Numerical Analysis of In Situ Conversion Process of Oil Shale Formation Based on Thermo-Hydro-Chemical Coupled Modelling
Abstract
:1. Introduction
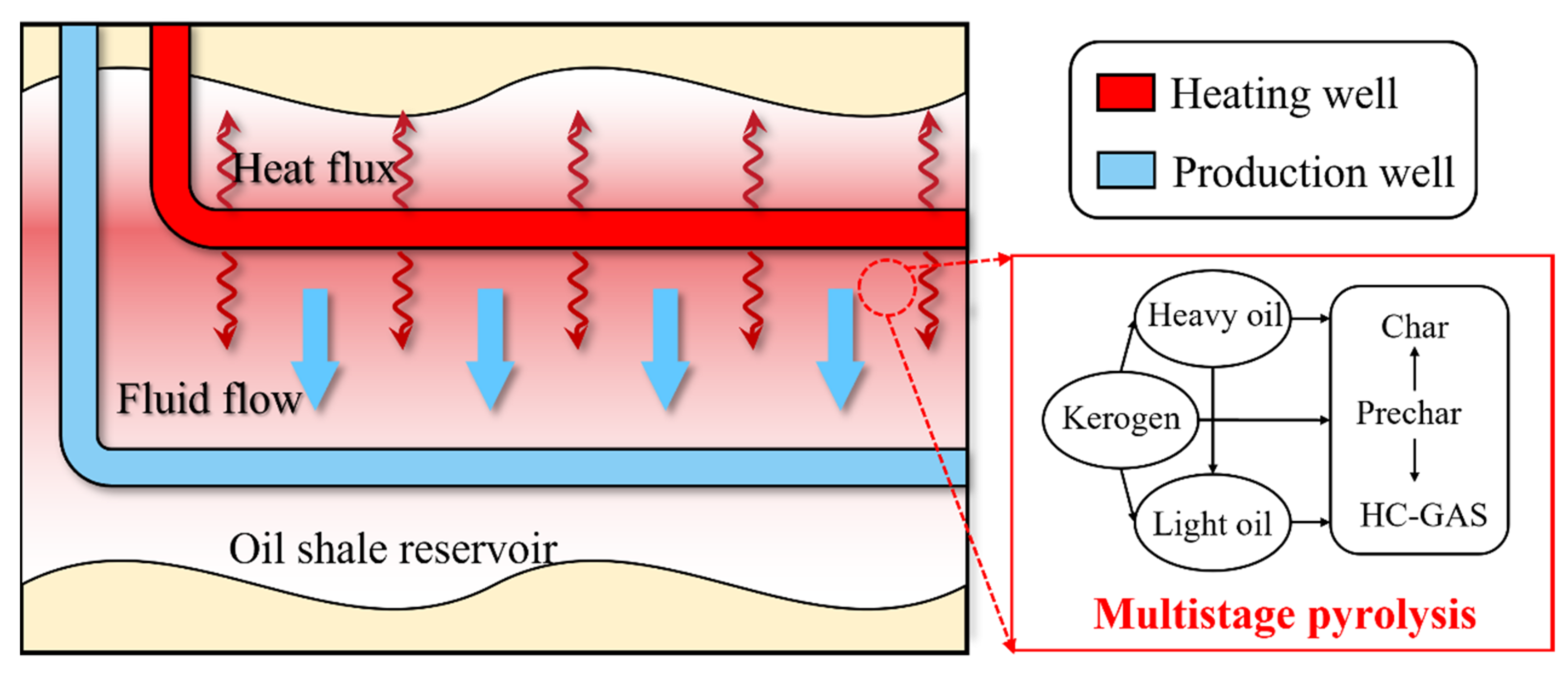
2. Model Description
2.1. Basic Assumptions
- (1)
- The flow of fluid products is considered as single-phase flow.
- (2)
- The deformation of oil shale is negligible.
- (3)
- The density of solid components is constant.
- (4)
- Due to the layered characteristics of oil shale, it is considered as a transversely isotropic material.
- (5)
- The porosity of oil shale is described as a function of temperature.
2.2. Pyrolytic Reaction Kinetics
2.3. Flow in Porous Media
2.4. Heat Transfer Model of Porous Media
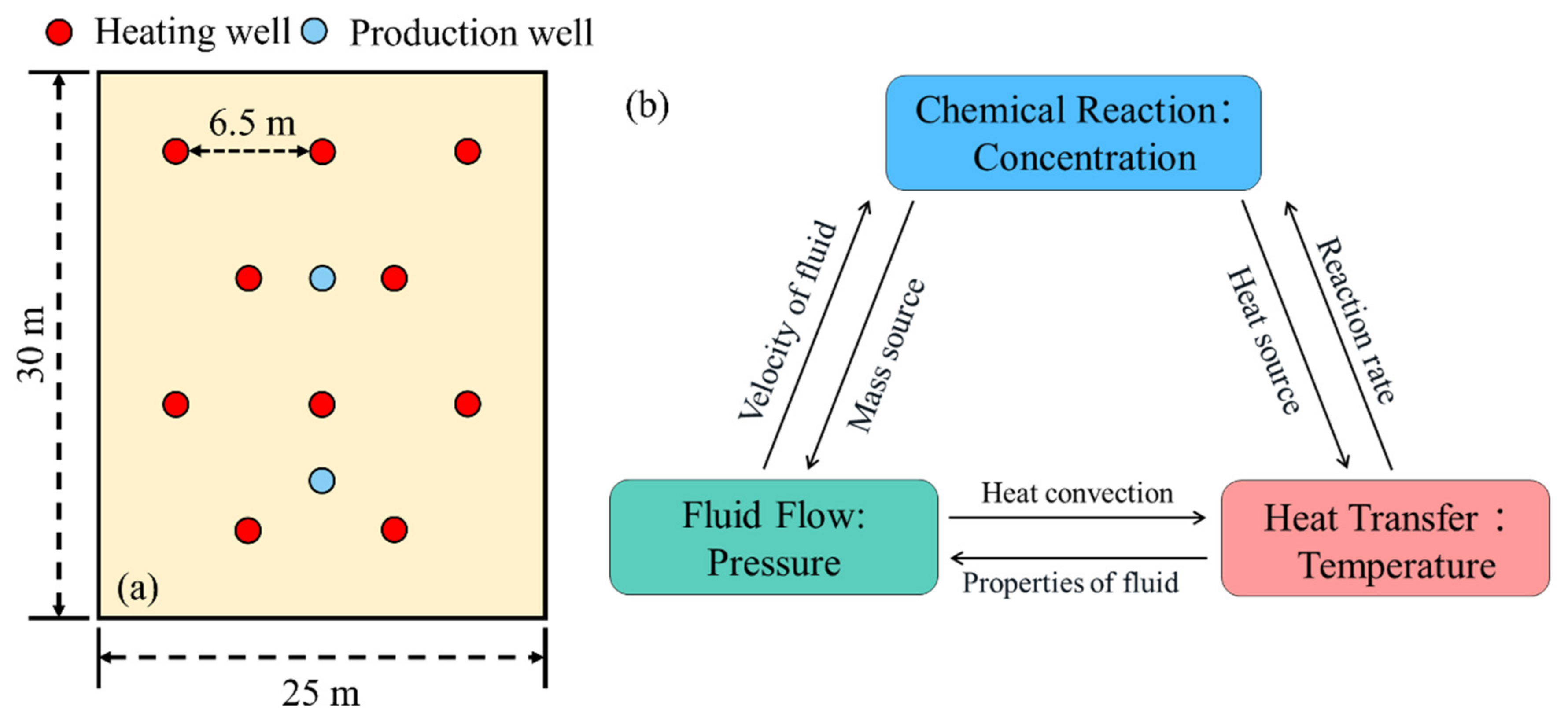
2.5. Numerical Model and Input Parameters
3. Results and Discussions
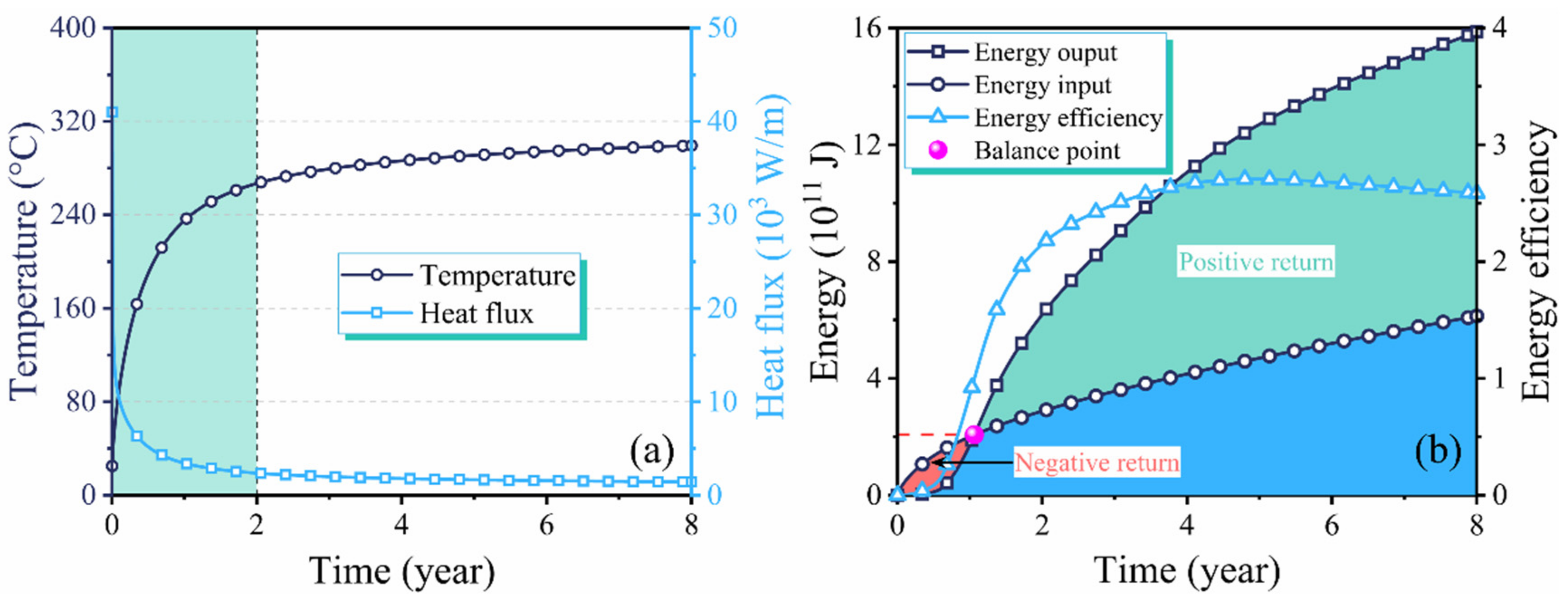
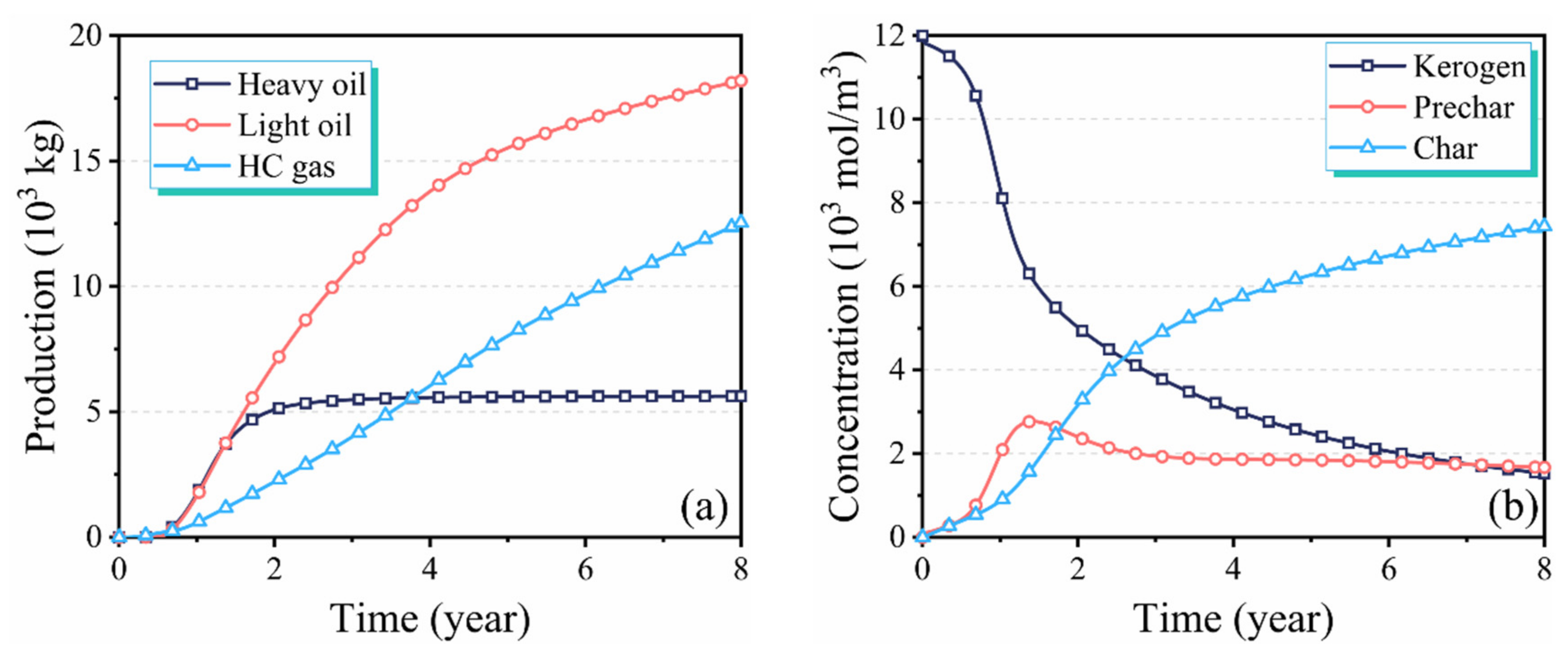
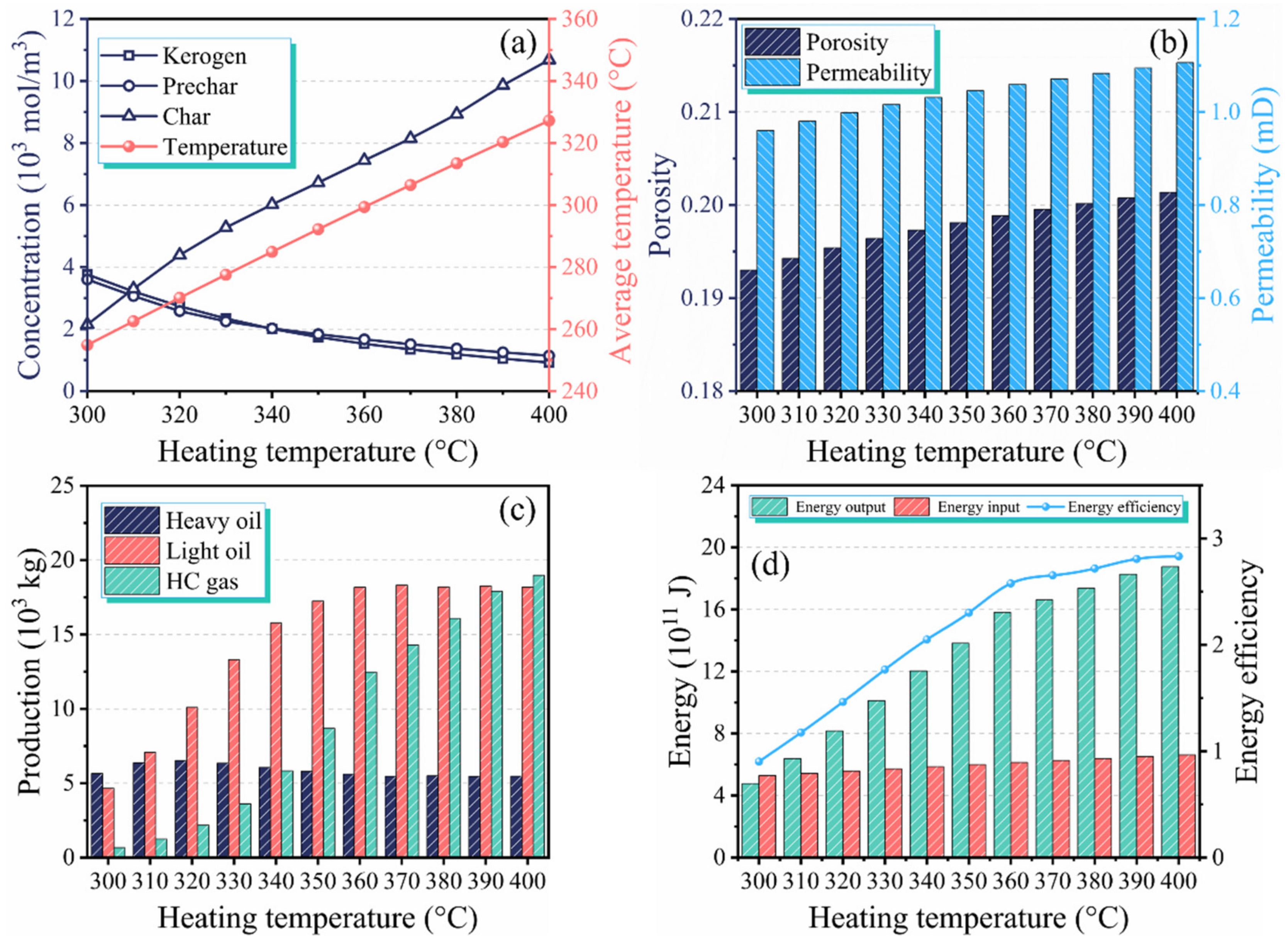
3.1. Performance Evaluation of In Situ Conversion Process
3.2. Effect of Heating Temperature on Production Performance
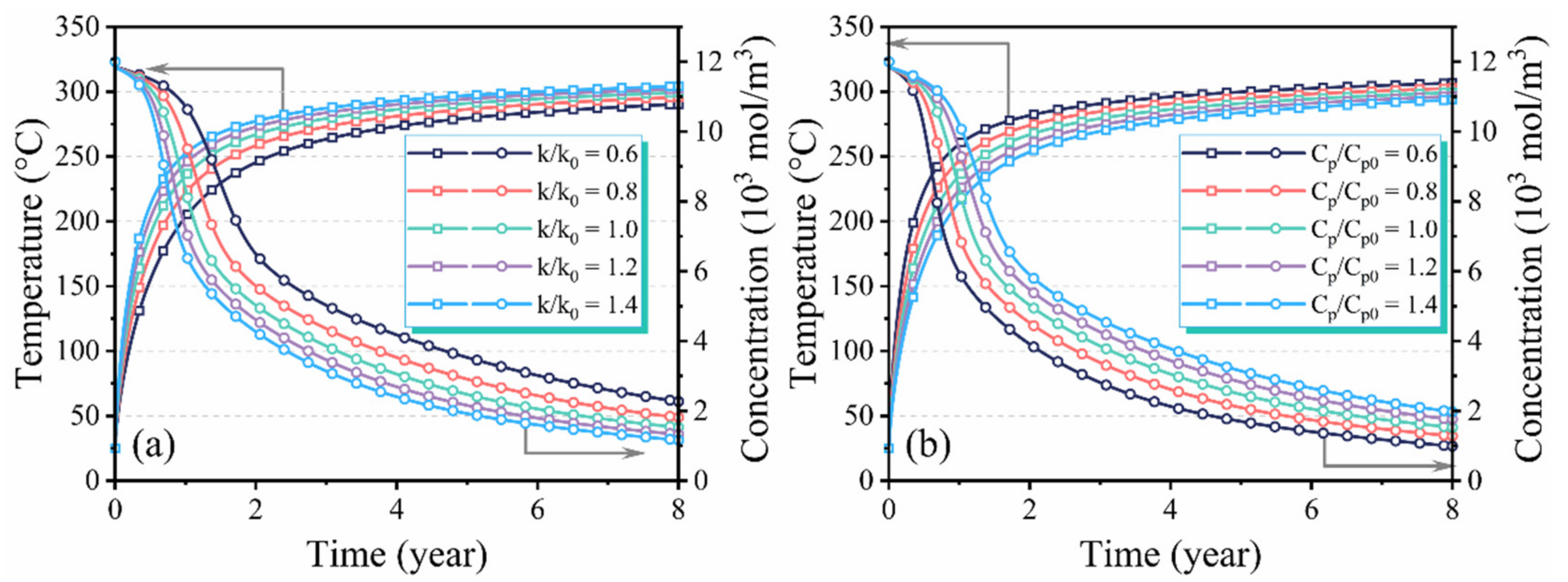
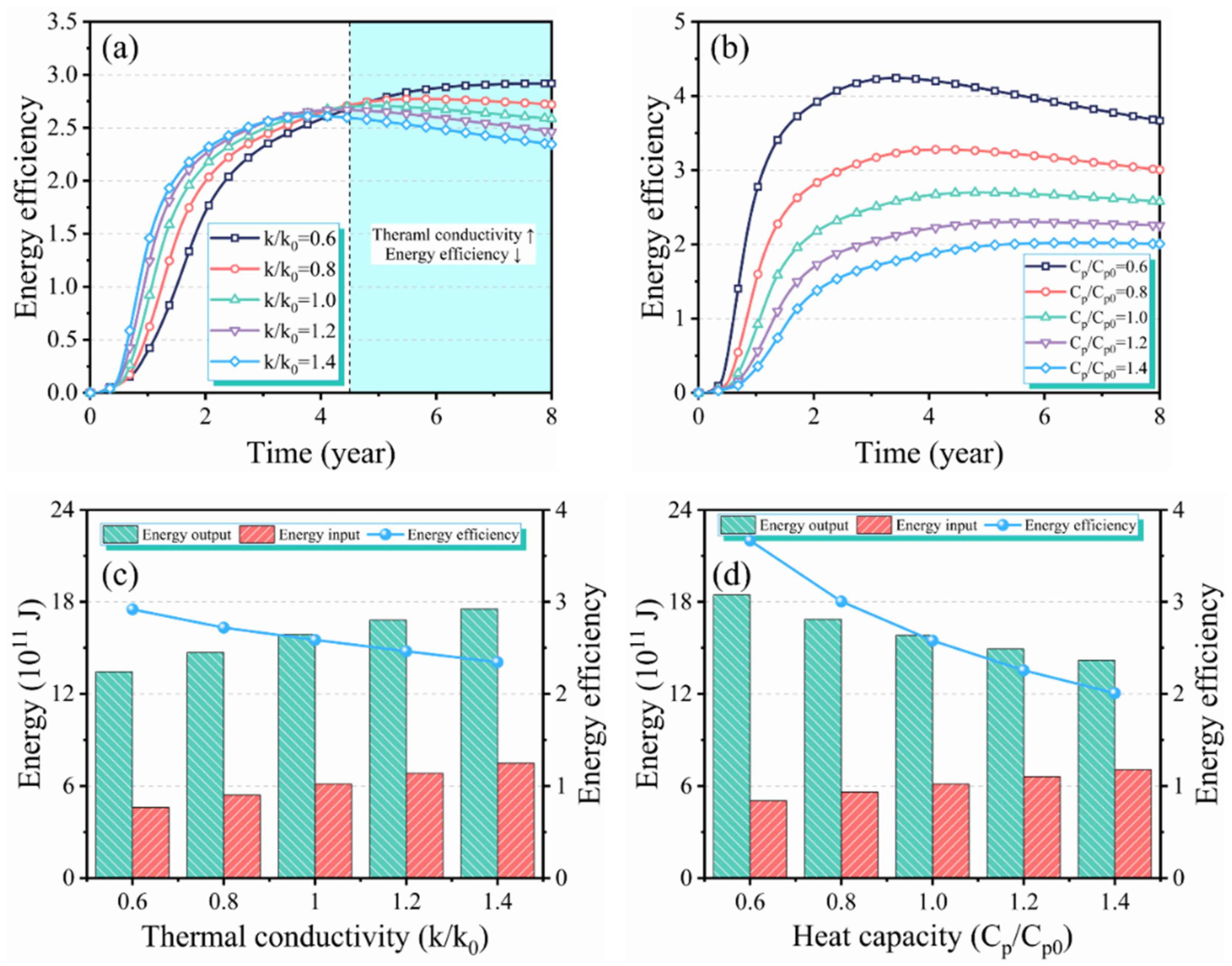
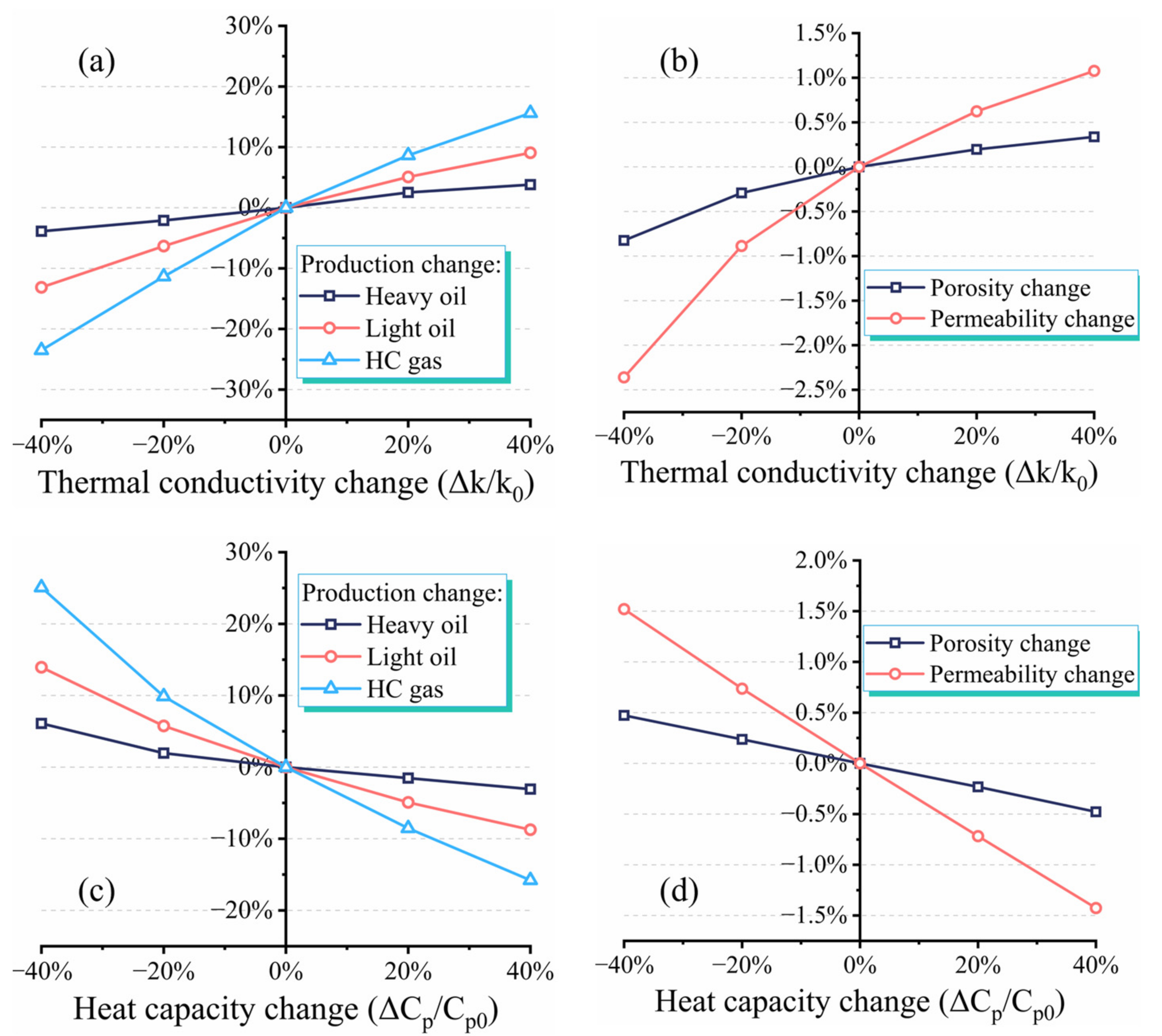
3.3. Effect of Thermophysical Properties on Production Performance
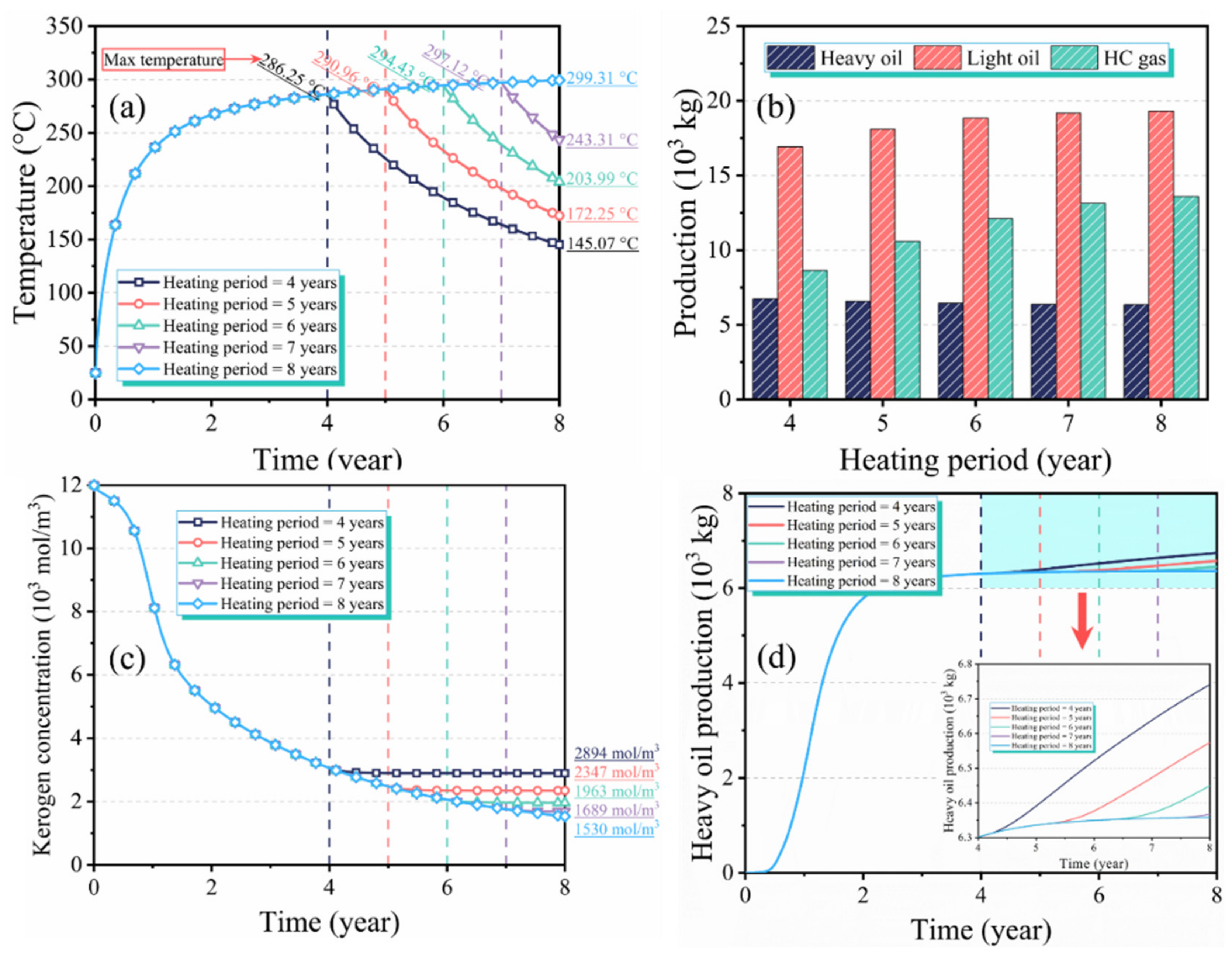
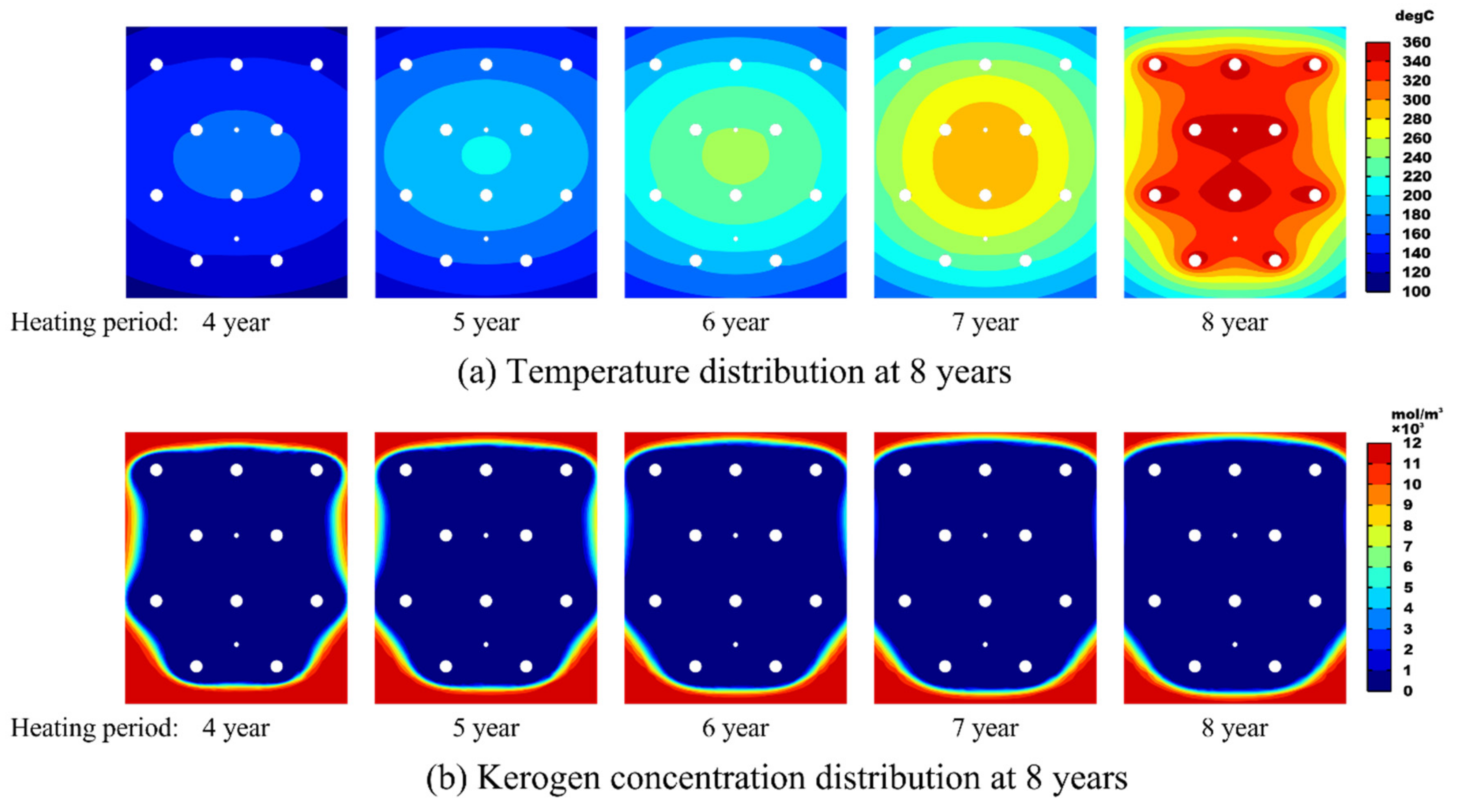
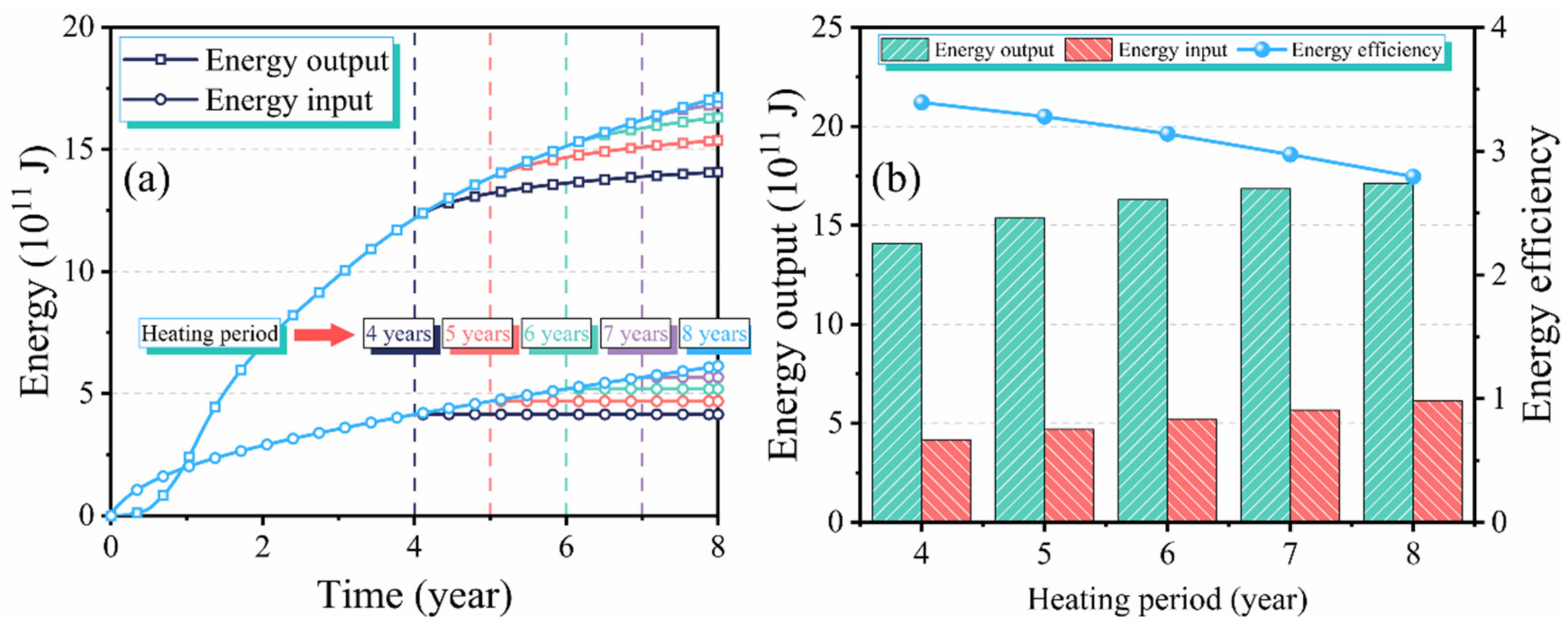
3.4. Effect of the Heating Period on Production Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Jiang, X.; Han, X.; Tong, J. Investigation of Chinese Oil Shale Resources Comprehensive Utilization Performance. Energy 2012, 42, 224–232. [Google Scholar] [CrossRef]
- World Energy Council. Survey of Energy Resources; World Energy Council: London, UK, 2010. [Google Scholar]
- Micheal, M.; Xu, W.; Jin, J.; Yu, H.; Liu, J.; Jiang, W.; Liu, H.; Wu, H. A Multi-Scale Quadruple-Continuum Model for Production Evaluation of Shale Gas Reservoirs Considering Complex Gas Transfer Mechanisms and Geomechanics. J. Pet. Sci. Eng. 2022, 213, 110419. [Google Scholar] [CrossRef]
- Li, Q.; Xu, S.; Zhang, L.; Chen, F.; Wu, S.; Bai, N. Shale Oil Enrichment Mechanism of the Paleogene Xingouzui Formation, Jianghan Basin, China. Energies 2022, 15, 4038. [Google Scholar] [CrossRef]
- Lee, K.J.; Moridis, G.J.; Ehlig-Economides, C.A. A Comprehensive Simulation Model of Kerogen Pyrolysis for the In-Situ Upgrading of Oil Shales. SPE J. 2016, 21, 1612–1630. [Google Scholar] [CrossRef]
- Hazra, K.G.; Lee, K.J.; Economides, C.E.; Moridis, G. Comparison of Heating Methods for In-Situ Oil Shale Extraction. In Proceedings of the IOR 2013-17th European Symposium on Improved Oil Recovery, Saint Petersburg, Russia, 16 April 2013; European Association of Geoscientists & Engineers: Bunnik, Netherlands, 2013; p. cp-342-00038. [Google Scholar]
- Micheal, M.; Xu, W.; Xu, H.; Zhang, J.; Jin, H.; Yu, H.; Wu, H. Multi-Scale Modelling of Gas Transport and Production Evaluation in Shale Reservoir Considering Crisscrossing Fractures. J. Nat. Gas Sci. Eng. 2021, 95, 104156. [Google Scholar] [CrossRef]
- Yu, H.; Chen, J.; Zhu, Y.; Wang, F.; Wu, H. Multiscale Transport Mechanism of Shale Gas in Micro/Nano-Pores. Int. J. Heat Mass Transf. 2017, 111, 1172–1180. [Google Scholar] [CrossRef]
- Li, X.; Zhou, H.; Wang, Y.; Qian, Y.; Yang, S. Thermoeconomic Analysis of Oil Shale Retorting Processes with Gas or Solid Heat Carrier. Energy 2015, 87, 605–614. [Google Scholar] [CrossRef]
- Han, X.X.; Jiang, X.M.; Cui, Z.G. Studies of the Effect of Retorting Factors on the Yield of Shale Oil for a New Comprehensive Utilization Technology of Oil Shale. Appl. Energy 2009, 86, 2381–2385. [Google Scholar] [CrossRef]
- Selberg, A.; Viik, M.; Pall, P.; Tenno, T. Environmental Impact of Closing of Oil Shale Mines on River Water Quality in North-Eastern Estonia. Oil Shale 2009, 26, 169–183. [Google Scholar] [CrossRef]
- Xu, H.; Yu, H.; Fan, J.; Xia, J.; Liu, H.; Wu, H. Formation Mechanism and Structural Characteristic of Pore-Networks in Shale Kerogen during in-Situ Conversion Process. Energy 2022, 242, 122992. [Google Scholar] [CrossRef]
- Brandt, A.R. Converting Oil Shale to Liquid Fuels: Energy Inputs and Greenhouse Gas Emissions of the Shell in Situ Conversion Process. Environ. Sci. Technol. 2008, 42, 7489–7495. [Google Scholar] [CrossRef]
- Hui, H.; Ning-Ning, Z.; Cai-Xia, H.; Yan, L.; Qing-Yong, L.; Na, D.; Xiao-Yan, H. Numerical Simulation of In Situ Conversion of Continental Oil Shale in Northeast China. Oil Shale 2016, 33, 45. [Google Scholar] [CrossRef]
- Kang, Z.; Zhao, Y.; Yang, D. Review of Oil Shale In-Situ Conversion Technology. Appl. Energy 2020, 269, 115121. [Google Scholar] [CrossRef]
- Raukas, A.; Punning, J.-M. Environmental Problems in the Estonian Oil Shale Industry. Energy Environ. Sci. 2009, 2, 723–728. [Google Scholar] [CrossRef]
- Kang, Z.; Zhao, Y.; Yang, D.; Tian, L.; Li, X. A Pilot Investigation of Pyrolysis from Oil and Gas Extraction from Oil Shale by In-Situ Superheated Steam Injection. J. Pet. Sci. Eng. 2020, 186, 106785. [Google Scholar] [CrossRef]
- Crawford, P.; Biglarbigi, K.; Dammer, A.; Knaus, E. Advances in World Oil-Shale Production Technologies. In Proceedings of the SPE Annual Technical Conference and Exhibition, OnePetro, Denver, CO, USA, 21–24 September 2008. [Google Scholar]
- Crawford, P.M.; Killen, J.C. New Challenges and Directions in Oil Shale Development Technologies. Oil Shale Solut. Liq. Fuel Dilemma 2010, 1032, 21–60. [Google Scholar]
- Fowler, T.D.; Vinegar, H.J. Oil Shale ICP—Colorado Field Pilots. In Proceedings of the SPE Western Regional Meeting, San Jose, CA, USA, 24 March 2009; SPE: San Jose, CA, USA, 2009; p. SPE-121164-MS. [Google Scholar]
- Yu, H.; Fan, J.; Xia, J.; Liu, H.; Wu, H. Multiscale Gas Transport Behavior in Heterogeneous Shale Matrix Consisting of Organic and Inorganic Nanopores. J. Nat. Gas Sci. Eng. 2020, 75, 103139. [Google Scholar] [CrossRef]
- Yu, H.; Xu, H.; Fan, J.; Zhu, Y.-B.; Wang, F.; Wu, H. Transport of Shale Gas in Microporous/Nanoporous Media: Molecular to Pore-Scale Simulations. Energy Fuels 2021, 35, 911–943. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, W.; Sun, Y.; Li, Q.; Deng, S.; Wang, Y.; Cui, G. Reaction Mechanism and Reservoir Simulation Study of the High-Temperature Nitrogen Injection in-Situ Oil Shale Process: A Case Study in Songliao Basin, China. Fuel 2022, 316, 123164. [Google Scholar] [CrossRef]
- Ryan, R.C.; Fowler, T.D.; Beer, G.L.; Nair, V. Shell’s in Situ Conversion Process− from Laboratory to Field Pilots. In Oil Shale: A Solution to the Liquid Fuel Dilemma; ACS Publications: Washington, DC, USA, 2010; pp. 161–183. ISBN 1947-5918. [Google Scholar]
- Wang, G.; Yang, D.; Zhao, Y.; Kang, Z.; Zhao, J.; Huang, X. Experimental Investigation on Anisotropic Permeability and Its Relationship with Anisotropic Thermal Cracking of Oil Shale under High Temperature and Triaxial Stress. Appl. Therm. Eng. 2019, 146, 718–725. [Google Scholar] [CrossRef]
- Al-Ayed, O.S.; Matouq, M.; Anbar, Z.; Khaleel, A.M.; Abu-Nameh, E. Oil Shale Pyrolysis Kinetics and Variable Activation Energy Principle. Appl. Energy 2010, 87, 1269–1272. [Google Scholar] [CrossRef]
- Burnham, A.K.; Braun, R.L. Global Kinetic Analysis of Complex Materials. Energy Fuels 1999, 13, 1–22. [Google Scholar] [CrossRef]
- Kuang, W.; Lu, M.; Yeboah, I.; Qian, G.; Duan, X.; Yang, J.; Chen, D.; Zhou, X. A Comprehensive Kinetics Study on Non-Isothermal Pyrolysis of Kerogen from Green River Oil Shale. Chem. Eng. J. 2019, 377, 120275. [Google Scholar] [CrossRef]
- Bouamoud, R.; Moine, E.C.; Mulongo-Masamba, R.; El Hamidi, A.; Halim, M.; Arsalane, S. Type I Kerogen-Rich Oil Shale from the Democratic Republic of the Congo: Mineralogical Description and Pyrolysis Kinetics. Pet. Sci. 2020, 17, 255–267. [Google Scholar] [CrossRef]
- Hubbard, A.B.; Robinson, W.E. A Thermal Decomposition Study of Colorado Oil Shale; US Department of the Interior, Bureau of Mines: Washington, DC, USA, 1950; Volume 4744. [Google Scholar]
- Anthony, D.B.; Howard, J.B. Coal Devolatilization and Hydrogastification. AIChE J. 1976, 22, 625–656. [Google Scholar] [CrossRef]
- Campbell, J.H.; Gallegos, G.; Gregg, M. Gas Evolution during Oil Shale Pyrolysis. 2. Kinetic and Stoichiometric Analysis. Fuel 1980, 59, 727–732. [Google Scholar] [CrossRef]
- Braun, R.L.; Burnham, A.K. PMOD: A Flexible Model of Oil and Gas Generation, Cracking, and Expulsion. Org. Geochem. 1992, 19, 161–172. [Google Scholar] [CrossRef]
- Shen, C. Reservoir Simulation Study of An In-Situ Conversion Pilot of Green-River Oil Shale. In Proceedings of the SPE Rocky Mountain Petroleum Technology Conference, Denver, CO, USA, 14 April 2009; SPE: Denver, CO, USA, 2009; p. SPE-123142-MS. [Google Scholar]
- Fan, Y.; Durlofsky, L.; Tchelepi, H.A. Numerical Simulation of the In-Situ Upgrading of Oil Shale. SPE J. 2010, 15, 368–381. [Google Scholar] [CrossRef]
- Song, X.; Zhang, C.; Shi, Y.; Li, G. Production Performance of Oil Shale In-Situ Conversion with Multilateral Wells. Energy 2019, 189, 116145. [Google Scholar] [CrossRef]
- Wang, G.; Liu, S.; Yang, D.; Fu, M. Numerical Study on the In-Situ Pyrolysis Process of Steeply Dipping Oil Shale Deposits by Injecting Superheated Water Steam: A Case Study on Jimsar Oil Shale in Xinjiang, China. Energy 2022, 239, 122182. [Google Scholar] [CrossRef]
- Wang, G.; Yang, D.; Kang, Z.; Zhao, J. Anisotropy in Thermal Recovery of Oil Shale—Part 1: Thermal Conductivity, Wave Velocity and Crack Propagation. Energies 2018, 11, 77. [Google Scholar] [CrossRef]
- Pei, S.; Wang, Y.; Zhang, L.; Huang, L.; Cui, G.; Zhang, P.; Ren, S. An Innovative Nitrogen Injection Assisted In-Situ Conversion Process for Oil Shale Recovery: Mechanism and Reservoir Simulation Study. J. Pet. Sci. Eng. 2018, 171, 507–515. [Google Scholar] [CrossRef]
- Braun, R.L.; Burnham, A.K. Mathematical Model of Oil Generation, Degradation, and Expulsion. Energy Fuels 1990, 4, 132–146. [Google Scholar] [CrossRef]
- Zhu, J.; Yi, L.; Yang, Z.; Li, X. Numerical Simulation on the in Situ Upgrading of Oil Shale Reservoir under Microwave Heating. Fuel 2021, 287, 119553. [Google Scholar] [CrossRef]
- Zhu, J.; Yi, L.; Yang, Z.; Duan, M. Three-Dimensional Numerical Simulation on the Thermal Response of Oil Shale Subjected to Microwave Heating. Chem. Eng. J. 2021, 407, 127197. [Google Scholar] [CrossRef]
- Whitaker, S. Flow in Porous Media I: A Theoretical Derivation of Darcy’s Law. Transp. Porous Media 1986, 1, 3–25. [Google Scholar] [CrossRef]
- Xu, W.; Yu, H.; Micheal, M.; Huang, H.; Liu, H.; Wu, H. An Integrated Model for Fracture Propagation and Production Performance of Thermal Enhanced Shale Gas Recovery. Energy 2023, 263, 125682. [Google Scholar] [CrossRef]
- Geng, Y.; Liang, W.; Liu, J.; Cao, M.; Kang, Z. Evolution of Pore and Fracture Structure of Oil Shale under High Temperature and High Pressure. Energy Fuels 2017, 31, 10404–10413. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, D.; Kang, Z.; Feng, Z. A Micro-CT Study of Changes in the Internal Structure of Daqing and Yan’an Oil Shales at High Temperatures. Oil Shale 2012, 29, 357. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Yang, D.; Kang, Z.; Zhao, J. Effect of Pyrolysis on Oil Shale Using Superheated Steam: A Case Study on the Fushun Oil Shale, China. Fuel 2019, 253, 1490–1498. [Google Scholar] [CrossRef]
- Saif, T.; Lin, Q.; Singh, K.; Bijeljic, B.; Blunt, M.J. Dynamic Imaging of Oil Shale Pyrolysis Using Synchrotron X-Ray Microtomography. Geophys. Res. Lett. 2016, 43, 6799–6807. [Google Scholar] [CrossRef]
- Pei, S.; Cui, G.; Wang, Y.; Zhang, L.; Wang, Q.; Zhang, P.; Huang, L.; Ren, S. Air Assisted in Situ Upgrading via Underground Heating for Ultra Heavy Oil: Experimental and Numerical Simulation Study. Fuel 2020, 279, 118452. [Google Scholar] [CrossRef]
- Wang, G.; Yang, D.; Kang, Z.; Zhao, J.; Lv, Y. Numerical Investigation of the in Situ Oil Shale Pyrolysis Process by Superheated Steam Considering the Anisotropy of the Thermal, Hydraulic, and Mechanical Characteristics of Oil Shale. Energy Fuels 2019, 33, 12236–12250. [Google Scholar] [CrossRef]
- Speight, J.G. Lange’s Handbook of Chemistry; McGraw-Hill Education: New York, NY, USA, 2017; ISBN 1-259-58609-X. [Google Scholar]


| Reactions | Frequency Factor (1/s) | Activation Energy (kJ/mol) | Enthalpy (kJ/mol) |
|---|---|---|---|
| Kerogen → 0.010699 Heavy oil + 0.009722 Light oil + 0.007131 HC gas + 0.641083 Prechar | 3.0 × 1013 | 213.384 | −335 |
| Heavy oil → 0.661282 Light oil + 1.503765 HC gas + 13.4175 Prechar | 1.0 × 1013 | 226.09 | −46.5 |
| Light oil → 3.237828 HC gas+ 5.182242 Prechar | 5.0 × 1011 | 226.09 | −46.5 |
| Prechar → 0.017177 HC gas + 0.99021 Char | 1.0 × 1013 | 226.09 | −46.5 |
| Parameter | Value | Source |
|---|---|---|
| Initial reservoir temperature | 25 °C | - |
| Initial reservoir pressure | 8 MPa | - |
| Heating temperature | 360 °C | - |
| Bottom hole pressure | 1 MPa | - |
| Initial thermal conductivity of the fluid mixture | 0.1 W/(m·K) | - |
| Initial porosity of oil shale | 0.019 | [46] |
| Initial permeability of oil shale | 0.001 mD | - |
| Initial heat capacity of the fluid mixture | 2050 J/(kg·K) | [51] |
| Initial dynamic viscosity of the fluid mixture | 1.2 × 10−3 Pa·s | [51] |
| Initial density of the fluid¥mixture | 700 kg/m3 | [51] |
| Density of oil shale | 2520 kg/m3 | [35] |
| Molecular weight of kerogen | 14.70 g/mol | [39] |
| Molecular weight of heavy oil | 382.4 g/mol | [39] |
| Molecular weight of light oil | 215.7 g/mol | [39] |
| Molecular weight of HC gas | 46.3 g/mol | [39] |
| Molecular weight of prechar | 12.7 g/mol | [39] |
| Molecular weight of char | 12.0 g/mol | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Jiang, W.; Liu, J.; Shi, J.; Zhang, X.; Cheng, W.; Yu, Z.; Chen, W.; Ye, T. Numerical Analysis of In Situ Conversion Process of Oil Shale Formation Based on Thermo-Hydro-Chemical Coupled Modelling. Energies 2023, 16, 2103. https://doi.org/10.3390/en16052103
Jin J, Jiang W, Liu J, Shi J, Zhang X, Cheng W, Yu Z, Chen W, Ye T. Numerical Analysis of In Situ Conversion Process of Oil Shale Formation Based on Thermo-Hydro-Chemical Coupled Modelling. Energies. 2023; 16(5):2103. https://doi.org/10.3390/en16052103
Chicago/Turabian StyleJin, Juan, Weidong Jiang, Jiandong Liu, Junfeng Shi, Xiaowen Zhang, Wei Cheng, Ziniu Yu, Weixi Chen, and Tingfu Ye. 2023. "Numerical Analysis of In Situ Conversion Process of Oil Shale Formation Based on Thermo-Hydro-Chemical Coupled Modelling" Energies 16, no. 5: 2103. https://doi.org/10.3390/en16052103