery cycling. Electrochim. Acta 2014, 127, 266–275. [Google Scholar] [CrossRef]





| Substrate | Base Pressure | Working Pressure | Chamber Atmosphere | Temperature | Growth Time |
|---|---|---|---|---|---|
| Cu foil | 5 × 10−5 Torr | 4 × 10−2 Torr | H2:CH4 = 2:1 | 550 °C | 20 min |
| Target | Cu | Si |
|---|---|---|
| RF power | 100 W | 100 W |
| Base pressure | 1 × 10−5 Torr | 1 × 10−5 Torr |
| Working pressure | 3 × 10−3 Torr | 3 × 10−3 Torr |
| Sputtering gas | Ar: 40 sccm | Ar: 40 sccm |
| Rotation speed | 1700 rph | 1700 rph |
| Deposition time | 10 min | 25 min |
| Deposition temperature | Room temperature | Room temperature |
| Sample | Weight % | Atomic % | ||
|---|---|---|---|---|
| Cu/CNW | C | Cu | C | Cu |
| 87.64 | 12.36 | 97.39 | 2.61 | |
| Si/CNW | C | Si | C | Si |
| 92 | 8 | 98 | 1.62 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thi, M.T.; Kim, C.; Kwon, S.; Kang, H.; Ko, J.M.; Kim, J.; Choi, W. Investigation of the Properties of Anode Electrodes for Lithium–Ion Batteries Manufactured Using Cu, and Si-Coated Carbon Nanowall Materials. Energies 2023, 16, 1935. https://doi.org/10.3390/en16041935
Thi MT, Kim C, Kwon S, Kang H, Ko JM, Kim J, Choi W. Investigation of the Properties of Anode Electrodes for Lithium–Ion Batteries Manufactured Using Cu, and Si-Coated Carbon Nanowall Materials. Energies. 2023; 16(4):1935. https://doi.org/10.3390/en16041935
Chicago/Turabian StyleThi, May Tran, Chulsoo Kim, Seokhun Kwon, Hyunil Kang, Jang Myoun Ko, Junghyun Kim, and Wonseok Choi. 2023. "Investigation of the Properties of Anode Electrodes for Lithium–Ion Batteries Manufactured Using Cu, and Si-Coated Carbon Nanowall Materials" Energies 16, no. 4: 1935. https://doi.org/10.3390/en16041935






