Anode Nitrogen Concentration Estimation Based on Voltage Variation Characteristics for Proton Exchange Membrane Fuel Cell Stacks
Abstract
:1. Introduction
2. Experimental and Method
2.1. Fuel Cell Stack and Fuel Cell Test System
2.2. Experimental Procedure
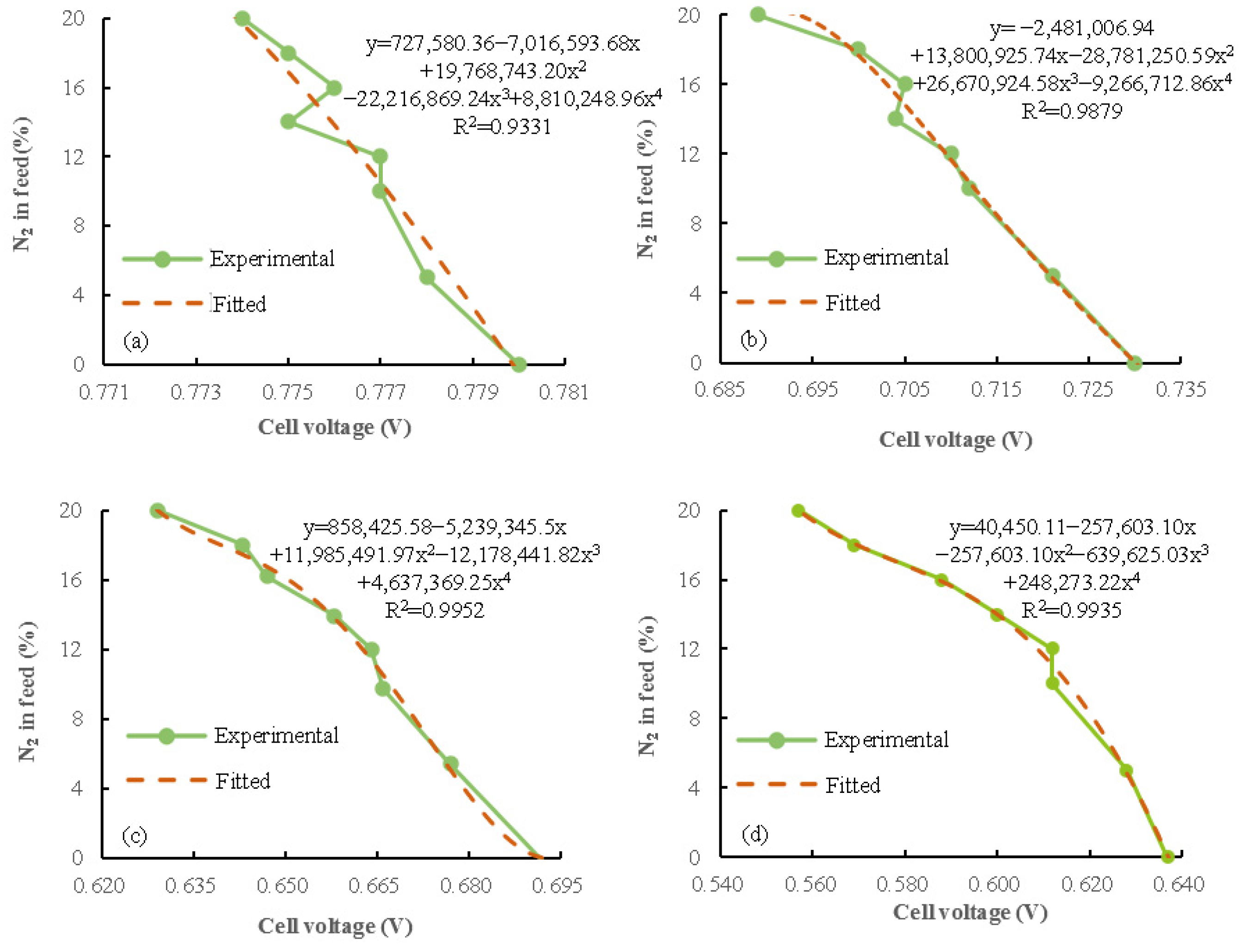
2.3. Data Fitting Method and Error Analysis
3. Results and Discussion
3.1. Effect of Nitrogen Concentration on the Mean Voltage of the Stack
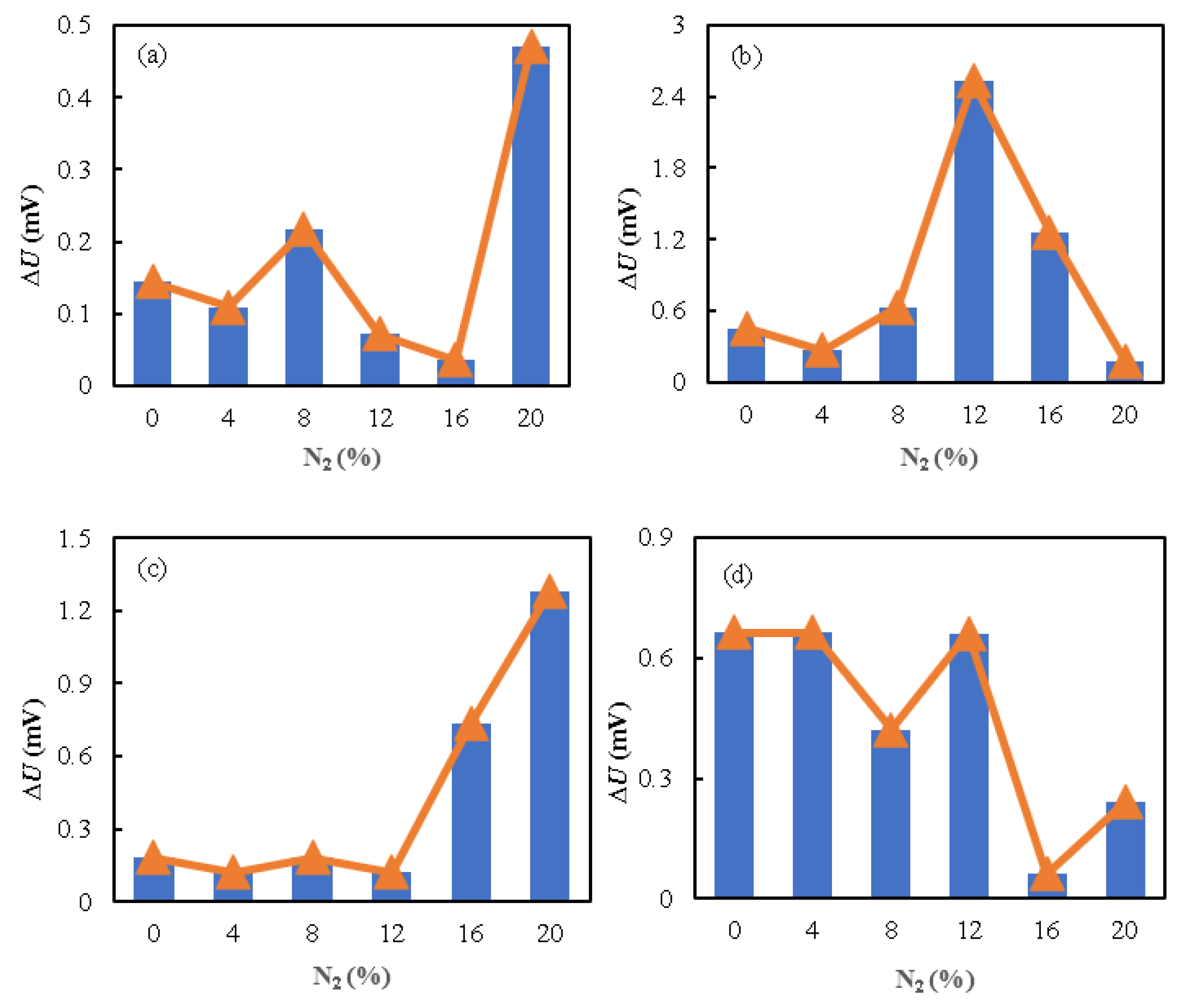
3.2. Effect of Nitrogen Concentration on the Voltage of a Specific Single Cell
3.3. Nitrogen Concentration Prediction and Validation
4. Conclusions
- The influence of reducing hydrogen concentration on the performance of PEMFCs at varied current densities was investigated. Although a decrease in hydrogen concentration can significantly affect the overall fuel cell performance, to what extent it affects the fuel cell performance varies for a specific cell. With a current density of 1.4 A·cm−2 and an increase in nitrogen concentration from 0 to 20%, the average stack voltage, cell 11’s voltage, and cell 4’s voltage drop are 3.4%, 9.8%, and 2.8%, respectively. This may be due to the fact that the consistency of fuel cells is influenced by various aspects, like structural layout, component materials, and manufacturing processes.
- An estimation method of nitrogen concentration in the anodes of PEMFC is proposed on the basis of voltage variation characteristics. The nitrogen concentration of the anode could be evaluated more accurately by analyzing the decreasing pattern of fuel cell voltage, and the prediction of anode nitrogen concentration based on voltage variation characteristics becomes increasingly accurate with the increase in data amount. The maximum absolute percentage error obtained by this method is only 0.35%, which has high accuracy.
- Due to the non-uniform distribution of gases within the anode, the anode nitrogen concentration may not be accurately estimated using a single-cell voltage. Under different current densities, the relative mean maximum values of the absolute residual values are 0.350%, 1.980%, and 1.224% for the average voltage, cells 11 and 4, respectively. Therefore, the anode nitrogen concentration is estimated using the mean cell voltage. The accumulation of nitrogen in the anode at varying current densities can be expressed as a function of the working voltage, which can be programmed into the controller for PEMFC management.
- Anode nitrogen concentration estimation and nitrogen purge algorithms have been the focus of PEMFC research. This paper uses experimental and data analysis methods to evaluate the nitrogen concentration of an anode, and this method has less error in estimating the steady-state nitrogen concentration and requires less calculation. However, at the same time, this method does not consider the water and thermal characteristics of the stack, and it is very difficult to operate at a constant current density because the fuel cell has frequent load changes under on-vehicle operation, so this method is more suitable for operating under conditions where the load is constant for a long time. A combination of modeling and actual operating data analysis can be investigated in the future to more accurately evaluate the nitrogen concentration and to decrease the nitrogen concentration in the anode manifold by purge valve nitrogen purging.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hren, R.; Vujanović, A.; Van Fan, Y.; Klemeš, J.J.; Krajnc, D.; Čuček, L. Hydrogen production, storage and transport for renewable energy and chemicals: An environmental footprint assessment. Renew. Sustain. Energy Rev. 2023, 173, 113113. [Google Scholar] [CrossRef]
- Sallam, E.R.; Khairy, H.M.; Elshobary, M.; Fetouh, H.A. Application of Algae for Hydrogen Generation and Utilization. In Handbook of Research on Algae as a Sustainable Solution for Food, Energy, and the Environment; IGI Global: Hershey, PA, USA, 2022; pp. 354–378. [Google Scholar] [CrossRef]
- Sallam, E.R.; Fetouh, H.A. Comparative Study for the Production of Sustainable Electricity from Marine Sediment Using Recyclable Low-Cost Solid Wastes Aluminum Foil and Graphite Anodes. ChemistrySelect 2022, 7, e202103972. [Google Scholar] [CrossRef]
- Sallam, E.R.; Khairy, H.M.; Elnouby, M.S.; Fetouh, H.A. Sustainable electricity production from seawater using Spirulina platensis microbial fuel cell catalyzed by silver nanoparticles-activated carbon composite prepared by a new modified photolysis method. Biomass Bioenergy 2021, 148, 106038. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Pang, L.; Geng, T.; Guo, Y.; Jiang, L.; Kang, K.; Wang, X.; Liu, Z. Study on the effect of purging time on the performance of PEMFC with dead-ended anode under gravity. Renew. Energy 2022, 200, 1141–1151. [Google Scholar] [CrossRef]
- Li, Y.; Yang, F.; Chen, D.; Hu, S.; Xu, X. Thermal-physical modeling and parameter identification method for dynamic model with unmeasurable state in 10-kW scale proton exchange membrane fuel cell system. Energy Convers. Manag. 2023, 276, 116580. [Google Scholar] [CrossRef]
- İnci, M. Future vision of hydrogen fuel cells: A statistical review and research on applications, socio-economic impacts and forecasting prospects. Sustain. Energy Technol. Assess. 2022, 53, 102739. [Google Scholar] [CrossRef]
- Ahluwalia, R.; Wang, X.; Papadias, D.; Star, A. Performance and Total Cost of Ownership of a Fuel Cell Hybrid Mining Truck. Energies 2022, 16, 286. [Google Scholar] [CrossRef]
- Ren, P.; Pei, P.; Li, Y.; Chen, D.; Fu, X.; Zhu, Z.; Zhang, L.; Wang, M. In-situ characterization of gas distribution in proton exchange membrane fuel cell stacks. Energy Convers. Manag. 2022, 269, 116143. [Google Scholar] [CrossRef]
- Deng, X.; Huang, C.; Pei, X.; Hu, B.; Zhou, W. Recent progresses and remaining issues on the ultrathin catalyst layer design strategy for high-performance proton exchange membrane fuel cell with further reduced Pt loadings: A review. Int. J. Hydrogen Energy 2022, 47, 1529–1542. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Alanazi, A.; Vichare, P.; Sayed, E.T.; Maghrabie, H.M.; Elsaid, K.; Abdelkareem, M.A. Novel Trends in Proton Exchange Membrane Fuel Cells. Energies 2022, 15, 4949. [Google Scholar] [CrossRef]
- Pourrahmani, H.; Siavashi, M.; Yavarinasab, A.; Matian, M.; Chitgar, N.; Wang, L.; Van herle, J. A Review on the Long-Term Performance of Proton Exchange Membrane Fuel Cells: From Degradation Modeling to the Effects of Bipolar Plates, Sealings, and Contaminants. Energies 2022, 15, 5081. [Google Scholar] [CrossRef]
- Fan, L.; Xing, L.; Tu, Z.; Hwa Chan, S. A breakthrough hydrogen and oxygen utilization in a H2-O2 PEMFC stack with dead-ended anode and cathode. Energy Convers. Manag. 2021, 243, 114404. [Google Scholar] [CrossRef]
- Chen, L.; Xu, K.; Yang, Z.; Yan, Z.; Dong, Z. Optimal Design and Operation of Dual-Ejector PEMFC Hydrogen Supply and Circulation System. Energies 2022, 15, 5427. [Google Scholar] [CrossRef]
- Ding, R.; Chen, Y.; Chen, P.; Wang, R.; Wang, J.; Ding, Y.; Yin, W.; Liu, Y.; Li, J.; Liu, J. Machine Learning-Guided Discovery of Underlying Decisive Factors and New Mechanisms for the Design of Nonprecious Metal Electrocatalysts. ACS Catal. 2021, 11, 9798–9808. [Google Scholar] [CrossRef]
- Wang, J.; Ding, R.; Cao, F.; Li, J.; Dong, H.; Shi, T.; Xing, L.; Liu, J. Comparison of state-of-the-art machine learning algorithms and data-driven optimization methods for mitigating nitrogen crossover in PEM fuel cells. Chem. Eng. J. 2022, 442, 136064. [Google Scholar] [CrossRef]
- Legala, A.; Zhao, J.; Li, X. Machine learning modeling for proton exchange membrane fuel cell performance. Energy AI 2022, 10, 100183. [Google Scholar] [CrossRef]
- Mao, X.; Liu, S.; Tan, J.; Hu, H.; Lu, C.; Xuan, D. Multi-objective optimization of gradient porosity of gas diffusion layer and operation parameters in PEMFC based on recombination optimization compromise strategy. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Chen, D.; Pei, P.; Meng, Y.; Ren, P.; Li, Y.; Wang, M.; Wang, X. Novel extraction method of working condition spectrum for the lifetime prediction and energy management strategy evaluation of automotive fuel cells. Energy 2022, 255, 124523. [Google Scholar] [CrossRef]
- Omrani, R.; Shabani, B. An analytical model for hydrogen and nitrogen crossover rates in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2020, 45, 31041–31055. [Google Scholar] [CrossRef]
- Tang, Q.; Li, B.; Yang, D.; Ming, P.; Zhang, C.; Wang, Y. Review of hydrogen crossover through the polymer electrolyte membrane. Int. J. Hydrogen Energy 2021, 46, 22040–22061. [Google Scholar] [CrossRef]
- Zhou, Z.; Ye, L.; Qiu, D.; Peng, L.; Lai, X. Experimental investigation and decoupling of voltage losses distribution in proton exchange membrane fuel cells with a large active area. Chem. Eng. J. 2023, 452, 139497. [Google Scholar] [CrossRef]
- Goo, B.-H.; Paek, S.Y.; Al Munsur, A.Z.; Choi, O.; Kim, Y.; Kwon, O.J.; Lee, S.Y.; Kim, H.-J.; Kim, T.-H. Polyamide-coated Nafion composite membranes with reduced hydrogen crossover produced via interfacial polymerization. Int. J. Hydrogen Energy 2022, 47, 1202–1216. [Google Scholar] [CrossRef]
- Zhu, K.-Q.; Ding, Q.; Xu, J.-H.; Yang, Y.-R.; Yang, C.; Zhang, J.; Zhang, Y.; Huang, T.-M.; Yan, W.-M.; Wan, Z.-M.; et al. Optimization of gas diffusion layer thickness for proton exchange membrane fuel cells under steady-state and load-varying conditions. Energy Convers. Manag. 2022, 267, 115915. [Google Scholar] [CrossRef]
- Chen, D.; Pei, P.; Ren, P.; Song, X.; Wang, H.; Zhang, L.; Wang, M. Analytical methods for the effect of anode nitrogen concentration on performance and voltage consistency of proton exchange membrane fuel cell stack. Energy 2022, 258, 124850. [Google Scholar] [CrossRef]
- Zhou, M.; Frensch, S.; Liso, V.; Li, N.; Sahlin, S.L.; Cinti, G.; Simon Araya, S. Modeling the Performance Degradation of a High-Temperature PEM Fuel Cell. Energies 2022, 15, 5651. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, H.; He, S.; Meng, K.; Liu, Y.; Cai, Y. Numerical simulation on purge strategy of proton exchange membrane fuel cell with dead-ended anode. Energy 2021, 234, 121265. [Google Scholar] [CrossRef]
- Jiao, J.; Chen, F. Humidity estimation of vehicle proton exchange membrane fuel cell under variable operating temperature based on adaptive sliding mode observation. Appl. Energy 2022, 313, 118779. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, J.; Chen, Y.; Liu, S.; Wang, Z. Effects of gas permeation on the sealing performance of PEMFC stacks. Int. J. Hydrogen Energy 2021, 46, 36424–36435. [Google Scholar] [CrossRef]
- Shen, K.-Y.; Park, S.; Kim, Y.-B. Hydrogen utilization enhancement of proton exchange membrane fuel cell with anode recirculation system through a purge strategy. Int. J. Hydrogen Energy 2020, 45, 16773–16786. [Google Scholar] [CrossRef]
- Baik, K.D.; Kim, M.S. Characterization of nitrogen gas crossover through the membrane in proton-exchange membrane fuel cells. Int. J. Hydrogen Energy 2011, 36, 732–739. [Google Scholar] [CrossRef]
- Ziogou, C.; Voutetakis, S.; Papadopoulou, S.; Georgiadis, M.C. Modeling, simulation and experimental validation of a PEM fuel cell system. Comput. Chem. Eng. 2011, 35, 1886–1900. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Liu, H.; Yan, C.; Hou, Y.; He, Q.; Zhang, J.; Hissel, D. Anode purge management for hydrogen utilization and stack durability improvement of PEM fuel cell systems. Appl. Energy 2020, 275, 115110. [Google Scholar] [CrossRef]
- Piffard, M.; Gerard, M.; Fonseca, R.D.; Massioni, P.; Bideaux, E. Sliding mode observer for proton exchange membrane fuel cell: Automotive application. J. Power Sources 2018, 388, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Chen, D.; Li, Y.; Hong, J.; Xu, X. Methods for estimating the accumulated nitrogen concentration in anode of proton exchange membrane fuel cell stacks based on back propagation neural network. Int. J. Energy Res. 2022, 46, 22530–22540. [Google Scholar] [CrossRef]
- Karimäki, H.; Pérez, L.C.; Nikiforow, K.; Keränen, T.M.; Viitakangas, J.; Ihonen, J. The use of on-line hydrogen sensor for studying inert gas effects and nitrogen crossover in PEMFC system. Int. J. Hydrogen Energy 2011, 36, 10179–10187. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Yang, C.-W.; Lee, J.-Y. Implementation and evaluation for anode purging of a fuel cell based on nitrogen concentration. Appl. Energy 2014, 113, 1519–1524. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Z.; Fang, C.; Li, J.; Hong, P.; Jiang, H.; Guo, D.; Ouyang, M. Anode state observation of polymer electrolyte membrane fuel cell based on unscented Kalman filter and relative humidity sensor before flooding. Renew. Energy 2021, 168, 1294–1307. [Google Scholar] [CrossRef]










| Parameters | Value |
|---|---|
| Membrane active area | 301 cm2 |
| Power | 5 kW |
| Number of cells | 16 |
| Anode (Pt) | 0.1 mg∙cm−2 |
| Cathode (Pt) | 0.4 mg∙cm−2 |
| Bipolar plate | 316 L stainless steel |
| Excess ratio λ1 of hydrogen/nitrogen flow channel | 1.498 |
| Inlet pressure P1 of hydrogen/nitrogen flow channel | 260 kPa(abs.) |
| Humidifier dew temperature T1 of hydrogen/nitrogen flow channel | 60 °C |
| Excess ratio λ2 of air flow channel | 1.697 |
| Inlet pressure P2 of air flow channel | 250 kPa(abs.) |
| Humidifier dew temperature T2 of air flow channel | 60 °C |
| Coolant inlet temperature T3 | 74 °C |
| Current Density (A·cm−2) | Serial Number | ΔUmax (mV) | ΔUmin (mV) | ΔUa (mV) | δr (%) |
|---|---|---|---|---|---|
| 0.2 | Mean Cell Voltage | 0.497 | 0.090 | 0.241 | 0.308 |
| Cell Voltage 11 | 0.689 | 0.109 | 0.368 | 0.474 | |
| Cell Voltage 4 | 0.470 | 0.036 | 0.175 | 0.224 | |
| 0.6 | Mean Cell Voltage | 0.242 | 0.061 | 0.171 | 0.236 |
| Cell Voltage 11 | 4.699 | 0.180 | 1.386 | 1.980 | |
| Cell Voltage 4 | 2.530 | 0.181 | 0.889 | 1.224 | |
| 1.0 | Mean Cell Voltage | 0.273 | 0.091 | 0.152 | 0.219 |
| Cell Voltage 11 | 0.651 | 0.072 | 0.361 | 0.465 | |
| Cell Voltage 4 | 1.281 | 0.122 | 0.437 | 0.628 | |
| 1.4 | Mean Cell Voltage | 0.361 | 0.090 | 0.226 | 0.350 |
| Cell Voltage 11 | 2.892 | 0.359 | 1.144 | 1.867 | |
| Cell Voltage 4 | 0.663 | 0.061 | 0.452 | 0.691 |
| Nitrogen Concentration Estimation Method | Advantage | Disadvantage | References |
|---|---|---|---|
| Use of designed on-line hydrogen sensors | The real-time concentration of anode nitrogen can be obtained directly with a time delay of maximum 1.1 s. | Additional parts added, not suitable for installation. | [36] |
| Using mass spectrometry | Can obtain high-precision nitrogen concentration data | The gas concentration is measured for a period of 30 min or more while maintaining a certain working condition. | [31] |
| Using the integration of electrical density | A simple method can be used to estimate the nitrogen concentration and control the nitrogen concentration within 15%. | Cannot accurately estimate the nitrogen concentration in the flow channel. | [37] |
| Using a modeling approach | More accurate data can be obtained without adding additional components by reducing the nitrogen concentration from 20% to 2%. | Complex, computationally intensive, and difficult to ascertain parameters. | [32,38] |
| This Study | Simple method, easy to repeat, and more accurate results. | Lower accuracy compared to using a mass spectrometer and a modeling approach. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, R.; Chen, D.; Li, Y.; Wu, W.; Hu, S.; Xu, X. Anode Nitrogen Concentration Estimation Based on Voltage Variation Characteristics for Proton Exchange Membrane Fuel Cell Stacks. Energies 2023, 16, 2111. https://doi.org/10.3390/en16052111
Guo R, Chen D, Li Y, Wu W, Hu S, Xu X. Anode Nitrogen Concentration Estimation Based on Voltage Variation Characteristics for Proton Exchange Membrane Fuel Cell Stacks. Energies. 2023; 16(5):2111. https://doi.org/10.3390/en16052111
Chicago/Turabian StyleGuo, Ruifeng, Dongfang Chen, Yuehua Li, Wenlong Wu, Song Hu, and Xiaoming Xu. 2023. "Anode Nitrogen Concentration Estimation Based on Voltage Variation Characteristics for Proton Exchange Membrane Fuel Cell Stacks" Energies 16, no. 5: 2111. https://doi.org/10.3390/en16052111
APA StyleGuo, R., Chen, D., Li, Y., Wu, W., Hu, S., & Xu, X. (2023). Anode Nitrogen Concentration Estimation Based on Voltage Variation Characteristics for Proton Exchange Membrane Fuel Cell Stacks. Energies, 16(5), 2111. https://doi.org/10.3390/en16052111









