Generalised Isentropic Relations in Thermodynamics
Abstract
:1. Introduction
2. Isentropic Exponents for the Real Gas Thermodynamic Region
2.1. Generalised Isentropic Relations
2.2. Limits of the Generalised Isentropic Exponents for Ideal Gases and Incompressible Liquids
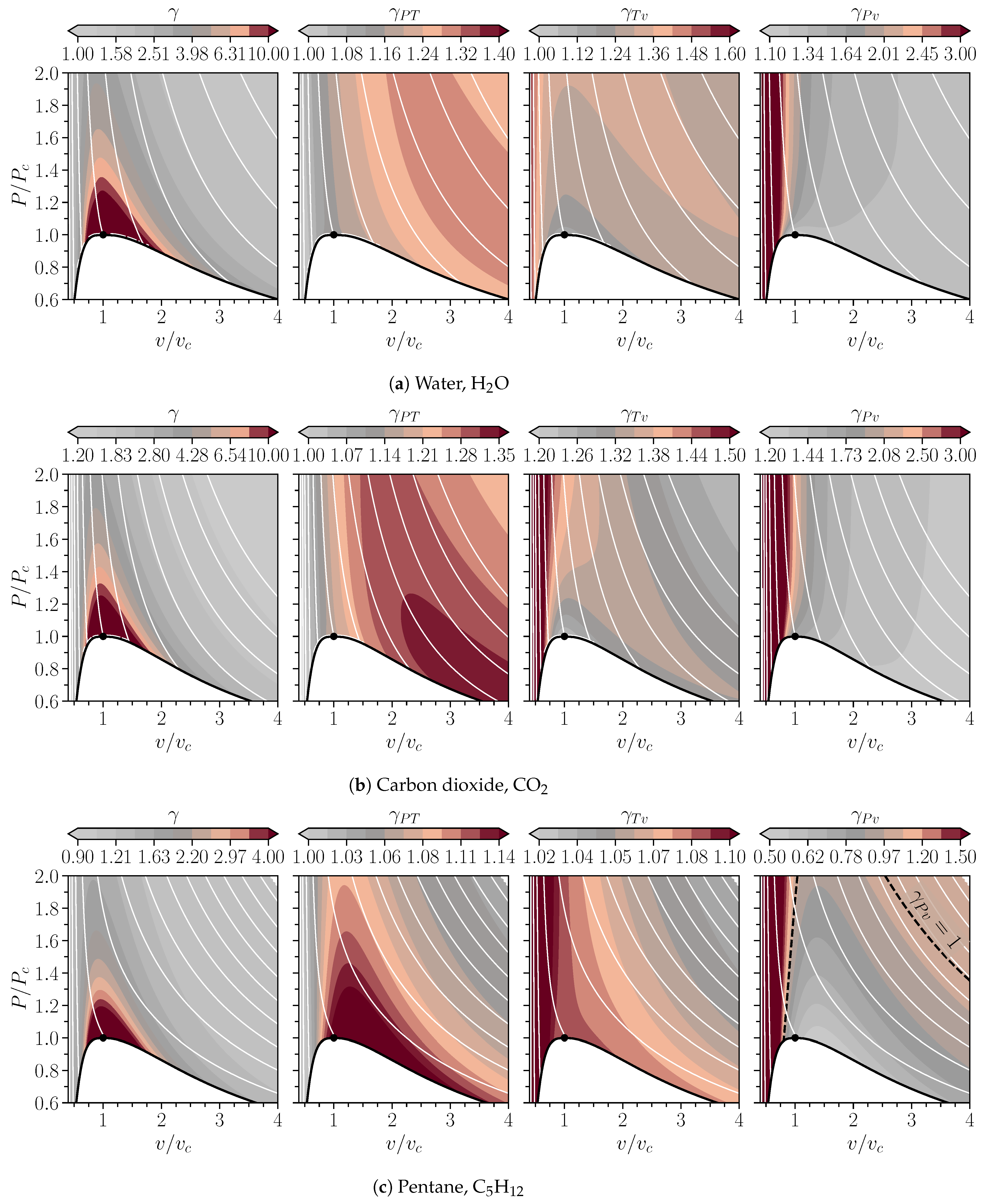
2.3. Isentropic Exponents Plotted in the Pv-Plane for Water, Carbon Dioxide, and Pentane
3. Generalised Speed of Sound, Isentropic Flows Transformations, and Isentropic Work
3.1. Generalised Speed of Sound in Fluids
3.2. Isentropic Transformations for Non-Ideal Compressible Isentropic Flows
3.3. Isentropic Work in Compression and Expansion
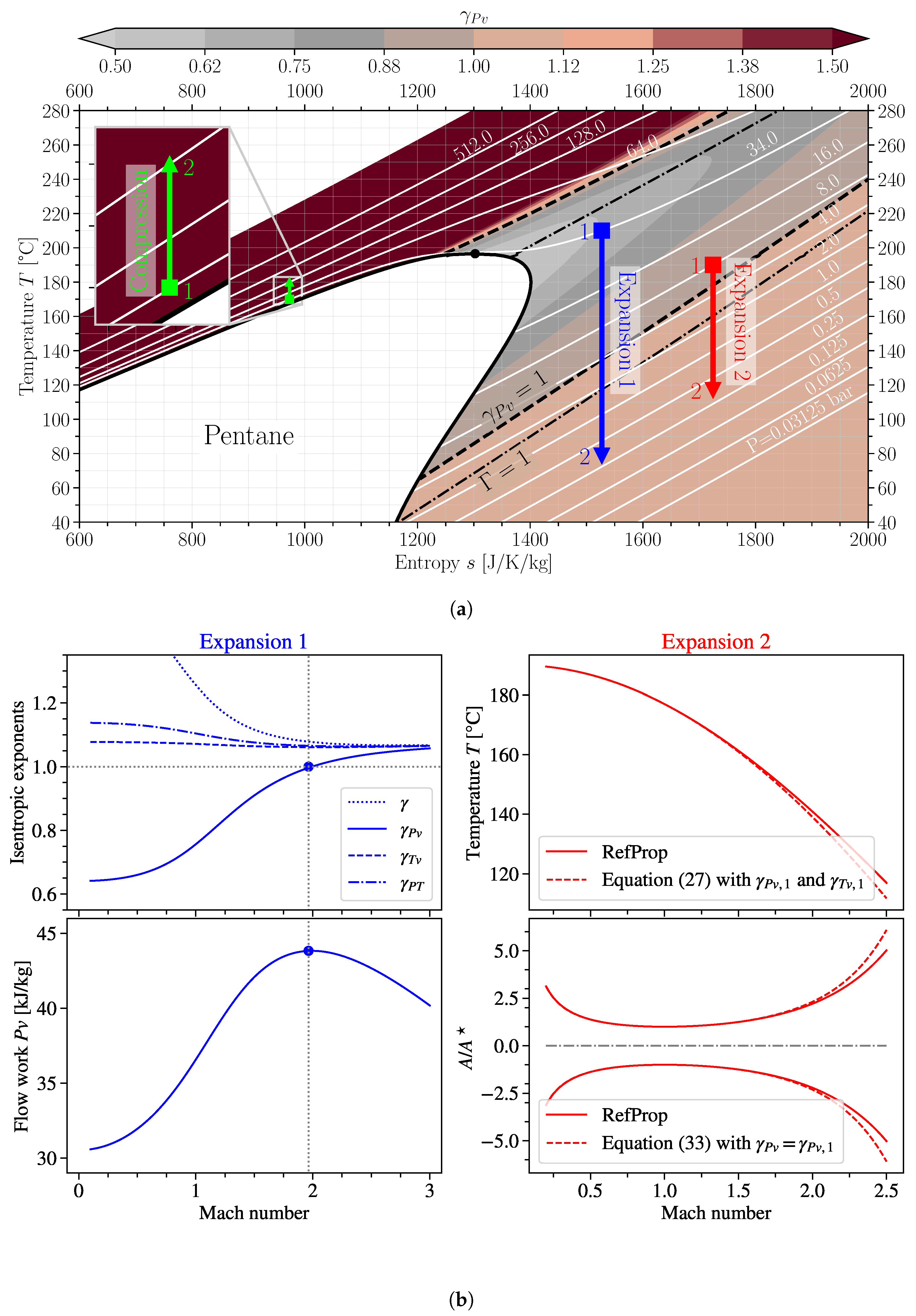
4. Considerations for Non-Ideal Isentropic Relations in Practical Applications
- Compression in the liquid region;
- Expansion 1 in the dense vapour region close to the vapour saturation line, starting from the critical pressure to a low pressure while crossing the line;
- Expansion 2 in the vapour region, again crossing the line, but with a lower pressure ratio.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Liquids | Real Gases | Ideal Gas |
|---|---|---|
| Limits of the isentropic exponents | ||
| Generalised speed of sound relation | ||
| Generalised Bernoulli equation | ||
| Generalised isentropic relations | ||
| Isentropic compression and expansion work | ||
References
- Nan, X.; Himeno, T.; Watanabe, T. The Real Gas Effect on the Stagnation Properties for Supercritical Carbon Dioxide Flows. Int. J. Gas Turbine Propuls. Power Syst. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Yao, H.; Zhao, M.; Liu, Z. A New Method for Impeller Inlet Design of Supercritical CO2 Centrifugal Compressors in Brayton Cycles. Energies 2020, 13, 5049. [Google Scholar] [CrossRef]
- Uysal, S.C.; Liese, E. Radial Compressor Design and Off-Design for Trans-critical CO2 Operating Conditions. In Proceedings of the 7th International Supercritical CO2 Power Cycles Symposium, San Antonio, TX, USA, 21–24 February 2022. [Google Scholar]
- Blondel, Q.; Tauveron, N.; Caney, N.; Voeltzel, N. Experimental Study and Optimization of the Organic Rankine Cycle with Pure NovecTM649 and Zeotropic Mixture NovecTM649/HFE7000 as Working Fluid. Appl. Sci. 2019, 9, 1865. [Google Scholar] [CrossRef] [Green Version]
- Ikäheimo, J.; Weiss, R.; Kiviluoma, J.; Pursiheimo, E.; Lindroos, T.J. Impact of power-to-gas on the cost and design of the future low-carbon urban energy system. Appl. Energy 2022, 305, 117713. [Google Scholar] [CrossRef]
- Bell, I.H.; Wronski, J.; Quoilin, S.; Lemort, V. Pure and Pseudo-pure Fluid Thermophysical Property Evaluation and the Open-Source Thermophysical Property Library CoolProp. Ind. Chem. Res. 2014, 53, 2498–2508. [Google Scholar] [CrossRef] [Green Version]
- Lemmon, E.W.; Bell, I.H.; Huber, M.L.; McLinden, M.O. Standard Reference Database 23, NIST Reference Fluid Thermodynamic and Transport Properties, version 10.0; Standard Reference Data Program: Gaithersburg, Maryland, 2018. [Google Scholar]
- Kouremenos, D.A.; Kakatsios, X.K. The Three Isentropic Exponents of Dry Steam. Forsch. Im Ingenieurwesen 1985, 51, 117–122. [Google Scholar] [CrossRef]
- Kouremenos, D.A.; Kakatsios, X.K. Ideal Gas Relations for The Description of The Real Gas Isentropic Changes. Forsch. Im Ingenieurwesen 1985, 51, 169–174. [Google Scholar] [CrossRef]
- Kouremenos, D.A.; Antonopoulos, K.A.; Kakatsios, X.K. A Correlation of the Isentropic Exponents of Real Gases. Int. J. Heat Fluid Flow 1988, 9, 410–414. [Google Scholar] [CrossRef]
- Kouremenos, D.; Antonopoulos, K. Generalized and exact solutions for oblique shock waves of real gases with application to real air. Int. J. Heat Fluid Flow 1989, 10, 328–333. [Google Scholar] [CrossRef]
- Baltadjiev, N.D. An Investigation of Real Gas Effects in Supercritical CO2 Compressors. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2012. [Google Scholar]
- Nederstigt, P. Real Gas Thermodynamics. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 2017. [Google Scholar]
- Orlando, G.; Barbante, P.F.; Bonaventura, L. An efficient IMEX-DG solver for the compressible Navier-Stokes equations for non-ideal gases. J. Comput. Phys. 2022, 471, 111653. [Google Scholar] [CrossRef]
- Callen, H.B. Thermodynamics and an Introduction to Thermostatistics, 2nd ed.; John Wiley&Sons: Hoboken, NJ, USA, 1985; p. 485. [Google Scholar]
- Pippard, A.B. Elements of Classical Thermodynamics for Advanced Students of Physics, 4th ed.; Cambridge University Press: Cambridge, UK, 1957; p. 165. [Google Scholar]
- Thompson, P.A. A Fundamental Derivative in Gasdynamics. Phys. Fluids 1971, 14, 1843–1849. [Google Scholar] [CrossRef]
- Thompson, P.A. Compressible Fluid Dynamics, 1st ed.; McGraw-Hill: New York, NY, USA, 1972. [Google Scholar]
- Giuffre, A.; Pini, M. Design guidelines for axial turbines operating with non-ideal compressible flows. J. Eng. Gas Turbines Power 2021, 143. [Google Scholar] [CrossRef]
- Wagner, W.; Pruß, A. The IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use. J. Phys. Chem. Ref. Data 2002, 31, 387–535. [Google Scholar] [CrossRef] [Green Version]
- Kunz, O.; Klimeck, R.; Wagner, W.; Jaeschke, M. The GERG-2004 Wide-Range Equation of State for Natural Gases and Other Mixtures. J. Chem. Eng. Data 2012, 57, 3032–3091. [Google Scholar] [CrossRef]
- Rivera-Alvarez, A.; Abakporo, O.I.; Osorio, J.D.; Hovsapian, R.; Ordonez, J.C. Predicting the Slope of the Temperature–Entropy Vapor Saturation Curve for Working Fluid Selection Based on Lee–Kesler Modeling. Ind. Eng. Chem. Res. 2020, 59, 956–969. [Google Scholar] [CrossRef]
- Span, R.; Wagner, W. A New Equation of State for Carbon Dioxide Covering the Fluid Region from the Triple-Point Temperature to 1100K at Pressures up to 800MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef] [Green Version]
- Thol, M.; Dubberke, F.H.; Baumhögger, E.; Vrabec, J.; Span, R. Speed of Sound Measurements and Fundamental Equations of State for Octamethyltrisiloxane and Decamethyltetrasiloxane. J. Chem. Data 2017, 62, 2633–2648. [Google Scholar] [CrossRef]
- Zucrow, M.J.; Hoffman, J.D. Gas Dynamics, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 1976; Volume 1, p. 772. [Google Scholar]
- Kouremenos, D.A. The Normal Shock Waves of Real Gases and The Generalized Isentropic Exponents. Forsch. Im Ingenieurwesen 1986, 52, 23–31. [Google Scholar] [CrossRef]
- Kouremenos, D.A.; Antonopoulos, K.A. Sound velocity and isentropic exponents for gases with different acentric factors by using the Redlich-Kwong-Soave equation of state. Acta Mech. 1987, 66, 177–189. [Google Scholar] [CrossRef]
- Douhéret, G.; Davis, M.I.; Reis, J.C.R.; Blandamer, M.J. Isentropic Compressibilities—Experimental Origin and the Quest for their Rigorous Estimation in Thermodynamically Ideal Liquid Mixtures. ChemPhysChem 2001, 2, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, H.; Burton, R.; Schoenau, G. Fluid Bulk Modulus: A Literature Survey. Int. J. Fluid Power 2011, 12, 5–15. [Google Scholar] [CrossRef]
| Liquids | Real Gases | Ideal Gases | |||
|---|---|---|---|---|---|
| Limits of derivatives and isentropic exponents | |||||
| (a) Thermodynamic states | |||
|---|---|---|---|
| RefProp | Isentropic transformations Equation (2) with and (rel. error) | ||
| State | 1 | 2 | 2 |
| P [bar] | 34 | 128 | |
| T [K] | 443.15 | 453.27 | 450.60 (0.6%) |
| [kg/m] | 433.76 | 480.36 | 472.89 (1.56%) |
| 1.47 | 1.28 | ||
| 1.012 | 1.023 | ||
| 1.193 | 1.250 | ||
| (b) Enthalpy change | |||
| RefProp | (rel. error) | Isentropic Work Equation (41) with (rel. error) | |
| [kJ/kg] | 20.43 | 21.67 (6.1%) | 20.57 (0.69%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nederstigt, P.; Pecnik, R. Generalised Isentropic Relations in Thermodynamics. Energies 2023, 16, 2281. https://doi.org/10.3390/en16052281
Nederstigt P, Pecnik R. Generalised Isentropic Relations in Thermodynamics. Energies. 2023; 16(5):2281. https://doi.org/10.3390/en16052281
Chicago/Turabian StyleNederstigt, Pim, and Rene Pecnik. 2023. "Generalised Isentropic Relations in Thermodynamics" Energies 16, no. 5: 2281. https://doi.org/10.3390/en16052281