Dimensioning Air Reactor and Fuel Reactor of a Pressurized Chemical Looping Combustor to Be Coupled to a Gas Turbine: Part 1, the Air Reactor
Abstract
:1. Introduction
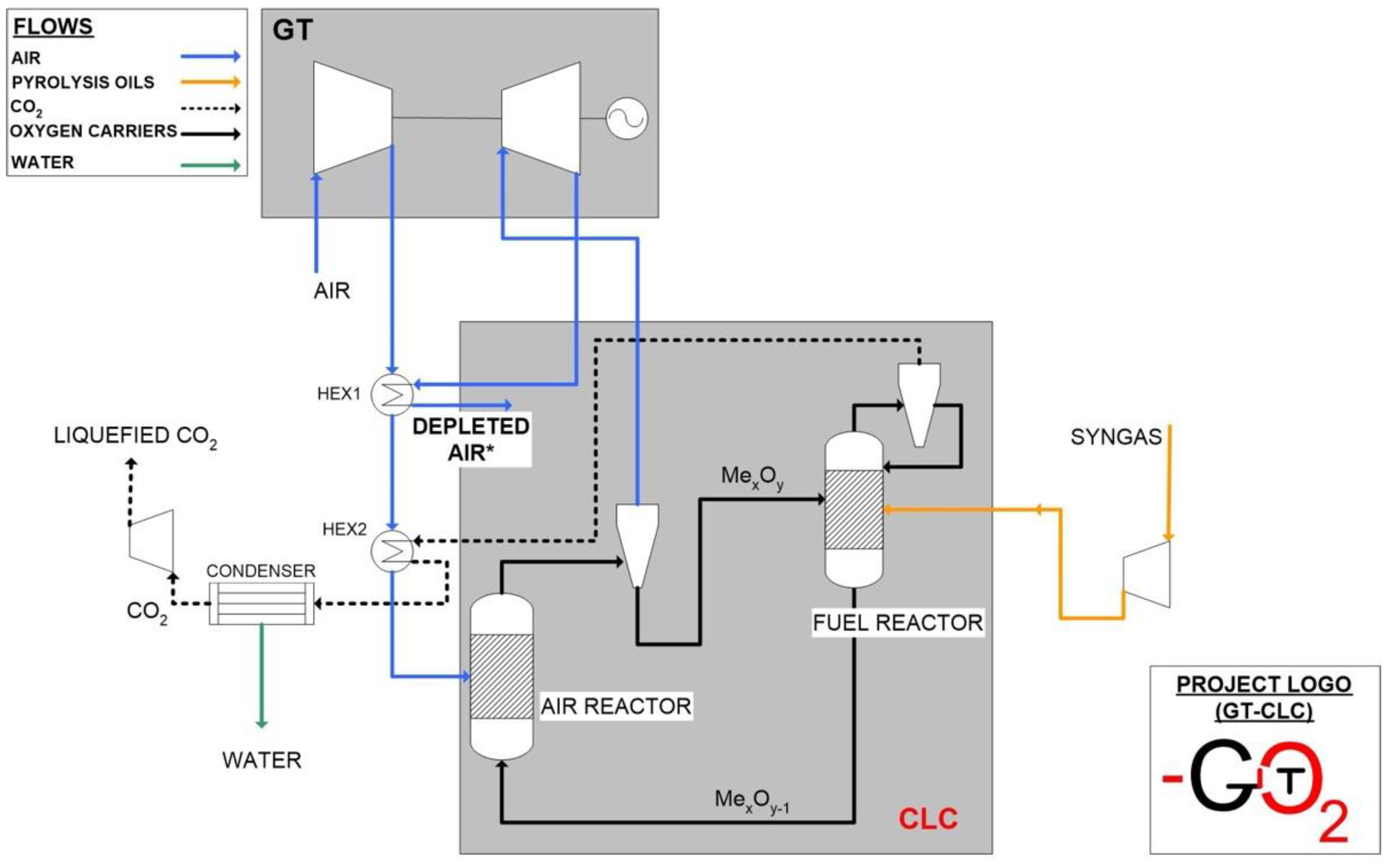
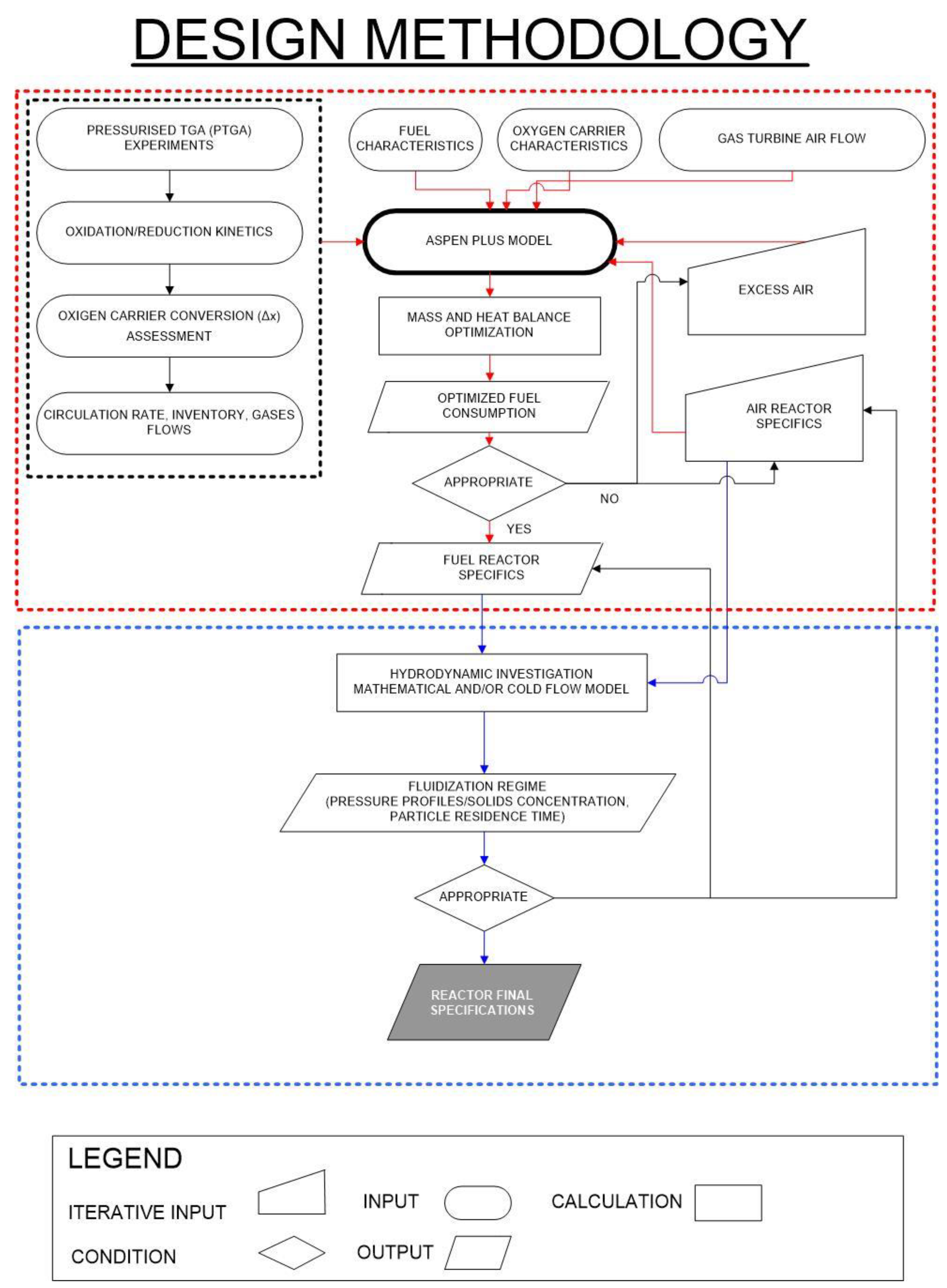
Pressurised Chemical Looping Combustor Design Methodology
- It can grant effective solid material circulation between the two reactors, ensuring oxygen to the fuel reactor and heat distribution among the two reactors.
- Through an accurate management of the solids inventory across the height of both the reactors’ bodies, a satisfactory conversion of the gases fed to the air and to the fuel reactor can be achieved.
- This is one of the more promising configurations that minimizes gas leakages between the two reactors through the presence of a loop seal, which enables to handle pressure differences among them.
- Firstly the power capacity of the gas turbine is considered and based on this the air mass flow is calculated, using Aspen Plus and assuming an excess air value which is iteratively optimized trying to reduce fuel consumption and maintaining a Turbine Inlet Temperature constant at 1200 °C;
- Then from the stoichiometric air flow the fuel consumption is calculated (and it is used iteratively to optimize excess air consumption);
- From the optimization process the air reactor and fuel reactor specification are determined, which are used to calculate the mass and energy balances of the air reactor and fuel reactor;
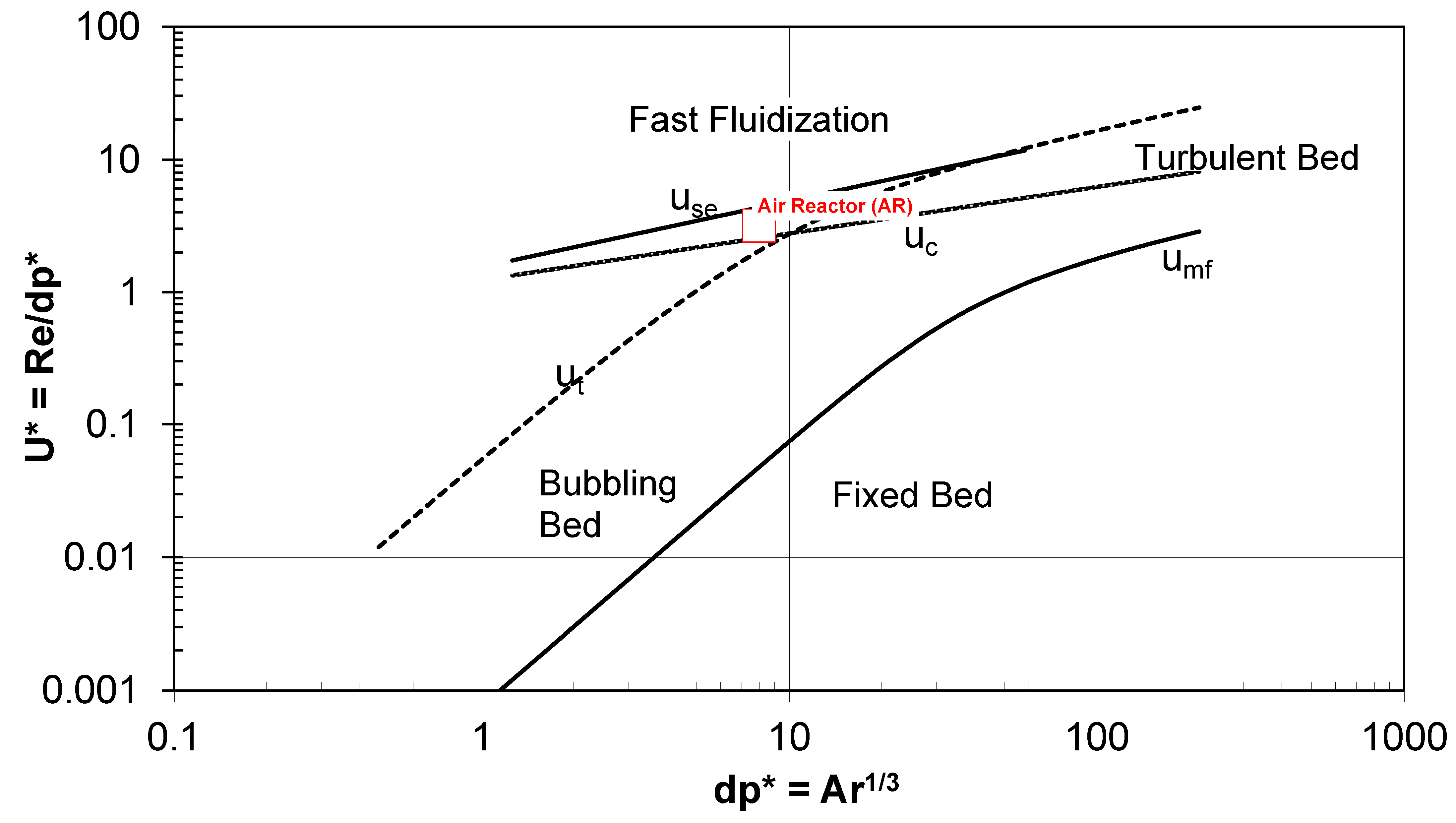
- Then from the box delimited with red dotted lines which is about mass and energy balances optimization we pass to the box delimited with blue dotted lines which is instead on the hydrodynamics optimization we want in fact that both the air reactor and the fuel reactor are close to the fast fluidization regime which allows us to reduce the inventory and so also the operating costs of the plant. This is done by drawing the Grace diagram as it will be shown in the last paragraphs of this paper.
2. Materials and Methods
2.1. Fuel Mass Flow Calculation
2.2. Solids Circulation Rate in the Air Reactor
- -
- br is the stoichiometric factor in the reduction reactions (see number 3 and 4), expressed in mol of solid reacting per mol of fuel gas. In particular, if we refer to the fuel reactor, we will have to consider the following reactions:
- -
- In Equation (2), M is the molecular weight of the material, expressed in g per mole;
- -
- Ff is the molar flow of the fuel gas, expressed in moles/s;
- -
- ΔXf is the conversion rate of the fuel gas, which is assumed to be equal to 1;
- -
- xNiO is the mass fraction of NiO in the fully oxidized sample; this is indicated in many cases in the name of the catalyst, for example if we take into consideration the oxygen carrier Fe20γAl (which is used in [41]), this contains 20% in mass of active iron while the remaining is represented by the support (which in this case is gamma alumina). The content of metal oxide is usually measured with ICP-AES. In this specific case, we will consider Ni40Al-FG (where the two last letters mean freeze granulation) because it has been tested in pressurized conditions at the Instituto de Carboquimica, see [17];
- -
- ΔXs represents the conversion of the solids. In fact, not all the solids that are circulating in the reactor are fully converted; we can assume that 0.3 of the solids is converted, as reported for example in [37].
- -
- XS,o is the solids conversion in the oxidation reaction and can be calculated from Equation (9).where m is the mass of the sample. In reality, in this case, we assume that:
- -
- in the air reactor, the oxygen carrier enters with Xo = 0.5 and exits with Xo = 0.8;
- -
- in the fuel reactor, the oxygen carrier enters with Xr = 0.2 and exits with Xr = 0.5
- -
- the solids conversion rate for reduction (ΔXr) and the solids conversion rate for oxidation (ΔXo) are both equal to 0.3.
2.3. Solids Inventory Calculation for the Chemical Looping Combustion Plant
- -
- mOX,FR and mOX,AR are the inventory in the fuel reactor and in the air reactor, respectively.
- -
- ρNiO is the density of the oxygen carrier, as reported in Table 2;
- -
- and are the average reaction rates of the oxygen carrier per unit volume of the reacting solid, which can be calculated as follows:
- -
- and are the mean residence times in the two reactors (respectively, fuel and air reactor) and depend on the circulation rate and the reactor volume, assuming that the solids circulate with plug flow behavior.
- -
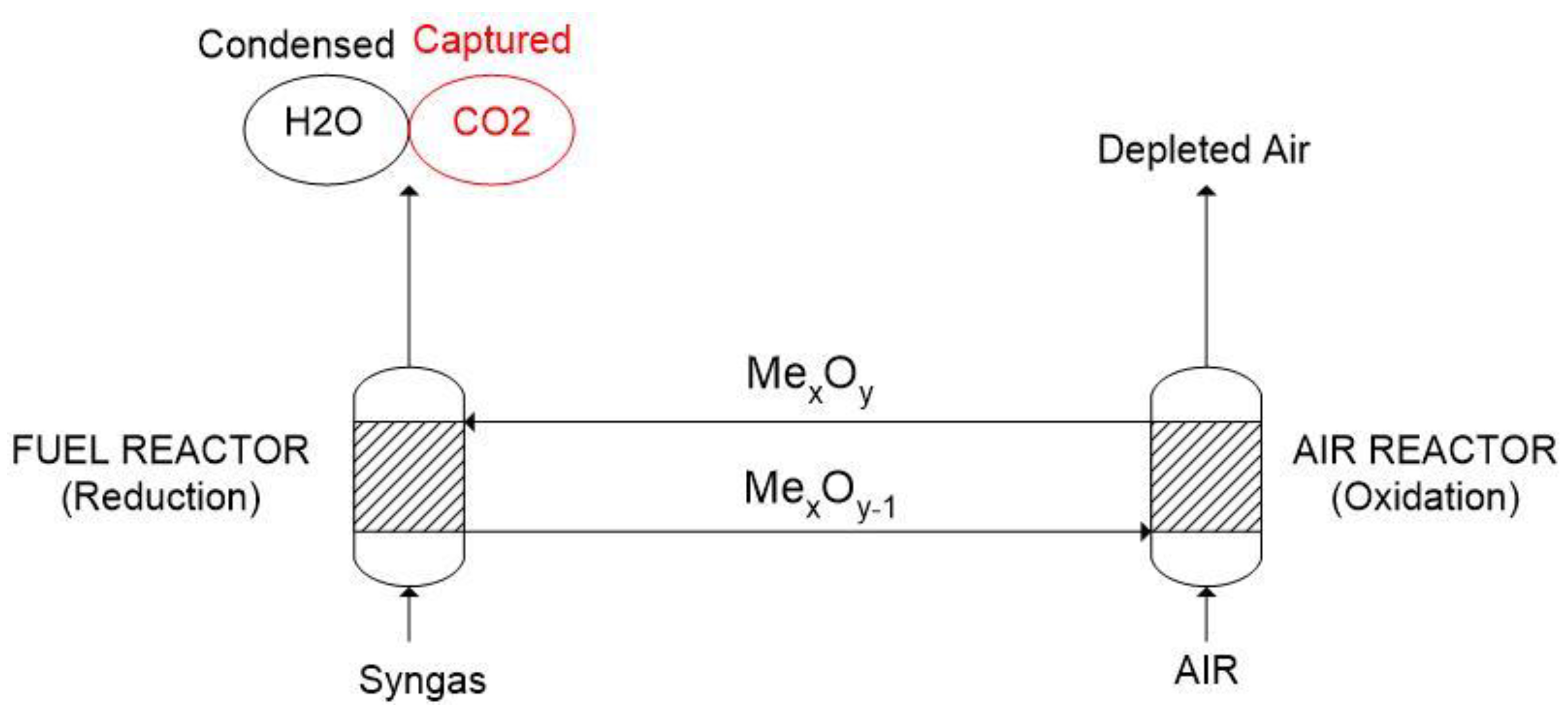
- To first define tr and to, we must make the assumption that the oxygen carrier that enters the fuel and air reactor is already partially converted (as it was also shown in Figure 4). The values of conversion at the inlet of the reactors are indicated, respectively, as: and and are higher than 0. Once this has been cleared, the quantities of tr and to are defined as the time needed for a particle to react from conversion equal to zero to the maximum variation of oxygen conversion (i.e., 1 − and 1 − ). The two equations indicating tr and to are presented below.
- -
- yg can be considered equal to 1.
- -
- is equal to 0.999.
- -
- R0 (as already said) is a parameter which is typical of the oxygen carrier.
- -
- d is typical of the reaction.
- -
- Mo is the molecular mass of oxygen (so it is 16).
2.4. Particle Size Distribution inside the Air Reactor
2.5. Transport Disengaging Height (TDH) and Elutriation Models: Influence on Circulation Rate and on Air Velocity Inside the Reactor
- -
- ki,∞ is the elutriation coefficient, expressed in kg/(m2s);
- -
- ρG, is the gas density expressed in kg/m3;
- -
- ut,I is the particle terminal velocity, expressed in m/s;
- -
- u is the fluidizing velocity, expressed in m/s.
- -
- the first is calculated from correlation;
- -
- the second is calculated based on the volume fraction profile.
3. Results
3.1. Final Design of the Air Reactor
- -
- Transport Disengaging Height calculated by correlation;
- -
- Transport Disengaging Height based on solids volume fraction profile;
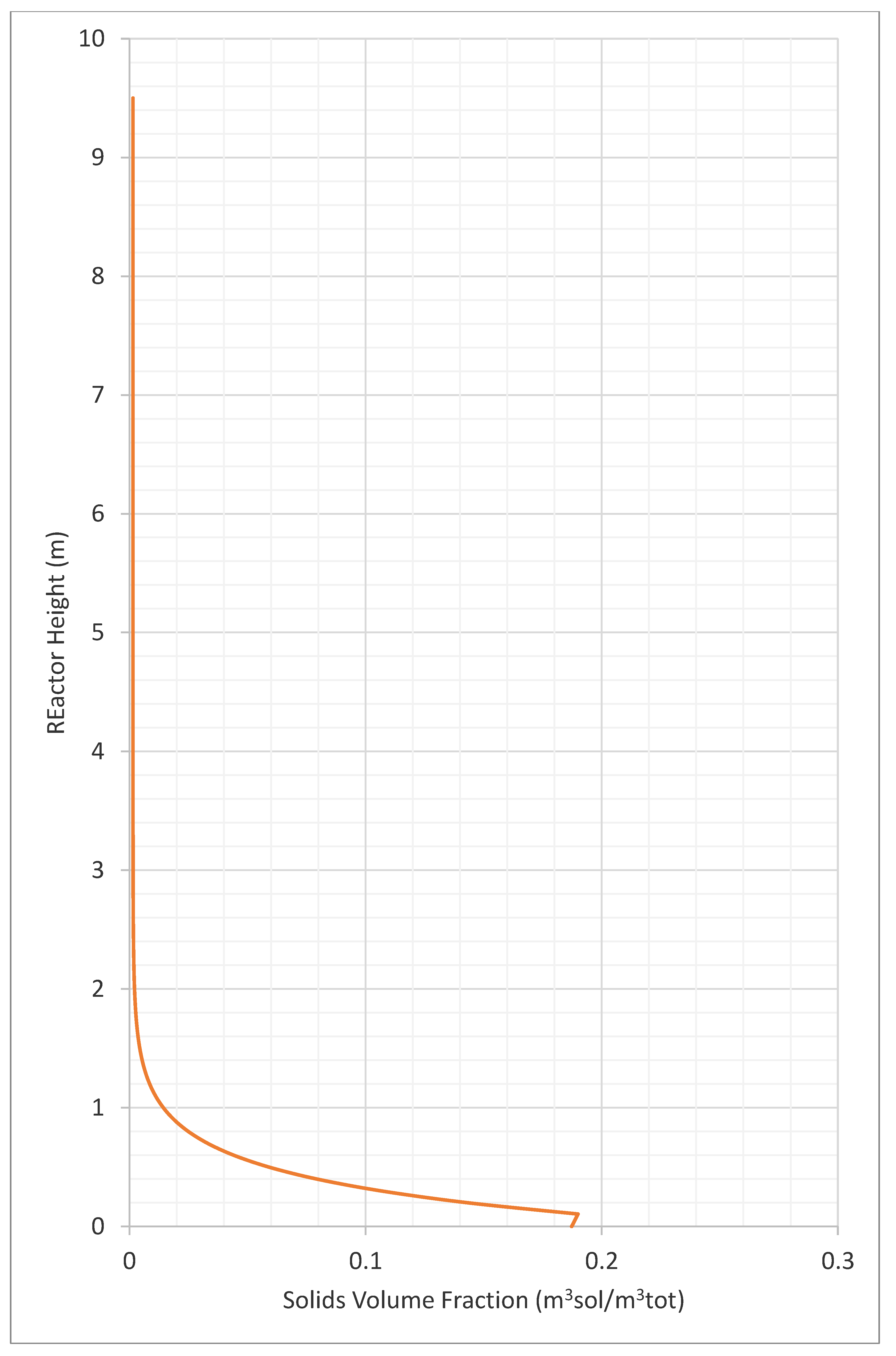
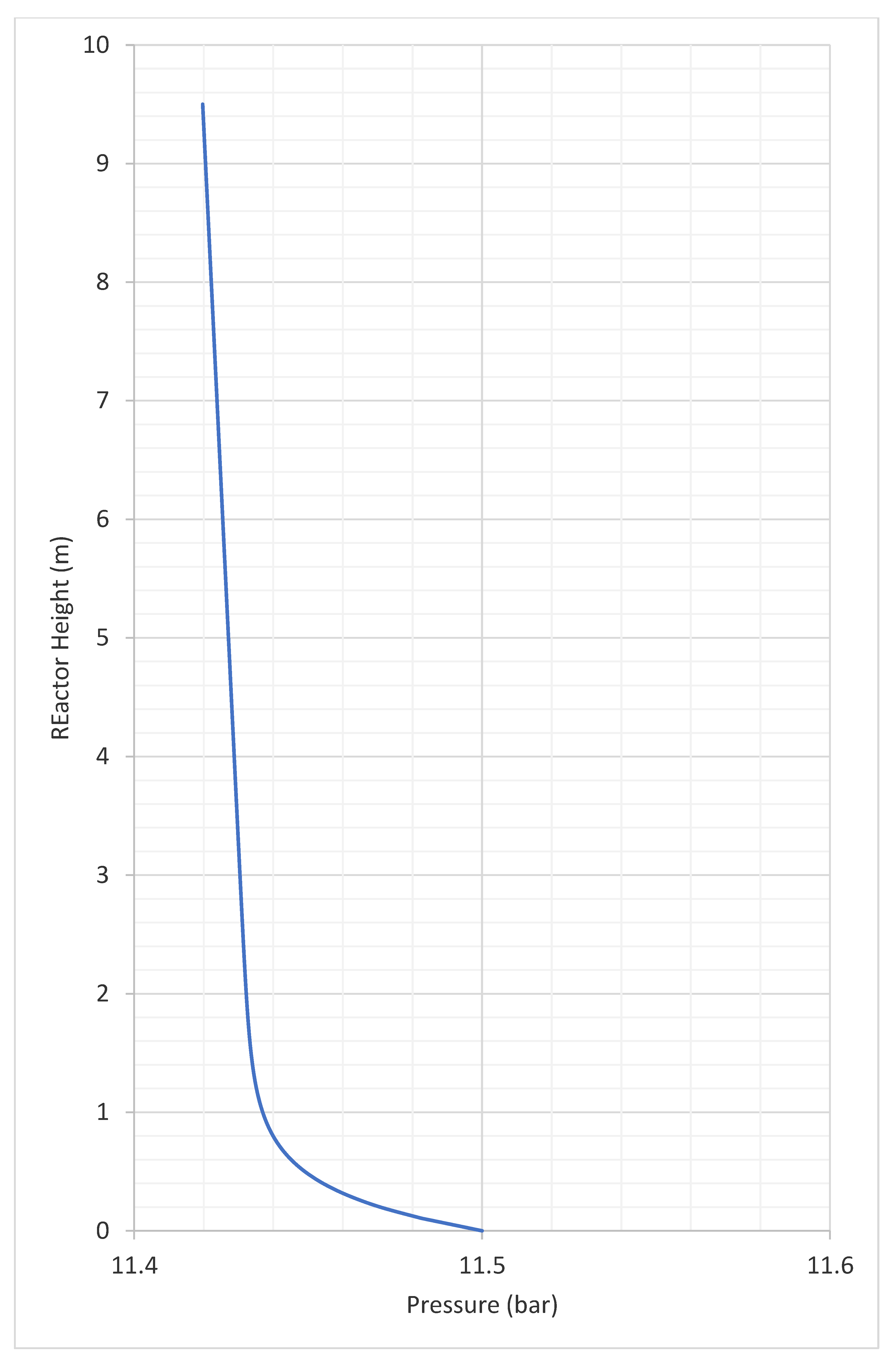
3.2. Profiles of Velocity, Solids Volume Fraction
- -
- superficial velocity (m/s)
- -
- interstitial velocity (m/s)
- -
- solids volume fraction (-)
- -
- pressure (bar).
- -
- ρg is the density of the gas;
- -
- u is the velocity of the gas (m/s);
- -
- dp is the diameter of the particles (m);
- -
- μg is he gas viscosity (cp).
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fridahl, M.; Lehtveer, M. Bioenergy with carbon capture and storage (BECCS): Global potential, investment preferences, and deployment barriers. Energy Res. Soc. Sci. 2018, 42, 155–165. [Google Scholar] [CrossRef]
- Rydén, M.; Lyngfelt, A.; Langørgen, Ø.; Larring, Y.; Brink, A.; Teir, S.; Havåg, H.; Karmhagen, P. Negative CO2 emissions with chemical-looping combustion of biomass–a Nordic energy research flagship project. Energy Procedia 2017, 114, 6074–6082. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, H.; Sikarwar, V.S.; Zhao, M.; Park, A.-H.A.; Fennell, P.S.; Shen, L.; Fan, L.-S. Biomass-based chemical looping technologies: The good, the bad and the future. Energy Environ. Sci. 2017, 10, 1885–1910. [Google Scholar] [CrossRef] [Green Version]
- Lyngfelt, A.; Brink, A.; Langørgen, Ø.; Mattisson, T.; Rydén, M.; Linderholm, C. 11,000 h of chemical-looping combustion operation—Where are we and where do we want to go? Int. J. Greenh. Gas Control 2019, 88, 38. [Google Scholar]
- Bhui, B.; Vairakannu, P. Prospects and issues of integration of co-combustion of solid fuels (coal and biomass) in chemical looping technology. J. Environ. Manag. 2019, 231, 1241–1256. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhou, W.; Luo, Z.; Wang, H.; Liu, W.; Yin, K. Developing Oxygen Carriers for Chemical Looping Biomass Processing: Challenges and Opportunities. Adv. Sustain. Syst. 2020, 4, 2000099. [Google Scholar] [CrossRef]
- Osman, M.; Khan, M.N.; Zaabout, A.; Cloete, S.; Amini, S. Review of pressurized chemical looping processes for power generation and chemical production with integrated CO2 capture. Fuel Processing Technol. 2021, 214, 10668. [Google Scholar]
- Kronberger, B.; Lyngfelt, A.; Löffler, G.; Hofbauer, H. Design and Fluid Dynamic Analysis of a Bench-Scale Combustion System with CO2 Separation−Chemical-Looping Combustion. Ind. Eng. Chem. Res. 2005, 44, 546–556. [Google Scholar] [CrossRef]
- De Leeuwe, C.; Zaheer Abbas, S.; Alexandros Argyris, P.; Zaidi, A.; Amiero, A.; Poultson, S.; Wails, D.; Spallina, V. Thermochemical syngas generation via solid looping process: An experimental demonstration using Fe-based material. Chem. Eng. J. 2022, 453, 139791. [Google Scholar] [CrossRef]
- Abbas, S.Z.; Fernández, J.R.; Amieiro, A.; Rastogi, M.; Brandt, J.; Spallina, V. Lab-scale experimental demonstration of CaCu chemical looping for hydrogen production and in-situ CO2 capture from a steel-mill. Fuel Processing Technol. 2022, 237, 107475. [Google Scholar] [CrossRef]
- Alexandros Argyris, P.; de Leeuwe, C.; Abbas, S.Z.; Amieiro, A.; Poultson, S.; Wails, D.; Spallina, V. Chemical looping reforming for syngas generation at real process conditions in packed bed reactors: An experimental demonstration. Chem. Eng. J. 2022, 435, 134883. [Google Scholar] [CrossRef]
- Argyris, P.A.; Wright, A.; Taheri Qazvini, O.; Spallina, V. Dynamic behaviour of integrated chemical looping process with pressure swing adsorption in small scale on-site H2 and pure CO2 production. Chem. Eng. J. 2022, 428, 132606. [Google Scholar] [CrossRef]
- Ugwu, A.; Arnaiz del Pozo, C.; Zaabout, A.; Nazir, S.M.; Kalendar, N.U.; Cloete, S.; Szima, S.; Fogarasi, S.; Donat, F.; van Diest, G.; et al. Gas switching technology: Economic attractiveness for chemical looping applications and scale up experience to 50 kWth. Int. J. Greenh. Gas Control 2022, 114, 103593. [Google Scholar] [CrossRef]
- Bischi, A.; Langørgen, Ø.; Saanum, I.; Bakken, J.; Seljeskog, M.; Bysveen, M.; Morin, J.-X.; Bolland, O. Design study of a 150 kWth double loop circulating fluidized bed reactor system for chemical looping combustion with focus on industrial applicability and pressurization. Int. J. Greenh. Gas Control 2011, 5, 467–474. [Google Scholar]
- Bartocci, P.; Abad, A.; Cabello Flores, A.; de las Obras Loscertales Navarro, M.; Pelucchi, M.; Andrea, T.; Wang, L.; Haiping, Y.; Haibo, Z.; Qing, Y. Integration of multiphase CFD models with detailed kinetics to understand the behavior of oxygen carriers under pressurized conditions. In Proceedings of the 13th International Conference on Applied Energy (ICAE2021), Online, 29 November–2 December 2021; pp. 1–6. [Google Scholar]
- García-Labiano, F.; Adánez, J.; de Diego, L.F.; Gayán, P.; Abad, A. Effect of Pressure on the Behavior of Copper-, Iron-, and Nickel-Based Oxygen Carriers for Chemical-Looping Combustion. Energy Fuels 2006, 20, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Abad, A.; García-Labiano, F.; de Diego, L.F.; Gayán, P.; Adánez, J. Reduction Kinetics of Cu-, Ni-, and Fe-Based Oxygen Carriers Using Syngas (CO + H2) for Chemical-Looping Combustion. Energy Fuels 2007, 21, 1843–1853. [Google Scholar] [CrossRef] [Green Version]
- Siriwardane, R.; Poston, J.; Chaudhari, K.; Zinn, A.; Simonyi, T.; Robinson, C. Chemical-Looping Combustion of Simulated Synthesis Gas Using Nickel Oxide Oxygen Carrier Supported on Bentonite. Energy Fuels 2007, 21, 1582–1591. [Google Scholar] [CrossRef]
- Gu, H.; Shen, L.; Xiao, J.; Zhang, S.; Song, T.; Chen, D. Evaluation of the Effect of Sulfur on Iron-Ore Oxygen Carrier in Chemical-Looping Combustion. Ind. Eng. Chem. Res. 2013, 52, 1795–1805. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, R.; Zheng, W. Comparative study between fluidized-bed and fixed-bed operation modes in pressurized chemical looping combustion of coal. Appl. Energy 2014, 130, 181–189. [Google Scholar] [CrossRef]
- Xiao, R.; Song, Q.; Zhang, S.; Zheng, W.; Yang, Y. Pressurized Chemical-Looping Combustion of Chinese Bituminous Coal: Cyclic Performance and Characterization of Iron Ore-Based Oxygen Carrier. Energy Fuels 2010, 24, 1449–1463. [Google Scholar] [CrossRef]
- Xiao, R.; Song, Q.; Song, M.; Lu, Z.; Zhang, S.; Shen, L. Pressurized chemical-looping combustion of coal with an iron ore-based oxygen carrier. Combust. Flame 2010, 157, 1140–1153. [Google Scholar] [CrossRef]
- Luo, S.; Zeng, L.; Xu, D.; Kathe, M.; Chung, E.; Deshpande, N.; Qin, L.; Majumder, A.; Hsieh, T.-L.; Tong, A. Shale gas-to-syngas chemical looping process for stable shale gas conversion to high purity syngas with a H 2: CO ratio of 2: 1. Energy Environ. Sci. 2014, 7, 4104–4117. [Google Scholar]
- Deshpande, N.; Majumder, A.; Qin, L.; Fan, L.-S. High-pressure redox behavior of iron-oxide-based oxygen carriers for syngas generation from methane. Energy Fuels 2015, 29, 1469–1478. [Google Scholar]
- Hamers, H.; Gallucci, F.; Williams, G.; Cobden, P.; van Sint Annaland, M. Reactivity of oxygen carriers for chemical-looping combustion in packed bed reactors under pressurized conditions. Energy Fuels 2015, 29, 2656–2663. [Google Scholar]
- San Pio, M.; Gallucci, F.; Roghair, I.; van Sint Annaland, M. Gas-solids kinetics of CuO/Al2O3 as an oxygen carrier for high-pressure chemical looping processes: The influence of the total pressure. Int. J. Hydrog. Energy 2017, 42, 12111–12121. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Rahman, R.A.; Lu, D.Y.; Ridha, F.N.; Duchesne, M.A.; Tan, Y.; Hughes, R.W. Pressurized chemical looping combustion with CO: Reduction reactivity and oxygen-transport capacity of ilmenite ore. Appl. Energy 2016, 184, 132–139. [Google Scholar]
- Tan, Y.; Ridha, F.N.; Lu, D.Y.; Hughes, R.W. Reduction kinetics of ilmenite ore for pressurized chemical looping combustion of simulated natural gas. Energy Fuels 2017, 31, 14201–14210. [Google Scholar] [CrossRef]
- Tan, Y.; Ridha, F.N.; Duchesne, M.A.; Lu, D.Y.; Hughes, R.W. Reduction kinetics of ilmenite ore as an oxygen carrier for pressurized chemical looping combustion of methane. Energy Fuels 2017, 31, 7598–7605. [Google Scholar] [CrossRef]
- Chen, L.; Kong, L.; Bao, J.; Combs, M.; Nikolic, H.S.; Fan, Z.; Liu, K. Experimental evaluations of solid-fueled pressurized chemical looping combustion–The effects of pressure, solid fuel and iron-based oxygen carriers. Appl. Energy 2017, 195, 1012–1022. [Google Scholar] [CrossRef] [Green Version]
- Tian, Q.; Su, Q. Performance of a Cu-based oxygen carrier with pressurized CH4 in a fixed bed CLC process. In Proceedings of the 2017 2nd International Conference on Civil, Transportation and Environmental Engineering (ICCTE 2017), Shenzhen, China, 10–11 May 2017. [Google Scholar]
- Lee, D.; Nam, H.; Kim, H.; Hwang, B.; Baek, J.-I.; Ryu, H.-J. Experimental screening of oxygen carrier for a pressurized chemical looping combustion. Fuel Processing Technol. 2021, 218, 106860. [Google Scholar] [CrossRef]
- Nordness, O.; Han, L.; Zhou, Z.; Bollas, G.M. High-pressure chemical-looping of methane and synthesis gas with Ni and Cu oxygen carriers. Energy Fuels 2016, 30, 504–514. [Google Scholar] [CrossRef]
- Guo, X.; Li, Y.; Zhu, Q.; Hu, X.; Ma, J.; Guo, Q. Reactivity of iron-based oxygen carriers with coal ash in pressurized chemical looping gasification. Fuel Processing Technol. 2021, 219, 106890. [Google Scholar]
- Díez-Martín, L.; Grasa, G.; Murillo, R.; Martini, M.; Gallucci, F.; van Sint Annaland, M. Determination of the oxidation kinetics of high loaded CuO-based materials under suitable conditions for the Ca/Cu H2 production process. Fuel 2018, 219, 76–87. [Google Scholar]
- Rana, S.; Sun, Z.; Mehrani, P.; Hughes, R.; Macchi, A. Ilmenite oxidation kinetics for pressurized chemical looping combustion of natural gas. Appl. Energy 2019, 238, 747–759. [Google Scholar] [CrossRef]
- García-Labiano, F.; de DIEGO, L.F.; Adánez, J.; Abad, A.; Gayán, P. Reduction and oxidation kinetics of a copper-based oxygen carrier prepared by impregnation for chemical-looping combustion. Ind. Eng. Chem. Res. 2004, 43, 8168–8177. [Google Scholar]
- Abad, A.; Adánez, J.; García-Labiano, F.; Luis, F.; Gayán, P.; Celaya, J. Mapping of the range of operational conditions for Cu-, Fe-, and Ni-based oxygen carriers in chemical-looping combustion. Chem. Eng. Sci. 2007, 62, 533–549. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.-H.; Lee, C.-H.; Lee, J.-C. Performance and Cost Analysis of Natural Gas Combined Cycle Plants with Chemical Looping Combustion. ACS Omega 2021, 6, 21043–21058. [Google Scholar] [CrossRef]
- Ji, C.; Xin, G.; Wang, S.; Cong, X.; Meng, H.; Chang, K.; Yang, J. Effect of ammonia addition on combustion and emissions performance of a hydrogen engine at part load and stoichiometric conditions. Int. J. Hydrog. Energy 2021, 46, 40143–40153. [Google Scholar] [CrossRef]
- Cabello, A.; Abad, A.; García-Labiano, F.; Gayán, P.; de Diego, L.F.; Adánez, J. Kinetic determination of a highly reactive impregnated Fe2O3/Al2O3 oxygen carrier for use in gas-fueled Chemical Looping Combustion. Chem. Eng. J. 2014, 258, 265–280. [Google Scholar] [CrossRef] [Green Version]
- Cho, P.; Mattisson, T.; Lyngfelt, A. Comparison of iron-, nickel-, copper- and manganese-based oxygen carriers for chemical-looping combustion. Fuel 2004, 83, 1215–1225. [Google Scholar] [CrossRef]
- Abad, A.; Adánez, J.; García-Labiano, F.; de Diego, L.F.; Gayán, P. Modeling of the chemical-looping combustion of methane using a Cu-based oxygen-carrier. Combust. Flame 2010, 157, 602–615. [Google Scholar] [CrossRef] [Green Version]
- Schuhmann, R. Technical Publication 1189; American Institute of Mining and Metallurgical Engineers: New York, NY, USA, 1940. [Google Scholar]
- Tasirin, S.; Geldart, D. The elutriation of fine and cohesive particles from gas fluidized beds. Chem. Eng. Commun. 1999, 173, 175–195. [Google Scholar] [CrossRef]
- Do, H.T.; Grace, J.R.; Clift, R. Particle ejection and entrainment from fluidised beds. Powder Technol. 1972, 6, 195–200. [Google Scholar] [CrossRef]
- Brems, A.; Chan, C.W.; Seville, J.P.; Parker, D.; Baeyens, J. Modelling the transport disengagement height in fluidized beds. Adv. Powder Technol. 2011, 22, 155–161. [Google Scholar]
- Geldart, D.; Broodryk, N.; Kerdoncuff, A. Studies on the flow of solids down cyclone diplegs. Powder Technol. 1993, 76, 175–183. [Google Scholar]
- George, S.; Grace, J. Heat transfer to horizontal tubes in the freedboard region of a gas fluidized bed. AIChE J. 1982, 28, 759–765. [Google Scholar] [CrossRef]
- Baeyens, J.; van Puyvelde, F. Fluidized bed incineration of sewage sludge: A strategy for the design of the incinerator and the future for incinerator ash utilization. J. Hazard. Mater. 1994, 37, 179–190. [Google Scholar]
- Ergun, S. Fluid flow through packed columns. Chem. Eng. Prog. 1952, 48, 89–94. [Google Scholar]
- George, S. Entrainment of particles from aggregative fluidized beds. AIChE Symp. Ser. Fluid. 1978, 176, 67–74. [Google Scholar]
- Tasirin, S.; Geldart, D. Entrainment of FCC from fluidized beds—A new correlation for the elutriation rate constants Ki∞. Powder Technol. 1998, 95, 240–247. [Google Scholar]
- Cahyadi, A.; Neumayer, A.H.; Hrenya, C.M.; Cocco, R.A.; Chew, J.W. Comparative study of Transport Disengaging Height (TDH) correlations in gas–solid fluidization. Powder Technol. 2015, 275, 220–238. [Google Scholar]
- Bartocci, P.; Bidini, G.; Abad, A.; Bischi, A.; Cabello, A.; Loscertales, M.D.L.O.; Zampilli, M.; Massoli, S.; Garlatti, S.; Fantozzi, F. Pressurised Chemical Looping Combustion (PCLC): Air Reactor design. J. Phys. Conf. Ser. 2022, 2385, 012127. [Google Scholar] [CrossRef]
- Bi, H.T.; Grace, J.R. Flow regime diagrams for gas-solid fluidization and upward transport. Int. J. Multiph. Flow 1995, 21, 1229–1236. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, L.; Bollas, G. Overview of chemical-looping reduction in fixed bed and fluidized bed reactors focused on oxygen carrier utilization and reactor efficiency. Aerosol Air Qual. Res. 2014, 14, 559–571. [Google Scholar]
- Kolbitsch, P.; Pröll, T.; Bolhar-Nordenkampf, J.; Hofbauer, H. Design of a chemical looping combustor using a dual circulating fluidized bed (DCFB) reactor system. Chem. Eng. Technol. 2009, 32, 398–403. [Google Scholar]
- Lim, K.; Zhu, J.; Grace, J. Hydrodynamics of gas-solid fluidization. Int. J. of Multiph. Flow 1995, 21, 141–193. [Google Scholar] [CrossRef]


| Group | Fuel | OC | Source |
|---|---|---|---|
| CSIC, Spain | Syngas | CuO/Al2O3, Fe2O3/Al2O3, NiO/Al2O3 | [16,17] |
| USDOE | Syngas | NiO | [18] |
| Southeast University | CO, Coal | Hematite | [19,20,21,22] |
| The Ohio State University | CH4, H2 | Fe2TiO5 | [23,24] |
| Eindhoven University of Technology | CO, H2 | CuO/Al2O3 NiO/CaAl2O4 | [25,26] |
| Canmet ENERGY & North China Electric Power University | CO | FeTiO3 | [27,28,29] |
| University of Kentucky | Coal char | FeTiO3, Red mod | [30] |
| University of Science and Technology Beijing | CH4 | Cu-based | [31] |
| Korea Institute of Energy Research | CH4 | NiO, Mn3O4, CuO, Fe2O3 | [32] |
| University of Connecticut | CH4 | Ni & Cu Ocs | [33] |
| Ningxia University | Coal | Fe2O3/Al2O3 | [34] |
| Parameter | Definition | Units of Measure |
|---|---|---|
| CLC Plant Inventory | Amount of solids contained in both the reactors (air and fuel reactor) | kg OC MWf−1 |
| Solids circulation rate | Is the mass flow of OC totally oxidized | kg OC s−1MWf−1 |
| Parameter | Value | Unit of Measure |
|---|---|---|
| Active NiO content | 40 | wt% |
| Oxygen transport capacity, R0 | 0.084 | - |
| Particle size | 0.2 | (μm) |
| Porosity | 0.36 | % |
| Specific surface area (BET) | 0.8 | m2/g |
| Solid density | 5380 | kg/m3 |
| Interval | Lower Limit | Upper Limit | Weight Fraction | Cumulative Weight Fraction |
|---|---|---|---|---|
| 1 | 0 | 1 | 0.0316228 | 0.0316228 |
| 2 | 1 | 2 | 0.0578199 | 0.0894427 |
| 3 | 2 | 3 | 0.074874 | 0.164317 |
| 4 | 3 | 4 | 0.0886654 | 0.252982 |
| 5 | 4 | 5 | 0.100571 | 0.353553 |
| 6 | 5 | 6 | 0.111205 | 0.464758 |
| 7 | 6 | 7 | 0.120904 | 0.585662 |
| 8 | 7 | 8 | 0.12988 | 0.715542 |
| 9 | 8 | 9 | 0.138273 | 0.853815 |
| 10 | 9 | 10 | 0.146185 | 1 |
| Parameter | Value | Unit of Measure |
|---|---|---|
| Voidage at minimum fluidization | 0.5 | - |
| Geldart classification | B | - |
| Minimum fluidization velocity calculation method | Ergun [51] | - |
| Transport disengagement Height Model | George and Grace [52] | - |
| Maximum dCv/dh | 1 × 10−5 | - |
| Elutriation model | Tasirin & Geldart [53] | - |
| Decay constant | 3 | - |
| TG parameter A1 | 23.7 | - |
| TG parameter A2 | 14.5 | - |
| TG parameter B1 | 2.5 | - |
| TG parameter B2 | 2.5 | - |
| TG parameter C1 | −5.4 | - |
| TG parameter C2 | −5.4 | - |
| Constant diameter | - | - |
| Cross section | circular | - |
| Solids discharge location | 95% of total height | - |
| Gas distribution | Bubble cap nozzle | - |
| Distributor pressure drop | 0.04 | bar |
| Parameter | Air Reactor | Unit of Measure |
|---|---|---|
| Total reactor height | 9.5 | m |
| Reactor diameter | 1.8 | m |
| Inventory | 10,880 | kg |
| Circulation rate | 111 | kg/s |
| Operating pressure | 12 | bar |
| Height of bottom zone | 1.8 | m |
| Height of freeboard | 7.7 | m |
| Transport Disengaging Height calculated by correlation | 18.4 | m |
| Transport Disengaging Height based on solids volume fraction profile | 3.7 | m |
| Number of particles in bed | 1.1 × 1011 | - |
| Surface area | 21,864 | sqm |
| Minimum fluidization velocity | 0.1 | m/s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartocci, P.; Abad, A.; Bischi, A.; Wang, L.; Cabello, A.; de Las Obras Loscertales, M.; Zampilli, M.; Yang, H.; Fantozzi, F. Dimensioning Air Reactor and Fuel Reactor of a Pressurized Chemical Looping Combustor to Be Coupled to a Gas Turbine: Part 1, the Air Reactor. Energies 2023, 16, 2102. https://doi.org/10.3390/en16052102
Bartocci P, Abad A, Bischi A, Wang L, Cabello A, de Las Obras Loscertales M, Zampilli M, Yang H, Fantozzi F. Dimensioning Air Reactor and Fuel Reactor of a Pressurized Chemical Looping Combustor to Be Coupled to a Gas Turbine: Part 1, the Air Reactor. Energies. 2023; 16(5):2102. https://doi.org/10.3390/en16052102
Chicago/Turabian StyleBartocci, Pietro, Alberto Abad, Aldo Bischi, Lu Wang, Arturo Cabello, Margarita de Las Obras Loscertales, Mauro Zampilli, Haiping Yang, and Francesco Fantozzi. 2023. "Dimensioning Air Reactor and Fuel Reactor of a Pressurized Chemical Looping Combustor to Be Coupled to a Gas Turbine: Part 1, the Air Reactor" Energies 16, no. 5: 2102. https://doi.org/10.3390/en16052102