Comparative Life Cycle Assessment of Catalytic Intermediate Pyrolysis of Rapeseed Meal
Abstract
:1. Introduction
2. Materials and Methods
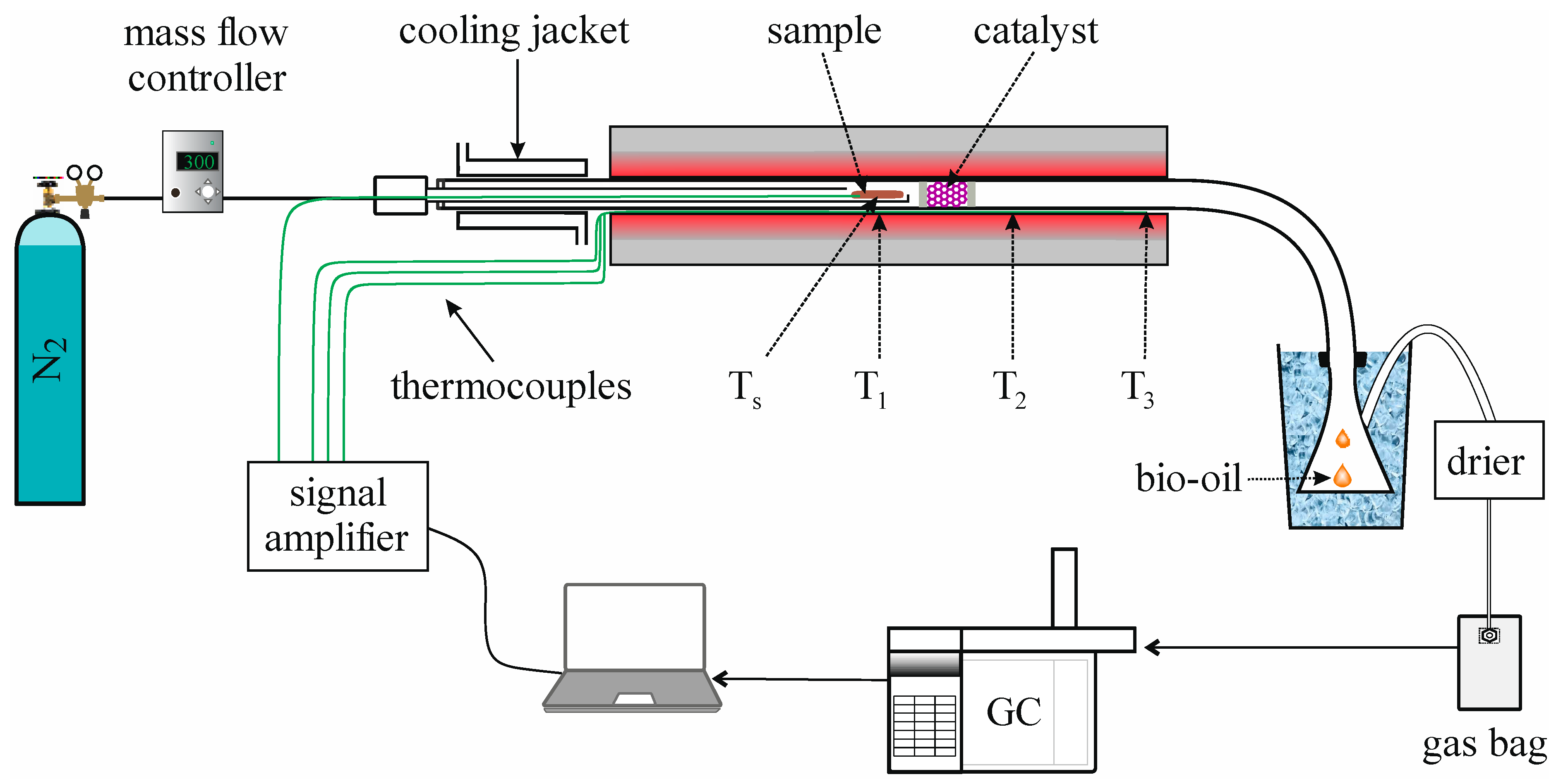
2.1. Description of Pyrolysis Experiments
2.1.1. Biomass Preparation
2.1.2. Catalysis
2.1.3. Pyrolysis
2.2. Life Cycle Assessment Methodology
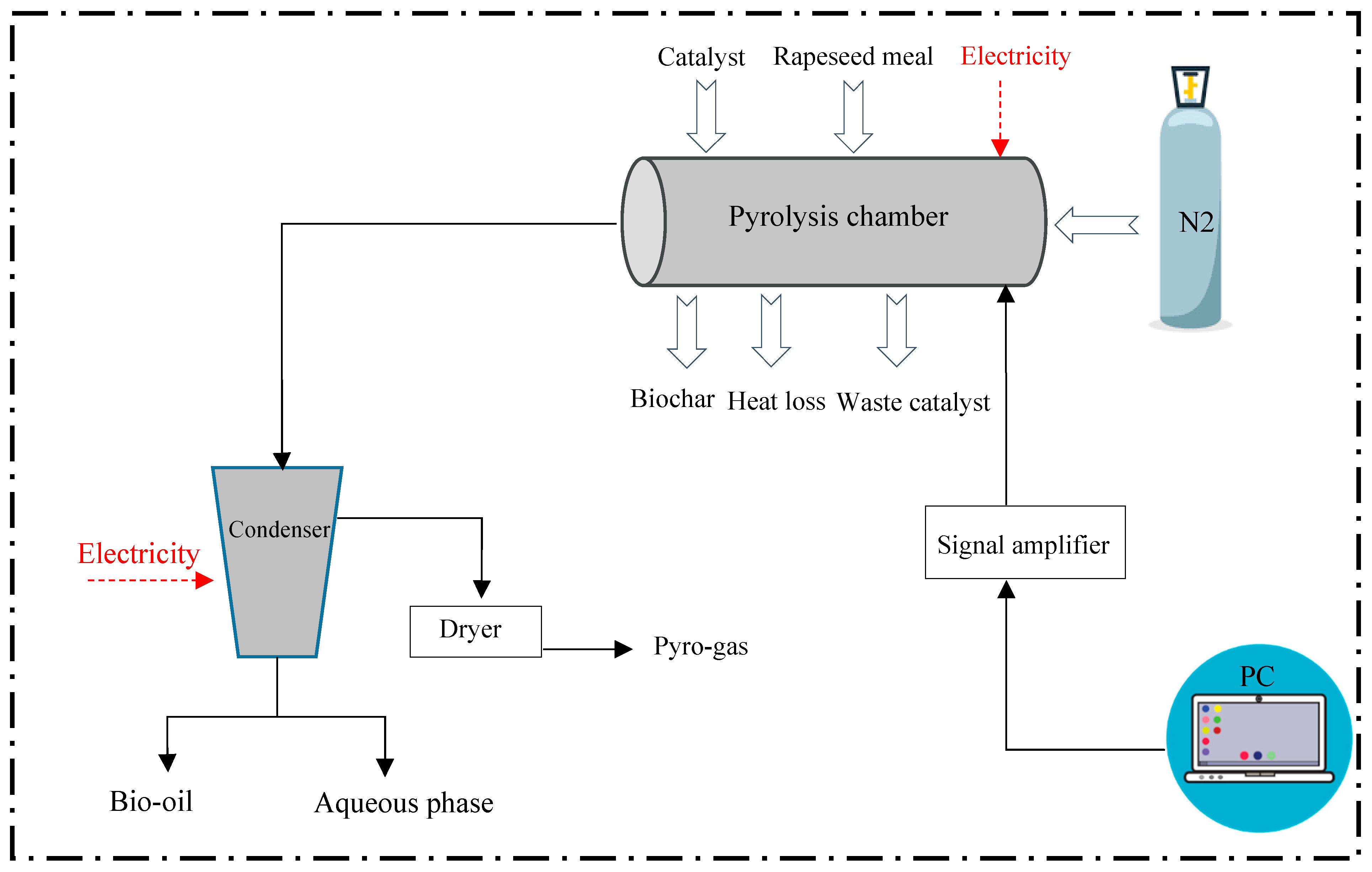
2.2.1. Goals and Scope Definition
2.2.2. Inventory Analysis
2.2.3. Impact Assessment
3. Results and Discussion
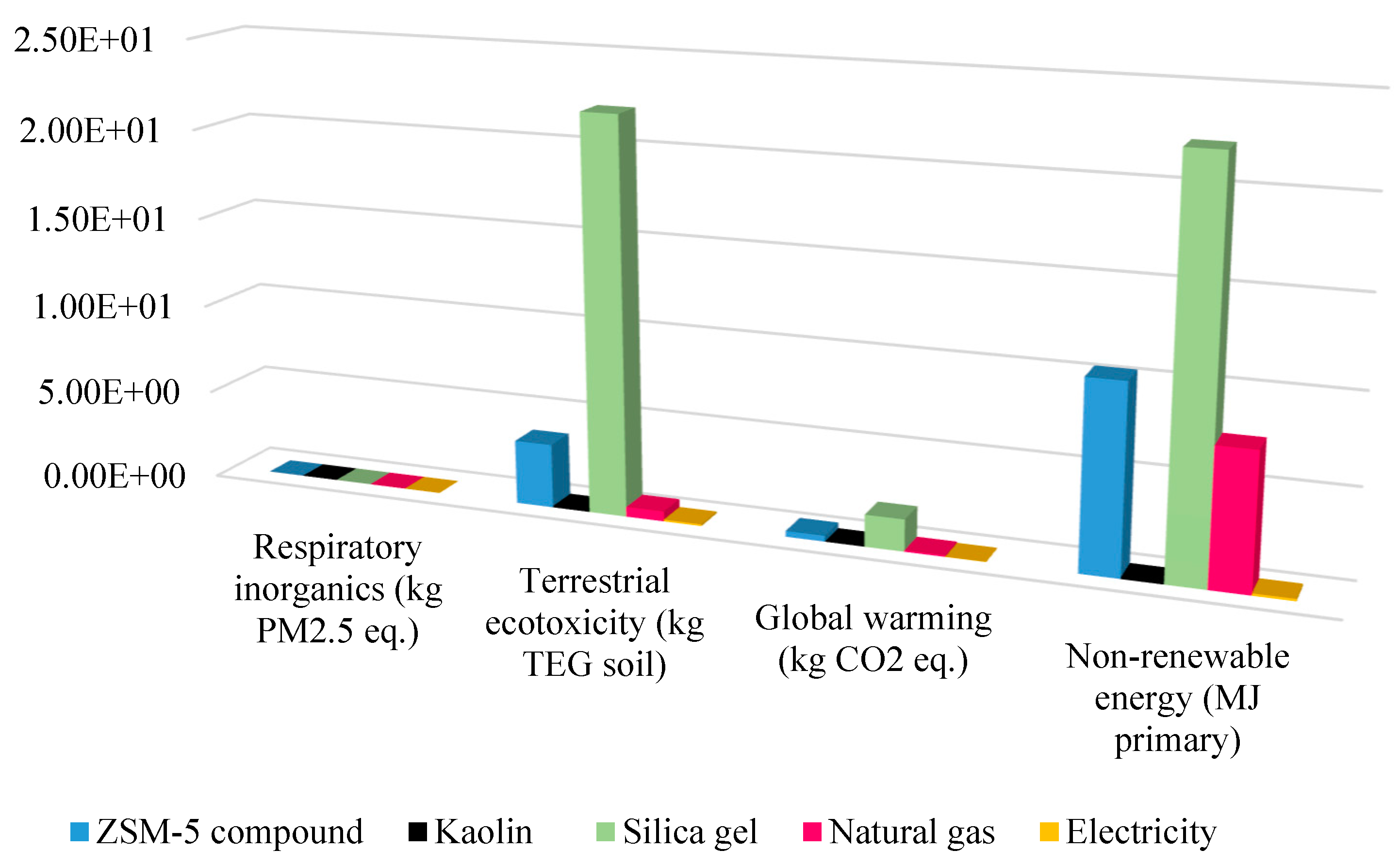
3.1. Environmental Impacts of the Catalytic Pyrolysis
- Global warming (GW)
- Non-renewable energy (NRE)
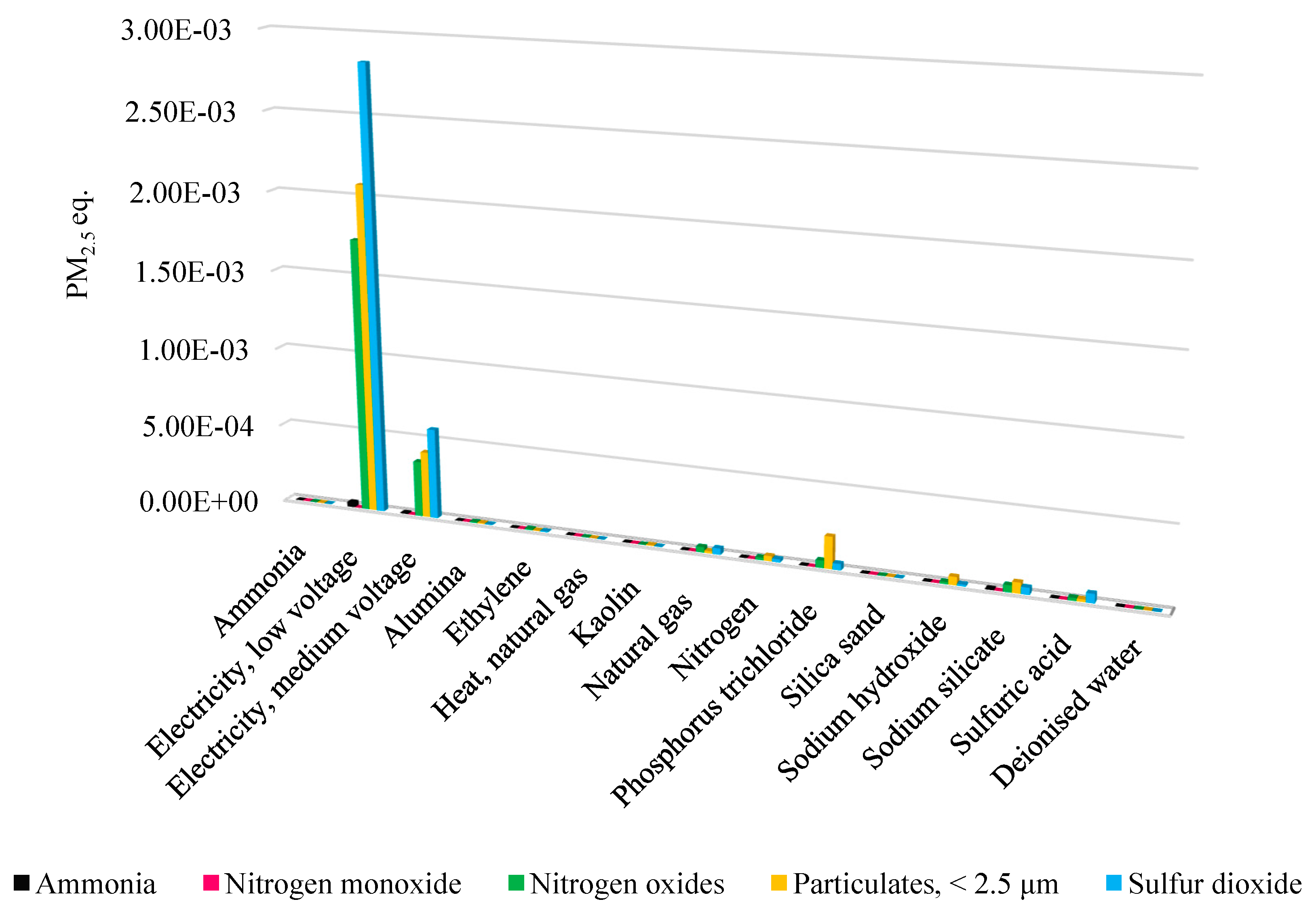
- Respiratory inorganics (RIOs)
3.2. Catalyst Impact on Life Cycle Assessment
4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasul, G. Managing the Food, Water, and Energy Nexus for Achieving the Sustainable Development Goals in South Asia. Environ. Dev. 2016, 18, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Bieber, N.; Ker, J.H.; Wang, X.; Triantafyllidis, C.; Van Dam, K.H.; Koppelaar, R.H.E.M.; Shah, N. Sustainable planning of the energy-water-food nexus using decision making tools. Energy Policy 2018, 113, 584–607. [Google Scholar] [CrossRef]
- IEA Bioenergy Annual Report 2021. Available online: https://www.ieabioenergy.com/blog/publications/iea-bioenergy-annual-report-2021/ (accessed on 16 January 2023).
- Soleymani Angili, T.; Grzesik, K.; Salimi, E.; Loizidou, M. Life Cycle Analysis of Food Waste Valorization in Laboratory-Scale. Energies 2022, 15, 7000. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Anguilano, L.; Ghazal, H.; Krzyżyńska, R.; Reynolds, A.J.; Spencer, N.; Jouhara, H. Potential of pyrolysis processes in the waste management sector. Therm. Sci. Eng. Prog. 2017, 3, 171–197. [Google Scholar] [CrossRef]
- D2.6 Report of the Laboratory Tests for Pyrolysis of Forestry Residues. 2018. Available online: https://agrocycle.eu/general-documents/ (accessed on 25 November 2022).
- Patel, A.D.; Meesters, K.; Den Uil, H.; De Jong, E.; Worrell, E.; Patel, M.K. Early-Stage Comparative Sustainability Assessment of New Bio-based Processes. ChemSusChem 2013, 6, 1724–1736. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of Slow Pyrolysis Biochars: Effects of Feedstocks and Pyrolysis Temperature on Biochar Properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef]
- Hornung, A. Intermediate pyrolysis of biomass. In Biomass Combustion Science, Technology and Engineering; Woodhead Publishing Limited: Sawston, UK, 2013; pp. 172–186. ISBN 9780857097439. [Google Scholar] [CrossRef]
- Jerzak, W.; Gao, N.; Kalemba-rec, I.; Magdziarz, A. Catalytic intermediate pyrolysis of post-extraction rapeseed meal by reusing ZSM-5 and Zeolite Y catalysts. Catal. Today 2022, 404, 63–77. [Google Scholar] [CrossRef]
- Greenhalf, C.E.; Nowakowski, D.J.; Harms, A.B.; Titiloye, J.O.; Bridgwater, A.V. A comparative study of straw, perennial grasses and hardwoods in terms of fast pyrolysis products. Fuel 2013, 108, 216–230. [Google Scholar] [CrossRef]
- Bridgwater, T. Challenges and Opportunities in Fast Pyrolysis of Biomass: Part II. Johns. Matthey Technol. Rev. 2018, 62, 150–160. [Google Scholar] [CrossRef]
- Butler, E.; Devlin, G.; Meier, D.; Mcdonnell, K. A review of recent laboratory research and commercial developments in fast pyrolysis and upgrading. Renew. Sustain. Energy Rev. 2011, 15, 4171–4186. [Google Scholar] [CrossRef] [Green Version]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Jaimes Figueroa, J.E.; Ardila, Y.C.; Lunelli, B.H.; Maciel Filho, R.; Maciel, M.W. Evaluation of Pyrolysis and Steam Gasification Processes of Sugarcane Bagasse in a Fixed Bed Reactor. Chem. Eng. Trans. 2013, 32, 925–930. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.; Ryu, C.; Jeon, J.; Shin, M.; Park, Y. Production and Utilization of Biochar: A Review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Parascanu, M.M. Life Cycle Assessment of Biomass Wastes Valorization through Thermochemical and Biochemical Process. Ph.D. Thesis, Faculty of Sciences and Chemical Technologies, University of Castilla-la Mancha, Ciudad Real, Spain, 2019. [Google Scholar]
- Oyeleke, O.O.; Ohunakin, O.S.; Adelekan, D.S. Catalytic Pyrolysis in Waste to Energy Recovery Applications: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1107, 012226. [Google Scholar] [CrossRef]
- Miskolczi, N.; Juzsakova, T.; Sója, J. Preparation and application of metal loaded ZSM-5 and y-zeolite catalysts for thermo-catalytic pyrolysis of real end of life vehicle plastics waste. J. Energy Inst. 2019, 92, 118–127. [Google Scholar] [CrossRef]
- Shun, T.; Zhijun, Z.; Jianping, S.; Qingwen, W. Recent progress of catalytic pyrolysis of biomass by HZSM-5. Chin. J. Catal. 2013, 34, 641–650. [Google Scholar] [CrossRef]
- van Ree, R.; van Zeeland, A.N.T. IEA Bioenergy Task42 Biorefining: Sustainable and Synergetic Processing of Biomass into Marketable Food & Feed Ingredients, Chemicals, Materials and Energy (Fuels, Power, Heat). 2014. Available online: https://edepot.wur.nl/313931, (accessed on 25 November 2022).
- Soleymani Angili, T.; Grzesik, K.; Rödl, A.; Kaltschmitt, M. Life Cycle Assessment of Bioethanol Production: A Review of Feedstock, Technology and Methodology. Energies 2021, 14, 2939. [Google Scholar] [CrossRef]
- Ahlgren, S.; Björklund, A.; Ekman, A.; Karlsson, H.; Berlin, J.; Börjesson, P.; Ekvall, T.; Finnveden, G.; Janssen, M.; Strid, I. Review of methodological choices in LCA of biorefi nery systems-key issues and recommendations. Biofuels Bioprod. Biorefining 2015, 9, 606–619. [Google Scholar] [CrossRef] [Green Version]
- Mu, D.; Seager, T.; Rao, P.S.; Zhao, F. Comparative Life Cycle Assessment of Lignocellulosic Ethanol Production: Biochemical Versus Thermochemical Conversion. Environ. Manag. 2010, 46, 565–578. [Google Scholar] [CrossRef]
- Curran, M.A.; Young, S.B. Critical Review: A summary of the current state-of-practice. Int. J. Life Cycle Assess. 2014, 19, 1667–1673. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Hamelin, L. Framework for consequential life cycle assessment of pyrolysis biorefineries: A case study for the conversion of primary forestry residues. Renew. Sustain. Energy Rev. 2021, 138, 110549. [Google Scholar] [CrossRef]
- Patel, A.D.; Zabeti, M.; Seshan, K.; Patel, M.K. Economic and Environmental Assessment of Catalytic and Thermal Pyrolysis Routes for Fuel Production from Lignocellulosic Biomass. Process 2020, 8, 1612. [Google Scholar] [CrossRef]
- Chhabra, V.; Parashar, A.; Shastri, Y.; Bhattacharya, S. Techno-Economic and Life Cycle Assessment of Pyrolysis of Unsegregated Urban Municipal Solid Waste in India. Ind. Eng. Chem. Res. 2021, 60, 1473–1482. [Google Scholar] [CrossRef]
- Li, C.; Yuan, X.; Sun, Z.; Suvarna, M.; Hu, X.; Wang, X.; Ok, Y.S. Pyrolysis of waste surgical masks into liquid fuel and its life-cycle assessment. Bioresour. Technol. 2022, 346, 126582. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Patel, P.; Mondal, P. Life cycle analysis (LCA) and economic evaluation of catalytic fast pyrolysis: Implication of coproduct’s end-usage, catalyst type, and process parameters. Sustain. Energy Fuels 2022, 6, 2970–2988. [Google Scholar] [CrossRef]
- Monteiro, A.D.R.D.; de Miranda, D.M.V.; da Silva Pinto, J.C.C.; Soto, J.J. Life Cycle Assessment of the Catalytic Pyrolysis of High-Density Polyethylene (HDPE) and High-Impact Polystyrene (HIPS). Macromol. React. Eng. 2022, 16, 202200037. [Google Scholar] [CrossRef]
- Rapeseed Oil Market: Industrial Applications of Rapeseed Oil in Biodiesel Production to Compete with Its Use in Food Processing. 2018. Available online: https://www.futuremarketinsights.com/reports/rapeseed-oil-market (accessed on 5 December 2022).
- Di Lena, G.; Sanchez del Pulgar, J.; Lucarini, M.; Durazzo, A.; Ondrejíčková, P.; Oancea, F.; Frincu, R.M.; Aguzzi, A.; Ferrari Nicoli, S.; Casini, I.; et al. Valorization Potentials of Rapeseed Meal in a Biorefinery Perspective: Focus on Nutritional and Bioactive Components. Molecules 2021, 26, 6787. [Google Scholar] [CrossRef]
- Krautgartner, R.; Audran, X.; Fischer, J.; Mila, B.; Flach, B.; Wilson, J.; Faniadis, D.; Guerrero, M.; Bolla, S. EU Oilseeds Annual. 2021. Available online: https://gain.fas.usda.gov/ (accessed on 9 December 2022).
- Azapagic, A. Life cycle assessment and its application to process selection, design and optimisation. Chem. Eng. J. 1999, 73, 1–21. [Google Scholar] [CrossRef]
- Gahane, D.; Biswal, D.; Mandavgane, S.A. Life Cycle Assessment of Biomass Pyrolysis. BioEnergy Res. 2022, 15, 1387–1406. [Google Scholar] [CrossRef]
- Dutta, A.; Sahir, A.; Tan, E.; Humbird, D.; Snowden-swan, L.J.; Meyer, P.; Ross, J.; Sexton, D.; Yap, R.; Lukas, J.; et al. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbon Fuels, Thermochemical Research Pathways with In Situ and Ex Situ Upgrading of Fast Pyrolysis Vapors; U.S. Department of Energy: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Benavides, P.T.; Cronauer, D.C.; Adom, F.; Wang, Z.; Dunn, J.B. The influence of catalysts on biofuel life cycle analysis (LCA). Sustain. Mater. Technol. 2017, 11, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Widayat, W.; Annisa, A.N. Synthesis and Characterization of ZSM-5 Catalyst at Different Temperatures. IOP Conf. Ser. Mater. Sci. Eng. 2017, 214, 012032. [Google Scholar] [CrossRef] [Green Version]
- Abu Suleiman, L.; Haddadin, R.; Hodali, H.A. Antimicrobial Activity of Metal-Loaded Zeolites Against “S. aureus” and “E. coli”. Jordan J. Chem. 2019, 14, 61–68. [Google Scholar]
- Wang, Z.; Benavides, P.T.; Dunn, J.B.; Cronauer, D.C. Development of Greet Catalyst Module; U.S. Department of Energy: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Saavedra, A.C.; Timoshev, V.; Hauck, M.; Hassan Nejad, M.; Dang, T.T.; Vu, X.H.; Seifert, M.; Busse, O.; Weigand, J.J. Binder Selection to Modify Hydrocarbon Cracking Properties of Zeolite-Containing Composites. ACS Omega 2022, 7, 16430–16441. [Google Scholar] [CrossRef]
- Guinée, J.B.; Gorrée, M.; Heijungs, R.; Huppes, G.; Kleijn, R.; de Koning, A.; van Oers, L.; Wegener Sleeswijk, A.; Suh, S.; Udo de Haes, H.A. Life Cycle Assessment: An Operational Guide to the ISO Standards; Leiden University: Leiden, The Netherland, 2002. [Google Scholar]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. ISO (International Organization for Standardization): Geneva, Switzerland, 2006.
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. ISO (International Organization for Standardization): Geneva, Switzerland, 2006.
- PRé Consultants. SimaPro (8.5.2.0); PRé Consultants BV: Amersfoort, The Netherlands, 2017. [Google Scholar]
- Kralisch, D.; Ott, D.; Gericke, D. Rules and benefits of Life Cycle Assessment in green chemical process and synthesis design: A tutorial review. Green Chem. 2015, 17, 123–145. [Google Scholar] [CrossRef]
- Dunn, J.B.; Adom, F.; Sather, N.; Han, J.; Snyder, S.; He, C.; Gong, J.; Yue, D.; You, F. Life-Cycle Analysis of Bioproducts and Their Conventional Counterparts in GREETTM; U.S. Department of Energy: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle Assess. 2016, 3, 1218–1230. [Google Scholar] [CrossRef]
- Hertwich, E.G.; Hammitt, J.K. A decision-analytic framework for impact assessment part I: LCA and decision analysis. Int. J. Life Cycle Assess. 2001, 6, 5–12. [Google Scholar] [CrossRef]
- Pennington, D.W.; Potting, J.; Finnveden, G.; Lindeijer, E.; Jolliet, O.; Rydberg, T.; Rebitzer, G. Life cycle assessment Part 2: Current impact assessment practice. Environ. Int. 2004, 30, 721–739. [Google Scholar] [CrossRef]
- Humbert, S.; De Schryver, A.; Bengoa, X.; Margni, M.; Jolliet, O. IMPACT 2002+: User Guide. Draft for Version Q2.21. 2014. Available online: https://quantis.com/pdf/IMPACT2002+_UserGuide_for_vQ2.21_30April2014a.pdf (accessed on 1 August 2022).
- Dang, Q.; Yu, C.; Luo, Z. Environmental life cycle assessment of bio-fuel production via fast pyrolysis of corn stover and hydroprocessing. Fuel 2014, 131, 36–42. [Google Scholar] [CrossRef]
- Steele, P.; Puettmann, M.E.; Penmetsa, V.K.; Cooper, J.E. Life-Cycle Assessment of Pyrolysis Bio-Oil Production. For. Prod. J. 2012, 62, 326–334. [Google Scholar] [CrossRef] [Green Version]



| Parameters | Value |
|---|---|
| Proximate analysis (%) | |
| Moistur | 5.66 |
| Volatile Matter | 73.99 |
| Ash | 6.80 |
| Fixed Carbon | 13.55 |
| Ultimate analysis (%) | |
| Carbon | 42.57 |
| Hydrogen | 6.40 |
| Nitrogen | 5.80 |
| Sulphur | 0.61 |
| Oxygen a | 37.82 |
| Component analysis (%) | |
| Cellulose | 8.91 |
| Hemicellulose | 8.09 |
| Lignin | 7.23 |
| Extractives | 75.77 |
| Framework Code | Zeolite Type | SiO2 % | Al2O3 % | Si/Al % | Na2O % |
|---|---|---|---|---|---|
| MFI (Pentasil family) | ZSM-5 | 92.13 | 5.67 | 13.8 | 2.21 |
| FAU (Faujasite family) | Zeolite Y | 60.04 | 28.30 | 1.8 | 11.64 |
| Data Source | Data Type |
|---|---|
| i. Literature | i. Measured |
| ii. Experiments | ii. Calculated |
| iii. Consultation | iii. Estimated iv. Average value |
| Value | Unit | |
|---|---|---|
| Pyrolysis process | ||
| RM | 1 | kg |
| ZSM-5/Zeolite Y | 100 | g |
| N2 | 240 | dm3 |
| Electricity | 7.86 | kWh |
| * Substances/energy per ton ZSM-5 catalyst production | ||
| ZSM-5 compound | 0.5 | ton |
| SiO2 gel | 0.25 | ton |
| Kaolin | 0.25 | ton |
| Natural gas | 60.22 | mmBtu |
| Electricity | 0.36 | mmBtu |
| * Substances/energy per 1 kg ZY production | ||
| Quartz | 1.291 | kg |
| Bauxite | 0.489 | kg |
| Rock salt | 1.212 | kg |
| Limestone | 0.878 | kg |
| Sodium silicate solution | 4.498 | kg |
| Aluminum hydroxide | 0.389 | kg |
| NaOH | 0.220 | kg |
| Soda | 0.670 | kg |
| Energy consumption | 46.259 | MJ |
| Impact Category | Midpoint Reference Substance | Abbr. |
|---|---|---|
| Respiratory inorganics | kg PM2.5 into air-eq | RIOs |
| Terrestrial ecotoxicity | kg Triethylene glycol into soil-eq | TE |
| Global warming | kg CO2 equivalent | GW |
| Non-renewable energy | MJ primary | NRE |
| Material/Energy | Value (MJ Primary) |
|---|---|
| Electricity | 118.99 |
| Natural gas | 11.77 |
| Crude alumina (Al2O3) | 0.18 |
| Ethylene (C2H4) | 1.80 |
| Heat | 0.13 |
| Kaolin | 0.08 |
| Phosphorus trichloride (PCl3) | 2.50 |
| Silica sand (SiO2) | 0.02 |
| Sodium hydroxide (NaOH) | 0.61 |
| Sodium silicate (Na2SiO3) | 1.60 |
| Sulfuric acid (H2SO4) | 0.88 |
| Syngas | 0.51 |
| Ammonia | 0.16 |
| Deionized water | 0.03 |
| Nitrogen (N2) | 1.62 |
| Compartment | Number of Substances | Pyrolysis Involved ZSM-5 (kg TEG Soil) | Pyrolysis Involved Zeolite Y (kg TEG Soil) |
|---|---|---|---|
| Soil | 164 | 85.73 | 84.04 |
| Air | 129 | 39.90 | 34.47 |
| Water | 73 | 6.84 × 10−7 | 1.30 × 10−7 |
| Substance | Compartment | Pyrolysis Involved ZSM-5 (kg TEG Soil) | Pyrolysis Involved Zeolite Y (kg TEG Soil) |
|---|---|---|---|
| Aluminum | Air | 17.07 | 14.71 |
| Aluminum | Soil | 33.34 | 32.41 |
| Arsenic | Air | 0.67 | 0.63 |
| Cadmium | Air | 0.36 | 0.33 |
| Cadmium | Soil | 0.16 | 0.16 |
| Chromium | Air | 0.83 | 0.65 |
| Chromium | Soil | 0.92 | 0.91 |
| Chromium VI | Soil | 8.96 | 8.96 |
| Copper | Air | 5.27 | 4.76 |
| Copper | Soil | 20.37 | 20.33 |
| Lead | Air | 0.50 | 0.44 |
| Mercury | Air | 2.76 | 2.77 |
| Nickel | Air | 2.11 | 1.94 |
| Nickel | Soil | 0.37 | 0.36 |
| Zinc | Air | 10.21 | 8.13 |
| Zinc | Soil | 21.40 | 20.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soleymani Angili, T.; Grzesik, K.; Jerzak, W. Comparative Life Cycle Assessment of Catalytic Intermediate Pyrolysis of Rapeseed Meal. Energies 2023, 16, 2004. https://doi.org/10.3390/en16042004
Soleymani Angili T, Grzesik K, Jerzak W. Comparative Life Cycle Assessment of Catalytic Intermediate Pyrolysis of Rapeseed Meal. Energies. 2023; 16(4):2004. https://doi.org/10.3390/en16042004
Chicago/Turabian StyleSoleymani Angili, Tahereh, Katarzyna Grzesik, and Wojciech Jerzak. 2023. "Comparative Life Cycle Assessment of Catalytic Intermediate Pyrolysis of Rapeseed Meal" Energies 16, no. 4: 2004. https://doi.org/10.3390/en16042004
APA StyleSoleymani Angili, T., Grzesik, K., & Jerzak, W. (2023). Comparative Life Cycle Assessment of Catalytic Intermediate Pyrolysis of Rapeseed Meal. Energies, 16(4), 2004. https://doi.org/10.3390/en16042004






